Abstract
Clinical impact of biotechnology has been constrained by the limitations of traditional hypodermic injection of biopharmaceuticals. Microneedle patches have been proposed as a minimally invasive alternative. In this study, we assess the translation of a dissolving microneedle patch designed for simple, painless self-administration of biopharmacetucials that generates no sharp biohazardous waste. To study pharmacokinetics and safety of this approach, human growth hormone (hGH) was encapsulated in 600 μm long dissolving microneedles composed of carboxymethylcellulose and trehalose using an aqueous, moderate-temperature process that maintained complete hGH activity after encapsulation and retained most activity after storage for up to 15 months at room temperature and humidity. After manual insertion into the skin of hairless rats, hGH pharmacokinetics were similar to conventional subcutaneous injection. After patch removal, the microneedles had almost completely dissolved, leaving behind only blunt stubs. The dissolving microneedle patch was well tolerated, causing only slight, transient erythema. This study suggests that a dissolving microneedle patch can deliver hGH and other biopharmaceuticals in a manner suitable for self-administration without sharp biohazardous waste.
1. Introduction
The impact of biotechnology on medicine has been limited by the need for hypodermic injection of protein therapeutics and other macromolecular drugs.[1-3] The large molecular size and enzymatic sensitivity of these drugs precludes oral administration, conventional transdermal delivery or absorption via mucosal routes in most cases. Thus, many patients need to visit the clinic for injection by medical personnel which is an inefficient use of resources. Alternatively, patients must be trained to inject themselves, which requires a significant initial investment of time and brings the risks of biohazardous sharp needle waste into patient households[4] and requires many patients to overcome their fear and apprehension of hypodermic needles.[5, 6]
For example, children with short stature or other endocrine disorders require human growth hormone (hGH), which is injected subcutaneously multiple times per week for months to years.[7, 8] As mentioned above, the major clinical problem associated with hGH injection is patient non-compliance or refusal to inject due to needle-phobia.[9] A much better way to administer hGH would enable self-administration without special training, eliminate pain and apprehension, and avoid the generation of sharp, biohazardous waste. Previous attempts to administer hGH without a hypodermic needle, for example using conventional transdermal delivery or intranasal delivery, have had very low bioavailability.[10-12] Here, we present the translation of a dissolving microneedle patch designed for simple, painless self-administration of hGH that generates no sharp biohazardous waste.
Microneedle patches have been developed to combine the convenience and safety of transdermal patches with the hypodermic needle's ability to deliver macromolecules.[13, 14] These microneedles measure hundreds of microns long, which is sufficient to cross the skin's permeability barrier of stratum corneum[15], but is short enough to avoid causing pain.[16] Previous generations of microneedles have been made of non-water-soluble materials such as silicon, metals, and organic polymers and have been used either to pierce the skin to increase skin's permeability or prepared with a drug coating that dissolves off upon insertion into skin.[17-21] Initial efforts to encapsulate drugs within microneedles required harsh processing conditions incompatible with sensitive biomolecules. Recently, we and others have developed microneedles made of water-soluble materials that dissolve in the skin and thereby leave no sharp biohazardous waste.[22-25]
In this study, we seek to utilize our dissolving microneedle technology to assess its translation into a patch for administration of hGH. We selected carboxymethylcellulose (CMC) and trehalose as water-soluble matrix materials of the microneedle patch, both of which are safely used in the body in FDA-approved formulations.[26, 27] CMC was included in the formulation to provide mechanical strength and trehalose was added to increase microneedle dissolution rate. Using this approach, this study presents hGH delivery using a dissolving microneedle patch designed for simple, painless self-administration that generates no biohazardous sharp waste. By addressing questions about hGH stability, bioavailability and safety, we seek to translate the use of dissolving microneedle patches for eventual clinical administration of hGH, as well as other protein therapeutics.
2. Results
2.1. Fabrication of dissolving microneedle patch encapsulating hGH
Design and fabrication of a dissolving microneedle patch for hGH administration required an interdisciplinary approach involving microfabrication and pharmaceutics in order to enable gentle encapsulation, self-administration, effective delivery into skin, and safe disposal. We first prepared a master microneedle structure using UV lithography and reactive ion etching, as shown in Figure 1A-1B. The master structure was then used to cast an inverse mold out of polydimethylsiloxane (PDMS). To facilitate reliable insertion into skin, each microneedle was designed to be 600 μm long with a tip radius measuring less than 10 μm. To provide sufficient mechanical strength, microneedles tapered down to a base measuring 300 μm wide.
Figure 1.
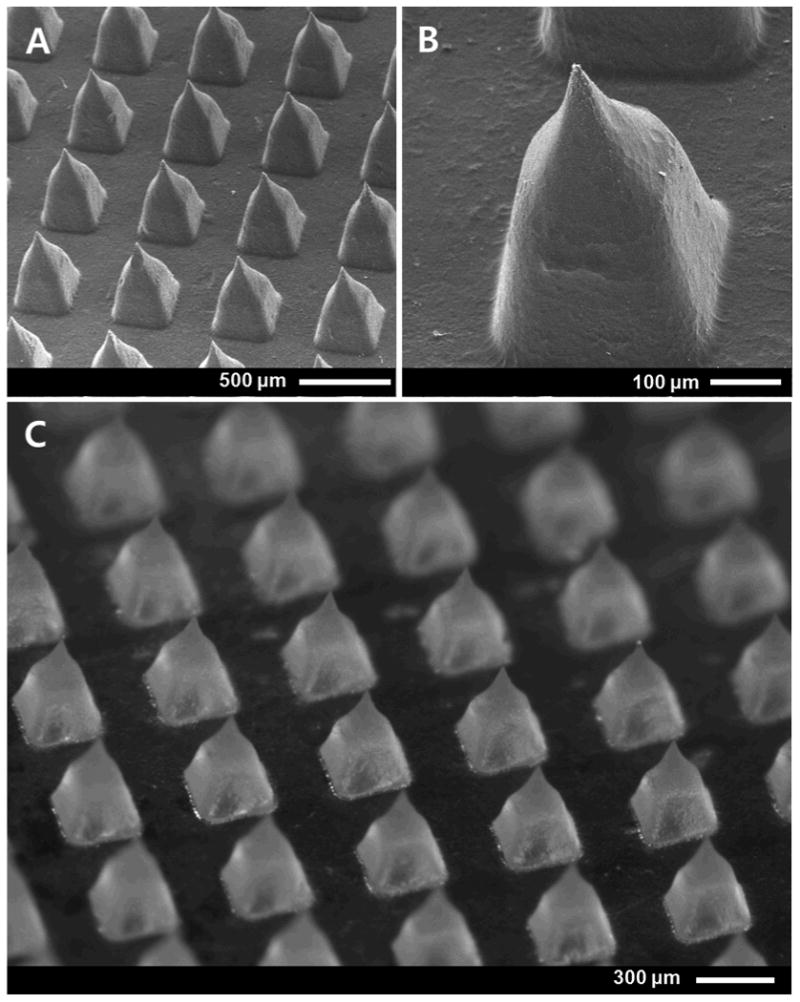
Dissolving microneedles patch. Scanning electron micrograph of (A) an array of microneedles and (B) a further magnified view of a single microneedle in a master structure microneedle patch. (C) Brightfield micrograph of a CMC/trehalose dissolving microneedle patch encapsulating hGH, which was molded from the master structure.
Micromolds were then cast with an aqueous solution of hGH in CMC or CMC-plus-trehalose and allowed to dry at 35°C under centrifugation in order to remove voids formed during water evaporation[24], as shown in Figure 1C. Microneedles were prepared this way either as a one-step process, in which hGH was encapsulated within the microneedles and the patch backing, or as a two-step process, in which the cavities of the mold were first filled with a casting solution containing hGH to form the microneedles and then covered with a casting solution lacking hGH to form the patch backing. This mild-temperature, water-based fabrication method was designed to minimize damage to hGH during processing and to serve as a low-cost approach suitable for scale up to mass production.
2.2. Functional activity of hGH
To assess biocompatibility of the fabrication processes, the stability of hGH encapsulated in dissolving microneedle patches was assessed by an established cell proliferation assay.[28, 29] As shown in Figure 2, exposure of Nb2 cells to unprocessed hGH (positive control) stimulated cell growth as a function of hGH concentration. Addition of CMC to unprocessed hGH stimulated cell growth equally well, which indicates that CMC had no deleterious effect on hGH activity or cell proliferation. As a negative control, reconstitution of CMC microneedles (containing no hGH) and incubation with Nb2 cells did not stimulate cell growth, further indicating that CMC was inert.
Figure 2.
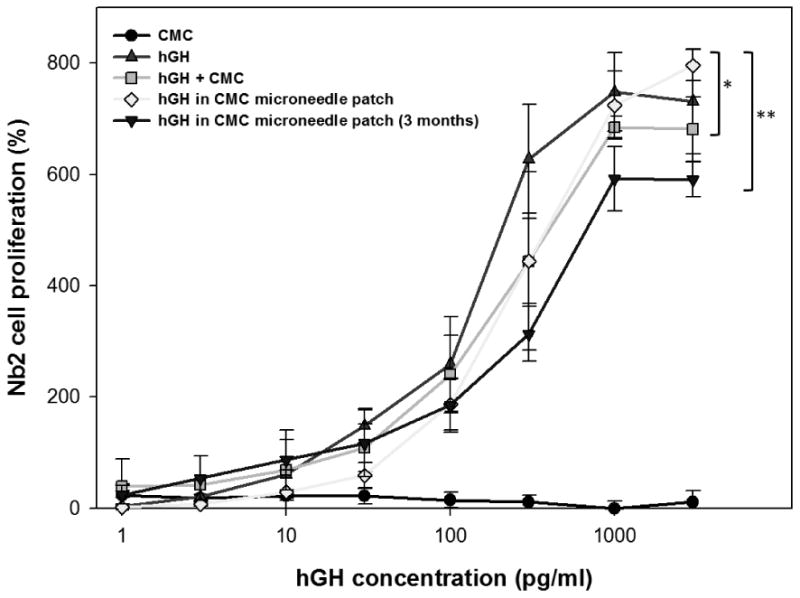
hGH stability after encapsulation in a dissolving microneedle patch. The addition of hGH to Nb2 cell culture in the stationary phase stimulated the proliferation of Nb2 cells, which was recorded at 3 days after hGH treatment and used as a measure of the functional activity of hGH after encapsulation in dissolving microneedles. Five experimental groups were studied: CMC solution by the reconstitution of a placebo CMC microneedle patch (CMC, negative control, ●), hGH solution (hGH, positive control, ▲), hGH solution mixed with CMC reconstituted from a placebo CMC microneedle patch (hGH + CMC, ▪), hGH and CMC solution reconstituted from a CMC microneedle patch encapsulating hGH (hGH in CMC microneedle patch, ◆), and hGH and CMC solution reconstituted from a CMC microneedle patch encapsulating hGH after storage for 3 months at ambient conditions (hGH in CMC microneedle patch (3 months), ▾). All groups contain CMC and hGH at the same mass ratio (5 hGH: 95 CMC) at all hGH concentrations, except the positive control. Asterisk indicates comparison with the hGH positive control (two-way ANOVA, p > 0.05 for * and p < 0.05 for **). Data points represent the average ± standard deviation for n=4 replicates for all groups.
Reconstitution of CMC microneedle patches encapsulating hGH was not significantly different from the positive control, showing that the encapsulation and release of hGH from the CMC microneedle patches was not detrimental to hGH activity. Moreover, storage of CMC microneedle patches encapsulating hGH for 3 months in air at ambient temperature (23°C) and relative humidity (∼30%), caused only a 15% loss of hGH activity. We expect that storage under vacuum, refrigeration and/or dehumidification could further minimize loss of hGH activity. Altogether, this demonstrates the suitability of the formulation and fabrication process to protect hGH activity for subsequent administration to the skin.
2.3. Pharmacokinetics of hGH administered by dissolving microneedle patch
This study hypothesized that a dissolving microneedle patch can administer hGH via the skin after insertion and dissolution of the microneedle matrix. To assess this, we administered hGH via five different methods and measured plasma concentration of hGH over time in the hairless rat.
As shown in Figure 3, hGH administered by any of the methods quickly rose to a peak hGH plasma concentration at tmax ≈ 0.7 h (ANOVA, p > 0.05) and then sharply decreased over the next 6 hours with a half-life of t½ ≈ 1.1 h (ANOVA, p > 0.05) (Table 1). Although the shape of the pharmacokinetic profile was the same by each method, the absolute hGH concentrations, and hence bioavailability differed.
Figure 3.
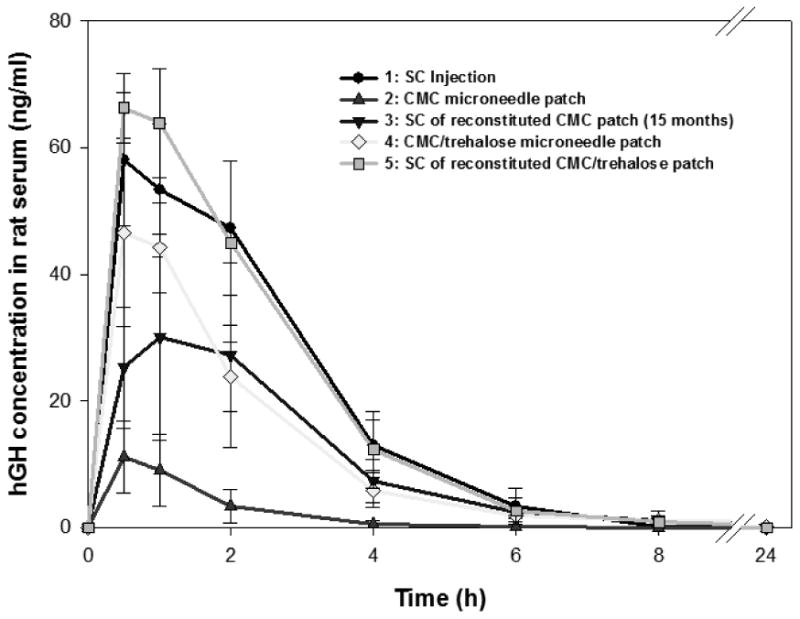
Pharmacokinetic profile of hGH in rat serum after hGH administration using microneedles. Five experimental groups were studied to assess different methods of hGH administration: (1) subcutaneous injection (●), (2) CMC microneedle patch (▲), (3) subcutaneous injection of reconstituted CMC microneedle patch after 15 months storage at ambient conditions (▾), (4) CMC/trehalose microneedle patch (◆), and (5) subcutaneous injection of reconstituted CMC/trehalose microneedle patch (▪). Data represent the average ± standard deviation, n=6 for all groups except Group 3 (n=3).
Table 1.
Pharmacokinetic parameters of hGH delivery to rats.
| Group[g] | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Administered dose (μg)[a] | 169 ± 10 | 148 ± 5 | 162[h] | 164 ± 14 | 167 ± 15 |
| Cmax (ng/ml)[b] | 60 ± 12 | 12 ± 5* | 31 ± 15* | 49 ± 12 | 68 ± 7 |
| tmax (h)[c] | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.8 ± 0.3 | 0.6 ± 0.2 | 0.7 ± 0.3 |
| t1/2 (h)[d] | 1.2 ± 0.2 | 0.9 ± 0.3 | 1.4 ± 0.1 | 1.1 ± 0.2 | 1.1 ± 0.3 |
| AUC (h·ng/ml)[e] | 174 ± 35 | 20 ± 13* | 101 ± 49* | 120 ± 28* | 181 ± 26 |
| BA (%)[f] | 100 ± 20 | 13 ± 8* | 61 ± 29* | 71 ± 17* | 106 ± 15 |
The applied dose was measured with ELISA (n=4).
Cmax is the maximum hGH concentration.
tmax is the time recorded as the peak concentration of hGH, Cmax
t1/2 is half-life of hGH concentration relative to Cmax.
Area under the curve (AUC) was calculated from Fig. 3 using the trapezoid method.
Bioavailability (BA) was calculated as (AUCgroup/Dosegroup)/(AUCgroup 1./Dosegroup 1)
The group numbers are the same as in Figure 3: (1) SC injection, (2) CMC microneedle patch, (3) SC injection of reconstituted CMC patch (after 15 months storage), (4) CMC/trehalose microneedle patch, (5) SC injection of reconstituted CMC/trehalose patch.
The Group 3 dose is the original amount of hGH encapsulated in the microneedles on dry basis, not the amount of active hGH after storage.
Significantly different from Group 1 (Student's t-test, p < 0.05)
Subcutaneous injection of hGH was used as the positive control corresponding to 100% bioavailability (Figure 3). Dissolution of a microneedle patch in vitro followed by subcutaneous injection yielded a pharmacokinetic profile (ANOVA, p > 0.05) and bioavailability (Student's t-test, p > 0.05) indistinguishable from the positive control, which is consistent with our in vitro finding that the process of hGH encapsulation does not damage hGH bioactivity.
We next studies hGH administration using microneedle patches prepared with two different formulations. The first formulation contained only CMC as the microneedle material. In this case, plasma hGH concentration was significantly lower than the positive control, corresponding to a peak hGH concentration, Cmax, that was 20% of the positive control (Student's t-test, p < 0.05) and a bioavailability of 13%.
We hypothesized that this low bioavailability may be due to slow and incomplete dissolution of the CMC microneedle matrix in the limited amount of the interstitial fluid in the skin, which thereby limited release of encapsulated hGH. To test this hypothesis, we prepared microneedles having a matrix formulated with a mixture of CMC and trehalose, which dissolves more quickly and with less fluid than CMC. Consistent with this hypothesis, CMC/trehalose microneedles achieved a Cmax that was four times higher and a bioavailability that was five times higher than CMC microneedles (Student's t-test, p < 0.05).
We next imaged microneedles after 24 h insertion in skin to determine if CMC/trehalose microneedles indeed dissolved more extensively. As shown in Figure 4, CMC microneedles were partially dissolved, such that only the tip was dissolved and much of the microneedle shaft remained. In contrast, CMC/trehalose microneedles were mostly dissolved, leaving just short stubs behind. These post-application microneedle patches were then dissolved in vitro and analyzed to determine residual hGH content, which was found to be 69±3% of the initial dose in CMC microneedle patches and 17±3% of the initial dose in CMC/trehalose microneedle patches.
Figure 4.
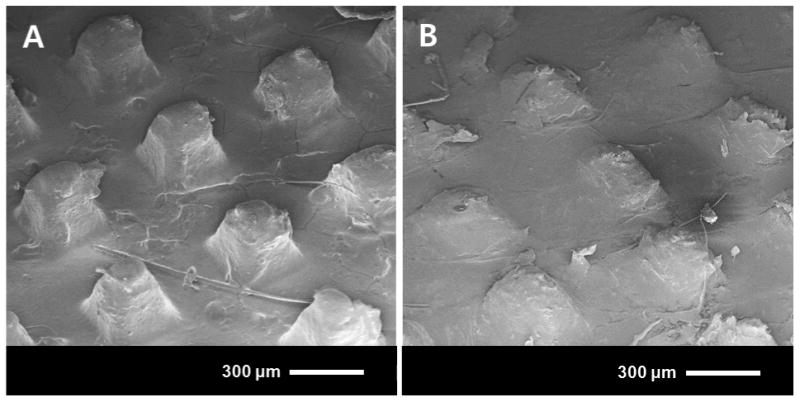
Scanning electron micrographs of dissolving microneedles after 24 h insertion into hairless rat skin in vivo/. (A) CMC microneedle patch. (B) CMC/trehalose microneedle patch.
This confirmed the expectation that CMC/trehalose microneedles were able to dissolve more completely and provides an explanation for the reduced bioavailability using microneedles. In future studies, microneedle design could be modified to increase insertion depth more fully into the skin, to incorporate a more water-soluble formulation or to localize hGH more toward the tip and away from the base. These, or other modifications, could increase hGH bioavailability using microneedles.
The final group in our pharmacokinetics study was designed to assess functional stability of microneedles during storage. We reconstituted hGH from a CMC microneedle patch after 15 months storage at room temperature and humidity and injected it subcutaneously, which produced a bioavailability of 61±29%. This remarkable stability may be due to storage of hGH by coupling to the rigid glassy microneedle matrix[30], which limits molecular mobility and contact with oxygen from the air. In contrast, commercially formulated hGH is lyophilized and must be stored under refrigeration without humidity or oxygen, both of which lead to degradation of hGH integrity.[31] Packaging microneedles without humidity or oxygen could further stabilize hGH during storage, as would storage at reduced temperature.
2.4. Skin resealing after microneedle administration
In this study, dissolving microneedles were used to pierce the skin barrier and deposit hGH in the skin. Previous studies have shown that skin reseals quickly after insertion of non-dissolving microneedles.[32] We therefore determined the kinetics of skin resealing after insertion of dissolving microneedles using skin electrical impedance as a measurement previously shown to correlate with the skin's permeability barrier.[33]
As shown in Figure 5, impedance of untreated skin was initially around 105 kΩ·cm2. In the negative control (Group A), which was untreated, but covered continuously for 24 h with a measurement electrode, skin impedance dropped to less than 102 kΩ·cm2 and remained low due to the known effect of increased skin hydration due to extended occlusion by the electrode[34]. After the occluding electrode was removed from the skin at 24 h, skin impedance returned to original levels because the electrode was only applied briefly at the time of each measurement.
Figure 5.
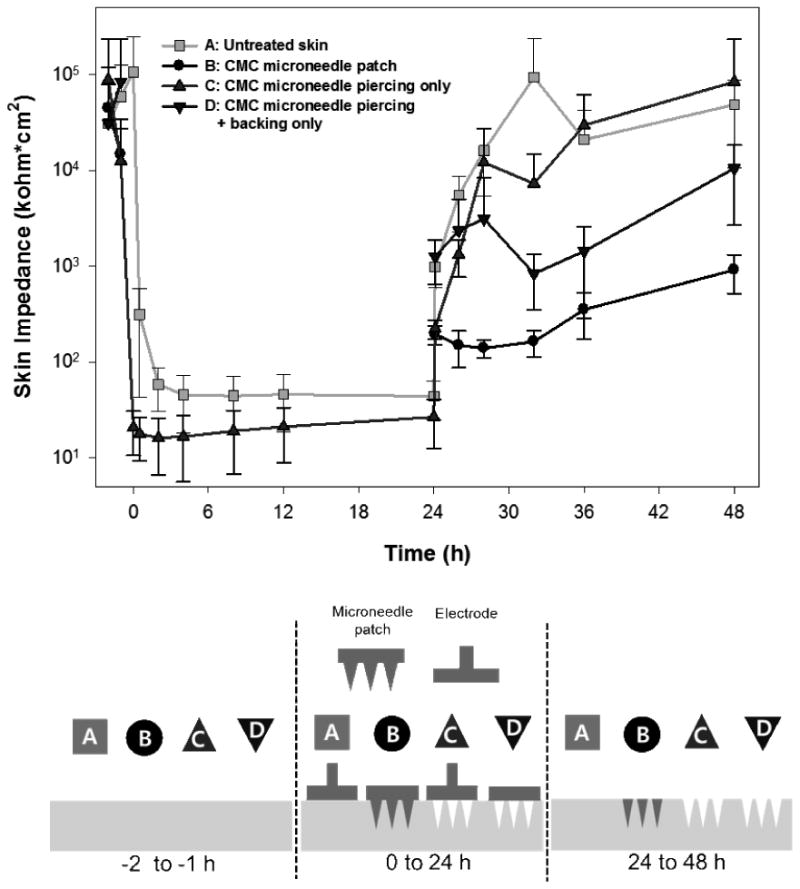
Recovery of skin barrier function after insertion of a dissolving microneedle patch into hairless rat skin in vivo. The electrical impedance of untreated skin was measured before treatment of each group to serve as an internal negative control. The experimental groups represent: (A) untreated skin (negative control) (B) skin treated with a CMC microneedle patch inserted for 24 h, (C) skin treated with a CMC microneedle patch inserted for 3 s and then removed and (D) skin treated with a CMC microneedle patch inserted for 3 s and removed, and then covered with a needle-free CMC patch backing. All groups were occluded for the first 24 h and unoccluded for the next 24 h. Data represent the average ± standard deviation, n=6 for each group.
Guided by this baseline measurement, a CMC microneedle patch (Group B) was applied to the skin for 24 h. Impedance measurements could not be made during this time because the patch was in the way. Immediately after removing the patch at 24 h, skin impedance had dropped to approximately 102 kΩ·cm2, due to the combined effects of microneedle penetration into the skin and extended occlusion by the patch. Over time after patch removal, skin impedance increased (ANOVA, p < 0.05), but always remained lower than untreated skin (ANOVA, p < 0.05) and did not return to pre-treatment values over the timescale of the study (Student's t-test, p < 0.05).
To better understand the effects of microneedle insertion into skin, we applied a CMC microneedle patch (Group C) to the skin and then immediately removed it. In this way, the microneedles punctured holes in the skin, but did not dissolve in the skin. The skin impedance of Group C decreased sharply from pre-treatment values and remained lower than the negative control, Group A, while occluded (ANOVA, p < 0.05) due to the increased skin permeability caused by insertion of microneedles. However, after the 24 h occlusion, skin impedance of Groups A and C increased rapidly and both groups were statistically indistinguishable (ANOVA, p > 0.05), indicating complete recovery of the skin barrier.
As a final comparison, we again pierced the skin transiently with a CMC microneedle patch and then occluded the skin with a separate needle-free CMC patch backing (Group D). In this way, the microneedles punctured holes in the skin, but did not dissolve in the skin, and the site remained occluded. Once again, impedance measurements could not be made during the first 24 h due to interference of the applied patch. After removal of the occlusion, skin impedance of Group D showed recovery that was significantly less than the untreated control, Group A (ANOVA, p < 0.05) and significantly more than the microneedle patch, Group B (ANOVA, p < 0.05). This indicates that the skin resealing is delayed not only by microneedle dissolution in the skin, but also by presence of the patch backing.
By comparing these different experimental groups, we can isolate the effects of (i) microneedles puncturing the skin, (ii) microneedles dissolving within those holes in the skin, (iii) a dissolving patch backing applied over holes in the skin, and (iv) the measurement artifact created by skin occlusion. By comparing Groups A and C, we find that puncturing skin with microneedles reduces the skin barrier, as expected, and recovers within the 24 h time resolution of this study design. Comparing Groups B and C shows that microneedle dissolution within the skin delays skin recovery, probably due to the presence of dissolved CMC at high concentration within the microneedle hole. We hypothesize that the use of CMC/trehalose microneedles might delay skin resealing to a lesser extent, due to the increased water solubility and diffusivity of trehalose compared to CMC, which could clear the microneedle hole of matrix material more quickly. Finally, comparison of Groups A and D shows that the patch backing delays barrier recovery more than occlusion with an electrode, which suggests that the patch backing may partially dissolve in the skin and thereby retard resealing. These findings suggest that the rate of skin barrier recovery can be manipulated based on microneedle patch design. Future studies will be required to determine if the long-lived skin barrier disruption caused by dissolving microneedles poses a possible increase risk of infection, or if the CMC-filled holes block entry of bacteria and viruses, which are orders of magnitude larger than the ions whose movement was assessed through our electrical impedance analysis.
2.5. Skin reaction to microneedle administration
As an additional assessment of safety, skin reaction to hGH delivery using microneedles was determined by measuring erythema and edema on a 4-point scale[35] and imaging the skin (Supplementary Figure S1). There was no skin edema observed in any of the experimental groups. As shown in Supplementary Figure S2, the subcutaneous injection groups also exhibited no erythema at any time from 24 h after injection. CMC and CMC/trehalose microneedle patches showed slight erythema upon patch removal (Student's t-test, p < 0.05), which decreased with time (ANOVA, p < 0.05) and returned to zero within 48 h after patch removal. The CMC microneedle patch initially caused more erythema than the CMC/trehalose formulation initially (ANOVA, p < 0.05), but there was no significant difference from 6 h after patch removal. In all cases, erythema was only slight and transient, and does not appear to be a significant safety or cosmetic concern, although additional studies, especially in humans, will be needed. Previous studies with non-dissolving microneedles have also shown slight erythema, but it has reversed more quickly.[16] Based on the lifetime of skin erythema (Supplementary Figure S2) and of skin impedance barrier disruption (Figure 5), we hypothesize that complete dissolution and dispersal of the microneedle dissolution products takes 1 – 2 days.
Histological sections from skin biopsies taken 24 h, 48 h, and 1 week after treatment showed no remarkable features in skin exposed to subcutaneous injection of hGH. Skin treated with CMC or CMC/trehalose microneedle patches exhibited signs of highly localized stratum corneum disruption and inflammatory response at the apparent sites of individual microneedle penetration and dissolution (Supplementary Figure S3 and S4). These micron-scale features are consistent with reduced stratum corneum barrier function and slight, punctuate erythema reported above.
3. Discussion
This work presents a detailed study of hGH delivery using a dissolving microneedle patch designed for simple, painless self-administration that generates no biohazardous sharp waste. Conventional administration of biopharmaceuticals like hGH requires hypodermic injection, which requires patients to either visit the clinic for each injection or receive training to self-administer the medication. Perhaps the greatest potential impact of the dissolving microneedle patch described in this study is the ability to replace hypodermic needles and thereby empower patients to self-administer biopharmaceuticals by reducing patient apprehension, removing the need for expert administration and minimizing safety concerns.
This study addressed delivery of hGH because this biopharmaceutical is conventionally administered to children by hypodermic injection multiple times per week for up to years. Currently, there are approximately 20,000 patients per year in the United States taking hGH and approximately 4,000 per year are estimated as new candidates for hGH treatment.[36] Thus, hGH delivery serves not only as a useful model compound to study delivery of biopharmaceuticals using dissolving microneedles, but also represents a drug that could significantly benefit from administration using a microneedle patch.
A dissolving microneedle patch can provide a number of safety advantages compared to hypodermic needles. First, dissolving microneedle patches generate no biohazardous sharp waste, because the microneedles dissolve in the skin and disappear (Figure 4). Indeed, they have the potential to generate no medical waste at all, because the patch backing is also made of water-soluble polymer that can easily be eliminated by dissolving in water in the toilet or sink. This safety feature removes the risk of accidental needle-stick injury or intentional reuse of needles, which is common in some developing countries and is responsible for close to one million deaths per year due to transmission of hepatitis B, HIV and other infectious diseases[4].
Safe use in the skin was addressed in this study and suggested that dissolving microneedles can be safely inserted into the skin. Only slight, transient erythema without edema was observed after microneedle treatment. However, it may be of concern that the skin barrier was slow to fully recover and associated with highly localized inflammatory responses, which we hypothesize, were due to slow clearance of residual microneedle matrix material in the skin. Changing microneedle formulation to comprise materials more readily cleared from the skin could increase the rate of local skin recovery.
A critical element of dissolving microneedle design was the encapsulation of hGH without damaging its functional integrity. By designing a moderate-temperature, water-based fabrication process, we found that hGH retained full activity after microneedle fabrication as assessed both in vitro and in vivo. After extended storage for months, some loss of activity was measured. This stability assessment was carried out using microneedles formulated only with CMC. The addition of trehalose to the formulation, which was done in this study primarily to expedite dissolution time, may also further increase hGH stability, because trehalose is known to stabilize biomolecules during storage.[37] For further reference, the suggested shelf life for commercial lyophilized hGH is 2 years at 2-8°C and the reconstituted solution can be stored for approximately 2 weeks at 2-8°C.[38] Mechanistic studies have shown that hGH is sensitive to degradation by oxidation, for example, storage of lyophilized hGH in the presence of approximately 0.75% oxygen at room temperature for 6 months resulted in 12% decomposition by oxidation.[31] In this study, hGH encapsulated in dissolving microneedle patches was exposed to air containing 21% oxygen at 23°C and lost 15% and 40% activity after 3 or 15 months, respectively. It therefore appears that hGH encapsulated in air-dried microneedles affords similar stability as conventional lyophilized hGH and that storage in inert gas or under vacuum that reduces oxidative damage could enable long-term stability of hGH in dissolving microneedle patches.
Bioavailability of hGH administered by a dissolving microneedle patch was approximately 30% lower than subcutaneously injected hGH, which was shown to be caused by incomplete insertion and dissolution of the microneedles in the skin. More complete microneedle dissolution could be achieved by improving the microneedle formulation by further increasing matrix material water solubility, by modifying microneedle geometry and insertion method to increase the depth of penetration into skin, and by localizing drug more toward the microneedle tip.
In conclusion, this study addresses the limitations of hypodermic injection of biopharmaceuticals by presenting a dissolving microneedle patch designed for safe and simple delivery of hGH for self-administration by patients. Microneedle patches were shown to encapsulate hGH without loss of functional activity and to exhibit good stability after storage up to 15 months in air at room temperature. Our data in rats demonstrated 71% bioavailability of hGH, where the remaining hGH was mostly accounted for in the patch due to incomplete dissolution of microneedles. Treatment with dissolving microneedles was well tolerated by the skin with only slight and transient erythema. The skin barrier stayed open for up to 2 days after patch removal, which was probably due to residual microneedle matrix in skin. Overall, this study demonstrates the feasibility of a dissolving microneedle patch for delivery of hGH and other biomolecules.
4. Experimental Section
4.1. Fabrication of dissolving microneedle patch
Dissolving microneedles were fabricated using molding techniques and a modified solvent-casting method described previously.[24, 39] First, microneedle master structures were fabricated by using UV photolithography processes with SU-8 photoresist (SU-8 2025, Microchem, Newton, MA), as shown in Figure 1A-1B. Then, an inverse mold was created by casting master structures in polydimethylsiloxane (PDMS, Sylgard 184, Dow Corning, Midland, MI).
To serve as the CMC microneedle matrix material, ultra-low viscosity carboxymethylcellulose (CMC, Cat No. 360384, Aldrich, Milwaukee, WI) was dissolved in deionized water and then dehydrated to form a viscous hydrogel, the concentration of which was approximately 25% w/v. To make CMC/trehalose microneedles, CMC and D-(+)-trehalose dihydrate (Cat No. T9531, Sigma, St. Louis, MO) were mixed at a ratio of 1:1 and similarly concentrated to approximately 25% w/v. Then, recombinant human growth hormone (hGH, Genotropin, Pfizer, Groton, CT) was added by hand mixing to form a homogeneous mixture in the concentrated hydrogel containing human growth hormone and matrix material at a mass ratio of 1:9.
To encapsulate hGH within both the microneedle shaft and the patch backing, the hydrogel containing hGH was cast onto the mold and dried under centrifugation, as described previously[24] and shown in Figure 1C. To encapsulate hGH within the microneedle shaft only, the hydrogel containing hGH was cast into the mold cavities only and then pure hydrogel without hGH was applied onto the mold surface to form the hGH-free patch backing, and then the system was dried under centrifugation. For the skin resealing study, patches without microneedles were made by applying only pure hydrogel without hGH onto a mold without microneedle cavities.
4.2. Animal model
Wild-type male hairless rats (10-11 weeks old, 280-340 g, CD Hairless Rat, Charles River, Wilmington, MA) were used for in vivo hGH delivery experiments, with approval by the Institutional Animal Care and Use Committee of Georgia Tech. They were anesthetized with isoflurane (ISOTHESIA, Butler Animal Health Supply, Dublin, OH) during insertion of microneedles, measurement of skin impedance for the skin resealing study and drawing blood for the pharmacokinetics study. For the skin reaction study, skin treated with microneedles was observed for up to 1 week and then excised after euthanasia, fixed using formalin, embedded with paraffin, and sectioned for histology.
4.3. Functional activity of hGH
hGH functional activity was determined by calibrated measurement of hGH-stimulated growth of Nb2 rat lymphoma cells[28, 29], purchased from Sigma-Aldrich. This study was composed of three phases; (1) cell growth in the growth-promoting culture medium to determine the normal growth rate of Nb2 cells for 3 days, (2) transfer of Nb2 cells to the stationary culture medium to suppress cell growth for 1 day and (3) addition of hGH test solutions to Nb2 cells incubated in the stationary culture medium to measure cell proliferation induced by hGH for 3 days. All types of culture medium were prepared by following the methods described previously.[28] The number of viable Nb2 cells at each phase was measured using a cell viability analyzer (Vi-CELL, Beckman Coulter, Miami, FL). Five different hGH test solutions were added to the Nb2 cell culture in phase 3; (i) Placebo solution having only CMC reconstituted from a microneedle patch (negative control), (ii) hGH solution (Genotropin, positive control), (iii) hGH solution mixed with CMC reconstituted from a microneedle patch, (iv) hGH and CMC solution reconstituted from a CMC microneedle patch encapsulating hGH, and (v) hGH and CMC solution reconstituted from a CMC microneedle patch encapsulating hGH after 3 months storage at ambient conditions (23±2°C and 38±5% relative humidity). For all groups, the mass ratio of hGH to CMC in the whole system is 5:95, which means that 5 wt% hGH was loaded into the CMC microneedle patch.
4.4. Pharmacokinetics of hGH
The pharmacokinetic study involved five groups of hairless rats, as summarized in Supplementary Figure S5. Group 1 was administered a subcutaneous injection of 169 ± 10 μg hGH in the Genotropin formulation as received from the manufacturer, which served as the positive control. Group 2 was administered a CMC microneedle patch encapsulating 148 ± 5 μg hGH. Group 3 was administered a subcutaneous injection of a reconstituted CMC microneedle patch encapsulating 162 μg hGH after 15 months storage at ambient conditions (23±2°C and 38±5% relative humidity). Group 4 was administered a CMC/trehalose microneedle patch encapsulating 164 ± 14 μg hGH. Finally, Group 5 was administered a subcutaneous injection of a reconstituted CMC/trehalose microneedle patch encapsulating 167 ± 15 μg hGH. Subcutaneous injections were performed using a 27G hypodermic needle (Becton Dickinson, Franklin Lakes, NJ) inserted into the center of the back and dissolving microneedle patches were inserted into the lower part of the back by gentle pressing with a thumb, as described previously.[24]
In groups 2 and 4, microneedle patches were covered with a dressing (Tegaderm, 3M Health Care, St. Paul, MN), and then the animal's torso was bandaged with self-adherent wrap (Coban, 3M Health Care) and affixed with adhesive tape (Zonas; Johnson and Johnson, Skillman, NJ). The microneedles were secured in this way for 24 h to facilitate drug delivery during microneedle dissolution and prevent patch removal or disturbance by the animals. In some cases, a rodent e-collar (404 ¼VS, Webster Veterinary, Sterling, MA) was placed around the animal's neck.
At 0, 0.5, 1, 2, 4, 6, 8, 12, and 24 h after hGH administration, 120 μl of blood was drawn from the saphenous vein in the tail by a minor incision with a surgical blade and collected in a CAPIJECT tube (T-MG, Terumo Medical, Elkton, MD). The collected blood was left at room temperature for 2 h and then spun at 1200×g for 10 min (5415 R centrifuge, Eppendorf, Westbury, NY) to isolate serum, which was transferred into a 0.5 ml conical tube. After storage at -70°C, hGH concentration was determined by enzyme-linked immunosorbent assay (ELISA) and a microplate reader (iMark, Bio-Rad, Hercules, CA) using a kit specific for hGH without cross-reaction with endogenous rat growth hormone (ACTIVE DSL-10-1900, Diagnostic Systems Laboratories, Webster, TX). Areas under the concentration curve (AUC) were computed by the trapezoid method and used to calculate bioavailability.
4.5. Skin impedance measurements
Skin resealing after insertion of CMC microneedle patches was monitored by measuring skin electrical impedance by adapting a method from a related study carried out in humans.[40] An electrical impedance meter, (Prep-Check EIM-105, General Devices, Ridgefield, NJ) applied 30 Hz AC current between a reference electrode (4.5 cm2) coated with highly conductive gel (Superior Silver Electrode with PermaGel, Uni-Patch, Wabasha, MN) and an adjacent, disposable Ag/AgCl dry electrode (0.8 cm2) (T3404, Thought Technology, Stens Corporation, San Rafael, CA) at the site of microneedle insertion. The skin impedance was measured in 4 groups. Group A received no treatment (negative control). Group B was administered a CMC microneedle patch left in place for 24 h. Group C was administered a CMC microneedle patch that was inserted and then removed within 3 s, i.e., before significant microneedle dissolution could occur. Finally, Group D was administered a CMC microneedle patch that was inserted and removed within 3 s, and then covered with a CMC patch backing (i.e., without microneedles attached) for 24 h. In all cases, the skin was occluded using bandages as described above for the first 24 h and then unoccluded for the second 24 h. In Groups A and C, skin impedance was monitored for the full 48 h experiment, where the measurement electrode was left in contact with the skin for the first 24 h under occlusion and only briefly contacted the skin for each measurement during the second 24 h without occlusion. In Groups B and D, skin impedance was measured only during the second 24 h period, because the presence of the microneedle patch backing prevented impedance measurements during the first 24 h.
4.6. Skin reaction
To study skin reactions after treatment with hGH dissolving microneedles, the insertion site was imaged by digital photography (FZ50, Panasonic, Tokyo, Japan) at 24, 27, 30, 36, and 48 h and then daily for 5 additional days to assess erythema and edema using a 0-4 point scale for each[35]. After euthanasia, treated skin sites were biopsied and prepared for histology by tissue fixing with 10% neutral buffered formalin, paraffin embedding, slicing into 1 μm thick sections with a rotary microtome, and staining with hematoxylin and eosin for viewing by brightfield microscopy (E600, Nikon, Tokyo, Japan).
4.7. Statistical analysis
Statistical analysis was performed by using one- and two-way analysis of variance (ANOVA) or Student's t-test with 95% confidence. For each statistical analysis, a p value less than 0.05 indicates that the two groups in comparison were significantly different.
Supplementary Material
Figure S1. Representative images of hairless rat skin in vivo during recovery after hGH administration. Each group is the same group as in Figure S5. The skin was occluded for the first 24 h and unoccluded afterwards.
Figure S2. Skin erythema rating after treatment with microneedles. Erythema scale: 0 = normal skin, 1 = slight, 2 = mild, 3 = moderate, and 4 = severe erythema. Each group is the same as defined in Figure S5. Skin erythema was not rated during the first 24 hours, because it was occluded and could not be seen. No edema was seen in any groups. Data points represent the average ± standard deviation for n ≥ 4 for up to 72 hours.
Figure S3. Representative histological images of skin biopsied from hairless rats in vivo. Each group is the same as described in Figure S5. At 24 h, Groups 2 and 4 show the sites of microneedle insertion (arrows) and appear to undergo highly localized inflammatory responses (see Figure S4 for magnified views).
Figure S4. Representative histological images under high magnification of skin biopsied from hairless rats in vivo. (A) Normal skin (negative control). (B) Subcutaneously injected skin (Group 1). Skin at sites of microneedle insertion 24 h after treatment with a (C) CMC microneedle patch (Group 2) and (D) CMC/trehalose microneedle patch (Group 4). Note the presence of cells at the sites of insertion in (C) and (D) (arrows), which appear to be associated with inflammation.
Figure S5. Summary of hGH formulation and administration. Group 1: subcutaneous injection of hGH, Group 2: CMC microneedle patch encapsulating hGH, Group 3: subcutaneous injection of reconstituted CMC microneedle patch encapsulating hGH after 15 months storage at ambient condition, Group 4: CMC/trehalose microneedle patch encapsulating hGH, and Group 5: subcutaneous injection of reconstituted CMC/trehalose microneedle patch encapsulating hGH.
Acknowledgments
We thank Dr. Seungkeun Choi for the help with the microneedle master fabrication and Prof. Ajay K. Banga for helpful demonstration of the design of in vivo administration of the patch to the animal skin. This work was carried out at the Georgia Tech Center for Drug Design, Development and Delivery and Institute for Bioengineering and Biosciences and was supported in part by the National Institutes of Health. M.R.P. serves as a consultant and is an inventor on patents licensed to companies developing microneedle-based products.
Footnotes
This possible conflict of interest has been disclosed and is being managed by Georgia Tech and Emory University.
Supplementary Information: Dissolving Microneedle Patch for Transdermal Delivery of Human Growth Hormone
Contributor Information
Dr. Jeong Woo Lee, School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, Atlanta, GA 30332
Dr. Seong-O Choi, School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, Atlanta, GA 30332
Prof. Eric I. Felner, Division of Pediatric Endocrinology, Hughes Spalding Children's Hospital, Emory University School of Medicine, Atlanta, GA 30322
Prof. Mark R. Prausnitz, Email: prausnitz@gatech.edu, School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, Atlanta, GA 30332.
References
- 1.Walsh G. Trends Biotechnol. 2005;23:553–558. doi: 10.1016/j.tibtech.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Walsh G. Nat Biotechnol. 2006;24:769–776. doi: 10.1038/nbt0706-769. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen L, Nielson HM. Delivery technologies for biopharmaceuticals: peptides, proteins, nucleic acids, and vaccines. John Wiley & Sons; West Sussex: 2009. [Google Scholar]
- 4.Hauri AM, Armstrong GL, Hutin YJF. Int J STD AIDS. 2004;15:7–16. doi: 10.1258/095646204322637182. [DOI] [PubMed] [Google Scholar]
- 5.Deacon B, Abramowitz J. J Anxiety Disorders. 2006;20:946–960. doi: 10.1016/j.janxdis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Hanas R. Pediatr Diabetes. 2004;5:102–111. doi: 10.1111/j.1399-543X.2004.00048.x. [DOI] [PubMed] [Google Scholar]
- 7.Hardin DS, Kemp SF, Allen DB. Clin Pediatr. 2007;46:279–286. doi: 10.1177/0009922806293924. [DOI] [PubMed] [Google Scholar]
- 8.Chatelain P, Carrascosa A, Bona G, Ferrandez-Longas A, Sippell W. Horm Res. 2007;68:300–309. doi: 10.1159/000107935. [DOI] [PubMed] [Google Scholar]
- 9.Smith SL, Hindmarsh PC, Brook CGD. Arch Dis Child. 1993;68:91–93. doi: 10.1136/adc.68.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YP, Shen YY, Guo X, Zhang CS, Yang WJ, Ma ML, Liu S, Zhang MB, Wen LP. Nat Biotechnol. 2006;24:455–460. doi: 10.1038/nbt1193. [DOI] [PubMed] [Google Scholar]
- 11.Leitner VM, Guggi D, Krauland AH, Bernkop-Schnurch A. J Controlled Release. 2004;100:87–95. doi: 10.1016/j.jconrel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Walvoord EC, de la Pena A, Park S, Silverman B, Cuttler L, Rose SR, Cutler G, Drop S, Chipman JJ. J Clin Endocrinol Metab. 2009;94:2052–2059. doi: 10.1210/jc.2008-1897. [DOI] [PubMed] [Google Scholar]
- 13.Barry BW. Eur J Pharm Sci. 2001;14:101–114. doi: 10.1016/s0928-0987(01)00167-1. [DOI] [PubMed] [Google Scholar]
- 14.Prausnitz MR. Adv Drug Del Rev. 2004;56:581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Prausnitz MR, Langer R. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill HS, Denson DD, Burris BA, Prausnitz MR. Clin J Pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Proc Natl Acad Sci U S A. 2003;100:13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verbaan FJ, Bal SM, van den Berg DJ, Groenink WHH, Verpoorten H, Luttge R, Bouwstra JA. J Controlled Release. 2007;117:238–245. doi: 10.1016/j.jconrel.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, Daddona PE. Pharm Res. 2002;19:63–70. doi: 10.1023/a:1013607400040. [DOI] [PubMed] [Google Scholar]
- 20.Park JH, Allen MG, Prausnitz MR. J Controlled Release. 2005;104:51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Koutsonanos DG, Martin MD, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, Skountzou I. Plos One. 2009;4:e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyano T, Tobinaga Y, Kanno T, Matsuzaki Y, Takeda H, Wakui M, Hanada K. Biomed Microdevices. 2005;7:185–188. doi: 10.1007/s10544-005-3024-7. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y, Yoshimitsu JI, Shiroyama K, Sugioka N, Takada K. J Drug Targeting. 2006;14:255–261. doi: 10.1080/10611860600785080. [DOI] [PubMed] [Google Scholar]
- 24.Lee JW, Park JH, Prausnitz MR. Biomaterials. 2008;29:2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan SP, Murthy N, Prausnitz MR. Adv Mater. 2008;20:933–938. doi: 10.1002/adma.200701205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appel RA, Dmochowski RR, Herschorn S. BJU Int. 2006;98:27–30. doi: 10.1111/j.1464-410X.2006.06305.x. [DOI] [PubMed] [Google Scholar]
- 27.Cohen MH, Gootenberg J, Keegan P, Pazdur R. Oncologist. 2007;12:356–361. doi: 10.1634/theoncologist.12-3-356. [DOI] [PubMed] [Google Scholar]
- 28.Gout PW, Beer CT, Noble RL. Cancer Res. 1980;40:2433–2436. [PubMed] [Google Scholar]
- 29.Tanaka T, Shiu RPC, Gout PW, Beer CT, Noble RL, Friesen HG. J Clin Endocrinol Metab. 1980;51:1058–1063. doi: 10.1210/jcem-51-5-1058. [DOI] [PubMed] [Google Scholar]
- 30.Pikal MJ, Rigsbee D, Roy ML, Galreath D, Kovach KJ, Wang BQ, Carpenter JF, Cicerone MT. J Pharm Sci. 2008;97:5106–5121. doi: 10.1002/jps.21374. [DOI] [PubMed] [Google Scholar]
- 31.Pikal MJ, Dellerman KM, Roy ML, Riggin RM. Pharm Res. 1991;8:427–436. doi: 10.1023/a:1015834724528. [DOI] [PubMed] [Google Scholar]
- 32.Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, Morrissey A, Birchall JC. Biomed Microdevices. 2009;11:35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 33.Karande P, Jain A, Mitragotri S. J Controlled Release. 2006;110:307–313. doi: 10.1016/j.jconrel.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Leveque JL. Cutaneous Investigation in Health and Disease. Marcel Dekker; New York: 1989. [Google Scholar]
- 35.Barry BW, Woodford R. Br J Dermatol. 1974;91:323–338. doi: 10.1111/j.1365-2133.1974.tb12903.x. [DOI] [PubMed] [Google Scholar]
- 36.Eledrisi MS. eMedicine. 2008 http://emedicine.medscape.com/article/120767-overview.
- 37.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. J Controlled Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang YJ, Pearlman R. Stability and Characterization of Protein and Peptide Drugs. Vol. 5. Plenum Publishing Corporation; New York: 1993. [Google Scholar]
- 39.Park JH, Yoon YK, Choi SO, Prausnitz MR, Allen MG. IEEE Trans Biomed Eng. 2007;54:903–913. doi: 10.1109/TBME.2006.889173. [DOI] [PubMed] [Google Scholar]
- 40.Gupta J, Prausnitz MR. Ultrasound Med Biol. 2009;35:1405–1408. doi: 10.1016/j.ultrasmedbio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Representative images of hairless rat skin in vivo during recovery after hGH administration. Each group is the same group as in Figure S5. The skin was occluded for the first 24 h and unoccluded afterwards.
Figure S2. Skin erythema rating after treatment with microneedles. Erythema scale: 0 = normal skin, 1 = slight, 2 = mild, 3 = moderate, and 4 = severe erythema. Each group is the same as defined in Figure S5. Skin erythema was not rated during the first 24 hours, because it was occluded and could not be seen. No edema was seen in any groups. Data points represent the average ± standard deviation for n ≥ 4 for up to 72 hours.
Figure S3. Representative histological images of skin biopsied from hairless rats in vivo. Each group is the same as described in Figure S5. At 24 h, Groups 2 and 4 show the sites of microneedle insertion (arrows) and appear to undergo highly localized inflammatory responses (see Figure S4 for magnified views).
Figure S4. Representative histological images under high magnification of skin biopsied from hairless rats in vivo. (A) Normal skin (negative control). (B) Subcutaneously injected skin (Group 1). Skin at sites of microneedle insertion 24 h after treatment with a (C) CMC microneedle patch (Group 2) and (D) CMC/trehalose microneedle patch (Group 4). Note the presence of cells at the sites of insertion in (C) and (D) (arrows), which appear to be associated with inflammation.
Figure S5. Summary of hGH formulation and administration. Group 1: subcutaneous injection of hGH, Group 2: CMC microneedle patch encapsulating hGH, Group 3: subcutaneous injection of reconstituted CMC microneedle patch encapsulating hGH after 15 months storage at ambient condition, Group 4: CMC/trehalose microneedle patch encapsulating hGH, and Group 5: subcutaneous injection of reconstituted CMC/trehalose microneedle patch encapsulating hGH.


