Abstract
Objective
To evaluate associations of perinatal HIV infection (PHIV), HIV disease severity, and combination antiretroviral treatment with age at pubertal onset.
Design
Analysis of data from two U.S. longitudinal cohort studies [IMPAACT 219C and PHACS AMP], conducted 2000–2012, including PHIV and HIV-exposed uninfected (HEU) youth. Tanner stage assessments of pubertal status (breast and pubic hair in girls; genitalia and pubic hair in boys) were conducted annually.
Methods
We compared the timing of pubertal onset (Tanner stage ≥2) between PHIV and HEU youth using interval-censored models. For PHIV youth, we evaluated associations of HIV disease severity and combination antiretroviral treatment with age at pubertal onset, adjusting for race/ethnicity and birth cohort.
Results
The mean age at pubertal onset was significantly later for the 2086 PHIV youth compared to 453 HEU children (10.3 vs 9.6, 10.5 vs 10.0, 11.3 vs 10.4, and 11.5 vs 10.7 years according to female breast, female pubic hair, male genitalia, and male pubic hair staging, respectively, all p<0.001). PHIV youth with HIV-1 RNA viral load >10,000 copies/mL (vs ≤10,000 copies/mL) or CD4% <15% (vs ≥15%) had significantly later pubertal onset (by 4–13 months). Each additional year of combination antiretroviral treatment was associated with a 0.6- to1.2-month earlier mean age at pubertal onset, but this trend did not persist after adjustment for birth cohort.
Conclusions
Pubertal onset occurs significantly later in PHIV than in HEU youth, especially among those with more severe HIV disease. However, in the current era, combination antiretroviral treatment may result in more normal timing of pubertal onset.
Keywords: puberty, antiretroviral therapy, protease inhibitors, pediatrics, CD4, viral load, statistics, interval-censored, Tanner stage, BMI
INTRODUCTION
Puberty is a complex biological process involving physical and hormonal changes in which the body transitions to sexual maturity. Epidemiological studies have established a trend over the past two decades toward earlier pubertal onset, particularly in girls [1–5]. These changes have occurred against a backdrop of increasing prevalence of childhood obesity in parallel with changing dietary patterns and declining physical activity [6]. In addition, environmental exposures may play a role in trends of earlier pubertal onset [1,7].
Perinatally HIV-infected (PHIV) youth have historically demonstrated decreased growth [8–9] and delays in pubertal onset, particularly among those with more advanced HIV disease [10–16]. Metabolic and endocrine abnormalities in PHIV youth may also play a role in deficient growth and delayed pubertal onset [17–24]. Combination antiretroviral (ARV) treatment has been associated with improvement in growth [25–28] which could reduce the risk for pubertal delay. However, few studies have addressed the effect of combination treatment on pubertal onset. Buchacz et al observed significantly earlier pubertal onset among boys with prior protease inhibitor (PI) use, but no clear association with PI use among girls [14]. This study was conducted prior to 2000, at a time when mono- or dual-agent therapy as a standard of care was transitioning to more effective combination regimens, including the use of PIs. Two recent studies observed later pubertal onset among HIV-infected youth with lower CD4+ cells, but neither addressed the association with antiretroviral regimens [16,29].
We used data collected from two U.S.-based longitudinal cohort studies, the International Maternal Pediatric and Adolescent Clinical Trials (IMPAACT) 219/219C study (219C) and the Adolescent Master Protocol (AMP) study of the Pediatric HIV/AIDS Cohort Study (PHACS) network, to compare the timing of pubertal onset among PHIV children to that of perinatally HIV-exposed but uninfected (HEU) children. In addition, we evaluated the association of HIV disease severity and ARV treatment with pubertal onset in children with perinatal HIV infection.
METHODS
Description of Protocols and Study Population
This investigation included children born to HIV-infected women who had pubertal staging assessed at age 7 years or older in the 219C or AMP studies. The 219C study was a large prospective study conducted at over 80 U.S. clinical research sites between 1993 and 2007 to evaluate the long-term effects of HIV infection and in utero ARV exposure [30–31]. AMP is a smaller ongoing prospective cohort study which opened in March 2007 [32]. Subjects were eligible for AMP (whether in 219C or not) if they were perinatally HIV-infected or HIV-exposed, and 7 to <17 years old. Both studies were approved by the site Institutional Review Boards and written informed consent was obtained from each parent or legal guardian, with assent from children as appropriate.
At each study visit, we obtained medical histories through chart reviews and ascertained health status through physical and laboratory evaluations. HIV disease severity information for HIV-infected youth was collected at scheduled visits (every 3 months for 219C, every 6–12 months for AMP). Race and ethnicity were self-reported at study entry and categorized as white Non-Hispanic, black non-Hispanic, Hispanic, or “other”. We excluded participants of PACTG 219 who did not participate in 219C and youth who were judged to be at later stages of pubertal maturity (Tanner stage 3 to 5) at their first study visit.
Pubertal Staging Measures
Study clinicians trained to evaluate child growth and development assessed pubertal staging annually in 219C and at each study visit in AMP (initially every 6 months and then annually after August 2010) by visual inspection according to criteria of Tanner and Whitehouse [33], ranging from 1 (pre-pubertal) to 5 (sexually mature). Pubertal onset was defined for each Tanner measure as attaining stage 2 or higher, with separate indicators for breast development and pubic hair in girls, and for genitalia and pubic hair in boys.
Statistical Methods
The date of the first pubertal staging assessment at age ≥7 years was considered to define the start of longitudinal follow-up for pubertal onset. The last available measures of height and body mass index (BMI, kg/m2) prior to this index date were obtained, and Centers for Disease Control and Prevention (CDC) 2000 growth standards were used to calculate age- and sex-adjusted Z-scores [34–38]. For HIV-infected youth, latest measures of CD4 T-lymphocyte percent (CD4%), CD4 count, HIV-1 RNA viral load, and CDC clinical classification were identified prior to or at the index date. Nadir CD4% and peak viral load were based on the lowest CD4% and highest viral load measure, respectively, prior to or at the first pubertal assessment. For those with at least two viral load measures before their first pubertal assessment, a cumulative measure of copy-years viremia was calculated as described by Cole et al [39]. Combination antiretroviral treatment was defined as concurrent use of at least three drugs from at least two drug classes, and cumulative ARV history was reflected by years of receipt of combination treatment, PIs, and non-nucleoside reverse transcriptase inhibitors (NNRTIs) prior to the index date. We summarized characteristics of the participants by HIV infection status. The percentage of children with delayed pubertal onset, defined as not attaining Tanner stage 2 by age 12 in girls (by both breast and pubic hair staging) or by age 13 in boys (by both genitalia and pubic hair staging) was assessed by birth cohort and HIV infection status.
Interval-censored approaches under an assumed normal distribution were used to estimate the mean age at pubertal onset separately for each Tanner measure, by HIV infection status, race/ethnicity, and birth cohort (pre-1990, 1990–1992, 1993–1996, 1997 or later). The interval-censoring approach accounts for whether pubertal onset occurred prior to the first pubertal assessment (left-censored), between study visits (interval-censored), or had not occurred by the last study visit (right-censored); this is the most appropriate statistical approach for evaluating pubertal onset based on longitudinal data [40]. The mean age at pubertal onset was compared between HIV-infected and HEU youth in unadjusted models and with adjustment for race/ethnicity and birth cohort. Further adjustment for BMI and height Z-scores was performed, but since these measures may be on the causal pathway between HIV infection and pubertal onset, results of these sensitivity analyses are presented only in online supplemental tables. Caregiver education level was considered but not found to be associated with timing of pubertal onset. We also qualitatively compared the estimated mean ages at pubertal onset with the general US population as reflected by the National Health and Nutrition Examination Survey (NHANES) III (1988–1994) [4,5,40].
For perinatally HIV-infected youth, interval-censored models were fit to evaluate each HIV disease severity measure and ARV treatment characteristic separately, in unadjusted models and after adjustment for race/ethnicity and birth cohort. ARV regimens were classified as combination treatment with PI, combination treatment without PI, or not on combination treatment (not on ARVs, or on other regimen). Sensitivity analyses were conducted to examine effects of further adjustment for potential intermediates between combination antiretroviral treatment and pubertal onset, including BMI and height Z-scores and CD4% <15%. Duration of prior use of combination treatment and specific drug classes (PIs and NNRTIs) were considered as continuous predictors after evaluation of linearity assumptions. A sensitivity analysis was also conducted to examine consistency of results when restricting the study population to only pre-pubertal youth.
RESULTS
Characteristics of the Study Population
We evaluated 3006 children from 219C or AMP who were born to HIV-infected women and who had pubertal staging at age 7 years or older. We excluded 467 youth who had pubertal onset prior to study entry and were at Tanner stage 3 or higher at their first visit, leaving 2539 in analyses of pubertal onset (1253 girls and 1286 boys). Demographic characteristics are shown in Table 1 by HIV infection status; 56% were Black non-Hispanic and 29% were Hispanic.
Table 1.
Characteristics of 2539 Youth Evaluated for Pubertal Onset from the IMPAACT 219C and PHACS AMP Studies, and HIV-related Characteristics as of the First Pubertal Assessment* for 2086 Perinatally HIV-infected Children
| Characteristic | HIV Infection Status
|
|
|---|---|---|
| HIV-exposed Uninfected (N=453) | Perinatally HIV-infected (N=2086) | |
| Age at 1st Pubertal Assessment, Median (IQR) | 7.60 (7.20, 8.40) | 8.00 (7.40, 9.70) |
| Study Participation | ||
| PHACS AMP & PACTG 219C | 101 (22%) | 296 (14%) |
| PACTG 219C only | 265 (58%) | 1,718 (82%) |
| PHACS AMP only | 87 (19%) | 72 (3%) |
| Sex | ||
| Boys | 232 (51%) | 1,054 (51%) |
| Girls | 221 (49%) | 1,032 (49%) |
| Birth Cohort | ||
| Before 1990 | 0 (0%) | 500 (24%) |
| 1990–1992 | 41 (9%) | 654 (31%) |
| 1993–1996 | 150 (33%) | 644 (31%) |
| 1997 or later | 262 (58%) | 288 (14%) |
| Race/Ethnicity | ||
| White Non-Hispanic | 53 (12%) | 266 (13%) |
| Black Non-Hispanic | 230 (51%) | 1,197 (57%) |
| Hispanic | 162 (36%) | 581 (28%) |
| Other/unknown | 8 (2%) | 42 (2%) |
| Body Mass Index (BMI) Z-score; Mean (SD)a | 0.76 (1.24) | 0.28 (1.04) |
| Height Z-score; Mean (SD)a | 0.20 (1.08) | −0.64 (1.20) |
| Primary Caregiver High School Graduate | 286 (63%) | 1,366 (65%) |
| HIV-related Characteristics, Perinatally HIV-infected only | ||
| CD4 T-lymphocyte percentage (CD4%) | ||
| Median (IQR) | 30 (22, 37) | |
| <15% | 257 (12%) | |
| 15–25% | 462 (23%) | |
| >25% | 1,348 (65%) | |
| Not available | 19 | |
| CD4 T-lymphocyte count (cells/mm3) | ||
| Median (IQR) | 748 (456, 1,032) | |
| 0–200 | 346 (17%) | |
| 201–350 | 233 (11%) | |
| >350 | 1,487 (72%) | |
| Not available | 20 | |
| Nadir CD4%; Median (IQR)b | 20 (12, 28) | |
| HIV-1 Plasma RNA Viral Load | ||
| Median log HIV-1 RNA (IQR) | 2.90 (2.60, 4.10) | |
| <400 copies/mL | 699 (45%) | |
| 401–10,000 copies/mL | 434 (28%) | |
| >10,000 copies/mL | 417 (27%) | |
| Not available | 536 | |
| Peak Viral Load (copies/mL); Median (IQR)b | 28,536 (1,844, 161,881) | |
| Log Copy-years Viremia; Median (IQR)b | 4.27 (3.38, 4.97) | |
| CDC Class C Condition, N (%) | 667 (32%) | |
| ARV treatment at first pubertal assessment, N (%) | ||
| Combination treatment with PI | 1,141 (55%) | |
| Combination treatment without PI | 193 (9%) | |
| Not on combination treatment | 752 (36%) | |
| Duration of prior treatment (years)c, Median (IQR) | ||
| Years on combination treatment, among prior users | 3.24 (1.62, 5.20) | |
| Years on any PI-containing regimen | 3.12 (1.66, 4.99) | |
| Years on any NNRTI-containing regimen | 1.65 (078, 3.06) | |
IQR = interquartile range (displayed as 25th to 75th percentile); PHACS = Pediatric HIV/AIDS Cohort Study; AMP = Adolescent Master Protocol; PACTG = Pediatric AIDS Clinical Trials Group; SD = standard deviation; ARV = antiretroviral; PI = protease inhibitor; NNRTI = non-nucleoside reverse transcriptase inhibitor; CDC = Centers for Disease Control and Prevention
All HIV-disease related characteristics are based on measures available prior to or at the first pubertal assessment, at age 7 years or older.
BMI and height Z-scores are sex- and age-adjusted Z-scores based on CDC 2000 growth standards using the latest BMI and height measured as of the first pubertal assessment. Measures of height and BMI were unavailable for 1 HIV-exposed uninfected child and 35 HIV-infected children.
Measures of past HIV disease severity were unavailable for 19 children for nadir CD4%, and for 536 children for peak viral load. Lack of two viral load measures prevented calculation of copy-years viremia for 989 children.
Duration of prior ARV summarized among the subset of youth with any prior use of regimen, and include 1464 with prior combination ARV treatment, 1400 with prior PI use, and 938 with prior NNRTI use; each regimen is considered separately and categories are not mutually exclusive.
The 2086 PHIV youth more often were born in earlier years than the 453 HEU children and had lower mean Z-scores for both BMI and height at the time of their first pubertal assessment. Among HIV-infected youth, 12% had CD4% <15%, and 27% had viral load >10,000 copies/mL proximate to the first pubertal assessment (Table 1). Males more often had low CD4% and high viral load than females (Supplemental Digital Content 1). At the time of the first pubertal assessment, 64% were on combination ARV therapy (55% including a PI and 9% without a PI); the median duration of prior combination treatment among those with any prior use was 3.24 years. Of the 752 children not on combination treatment, 82% received mono- or dual-agent therapy, 6% were on three or more NRTIs, and 12% were not on any ARV treatment.
Age at Pubertal Onset by HIV Status, Race/Ethnicity, and Birth Cohort
HEU girls had pubertal onset at mean ages of 9.6 and 10.0 years according to breast and pubic hair stages respectively, while boys had onset at mean ages of 10.4 and 10.7 years based on genitalia and pubic hair, respectively (Table 2). Consistent with most studies in girls, thelarche (reflected by breast staging) occurred earlier on average than pubarche (reflected by pubic hair growth) [40–43]. Mean ages at pubertal onset by race/ethnicity were similar for HEU youth to those based on NHANES III data for girls [40–43], but were later than NHANES III among both Black non-Hispanic (10.0 vs 9.2–9.5 years) and white non-Hispanic boys (11.6 vs 10.0 years) [3–5,40].
Table 2.
Estimated Mean Ages in Years (and 95% Confidence Intervals) at Pubertal Onset by HIV Infection status and Race/Ethnicity for Perinatally HIV-exposed Youth from the IMPAACT 219C and PHACS AMP Studies.
| Girls | Boys | |||
|---|---|---|---|---|
|
| ||||
| Breast (N=1222) | Pubic hair (N=1185) | Genitalia (N=1259) | Pubic Hair (N=1182) | |
|
| ||||
| Estimated Mean (95 % CI) | Estimated Mean (95 % CI) | Estimated Mean (95 % CI) | Estimated Mean (95 % CI) | |
| By HIV Infection Status | ||||
| HIV-exposed uninfected | 9.61 (9.38, 9.84) | 9.98 (9.72, 10.23) | 10.36 (10.09, 10.63) | 10.72 (10.43, 11.01) |
| HIV-infected | 10.28 (10.18, 10.38)** | 10.46 (10.34, 10.57)** | 11.25 (11.13, 11.36)** | 11.54 (11.42, 11.66)** |
| By Race/Ethnicity | ||||
| White Non-Hispanic | 10.75 (10.49, 11.01) | 11.01 (10.73, 11.29) | 11.68 (11.36, 12.00) | 12.07 (11.74, 12.40) |
| Black Non-Hispanic | 9.92 (9.80, 10.05)** | 10.03 (9.90, 10.16)** | 10.90 (10.76, 11.05)** | 11.19 (11.04, 11.34)** |
| Hispanic | 10.42 (10.24, 10.60)* | 10.80 (10.61, 10.99) | 11.29 (11.09, 11.48)* | 11.59 (11.38, 11.79)* |
| Other/Unknown | 9.75 (9.13, 10.37)* | 10.29 (9.66, 10.91)* | 11.15 (10.31, 12.00) | 11.64 (10.76, 12.51) |
| By Race/Ethnicity and HIV Infection Status | ||||
| Among HIV-exposed Uninfected | (n=218) | (n=216) | (n=226) | (n=210) |
| White Non-Hispanic | 10.48 (9.81, 11.16) | 11.27 (10.44, 12.10) | 11.61 (10.68, 12.54) | 11.92 (10.89, 12.95) |
| Black Non-Hispanic | 9.15 (8.85, 9.45)** | 9.55 (9.20, 9.89)** | 10.03 (9.69,10.36)* | 10.43 (10.03, 10.82)* |
| Hispanic | 9.95 (9.56, 10.35) | 10.15 (9.71, 10.58)* | 10.40 (10.00,10.81)* | 10.80 (10.33, 11.27)* |
| Other/Unknown | 9.93 (8.75, 11.11) | 11.07 (9.60, 12.53) | N/A | N/A |
| Among Perinatally HIV-infected | (n=1004) | (n=969) | (n=1031) | (n=970) |
| White Non-Hispanic | 10.78 (10,51, 11.06) | 10.97 (10.68, 11.27) | 11.67 (11.34, 12.00) | 12.08 (11.74, 12.42) |
| Black Non-Hispanic | 10.06 (9.93, 10.20)** | 10.11 (9.97, 10.25)** | 11.04 (10.89, 11.19)** | 11.30 (11.14, 11.46)** |
| Hispanic | 10.52 (10.33, 10.71) | 10.95 (10.74, 11.15) | 11.47 (11.26, 11.68) | 11.75 (11.53, 11.97) |
| Other/Unknown | 9.69 (9.00, 10.39)* | 10.11 (9.42, 10.79)* | 11.07 (10.23, 11.91) | 11.57 (10.69, 12.44) |
Statistical comparisons with reference group (listed first within group) indicated by ** denoting p<0.001 and *denoting p<0.05. CI=confidence interval
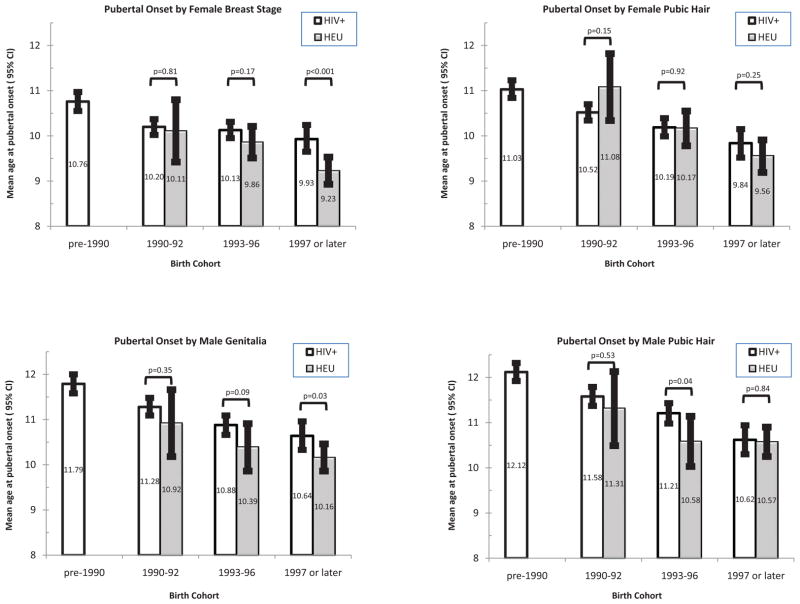
Compared to the HEU youth, PHIV children had significantly later estimated mean ages at onset, representing delays of about 6 to 11 months (Table 2). The mean age at pubertal onset for PHIV as compared to HEU was 10.3 vs 9.6 years, 10.5 vs 10.0, 11.3 vs 10.4, and 11.5 vs 10.7 years according to female breast, female pubic hair, male genitalia, and male pubic hair staging, respectively (all p<0.001). Estimated mean ages by race/ethnicity indicated significantly earlier pubertal onset for black non-Hispanic youth (by 9 to 12 months) and for Hispanics (by 3 to 4 months) than for white non-Hispanic youth, consistent with previous studies [3–5, 40–43]. Striking trends for earlier pubertal onset with more recent birth year were observed in both PHIV and HEU youth; however, the mean ages at onset were typically later for PHIV than HEU within each birth cohort (Figure 1). In interval-censored models, this shift was attenuated after adjustment for race/ethnicity and birth cohort, with 4–6 months later onset for PHIV youth on average (Table 3) according to breast, genitalia, and male pubic hair staging measures. Higher Z-scores for pre-pubertal height and BMI were associated with significantly earlier pubertal onset, but further adjustment for these measures had little effect on differences by HIV infection status. In sensitivity analyses excluding subjects at Tanner stage 2 at their first pubertal assessment (10% HEU, 11% PHIV), estimated mean ages at onset were slightly later (~2 months), but differences by HIV infection status were similar to those reported in Table 3.
Figure 1.
Mean age at pubertal onset by Tanner measure and birth cohort, for HIV-infected youth (unshaded bars) and HIV-exposed uninfected youth (shaded bars). P-values for statistical significance based on interval-censored models for the effect of HIV infection status fit separately within each birth cohort.
Table 3.
Estimated Shifts (months) in Mean Ages at Pubertal Onset by HIV infection status in Perinatally HIV-exposed Youth from the IMPAACT 219C and PHACS AMP Studies and by HIV Disease Severity and ARV Treatment Measures as of First Pubertal Assessment among Perinatally HIV-infected Youth
| Characteristic | Unadjusted* | Adjusted for Race/Ethnicity and Birth Cohort** | Unadjusted* | Adjusted for Race/Ethnicity and Birth Cohort** | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean Shift (95% CI) | p-value | Mean Shift (95% CI) | p-value | Mean Shift (95% CI) | p-value | Mean Shift (95% CI) | p-value | |
| (a) Estimated Shifts (months) in Mean Ages at Pubertal Onset in Girls | ||||||||
|
| ||||||||
| Breast Development | Pubic Hair | |||||||
|
| ||||||||
| Among all Girls, by HIV Infection status | ||||||||
|
| ||||||||
| (N=1222) | (N=1185) | |||||||
| HIV-infected vs uninfected | 8.09 (5.05, 11.13) | <0.001 | 5.55 (2.38, 8.72) | <0.001 | 5.75 (2.42, 9.07) | <0.001 | 1.48 (−1.87, 4.83) | 0.39 |
|
| ||||||||
| Among HIV-infected Girls: by HIV Disease Severity and Antiretroviral Treatment | ||||||||
|
| ||||||||
| (N=1004) | (N=969) | |||||||
| CD4% <15% | 7.17 (3.11, 11.23) | <0.001 | 4.78 (0.71, 8.85) | 0.021 | 10.91 (6.53, 15.30) | <0.001 | 7.67 (3.40, 11.94) | <0.001 |
| CD4 count < 200 cells/mm3 | 7.93 (4.48, 11.38) | <0.001 | 5.66 (2.18, 9.14) | 0.001 | 9.68 (5.96, 13.39) | <0.001 | 6.28 (2.64, 9.92) | <0.001 |
| Nadir CD4% <15% | 1.58 (−1.19, 4.34) | 0.26 | 0.07 (−2.62, 2.77) | 0.96 | 5.54 (2.57, 8.51) | <0.001 | 2.97 (0.14, 5.80) | 0.040 |
| viral load >10,000 copies/mL | 5.64 (2.30, 8.98) | <0.001 | 3.54 (0.15, 6.92) | 0.041 | 3.94 0.10, 7.77) | 0.045 | 0.61 (−3.13, 4.35) | 0.75 |
| Peak viral load >100,000 copies/mL | 0.51 (−2.62, 3.65) | 0.75 | 0.99 (−2.04, 4.01) | 0.52 | −1.34 (−4.86, 2.18) | 0.45 | −0.34 (−3.60, 2.93) | 0.84 |
| Log10 Copy-years Viremia | 1.60 (−0.06, 3.26) | 0.059 | 1.33 (−0.23, 2.89) | 0.094 | 1.52 (−0.35, 3.40) | 0.11 | 1.00 (−0.72, 2.71) | 0.25 |
| CDC Class C | 1.50 (−1.15, 4.14) | 0.27 | 1.96 (−0.57, 4.50) | 0.13 | 0.84 (−2.00, 3.68) | 0.56 | 1.18 (−1.45, 3.81) | 0.38 |
| ARV regimen as of first pubertal assessment | ||||||||
| Combination with PI | −2.67 (−5.25, −0.08) | 0.044 | 0.40 (−2.36, 3.16) | 0.78 | −3.68 (−6.45, −0.91) | 0.009 | 0.65 (−2.21, 3.51) | 0.65 |
| Combination without PI | −1.76 (−6.46, 2.93) | 0.46 | 0.79 (−3.86, 5.43) | 0.74 | −2.64 (−7.62, 2.33) | 0.30 | 2.05 (−2.74, 6.85) | 0.40 |
| Not on combination regimen | (ref) | --- | (ref) | --- | (ref) | --- | (ref) | --- |
| Each additional year of ART use by regimen or drug class (not mutually exclusive) | ||||||||
| Combination regimen | −0.60 (−1.09, −0.12) | 0.015 | 0.17 (−0.50, 0.84) | 0.62 | −1.15 (−1.67, −0.64) | <0.001 | 0.14 (−0.57, 0.85) | 0.71 |
| Combination regimen with PI | −0.65 (−1.17, −0.14) | 0.012 | 0.04 (−0.60, 0.67) | 0.91 | −1.22 (−1.76, −0.67) | <0.001 | −0.08 (−0.75, 0.59) | 0.83 |
| Any PI-containing regimen | −0.58 (−1.09, −0.07) | 0.025 | 0.18 (−0.44, 0.81) | 0.57 | −1.19 (−1.74, −0.65) | <0.001 | −0.05 (−0.71, 0.61) | 0.89 |
| NNRTI-containing regimen | −0.72 (−1.51, 0.06) | 0.069 | −0.15 (−0.96, 0.66) | 0.72 | −1.46 (−2.28, −0.65) | <0.001 | −0.39 (−1.22, 0.44) | 0.36 |
|
| ||||||||
| 3(b) Estimated Shifts (months) in Mean Ages at Pubertal Onset in Boys | ||||||||
|
| ||||||||
| Genitalia | Pubic Hair | |||||||
|
| ||||||||
| Among all Boys, by HIV Infection status | ||||||||
|
| ||||||||
| (N=1259) | (N=1182) | |||||||
| HIV-infected vs uninfected | 10.61 (7.08, 14.14) | <0.001 | 6.02 (2.15, 9.90) | 0.002 | 9.78 (6.03, 13.54) | <0.001 | 3.92 (−0.14, 7.98) | 0.058 |
|
| ||||||||
| Among HIV-infected Boys: by HIV Disease Severity and Antiretroviral Treatment | ||||||||
|
| ||||||||
| (N=1031) | (N=970) | |||||||
| CD4% <15% | 12.20 (8.41, 15.99) | <0.001 | 8.95 (5.06, 12.84) | <0.001 | 12.78 (8.95, 16.62) | <0.001 | 9.04 (5.16, 12.92) | <0.001 |
| CD4 count < 200 cells/mm3 | 10.23 (6.78,13.68) | <0.001 | 6.98 (3.40,10.56) | <0.001 | 11.85 (8.37,15.33) | <0.001 | 8.36 (4.79, 11.94) | <0.001 |
| Nadir CD4% < 15% | 8.13 (5.21, 11.06) | <0.001 | 5.74 (2.85, 8.64) | <0.001 | 8.70 (5.70, 11.70) | <0.001 | 5.94 (3.01, 8.88) | <0.001 |
| Viral load >10,000 copies/mL | 9.36 (5.88, 12.83) | <0.001 | 5.07 (1.52, 8.62) | 0.005 | 10.18 (6.60, 13.75) | <0.001 | 5.50 (1.90, 9.10) | 0.003 |
| Peak viral load >100,000 copies/mL | 4.60 (1.13, 8.06) | 0.009 | 4.47 (1.18, 7.76) | 0.008 | 3.53 (−0.04, 7.10) | 0.053 | 4.15 (0.83, 7.48) | 0.014 |
| Log10 Copy-years Viremia | 3.23 (1.27, 5.19) | 0.001 | 2.04 (0.24, 3.85) | 0.026 | 4.47 (2.43, 6.51) | <0.001 | 3.09 (1.23, 4.95) | 0.001 |
| CDC Class C | 5.30 (2.27, 8.33) | <0.001 | 5.39 (2.51, 8.27) | <0.001 | 3.59 (0.43, 6.74) | 0.026 | 3.20 (0.25, 6.15) | 0.034 |
| ARV regimen as of first pubertal assessment | ||||||||
| Combination with PI | −0.97 (−3.98, 2.04) | 0.53 | 3.98 (0.88, 7.08) | 0.012 | −3.17 (−6.25, −0.09) | 0.044 | 1.75 (−1.38, 4.89) | 0.27 |
| Combination without PI | 0.05 (−5.24, 5.35) | 0.98 | 3.46 (−1.53, 8.46) | 0.17 | 1.82 (−3.53, 7.17) | 0.51 | 4.90 (−0.08, 9.88) | 0.054 |
| Not on combination regimen | (ref) | --- | (ref) | --- | (ref) | --- | (ref) | --- |
| Each additional year of ARV treatment by regimen or drug class (not mutually exclusive) | ||||||||
| Combination regimen | −0.62 (−1.19, −0.04) | 0.035 | 0.98 (0.27, 1.70) | 0.007 | −0.89 (−1.48, −0.31) | 0.003 | 0.90 (0.18, 1.63) | 0.014 |
| Combination regimen with PI | −0.61 (−1.22, −0.01) | 0.046 | 0.92 (0.20, 1.64) | 0.012 | −0.99 (−1.60, −0.37) | 0.002 | 0.71 (−0.03, 1.44) | 0.059 |
| Any PI-containing regimen | −0.57 (−1.16, 0.03) | 0.062 | 0.96 (0.26, 1.66) | 0.007 | −0.92 (−1.53, −0.31) | 0.003 | 0.75 (0.04, 1.46) | 0.039 |
| NNRTI-containing regimen | −1.30 (−2.20, −0.40) | 0.005 | −0.24 (−1.16, 0.68) | 0.61 | −1.63 (−2.56, −0.71) | <0.001 | −0.39 (−1.32, 0.54) | 0.41 |
CI = confidence interval; IQR = interquartile range; ARV = antiretroviral; PI = protease inhibitor; NNRTI = non-nucleoside reverse transcriptase inhibitor; CDC = Centers for Disease Control and Prevention
All estimated shifts in mean age at pubertal onset are based on interval-censored models; and reflect the estimated shift in mean age at pubertal onset for those with versus without a characteristic, or for each 1-unit change in continuous measures.
Interval-censored models for covariate of interest adjusted for Race/Ethnicity (classified as White non-Hispanic, Black non-Hispanic, Hispanic, or other/unknown race) and birth cohort (classified as pre-1990, 1990-1992, 1993-1996, and 1997 or later.
The percent of PHIV youth with delay in pubertal onset was 4.1% overall, but showed substantial decreases over time (11.2%, 3.1%, 1.7%, and 0.4% for those born before 1990, 1990–1992, 1993–1996, and 1997 or later, respectively; trend test p-value<0.001). In contrast, delay in pubertal onset was rare among HEU youth (2/453, 0.4%).
Association of HIV Disease Severity with Age at Pubertal Onset
Mean ages at pubertal onset were significantly later for youth with more advanced HIV disease status at the first pubertal assessment (Table 3). For both girls and boys, there was a significant association of low CD4 (as reflected by CD4% <15% and CD4 count <200 cells/mm3) and high viral load (>10,000 cp/mL) with later pubertal onset, both with and without adjustment for race/ethnicity and birth cohort, with the exception of female pubic hair (Table 3). Associations remained significant for most Tanner measures after further adjustment for BMI and height Z-scores (Supplemental Digital Content 2). Measures of past HIV disease severity showed stronger associations with timing of pubertal onset in boys than in girls. Boys with CDC Class C (prior AIDS-defining condition), low nadir CD4%, or higher peak viral load had significantly later pubertal onset than those with milder classifications. Cumulative viral burden reflected by copy-years viremia was associated with significantly later age at pubertal onset in boys after adjustment for race/ethnicity and birth cohort, but not in girls. No association with peak viral load or CDC class was observed among girls.
Association of Combination Treatment and ARV Drug Classes with Age at Pubertal Onset
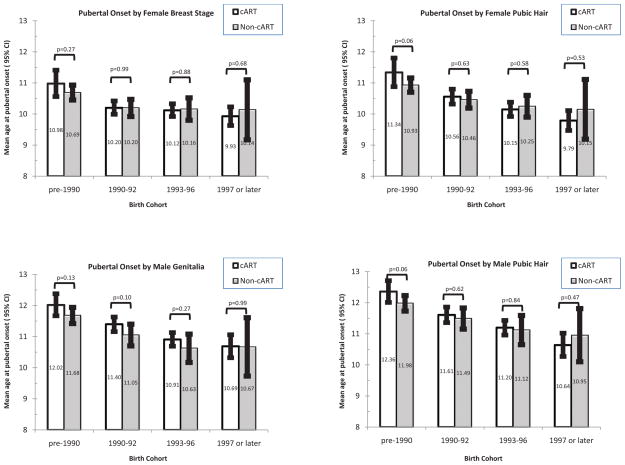
In unadjusted models, youth on combination treatment with a PI at the first pubertal assessment had a significantly earlier mean age at pubertal onset (by 2.7–3.7 months) than did youth not on combination treatment, with the exception of male genitalia (Table 3). In contrast, there was no significant difference in age at onset for those on combination regimens without PIs as compared to those youth unexposed to combination regimens for any Tanner measure. Associations for combination treatment with PI were non-significant in females and reversed direction in males when further adjusted for birth cohort. Examination of differences by birth cohort revealed that mean ages at pubertal onset were earlier for those exposed to combination treatment among those born since 1997 for all measures except male genitalia, but later for those exposed to combination treatment among those born prior to 1990 for all four staging measures (Figure 2); however, statistical tests for interaction were not significant.
Figure 2.
Mean age at pubertal onset by Tanner measure and birth cohort, for HIV-infected youth on combination antiretroviral treatment (cART, unshaded bars) as compared to those not on cART (non-cART, shaded bars) at the time of the first pubertal assessment. P-values for statistical significance based on interval-censored models for the effect of cART fit separately within each birth cohort.
Duration of prior combination treatment, overall or with a PI, at the time of the first pubertal assessment also showed significant associations with earlier age at pubertal onset based on all four staging measures in unadjusted analyses (Table 3). Mean ages at onset ranged from 0.6 to 1.2 months earlier for each additional year on combination treatment, depending on staging measure. However, after adjustment for race/ethnicity and birth cohort, there was no association of duration of combination treatment (overall or with PI) in girls, and the direction of effect reversed for boys. Similar associations were observed for prior years of use of PIs and NNRTIs (Table 3). Further adjustment for BMI and height Z-scores and for HIV-disease status as reflected by CD4% <15% had little effect on estimated mean ages of pubertal onset (Supplemental Digital Content 2).
DISCUSSION
We confirmed a significant delay in the mean age at pubertal onset for PHIV children compared to uninfected but perinatally HIV-exposed youth, ranging from a 6- to 8-month later mean age at onset in girls and a 10 to 11-month later onset in boys. The later average age at onset corresponded to an increased prevalence of delayed onset for PHIV vs HEU youth, but clinical delay among youth born after 1997 was rare regardless of HIV status. The PHIV youth in our cohort were more often born in earlier years than the HEU youth, notable given the secular trends in timing of pubertal onset [1–5]; however, the later pubertal onset for PHIV youth persisted even after adjustment for race/ethnicity and birth cohort for all measures except pubic hair in girls. PHIV girls and boys with more advanced HIV disease were at greatest risk of delay in pubertal onset, and these associations persisted for most staging measures after adjustment for birth cohort. Boys had stronger associations with past measures of disease severity than did girls, which could be attributable to either poorer initial immunological status or increased sensitivity of hormonal pathways in boys.
While both prior combination antiretroviral treatment with PIs and longer duration of combination treatment appeared to be protective in restoring earlier timing of pubertal onset, these associations did not persist after adjustment for birth cohort. Descriptively, the mean age at pubertal onset was earlier for those on a combination regimen compared to those not on combination regimens for those born in 1997 or later, while the reverse was generally true for those born earlier. We did not observe a significant benefit of combination regimens in models adjusting for birth cohort, possibly due to confounding by indication resulting from only the sickest HIV-infected children receiving combination treatment in the earlier birth cohorts (33% of those in the pre-1990 cohort), while combination treatment was widespread among HIV-infected youth in the later birth cohorts (88% among those born 1997 or later). While there were no statistically significant interaction effects between combination treatment and birth cohort for any of the four staging measures, power for testing interaction may have been limited.
The mean ages at pubertal onset across the four staging measures and by race/ethnicity in our HEU youth from 219C and AMP were generally similar to other studies using NHANES III (1988–1994) data. Among PHIV youth, our estimated mean ages at onset are similar to those reported by Buchacz et al [14] of 10.7 years for girls and 11.8 years for boys (their study population was 52% black non-Hispanic, 33% Hispanic), but substantially earlier than mean ages at onset of 12.1–12.9 years among Caucasian HIV-infected children in an Italian study reported by DeMartino et al [13].
The etiology of delayed puberty in adolescents with HIV infection is not well-understood. It has been ascribed to the general effects of chronic illness mediated through cytokine-induced inhibition of gonadotropin secretion [26, 44,45]. It has also been suggested that HIV infection directly or indirectly affects production or secretion of hormones that regulate or control pubertal initiation and tempo (e.g., leptin produced in adipose tissue) [14]. Delayed pubertal development in HIV-infected children has been attributed in part to reduced adrenal androgen secretion [23,46].
Implications of altered pubertal timing in the general population have received more attention for early maturation, which has been associated with increased incidence of antisocial behaviors and substance use. A general trend of earlier pubertal onset is thus not necessarily desirable, given the potential adverse social and clinical consequences of early puberty noted in the literature [1]. However, while youth in many developed countries are attaining pubertal onset earlier than in previous decades, those with perinatally-acquired HIV still tend to have later onset than US norms based on NHANES. Later maturation may also be associated with risk for psychosocial problems, including lower self-esteem and depression, and may have implications for reproductive health [15,47]. Thus, the implications of our study findings in perinatally infected youth focus at the other end of the spectrum, in the benefits of reducing the risk for delayed pubertal onset along with associated psychosocial and reproductive consequences. Finally, our study findings may have particular relevance for low-resource settings such as sub-Saharan Africa, where rates of vertical HIV transmission remain relatively high and thus the population of youth with perinatally-acquired HIV remains large [48]. Despite wider availability and earlier initiation of ARV treatment in South Africa and other African countries over the past decade, it has been documented that the majority of children still have severe immunodeficiency before starting treatment, increasing the risk of delayed pubertal onset [49]. Similar studies are warranted to evaluate the impact of early ARV treatment initiation on pubertal onset and maturation in low-resource settings.
We recognize several limitations in our analysis. Our cohort of HEU youth was relatively small and included few white non-Hispanic youth and no uninfected youth born before 1990. Like all studies utilizing Tanner staging measures, there is potential for misclassification, particularly for breast staging which may be confounded by increased body fat deposition [1,40]. Although orchidometers were not used in 219C, they were used in AMP and may provide better accuracy in future evaluations of pubertal progression and sexual maturation. We lacked information on birth weight or other early life exposures because our cohorts were not followed from birth. As an observational study, our analysis was subject to confounding by indication, particularly for evaluating the association of combination treatment with pubertal onset for the earlier birth cohorts; lack of information for most participants on viral load or CD4 prior to combination treatment initiation precluded our ability to evaluate and adjust for such confounding.
Despite these limitations, this is the largest study to date evaluating the timing of pubertal onset, with over 2000 PHIV youth. We found that pubertal onset occurs significantly later in HIV-infected than in uninfected youth, with the greatest delays among those with more advanced HIV disease. Importantly, combination treatment may result in more normal timing of pubertal onset, as suggested for youth born since 1997 who were receiving PI-containing combination regimens. Further evaluation of pubertal onset and sexual maturation in the current era of widespread treatment with combination treatment will be needed to fully understand the impact of ARV treatment on sexual development of youth with HIV infection.
Supplementary Material
Supplemental Digital Content 1: (table) HIV-related Characteristics as of the First Pubertal Assessment* for 2086 Perinatally HIV-infected Children by Sex
Supplemental Digital Content 2: (table) Estimated Shifts (in months) in Mean Ages at Pubertal Onset by HIV Infection Status and by HIV Disease Severity and Antiretroviral Treatment Measures among perinatally HIV-infected Youth
Acknowledgments
FUNDING:
Support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases [U01 AI068632] and the Eunice Kennedy Shriver National Institute of Child Health and Human Development [contract N01-3-3345 and HHSN267200800001C]. This work was also supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute of Allergy and Infectious Diseases, the National Institute on Drug Abuse, the National Institute of Mental Health, the National Institute of Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (U01 HD052102-04) and the Tulane University School of Medicine (U01 HD052104-01). NIH representatives were part of the study team and therefore the sponsor was involved in study design, coordination, data collection, data analysis, data interpretation, and writing of the report.
We thank the children and families for their participation in PHACS AMP and PACTG 219C, and the individuals and institutions involved in the conduct of these studies.
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- AMP
Adolescent Master Protocol
- ARV
antiretroviral
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- HEU
HIV-exposed uninfected
- HIV
human immunodeficiency virus
- IMPAACT
International Maternal Pediatric and Adolescent AIDS Clinical Trials
- NHANES
National Health and Nutrition Examination Survey
- NRTI
nucleoside reverse transcriptase inhibitor
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- PHIV
perinatally HIV-infected
- PHACS
Pediatric HIV/AIDS Cohort Study
- PI
protease inhibitor
Footnotes
Contributions: PLW and MEG were the primary authors who conceived and designed the study. PLW had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. PLW was responsible for conducting all statistical analyses, and led the writing of the manuscript. JMO, RVD, PLW, MJA, RH, and GRS provided leadership and oversight of the IMPAACT 219C study; RVD, GRS, PLW, RH, DLJ, KP, and MEG provided leadership and oversight of the PHACS AMP study. All authors provided input on the study design, interpretation of analyses, and revisions to manuscript.
All authors state that they have no conflicts of interest related to this manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The following institutions, clinical site investigators and staff participated in conducting PHACS AMP in 2011 in alphabetical order: Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Baig, Anna Cintron; Children’s Diagnostic & Treatment Center: Ana Puga, Sandra Navarro, Doyle Patton, Deyana Leon; Children’s Hospital, Boston: Sandra Burchett, Nancy Karthas, Betsy Kammerer; Ann & Robert H. Lurie Children’s Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Molly Nozyce; St. Christopher’s Hospital for Children: Janet Chen, Latreca Ivey, Maria Garcia Bulkley, Mitzie Grant; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University Health Sciences Center: Margarita Silio, Medea Jones, Patricia Sirois; University of California, San Diego: Stephen Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Emily Barr, Robin McEvoy; University of Medicine and Dentistry of New Jersey: Arry Dieudonne, Linda Bettica, Susan Adubato; University of Miami: Gwendolyn Scott, Patricia Bryan, Elizabeth Willen.
The following institutions participated in PACTG 219/219C, in alphabetical order:
Baystate Medical Center; Boston Medical Center; Bronx-Lebanon Hospital Center; Children’s Diagnostic and Treatment Center of South Florida; Children’s Hospital at Albany Medical Center; Children’s Hospital Boston; Children’s Hospital of Columbus, Ohio; Children’s Hospital of the King’s Daughters; Children’s Hospital of Los Angeles; Children’s Hospital of Michigan; Children’s Hospital of Philadelphia; Children’s Hospital & Research Center Oakland; Children’s Hospital, Washington, DC; Children’s Medical Center of Dallas; Children’s Memorial Hospital University of Chicago; Columbia University Medical Center; Columbus Medical Center; Connecticut Children’s Medical Center; Cook County Hospital; Cornell University; Duke University School of Medicine; Emory University Hospital; Harbor - UCLA Medical Center; Harlem Hospital Center; Howard University Hospital; Incarnation Children’s Center; Jacobi Medical Center; Johns Hopkins University Hospital; LA County/University of Southern California Medical Center; Lincoln Medical & Mental Health Center; Long Beach Memorial Medical Center; Medical College of Georgia School of Medicine; Medical College of Virginia; Medical University of South Carolina; Metropolitan Hospital Center; Montefiore Medical Center - Albert Einstein College of Medicine; Mt. Sinai Hospital Medical Center; New York University Medical Center/Bellevue Hospital; North Shore University Hospital; Oregon Health Sciences University; Palm Beach County Health Department; Phoenix Children’s Hospital; Ramon Ruiz Arnau University Hospital; Robert Wood Johnson University Hospital; Sacred Heart Children’s Hospital/CMS of Florida; San Francisco General Hospital; San Juan City Hospital; Schneider Children’s Hospital; Seattle Children’s Hospital & Medical Center; St. Christopher’s Hospital for Children; St. Josephs Hospital and Medical Center; St. Jude Children’s Research Hospital; St. Luke’s-Roosevelt Hospital Center; SUNY at Stony Brook School of Medicine; SUNY Downstate Medical Center; SUNY Upstate Medical University; Texas Children’s Hospital (Baylor); Tulane University Health Sciences Center; University of Alabama at Birmingham School of Medicine; University of California Los Angeles Medical Center; University of California San Diego; University of California San Francisco Medical Center; University of Cincinnati; University of Colorado at Denver and Health Sciences; University of Florida College of Medicine; University of Florida Health Science Center Jacksonville; University of Illinois at Chicago; University of Maryland Medical Center; University of Massachusetts Memorial Children’s Medical School; University of Medicine and Dentistry of New Jersey; University of Miami Miller School of Medicine; University of Mississippi Medical Center; University of North Carolina at Chapel Hill School of Medicine; University of Puerto Rico; University of Rochester Medical Center; University of South Alabama College of Medicine; University of South Florida; Vanderbilt University Medical Center; Washington University, St. Louis Children’s Hospital; Yale University School of Medicine.
References
- 1.Sørensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A. Recent secular trends in pubertal timing: Implications for evaluation and diagnosis of precocious puberty. Horm Res Paediatr. 2012;77:137–145. doi: 10.1159/000336325. [DOI] [PubMed] [Google Scholar]
- 2.Anderson SE, Dallal GE, Must A. Relative weight and race influence average age of menarche: Results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics. 2003;111:844–50. doi: 10.1542/peds.111.4.844. [DOI] [PubMed] [Google Scholar]
- 3.Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, et al. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the pediatric research in office settings network. Pediatrics. 1997;99:505–12. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 4.Herman-Giddens ME, Wang L, Koch G. Secondary sexual characteristics in boys. Estimates from the National Health and Nutrition Examination Survey III, 1988–1994. Arch Pediatr Adolesc Med. 2001;155:1022–28. doi: 10.1001/archpedi.155.9.1022. [DOI] [PubMed] [Google Scholar]
- 5.Karpati AM, Rubin, Kieszak S, Marcus M, Troiano RP. Stature and pubertal stage assessment in American Boys: The 1988–1994 Third National Health and Nutrition Examination Survey. J Adolesc Health. 2002;30:205–12. doi: 10.1016/s1054-139x(01)00320-2. [DOI] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–49. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 7.Özen S, Darcan Ş. Effects of environmental endocrine disruptors on pubertal development. J Clin Res Pediatr Endocrinol. 2011;3:1–6. doi: 10.4274/jcrpe.v3i1.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschfeld S. Dysregulation of growth and development in HIV-infected children. J Nutr. 1996;126:2641–2650S. doi: 10.1093/jn/126.suppl_10.2641S. [DOI] [PubMed] [Google Scholar]
- 9.Isanaka S, Duggan C, Fawzi WW. Patterns of postnatal growth in HIV-infected and HIV-exposed children. Nutr Rev. 2009;67:343–59. doi: 10.1111/j.1753-4887.2009.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gertner JM, Kaufman FR, Donfield SM, Sleeper LA, Shapiro AD, Howard C, et al. Delayed somatic growth and pubertal development in human immunodeficiency virus-infected hemophiliac boys: Hemophilia Growth and Development study. J Pediatr. 1994;124:896–902. doi: 10.1016/s0022-3476(05)83177-4. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman FR, Gertner JM, Sleeper LA, Donfield SM the Hemophilia Growth and Development Study. Growth hormone secretion in HIV-positive versus HIV-negative hemophilic males with abnormal growth and pubertal development. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:137–44. doi: 10.1097/00042560-199706010-00007. [DOI] [PubMed] [Google Scholar]
- 12.Mahoney EM, Donfield SM, Howard C, Kaufman F, Gertner JM the Hemophilia Growth and Development Study. HIV-associated immune dysfunction and delayed pubertal development in a cohort of young hemophiliacs. J Acquir Immune Defic Syndr. 1999;21:333–37. doi: 10.1097/00126334-199908010-00012. [DOI] [PubMed] [Google Scholar]
- 13.De Martino M, Tovo PA, Galli L, Gabiano C, Chiarelli F, Zappa M, et al. for the Italian Register for HIV Infection in Children. Puberty in perinatal HIV-infection: a multicentre longitudinal study of 212 children. AIDS. 2001;15:1527–34. doi: 10.1097/00002030-200108170-00010. [DOI] [PubMed] [Google Scholar]
- 14.Buchacz K, Rogol AD, Lindsey JC, Wilson CM, Hughes MD, Seage GR, et al. for the Pediatric AIDS Clinical Trials Group 219 Study Team. Delayed onset of pubertal development in children and adolescents with perinatally acquired HIV infection. J Acquir Immune Defic Syndr. 2003;33:56–65. doi: 10.1097/00126334-200305010-00009. [DOI] [PubMed] [Google Scholar]
- 15.Majaliwa ES, Mohn A, Chiarelli F. Growth and puberty in children with HIV infection. J Endocrinol Invest. 2009;32:85–90. doi: 10.1007/BF03345686. [DOI] [PubMed] [Google Scholar]
- 16.Stagi S, Galli L, Cecchi C, Chiappini E, Losi S, Gattinara CG, et al. Final height in patients perinatally infected with the human immunodeficiency virus. Horm Res Paediatr. 2010;74:165–71. doi: 10.1159/000281018. [DOI] [PubMed] [Google Scholar]
- 17.Geffner ME, Yeh EY, Landaw EM, Scott ML, Stiehm ER, Bryson YJ, Israele V. In vitro insulin-like growth factor-1, growth hormone, and insulin resistance occurs in symptomatic human immunodeficiency virus-1 infected children. Pediatr Res. 1993;34:66–73. doi: 10.1203/00006450-199307000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Dimock D, Thomas V, Cushing A, Purdy JB, Worrell C, Kopp JB, et al. Longitudinal assessment of metabolic abnormalities in adolescents and young adults with HIV-infection acquired perinatally or in early childhood. Metabolism. 2011;60:874–80. doi: 10.1016/j.metabol.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tassiopoulos K, Williams PL, Seage GR, Crain M, Farley J. Association of hypercholesterolemia incidence with antiretroviral treatment, including protease inhibitors, among perinatally HIV-infected children. J Acquir Immune Defic Syndr. 2008;47:607–14. doi: 10.1097/QAI.0b013e3181648e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson DL, Patel K, Siberry GK, Van Dyke RB, DiMeglio LA, Geffner ME, et al. for the Pediatric HIV/AIDS Cohort Study. Body fat distribution in perinatally HIV-infected and HIV-exposed but uninfected children in the era of highly active antiretroviral therapy: Outcomes from the Pediatric HIV/AIDS Cohort Study (PHACS) Amer J Clin Nutr. 2011;94:1485–95. doi: 10.3945/ajcn.111.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geffner ME, Patel K, Miller TL, Hazra R, Silio M, Van Dyke RB, et al. for the Pediatric HIV/AIDS Cohort Study. Factors associated with insulin resistance among children and adolescents perinatally-infected with HIV-1 in the Pediatric HIV/AIDS Cohort Study (PHACS) Horm Res Paediatr. 2011;76:386–91. doi: 10.1159/000332957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viganò A, Brambilla P, Pattarino G, Stucchi S, Fasan S, Raimondi C, et al. Long-term evaluation of glucose homeostatis in a cohort of HAART-treatment HIV-infected children: a longitudinal, observational cohort study. Clinical Drug Invest. 2009;29:101–09. doi: 10.2165/0044011-200929020-00004. [DOI] [PubMed] [Google Scholar]
- 23.Chantry CJ, Frederick MM, Meyer WA, 3rd, Handelsman E, Rich K, Paul ME, et al. Endocrine abnormalities and impaired growth in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2007;26:53–60. doi: 10.1097/01.inf.0000247131.76584.af. [DOI] [PubMed] [Google Scholar]
- 24.Lee PA, Gollenberg AL, Hediger ML, Himes JH, Zhang Z, Buck Louis GM. Luteinizing hormone, testosterone and inhibin B levels in the peripubertal period and racial/ethnic differences among boys aged 6–11 years: analyses from NHANES III, 1988–1994. Clin Endocrinol. 2010;73:744–751. doi: 10.1111/j.1365-2265.2010.03866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dreimane D, Nielsen K, Deveikis A, Bryson YJ, Geffner ME. Effect of protease inhibitors combined with standard antiretroviral therapy on linear growth and weight gain in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J. 2001;20:315–16. doi: 10.1097/00006454-200103000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Dreimane D, Geffner ME. Endocrinologic problems. In: Zeichner SL, Read JS, editors. Textbook of Pediatric HIV Care. 2. Cambridge University Press; Cambridge UK: 2006. [Google Scholar]
- 27.Nachman SA, Lindsey JC, Moye J, Stanley KE, Johnson GM, Krogstad PA, Wiznia AA for the Pediatric AIDS Clinical Trials Group 377 Study Team. Growth of human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2005;24:352–57. doi: 10.1097/01.inf.0000157095.75081.43. [DOI] [PubMed] [Google Scholar]
- 28.McGrath CJ, Chung MH, Richardson BA, Benki-Nugen S, Warui D, John-Stewart GC. Younger age at HAART initiation is associated with more rapid growth reconstitution. AIDS. 2011;25:345–55. doi: 10.1097/QAD.0b013e32834171db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Bianco G, Heresi G, Frederick T, Wheeling T, Bohannon B, Dominguez K, Siberry G. Onset of puberty in perinatally HIV-infected children and adolescents in the United States during the HAART era. Abstract presented at IDSA; October 2010; http://idsa.confex.com/idsa/2010/webprogram/Paper2859.html. [Google Scholar]
- 30.Brady MT, Oleske JM, Williams PL, Elgie C, Mofenson LM, Dankner W, Van Dyke RB for the PACTG 219/219C Team. Declines in mortality rates and changes in causes of death in HIV-1 infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53:86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brogly S, Williams PL, Seage GR, 3rd, Oleske JM, Van Dyke R, McIntosh K for the PACTG 219C Team. Antiretroviral treatment in pediatric HIV infection in the United States: from clinical trials to clinical practice. JAMA. 2005;18:2213–20. doi: 10.1001/jama.293.18.2213. [DOI] [PubMed] [Google Scholar]
- 32.Van Dyke RB, Patel K, Siberry GK, Burchett SK, Spector SA, Chernoff MC, et al. for the Pediatric HIV/AIDS Cohort Study. Antiretroviral treatment of US children with perinatally acquired HIV infection: temporal changes in therapy between 1991 and 2009 and predictors of immunologic and virologic response. J Acquir Immune Defic Syndr. 2011;57:165–73. doi: 10.1097/QAI.0b013e318215c7b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51:170–79. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. 1994 revised classification for human immunodeficiency virus infection in children less than 13 years of age. Morb Mortal Wkly Rep CDC Surveill Summ. 1994;43:1. [Google Scholar]
- 35.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morb Mortal Wkly Rep. 1992:41. [PubMed] [Google Scholar]
- 36.Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT Centers for Disease Control and Prevention (CDC) Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years--United States, 2008. MMWR Recomm Rep. 2008;57(RR-10):1–12. [PubMed] [Google Scholar]
- 37.Kalish LA, McIntosh K, Read JS, Diaz C, Landesman SH, Pitt J, Rich KC, Shearer WT, Davenny K, Lew JF. Evaluation of human immunodeficiency virus (HIV) type 1 load, CD4 T cell level, and clinical class as time-fixed and time-varying markers of disease progression in HIV-1-infected children. J Infect Dis. 1999;180:1514–20. doi: 10.1086/315064. [DOI] [PubMed] [Google Scholar]
- 38.Rich KC, Fowler MG, Mofenson LM, Abboud R, Pitt J, Diaz C, Hanson IC, Cooper E, Mendez H. Maternal and infant factors predicting disease progression in human immunodeficiency virus type 1-infected infants. Women and Infants Transmission Study Group. Pediatrics. 2000;105:e8. doi: 10.1542/peds.105.1.e8. [DOI] [PubMed] [Google Scholar]
- 39.Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron JJ, Jr, Saag MS. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol. 2010;171:198–205. doi: 10.1093/aje/kwp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorensen TIA, et al. Examination of US Puberty-Timing data from 1940 to 1994 for secular trends: Panel findings. Pediatrics. 2008;121:S172. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 41.Wu T, Mendola P, Buck GM. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: The Third National Health and Nutrition Examination Survey, 1988–1994. Pediatrics. 2002;110:752–57. doi: 10.1542/peds.110.4.752. [DOI] [PubMed] [Google Scholar]
- 42.Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, et al. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110:911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- 43.Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123:84. doi: 10.1542/peds.2008-0146. [DOI] [PubMed] [Google Scholar]
- 44.Laue L, Cutler GB., Jr . Abnormalities in growth and development. In: Pizzo PA, Wilfert CM, editors. Pediatric AIDS. 2. Williams & Wilkins; Baltimore MD: 1994. [Google Scholar]
- 45.Zeitler PS, Travers S, Kappy MS. Advances in the recognition and treatment of endocrine complications in children with chronic illness. Adv Pediatr. 1999;46:101–49. [PubMed] [Google Scholar]
- 46.Ratner Kaufman F, Gertner JM, Sleeper LA, Donfield SM. Growth hormone secretion in HIV-positive versus HIV-negative hemophilic males with abnormal growth and pubertal development. The Hemophilia Growth and Development Study. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:137–44. doi: 10.1097/00042560-199706010-00007. [DOI] [PubMed] [Google Scholar]
- 47.Brogly SB, Watts DH, Ylitalo N, Franco EL, Seage GR, III, Oleske J, et al. Reproductive health of adolescent girls perinatally infected with HIV. Amer J Public Health. 2007;97:1047–52. doi: 10.2105/AJPH.2005.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mofenson LM. Protecting the next generation: eliminating perinatal HIV-1 infection. N Engl J Med. 2010;362:2316–18. doi: 10.1056/NEJMe1004406. [DOI] [PubMed] [Google Scholar]
- 49.Fatti G, Bock P, Eley B, Mothibi E, Grimwood A. Temporal trends in baseline characteristics and treatment outcomes of children starting antiretroviral treatment: An analysis in four provinces in South Africa, 2004–2009. J Acquir Immune Defic Syndr. 2011;58:e60–67. doi: 10.1097/QAI.0b013e3182303c7e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1: (table) HIV-related Characteristics as of the First Pubertal Assessment* for 2086 Perinatally HIV-infected Children by Sex
Supplemental Digital Content 2: (table) Estimated Shifts (in months) in Mean Ages at Pubertal Onset by HIV Infection Status and by HIV Disease Severity and Antiretroviral Treatment Measures among perinatally HIV-infected Youth