Abstract
Regulatory T cells (Tregs), a subset of CD4+ T cells plays a pivotal role in regulating the immune system. An increase in Treg numbers enables cancer progression by dampening the immune system and allowing tumor cells to evade immune detection and destruction. An increase in Treg numbers and expression of inhibitory cytokines including TGF-β and IL-10 are mechanisms by which Tregs exert their immune suppressive function. However, the presence of Tregs and inhibitory cytokines in oral cancer patients is still unclear. In this study, the presence of circulating Tregs in 39 oral cancer patients and 24 healthy donors was examined by studying the presence of the CD4+CD25hiCD127low cell population in their peripheral blood mononuclear cells using flow cytometry. Serum levels of TGF-β and IL-10 were measured by ELISA. T cell subsets of OSCC patients were found to differ significantly from healthy donors where a decrease in CD8+ cytotoxic T cells and an increase in Tregs (CD4+CD25hiCD127low) were observed. Further, the ratio of CD8+ T cells/Tregs was also decreased in patients compared to healthy donors. The presence of Tregs was accompanied by a decrease in IL-10 but not TGF-β secretion in OSCC patients when compared to donors; in addition, the analysis also revealed that an increased presence of Tregs was accompanied by better patient survival. Amongst OSCC patients, smokers had significantly higher levels of TGF-β. It is apparent that the immune system is compromised in OSCC patients and the characterization of the Treg subpopulation could form a basis for improving our understanding of the perturbations in the immune system that occur during OSCC tumorigenesis.
Introduction
The inability of the immune system to eradicate established tumors is a well-recognized hallmark of cancer [1], [2]. Tumors can employ numerous mechanisms to suppress host immunity and in particular, many studies have focused on the role of regulatory T cells (Tregs) in helping tumors evade immune surveillance [3]–[6]. Whilst Tregs are important regulators of immune-mediated inflammation, they have been demonstrated to be equally critical as mediators of active immune evasion; the depletion of these cells can improve endogenous anti-tumor immunity and efficacy of immunotherapy [7], [8]. This realization has resulted in the development of strategies to target the immunosuppressive arm of the immune system [9], [10].
Treg-mediated suppression can occur through several mechanisms, either by cell-cell contact or by secretion of cytokines (TGF-β and IL-10) [11]. The involvement of Tregs in tumor progression in patients with lung, head and neck, prostate, and breast cancers has been demonstrated, where there are increased levels of these cells in the peripheral blood [12]–[15]. Further, levels of IL-10 which mediates immune suppression by down-regulating MHC class I expression or by inhibiting T cell activation were shown to be increased in many types of cancers including melanoma, head and neck, pancreatic, gastric, lung, and breast [16]–[23]. TGF-β is a potent suppressor of the immune system and it inhibits the secretion of immunoglobulin (Ig) M, IgG1, IgG2a and IgG3 [24]. Neutralizing antibodies to TGF-β have been shown to reverse Treg-mediated suppression in inflammatory bowel disease (IBD) in mice and thyroiditis in rats [25], [26]. In the main, the presence of Tregs has been associated with poor disease outcome, however, contradictory reports exists [12], [13], [15], [27]–[34]. The discrepancies happen in part because of the multiple markers that are used to identify the Treg population. Markers delineating these cells include CD25, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR), lymphocyte activation gene-3 (LAG-3), CD127 and forkhead / winged-helix transcription factor box P3 (FOXP3) [26], [35]–[39]. As many of these markers are also markers of T cell activation [40], CD4+CD25+FOXP3+ and CD4+CD25+CD127low remain the most well accepted markers for the identification of Tregs, as cells with these phenotypes are immunosuppressive [41]–[43].
Head and neck cancers pose a significant global burden [44], [45] and oral cancer, a subset of head and neck cancers occurs in about 400,000 individuals world-wide, and contribute to more than 222,000 deaths annually [44], [46]. Moreover 50% of oral cancer patients suffer from disease recurrence or secondary tumors [47]–[49]. As we begin to identify key genetic drivers through the compilation of gene expression, chromosomal copy number and sequencing data for many types of solid tumors [50], [51], the development of novel treatments (especially immunotherapy) to target some of the mutations arising from these genetic events is currently possible [52]. However, the presence of immune suppression in patients could still hinder the success of these novel treatment modalities. In this study, the immunological status of oral cancer patients was characterized by looking at their lymphocyte subsets, and the levels of IL-10 and TGF-β with the hypothesis that OSCC patients would have a compromised immune system with an increased presence of Tregs and levels of IL-10 and TGF-β. A significant increase in CD4+CD25hiCD127low sub-population of Tregs in OSCC patients and a novel subpopulation of CD4+CD25hi that completely lacked of CD127 expression was found in OSCC patients. Further, the presence of regulatory T cells was accompanied by a decrease in IL-10 but not TGF-β secretion. Our results show that the level of Tregs differs between OSCC patients and normal individuals suggesting that these cells may play a major role in oral carcinogenesis.
Materials and Methods
Peripheral Blood Mononuclear Cell (PBMC) isolation
Thirty-nine OSCC patients (no prior treatment) and 24 healthy donors were included in this study. Healthy donors were recruited from relatives or friends who accompanied the patient and at blood donation drives. All individuals were briefed regarding the project prior to obtaining written consent; in the event of an individual who was unable to provide written consent due to the inability to write, written consent was either obtained in the form of a thumb print or from the next of kin. All consent documents were filed and kept for future reference in the Oral Cancer Research & Coordinating Centre (OCRCC), Faculty of Dentistry, University of Malaya. All relevant documents and procedures were approved by the institutional review board of the Faculty of Dentistry Medical Ethics Committee, University Malaya (Medical ethics number: DF OS0910/0049(L). Eight to thirty milliliters of peripheral blood was collected from each of these individuals in BD Vacutainer CPT tubes (Becton Dickinson, NJ, USA). Peripheral blood mononuclear cells (PBMC) were isolated from the peripheral blood, washed in Hanks balanced salt solution (HBSS; Gibco, Life Technologies, California, USA) and re-suspended in complete culture medium [Roswell Park Memorial Institute Medium (RPMI; Gibco, Life Technologies, California, USA) supplemented with 5% heat-inactivated human AB serum (Gemini Bio-Product, California, USA) and 1× penicillin/streptomycin (Gibco, Life Technologies, California, USA)]. PBMC (1×106)were used for the assays as described below.
Identifying circulating T cell subsets
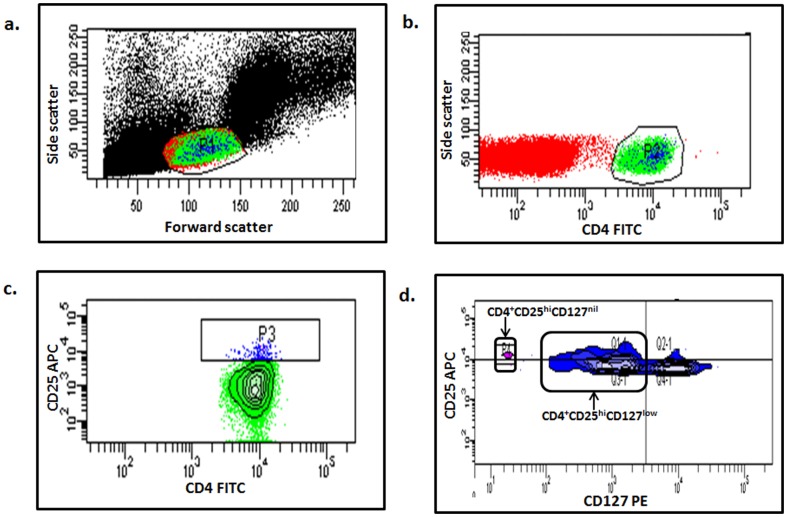
The presence of Tregs was determined in 35 OSCC patients and 14 healthy donors by measuring the following markers: CD4 (BD Biosciences-Pharmingen, California, USA), CD25 (e-Bioscience, CA, USA) and CD127 (BD Biosciences-Pharmingen, California, USA). Briefly, 5×105 fresh PBMCs were incubated with specific antibodies conjugated with fluorochrome (0.42 µg/ml of CD25-APC, 0.33 µg/ml of CD4-FITC and 0.33 µg/ml of CD127-PE) for 30 minutes at 4°C. The cells were then washed using phosphate buffered saline (PBS), resuspended in Pharmingen Stain Buffer (PSB) and analyzed using a flow cytometer (FACSCalibur, BD Biosciences, California, USA). The presence of Tregs was then analyzed using FACSDiva analysis software (BD Biosciences, California, USA). Briefly, 50,000–100,000 cells were collected and lymphocyte population was gated based on cell size (Figure 1a) and the presence of helper T cells (CD4+), CD4+CD25hi and CD4+CD25hiCD127low cells were further determined from the CD4+ cell population (Figure 1b, c, d). In addition, the presence of CD4+CD25hiCD127nil cells were also identified (Figure 1d). Similarly, CD4+ and CD8+ T cell subsets were detected with TCR-APC (Miltenyi Biotec, Surrey, UK), CD4-FITC (BD Biosciences-Pharmingen, California, USA) and CD8-PE (Invitrogen, NY, USA) antibodies and stained as described above in 35 OSCC patients, 9 healthy donors and 27 OSCC patients and 9 healthy donors respectively.
Figure 1. Gating used for the identification of circulating T cell subsets in human.
Multiparameter flow cytometry analyses of CD4+CD25hi, CD4+CD25hiCD127low and CD4+CD25hiCD127nil regulatory T cells subsets. Gates were set on (a) T lymphocytes (based on forward and side scatter), (b) CD4+ T cells, (c) CD4+CD25hi and (d) CD4+CD25hiCD127low and CD4+CD25hiCD127nil.
Measurement of IL-10 and TGF-β by Enzyme-linked Immunosorbent Assay (ELISA)
The levels of two cytokines (IL-10 and TGF-β) which are known to be involved in the immunosuppressive function of Tregs were analyzed in 30 OSCC patients and 16 healthy donors using ELISA, according to the manufacturer's protocol (Invitrogen, New York, USA). Prior to analyzing TGF-β levels, 100 µl of serum was activated by adding 4 µl of 1N HCl and incubated at room temperature for 15 minutes and neutralized using 3 µl 1N NaOH. The activated serum was then diluted 4× with assay buffer and dispensed into 96 well plates which were pre-coated with TGF-β antibody, this was followed by the addition of the detection antibody and streptavidin-HRP conjugate. The specific binding was visualized after adding the substrate solution. A standard curve (log-log curve fit) was generated using respective recombinant human cytokine and the absolute concentration of cytokine in the serum was determined from this curve. Detection of IL-10 levels was performed as described above without the serum activation procedure.
Statistical analysis
All statistical analyses were performed using the statistical software package SPSS 16 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism software (GraphPad Inc., CA, USA). Independent t-test was used to compare the differences of immune cells/cytokines levels in patients and healthy donors. The correlation between immune cells subsets and cytokines levels with clinico-pathological factors was estimated by Fisher's exact test. Kaplan-Meier survival analysis was used to correlate survival rates with the level of CD4+CD25hiCD127low regulatory T cells, the survival probability differences were compared by log-rank test. A p-value of <0.05 was considered to be statistically significant.
Results
Demographic and clinical characteristics of OSCC patients
The demographic and clinical characteristics of the patients included in this study are tabulated in Table 1. The mean age of the OSCC patients was 58±13 years (range: 26–76 years). The ethnic distribution of the patients was representative of the OSCC patient population in Malaysia where the majority of the patients were of Indian ethnicity. More than 50% of the patients were diagnosed with late stage disease (stage III & IV; Table 1). The mean age of healthy donors was 32±8 years (range 24–49 years), but other demographic information on these individuals were not available. Survival information was available for 32 of 39 patients, in which the length of follow up ranged from 2 to 85 months until May of 2013, with a median survival of 24 months.
Table 1. Patients' demographic data.
| Variables | OSCC | |||
| Age (year) | Mean ± SD: 58±13 | n | % | |
| Gender | Male | 8 | 21 | |
| Female | 31 | 79 | ||
| Ethnic | Malay | 3 | 8 | |
| Chinese | 4 | 10 | ||
| Indian | 31 | 79 | ||
| Others* | 1 | 3 | ||
| Habits | Ever Smoker | 6 | 15 | |
| Ever Drinker | 5 | 13 | ||
| Ever Chewer | 23 | 59 | ||
| No habit | 10 | 26 | ||
| Stage | Early (stage I & II) | 13 | 33 | |
| Late (stage III & IV) | 23 | 59 | ||
| Data not available | 3 | 8 | ||
| Disease sites | Cheek | 16 | 41 | |
| Tongue | 10 | 26 | ||
| Gingiva | 5 | 13 | ||
| Others** | 7 | 18 | ||
| Data not available | 1 | 2 | ||
*Others: Indigenous population.
** Others: Lip, palate, floor of mouth.
Comparison of circulating T cell subsets in OSCC patients and healthy donors
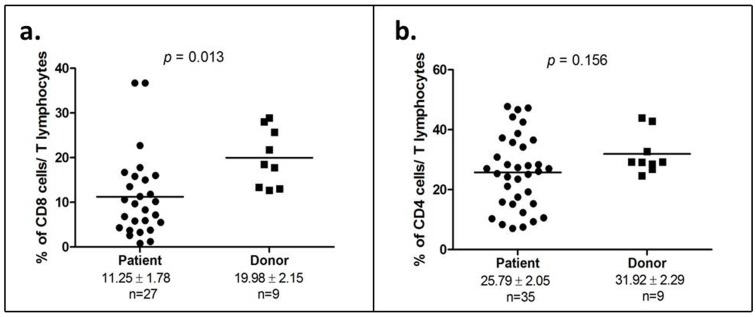
T lymphocyte subpopulations in OSCC patients and healthy donors were analyzed using flow cytometry. No difference was found in the average percentage of T lymphocytes between OSCC patients and healthy donors (data not shown). However, the analysis of sub-populations of T lymphocytes demonstrated a significant reduction in the cytotoxic T cells population (CD8+) in OSCC patients compared to healthy donors (11.25±1.78 vs 19.98±2.15; p = 0.013; Figure 2a). Further, a decrease in helper T cells (CD4+) count was also seen in OSCC patients compared to healthy donors but this difference was not statistically significant (25.79±2.05 vs 31.92±2.29; p = 0.156; Figure 2b).
Figure 2. Reduction of cytotoxic T cells (CD8+) is a characteristic of OSCC patients.
(a) Percentage of cytotoxic T lymphocytes (CD8+) in both OSCC patients and healthy donors; (b) Percentage of helper T cells (CD4+) in both OSCC patients and healthy donors. Each symbol represents an individual and the narrow bar represents the mean percentage of the specific cell population.
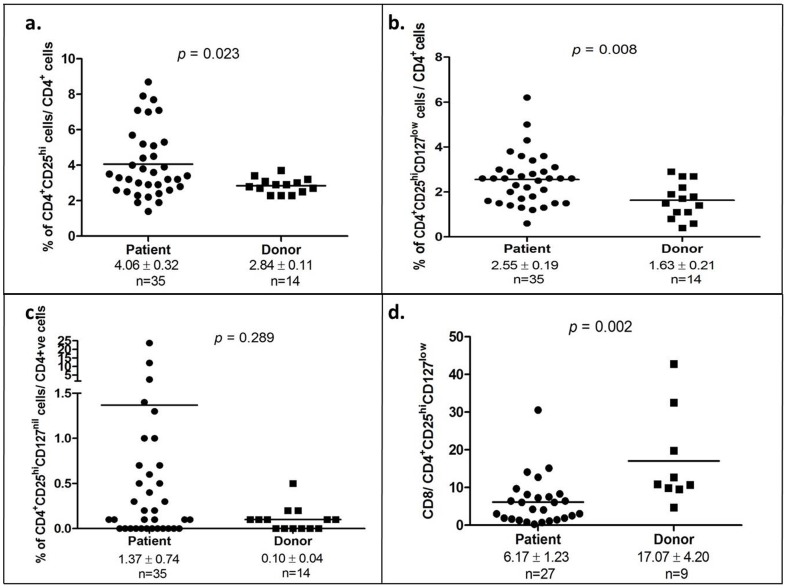
The percentage of CD4+CD25hi was significantly elevated in OSCC patients compared to healthy donors (4.06±0.32 vs 2.84±0.11; p = 0.023; Figure 3a). Notably, the expanded subset of the CD4+CD25hi cells, the CD4+CD25hi CD127low T cells (which are indicative of defined regulatory T cells) was prominently higher in OSCC patients compared to healthy donors (2.55±0.19 vs 1.63±0.21; p = 0.008; Figure 3b). Of note, another population of cells that totally lacked the expression of CD127 was detected and designated here as the CD4+CD25hiCD127nil population; this subpopulation of CD4+CD25hi cells demonstrated a diverse presence in patients ranging from 0%–23.7% of the CD4+ T cell population, whilst this diversity was not observed in healthy donors (0%–0.4% of CD4+ T cells). However, when comparing the average levels of CD4+CD25hiCD127nil levels, no significant difference was observed between OSCC patients and healthy donors (1.37±0.74 vs 0.10±0.04; p = 0.289; Figure 3c). Given that a significant reduction in CD8+ T cell population and a prominent increment of the of CD4+CD25hi CD127low regulatory T cells were observed, the ratio of CD8+ T cell to CD4+CD25hi CD127low regulatory T cells was further determined; OSCC patients demonstrated a 3-fold decrease in the ratio as compared to healthy donors (6.17±1.23 vs 17.07±4.20; p = 0.002).
Figure 3. CD4+CD25hiCD127low regulatory T cells were up-regulated in OSCC patients.
(a) Percentage of CD4+CD25hi T cells amongst CD4+T cells in OSCC patients and healthy donors; (b) Percentage of CD4+CD25hiCD127low T cells amongst CD4+ T cells in OSCC patients and healthy donors; (c) Percentage of CD4+CD25hi CD127nil T cells amongst CD4+ T cells in OSCC patients and healthy donors; (d) Ratio of CD8 T cells/CD4+CD25hiCD127low T cells in OSCC patients and healthy donors; Each symbol represents an individual person and the narrow bar represents the mean percentage of the specific cell population.
The presence of TGF-β and IL-10 in the serum of OSCC patients and healthy donors
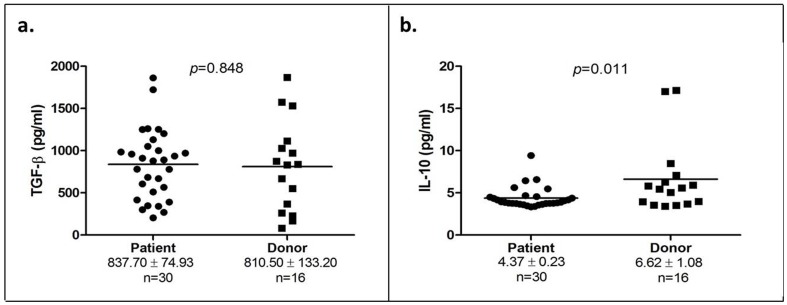
We did not find any difference in the level of TGF-β in OSCC patients compared to healthy donors (837.704±74.93 pg/ml vs 810.50±133.20 pg/ml; p = 0.848; Figure 4a). However, our results exhibited a significant decrease in the IL-10 level in OSCC patients as compared to healthy individuals (4.37±0.23 pg/ml vs 6.62±1.08 pg/ml samples; p = 0.011 Figure 4b).
Figure 4. TGF-β and IL-10 levels in OSCC patients and healthy donors.
(a) TGF-β concentration of 30 OSCC patients and 16 healthy donors; (b) IL-10 concentration of 30 OSCC patients and 16 healthy donors. Each symbol represents an individual person and the narrow bar represents the mean percentage of the specific cell population.
Correlation of circulating T cell subsets and cytokines with patient clinico-pathological factors
We analyzed if there were any correlation between of the percentage of Tregs (CD4+CD25hiCD127low) and the levels of TGF-β/IL-10 with patient's clinico-pathological parameters. Our data showed that OSCC patients who smoke have significantly higher TGF-β levels compared to non-smokers (p = 0.001; Table 2A) but no correlation between IL-10 levels with patients' clinico-pathological parameters was observed (Table 2B). Furthermore, patients who were at early stages of oral cancer had higher levels CD4+CD25hiCD127low regulatory T cells (p = 0.027, Table 3A) but this correlation was not seen for the CD8+ T cells/Tregs ratio (Table 3B). In addition, no association between the levels of Tregs, IL-10 and TGF-β was found (data not shown).
Table 2. The correlation between TGF-β (A) and IL-10 (B) levels with patient clinico-pathological factors.
| A | |||
| Factor | N | TGF-β | p value |
| Mean ± SD | |||
| Staging | 29 | ||
| Early | 10 | 882.50±562.78 | |
| Late | 19 | 822.93±331.82 | 0.721 |
| Ever chewer | 30 | ||
| Yes | 18 | 818.88±391.54 | |
| No | 12 | 865.87±453.55 | 0.765 |
| Ever smoker | 30 | ||
| Yes | 5 | 1339.58±296.05 | |
| No | 25 | 737.30±354.90 | 0.001 |
| Ever drinker | 30 | ||
| Yes | 4 | 955.53±385.28 | |
| No | 26 | 819.55±418.35 | 0.547 |
| Mean Age | 30 | ||
| ≥58 | 22 | 795.83±402.34 | |
| <58 | 8 | 953.76±437.67 | 0.363 |
Table 3. The correlation between specific T cell populations and patient clinico-pathological factors.
| A | |||
| Factor | N | CD4+CD25hiCD127low | p value |
| Mean ± SD | |||
| Staging | 33 | ||
| Early | 12 | 3.08±1.41 | |
| Late | 21 | 2.01±0.80 | 0.027 |
| Ever chewer | 35 | ||
| Yes | 19 | 2.37±1.16 | |
| No | 16 | 2.78±1.10 | 0.299 |
| Ever smoker | 35 | ||
| Yes | 6 | 2.73±1.04 | |
| No | 29 | 2.52±1.17 | 0.678 |
| Ever drinker | 35 | ||
| Yes | 4 | 2.45±0.97 | |
| No | 31 | 2.57±1.17 | 0.849 |
| Mean Age | 35 | ||
| ≥58 | 22 | 2.53±1.26 | |
| <58 | 13 | 2.59±0.93 | 0.882 |
High levels of CD4+CD25hiCD127low Tregs is associated with better survival
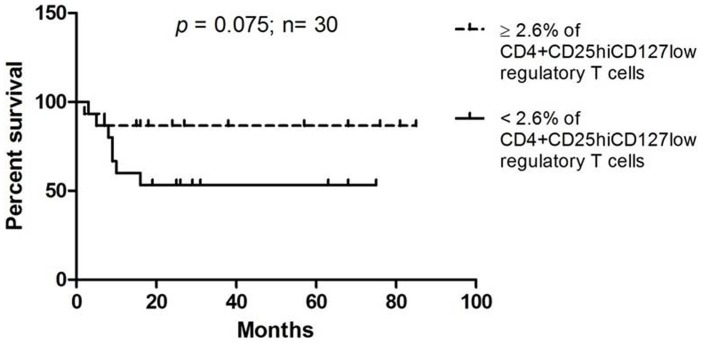
To evaluate the correlation of the presence of CD4+CD25hiCD127low regulatory T cells to patient survival, we used the mean percentage of CD4+CD25hiCD127low regulatory T cells (2.6%) of all patients as cut off point and divided patients into those who have equal or more than 2.6% of CD4+CD25hiCD127low regulatory T cells and those who have less than 2.6% of CD4+CD25hiCD127low regulatory T cells. Using Kaplan-Meier analysis, patients who had higher levels of CD4+CD25hiCD127low regulatory T cells were found to have better survival probability compared to patients with lower CD4+CD25hiCD127low regulatory T cells, however this did not reach statistical significance (p = 0.075; Figure 5).
Figure 5. Disease-specific Kaplan-Meier survival estimates based on the levels of CD4+CD25hiCD127low regulatory T cells.
Discussion
The homeostasis between immune activation and immune suppression is important in preventing tumorigenesis. The immune system functions either to eliminate cancer cells or to keep cancer cells in check by maintaining an equilibrium; if there is a malfunction in the immune system, immune escape may happen which then allows cancer cells to grow into clinically apparent tumors [53]. The mechanisms by which tumor escapes immune-surveillance include deregulation of the function of Tregs and/or immune-regulatory cytokines.
In our study, the percentage of T lymphocytes in both the OSCC patients and healthy donors were not significantly different. Previous reports have demonstrated a reduction of T lymphocytes in cancer patients, the main difference between our study and these may lie in the fact that in our study, treatment naïve patients were recruited whereas most other studies included post-treated cancer patients who had undergone chemo/radiotherapy which could result in lymphodepletion [54], [55]. Looking at the subsets of T cells, a reduction in the CD8+ T cell population was observed, which suggests a compromised immune system, as CD8+ T cells are involved in adaptive immunity that is responsible for eliminating tumor cells when the cancer develops [56]. This data is consistent with a previous report where a reduction in the CD8+ T cell population was also observed in head and neck cancer patients [57].
Tregs have been reported to be increased in the peripheral blood of various cancers [7], [13], [58]–[60]. While the levels of circulating Tregs have initially been reported to be increased in head and neck cancers [60], [61], emerging data showed no differences in the Treg numbers between head and neck cancer patients and healthy controls [43], [62]. These discrepancies are likely due to the differences in patient cohorts where a variety of anatomical sub-sites were analyzed, and the utilization of different markers in defining the Treg cell populations. While we observed a significant increase in the Treg cell populations in OSCC patients compared to healthy donors, Drennan et al. found no difference despite using identical markers in identifying Tregs (CD4+CD25highCD127low/−) [43]. The possible reason for this discrepancy is that the current study analyzed OSCC patients while Drennan and colleagues conducted their study purely on patients with laryngeal and oropharyngeal cancers. Differences in OSCC and oropharyngeal are well established, and the most apparent is the involvement of HPV where up to 60% of oropharyngeal cancer have been reported to be HPV positive [63]. By contrast, the prevalence of HPV infection in OSCC is low [64] and emerging data from our laboratory indicate that the prevalence of HPV infection in Malaysia OSCC patients is in the range of 1.5% (TG Kallarakkal et al; unpublished data), suggesting that this could be one of the reasons for the discrepancy that is observed. In our study, high levels of CD4+CD25hiCD127low cells were found to be significantly increased in early stage OSCC patients compared to late stage patients and further found to trend with better patient survival. This seemingly contradictory observation of a better prognosis associated with higher levels of Tregs could be explained by their role in reducing tumor-specific immune-mediated inflammation which has been shown to drive the progression of tumors in certain cases [65]–[67]. This finding is in line with other studies demonstrating that the presence of Tregs correlated with better loco-regional control in head and neck cancer and the presence of Treg is an indicator of better overall survival in Hodgkin's lymphoma [12], [68]. Indeed, adoptive transfer of Tregs in mice has been shown to suppress colitis-associated colon cancer and intestinal adenomas [69], [70] and this could likely be due to the ability of Tregs in reducing the effects of chronic inflammation by down-regulating the expression of cyclooxygenase-2 and pro-inflammatory cytokines including TNF-α and IFN-γ. This effect on other solid tumors remains to be tested. Further, the CD8+ T cell/Treg ratio has been shown to be significantly associated with patient survival [5], [71], although a meaningful reduction of this ratio in OSCC patients was observed, correlation with patient survival was not significant. In addition a subpopulation where CD127 is not expressed (CD4+CD25hiCD127nil) was detected, this population has not yet been reported and the significance and function if any, of this cell population remains unclear.
In addition to the up-regulation of Tregs, the secretion of immune regulatory cytokines particularly TGF-β and IL-10 is also known to be a mechanism of immune suppression [72], [73]. The increase expression of IL-10 in head and neck squamous cell carcinoma patients remains controversial. Several studies have suggested that patients with advanced head and neck cancer have elevated serum levels of IL-10 and this finding is associated with poor prognosis [74], [75]. However, others failed to detect a differential expression of IL-10 in these patients [76], [77]. The main reason for these could be the inherent differences in tumours from different sites of the head and neck where patients with oral cavity cancers are more likely to have undetectable levels of IL-10 compared to other sub-sites [74]. In the present study, the IL-10 levels were decreased or undetectable in OSCC patients and were not correlated with any patient's demographic information. This is consistent with the findings of Green et al. where IL-10 levels were not associated with any clinicopathological parameters nor with overall patient survival [78]. As for the serum levels of TGF-β, both patients and healthy donors were found to have similar levels of this cytokine. However, further analysis showed that smokers had significantly higher levels of TGF-β compared with non-smokers who incidentally were mainly betel quid chewers. TGF-β is known to have complex and contradictory growth effects on cancer cells [79] but the relationship between smoking and increase in TGF-β levels is still not well understood and warrants further investigation. Perhaps the lack of TGF-β and IL-10 is not too surprising as others have demonstrated that in head and neck cancers, Tregs that primarily secrete IL-10 and TGF-β are those within the population of tumor infiltrating lymphocytes and are not those in circulation [80].
In summary, the current findings are consistent with other studies, OSCC patients have a compromised immune status where the CD8+ T cell levels are reduced and the Treg population is elevated. Within the OSCC patient cohort however, higher levels of Treg were associated with better survival. A new group of T cells that is CD4+CD25hiCD127nil have also been demonstrated which has not been reported previously. The presence of this population of cells particularly within the OSCC patients warrants further investigation.
Acknowledgments
We thank our collaborating partners in Oral Cancer Research and Co-ordinating Centre, University Malaya (OCRCC) and the Ministry Of Health Malaysia for the collection of tissue and data under the Malaysian Oral Cancer Database and Tissue Bank System (MOCDTBS). CARIF is a non-profit research organization. We are committed to an understanding of cancer prevention, diagnosis and treatment through a fundamental research programme.
Funding Statement
This study was funded by the University of Malaya Research Grant (UMRG), RG289/11HTM, the Ministry of Science, Technology and Innovation (MOSTI) of Malaysia (e-Science Fund, 02-04-03-SF0011) and other sponsors of the Cancer Research Initiatives Foundation (CARIF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 3. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, et al. (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313: 1960–1964. [DOI] [PubMed] [Google Scholar]
- 4. Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, et al. (2009) Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer immunology, immunotherapy: CII 58: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, et al. (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America 102: 18538–18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang YL, Li J, Mo HY, Qiu F, Zheng LM, et al. (2010) Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Molecular cancer 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, et al. (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine 10: 942–949. [DOI] [PubMed] [Google Scholar]
- 8. Yu P, Lee Y, Liu W, Krausz T, Chong A, et al. (2005) Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. The Journal of experimental medicine 201: 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Julie R Brahmer LH, Scott Antonia, David R Spigel, Leena Gandhi, Lecia V Sequist, et al. (2012) Clinical activity and safety of anti-PD1 (BMS-936558, MDX-1106) in patients with advanced non-small-cell lung cancer (NSCLC). Journal of clinical oncology: official journal of the American Society of Clinical Oncology 30 : suppl; abstr 7509. [Google Scholar]
- 10. Robert C, Thomas L, Bondarenko I, O'Day SJ, McDermott DF, et al. (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England journal of medicine 364: 2517–2526. [DOI] [PubMed] [Google Scholar]
- 11. Menetrier-Caux C, Curiel T, Faget J, Manuel M, Caux C, et al. (2012) Targeting regulatory T cells. Targeted oncology 7: 15–28. [DOI] [PubMed] [Google Scholar]
- 12. Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, et al. (2006) Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clinical cancer research: an official journal of the American Association for Cancer Research 12: 465–472. [DOI] [PubMed] [Google Scholar]
- 13. Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ (2005) Regulatory T cells in ovarian cancer: biology and therapeutic potential. American journal of reproductive immunology 54: 369–377. [DOI] [PubMed] [Google Scholar]
- 14. Fox SB, Launchbury R, Bates GJ, Han C, Shaida N, et al. (2007) The number of regulatory T cells in prostate cancer is associated with the androgen receptor and hypoxia-inducible factor (HIF)-2alpha but not HIF-1alpha. The Prostate 67: 623–629. [DOI] [PubMed] [Google Scholar]
- 15. Okita R, Saeki T, Takashima S, Yamaguchi Y, Toge T (2005) CD4+CD25+ regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer. Oncology reports 14: 1269–1273. [PubMed] [Google Scholar]
- 16. Asadullah K, Sterry W, Volk HD (2003) Interleukin-10 therapy—review of a new approach. Pharmacological reviews 55: 241–269. [DOI] [PubMed] [Google Scholar]
- 17. Cacev T, Radosevic S, Krizanac S, Kapitanovic S (2008) Influence of interleukin-8 and interleukin-10 on sporadic colon cancer development and progression. Carcinogenesis 29: 1572–1580. [DOI] [PubMed] [Google Scholar]
- 18. Chan SL, Mo FK, Wong CS, Chan CM, Leung LK, et al. (2012) A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer 118: 3984–3992. [DOI] [PubMed] [Google Scholar]
- 19. Fujieda S, Sunaga H, Tsuzuki H, Fan GK, Saito H (1999) IL-10 expression is associated with the expression of platelet-derived endothelial cell growth factor and prognosis in oral and oropharyngeal carcinoma. Cancer letters 136: 1–9. [DOI] [PubMed] [Google Scholar]
- 20. Gaiolla RD, Domingues MA, Niero-Melo L, de Oliveira DE (2011) Serum levels of interleukins 6, 10, and 13 before and after treatment of classic Hodgkin lymphoma. Archives of pathology & laboratory medicine 135: 483–489. [DOI] [PubMed] [Google Scholar]
- 21. Gholamin M, Moaven O, Memar B, Farshchian M, Naseh H, et al. (2009) Overexpression and interactions of interleukin-10, transforming growth factor beta, and vascular endothelial growth factor in esophageal squamous cell carcinoma. World journal of surgery 33: 1439–1445. [DOI] [PubMed] [Google Scholar]
- 22. Hatanaka H, Abe Y, Kamiya T, Morino F, Nagata J, et al. (2000) Clinical implications of interleukin (IL)-10 induced by non-small-cell lung cancer. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 11: 815–819. [DOI] [PubMed] [Google Scholar]
- 23. Heckel MC, Wolfson A, Slachta CA, Schwarting R, Salgame P, et al. (2011) Human breast tumor cells express IL-10 and IL-12p40 transcripts and proteins, but do not produce IL-12p70. Cellular immunology 266: 143–153. [DOI] [PubMed] [Google Scholar]
- 24. Coffman RL, Lebman DA, Shrader B (1989) Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. The Journal of experimental medicine 170: 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seddon B, Mason D (1999) Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor beta and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4(+)CD45RC- cells and CD4(+)CD8(−) thymocytes. The Journal of experimental medicine 189: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Read S, Malmstrom V, Powrie F (2000) Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. The Journal of experimental medicine 192: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, et al. (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 24: 5373–5380. [DOI] [PubMed] [Google Scholar]
- 28. Liu L, Yao J, Ding Q, Huang S (2006) CD4+CD25high regulatory cells in peripheral blood of NSCLC patients. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban 26: 548–551. [DOI] [PubMed] [Google Scholar]
- 29. Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, et al. (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. Journal of immunology 169: 2756–2761. [DOI] [PubMed] [Google Scholar]
- 30. Matsuura K, Yamaguchi Y, Ueno H, Osaki A, Arihiro K, et al. (2006) Maturation of dendritic cells and T-cell responses in sentinel lymph nodes from patients with breast carcinoma. Cancer 106: 1227–1236. [DOI] [PubMed] [Google Scholar]
- 31. Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, et al. (2008) Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood 112: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Powell DJ Jr, Attia P, Ghetie V, Schindler J, Vitetta ES, et al. (2008) Partial reduction of human FOXP3+ CD4 T cells in vivo after CD25-directed recombinant immunotoxin administration. Journal of immunotherapy 31: 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A (2003) CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 98: 1089–1099. [DOI] [PubMed] [Google Scholar]
- 34. Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, et al. (2008) Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica 93: 193–200. [DOI] [PubMed] [Google Scholar]
- 35. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M (1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Journal of immunology 155: 1151–1164. [PubMed] [Google Scholar]
- 36. McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, et al. (2002) CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16: 311–323. [DOI] [PubMed] [Google Scholar]
- 37. Huang CT, Workman CJ, Flies D, Pan X, Marson AL, et al. (2004) Role of LAG-3 in regulatory T cells. Immunity 21: 503–513. [DOI] [PubMed] [Google Scholar]
- 38. Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, et al. (2006) Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. The Journal of experimental medicine 203: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057–1061. [DOI] [PubMed] [Google Scholar]
- 40. Corthay A (2009) How do regulatory T cells work? Scandinavian journal of immunology 70: 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, et al. (2006) CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. The Journal of experimental medicine 203: 1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ziegler SF (2006) FOXP3: of mice and men. Annual review of immunology 24: 209–226. [DOI] [PubMed] [Google Scholar]
- 43. Drennan S, Stafford ND, Greenman J, Green VL (2013) Increased frequency and suppressive activity of CD127(low/-) regulatory T cells in the peripheral circulation of patients with head and neck squamous cell carcinoma are associated with advanced stage and nodal involvement. Immunology 140: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer Journal international du cancer 127: 2893–2917. [DOI] [PubMed] [Google Scholar]
- 45. Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA: a cancer journal for clinicians 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 46. Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 47. Dhooge IJ, De Vos M, Van Cauwenberge PB (1998) Multiple primary malignant tumors in patients with head and neck cancer: results of a prospective study and future perspectives. The Laryngoscope 108: 250–256. [DOI] [PubMed] [Google Scholar]
- 48. Mashberg A, Samit AM (1989) Early detection, diagnosis, and management of oral and oropharyngeal cancer. CA Cancer J Clin 39: 67–88. [DOI] [PubMed] [Google Scholar]
- 49. Silverman S Jr, Gorsky M (1990) Epidemiologic and demographic update in oral cancer: California and national data–1973 to 1985. Journal of the American Dental Association 120: 495–499. [DOI] [PubMed] [Google Scholar]
- 50. Cancer Genome Atlas Research N, Weinstein JN, Collisson EA, Mills GB, Shaw KR, et al (2013) The Cancer Genome Atlas Pan-Cancer analysis project. Nature genetics 45: 1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, et al. (2012) The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483: 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, et al. (2014) Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344: 641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, et al. (2007) Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450: 903–907. [DOI] [PubMed] [Google Scholar]
- 54. Zitvogel L, Apetoh L, Ghiringhelli, Kroemer G (2008) Immunological aspects of cancer chemotherapy. Nature reviews Immunology 8: 59–73. [DOI] [PubMed] [Google Scholar]
- 55. Laurent J, Speiser DE, Appay V, Touvrey C, Vicari M, et al. (2010) Impact of 3 different short-term chemotherapy regimens on lymphocyte-depletion and reconstitution in melanoma patients. Journal of immunotherapy 33: 723–734. [DOI] [PubMed] [Google Scholar]
- 56. Gattinoni L, Powell DJ Jr, Rosenberg SA, Restifo NP (2006) Adoptive immunotherapy for cancer: building on success. Nature reviews Immunology 6: 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL (2004) Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clinical cancer research: an official journal of the American Association for Cancer Research 10: 3755–3762. [DOI] [PubMed] [Google Scholar]
- 58. Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, et al. (2010) High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. International journal of cancer Journal international du cancer 126: 2635–2643. [DOI] [PubMed] [Google Scholar]
- 59. Ling KL, Pratap SE, Bates GJ, Singh B, Mortensen NJ, et al. (2007) Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer immunity 7: 7. [PMC free article] [PubMed] [Google Scholar]
- 60. Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, et al. (2005) Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. British journal of cancer 92: 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL (2007) The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clinical cancer research: an official journal of the American Association for Cancer Research 13: 6301–6311. [DOI] [PubMed] [Google Scholar]
- 62. Gasparoto TH, de Souza Malaspina TS, Benevides L, de Melo EJ Jr, Costa MR, et al. (2010) Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer immunology, immunotherapy: CII 59: 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van Monsjou HS, Balm AJ, van den Brekel MM, Wreesmann VB (2010) Oropharyngeal squamous cell carcinoma: a unique disease on the rise? Oral oncology 46: 780–785. [DOI] [PubMed] [Google Scholar]
- 64. Ribeiro KB, Levi JE, Pawlita M, Koifman S, Matos E, et al. (2011) Low human papillomavirus prevalence in head and neck cancer: results from two large case-control studies in high-incidence regions. International journal of epidemiology 40: 489–502. [DOI] [PubMed] [Google Scholar]
- 65. Kmieciak M, Knutson KL, Dumur CI, Manjili MH (2007) HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. European journal of immunology 37: 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Manjili MH, Egilmez N, Knutson KL, Selvan SR, Ostberg JR (2012) Tumor escape and progression under immune pressure. Clinical & developmental immunology 2012: 641079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reiman JM, Kmieciak M, Manjili MH, Knutson KL (2007) Tumor immunoediting and immunosculpting pathways to cancer progression. Seminars in cancer biology 17: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF, et al. (2005) Outcome in Hodgkin's lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clinical cancer research: an official journal of the American Association for Cancer Research 11: 1467–1473. [DOI] [PubMed] [Google Scholar]
- 69. Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, et al. (2003) CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. The American journal of pathology 162: 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, et al. (2005) CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer research 65: 3998–4004. [DOI] [PubMed] [Google Scholar]
- 71. Jordanova ES, Gorter A, Ayachi O, Prins F, Durrant LG, et al. (2008) Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clinical cancer research: an official journal of the American Association for Cancer Research 14: 2028–2035. [DOI] [PubMed] [Google Scholar]
- 72. Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G (2002) Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected]. The Journal of experimental medicine 196: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, et al. (2002) Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. The Journal of experimental medicine 196: 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Alhamarneh O, Agada F, Madden L, Stafford N, Greenman J (2011) Serum IL10 and circulating CD4(+) CD25(high) regulatory T cell numbers as predictors of clinical outcome and survival in patients with head and neck squamous cell carcinoma. Head & neck 33: 415–423. [DOI] [PubMed] [Google Scholar]
- 75. Sparano A, Lathers DM, Achille N, Petruzzelli GJ, Young MR (2004) Modulation of Th1 and Th2 cytokine profiles and their association with advanced head and neck squamous cell carcinoma. Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery 131: 573–576. [DOI] [PubMed] [Google Scholar]
- 76. Hoffmann TK, Sonkoly E, Homey B, Scheckenbach K, Gwosdz C, et al. (2007) Aberrant cytokine expression in serum of patients with adenoid cystic carcinoma and squamous cell carcinoma of the head and neck. Head & neck 29: 472–478. [DOI] [PubMed] [Google Scholar]
- 77.Kaskas NM, Moore-Medlin T, McClure GB, Ekshyyan O, Vanchiere JA, et al.. (2013) Serum Biomarkers in Head and Neck Squamous Cell Cancer. JAMA otolaryngology–head & neck surgery. [DOI] [PubMed]
- 78. Green VL, Irune E, Prasai A, Alhamarneh O, Greenman J, et al. (2012) Serum IL10, IL12 and circulating CD4+CD25high T regulatory cells in relation to long-term clinical outcome in head and neck squamous cell carcinoma patients. International journal of oncology 40: 833–839. [DOI] [PubMed] [Google Scholar]
- 79. Katz LH, Li Y, Chen JS, Munoz NM, Majumdar A, et al. (2013) Targeting TGF-beta signaling in cancer. Expert opinion on therapeutic targets 17: 743–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, et al. (2007) A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clinical cancer research: an official journal of the American Association for Cancer Research 13: 4345–4354. [DOI] [PubMed] [Google Scholar]