SUMMARY
The common γ-chain (γc) plays a central role in signaling by IL-2 and other γc–dependent cytokines. Here we report that activated T cells produce an alternatively spliced form of γc mRNA that results in protein expression and secretion of the γc extracellular domain. The soluble form of γc (sγc) is present in serum and directly binds to IL-2Rβ and IL-7Rα proteins on T cells to inhibit cytokine signaling and promote inflammation. Sγc suppressed IL-7 signaling to impair naïve T cell survival during homeostasis and exacerbated Th17-cell-mediated inflammation by inhibiting IL-2 signaling upon T cell activation. Reciprocally, the severity of Th17-cell-mediated inflammatory diseases was markedly diminished in mice lacking sγc. Thus, sγc expression is a naturally occurring immunomodulator that regulates γc cytokine signaling and controls T cell activation and differentiation.
INTRODUCTION
Cytokines of the common γ-chain (γc) family are critical for development of T lineage cells and depend on γc for cellular signaling (Rochman et al., 2009). γc-deficiency results in a paucity of both mature B- and T-cells because of impaired signaling by the γc cytokine IL-7 (Noguchi et al., 1993). In fact, IL-7 signaling is necessary for developing lymphocytes to proceed through the pre-pro-B cell stage of B cell differentiation and the CD4−CD8− double negative (DN) stage of thymopoiesis (Di Santo et al., 1995; Peschon et al., 1994; von Freeden-Jeffry et al., 1997). Impaired IL-7 signaling also profoundly impairs mature T cell survival and homeostasis (Schluns et al., 2000; Tan et al., 2001). Because γc is also required for IL-2 and IL-15 signaling, γc-deficiency impairs development of FoxP3+ regulatory T cells and NK cells (Heaney and Golde, 1996; Ma et al., 2006; Vosshenrich et al., 2005), and also alters CD4 T-helper lineage fate and CD8 memory cell differentiation (Rochman et al., 2009). Thus, γc governs the generation, differentiation, and homeostasis of all lymphocyte subsets in the adaptive immune system.
Whereas γc expression is necessary for γc cytokine signaling, γc alone cannot bind cytokines and cannot trigger downstream signaling (Minami et al., 1993). Rather, γc cytokines induce γc membrane proteins to complex with proprietary cytokine receptor subunits, such as the IL-7-specific IL-7 receptor α-chain (IL-7Rα) and the IL-2-specific IL-2 receptor β-chain (IL-2Rβ), to transduce cytokine receptor signals. Importantly, the magnitude and kinetics of γc cytokine responses are thought to be controlled by the proprietary cytokine-specific receptor subunits and not by γc (Rochman et al., 2009). For example, IL-7 stimulation affects IL-7Rα expression but does not affect γc expression (Park et al., 2004), and IL-2 stimulation affects IL-2Rα and IL-2Rβ without affecting γc expression (Depper et al., 1985; Siegel et al., 1987). Consequently, modulation of γc expression is thought to be irrelevant to either the kinetics or magnitude of cytokine signaling. Instead, γc expression levels are thought to be developmentally set and to remain constant during T cell activation and differentiation (Rochman et al., 2009).
In contrast to this prevailing view, we now report that modulation of γc expression actively contributes to the regulation of cytokine responses. Interestingly, γc exerts its regulatory effects not as a conventional membrane receptor protein but as a secreted protein induced by T cell stimulation. Specifically, we found that activated T cells expressed a γc mRNA splice isoform which resulted in production and secretion of soluble γc ectodomain proteins. Soluble γc inhibited cell signaling by γc cytokines and exacerbated inflammatory responses by promoting differentiation of pathogenic Th17 CD4+ T cells both in vitro and in vivo. Conversely, the severity of Th17 cell-mediated inflammatory autoimmune disease was markedly diminished in mice lacking sγc. Thus, this study identifies a role for γc as an active immunoregulatory molecule whose alternatively spliced form is secreted to dampen signaling by γc cytokines and promote inflammatory T cell immune responses in vivo.
RESULTS
Identification of a γc mRNA splice isoform that produces a secreted form of γc
T cell activation induces expression of soluble factors such as IL-2 that control immune responses both in trans and in cis (Malek, 2008). To further understand the role of such soluble factors, we analyzed culture supernatants of TCR and CD28-stimulated T cells. We found that culture supernatants from activated T cells not only contained IL-2 and TNF-α, but surprisingly also contained large amounts of a secreted form of membrane γc proteins (Figure 1A). Although shedding is a classical mechanism of producing soluble forms of membrane proteins (Heaney and Golde, 1996), this was not the explanation for γc protein secretion as inhibition of membrane metalloproteases by TAPI2 treatment to prevent membrane protein shedding failed to prevent expression of soluble γc (Figure 1A). Instead, we found that activated T cells upregulated expression of a novel γc mRNA species that encoded a soluble form of γc (sγc; soluble γc) rather than the conventional membrane form of γc (Figure S1A, S1B and S1C).
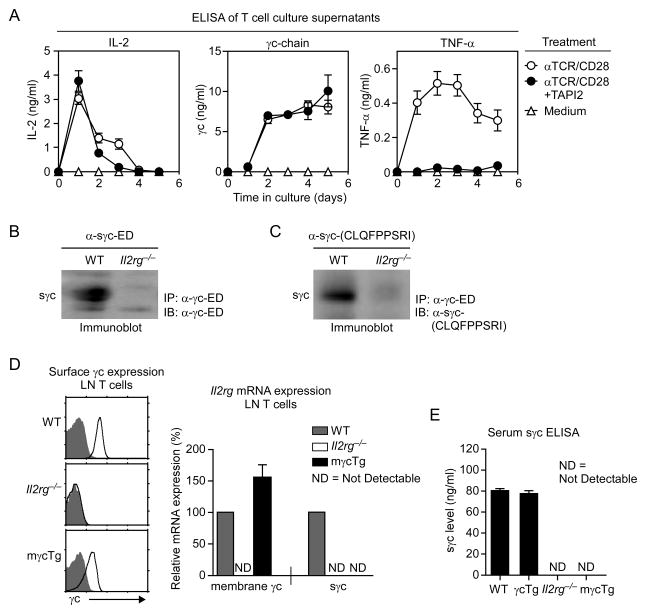
Figure 1. Soluble γc proteins are products of alternative splicing.
(A) Activated T cells express soluble γc proteins. T cells were stimulated with α-TCR and α-CD28 in the presence or absence of the metalloprotease inhibitor TAPI2. Culture supernatants were harvested at indicated days and assessed for IL-2, TNF-α and soluble γc. Results are the summary of three independent experiments.
(B) Immunoblot detection of sγc proteins in WT and Il2rg−/− sera. Serum samples were immunoprecipitated (IP) with γc-ED-specific antibodies and probed with the same antibodies in immunoblots (IB). Data are representative of five independent experiments.
(C) Immunoblot detection of the sγc C-terminal 9 a.a. epitope. α-γc-ED immunprecipitated samples were probed with custom generated sγc C-terminal 9 a.a.-specific antibodies. Data are representative of five independent experiments.
(D) Generation of T cells that exclusively produces membrane γc proteins. LN T cells from Il2rg−/− and mγcTg were assessed for γc surface (left) and mRNA expression (right). Cell surface γc expression (open histogram) versus control antibody staining (shaded histogram) is shown for Il2rg−/− and mγcTg LN T cells. Relative sγc and mγc mRNA expression were determined by qRT-PCR and normalized to expression in WT LN T cells, which was set to 100%. Data are summary of three independent experiments, ND (not detectable).
(E) Serum sγc levels in WT, γcTg, Il2rg−/−, and mγcTg mice. Data are the summary of three independent experiments. Error bars represent mean and SEM.
The gene encoding γc (Il2rg) is composed of 8 exons, and the entire transmembrane (TM) domain is encoded in exon 6 (Cao et al., 1993). The new γc isoform was generated by alternative RNA splicing that excluded exon 6 to encode a γc protein product with the full length γc extracellular domain (ED) but without the TM and intracellular domain (ID) (Figure S1A and S1B). Mechanistically, an open reading frame shift at the splice junction of exon 5 to exon 7 created a new 9 amino acid epitope followed by a stop codon. Notably, sγc transcripts were present at low levels in resting T and B cells, and were highly expressed in immature thymocytes and NK cells as well as in activated T cells (Figure S1D). Interestingly, we also identified alternatively spliced sγc transcripts in human T cells, indicating an evolutionarily conserved mechanism of soluble γc expression and regulation (Figure S1E).
To examine whether sγc is expressed in vivo, we tested serum for sγc proteins. C57Bl/6 wildtype (WT) and γc-deficient (Il2rg−/−) sera were immunoprecipitated with total γc-specific antibodies and then probed with γc extracellular domain-specific antibodies (α-γc-ED). As shown in Figure 1B, sγc proteins were detected in WT but not in Il2rg−/− serum. To determine whether serum sγc is a product of alternative splicing, we generated antibodies specific for the 9 residue C-terminal neoepitope, CLQFPPSRI (Figure 1C), and used these antibodies to probe anti-γc serum immunoprecipitates. Of note, the 9 residue neoepitope is not present in the mouse proteome (NCBI Blastp analysis, data not shown), and it is unique to the alternatively spliced sγc product. Immunoblot analysis showed immunoreactivity of CLQFPPSRI-specific antibodies with WT but not Il2rg−/− serum (Figure 1C). To further examine if shedding contributes to sγc expression in vivo, we generated mice that were unable to produce alternatively spliced sγc. We achieved this by generating a human CD2 mini-cassette driven membrane γc transgene (γcTg) and then breeding the transgene into Il2rg−/− mice to generate Il2rg−/−γcTg mice (referred to as mγcTg mice). In mγcTg mice, all γc proteins are expressed from a pre-spliced, transgenic γc cDNA that only encodes the full-length membrane γc and which cannot undergo splicing to produce the splice isoform. mγcTg T cells expressed large amounts of membrane γc mRNA and full length γc protein, but no alternatively spliced sγc transcripts (Figures 1D). Strikingly, serum from such mγcTg mice lacked circulating sγc proteins, revealing that virtually all serum sγc is generated by alternative splicing, with little or no contribution from shedding (Figure 1E). Thus, mγcTg mice are sγc-deficient, and serum sγc is the product of a post-transcriptional γc splice isoform.
Serum sγc expression is upregulated upon immune activation in vivo
Having identified sγc produced by activated T cells in vitro, we wished to know if T cell activation would increase sγc expression in vivo. To this end, we assessed serum sγc amounts in BALB/c mice injected with CD3 antibodies (α-CD3) to induce acute polyclonal T cell stimulation in vivo. In fact, serum sγc titers were increased overnight after CD3 antibody injection concomitant with T cell activation even as total T cell numbers were not increased (Figure 2A; Figure S2A and S2B).
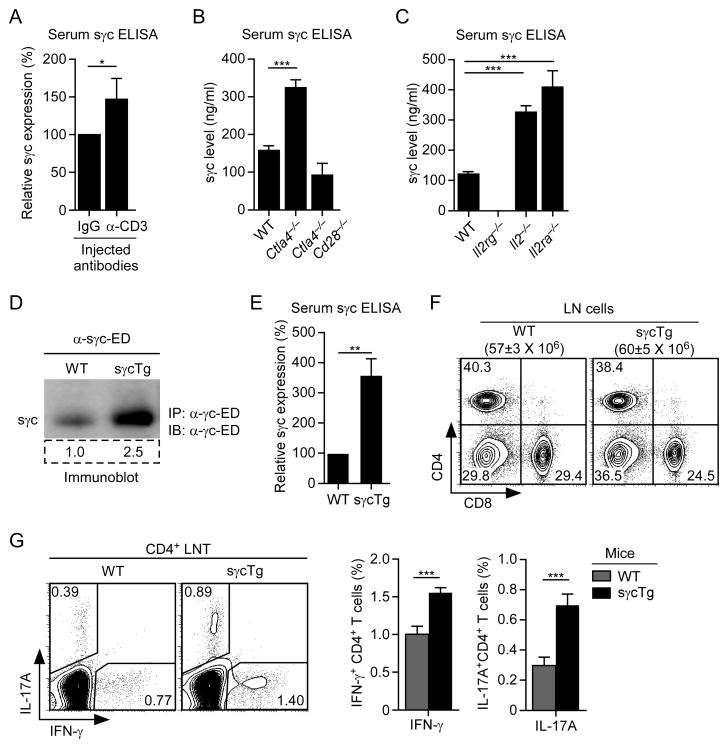
Figure 2. sγc expression is induced by T cell activation in vivo.
(A) Serum sγc levels in BALB/c mice injected overnight with α-CD3 antibodies. ELISA data are summary of two independent experiments with two mice per group.
(B and C) Serum sγc levels in Ctla4−/− and Ctla4−/−Cd28−/−, and in Il2−/− and Il2ra−/− mice. ELISA data are summary of three independent experiments.
(D) sγc protein expression in WT and sγcTg mice sera. Serum samples were immunoprecipitated with γc-ED-specific antibodies and probed with the same antibodies in immunoblots. Numbers in box indicate densitometric quantification of sγc expression. Data are representative of five independent experiments.
(E) Relative sγc levels in WT and sγcTg serum. ELISA data are summary of five independent experiments.
(F) LN cell profiles of WT and sγcTg mice. Data are representative of seventeen independent experiments.
(G) IFN-γ and IL-17A expression in sγcTg CD4+ LN T cells. CD4+ LN T cells from WT and sγcTg mice were stimulated for 3 hrs with PMA and ionomycin and assessed for IFN-γ and IL-17A expression by intracellular staining. IFN-γ, IL-17A profile is representative of six independent experiments (left). Bar graph shows percent (%) IFN-γ or IL-17A-producing CD4+ T cells. Error bars represent mean and SEM.
Next, we wished to analyze serum sγc titer in mice with chronically activated T cells. Ctla4−/− mice contain chronically activated T cells that mediate a lethal lymphoproliferative autoimmune disease (Waterhouse et al., 1995). We found the amount of serum sγc to be larger in Ctla4−/− mice than in WT mice (Figure 2B). Importantly, elevated sγc titer was dependent on the presence of activated T cells and not on Ctla4-deficiency itself, since sγc expression was not elevated in non-autoimmune Ctla4−/−Cd28−/− mice that lack activated T cells and consequently are disease-free (Figure 2B) (Tai et al., 2007). To further assess sγc expression during in vivo immune activation, we also analyzed autoimmune Il2−/− and CD25-deficient (Il2ra−/−) mice. Both mouse strains have defective CD4+ regulatory T cell generation which results in massive in vivo T cell activation and lethal autoimmunity (Sadlack et al., 1993; Willerford et al., 1995). We found that both Il2−/− and Il2ra−/− mice expressed increased amounts of serum sγc, confirming that sγc expression was elevated during in vivo immune activation (Figure 2C). Collectively, our results demonstrate that serum sγc protein levels are increased during T cell activation in vivo.
Generation of sγc transgenic mice
To understand the effect of increased sγc expression, we generated transgenic mice overexpressing sγc in T cells (sγcTg). In sγcTg mice, serum sγc levels were substantially increased (Figures 2D and 2E), but T cell numbers and phenotypes were unchanged from WT mice (Figures 2F; Figure S2C and S2D). To assess if in vivo sγc upregulation had any effect on T cells, we stimulated sγcTg CD4+ T cells with PMA/ionomycin and examined their ex vivo cytokine expression profiles. γc cytokines play an instructive role in CD4 effector T cell differentiation (Zhu et al., 2010), and we wished to know how in vivo exposure to increased sγc would affect T cell function. Interestingly, sγcTg CD4+ T cells contained higher proportions of IFN-γ producers than WT CD4+ T cells (Figure 2G). Expression of the Th2 cytokine IL-4, however, was not affected (Figure S2E). Notably, sγcTg CD4+ T cells contained a significantly larger population of IL-17-producing cells (P< 0.001), suggesting that increased sγc level promoted a Th1- and Th17-cell-prone pro-inflammatory environment (Figure 2G), but this could also reflect an increased presence of memory cells. Importantly, such settings were not created by defects in FoxP3+ CD4+ Treg cells since both development and function of Treg cells were unaffected in sγcTg mice (Figures S2F and S2G).
sγc is pro-inflammatory and exacerbates EAE pathology
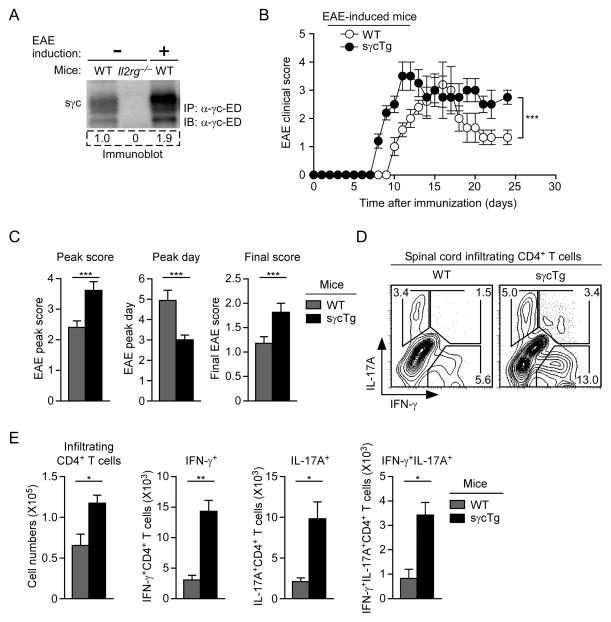
Since IL-17-producing Th17 cells are potent inducers of cell-mediated autoimmunity (Harrington et al., 2005; Park et al., 2005), we wished to know if sγc would promote autoimmunity. Experimental Autoimmune Encephalomyelitis (EAE) is a cell-mediated neuroimmunological disease that is largely mediated by Th17 cells and serves as an experimental model for human multiple sclerosis (Goverman, 2009). Injection of MOG peptides results in the activation and brain infiltration of autoreactive lymphocytes, which, in our hands, induced paralysis that peaked around day 5 after onset of the disease. Strikingly, when assessing serum sγc expression in EAE-induced mice, we found increased sγc levels in diseased mice (Figure 3A). To understand whether increased sγc expression contributed to or was the consequence of EAE pathology, we assessed disease progression and found sγcTg mice to be significantly more susceptible to EAE than control mice (P< 0.001) (Figure 3B). In fact, in every parameter examined, sγcTg mice displayed a more pronounced phenotype than WT mice. Specifically, we found in sγcTg mice that EAE severity worsened, disease progression accelerated, and disease recovery was substantially delayed (Figure 3C). Moreover, we found increased numbers of spinal cord infiltrating CD4+ T cells, which correlated with dramatically increased percentages and numbers of Th1 and Th17 cells and IL-17, IFN-γ-double-producing CD4+ T cells (Figures 3D and 3E). Taken together, these results indicate that sγc enhances inflammatory immune responses and increases differentiation of pathogenic Th17 CD4+ effector cells in vivo.
Figure 3. sγc is pro-inflammatory and exacerbates EAE disease progression.
(A) Serum sγc in EAE-induced mice. sγc was assessed by immunoprecipitation and immunoblot before (day 0) and after EAE induction (day 15) in WT mice. Blot shows representative serum sγc expression of four independent experiments.
(B) EAE induction in WT and sγcTg mice. EAE clinical scores are representative of seven independent EAE experiments with each five mice per group.
(C) EAE disease course in WT and sγcTg mice. Data are summary from seven independent experiments with each five mice per group.
(D) Th1 and Th17 cell subset analysis of spinal cord infiltrating CD4+ T cells in EAE-induced WT and sγcTg mice. IL-17A, IFN-γ profile is representative of two independent experiments with two mice per group.
(E) Spinal cord infiltrating CD4+ T cell numbers in EAE-induced mice. On day 14 after immunization, IFN-γ and/or IL-17A producing CD4+ T cells were enumerated from spinal cords of WT and sγcTg mice. Data are representative for two independent experiments with two mice per group. Error bars represent mean and SEM.
sγc impairs IL-2 signaling
Having observed that sγc expression exacerbated T cell mediated autoimmune diseases in vivo, we wished to determine the effect of soluble γc proteins on the generation of inflammatory Th17 cells. Th17 cell differentiation is negatively regulated by IL-2 signaling (Laurence et al., 2007) and is increased by inhibition of IL-2 signaling (Chen et al., 2011; Pandiyan et al., 2007). Thus, we wished to know if increased Th17 cell generation in sγcTg mice was the result of diminished IL-2 signaling. To this end, we produced sγc as recombinant proteins (rsγc) (Figure S3). Importantly, we found that rsγc proteins were expressed as disulfide-linked homodimers (Figure 4A), and that the same was true for endogenously produced sγc proteins in serum (Figure S4A). In fact, alternative splicing of γc in both humans and mice creates a C-terminal cysteine residue that promotes dimerization of sγc proteins (Figure S1A and S1E). Therefore, unlike membrane γc proteins, sγc proteins exist as dimers.
Figure 4. sγc inhibits IL-2 signaling.
(A) Dimer formation of recombinant sγc proteins. Recombinant sγc proteins were expressed in insect cells and were resolved in SDS-PAGE under reducing (+DTT) and non-reducing conditions. sγc was detected with antibodies against the γc-ED. Immunoblot is representative of four independent experiments.
(B) sγc binding affinities to IL-2Rβ determined using SPR. Binding sensorgrams of monomeric (left) and dimeric (right) sγc to immobilized IL-2Rβ. The insets show dose-response curves of the maximal responses (Rmax, depicted by the dashed boxes) for each sγc concentrations. Plots of Rmax versus sγc concentrations were non-linear fit to a single-site binding affinity model to determine Kd values.
(C) Recombinant sγc binding to mature
Il2rgfl/flE8IIICre thymocytes. Il2rgfl/flE8IIICrethymocytes (1.5 × 106 cells in 100 ul) were incubated for 3 hours with 2 ug rsγc in the presence or absence of IL-2 (200 ng) and assessed with anti-γc antibodies for surface γc staining. Histograms show surface γc staining on TCRβhi thymocytes.
(D) Titration of sγc binding to Il2rgfl/flE8IIICrethymocytes. Il2rgfl/flE8IIICrethymocytes were treated with increasing amounts of rsγc in the presence or absence of IL-2 (200 ng) as described above. Data are the summary of three independent experiments (mean and SEM).
(E) Recombinant sγc impairs proximal IL-2 signaling. CD8+ T cells were stimulated with IL-2 in the presence (4 ug/ml rsγc) or absence (Veh; vehicle) of rsγc, and assessed for pSTAT5 (left) or pAKT contents (right). Line graphs show fold induction of IL-2-induced STAT5 or AKT phosphorylation over Veh alone treated cells. Data are the summary of four independent experiments.
(F) Recombinant sγc inhibits IL-2-induced T cell proliferation. CFSE-labeled WT CD8+ LN T cells were stimulated with IL-2 in the presence or absence of rsγc. CFSE dilution was analyzed at the indicated time points. Histograms are representative of five independent experiments.
Because γc has no affinity for IL-2 (Minami et al., 1993), soluble γc would not diminish IL-2 signaling by binding free IL-2. Thus, we wished to know if sγc might diminish IL-2 signaling by binding to IL-2 receptor proteins on the cell surface. To test this idea, we performed surface plasmon resonance (SPR) studies with purified rsγc on immobilized IL-2Rβ extracellular domain (ED) proteins. Strikingly, SPR analysis showed that sγc proteins directly bound to IL-2Rβ-ED, and that dimeric sγc proteins bound much more strongly (Kd of 16.6 ± 5.5 uM) than monomeric sγc (Kd of 695 ± 76 uM) (Figure 4B). Of note, we consider that the theoretical affinity of sγc binding measured by in vitro SPR study only reflects the minimum potential affinity of sγc for IL-2Rβ, and that sγc would bind to actual cell surface IL-2Rβ with much higher affinity under physiological conditions.
To further assess if sγc directly binds to T cells, we utilized flow cytometry. To do so, we examined TCRβ+ mature thymocytes of mice with conditional deletion of γc in DP thymocytes (Il2rgfl/flE8IIICre) as they lack surface γc expression (McCaughtry et al., 2012) (Figure S4B). Notably, incubation of Il2rgfl/flE8IIICre thymocytes with rsγc proteins revealed direct binding of sγc to the surface, which we visualized by staining with γc-specific antibodies (Figure 4C) and which was still detectable at 0.06 ug (~10 nM) (Figure 4D). More importantly, addition of IL-2 significantly increased (P< 0.0001) sγc binding to Il2rgfl/flE8IIICre cells (Figure 4C and 4D), indicating that sγc affinity to IL-2Rβ is increased by IL-2. In sum, sγc proteins, especially dimeric sγc proteins, directly bind to surface IL-2Rβ chains, especially in the presence of IL-2.
To directly test if sγc binding to IL-2Rβ suppressed IL-2 signaling in T cells, we assessed IL-2-induced STAT5 and AKT phosphorylation in the presence or absence of rsγc (Malek, 2008). We found that rsγc effectively suppressed downstream IL-2 signaling (Figure 4E), and that rsγc-induced impairment of IL-2 signaling reduced IL-2’s biological effects on T cells. In mice, naïve CD8+ T cells respond to IL-2 by vigorous proliferation (Cho et al., 2010). We confirmed such IL-2-induced proliferation in purified CD8+ T cells, and found that rsγc suppressed IL-2-driven proliferation in the same cells (Figure 4F), demonstrating a direct inhibitory role of sγc in IL-2 signaling. Thus, by binding to IL-2Rβ proteins on the cell surface, sγc prevents association with membrane γc proteins to impair IL-2 signaling (Figure S4C).
sγc promotes Th17 cell differentiation by suppressing IL-2 signaling
To assess the IL-2 inhibitory effect of sγc on Th17 cell differentiation, we analyzed Th17 cell generation using in vitro cultures in the presence of rsγc. As previously described (Laurence et al., 2007), recombinant IL-2 potently suppressed, while antagonizing IL-2 antibodies substantially enhanced, Th17 cell differentiation (Figure 5A and 5B). Importantly, sγc treatment or sγc overexpression had the same effect as IL-2 antibodies as it increased both the proportion of IL-17-producing cells and overall IL-17 expression (Figures 5B and 5C). In fact, co-incubation of rsγc and antagonizing IL-2 antibodies did not have additive effects, indicating that the Th17 cell promoting mechanisms of rsγc and IL-2 antibodies were redundant (Figure 5D).
Figure 5. sγc promotes Th17 cell differentiation of CD4+ LN T cells.
(A) sγc promotes IL-17A expression. Sorted naïve CD4+ T cells were stimulated under Th17-cell-polarizing conditions and assessed for intracellular IL-17A and IFN-γ expression. Where indicated, recombinant human IL-2 (rhIL-2), neutralizing mouse IL-2-specific antibodies (α-mIL-2), recombinant sγc (4 ug/ml rsγc), or vehicle (Veh) were added at the beginning of the culture. Data are representative of ten independent experiments.
(B) Summary of IL-17A+CD4+ T cell differentiation under modified Th17-cell-polarizing conditions. Each symbol represents an individual experiment under the indicated condition as described above.
(C) IL-17A secretion in WT and sγcTg CD4+ T cells. Culture supernatants were collected at day 4 of Th0 or Th17 cell differentiation cultures. ELISA data are representative for five independent experiments.
(D) Redundant effect of rsγc and mIL-2-specific antibodies on Th17 cell differentiation. Naive CD4+ T cells were stimulated under Th17-cell-polarizing conditions in the presence of rsγc or mIL-2-specific antibodies. Each symbol represents an individual mouse.
(E) sγc effect on IL-17A expression under IL-2-deficient conditions. Naïve CD4+ T cells were stimulated under Th17-cell-polarizing conditions to assess intracellular IL-17A and IFN-γ expression. Where indicated, rsγc were added at the beginning of the differentiation culture. Contour plots are representative of four independent experiments (left). Bar graph shows percent (%) increase of IL-17A-producing CD4+ T cells by rsγc treatment compared to vehicle control. Error bars represent mean and SEM.
To further demonstrate that the sγc effect on Th17 cells was due to its inhibition of IL-2 signaling, we analyzed sγc effects on CD4+ T cells from Il2−/− mice. We stimulated naïve Il2−/− CD4+ T cells under Th17 conditions with or without rsγc and found that unlike the effect of sγc on WT T cells, sγc failed to increase IL-17 expression in Il2−/− T cells (Figure 5E). Identical results were observed in Il2−/−sγcTg CD4+ T cells where sγc transgene expression failed to enhance Th17 cell differentiation compared to Il2−/− cells (Figure 5E). Altogether, these results documents that sγc promotes Th17 cell differentiation by specifically impairing IL-2 signaling.
sγc-deficiency ameliorates Th17-cell-mediated inflammatory autoimmune diseases
Because sγc exacerbated IL-17 expression and inflammation, we wished to know if the absence of sγc would dampen inflammatory responses that otherwise occurred in WT mice. To this end, we assessed EAE-induction in mγcTg mice which only express transgenic membrane γc receptors and lack endogenous sγc proteins (Figure 1F). Strikingly, EAE disease progression in mγcTg mice was dramatically reduced compared to WT mice, with delayed disease onset and lower clinical scores (Figure 6A). Analysis of spinal cord and brain infiltrating T cells further revealed a significant reduction in inflammatory CD4+ T cell numbers and greatly diminished percentages of IL-17-IFN-γ-double-producers, demonstrating a protective effect of sγc-deficiency in mγcTg mice (Figure 6B and 6C). Importantly, the severity of inflammatory reactions was markedly reduced in mγcTg mice despite the presence of normal numbers and appearances of peripheral T cells (Figure S5A and S5B).
Figure 6. sγc deficiency is protective against inflammatory autoimmune diseases.
(A) EAE induction in WT and mγcTg mice. EAE clinical scores are summary of two independent EAE experiments with each four mice per group.
(B) Th1 and Th17 cell subset analysis of spinal cord and brain infiltrating CD4+ T cells in EAE-induced WT and mγcTg mice. Contour plots show representative IL-17A, IFN-γ profiles from four mice per group.
(C) Spinal cord and brain infiltrating CD4+ T cell numbers in EAE-induced mice. On day 15 after immunization, IFN-γ and/or IL-17A producing CD4+ T cells were enumerated from spinal cords and brains of WT and mγcTg mice. Data are representative of four mice per group.
(D) Body weight change of IBD-induced mice. Rag2−/− mice were injected with each 0.5 million WT or mγcTg CD4+CD45RBhi T cells and monitored for change in body weights. Data show results from four mice per group.
(E) Colon histology from Rag2−/− host mice injected with naïve CD4+ T cells from the indicated donor mice at 6 weeks after T cell transfer.
(F) Th1 and Th17 cell subset analysis in CD4+ LN T cells from IBD-induced WT and mγcTg mice (left). Data show results from three mice for each group (right). Error bars represent mean and SEM.
To further assess the ameliorative effect of sγc-deficiency, we utilized the CD4+CD45RBhi T cell transfer model of inflammatory bowel disease (IBD) (Powrie et al., 1993). Adoptive transfer of naïve T cells into immunodeficient Rag2−/− mice results in severe autoimmune inflammatory colitis that can be monitored by body weight loss and increased IBD clinical scores (Table S1). Notably, we found that Rag2−/− host mice injected with mγcTg CD4 T cells had markedly diminished IBD, while Rag2−/− mice injected with WT CD4+ T cells suffered from significant body weight loss, destruction of colon tissues, and rapid deterioration of health (Figure 6D and 6E; Figure S5C and S5D). Importantly, adoptively transferred mγcTg CD4+ T cells were still activated in vivo and produced large amounts of IFN-γ, but it was specifically the expression of pro-inflammatory IL-17 that was dramatically diminished in these cells (Figure 6F). Thus, sγc-deficient T cells were unable to induce severe autoimmune IBD because of reduced Th17 cell generation. Conversely, T cell expression or overexpression of sγc increased the severity of inflammatory disease (Figure S5D). These results reveal that sγc exacerbates in vivo inflammatory immune responses and contributes to the severity of in vivo autoimmune pathology.
Sγc overexpression impairs T cell development and naïve T cell homeostasis
While sγc directly binds to LN T cells (Figure 7A), resting T cells do not express high levels of IL-2Rβ. These data suggested that sγc also bound to receptors for cytokines other than IL-2. Specifically, we considered that sγc might bind to IL-7 receptors because they are highly expressed on all resting T cells. SPR analysis showed that this was indeed the case. We found that sγc proteins directly interacted with mouse IL-7Rα-ED proteins, and that dimeric sγc showed a dramatic increase of IL-7Rα-ED binding activity (Kd = 3.9 uM) over monomeric sγc proteins (Kd = 50.8 uM) (Figures 7B and Table S2).
Figure 7. sγc overexpression impairs T cell development and homeostasis.
(A) Surface γc chain staining on WT and sγcTg CD4+ T cells. Data are the summary of four independent experiments.
(B) sγc binding affinities to IL-7Rα determined using surface plasmon resonance. Binding sensorgrams and Kd of monomeric (left) and dimeric (right) sγc proteins to immobilized IL-7R α proteins.
(C) Cell surface γc staining of Il2rgfl/flE8IIICre thymocytes incubated with recombinant sγc. Il2rgfl/flE8IIICrethymocytes were incubated for 2 hours with 4 ug rsγc or Veh (BSA in PBS) and assessed with anti-γc antibodies for surface γc staining. Histograms show surface γc staining on DP and TCRβhi thymocytes.
(D) Cell surface γc staining of Il2rgfl/flE8IIICre and Il7rafl/flIl2rgfl/flE8IIICre thymocytes incubated with rsγc. Surface γc staining was assessed as mean fluorescence intensity (MFI) in TCRβhi thymocytes. Data are summary of two independent experiments.
(E) Total thymocyte numbers in WT and sγcTg mice. Results are the summary of eight independent experiments.
(F) T cell apoptosis in WT and sγcTg LN T cells. Caspase-3 activity was determined in naive CD44lo CD4+ or CD8+ LN T cells using CaspGLOW apoptosis kits. Histograms show caspase-3 activity in indicated cells (left). Bar graphs show summary of three independent experiments (right).
(G) Naïve and memory phenotype analysis of WT and sγcTg LN T cells. Contour plots show CD62L/CD44 and CD122/CD44 profiles and percentages of CD4+ and CD8+ LN T cells, respectively (left). Bar graph shows naive CD4+ and CD8+ LN T cells numbers from WT and sγcTg mice (right). Data are the summary of ten independent experiments. Error bars represent mean and SEM.
To further demonstrate sγc binding to surface IL-7Rα proteins, we incubated Il2rgfl/flE8IIICrethymocytes with rsγc proteins and assessed rsγc binding to thymocyte subpopulations. TCRβhi mature thymocytes express high levels of IL-7Rα and strongly bound sγc, while DP thymocytes do not express surface IL-7Rα and did not bind sγc (Figure 7C). To directly show that recombinant sγc specifically bound to IL-7Rα proteins, we examined γc-deficient TCRβhi thymocytes that were additionally deficient in IL-7Rα (McCaughtry et al., 2012). Indeed, IL-7Rα-deficiency substantially reduced sγc binding to sγc-deficient TCRβhi thymocytes (Figure 7D), documenting that sγc directly binds and interacts with surface IL-7Rα proteins.
IL-7 is critical for thymopoiesis and impaired IL-7 signaling results in reduced thymus cellularity (von Freeden-Jeffry et al., 1995). Notably, increased sγc expression in sγcTg mice resulted in decreased total thymocyte numbers (Figure 7E). CD25 is highly expressed on immature DN thymocytes but dilutes out with proliferation so that CD25 is normally absent on DP thymocytes. However, sγcTg DP thymocytes still expressed significant amounts of surface CD25 which indicated impaired IL-7 signaling and impaired expansion of DN cells (Figure S6A) (Crompton et al., 1994). These results demonstrate that sγc blocks in vivo IL-7 signaling. In further support of an sγc effect on IL-7 signaling, we observed increased T cell apoptosis and reduced percentages and numbers of naïve T cells in sγcTg mice (Figure 7F and 7G), even as their T cells expressed identical levels of IL-7Rα and other γc family receptors to WT mice (Figure S6B). Moreover, adoptive transfer of WT T cells into congenic sγcTg or WT host mice revealed that survival of CD44lo naïve donor T cells was substantially reduced in sγcTg hosts compared to WT hosts (Figure S6C). Finally, to directly demonstrate that sγc blocks IL-7-induced survival, we stimulated naïve T cells with IL-7 in vitro and assessed the effect of recombinant sγc on cell survival. As shown in Figure S6D, rsγc effectively blocked IL-7 induced T cells survival in vitro. Thus, sγc inhibited IL-7 signaling during both T cell development and peripheral T cell homeostasis.
DISCUSSION
Here we report a regulatory mechanism of γc signaling that tunes T cell responsiveness to IL-2 and IL-7, and potentially to other γc cytokines as well. This regulatory pathway was based on post-transcriptional generation and secretion of the γc extracellular domain which directly bound to γc cytokine proprietary receptors such as IL-2Rβ and IL-7Rα, especially in the presence of cytokines, and interfered with cytokine signaling. Importantly, secreted γc proteins required disulfide-linked homo-dimerization for high affinity binding and inhibition of cytokine signaling. During steady-state conditions, sγc interfered primarily with IL-7-dependent processes, resulting in impaired thymopoiesis and naïve T cell survival. During T cell activation, sγc inhibited IL-2 signaling and promoted the development of pro-inflammatory Th17 cell responses as demonstrated by increased Th17 cell differentiation in vitro and by exacerbation of EAE disease in sγc transgenic mice in vivo. Conversely, in vivo sγc-deficiency suppressed Th17 cell differentiation and dramatically reduced the severity of inflammatory autoimmune disease, resulting in markedly improved disease outcomes. Thus, this study identifies sγc proteins as endogenous immunomodulators that exacerbate in vivo inflammatory diseases by directly binding to cytokine receptors and dampening T cell responses to γc cytokines.
Cytokine signaling in T cells is tightly controlled at many levels. Expression of cytosolic inhibitory molecules, such as SOCS, limits the magnitude and duration of cytokine signaling (Yoshimura et al., 2007). Upregulation of surface cytokine receptor expression, on the other hand, positively affects cytokine signaling. Secretion of γc proteins now adds another layer of regulatory complexity because sγc expression is upregulated upon T cell activation but, unlike the upregulation of other IL-2 receptor chains which enhance IL-2 signaling, upregulation of sγc suppresses IL-2 signaling. Moreover, soluble γc is unique in its regulatory mechanism because it suppressed IL-2 signaling without binding to the IL-2 molecule itself. This is in contrast to other chains of the IL-2 receptor complex which regulate IL-2 responsiveness by direct interaction with IL-2 (Minami et al., 1993). Because IL-2 signaling requires membrane γc association with IL-2Rβ, sγc binding to IL-2Rβ proteins likely blocks IL-2 signaling by preventing recruitment of membrance γc proteins and assembly of signaling competent IL-2 receptor/membrane γc complexes.
The secreted γc protein is structurally identical to the membrane γc receptor, except for absent transmembrane and intracellular domains. Since ligand binding is mediated by the extracellular domain, it was initially unclear how soluble γc could compete with membrane γc for binding to proprietary receptor proteins. The mechanism was partly revealed by SPR analysis which showed that dimeric sγc proteins possessed >40 fold increased affinity of binding to IL-2Rβ proteins compared to monomeric sγc proteins which was further enhanced in the presence of cytokines. Thus, serum sγc proteins, which are mostly dimers, are vastly superior in binding to IL-2Rβ than membrane γc proteins which are monomers and which do not display any meaningful affinity to IL-2Rβ (Rickert et al., 2004). As a result, sγc proteins effectively outcompete membrane γc protein for binding to proprietary cytokine receptors IL-2Rβ and IL-7Rα. Therefore upon dimerization, sγc proteins acquire binding specificities that make them distinct from monomeric sγc and membrane γc proteins. In this regard, we think it is not a coincidence that alternative splicing of sγc pre-mRNA creates a new C-terminal cysteine residue that promotes disulfide linkage and dimerization of sγc proteins. Indeed, we found such de novo C-terminal cysteine residues in both mouse and human sγc proteins, indicating that dimerization of sγc proteins is an evolutionary conserved mechanism of regulating cytokine signaling.
Soluble cytokine receptors have been observed in earlier instances (Baran et al., 1988; Mortier et al., 2004; Nielsen et al., 1998). Expression of soluble cytokine receptors had been associated with inflammation, autoimmunity, and cancer (Heaney and Golde, 1996), but it was uncertain if their expression was the cause or the consequence of immune activation. In theory, soluble cytokine receptors might either potentiate or antagonize cytokine signaling (Heaney and Golde, 1996). Soluble IL-6Rα, for example, directly binds IL-6 and potently enhances IL-6 signaling (Rose-John, 2012), whereas soluble IL-15Rα sequesters IL-15 and prevents it from binding to membrane IL-15 receptors (Mortier et al., 2004). γc proteins, however, do not show significant binding affinity to free cytokines, so it was not immediately obvious what role soluble γc would play in cytokine signaling. Using recombinant sγc proteins and sγc transgenic mice, we have demonstrated that sγc antagonizes IL-2 and IL-7 signaling and increases inflammatory immune responses.
Since T cell activation induces sγc production and sγc in turn induces inflammatory cytokine expression, sγc production is a mechanism to reinforce pro-inflammatory immune responses downstream of T cell activation. In fact, sγc expression would limit IL-2 responses and promote effector T cell differentiation. Thus, our present results provide an explanation for earlier reports on soluble γc expression in which a significant correlation was found in Crohn’s disease patients between disease activity and serum γc levels (Nielsen et al., 1998). According to our present findings, increased soluble γc expression would dampen γc cytokine signaling which would enhance inflammatory cell activation to worsen the disease outcome. In agreement with our perspective, soluble γc is highly expressed under inflammatory conditions in both humans and mice. Soluble γc was abundantly expressed in synovial fluid of patients with rheumatoid arthritis and also highly induced in mice challenged with L. major (Meissner et al., 2001; Nishio et al., 2001). We also found that EAE disease outcome and in vivo inflammatory responses were exacerbated in sγc Tg mice. Collectively, these data indicate a role for sγc as an active inducer of pathogenic T cells and inflammation.
Because sγc promotes inflammation, it is interesting to speculate if neutralization of serum sγc expression would ameliorate inflammatory disease and dampen destructive T cell responses. The striking reduction in disease severity of EAE or IBD-induced mγcTg mice lacking sγc observed here suggests that depletion of sγc might be an effective therapy in patients suffering from inflammatory immune diseases. In this regard, the 9 a.a. C-terminal neoepitope CLQFPPSRI (and the 7 a.a. neoepitope RCPEFPP in humans) of sγc provides an excellent target for intervention with neutralizing antibodies. These peptide epitopes are generated only upon alternative splicing of γc pre-mRNA exon 5 to exon 7, and they are only found in sγc and not in membrane γc proteins. In contrast to conventional γc-specific antibodies, C-terminal sγc-specific antibodies will neutralize only alternatively spliced sγc proteins, making this epitope an attractive target to clear sγc from circulation without affecting membrane γc receptor function.
Discovery of the unique C-terminal sγc epitope was enlightening because it helped us identify the molecular origin of sγc. Specifically, the 9 a.a. C-terminal sequence is not found in membrane γc proteins so that its presence in serum directly demonstrated that sγc proteins are products of alternative RNA splicing. Soluble cytokine receptors are usually produced by one of the following two major mechanisms; proteolytic cleavage and shedding of membrane cytokine receptors or alternative splicing of cytokine receptor pre-mRNA (Levine, 2004). TNF-receptors are classical examples of cytokine receptors that are cleaved by transmembrane metalloproteinases and shed from cells (Hwang et al., 1993). Soluble IL-15 receptors, on the other hand, are usually generated by alternative splicing (Bulanova et al., 2007). Our data document that sγc is exclusively produced by alternative splicing. It is conceivable that genetic variations might also contribute to increased sγc expression. In fact, IL-7Rα gene polymorphisms can result in increased expression of soluble IL-7Rα (sIL7Rα) (Gregory et al., 2007), and sIL7Rα expression has been proposed as a significant risk factor for multiple sclerosis in humans (Harley, 2007; Lundstrom et al., 2013). Specifically, these single nucleotide polymorphisms (SNP) led to exclusion of exon 6 during IL-7Rα pre-mRNA splicing, and resulted in the deletion of the transmembrane domain to produce soluble IL-7R proteins (Goodwin et al., 1990; Gregory et al., 2007). It would be interesting to assess γc genes for genetic variations that increase exon 6 exclusion, and to test if such SNPs are associated with increased risk for MS or other autoimmune disease. Potentially, such γc genetic variations could predispose carriers to excessive inflammatory responses by increased levels of sγc.
In conclusion, the present study has identified sγc as an endogenous regulatory mechanism that dampens T cell signaling to γc cytokines and results in enhanced activity of Th17 cells. Enforced sγc expression constrained T cell survival under homeostatic conditions and promoted a pro-inflammatory environment under immune activation. Reciprocally, in vivo sγc-deficiency suppressed inflammatory Th17 cell differentiation and effectively reduced the severity of Th17-cell-mediated autoimmune disease. Thus, sγc represents a naturally occurring immunomodulator of T cells and introduces a target for intervention in inflammation and disease.
EXPERIMENTAL PROCEDURES
Animals
C57BL/6 (B6) mice and B6.Ly5.2 mice were obtained from the Jackson Laboratory. Ctla4−/−, Ctla4−/−Cd28−/−, Il2−/−, Il2ra−/−, Rag2−/−, and Il2rg−/− mice were bred in our colony. Il2rgfl/flE8IIICreand Il7rafl/flIl2rgfl/flE8IIICre mice were kindly provided by Dr. A. Singer, NCI. The γc- and sγc-transgenic constructs were generated by ligating a murine γc or sγc cDNA into human CD2 (hCD2) enhancer-promoter-based vectors, respectively, and injected into fertilized B6 oocytes to generate γcTg and sγcTg mice. Animal experiments were approved by the National Cancer Institute Animal Care and Use Committee (ACUC), and all mice were cared for in accordance with US National Institutes of Health guidelines.
Surface plasmon resonance (SPR)
Purified mouse IL-2Rβ ectodomain expressed from yeast was purchased from MyBiosource, Inc and used without further modification. IL-2Rβ was amine-coupled to a CM5 sensor chip after buffer exchange into PBS (pH 7.4) using a NAP-5 (GE Healthcare) column using similar methods described previously (McElroy et al., 2012). Concentration series of monomer and dimer forms of insect cell derived rsγc were injected over immobilized IL-2Rβ at a flow-rate of 25 μl/min using HBS-EP (pH 7.4). SPR experiments were performed at 25 C using a Biacore 3000 instrument (GE Healthcare). SPR sensorgrams were double-referenced and trimmed using BIAevaluation 4.1 (GE Healthcare). Dose-response curves (Rmax vs [rsγcmonomer or dimer]) were fit using Prism5.0 (GraphPad). Experiments were performed in triplicate.
Detection of soluble cytokine and receptor levels
Serum sγc was detected in a sandwich ELISA using γc-specific polyclonal antibodies (R&D systems) as capture antibodies and biotin-conjugated γc-specific monoclonal antibodies (4G3; BD) as detection antibodies. Recombinant sγc protein was used as positive control. IL-2, IFN-γ, TNF-α and IL-17A were analyzed by ELISA according to the manufacturer’s instructions (R&D systems).
In vitro CD4+ T helper differentiation
Naïve CD4+ T cells were electronically sorted by gating on CD62L+CD44loCD25− cells. Sorted cells were stimulated with plate-bound α-CD3 and α-CD28 and cultured under non-skewing Th0 cell conditions (medium alone) or were differentiated into Th17 cells with human TGF-β1 (5 ng/ml; Peprotech), mouse IL-6 (30 ng/ml; BD), anti-mouse IL-4 (10 μg/ml) and anti-mouse IFN-γ (10 μg/ml). Where indicated, human IL-2 (100 U/ml; NCI) or anti-mouse IL-2 (10 μg/ml; Becton Dickinson) or mouse rsγc (4 μg/ml) was added to the cell culture.
Cell proliferation assay
Naïve CD8 responder T cells were labeled with CFSE (carboxyfluorescein diacetate succinimidyl ester; Invitrogen) and incubated with human IL-2 (10 μg/ml; Peprotech) for 2 or 3 days.
CD4+CD45RBhi T cell transfer induced colitis
CD4+ T cells were purified from WT, sγcTg, and mγcTg mice using the EasySep mouse CD4+ T cell isolation kit (Stemcell Technologies). CD4+CD45RBhi cells were then electronically sorted by FACS. Rag2−/− mice were injected with 5 × 105 CD4+CD45RBhi T cells and monitored for 7 weeks for body weight change and clinical signs of colitis. Experimental mice showing clinical signs of severe colitis and a body weight loss of >20% were sacrificed according to the NCI ACUC guidelines.
Statistical analysis
Statistical differences were analyzed using Student’s two-tailed t-test. Clinical score comparisons between two groups were performed with non-parametric Mann-Whitney test. Curves of body weight change were fitted with nonlinear regression and group difference was examined by slope and intercept. P values of less than 0.05 were considered significant. *P < 0.05, **P < 0.01, ***P < 0.001, and NS (not significant) (Student’s two-tailed t-test).
Supplementary Material
HIGHLIGHTS.
Activated T cells upregulate expression of an alternatively spliced form of γc mRNA
γc splice isoform expression results in secretion of the γc extracellular domain
Soluble γc binds directly to surface IL-2Rβ and inhibits IL-2 signaling
Soluble γc promotes Th17 cell differentiation and exacerbates inflammatory disease
Acknowledgments
We thank Drs. A. Singer, C. Mackall, and M. Kimura for critical review of this manuscript. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research; and the National Institutes of Health (AI072142 to S.T.R.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baran D, Korner M, Theze J. Characterization of the soluble murine IL-2R and estimation of its affinity for IL-2. J Immunol. 1988;141:539–546. [PubMed] [Google Scholar]
- Bulanova E, Budagian V, Duitman E, Orinska Z, Krause H, Ruckert R, Reiling N, Bulfone-Paus S. Soluble Interleukin (IL)-15Rα is generated by alternative splicing or proteolytic cleavage and forms functional complexes with IL-15. J Biol Chem. 2007;282:13167–13179. doi: 10.1074/jbc.M610036200. [DOI] [PubMed] [Google Scholar]
- Cao X, Kozak CA, Liu YJ, Noguchi M, O’Connell E, Leonard WJ. Characterization of cDNAs encoding the murine interleukin 2 receptor (IL-2R) γ chain: chromosomal mapping and tissue specificity of IL-2R γ chain expression. Proc Natl Acad Sci USA. 1993;90:8464–8468. doi: 10.1073/pnas.90.18.8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Haines CJ, Gutcher I, Hochweller K, Blumenschein WM, McClanahan T, Hammerling G, Li MO, Cua DJ, McGeachy MJ. Foxp3+ regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity. 2011;34:409–421. doi: 10.1016/j.immuni.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Cho JH, Kim HO, Surh CD, Sprent J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity. 2010;32:214–226. doi: 10.1016/j.immuni.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton T, Moore M, MacDonald HR, Malissen B. Double-negative thymocyte subsets in CD3ζ chain-deficient mice: absence of HSA+CD44−CD25− cells. Eur J Immunol. 1994;24:1903–1907. doi: 10.1002/eji.1830240828. [DOI] [PubMed] [Google Scholar]
- Depper JM, Leonard WJ, Drogula C, Kronke M, Waldmann TA, Greene WC. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci USA. 1985;82:4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor γ chain. Proc Natl Acad Sci USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RG, Friend D, Ziegler SF, Jerzy R, Falk BA, Gimpel S, Cosman D, Dower SK, March CJ, Namen AE, et al. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990;60:941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SG, Schmidt S, Seth P, Oksenberg JR, Hart J, Prokop A, Caillier SJ, Ban M, Goris A, Barcellos LF, et al. Interleukin 7 receptor α chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat Genet. 2007;39:1083–1091. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- Harley JB. IL-7Rα and multiple sclerosis risk. Nat Genet. 2007;39:1053–1054. doi: 10.1038/ng0907-1053. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Heaney ML, Golde DW. Soluble cytokine receptors. Blood. 1996;87:847–857. [PubMed] [Google Scholar]
- Hwang C, Gatanaga M, Granger GA, Gatanaga T. Mechanism of release of soluble forms of tumor necrosis factor/lymphotoxin receptors by phorbol myristate acetate-stimulated human THP-1 cells in vitro. J Immunol. 1993;151:5631–5638. [PubMed] [Google Scholar]
- Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Levine SJ. Mechanisms of soluble cytokine receptor generation. J Immunol. 2004;173:5343–5348. doi: 10.4049/jimmunol.173.9.5343. [DOI] [PubMed] [Google Scholar]
- Lundstrom W, Highfill S, Walsh ST, Beq S, Morse E, Kockum I, Alfredsson L, Olsson T, Hillert J, Mackall CL. Soluble IL7Rα potentiates IL-7 bioactivity and promotes autoimmunity. Proc Natl Acad Sci USA. 2013;110:E1761–1770. doi: 10.1073/pnas.1222303110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- McCaughtry TM, Etzensperger R, Alag A, Tai X, Kurtulus S, Park JH, Grinberg A, Love P, Feigenbaum L, Erman B, et al. Conditional deletion of cytokine receptor chains reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus. J Exp Med. 2012;209:2263–2276. doi: 10.1084/jem.20121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy CA, Holland PJ, Zhao P, Lim JM, Wells L, Eisenstein E, Walsh ST. Structural reorganization of the interleukin-7 signaling complex. Proc Natl Acad Sci USA. 2012;109:2503–2508. doi: 10.1073/pnas.1116582109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner U, Blum H, Schnare M, Rollinghoff M, Gessner A. A soluble form of the murine common γ chain is present at high concentrations in vivo and suppresses cytokine signaling. Blood. 2001;97:183–191. doi: 10.1182/blood.v97.1.183. [DOI] [PubMed] [Google Scholar]
- Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- Mortier E, Bernard J, Plet A, Jacques Y. Natural, proteolytic release of a soluble form of human IL-15 receptor α-chain that behaves as a specific, high affinity IL-15 antagonist. J Immunol. 2004;173:1681–1688. doi: 10.4049/jimmunol.173.3.1681. [DOI] [PubMed] [Google Scholar]
- Nielsen OH, Kirman I, Johnson K, Giedlin M, Ciardelli T. The circulating common gamma chain (CD132) in inflammatory bowel disease. Am J Gastroenterol. 1998;93:323–328. doi: 10.1111/j.1572-0241.1998.00323.x. [DOI] [PubMed] [Google Scholar]
- Nishio J, Kohsaka H, Shimamura T, Hamuro J, Miyasaka N. Abundant expression of common cytokine receptor gamma chain (CD132) in rheumatoid joints. J Rheumatol. 2001;28:240–244. [PubMed] [Google Scholar]
- Noguchi M, Nakamura Y, Russell SM, Ziegler SF, Tsang M, Cao X, Leonard WJ. Interleukin-2 receptor γ chain: a functional component of the interleukin-7 receptor. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Rα transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- Rickert M, Boulanger MJ, Goriatcheva N, Garcia KC. Compensatory energetic mechanisms mediating the assembly of signaling complexes between interleukin-2 and its α, β, and γc receptors. J Mol Biol. 2004;339:1115–1128. doi: 10.1016/j.jmb.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by γc family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Siegel JP, Sharon M, Smith PL, Leonard WJ. The IL-2 receptor β chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987;238:75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- Tai X, Van Laethem F, Sharpe AH, Singer A. Induction of autoimmune disease in CTLA-4−/− mice depends on a specific CD28 motif that is required for in vivo costimulation. Proc Natl Acad Sci USA. 2007;104:13756–13761. doi: 10.1073/pnas.0706509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naïve T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Freeden-Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147–154. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, Di Santo JP. Roles for common cytokine receptor γ–chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations*. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.