Abstract
In filamentous fungi, intracellular signaling pathways which are mediated by changing calcium levels and/or by activated protein kinase C (Pkc), control fungal adaptation to external stimuli. A rise in intracellular Ca2+ levels activates calcineurin subunit A (CnaA), which regulates cellular calcium homeostasis among other processes. Pkc is primarily involved in maintaining cell wall integrity (CWI) in response to different environmental stresses. Cross-talk between the Ca2+ and Pkc-mediated pathways has mainly been described in Saccharomyces cerevisiae and in a few other filamentous fungi. The presented study describes a genetic interaction between CnaA and PkcA in the filamentous fungus Aspergillus nidulans. Overexpression of pkcA partially rescues the phenotypes caused by a cnaA deletion. Furthermore, CnaA appears to affect the regulation of a mitogen-activated kinase, MpkA, involved in the CWI pathway. Reversely, PkcA is involved in controlling intracellular calcium homeostasis, as was confirmed by microarray analysis. Furthermore, overexpression of pkcA in a cnaA deletion background restores mitochondrial number and function. In conclusion, PkcA and CnaA-mediated signaling appear to share common targets, one of which appears to be MpkA of the CWI pathway. Both pathways also regulate components involved in mitochondrial biogenesis and function. This study describes targets for PkcA and CnaA-signaling pathways in an A. nidulans and identifies a novel interaction of both pathways in the regulation of cellular respiration.
Introduction
Cellular responses to environmental stimuli are often mediated through G-proteins, which consist of a G-protein coupled receptor (GPCR) and the associated heterotrimeric G-proteins [1]. One such G-protein is phospholipase C which produces the second messengers diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3) from the cell membrane phospholipid phosphatidylinositol 4,5-bisphosphate. These second messengers subsequently cause an increase in intracellular Ca2+ levels [2]. The concentration of intracellular calcium ions (Ca2+) serves as a signal for the regulation of many cellular processes and is constantly altered in response to environmental cues and physiological signals [3]. In mammalian cells, a rise in intracellular Ca2+ levels causes the activation of the calcineurin phosphatase and the protein kinase C (Pkc) pathways [2]. Protein kinases and phosphatases act as key regulators of signal transduction by adding or removing phosphate groups to their protein targets hence directing the activity, location and function of many proteins [4]. In the filamentous fungus Aspergillus nidulans, PkcA was shown to be fundamental to the cell wall integrity (CWI) pathway, is essential for viability, and has been shown to be involved in penicillin production, polarized growth, morphogenesis, septum formation and the apoptosis [5]–[9]. Crosstalk between Pkc and the unfolded protein response (UPR) or other MAP (mitogen-activated protein) kinase cascades has been observed [10], [11].
The MAP-kinase (MAPK) cascades function via the sequential phosphorylation of three protein kinases (MAPKKK, MAPKK, MAPK) resulting in the activation of a multifunctional MAP kinase [12]. MAP kinase phosphorylation cascades are important for relaying, integrating and amplifying intracellular signals and are crucial signaling components involved in many cellular processes [12]. In Saccharomyces cerevisiae, MAP kinases control mating, the cellular response to high environmental osmolarity, pseudohyphal development, sporulation of diploid cells and the maintenance of CWI in response to stresses, such as heat stress and low osmolarity [13]. In S. cerevisiae, the CWI pathway is activated when Pkc1p phosphorylates Bck1p, a MAPKKK, which in turn activates the two MAPKKs, Mkk1p and Mkk2p, which then go on to phosphorylate the MAPK Slt2p. Mkk1p/2p and Slt2p regulate the expression of many downstream protein targets such as cell wall proteins and enzymes involved in cell wall biogenesis [14]. In A. nidulans, a similar mechanism operates in the activation of the CWI pathway. The orthologues of S. cerevisiae Bck1p and Slt2p, in A. nidulans, BckA (MAPKKK) and MpkA (MAPK), were shown to function downstream of PkcA and were involved in the suppression of apoptosis during heat stress [11].
A. nidulans PkcA contains a long conserved N-terminal regulatory region consisting of three subdomains (CN1, CN2 and CN3), which interact with cell membranes [15]. The CN3 subdomain has high similarity with the calcium-binding domain of mammalian PKCs, but the lack of an aspartate residue dramatically decreases the affinity for this ion [16]. In S. cerevisiae, the PkcA homologue, Pkc1p, is activated by the Ras-like GTPase Rho1p [17] which in turn is activated by the cell membrane stress sensors Wsc1p (cell wall integrity and stress response component) [18] and Mid2 (mating induced death). These membrane sensors interact with the Rho1p guanine nucleotide exchange factor Rom2 [19]. In contrast to mammalian and S. cerevisiae cells, the mechanism of PkcA activation in A. nidulans remains unknown.
In filamentous fungi, intracellular Ca2+ levels are essential for the regulation of hyphal morphology (branching) and growth (orientation) [20]–[22]. The two major mediators of Ca2+-mediated signaling are the Ca2+-binding protein calmodulin (CaM) and the Ca2+/calmodulin-dependent calcineurin, a serine/threonine protein phosphatase [23]. Calcineurin consists of a catalytic subunit A and a regulatory subunit B, which through its association renders the catalytic subunit inactive [21]. Upon Ca2+ and calmodulin binding, calcineurin subunit A dissociates from the regulatory subunit and becomes active [21]. In filamentous fungi, calcineurin mediates growth, cell morphology, mating, virulence and responses to antifungal drugs [21] [24]–[28]. One of the targets of calcineurin subunit A (CnaA) in A. nidulans is the transcription factor CrzA. Upon an increase in intracellular Ca2+ levels, CnaA becomes active and dephosphorylates CrzA which subsequently translocates to the nucleus [29]. CrzA regulates the expression of chsB, which encode a chitin synthase involved in cell wall synthesis [30], P-type Ca2+ ATPase-encoding genes, which are involved in Ca2+ efflux, and other genes involved in calcium metabolism [31], thus mediating calcium homeostasis.
Although an increase in intracellular Ca2+ levels does not directly activate protein kinase C in fungi, as is the case in mammalian cells, there is considerable crosstalk between Ca2+-signaling pathways and Pkc-mediated signaling. In S. cerevisiae, transcriptional regulation of FKS2, which encodes the catalytic subunit of glucan synthase, is governed by a combination of signals mediated by Pkc1p, calcineurin and the Kss1p MAPK pathway components Ste11p and Ste12p, in response to different environmental stimuli [32]. Furthermore, constitutively expressed calcineurin can partially suppress the lysing phenotypes caused by PKC1 mutations [33]. Similarly, in Candida albicans and Cryptococcus neoformans, calcineurin was shown to induce FKS1, which encodes a β-1,3-glucan synthase subunit also regulated by the CWI pathway [33].
The presented study provides further evidence for a crosstalk between the calcium- and PkcA-mediated signaling pathways in A. nidulans. Overexpression of pkcA in a ΔcnaA background partially suppressed the phenotypic effects caused by the cnaA deletion. Furthermore, PkcA seemed to be involved in maintaining intracellular calcium homeostasis through controlling the expression of genes encoding mitochondrial components. This work clearly states the involvement of protein kinase C in various calcium-regulated processes in a filamentous fungus.
Results
Genetic interaction between pkcA and cnaA
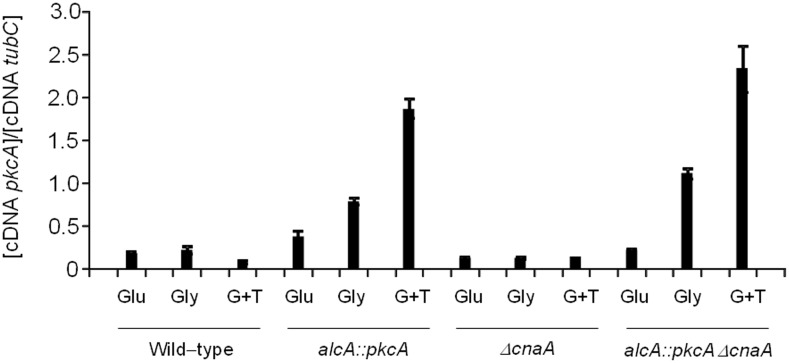
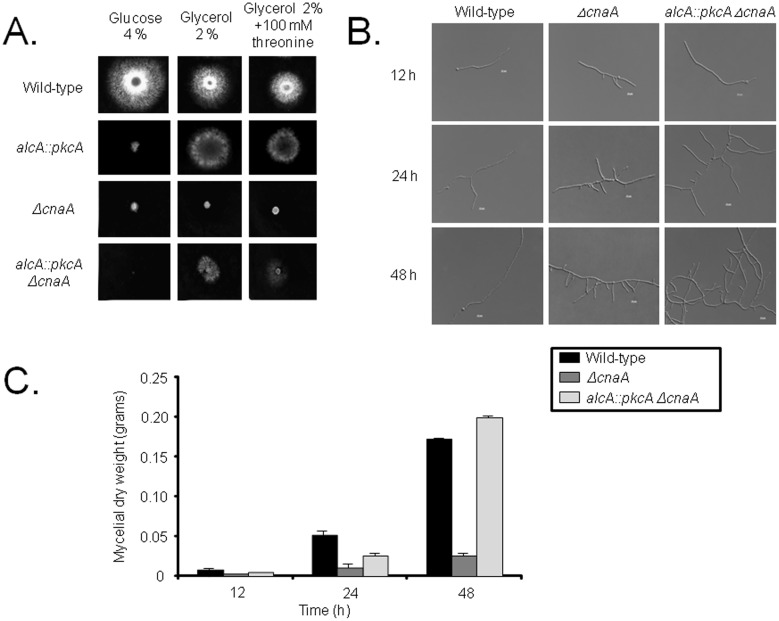
The deletion of the A. nidulans calcineurin phosphatase subunit A (CnaA) resulted in severe growth and conidiation defects, increased branching and septation [34], while both PkcA and CnaA are involved in maintaining cell wall integrity [32] [35], [36]. Therefore, a connection between these two proteins may exist. Hence, the alcA::pkcA ΔcnaA strain was constructed by sexually crossing an alcA::pkcA strain (in which the pkcA gene was placed under the regulatory control of the alcA promoter) with a ΔcnaA strain. Transcription of alcA is repressed in the presence of glucose, derepressed in the presence of glycerol and induced to high levels in the presence of ethanol or L-threonine [37]. The pkcA mRNA accumulation is increased about 3 to 4−fold when alcA::pkcA and alcA::pkcA ΔcnaA growth in 2% glycerol+100 mM threonine was compared to glucose 2% for both, respectively (Figure 1). The alcA::pkcA ΔcnaA strain showed a severe growth defect in the presence of glucose when compared to the wild-type strain, and worse than the ΔcnaA strain (Figure 2A). Growth of the alcA::pkcA ΔcnaA strain on solid media was partially restored in the presence of glycerol and glycerol plus threonine, when compared to the ΔcnaA strain (Figure 2A). The observed phenotypes were confirmed by measuring fungal biomass (dry weight) in liquid media containing 2% glycerol plus 100 mM threonine for 12, 24 and 48 hours at 37°C (Figure 2B). Nevertheless, a relation between branch emergence and septum formation may exist, as increased branching was observed in both the ΔcnaA and alcA::pkcA ΔcnaA strains (Figure 2B). After 48 hours of growth in the presence of glycerol plus threonine, the dry weight of alcA::pkaA ΔcnaA strain was slightly higher than, while the ΔcnaA strain was less than half, of the wild-type strain (Figure 2C).
Figure 1. The alcA::pkcA ΔcnaA strain has increased pkcA mRNA accumulation.
The wild−type, alcA::pkcA, ΔcnaA, and alcA::pkcA ΔcnaA strains were grown for 16 hours in minimal medium supplemented either with glucose 2% (G), glycerol 2% (Gly) or glycerol 2% plus 100 mM threonine (G+T), RNA extracted and RT-qPCR performed for pkcA.
Figure 2. Overexpression of pkcA suppresses the phenotype defects caused by the cnaA deletion.
(A) The wild-type, alcA::pkcA, ΔcnaA and alcA::pkcA ΔcnaA strains were grown for 72 hours at 37°C on agar plates containing either 4% glucose, 2% glycerol or 2% glycerol plus 100 mM threonine, or (B) in liquid media containing 2% glycerol plus 100 mM threonine for 12, 24 and 48 hours at 37°C, before their growth was assessed under the microscope (scale bars indicate 5 µm) and (C) mycelial dry weight measured for three biological replicates from each collected timepoint.
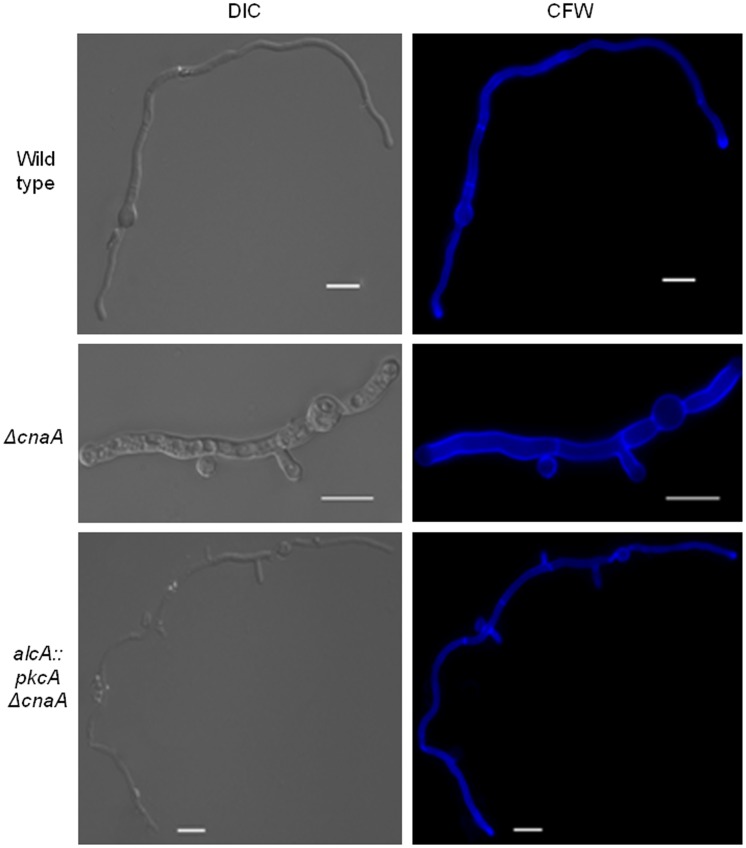
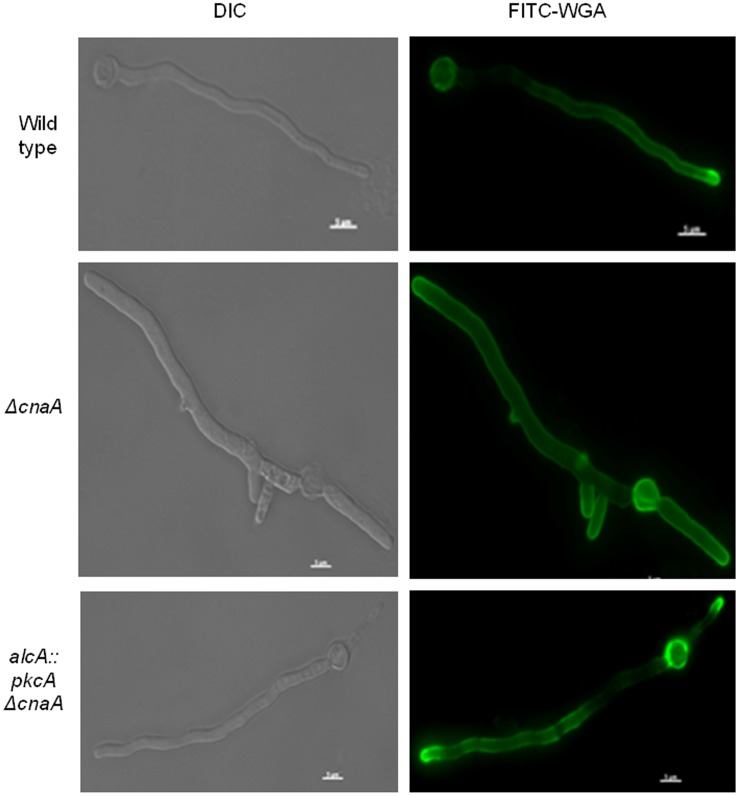
As previously observed [34], the ΔcnaA germlings contained a higher number of septa (5.9±0.88 septa in 100 germlings) than the wild-type and alcA::pkcA ΔcnaA (1.5±0.53 and 1.1±0.32 septa in 100 germlings) strains. The length between septa of the ΔcnaA was shorter than that of the wild− type (Figure 3). The length of the alcA:: pkcA ΔcnaA strain was restored under the PkcA-overexpression condition, and the restored length was similar to that of the wild−type (Figure 3). Septa are fungal cell walls which divide the non-apical part of the hypha into symmetrical compartments from which new branches can emerge [38]. One hypothesis is that septa function as a spatial cue for branch emergence in some fungi and that localized spikes in calcium levels may also specify branching sites, although substantial evidence for both theories is lacking [39]. Overexpression of pkcA restores the number of septa similar to the wild-type (Figure 3). In A. nidulans, FITC (fluorescein isothiocyanate)-conjugated WGA (wheat germ agglutinin) can be used to detect chitin that is deposited into the cell wall of the growing hyphal tip [40]. FITC-WGA staining of actively growing hyphae revealed that in approximately half (46%, 100 germlings; Figure 4) of the ΔcnaA hyphae, cell wall extension at the apex was lost when compared to the wild-type strain (100%, 100 germlings, Figure 2B). On the other hand, in the alcA::pkcA ΔcnaA strain, cell wall deposition was the same as in the wild-type strain (100%, 100 germlings; Figure 4). These results support the hypothesis of a genetic interaction between the signaling pathways that involve PkcA and CnaA.
Figure 3. Overexpression of pkcA in a ΔcnaA background rescues aberrant septa formation in a ΔcnaA background.
Wild-type, ΔcnaA and alcA::pkcA ΔcnaA strains were grown in minimal medium supplemented with 2% (w/v) glycerol plus 100 mM threonine for 16 hours at 30°C before being fixed for 30 minutes at room temperature (RT) and stained with calcofluor white (CFW) for 5 minutes at RT. Mycelial fluorescence was then assessed under the microscope (scale bars indicate 5 µm).
Figure 4. Overexpression of pkcA in a ΔcnaA background rescues aberrant cell wall deposition in a ΔcnaA background.
Wild-type, ΔcnaA and alcA::pkcA ΔcnaA strains were grown in minimal medium supplemented with 2% (w/v) glycerol plus 100 mM threonine for 16 hours at 30°C before being fixed for 30 minutes at room temperature (RT) and stained with fluorescein isothiocyanate wheat germ agglutinin (FITC-WGA) for 5 minutes at RT. Mycelial fluorescence was then assessed under the microscope (scale bars indicate 5 µm).
MpkA is targeted by calcineurin-mediated signaling
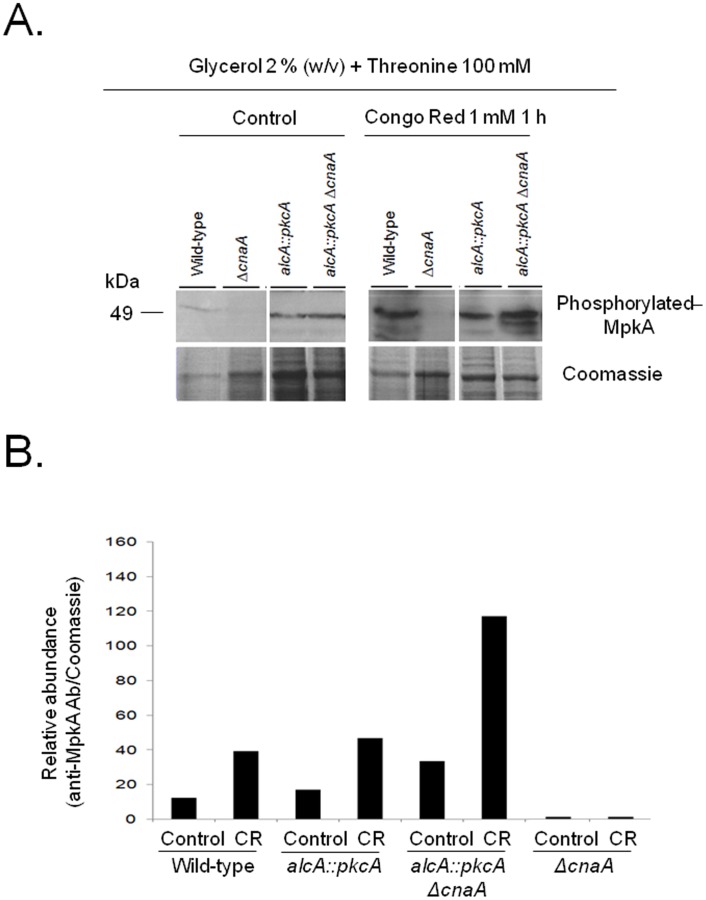
In S. cerevisiae, Pkc1p (the homologue of A. nidulans PkcA), is involved in the regulation of cell wall construction through indirectly phosphorylating the MAP kinase Mpk1p, which in turn activates the transcription factor Rlm1p. This transcription factor regulates the expression of genes whose products are important for cell wall biosynthesis [14]. The A. nidulans rlmA and mpkA are functional orthologs of S. cerevisiae RLM1 and MPK1 [41]. Cell wall stress, as experienced during hyphal extension and the addition of Congo red (CR) to liquid cultures, results in the phosphorylation of MpkA [10] [41]. Hence, the MpkA phosphorylation state in the ΔcnaA and alcA::pkcA ΔcnaA mutant strains was investigated. The wild-type strain contained a basal level of phosphorylated MpkA in the presence of 2% glycerol plus threonine, while the addition of CR resulted in a ∼4-fold increase of MpkA phosphorylation levels (Figures 5A and 5B). In the ΔcnaA strain, MpkA phophorylation levels were roughly tenfold less than in the wild-type strain and addition of CR did not result in an increase in MpkA phosphorylation levels (Figures 5A and 5B). In agreement with previous results [10], pkcA overexpression increases MpkA phosphorylation levels by around 20%, when compared to the wild-type strain, at both the basal level and upon addition of CR (Figures 5A and 5B). In the alcA::pkcA ΔcnaA strain the amount of MpkA phosphorylation was higher (18- to 60-fold) than in the wild-type and ΔcnaA strains in all conditions tested (Figures 5A and 5B). These results confirm the role of pkcA in the CWI pathway in A. nidulans and demonstrate that its overexpression can partially suppress the growth defects associated with the ΔcnaA strain. In addition, these results also indicate that MpkA is targeted by calcineurin-mediated signaling.
Figure 5. Overexpression of pkcA in a ΔcnaA background restores the levels of phosphorylated MpkA.
Western blots (A) of the protein crude extract from the wild-type, ΔcnaA, alcA::pkcA and alcA::pkcA ΔcnaA strains, when grown in 2% glycerol plus 100 mM threonine for 16 hours at 30°C before being treated with Congo Red (CR) for 1 hour. Phosphorylated MpkA (49 kDa band) was probed for with anti-MpkA antibodies (B) and signal intensities were quantified using the Image J software by dividing the intensity of Western band by a the Coomassie stained protein bands. kDa, kilo Daltons. These results are from a single representative experiment from three different repetitions that provided comparable results.
PkcA overexpression maintains intracellular calcium homeostasis in the absence of CnaA
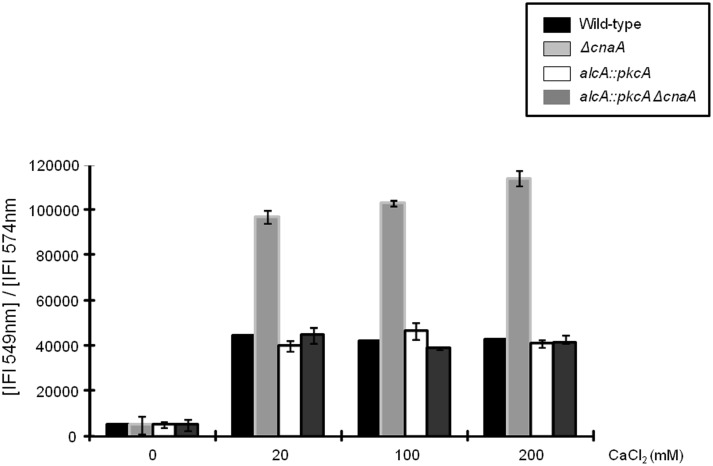
Intracellular calcium homeostasis is mediated through the products of a variety of genes, encoding proteins involved in calcium transport and metabolism, which are regulated by the transcription factor CrzA [29]. Appropriate calcium levels are vital for cell viability, as Ca2+ ions sequester phosphate molecules which can cause abnormal intracellular signaling [42]. Hence, to determine if pkcA overexpression could restore normal intracellular calcium levels in the absence of CnaA, the wild-type, alcA::pkcA, ΔcnaA and alcA::pkcA ΔcnaA strains were treated with different concentrations of CaCl2 for 10 minutes before Calcium Orange fluorophores (COF) were added for 30 min. Calcium orange chelates Ca2+ ions and upon binding exhibits increased fluorescence, allowing for the detection of the amount of calcium present (Life Technologies). The wild-type, alcA::pkcA and alcA::pkcA ΔcnaA strains had reduced intracellular calcium levels after 30 minutes of COF addition (Figure 6). In contrast, the ΔcnaA strain was unable to reduce Ca2+ levels after 30 minutes of COF addition and had approximately 2.5 times more intracellular Ca2+ than the wild-type, alcA::pkcA and alcA::pkcA ΔcnaA strains (Figure 6). These results indicate that pkcA overexpression can contribute to maintaining intracellular Ca2+ homeostasis in the absence of CnaA-associated signaling.
Figure 6. Overexpression of pkcA in a ΔcnaA background strain enables the cell to return to normal intracellular calcium levels after exposure to high concentrations of CaCl2.
The wild-type, alcA::pkcA, ΔcnaA and alcA::pkcA ΔcnaA strains were grown for 5 hours at 37°C before being exposed to 20 mM, 100 mM and 200 mM of CaCl2 for 30 minutes. The fluorophoric calcium chelator Calcium Orange was added then added for 30 minutes and fluorescence was assessed with a fluorometer at 579 nm.
Induction of alcA::pkcA in the ΔcnaA background modulates transcription of calcium controlled processes
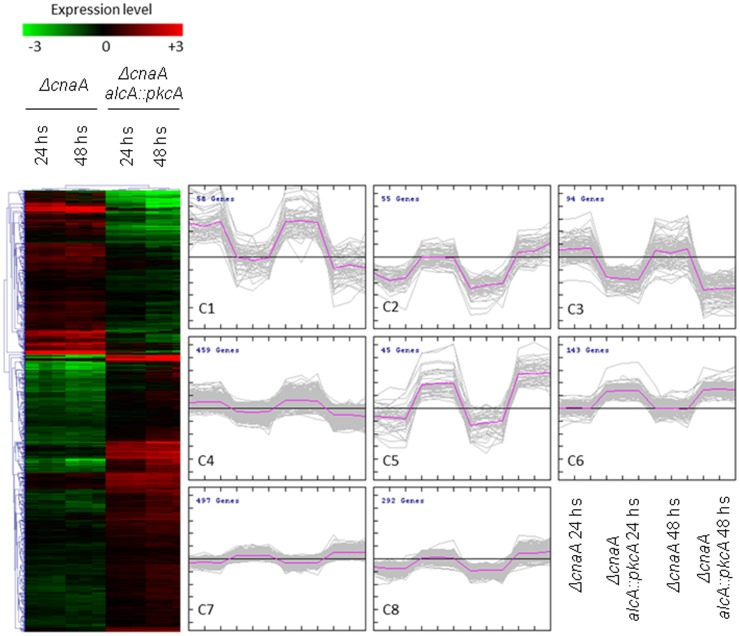
Microarray hybridizations were utilized to investigate in more detail which genes were being influenced by pkcA overexpression in the alcA::pkcA ΔcnaA strain, when compared to the ΔcnaA strain (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE48535). Both ΔcnaA and alcA::pkcA ΔcnaA strains were grown in minimal medium (MM) in the presence of glycerol plus threonine for 24 h and 48 h. The wild-type strain was used as an internal reference for both strains. A total of 1,643 genes were differentially expressed (t-test p<0.001) between the ΔcnaA and alcA::pkcA ΔcnaA strains. Hierarchical clustering and KMC (K-means clustering) identified 1,032 genes which demonstrated reduced expression in the ΔcnaA strain but had expression levels similar to the wild-type strain in the alcA::pkcA ΔcnaA strain (Figure 7; clusters 2, 5–8; Table S1). FetGOat analysis of these genes allowed determination of the functions of the proteins they encode and enabled the identification of overrepresented (p<0.05) gene ontologies (GO) (Tables S1, S2, S3). As shown in Table 1, the majority of these genes encoded proteins involved in cytoskeletal organization and trafficking, and, in particular, in mitochondria-related functions including oxidative phosphorylation. Genes encoding components of complex III of the respiratory chain and mitochondrial ribosomes were overrepresented within this dataset. The remaining 611 genes had increased expression levels in the ΔcnaA when compared to the alcA::pkcA ΔcnaA strain (Figure 7, clusters 1, 3 and 4). However, upon FetGOat analysis, only two GO terms were overrepresented and both described proteasomal components (GO:0000502 and GO:0008540; Table S4).
Figure 7. Hierarchal and k-means (KM) clustering of the genes which were differentially (t-test p<0.001) expressed between the Δcna and alcA::pkcA ΔcnaA strains.
Microarrays were performed on RNA extracted from both strains when grown in glucose or glycerol and threonine for 24 and 48 hours.
Table 1. Number of genes and the associated functions of the proteins they encode that had restored or elevated expression in the alcA::pkcA ΔcnaA strain when compared to the ΔcnaA strain.
| GO term | Annotation | P-value | sP |
| Mitochondrial components, function and energy conversion | |||
| GO:0006122 | mitochondrial electron transport, ubiquinol to cytochrome c | 1.12E-04 | 6 |
| GO:0055114 | oxidation-reduction process | 5.11E-05 | 32 |
| GO:0042773 | ATP synthesis coupled electron transport | 2.99E-05 | 9 |
| GO:0006626 | protein targeting to mitochondrion | 9.24E-07 | 17 |
| GO:0032543 | mitochondrial translation | 7.89E-04 | 10 |
| GO:0033617 | mitochondrial respiratory chain complex IV assembly | 3.85E-04 | 6 |
| GO:0008535 | respiratory chain complex IV assembly | 3.85E-04 | 6 |
| GO:0097034 | mitochondrial respiratory chain complex IV biogenesis | 3.85E-04 | 6 |
| GO:0007005 | mitochondrion organization | 1.40E-20 | 62 |
| GO:0033108 | mitochondrial respiratory chain complex assembly | 6.05E-07 | 10 |
| GO:0007006 | mitochondrial membrane organization | 2.93E-06 | 13 |
| GO:0042775 | mitochondrial ATP synthesis coupled electron transport | 2.99E-05 | 9 |
| GO:0022904 | respiratory electron transport chain | 2.99E-05 | 9 |
| GO:0007007 | inner mitochondrial membrane organization | 2.99E-05 | 9 |
| GO:0000002 | mitochondrial genome maintenance | 6.75E-05 | 13 |
| GO:0072655 | establishment of protein localization in mitochondrion | 4.87E-07 | 18 |
| GO:0022900 | electron transport chain | 2.99E-05 | 9 |
| GO:0006839 | mitochondrial transport | 6.64E-08 | 22 |
| GO:0045039 | protein import into mitochondrial inner membrane | 1.80E-03 | 5 |
| GO:0015980 | energy derivation by oxidation of organic compounds | 1.28E-07 | 32 |
| GO:0070585 | protein localization in mitochondrion | 4.87E-07 | 18 |
| GO:0045333 | cellular respiration | 9.65E-12 | 32 |
| GO:0009060 | aerobic respiration | 4.64E-12 | 30 |
| GO:0030150 | protein import into mitochondrial matrix | 1.72E-05 | 10 |
| GO:0006119 | oxidative phosphorylation | 5.19E-04 | 12 |
| GO:0006099 | tricarboxylic acid cycle | 7.89E-04 | 10 |
| Cytoskeleton-related transport | |||
| GO:0007018 | microtubule-based movement | 9.65E-06 | 11 |
| GO:0072384 | organelle transport along microtubule | 3.99E-06 | 11 |
| GO:0031109 | microtubule polymerization or depolymerization | 2.15E-03 | 6 |
| GO:0030473 | nuclear migration along microtubule | 7.16E-05 | 9 |
| GO:0010970 | microtubule-based transport | 3.99E-06 | 11 |
| GO:0007017 | microtubule-based process | 2.82E-05 | 22 |
| GO:0030705 | cytoskeleton-dependent intracellular transport | 9.03E-07 | 12 |
| GO:0046907 | intracellular transport | 2.29E-07 | 91 |
| GO:0017038 | protein import | 1.26E-07 | 29 |
| GO:0006810 | transport | 5.62E-04 | 136 |
| GO:0071806 | protein transmembrane transport | 2.23E-05 | 13 |
| GO:0065002 | intracellular protein transmembrane transport | 2.23E-05 | 13 |
| GO:0006886 | intracellular protein transport | 3.91E-04 | 41 |
| GO:0015031 | protein transport | 8.09E-04 | 41 |
| GO:0007097 | nuclear migration | 1.20E-05 | 13 |
| Cellular organisation | |||
| GO:0051656 | establishment of organelle localization | 2.15E-05 | 23 |
| GO:0034613 | cellular protein localization | 9.88E-04 | 46 |
| GO:0006605 | protein targeting | 9.38E-04 | 36 |
| GO:0006996 | organelle organization | 9.06E-08 | 133 |
| GO:0051647 | nucleus localization | 1.20E-05 | 13 |
| GO:0008104 | protein localization | 1.77E-03 | 50 |
| GO:0051648 | vesicle localization | 1.80E-03 | 5 |
| GO:0051641 | cellular localization | 6.37E-08 | 108 |
| GO:0045184 | establishment of protein localization | 9.46E-04 | 42 |
| GO:0040023 | establishment of nucleus localization | 1.20E-05 | 13 |
| GO:0051234 | establishment of localization | 1.82E-04 | 142 |
| GO:0051649 | establishment of localization in cell | 3.23E-08 | 99 |
| GO:0033036 | macromolecule localization | 1.80E-03 | 61 |
| GO:0051179 | localization | 1.01E-04 | 152 |
| GO:0051640 | organelle localization | 3.10E-05 | 26 |
| GO:0070727 | cellular macromolecule localization | 6.01E-04 | 48 |
| Metabolism | |||
| GO:0071840 | cellular component organization or biogenesis | 4.68E-07 | 205 |
| GO:0071841 | cellular component organization or biogenesis at cellular level | 1.49E-07 | 192 |
| GO:0006364 | rRNA processing | 2.48E-03 | 35 |
| GO:0006091 | generation of precursor metabolites and energy | 6.90E-06 | 44 |
| GO:0044085 | cellular component biogenesis | 1.53E-03 | 92 |
| GO:0016043 | cellular component organization | 2.78E-05 | 165 |
| GO:0043623 | cellular protein complex assembly | 7.16E-05 | 25 |
| GO:0071842 | cellular component organization at cellular level | 9.03E-06 | 146 |
| GO:0006744 | ubiquinone biosynthetic process | 9.95E-04 | 6 |
| GO:0006084 | acetyl-CoA metabolic process | 9.86E-04 | 11 |
| GO:0046356 | acetyl-CoA catabolic process | 7.89E-04 | 10 |
| GO:0006743 | ubiquinone metabolic process | 9.95E-04 | 6 |
| GO:0045426 | quinone cofactor biosynthetic process | 9.95E-04 | 6 |
| GO:0042375 | quinone cofactor metabolic process | 9.95E-04 | 6 |
| Signal transduction | |||
| GO:0031684 | heterotrimeric G-protein complex cycle | 7.04E-04 | 4 |
sP is number of genes in each category.
Overexpression of pkcA increases the mitochondria in the ΔcnaA genetic background
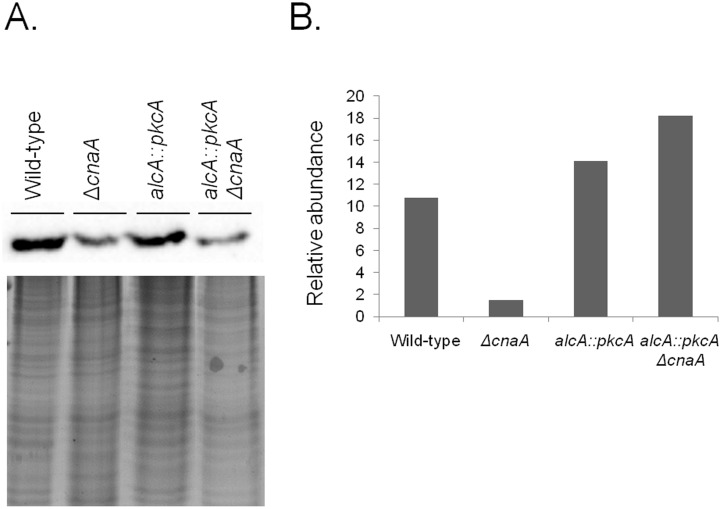
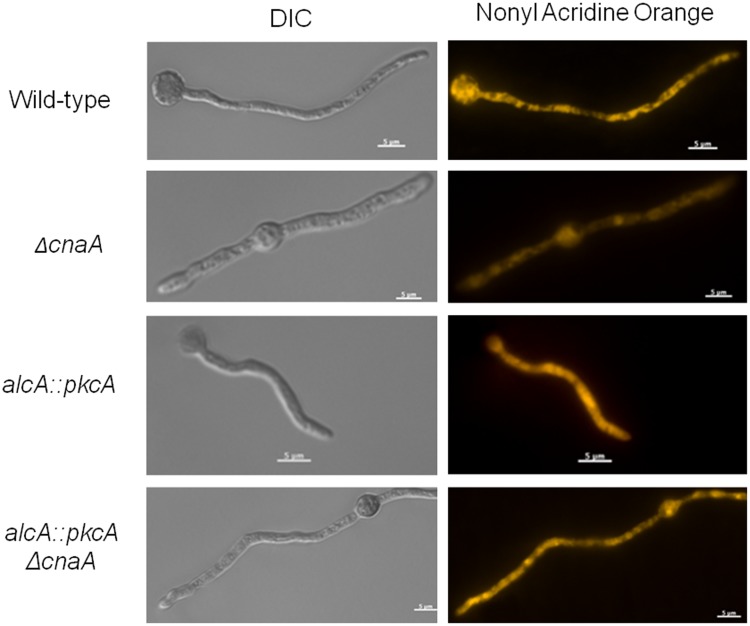
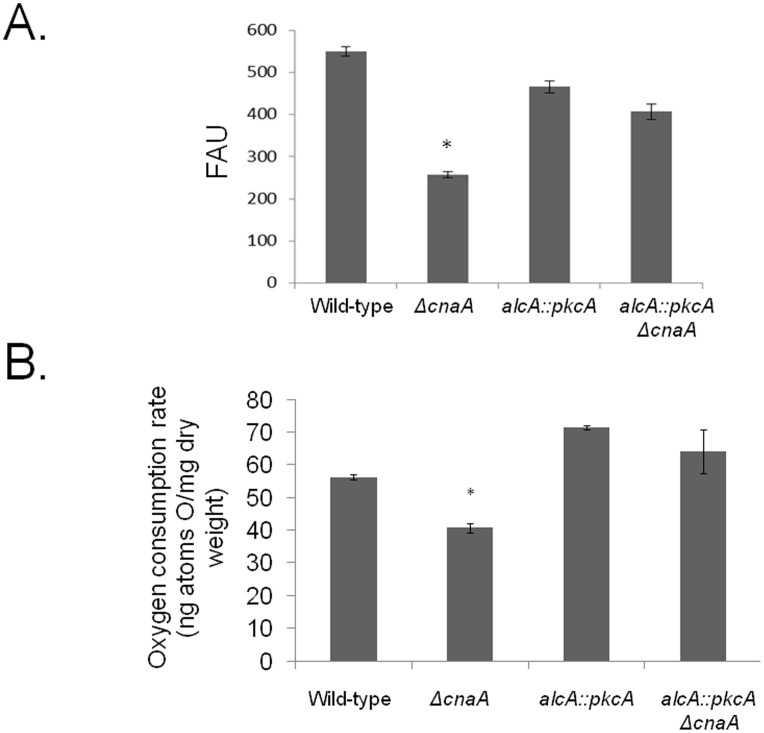
The microarray data showed a difference in the expression of genes which encoded for proteins involved in mitochondrial functions, between the ΔcnaA and alcA::pkcA ΔcnaA strains. Subsequently, to validate these results, mitochondrial mass was evaluated. Protein extracts from the wild-type, ΔcnaA, alcA::pkcA and alcA::pkcA ΔcnaA strains, when grown in MM supplemented with glycerol plus threonine, were incubated with anti-cytochrome c antibody (Figure 8A). Cytochrome c is a small heme protein found within the cristae of the inner mitochondrial membranes and is an essential component of the electron transport chain [43]. The abundance of cytochrome c was less in the ΔcnaA strain than the wild-type and alcA::pkcA strains (Figure 8B). Upon pkcA overexpression, in the alcA::pkcA ΔcnaA strain, cytochrome c levels were approximately ten times that observed in the ΔcnaA strain and were also higher than those observed in the wild-type strain (Figure 8B). These results were in agreement with the fluorescent microscopy data and flow cytometric analyses (FCA), where hyphal mitochondria were stained with Nonyl Acridine Orange, observed under the microscope and subsequently counted, revealing a decrease in mitochondrial mass in ΔcnaA strain (Figures 9 and 10A). In addition, the rate of oxygen consumption was measured in all strains. The ΔcnaA strain consumed approximately 30% less oxygen than the wild-type strain, whereas pkcA overexpression in the alcA::pkcA ΔcnaA strain restored normal levels of oxygen consumption, similar to the wild-type strain (Figure 10B). These data indicate that pkcA overexpression restores the number of mitochondria within the fungal cells, allowing normal levels of respiration to take place.
Figure 8. Overexpression of pkcA in a ΔcnaA background restores cytochrome c expression.
(A) Western blots of the protein extracts from the wild-type, ΔcnaA, alcA::pkcA and alcA::pkcA ΔcnaA strains when grown in the presence of 2% glycerol plus 100 mM threonine for 16 hours at 37°C. Signal intensities were quantified using the Image J software by dividing the intensity of Western band of the cytochrome c by the Coomassie stained protein bands (B). These results are from a single representative experiment from three different repetitions that provided comparable results.
Figure 9. Overexpression of pkcA in a ΔcnaA background restores mitochondrial number.
Wild-type, ΔcnaA, alcA::pkcA and alcA::pkcA ΔcnaA germlings were grown in minimal medium supplemented with 2% glycerol plus 100 mM threonine for 16 hours at 37 C and stained with nonyl acridine orange for 10 minutes at 37°C. Mycelial fluorescence was subsequently assessed under the microscope (scale bars indicate 5 µm).
Figure 10. Overexpression of pkcA in a ΔcnaA background restores oxygen consumption.
(A) Wild-type, ΔcnaA, alcA::pkcA and alcA::pkcA ΔcnaA germlings were grown in minimal medium supplemented with 2% glycerol plus 100 mM threonine for 16 hours at 37 C and stained with nonyl acridine orange for 10 minutes at 37°C. Mycelial fluorescence was subsequently assessed under the microscope (scale bars indicate 5 µm). Flow cytometric analyses (FCA) is shown. The results are expressed as mean ± SD (standard deviation) of three independent biological replicates and were considered statistically different (*) when p<0.05. P-values were determined by a Student t test using GraphPad Prism software version 5 (GraphPad Software). DIC, differential interference contrast; FAU, fluorescent arbitrary units. (B) Oxygen consumption rates of the wild-type, ΔcnaA, alcA::pkcA and alcA::pkcA ΔcnaA strains when grown in minimal medium supplemented with 2% glycerol plus 100 mM threonine for 16 hours at 37°C.
Discussion
Cellular signaling governs the cell's responses to environmental stimuli, thus allowing an organism to adapt to different conditions. Cells have evolved a complex network of signaling pathways mediated by a great number of proteins, such as protein kinases and phosphatases, which add or remove phosphate groups from target proteins, therefore directing their activity, location and function [4]. In mammalian cells, protein kinase C (Pkc) regulates gene transcription, membranes and cell growth, while being activated upon an increase in cellular Ca2+ levels [2]. In fungi, Pkc plays an important role in growth through activating the cell wall integrity (CWI) pathway which is mediated by a MAP (mitogen activated protein) kinase cascade [11] [14]. In S. cerevisiae, cell wall stress sensors activate the Rho GTPase Rho1p which in turn activates Pkc1p that then activates the CWI MAP pathway [17]. In the filamentous fungus A. nidulans, the mechanisms for PkcA activation remain unknown. An increase in intracellular Ca2+ levels activates the calcineurin phosphatase, whose catalytic subunit A, encoded by cnaA in A. nidulans, modulates the transcription of genes involved in maintaining cellular calcium homeostasis [29] [31]. Although crosstalk between the calcium- and Pkc-mediated pathways appears to take place in S. cerevisiae and other filamentous fungi [32], [33], knowledge about the interactions of these signaling pathways is limited in Aspergillus species. The genus Aspergillus contains many species of medical and industrial importance, thus elucidating signaling pathways involved in growth in these species could be relevant for many biotechnological applications.
The presented study describes a genetic interaction between the CnaA- and PkcA-mediated signaling pathways in A. nidulans. Previous investigations showed that cnaA is not an essential gene in A. nidulans and A. fumigatus, but that its deletion caused severe defects in apical extension and polarized growth in both fungi [26] [34]. Accordingly, the A. nidulans ΔcnaA strain showed reduced growth in solid and liquid media supplemented with different carbon sources. Furthermore, the cnaA deletion strain had a different number of septa and aberrant apical growth when compared to the wild-type strain. The intracellular localization of Ca2+ gradients participates in the determination of the site of germ tube emergence and maintains the axis of polarized growth [44]. In A. fumigatus, calcineurin localizes to the septum and the catalytic activity of calcineurin subunit A is required for maintaining normal hyphal growth [45]. Deletion of cnaA in A. fumigatus also resulted in abnormal cell wall architecture with decreased β-glucan and increased chitin contents [45]. In other fungi, calcineurin and Pkc were shown to be involved in the regulation of the cell wall constructing enzyme β-glucan synthase [32], [33]. Similar roles for CnaA in cell wall integrity could therefore be considered in A. nidulans.
Overexpressing pkcA in the ΔcnaA genetic background was able to partially rescue the observed growth ΔcnaA phenotypes. PkcA is involved in cell wall construction and maintenance through its involvement in the cell wall integrity pathway via MAPK signaling [11] [14]. In agreement, PkcA overexpression in the ΔcnaA strain restored MpkA (the CWI pathway MAPK) phosphorylation levels similar to the wild-type strain. Another explanation could be that both PkcA and calcineurin signaling affect cell cycle progression. In S. cerevisiae, a role for Pkc1p in G2/M cell cycle transition, separate from its role in the regulation of the MAPK pathway, has been previously suggested [46]. This could coordinate cell wall expansion with cell cycle progression. In Schizosaccaromyces pombe, calcineurin is activated by the cell cycle checkpoint kinase Cds1 and subsequently dephosphorylates Tip1, a protein involved in polarity factor transport, resulting in cell cycle progression delay [47]. All these results indicate that the CnaA- and PkcA-activated pathways share common targets, which could include MpkA, and in doing so coordinate the integrity of the cell wall during growth and in response to stress. This is not surprising as both proteins mediate signaling pathways involved in maintaining polarized hyphal growth.
Microarrays were utilized to provide an insight into the transcriptional impact of pkcA overexpression in the ΔcnaA strain. A comparision of the ΔcnaA and ΔcnaA pkcA overexpressing strains with the wild-type strain identified 1,643 genes as being differentially regulated between the two mutant strains, with 1,032 genes being down-regulated and 611 genes being up-regulated in the ΔcnaA strain when compared to alcA::pkcA ΔcnaA strain. The majority of the up-regulated genes encoded proteasomal components, suggesting an increase in the degradation of misfolded proteins within the ΔcnaA strain. Scrimale et al. [48] showed that induction of the CWI pathway in S. cerevisiae activated the UPR (unfolded protein response). The UPR is activated upon detection of misfolded proteins in the endoplasmic reticulum (ER), resulting from high throughput protein secretion causing cellular secretory stress [49]. Activation of the UPR results in the up-regulation of genes encoding proteins which control many cellular processes such as protein folding, cell wall architecture, protein trafficking, lipid biosynthesis and ERAD (ER-associated protein degradation) [48]. It appears that deletion of cnaA causes an increase in cellular stresses which subsequently activate the UPR and increase protein degradation. Indeed, Colabardini et al., [10] have previously shown crosstalk between PkcA and the UPR in A. nidulans in response to exposure to farnesol.
The majority of the down-regulated genes in the ΔcnaA strain, when compared to the ΔcnaA pkcA-overexpressing strain, encoded proteins involved in mitochondrial function and energy conversion, cytoskeleton-related transport, cellular organization and metabolism. Denis and Cyert [50] showed that the C2 domain of Pkc1p in S. cerevisiae is responsible for targeting the protein to the mitotic spindle, whereas the HR1 and C1 regulatory motifs ensure localization of Pkc1p to the bud tip and cell periphery. A. nidulans PkcA::GFP localizes to hyphal apices and growing septa, as well as to the conidiogenous apices of phialides, indicating a role for PkcA in polarized cell wall growth [5]. Calcineurin or calmodulin, activated through an increase in intracellular Ca2+ levels, have been shown to localize at the apex, at the Spitzenkörper or at the septa during polar growth in A. nidulans [51], [52] and A. fumigatus [45]. The Spitzenkörper is a sub-apical structure which coordinates polar growth that associates with and is surrounded by microtubules and actin filaments which shuttle vesicles carrying calcium (to ensure the tip-associated calcium gradient), signaling modules and cell wall components back and forth to the Spitzenkörper with the aid of motor proteins [53], [54]. The microarray data showed a reduction in the expression of genes encoding cytoskeletal components, cellular organization and transport in the ΔcnaA strain, while the overexpression of pkcA was able to restore the expression levels of such genes, further re-inforcing the idea of common targets for the PkcA- and CnaA-mediated signaling pathways, regarding hyphal growth.
Furthermore, genes encoding components of mitochondria were also down-regulated in the ΔcnaA strain when compared to the pkcA overexpressing strain. Further investigation showed that the deletion of calcineurin caused a decrease in mitochondria and respiration; a defect which was rescued through pkcA overexpression. Mitochondria sequester Ca2+ ions, therefore playing an important role in decreasing intracellular Ca2+ levels after it has carried out its signaling function [3]. Ca2+ ions can diffuse readily through the outer membrane of the mitochondria and are then transported through the inner membrane into the mitochondrial cytoplasm through highly selective ion channels and transporters [55]. An increase in mitochondrial Ca2+ levels boosts ATP production which as a consequence increases the concentration of cellular reactive oxygen species (ROS) which can lead to apoptosis [54]. In the presented study of A. nidulans, deletion of calcineurin caused an increase in cytoplasmic calcium levels, while previous work also showed that calcineurin regulates genes, encoding for proteins involved in calcium homeostasis, via the transcription factor CrzA [31]. It is therefore possible that increased cytosolic calcium levels in the ΔcnaA strain resulted in an increased uptake of Ca2+ ions by the mitochondria, resulting in increased mitochondrial death. Upon overexpressing pkcA, mitochondrial number and function was restored. PkcA-mediated signaling is therefore somehow involved in maintaining the mitochondrial copy number. Indeed, overexpression of pkcA upon exposure to farnesol, increases cell death through increasing metacaspase activity [10].
In summary, the presented study describes an interaction between calcium and protein kinase C signaling pathways. Deletion of the calcineurin catalytic subunit A caused growth abnormalities, increased cytosolic Ca2+ concentrations plus a reduction in mitochondrial number and function. These defects could all be rescued (at least partially) through the overexpression of pkcA. This is the first report in A. nidulans, which connects the PkcA- and calcineurin-mediated signaling pathways to mitochondrial function. Further work is required to elucidate at which step these pathways are interconnected, PkcA targets and to investigate in more detail how they link to the mitochondria.
Materials and Methods
Strains and media
All A. nidulans strains used in this study are listed in Table 2. Strains were cultivated at 37°C in either minimal medium [4% (w/v) glucose or 2% (w/v) glycerol or 2% (w/v) glycerol plus 100 mM threonine], 50 ml of a 20× salt solution (120 g/l NaNO3, 10.4 g/l KCl, 30 g/l KH2PO4, 10.4 g/l MgSO4), 1 ml of 5× Trace elements (22.0 g/l ZnSO4, 11 g/l boric acid, 5 g/l MnCl2, 5 g/l FeSO4, 1.6 g/l CoCl2, 1.6 g/l CuSO4, 1.1 g/l (NH4)2MoO4, 50 g/l EDTA)], pH 6.5; or in complete medium [2% (w/v) glucose, 0.5% (w/v) yeast extract, 1 ml of 5× trace elements (same as for the minimal medium)]. Minimal and complete media were supplemented with 0.12% (w/v) of each uridine and uracil, or pyrodoxine (0.5 µg/ml) depending on the genetic background. Solid media was made by adding 2% (w/v) agar to minimal or complete media. Unless otherwise stated, all chemicals were obtained from Sigma Aldrich (St. Louis, MO, USA).
Table 2. A. nidulans strains used in this work.
| Strain | Genotype | Reference |
| TNO2A3 (wild-type) | pyroA4, pyrG89, argB2, ΔnKuA::argB, cho1 | Nayak et al., 2006 (reference [58]) |
| alcA::pkcA | pyrG89; pyroA4; alcA::pkcA::pyr4; choA1 | Colabardini et al., 2010 (reference [10]) |
| ΔcnaA | pyroA4 pyrG89, ΔcnaA::pyro, wA3 | Soriani et al., 2008 (reference [34]) |
| alcA::pkcA ΔcnaA | alcA::pkcA ΔcnaA, cho1 | This work |
Mycelial staining and microscopy
Coverslips were inoculated with the different strains (see results section) in 5 ml of minimal media at 30°C.for 16 hours. Hyphae were then fixed [3.7% (v/v) formaldehyde, 50 mM sodium phosphate buffer pH 7.0 (NaH2PO4, Na2HPO4), 0.2% (v/v) Triton X-100)] for 30 min at room temperature, rinsed with phosphate buffered saline (PBS: 140 mM NaCl, 2 mM KCl, 10 mM NaHPO4, 1.8 mM KH2PO4, pH 7.4) and incubated for 5 minutes in a 100 ng ml−1 calcofluor white. Coverslips were again washed with PBS for 10 minutes at room temperature and rinsed with distilled water. FITC-WGA-staining was carried out by incubating coverslips in prewarmed (30°C) media containing 5 mg ml−1 FITC-WGA for 5 minutes [56]. Hyphae were fixed and washed as previously described.
Germlings were visualized on a Carl Zeiss (Jena, Germany) AxioObserver.Z1 fluorescent microscope equipped with a 100 W HBO mercury lamp, using a 100× magnification oil immersion objective (EC Plan-Neofluar, NA 1.3). Fluorescent and DIC (differential interference contrast) images were captured with an AxioCam camera (Carl Zeiss) and processed using the AxioVision software version 3.1. Further image processing was performed using Adobe Photoshop 7.0 (Adobe Systems Incorporated, CA).
RNA extraction
Mycelia were separated from the supernatant using Whatman filter paper. Fungal cell walls were broken through grinding the mycelia under liquid nitrogen and total RNA was extracted using RNeasy Plant Mini Kit (Qiagen) according to manufacturer's instructions. RNA samples were run on a 2.2 M formaldehyde, 1.2% (w/v) agarose gel to check RNA quality and quantified on the NanoDropH 2000 Thermo Scientific (Uniscience).
Microarray slides construction and gene expression analysis
Gene expression was compared between A. nidulans ΔcnaA and alcA::pkcA ΔcnaA strains when grown for 24 and 48 hours at 37°C in in minimal media supplemented with 2% glycerol plus 100 mg/ml threonine, before RNA was extracted. Microarray slide design, sample labeling and hybridization were performed as described previously [56].
The extraction of data from the TIFF files, generated through scanning the microarray slides, was performed using the Agilent Feature Extraction (FE) Software version 9.5.3.1 (Agilent Technologies). This software used the linear Lowess algorithm to subtract the background noise and obtain normalized intensity values. Normalized values were uploaded into the software Express Converter (version 2.1, TM4 platform available at http://www.tm4.org/utilities.html), which converts the Agilent file format to the multi-experiment viewer (mev) file format that is compatible with the TM4 software used for microarray analysis (available at http://www.tm4.org/). The mev files were uploaded into the MIDAS software (TM4 platform), where averages for each gene-replicate, from the biological replicates, were generated. Finally, analysis of the mev files was carried out using the TIGR MeV (TM4 platform, Multi Experiment Viewer, available at http://www.tigr.org/software/microarray.shtml) software. Differentially expressed genes were defined as those that had a mean log2 expression ratio statistically different from 0, identified through applying the one-class t-test (P>0.01).
Western blots
Intracellular proteins were extracted via grinding the mycelia under liquid nitrogen and then re-suspending the mycelial powder in extraction buffer [15 mM p-nitrophenylphosphate, 25 mM tris pH 7.4, 15 mM EGTA (ethylene glycol tetraacetic acid), 15 mM MgCl2 and protease inhibitor cocktail (Complete Mini; Roche)] by vortexing for 10 minutes. Samples were centrifuged for 10 minutes (13,000 rpm) and the supernatant collected. Total protein concentrations in the supernatants were determined using the Bio-Rad Protein Assay, according to manufacturer's instructions. 1× sample loading buffer (62.5 mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol and 5% bromophenol blue) was added to the samples and which were boiled at 100°C for 3 min. Samples were run on a 12.5% (w/v) SDS-PAGE gel. The gel was blotted onto a nitrocellulose membrane (0.2 mm; Bio-Rad), which was blocked with a 5% (w/v) dried milk and TBS-Tween buffer (10 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.05% Tween 20, pH 7.5) followed by the incubation with a 1∶1500 dilution of the anti-phospho-p44/42 MAPK antibody (Cell Signaling Technology, USA) overnight at 4°C. Primary antibodies were detected using a horseradish peroxidase (HRP)-conjugated second antibody (Kirkegaard and Perry Laboratories) at a 1∶5000 dilution in TBS-T plus 5% skimmed milk powder for 1 h, at room temperature. Chemiluminescence was detected using Super signal West Pico chemioluminescent substrate (Pierce), and signals were recorded using the Hyperfilm ECL (Amersham Biosciences).
Oxygen uptake measurements
Conidia (1×108) were inoculated in 15 ml of minimal medium supplemented with 2% glycerol plus 100 mM threonine for 5 hours at 37°C, 250 rpm. The germlings were harvested by centrifugation and washed twice with cold distilled water. Germlings were then washed three times with 0.7 mM sorbitol, 10 mM HEPES–KOH, pH 7.2 and subsequently kept on ice. Oxygen uptake was measured with a Clark-type electrode fitted to a Gilson oxygraph 1 (Gilson Medical Electronics, Inc.,2 Middleton, WI) in 1.8 ml of buffer containing 0.7 mM sorbitol, 10 mM HEPES–KOH, pH 7.2, 5 mM MgCl2 and 0.5 mM EGTA at 30°C [57]. The initial solubility of oxygen in the reaction buffer was considered to be 445 ng of O2 atoms/ml.
Mitochondrial mass measurements
Conidia from the different strains (1×108 ml−1) were incubated in 50 ml liquid minimal media at 37°C on a reciprocal shaker for 6 hours. Subsequently, conidia were washed with PBS and incubated with 5 nM Nonyl Acridine Orange (NAO; Invitrogen) diluted in PBS plus 5% fetal bovine serum (FBS) for 10 minutes at 37°C. Stained conidia were washed with PBS, resuspended in PBS plus 5% FBS, and then analyzed by flow cytometry. Propidium iodide (0.5 µM) staining of the nucleus was utilized to exclude dead cells. Flow cytometry was analyzed by guava Easycity 8 HH (Millipore) using 10,000 acquisitions for each analysis.
For cytochrome c measurements, 2×108 conidia/ml of each strain were inoculated in minimal media for 16 hours at 37°C. After this period, the cultures were filtered and the mycelia washed with 100 ml H2O, immediately frozen in liquid nitrogen and grinded. For every 0.1 g of dry weight, 1.3 ml HB buffer (150 mM NaCl, 30 mM KCl, 10 mM Na2HPO4, pH 7.0) was added. The suspension was centrifuged at 21,000 g at 4°C for 30 minutes. The supernatant was removed and centrifuged again for 10 minutes under the same conditions. After determining protein concentration, samples were prepared for SDS-PAGEas described above. Thirty micrograms of total protein from each sample were loaded into each lane of a 15% SDS-PAGE gel. After separation of the proteins, the gel was blotted onto a pure nitrocellulose membrane (0.2 µm; Bio-Rad) and after being blocked in 5% (w/v) dried milk in TBSTween, the membrane was probed with the rabbit anti-cyc1 antibody (CNAT against native cytochrome c from yeast; Sigma) at a 1∶200 dilution in TBS-Tween for 1 hour at room temperature. The membrane was washed four times with TBS-Tween for 5 minutes and then incubated with a 1∶5000 dilution of goat anti-rabbit IgG peroxidase-labeled (KPL) antibody for 1 hour. After being washed, the blot was developed by use of the SuperSignal Ultra chemiluminescence detection system (Pierce) and recorded by the use of Hyperfilm ECL (Amersham Biosciences).
Determination of intracellular calcium levels
To determine intracellular Ca2+ levels, wild-type, ΔcnaA, alcA::pkcA and alcA::pkcA ΔcnaA strains were first grown for 5 hours in minimal medium supplemented with 2% (w/v) glycerol plus 100 mM threonine at 37°C. Strains were then treated with 20 mM, 100 mM and 200 mM CaCl2 for 10 minutes. Mycelia were then washed 3 times with PBS and incubated with 1 µM Calcium Orange fluorophore at 37°C for 30 minutes. Mycelia were washed again and fluorescence was measured using a fluorometer at 549 nm excitation and 579 nm emission, according to manufacturer's instructions. The experiment was normalized by counting the number of cells in a Neubauer chamber.
Supporting Information
FetGOat analysis for genes with reduced mRNA accumulation in A. nidulans ΔcnaA (similar to the wild-type strain) when compared to the alcA::pkcA ΔcnaA strain.
(XLSX)
FetGOat analysis for genes with reduced mRNA accumulation in A. nidulans alcA::pkcA ΔcnaA (similar to the wild-type strain) when compared to the ΔcnaA strain.
(XLSX)
FetGOat analysis for genes with increased mRNA accumulation in A. nidulans alcA::pkcA ΔcnaA (similar to the wild-type strain) when compared to the ΔcnaA strain.
(XLSX)
FetGOat analysis for genes with increased mRNA accumulation in A. nidulans ΔcnaA when compared to the alcA::pkcA ΔcnaA strain.
(XLSX)
Acknowledgments
We would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for providing financial support, and the three anonymous reviewers for their suggestions and comments.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All the microarray hybridization data is at: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE48535.
Funding Statement
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS (2005) G-protein signaling: back to the future. Cell Mol Life Sci 62: 551–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson DL, Cox MM (2008) Lehninger Principles of Biochemistry, 5th edition, W.H. Freeman and Company, New York, USA. [Google Scholar]
- 3. Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signaling. Nature Reviews 4: 11–21. [DOI] [PubMed] [Google Scholar]
- 4. Hunter T (1995) Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell 80: 225–236. [DOI] [PubMed] [Google Scholar]
- 5. Teepe AG, Loprete DM, He Z, Hoggard TA, Hill TW (2007) The protein kinase C orthologue PkcA plays a role in cell wall integrity and polarized growth in Aspergillus nidulans . Fungal Genet Biol 44: 554–562. [DOI] [PubMed] [Google Scholar]
- 6. Herrmann M, Sprote P, Brakhage AA (2006) Protein kinase C (PkcA) of Aspergillus nidulans is involvedin penicillin production. Appl Env Microbiol 72: 2957–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ichinomiya M, Uchida H, Koshi Y, Ohta A, Horiuchi H (2007) A protein kinase C-encoding gene, pkcA, is essential to the viability of the filamentous fungus Aspergillus nidulans . Biosci Biotechnol Biochem 71: 2787–2799. [DOI] [PubMed] [Google Scholar]
- 8. Ronen R, Sharon H, Levdansky E, Romano J, Shadkchan Y, et al. (2007) The Aspergillus nidulans pkcA gene is involved in polarized growth, morphogenesis and maintenance of cell wall integrity. Curr Genet 51: 321–329. [DOI] [PubMed] [Google Scholar]
- 9. Munro CA, Selvaggini S, de Bruijn I, Walker L, Lenardon MD, et al. (2007) The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans . Mol Microbiol 63: 1399–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colabardini AC, de Castro PA, de Gouvea PF, Savoldi M, Malavazi I, et al. (2010) Involvement of Aspergillus nidulans protein kinase C with farnesol tolerance is related to the unfolded protein response. Molecular Microbiology 78: 1259–1279. [DOI] [PubMed] [Google Scholar]
- 11. Katayama T, Uchida H, Ohta A, Horiuchi H (2012) Involvement of protein kinase C in the suppression of apoptosis and in polarity establishment in Aspergillus nidulans under conditions of heat stress. PLoS One 7: e50503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pearson G, Robinson F, Gibson TB, Xu BE, Karandikar M, et al. (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrine Review 22: 153–183. [DOI] [PubMed] [Google Scholar]
- 13. Heinisch JJ, Lorberg A, Schmitz HP, Jacoby JJ (1999) The protein kinase C-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae . Mol Microbiol 32: 671–680. [DOI] [PubMed] [Google Scholar]
- 14. Jung US, Levin DE (1999) Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signaling pathway. Molecular Microbiology 34: 1049–1057. [DOI] [PubMed] [Google Scholar]
- 15. Morawetz R, Lendenfeld T, Mischak H, Mühlbauer M, Gruber F, et al. (1996) Cloning and characterisation of genes (pkc1 and pkcA) encoding protein kinase C homologues from Trichoderma reesei and Aspergillus niger . Mol Gen Genet 250: 17–28. [DOI] [PubMed] [Google Scholar]
- 16. Ponting CP, Parker PJ (1996) Extending the C2 domain family: C2s in PKCs delta, epsilon, eta, theta, phospholipases, GAPs, and perforin. Protein Sci 5: 162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drgonová J, Drgon T, Tanaka K, Kollár R, Chen GC, et al. (1996) Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science 272: 277–279. [DOI] [PubMed] [Google Scholar]
- 18. Lodder AL, Lee TK, Ballester R (1996) Characterisation of the Wsc1 protein, a putative receptor in the stress response of Saccharomyces cerevisiae . Genetics 152: 1487–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Philip B, Levin DE (2001) Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol Cell Biol 21: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson SL, Heath IB (1993) Roles of calcium ions in hyphal tip growth. Microbiol Rev 57: 367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fox DS, Heitman J (2002) Good fungi gone bad: the corruption of calcineurin. BioEssays 24: 894–903. [DOI] [PubMed] [Google Scholar]
- 22. Kraus PR, Heitman J (2003) Coping with stress: calmodulin and calcineurin in model and pathogenic fungi. Biochem Biophys Res Commun 311: 1151–1157. [DOI] [PubMed] [Google Scholar]
- 23. Carafoli E (2005) Calcium - a universal carrier of biological signals. FEBS J 272: 1073–1089. [DOI] [PubMed] [Google Scholar]
- 24. Cruz MC, Fox DS, Heitman J (2001) Calcineurin is required for hyphal elongation, during mating and haploid fruiting in Cryptococcus neoformans . The EMBO Journal 20: 1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cyert MS (2003) Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem Biophys Res Communications 311: 1143–1150. [DOI] [PubMed] [Google Scholar]
- 26. Steinback WJ, Cramer RA, Perfect BZ, Henn C, Nielsen K, et al. (2007) Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus . Antimicrobial Agents and Chemotherapy 51: 2979–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferreira MED, Heinekamp T, Hartl A, Brakhage AA, Semighini CP, et al. (2007) Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genetics and Biology 44: 219–230. [DOI] [PubMed] [Google Scholar]
- 28. Stie J, Fox D (2008) Calcineurin regulation in fungi and beyond. Eukaryot Cell 7: 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hernández-Ortiz P, Espeso EA (2013) Phospho-regulation and nucleocytoplasmic trafficking of CrzA in response to calcium and alkaline-pH stress in Aspergillus nidulans . Molecular Microbiology 89: 532–551. [DOI] [PubMed] [Google Scholar]
- 30. Spielvogel A, Findon H, Arst HN, Araújo-Bazán L, Hernández-Ortíz P, et al. (2008) Two zinc finger transcription factors, CrzA and SltA, are involved in cation homeostasis and detoxification in Aspergillus nidulans . Biochem J 414: 419–429. [DOI] [PubMed] [Google Scholar]
- 31. Hagiwara D, Kondo A, Fujioka T, Abe K (2008) Functional analysis of C2H2 zinc finger transcription factor CrzA involved in calcium signaling in Aspergillus nidulans . Current Genetics 54: 325–338. [DOI] [PubMed] [Google Scholar]
- 32. Wang X, Sheff MA, Simpson DM, Elion EA (2011) Ste11p MEKK signals through HOG, mating, calcineurin and PKC pathways to regulate the FKS2 gene. BMC Mol Biol 12: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fuchs BB, Mylokanis E (2009) Our paths might cross: the role of the fungal cell wall integrity pathway in stress response and cross talk with other stress response pathways. Eukaryotic cell 8: 1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soriani FM, Malavazi I, da Silva Ferreira ME, Savoldi M, Von Zeska Kress MR, et al. (2008) Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol Microbiol 67: 1274–1291. [DOI] [PubMed] [Google Scholar]
- 35. Munro CA, Selvaggini S, de Bruijn I, Walker L, Lenardon MD, et al. (2007) The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans . Molecular Microbiology 63: 1399–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thewes S (2014) Calcineurin-Crz1 signaling in lower eukaryotes. Eukaryot Cell (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Flipphi M, Kocialkowska J, Felenbok B (2002) Characteristics of physiological inducers of the ethanol utilization (alc) pathway in Aspergillus nidulans . Biochem J 364: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris SD (2010) Hyphal Growth and Polarity. In: Cellular and Molecular Biology of Filamentous Fungi, Chapter 18. Editors: KA Borkovich and DJ Ebbole. ASM Press, Washington DC, USA. [Google Scholar]
- 39. Harris SD (2008) Branching of fungal hyphae: regulation, mechanisms and comparison with other branching systems. Mycologia 100: 823–832. [DOI] [PubMed] [Google Scholar]
- 40. Harris SD (2005) Morphogenesis in germinating Fusarium graminearum macroconidia. Mycologia 97: 880–887. [DOI] [PubMed] [Google Scholar]
- 41. Fujioka T, Mizutani O, Furukawa K, Sato N, Yoshimi A, et al. (2007) MpkA-Dependent and -independent cell wall integrity signaling in Aspergillus nidulans . Eukaryot Cell 6: 1497–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clapham DE (2007) Calcium signaling. Cell 131: 1047–1058. [DOI] [PubMed] [Google Scholar]
- 43. Ow YLP, Green DR, Hao Z, Mak TW (2008) Cytochrome c: functions beyond respiration. Nature Reviews Molecular Cell Biology 9: 532–542. [DOI] [PubMed] [Google Scholar]
- 44. Brand A, Gow NAR (2009) Mechanisms of hypha orientation of fungi. Current Opinion in Microbiology 12: 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Juvvadi PR, Fortwendel JR, Rogg LE, Burns KA, Randell SH, et al. (2011) Localisation and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus . Molecular Microbiology 82: 1235–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Levin DE (2011) Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189: 1145–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kume K, Koyano T, Kanai M, Toda T, Hirata D (2011) Calcineurin ensures a link between the DNA replication checkpoint and microtubule-dependent polarized growth. Nature Cell Biology 13: 234–243. [DOI] [PubMed] [Google Scholar]
- 48. Scrimale T, Didone L, Bentley KLDM, Krysan DJ (2009) The unfolded protein response is induced by the cell wall integrity mitogen-activated protein kinase signaling cascade and is required for cell wall integrity in Saccharomyces cerevisiae . Molecular Biology of the Cell 20: 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Geysens S, Whyteside G, Archer DB (2009) Genomics of protein folding in the endoplasmic reticulum, secretion stress and glycosylation in the aspergilla. Fungal Genetics and Biology 46: S121–140. [Google Scholar]
- 50. Denis V, Cyert MS (2005) Molecular analysis reveals localization of Saccharomyces cerevisiae protein kinase C to sites of polarized growth and Pkc1p targeting to the nucleus and mitotic spindle. Eukaryotic Cell 4: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang G, Lu L, Zhang CY, Singapuri A, Yuan S (2006) Calmodulin concentrates at the apex of growing haphae and localizes to the Spitzenkörper in Aspergillus nidulans . Protoplasma 228: 159–166. [DOI] [PubMed] [Google Scholar]
- 52. Chen S, Song Y, Cao J, Wang G, Wei H, et al. (2010) Localisation of calmodulin in live-cells of Aspergillus nidulans . Fungal Genetics and Biology 47: 268–278. [DOI] [PubMed] [Google Scholar]
- 53. Xiang X, Plamann M (2003) Cytoskeleton and motor protein in filamentous fungi. Current Opinion in Microbiology 6: 628–633. [DOI] [PubMed] [Google Scholar]
- 54. Virag A, Harris SD (2006) The Spitzenkörper: a molecular perspective. Mycological Research 110: 4–13. [DOI] [PubMed] [Google Scholar]
- 55. Williams GS, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ (2013) Mitochondrial calcium uptake. Proc Natl Acad Sci USA 110: 10479–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Colabardini AC, Brown NA, Savoldi M, Goldman MH, Goldman GH (2013) Functional characterization of Aspergillus nidulans ypkA, a homologue of the mammalian kinase SGK. PLoS One 8: e57630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dinamarco TM, Pimentel Bde C, Savoldi M, Malavazi I, Soriani FM, et al. (2010) The roles played by Aspergillus nidulans apoptosis-inducing factor (AIF)-like mitochondrial oxidoreductase (AifA) and NADH-ubiquinone oxidoreductases (NdeA-B and NdiA) in farnesol resistance. Fungal Genetics and Biology 47: 1055–1069. [DOI] [PubMed] [Google Scholar]
- 58. Nayak T, Szewczyk E, Oakley CE, Osmani A, Ukil L, et al. (2006) A versatile and efficient gene-targeting system for Aspergillus nidulans . Genetics 172: 1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FetGOat analysis for genes with reduced mRNA accumulation in A. nidulans ΔcnaA (similar to the wild-type strain) when compared to the alcA::pkcA ΔcnaA strain.
(XLSX)
FetGOat analysis for genes with reduced mRNA accumulation in A. nidulans alcA::pkcA ΔcnaA (similar to the wild-type strain) when compared to the ΔcnaA strain.
(XLSX)
FetGOat analysis for genes with increased mRNA accumulation in A. nidulans alcA::pkcA ΔcnaA (similar to the wild-type strain) when compared to the ΔcnaA strain.
(XLSX)
FetGOat analysis for genes with increased mRNA accumulation in A. nidulans ΔcnaA when compared to the alcA::pkcA ΔcnaA strain.
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All the microarray hybridization data is at: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE48535.