Abstract
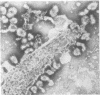
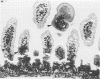
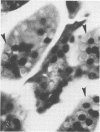
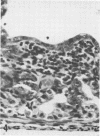
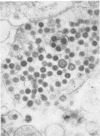
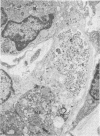
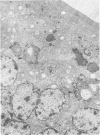
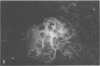
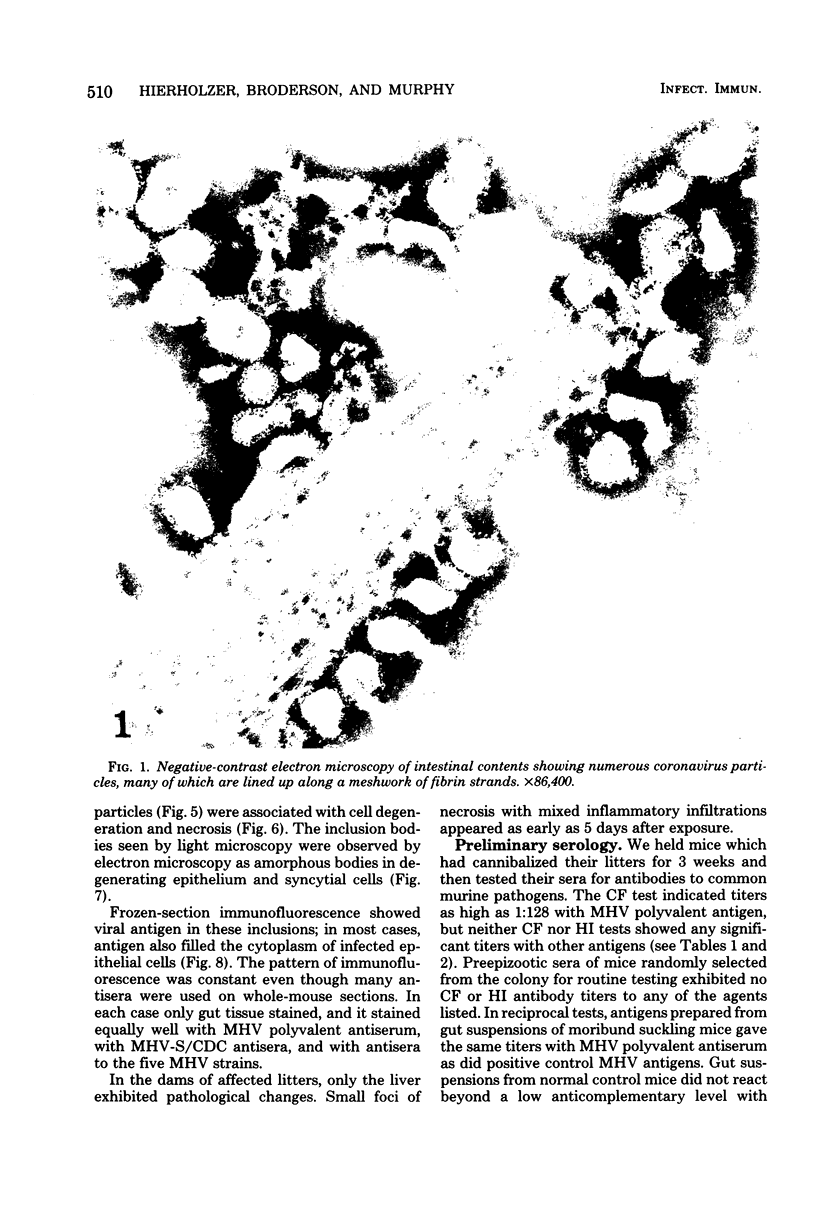
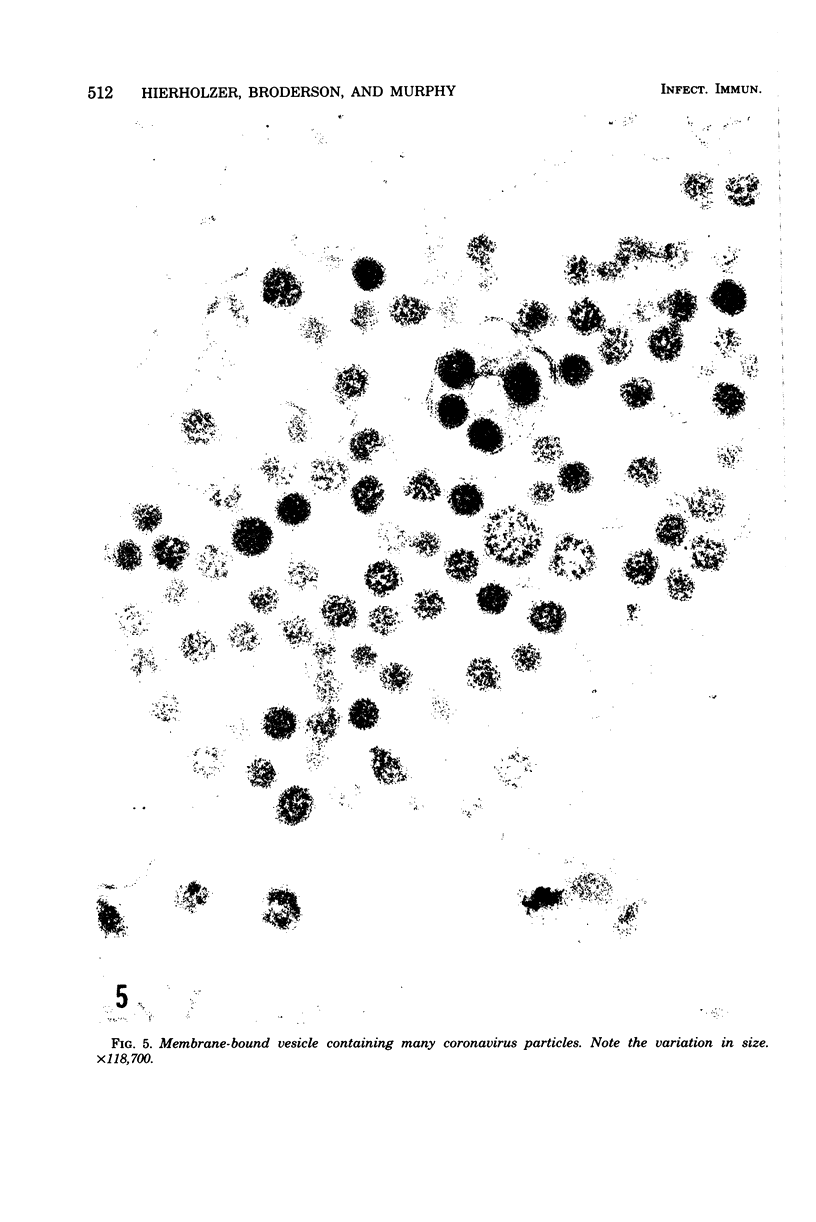
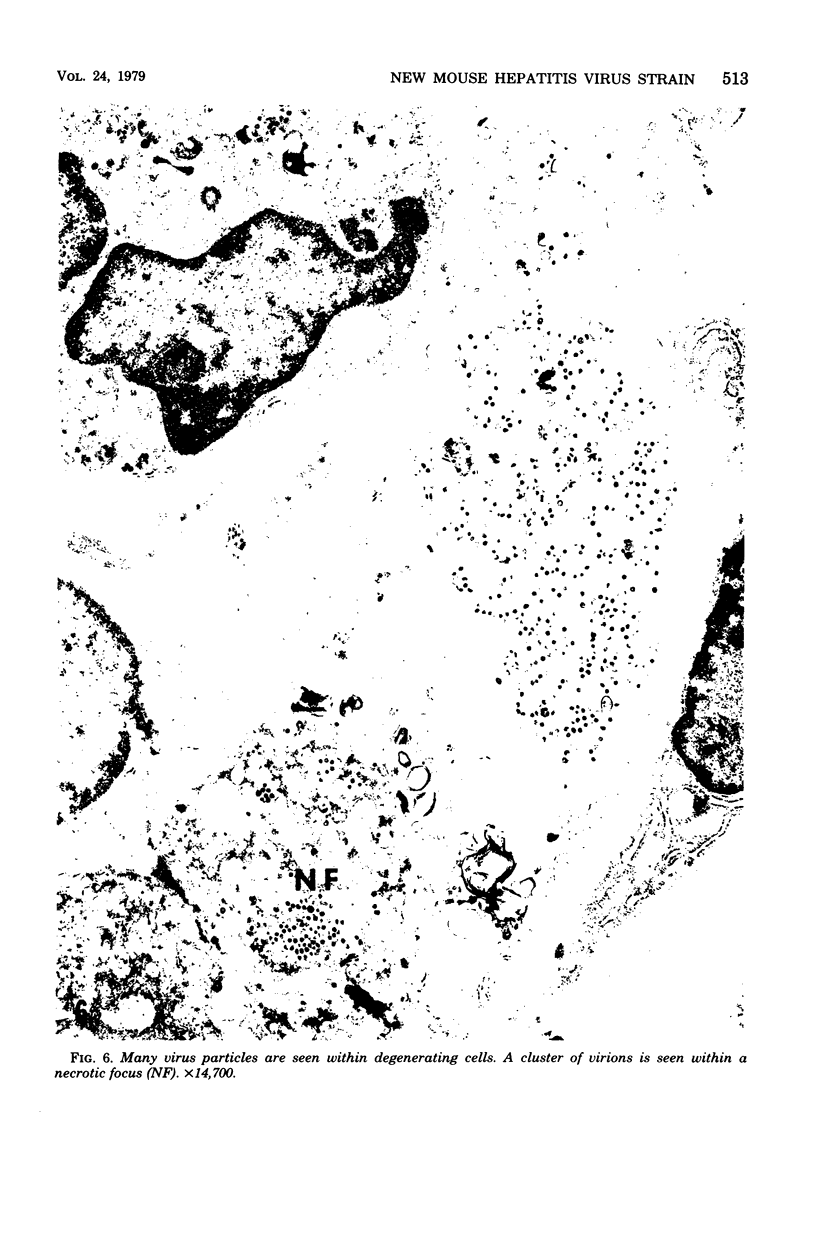
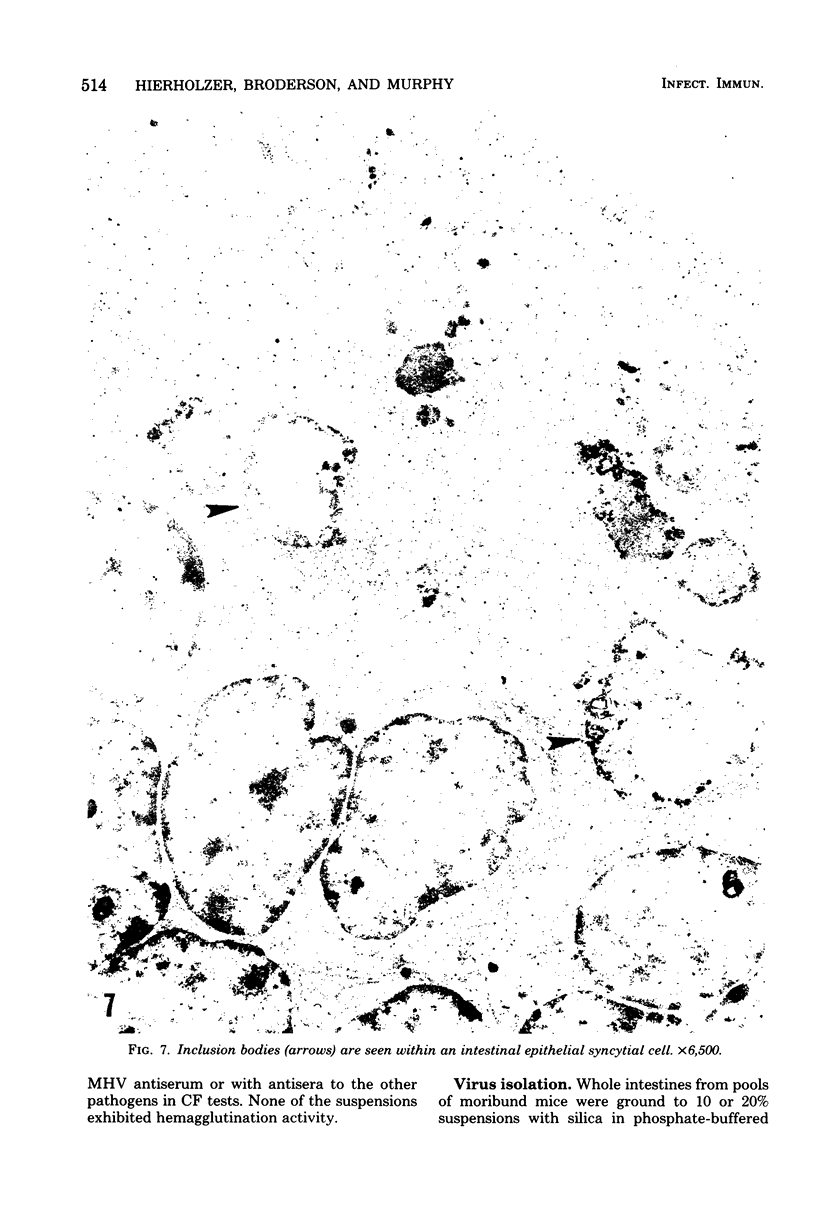
A new strain of mouse hepatitis virus (MHV) was isolated from pooled gut suspensions from an epizootic of lethal enteritis in newborn mice. Negative-contrast electron microscopy showed an abundance of coronavirus particles in the intestinal contents and intestinal epithelium of moribund mice. We found no other virus in the epizootic. Dams seroconverted to MHV polyvalent antigen and to the agent isolated, but did not develop antibodies to other known mouse pathogens. Virus propagated in NCTC-1469 tissue culture produced enteric disease in suckling mice but not fatal diarrhea; the dams of these mice also developed antibodies to MHV and to the isolates. By complement fixation, single radial hemolysis, and quantal neutralization tests, we found the isolates antigenically most closely related to MHV-S, unilaterally related to MHV-JHM, and more distantly related to MHV-1, MHV-3, MHV-A59, and human coronavirus OC-43. We also studied cross-reactions among the murine and human coronaviruses in detail. Tissues of infected newborn mice were examined by light microscopy, thin-section electron microscopy, and frozen-section indirect immunofluorescence, revealing that viral antigen, virus particles, and pathological changes were limited to the intestinal tract. We have designated our isolates as MHV-S/CDC.
Full text
PDF


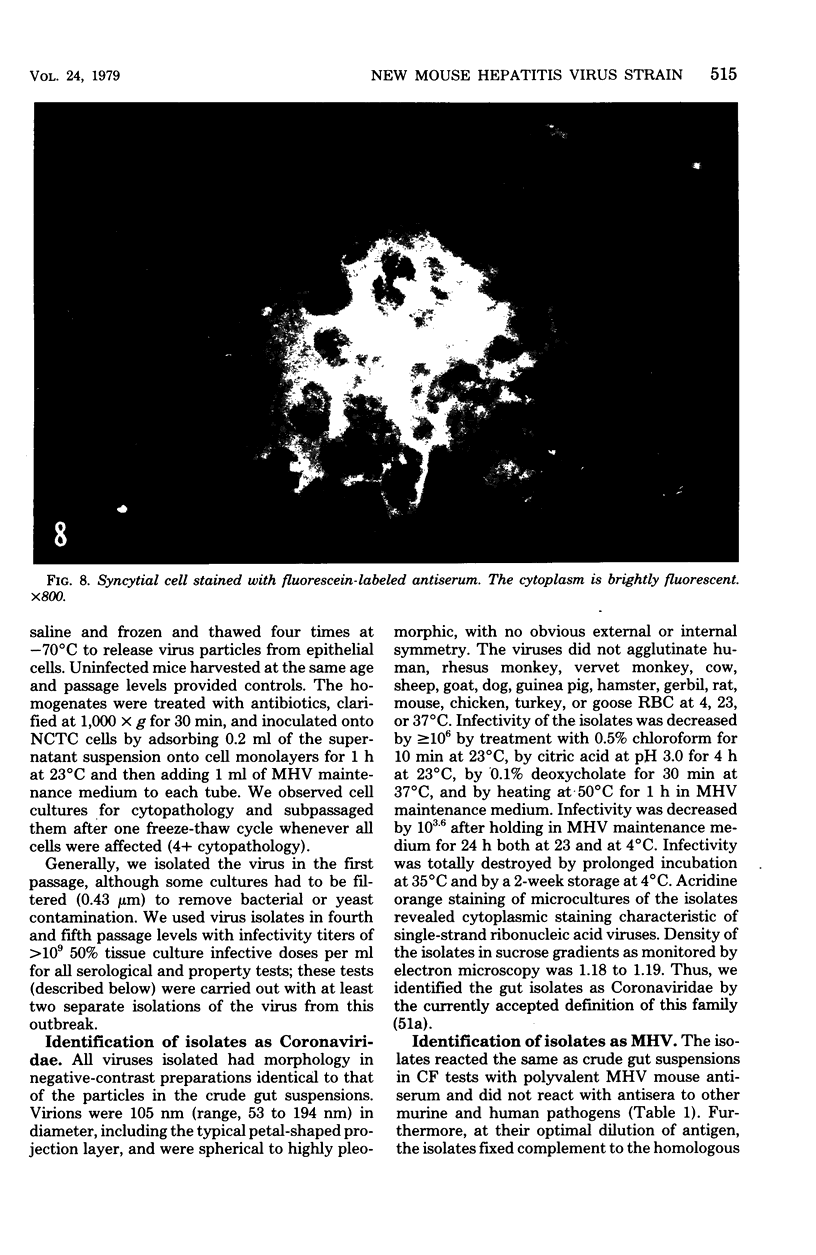
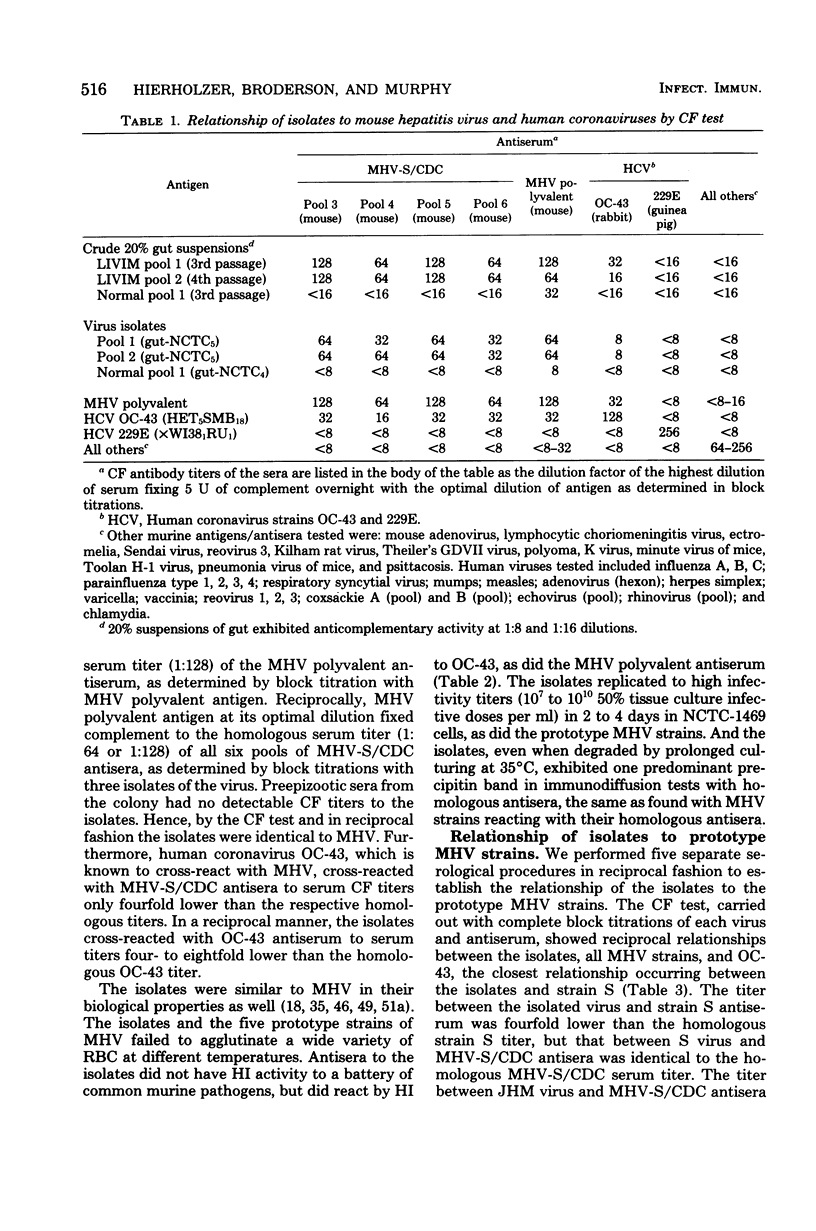
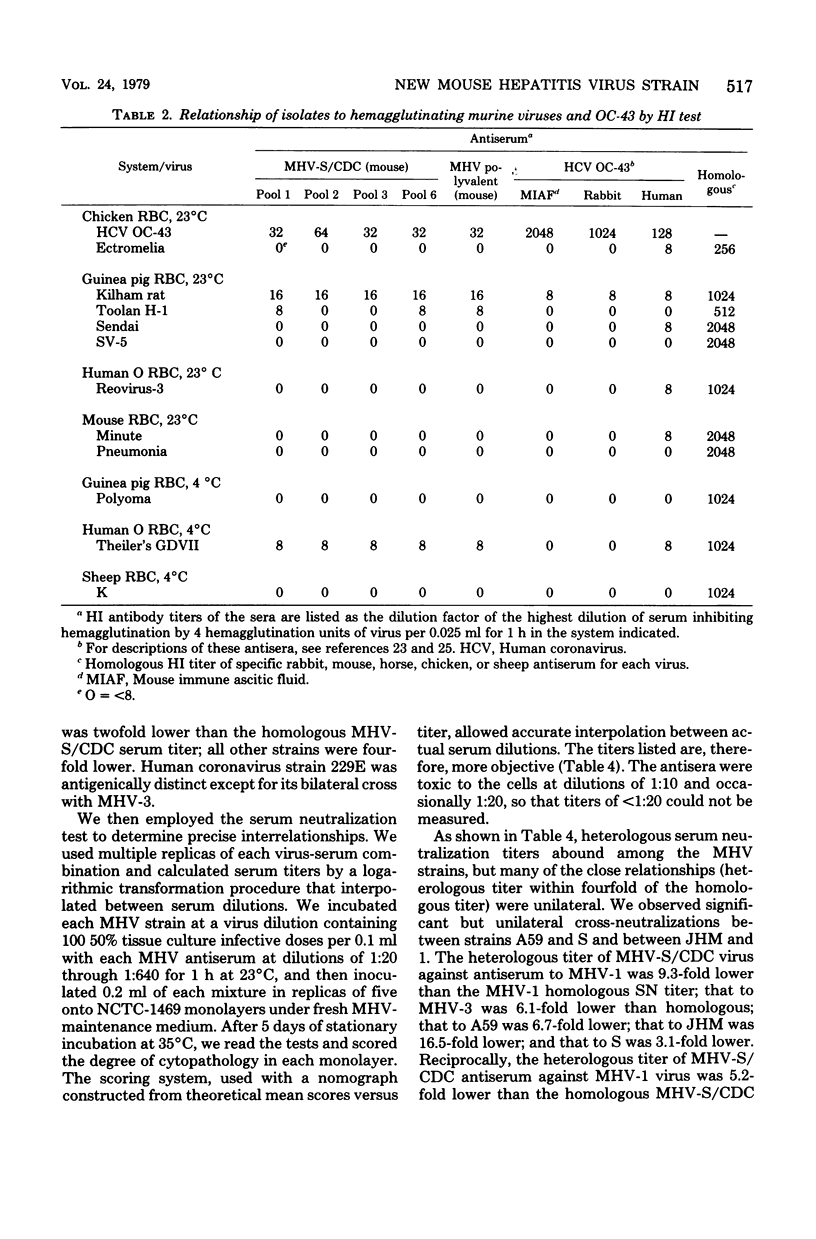
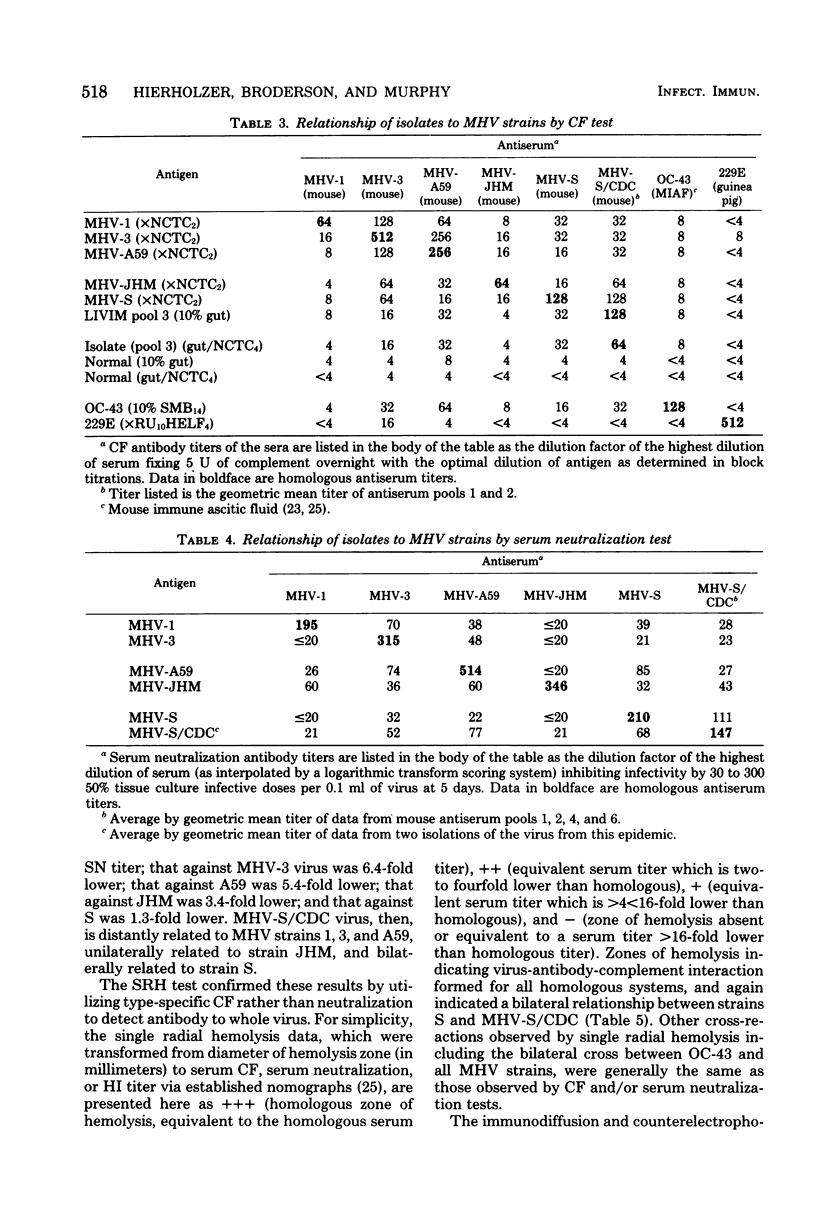



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apostolov K., Spasic P. Evidence of a viral aetiology in endemic (Balkan) nephropathy. Lancet. 1975 Dec 27;2(7948):1271–1273. doi: 10.1016/S0140-6736(75)90609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIGGERS D. C., KRAFT L. M., SPRINZ H. LETHAL INTESTINAL VIRUS INFECTION OF MICE (LIVIM). AN IMPORTANT NEW MODEL FOR STUDY OF THE RESPONSE OF THE INTESTINAL MUCOSA TO INJURY. Am J Pathol. 1964 Sep;45:413–422. [PMC free article] [PubMed] [Google Scholar]
- Bass E. P., Sharpee R. L. Coronavirus and gastroenteritis in foals. Lancet. 1975 Oct 25;2(7939):822–822. doi: 10.1016/S0140-6736(75)80058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S. P., Murphy F. A. Relationship of two arthropod-borne rhabdoviruses (kotonkan and Obodhiang) to the rabies serogroup. Infect Immun. 1975 Nov;12(5):1157–1172. doi: 10.1128/iai.12.5.1157-1172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P. N., Jacoby R. O., Jonas A. M. Respiratory infection in mice with sialodacryoadenitis virus, a coronavirus of rats. Infect Immun. 1977 Dec;18(3):823–827. doi: 10.1128/iai.18.3.823-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburne A. F. Antigenic relationships amongst coronaviruses. Arch Gesamte Virusforsch. 1970;31(3):352–364. doi: 10.1007/BF01253769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderson J. R., Murphy F. A., Hierholzer J. C. Lethal enteritis in infant mice caused by mouse hepatitis virus. Lab Anim Sci. 1976 Oct;26(5):824–824. [PubMed] [Google Scholar]
- Calisher C. H., Rowe W. P. Mouse hepatitis, reo-3, and the Theiler viruses. Natl Cancer Inst Monogr. 1966 Feb;20:67–75. [PubMed] [Google Scholar]
- Carthew P. Lethal intestinal virus of infant mice is mouse hepatitis virus. Vet Rec. 1977 Dec 3;101(23):465–465. [PubMed] [Google Scholar]
- Caul E. O., Egglestone S. I. Further studies on human enteric coronaviruses. Arch Virol. 1977;54(1-2):107–117. doi: 10.1007/BF01314383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh D. R., Sautter J. H., Patel B. L., Pomeroy B. S. Histopathology of fasting and bluecomb disease in turkey poults and embryos experimentally infected with bluecomb disease coronavirus. Avian Dis. 1976 Oct-Dec;20(4):631–640. [PubMed] [Google Scholar]
- Georgescu L., Diosi P., Butiu I., Plavosin L., Herzog G. Porcine coronavirus antibodies in endemic (Balkan) nephropathy. Lancet. 1978 Jan 21;1(8056):163–163. doi: 10.1016/S0140-6736(78)90471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLEY J. W., ROWE W. P., BLOOM H. H., TURNER H. C. ANTIBODIES TO MOUSE HEPATITIS VIRUSES IN HUMAN SERA. Proc Soc Exp Biol Med. 1964 Feb;115:414–418. doi: 10.3181/00379727-115-28928. [DOI] [PubMed] [Google Scholar]
- HARTLEY J. W., ROWE W. P. Tissue culture cytopathic and plaque assays for mouse hepatitis viruses. Proc Soc Exp Biol Med. 1963 Jun;113:403–406. doi: 10.3181/00379727-113-28378. [DOI] [PubMed] [Google Scholar]
- Haelterman E. O. On the pathogenesis of transmissible gastroenteritis of swine. J Am Vet Med Assoc. 1972 Feb 15;160(4):534–540. [PubMed] [Google Scholar]
- Hamre D., Procknow J. J. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966 Jan;121(1):190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Atuk N. O., Gwaltney J. M., Jr New human adenovirus isolated from a renal transplant recipient: description and characterization of candiate adenovirus type 34. J Clin Microbiol. 1975 Apr;1(4):366–376. doi: 10.1128/jcm.1.4.366-376.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Barme M. Counterimmunoelectrophoresis with adenovirus type-specific anti-hemagglutinin sera as a rapid diagnostic method. J Immunol. 1974 Mar;112(3):987–995. [PubMed] [Google Scholar]
- Hierholzer J. C., Palmer E. L., Whitfield S. G., Kaye H. S., Dowdle W. R. Protein composition of coronavirus OC 43. Virology. 1972 May;48(2):516–527. doi: 10.1016/0042-6822(72)90062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C. Purification and biophysical properties of human coronavirus 229E. Virology. 1976 Nov;75(1):155–165. doi: 10.1016/0042-6822(76)90014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Suggs M. T., Hall E. C. Standardized viral hemagglutination and hemagglutination-inhibition tests. II. Description and statistical evaluation. Appl Microbiol. 1969 Nov;18(5):824–833. doi: 10.1128/am.18.5.824-833.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Tannock G. A. Quantitation of antibody to non-hemagglutinating viruses by single radial hemolysis: serological test for human coronaviruses. J Clin Microbiol. 1977 Jun;5(6):613–620. doi: 10.1128/jcm.5.6.613-620.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantile enteritis viruses: morphogenesis and morphology. J Virol. 1975 Oct;16(4):937–943. doi: 10.1128/jvi.16.4.937-943.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Taguchi F., Lee Y. S., Yamada A., Tamura T., Fujiwara K. Isolation of mouse hepatitis virus from infant mice with fatal diarrhea. Lab Anim Sci. 1978 Jun;28(3):269–276. [PubMed] [Google Scholar]
- KRAFT L. M. An apparently new lethal virus disease of infant mice. Science. 1962 Jul 27;137(3526):282–283. doi: 10.1126/science.137.3526.282. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., James H. D., Kelly S. J., Vaughn A. L. Detection of coronavirus strain 692 by immune electron microscopy. Infect Immun. 1973 Jan;7(1):111–116. doi: 10.1128/iai.7.1.111-116.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye H. S., Yarbrough W. B., Reed C. J., Harrison A. K. Antigenic relationship between human coronavirus strain OC 43 and hemagglutinating encephalomyelitis virus strain 67N of swine: antibody responses in human and animal sera. J Infect Dis. 1977 Feb;135(2):201–209. doi: 10.1093/infdis/135.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye H. S., Yarbrough W. B., Reed C. J. Letter: Calf diarrhoea coronavirus. Lancet. 1975 Sep 13;2(7933):509–509. doi: 10.1016/S0140-6736(75)90591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft L. M. Epizootic diarrhea of infant mice and lethal intestinal virus infection of infant mice. Natl Cancer Inst Monogr. 1966 Feb;20:55–61. [PubMed] [Google Scholar]
- Lai Y. L., Jacoby R. O., Bhatt P. N., Jonas A. M. Keratoconjunctivitis associated with sialodacryoadenitis in rats. Invest Ophthalmol. 1976 Jul;15(7):538–541. [PubMed] [Google Scholar]
- McIntosh K., Chao R. K., Krause H. E., Wasil R., Mocega H. E., Mufson M. A. Coronavirus infection in acute lower respiratory tract disease of infants. J Infect Dis. 1974 Nov;130(5):502–507. doi: 10.1093/infdis/130.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Kapikian A. Z., Hardison K. A., Hartley J. W., Chanock R. M. Antigenic relationships among the coronaviruses of man and between human and animal coronaviruses. J Immunol. 1969 May;102(5):1109–1118. [PubMed] [Google Scholar]
- Mebus C. A., Stair E. L., Rhodes M. B., Twiehaus M. J. Neonatal calf diarrhea: propagation, attenuation, and characteristics of a coronavirus-like agent. Am J Vet Res. 1973 Feb;34(2):145–150. [PubMed] [Google Scholar]
- Mebus C. A., Stair E. L., Rhodes M. B., Twiehaus M. J. Pathology of neonatal calf diarrhea induced by a coronavirus-like agent. Vet Pathol. 1973;10(1):45–64. doi: 10.1177/030098587301000105. [DOI] [PubMed] [Google Scholar]
- Mengeling W. L., Cutlip R. C. Experimentally induced infection of newborn pigs with hemagglutinating encephalomyelitis virus strain 67N. Am J Vet Res. 1972 May;33(5):953–956. [PubMed] [Google Scholar]
- Murphy F. A., Whitfield S. G. Eastern equine encephalitis virus infection: electron microscopic studies of mouse central nervous system. Exp Mol Pathol. 1970 Oct;13(2):131–146. doi: 10.1016/0014-4800(70)90001-8. [DOI] [PubMed] [Google Scholar]
- Parker J. C., Cross S. S., Rowe W. P. Rat coronavirus (RCV): a prevalent, naturally occurring pneumotropic virus of rats. Arch Gesamte Virusforsch. 1970;31(3):293–302. doi: 10.1007/BF01253764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. C. Morphologic and physical characteristics of feline infectious peritonitis virus and its growth in autochthonous peritoneal cell cultures. Am J Vet Res. 1976 May;37(5):567–572. [PubMed] [Google Scholar]
- Pedersen N. C., Ward J., Mengeling W. L. Antigenic relationship of the feline infectious peritonitis virus to coronaviruses of other species. Arch Virol. 1978;58(1):45–53. doi: 10.1007/BF01315534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy K. A., Patel B. L., Larsen C. T., Pomeroy B. S. Combined immunofluorescence and transmission electron microscopic studies of sequential intestinal samples from turkey embryos and poults infected with turkey enteritis coronavirus. Am J Vet Res. 1978 Aug;39(8):1348–1354. [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., CAPPS W. I. Mouse hepatitis virus infection as a highly contagious, prevalent, enteric infection of mice. Proc Soc Exp Biol Med. 1963 Jan;112:161–165. doi: 10.3181/00379727-112-27980. [DOI] [PubMed] [Google Scholar]
- Reynolds D. J., Garwes D. J., Gaskell C. J. Detection of transmissible gastroenteritis virus neutralising antibody in cats. Arch Virol. 1977;55(1-2):77–86. doi: 10.1007/BF01314481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. A., Alexander D. J., Almeida J. D., Cunningham C. H., Easterday B. C., Garwes D. J., Hierholzer J. C., Kapikian A., Macnaughton M. R., McIntosh K. Coronaviridae: second report. Intervirology. 1978;10(6):321–328. doi: 10.1159/000148996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Smith M., Makin T., McCaughan C. Enteric coronavirus-like particles in sheep. Aust Vet J. 1978 Jun;54(6):320–321. doi: 10.1111/j.1751-0813.1978.tb02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Collins M. J., Jr, Parker J. C. Naturally occurring mouse hepatitis virus infection in the nude mouse. Lab Anim Sci. 1977 Jun;27(3):372–376. [PubMed] [Google Scholar]
- Waxler G. L. Lesions of transmissible gastroenteritis in the pig as determined by scanning electron microscopy. Am J Vet Res. 1972 Jul;33(7):1323–1328. [PubMed] [Google Scholar]