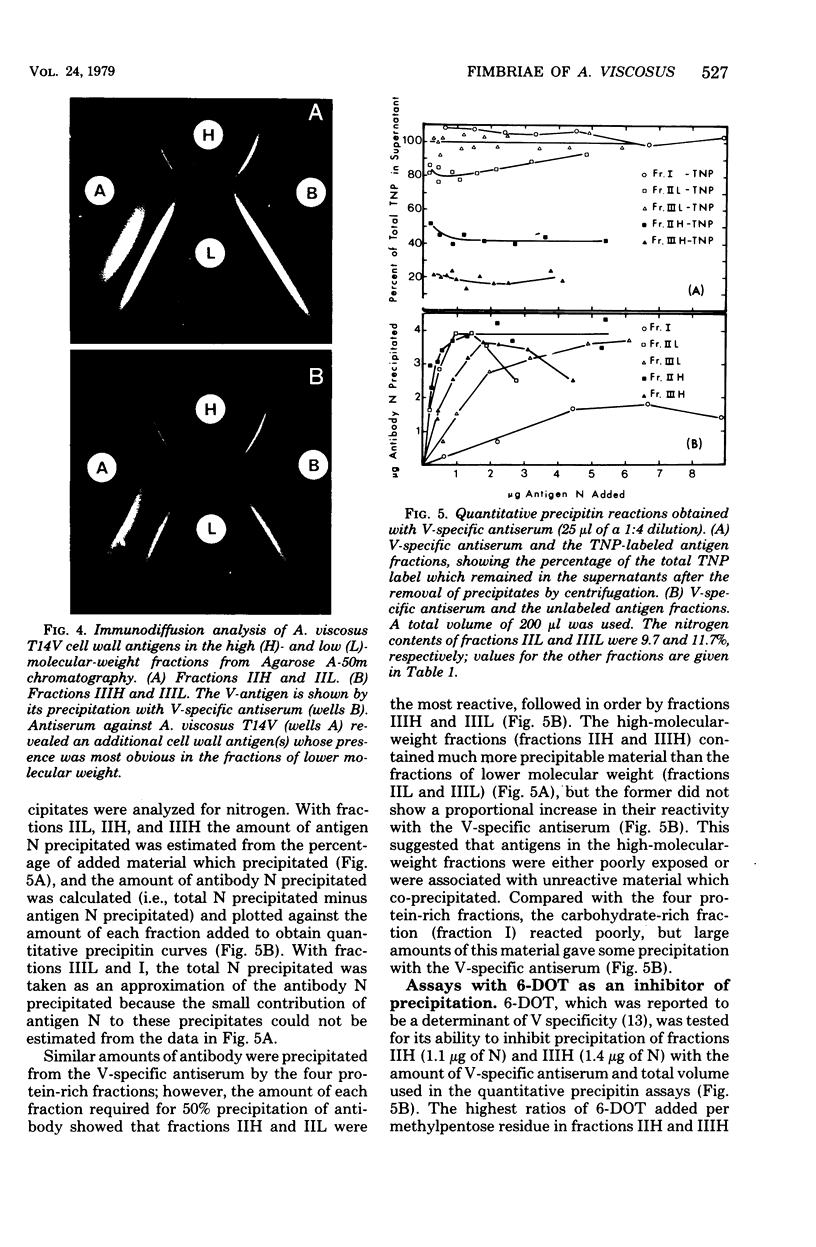
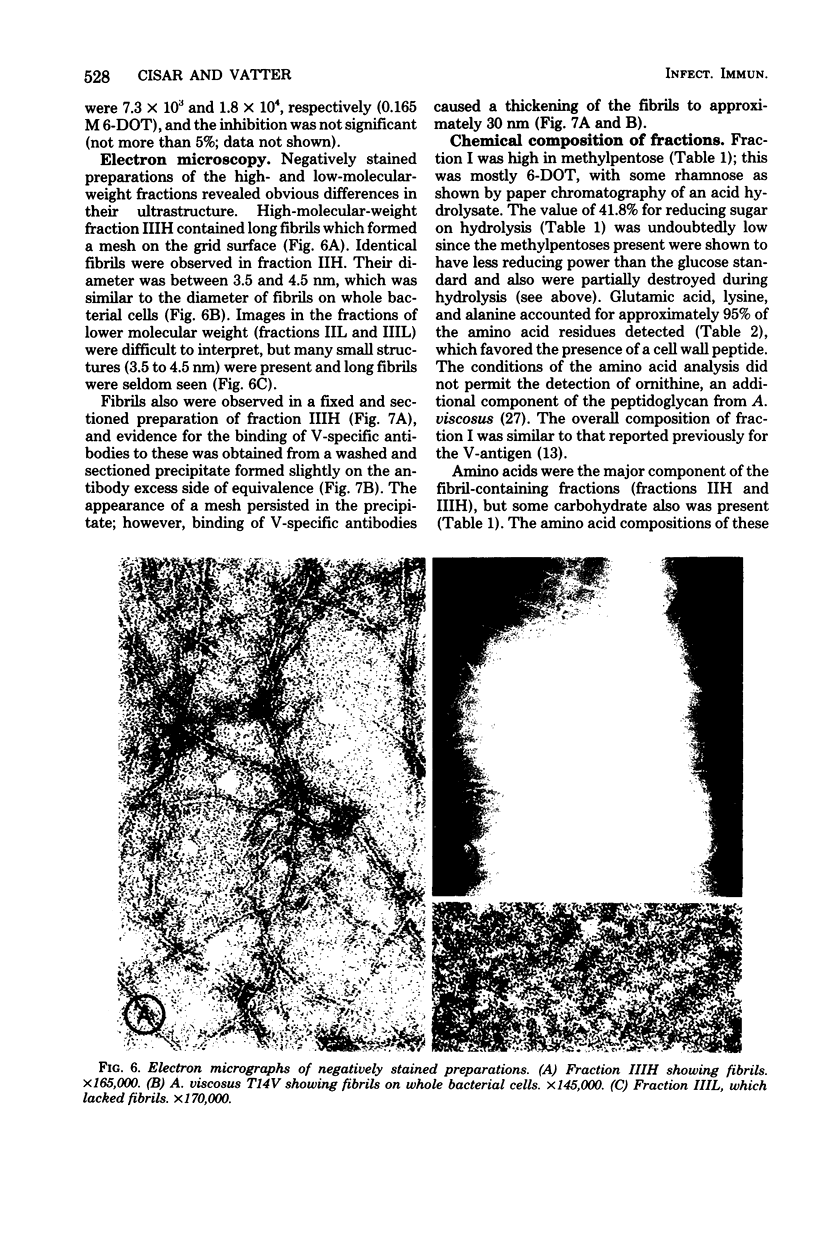
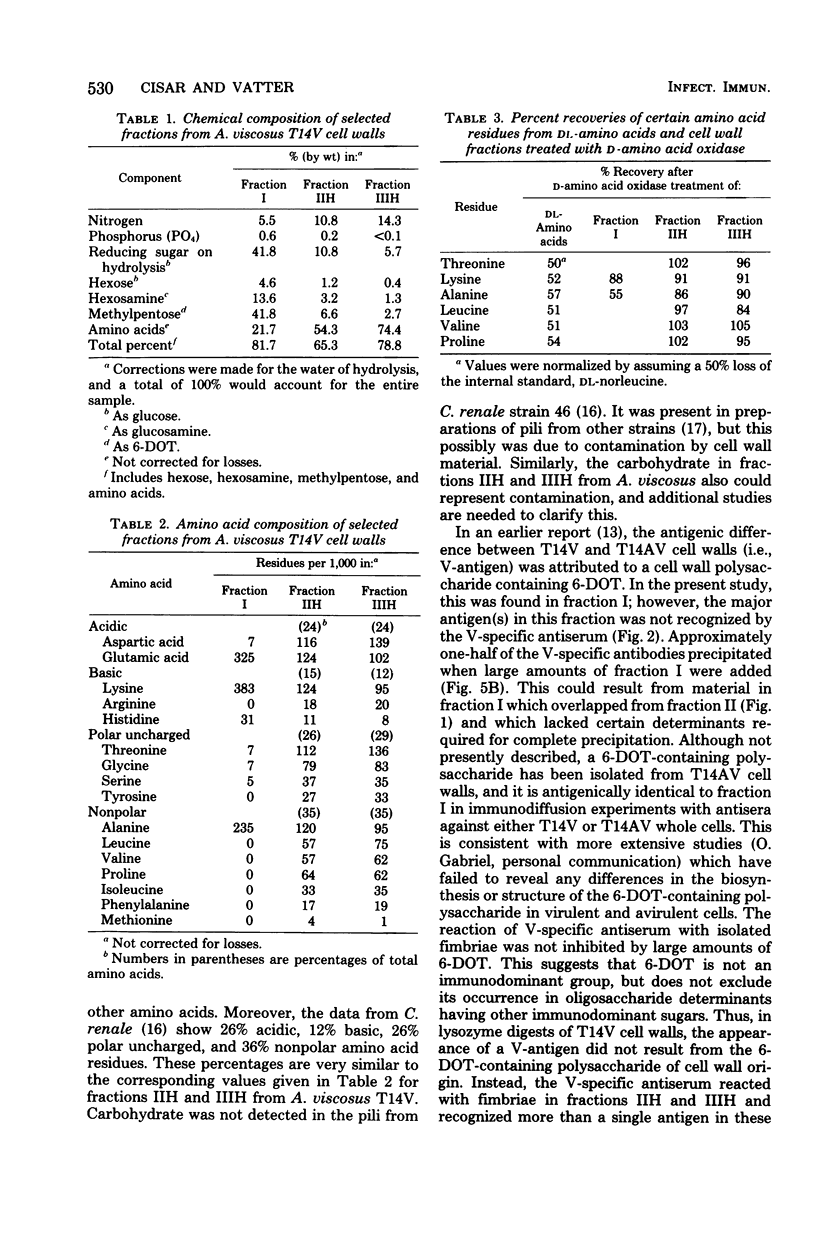
Abstract
Surface antigens of Actinomyces viscosus T14V were released from cell walls by digestion with lysozyme. These were separated by ion-exchange and gel filtration chromatography into fractions rich in carbohydrate or protein. The former contained a polysaccharide high in 6-deoxytalose, along with a peptide fragment from the cell wall. In the protein-rich fractions, material of high molecular weight was present, which contained some carbohydrate and up to 14.3% nitrogen. Aspartic acid, threonine, glutamic acid, lysine, alanine, and glycine were detected in these fractions, along with smaller amounts of 10 other amino acids. Most of the alanine was present as the L isomer and thus was not from peptidoglycan. Electron microscopy of the high-molecular-weight material revealed long fibrils, 3.5 to 4.5 nm in diameter, which resembled those seen on bacterial cells. V-specific antiserum, prepared by absorbing anti-A. viscosus T14V serum with cell walls of the avirulent strain (A. viscosus T14AV), did not react with the 6-deoxytalose polysaccharide but reacted well with isolated fibrils, and this was not inhibited by 6-deoxytalose.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
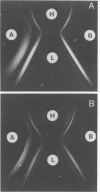
- Cisar J. O., Vatter A. E., McIntire F. C. Identification of the virulence-associated antigen on the surface fibrils of Actinomyces viscosus T14. Infect Immun. 1978 Jan;19(1):312–319. doi: 10.1128/iai.19.1.312-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J., Kabat E. A., Liao J., Potter M. Immunochemical studies on mouse myeloma proteins reactive with dextrans or with fructosans and on human antilevans. J Exp Med. 1974 Jan 1;139(1):159–179. doi: 10.1084/jem.139.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Walker D. L., Chan K. H. Association of long surface appendages with adherence-related functions of the gram-positive species Actinomyces naeslundii. J Bacteriol. 1978 Jun;134(3):1171–1175. doi: 10.1128/jb.134.3.1171-1175.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard A. E., Jacius B. H. Ultrastructure of Actinomyces viscosus and Actinomyces naeslundii. Arch Oral Biol. 1974 Jan;19(1):71–79. doi: 10.1016/0003-9969(74)90228-3. [DOI] [PubMed] [Google Scholar]
- Hammond B. F., Steel C. F., Peindl K. S. Antigens and surface components associated with virulence of Actinomyces viscosus. J Dent Res. 1976 Jan;55:A19–A25. doi: 10.1177/002203457605500111011. [DOI] [PubMed] [Google Scholar]
- Honda E., Yanagawa R. Agglutination of trypsinized sheep erythrocytes by the pili of Corynebacterium renale. Infect Immun. 1974 Dec;10(6):1426–1432. doi: 10.1128/iai.10.6.1426-1432.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa N., Yanagawa R. Chemical properties of the pili of Corynebacterium renale. Infect Immun. 1972 Jan;5(1):27–30. doi: 10.1128/iai.5.1.27-30.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa N., Yanagawa R. Comparison of the chemical and immunological properties of the pili of three types of Corynebacterium renale. Jpn J Microbiol. 1973 Jan;17(1):13–19. doi: 10.1111/j.1348-0421.1973.tb00698.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson D. M., Snetsinger D. C., Waibel P. E. Procedure for determination of D-amino acids. Anal Biochem. 1971 Feb;39(2):395–401. doi: 10.1016/0003-2697(71)90429-5. [DOI] [PubMed] [Google Scholar]
- MACLENNAN A. P. Composition of the cell wall of Actinomyces bovis: the isolation of 6-deoxy-L-talose. Biochim Biophys Acta. 1961 Apr 15;48:600–601. doi: 10.1016/0006-3002(61)90062-2. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Pabst M. J. Levan and levansucrase of Actinomyces viscosus. Infect Immun. 1977 Feb;15(2):518–526. doi: 10.1128/iai.15.2.518-526.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIFFMAN G., KABAT E. A., THOMPSON W. IMMUNOCHEMICAL STUDIES ON BLOOD GROUPS. XXX. CLEAVAGE OF A, B, AND H BLOOD-GROUP SUBSTANCES BY ALKALI. Biochemistry. 1964 Jan;3:113–120. doi: 10.1021/bi00889a018. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa R., Honda E. Presence of pili in species of human and animal parasites and pathogens of the genuscorynebacterium. Infect Immun. 1976 Apr;13(4):1293–1295. doi: 10.1128/iai.13.4.1293-1295.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]