Abstract
Uterine leiomyomas are characterized by an excessive extracellular matrix, increased mechanical stress, and increased active RhoA. Previously, we observed that mechanical signaling was attenuated in leiomyoma, but the mechanisms responsible remain unclear. Integrins, especially integrin β1, are transmembrane adhesion receptors that couple extracellular matrix stresses to the intracellular cytoskeleton to influence cell proliferation and differentiation. Here we characterized integrin and laminin to signaling in leiomyoma cells. We observed a 2.25 ± 0.32 fold increased expression of integrin β1 in leiomyoma cells, compared to myometrial cells. Antibody-mediated inhibition of integrin β1 led to significant growth inhibition in leiomyoma cells and a loss of cytoskeletal integrity. Specifically, polymerization of actin filaments and formation of focal adhesions were reduced by inhibition of integrin p1. Inhibition of integrin β1 in leiomyoma cells led to 0.81 ± 0.02 fold decrease in active RhoA, and resembled levels found in serum-starved cells. Likewise, inhibition of integrin β1 was accompanied by a decrease in phospho-ERK. Compared to myometrial cells, leiomyoma cells demonstrated increased expression of integrin α6 subunit to laminin receptor (1.91 ± 0.11 fold), and increased expression of laminin 5α (1.52±0.02), laminin 5β (3.06±0.92), and laminin 5γ (1.66 ± 0.06). Of note, leiomyoma cells grown on laminin matrix appear to realign themselves. Taken together, the findings reveal that the attenuated mechanical signaling in leiomyoma cells is accompanied by an increased expression and a dependence on integrin β1 signaling in leiomyoma cells, compared to myometrial cells.
Keywords: Uterine leiomyoma, Extracellular matrix, Integrin β1, Cytoskeletal integrity, RhoA, Laminins
1. Introduction
Uterine leiomyomata is a clinically important fibrotic disease that may result in a cost exceeding 34 billion dollars to the US health care system (Cardozo et al., 2012). The most common symptom is abnormal bleeding which may require surgical removal of the uterus due to symptom severity (Walker and Stewart, 2005; Sabry and Al-Hendy, 2012). The tumors are composed of excessive extracellular fibrous proteins including the collagens, proteoglycans and glycosamines (Wolańska et al., 1998; Nowak, 2001; Malik et al., 2010). It is the excessive amount of extracellular matrix (ECM) secreted by the leiomyoma cells that makes up the tumor bulk resulting in symptoms (Fujita, 1985; Wolańska et al., 1998; Mitropoulou et al., 2001). Proteins of the matrix bind to transmembrane cell surface adhesion receptors, called integrins, that connect the ECM to the intracellular actin cytoskeleton and via signaling molecules initiate intracellular cascades which control cell shape, migration, proliferation, differentiation and survival (Giancotti and Ruoslahti, 1999; Belkin and Stepp, 2000; Miranti and Brugge, 2002; Schwartz and Ginsberg, 2002).
Integrins are transmembrane α and β heterodimer receptors that bind specifically to ECM proteins and are important for the bidirectional signaling across the cell membrane. There are 18-α and 8-β subunits known, which combine in a wide array of heterodimers (Giancotti and Ruoslahti, 1999; Juliano, 2002; Margadant et al., 2011). A single ECM protein can associate with more than one integrin receptor (Giancotti and Ruoslahti, 1999). The most prevalent β subunit is the β1 integrin which has a broad pattern of expression (Hynes, 1992; Meredith et al., 1993; Tian et al., 2002; Wang et al., 2005) and forms a heterodimer with most α subunits (Shimaoka et al., 2002). Integrin β1 plays a critical role in a number of cellular functions such as cell adhesion and invasiveness (Arao et al., 2000; Whittard and Akiyama, 2001; Sawai et al., 2005; Lau et al., 2012), cell differentiation and proliferation (Streuli and Bissell, 1991; Streuli et al., 1991; Carroll et al., 1995; Faraldo et al., 2000; Mukhopadhyay et al., 2004; Lahlou and Muller, 2011), cell survival (Howlett et al., 1995; Wang et al., 2005) and morphogenesis (Fassler et al., 1995; Bagutti et al., 1996; Bouvard et al., 2001). Bissell and coworkers demonstrated reversion of malignant breast cancer cells to normal phenotype on inhibition of integrin β1 in 3-dimensional cultures (Weaver et al., 1997).
Integrins cluster into focal adhesions (FA) to coordinate extracellular matrix with intracellular cytoskeleton filaments (Schoenwaelder and Burridge, 1999; DeMali et al., 2003). Integrin activation directs polymerization and organization of intracellular actin filaments. Actin nucleation involves the Rho family GTPases that trigger stress fiber formation and assembly of FA (Ridley and Hall, 1992; Machesky and Hall, 1997; Schwartz and Shattil, 2000). Accordingly, Rho GTPases influence cytoskeletal re-arrangement, contractility and cell proliferation (Hall, 1994; Schwartz and Shattil, 2000; Etienne-Manneville and Hall, 2002; Wettschureck and Offermanns, 2002). The focal adhesions also provide the cell with attachment points through which the actin/stress filaments can generate mechanical stress through associated proteins such as filamins, focal adhesion kinases (FAKs) and integrin linked kinase complexes (Wickström et al., 2010). Importantly, altered states of mechanical stress can cause fibrosis by stimulating fibroblasts to deposit excessive extracellular matrix (Paszek and Weaver, 2004). In addition, fibrosis is also associated with increased laminin expression in liver (Plebani and Burlina, 1991; Neubauer et al., 2001; Rosa and Parise, 2008), bone marrow (Reilly, 1997; Wang, 2005), skin (Herrmann et al., 1990; Segarra et al., 2000) and lungs (Chilosi et al., 2006; Dekkers et al., 2010; Katayama et al., 2010).
Our previous studies examined the altered composition of ECM (Catherino et al., 2004) and altered structure of the ECM (Leppert et al., 2004) in leiomyoma. Furthermore, we observed that leiomyomas feature increased mechanical stress (Rogers et al., 2008), but paradoxically leiomyoma cells exhibited an attenuated response to mechanical cues, compared to myometrial cells (Norian et al., 2012). Collectively, these observations suggested that altered coupling of the ECM to internal mechanical signaling may contribute to leiomyoma growth.However, little is known about the integrins and the fidelity of their function in leiomyomas. Taylor and coworkers (Taylor et al., 1996) using immunohistochemistry demonstrated no differential expression in integrin distribution pattern in both leiomyomas and normal myometrium. However,Wolańska et al. (2001) showed that an increase in the uterine leiomyoma weight is accompanied by increased β1 integrin receptor expression and prolidase activity. Here we profiled the integrin α and β subunits in leiomyoma and patient matched myometrium cell lines, analyzed the differences in their expression, and suggest the role played by integrins in the actin cytoskeleton that could contribute to the mechanical stress.
2. Results
2.1. Integrin receptor subunits were upregulated in leiomyoma cells
The myometrial (M) and leiomyoma (L) immortalized cell lines expressed both α and β subunits of the integrin receptors that bind to structural ECM proteins, including collagen, laminin and fibronectin. As shown in Table 1, integrin receptor subunits were >10 fold greater in amount relative to the isotype controls for both the cell lines. The integrin subunit β3 was present in both the myometrial (2.1±0.06 fold) and leiomyoma (1.8±0.12 fold) cell lines but expression was significantly lower, compared to other integrins. We further analyzed the fold difference of each integrin in the leiomyoma versus myometrial cells. We observed a 2.25±0.32 fold (p=0.011) increased concentration of β1 subunits in the leiomyoma cells (Fig. 1a). A similar increase in β1 subunit was observed by cytoimmunofluorescence (Fig. 1b). As shown in Table 1, integrin subunit α6 was also elevated in leiomyoma cells compared to myometrial cells (1.91±0.11, p= 0.002). Integrin subunits α1 (0.71±0.06) and β2 (0.76±0.059) were significantly lower in leiomyoma cells (p<0.05) whereas other subunits were not significantly different between myometrial and leiomyoma cell lines. Fibronectin specific integrin receptor CD51/61(αvβ3) was significantly upregulated (1.42±0.06, p= 0.02) in leiomyoma cells.
Table 1.
Flow cytometric analysis and fold difference of the integrin α and β subunits in myometrial (M) and leiomyoma (L) cells lines. The median fold shift was calculated to unstained controls and is representative of a single experiment. The median fold difference (L:M) is based on 4 different experiments. Negative controls (IgG) demonstrated no background staining. Data on ECM ligands extracted and compiled from references (Hynes, 1992; Johansson et al., 1997; Mizejewski, 1999; Belkin and Stepp, 2000; Stupack, 2005).
| ECM ligands | Integrin subunit | Median fold difference (L:M) | Median fold shift | |
|---|---|---|---|---|
|
| ||||
| M | L | |||
| Collagens, laminins | CD49a integrin α 1 | 0.71±0.06 (p=0.04) | 20.18 | 16.99 |
| Collagens, laminins | CD49b integrin α 2 | 0.94±0.1 (p=0.6) | 15.53 | 15.49 |
| Collagens, laminins, fibronectin | CD49c integrin α 3 | 1.09±0.01 (p=0.07) | 13.68 | 15.12 |
| Fibronectin | CD49d integrin α 4 | 0.96±0.01 (p=0.032) | 14.82 | 14.26 |
| Fibronectin, laminins | CD49e integrin α 5 | 0.90±0.03 (p=0.029) | 69.69 | 69.20 |
| Laminins | CD49f integrin α 6 | 1.91±0.11 (p=0.002) | 38.40 | 72.50 |
| Collagens, laminins, fibronectin | CD29 integrin β 1 | 2.25±0.32 (p=0.011) | 15.83 | 62.57 |
| Fibronectin | CD18 integrin β 2 | 0.76±0.05 (p=0.02) | 18.83 | 15.34 |
| Laminins, fibronectin | CD61 integrin β 3 | 0.91±0.01 (p=0.01) | 2.00 | 1.80 |
| Fibronectin | FIB504 integrin β 7 | 0.99±0.03 (p=0.8) | 15.53 | 15.49 |
| Fibronectin, collagens, thrombospondin | CD51/61 αvβ3 | 1.42±0.06 (p=0.02) | 6.18 | 9.01 |
Fig. 1.
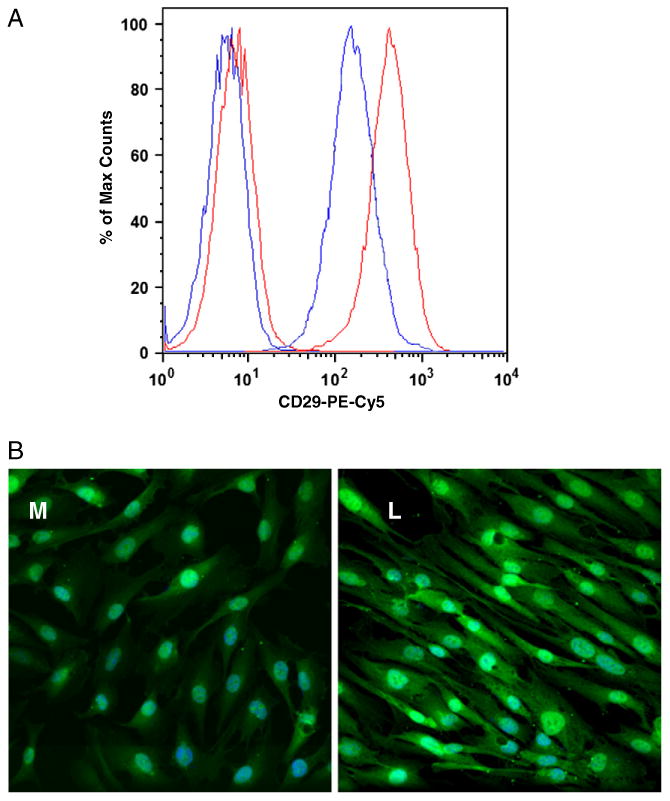
A. Representative (n=30 K) overlaid single-parameter histogram of flow cytometric analysis of the integrin β1subunit demonstrated a higher expression in leiomyoma cells (red) as compared to myometrial cells (blue). The first two peaks are the IgG isotype controls conjugated to PE-Cy5. B. Increased green fluorescence (Alexa 488) in leiomyoma cells (L) is representative of increased integrin β1subunit as compared to myometrial cells (M). Magnification=40×; experiment was repeated 4 times.
2.2. Functional inhibition of integrin β1 inhibited cell proliferation
Myometrial and leiomyoma cell lines demonstrated a time and concentration dependent decrease in cell proliferation in the presence of a β1 integrin function inhibiting antibody (Fig. 2). The difference in proliferation of myometrial and leiomyoma cell lines was observed at concentrations of ≥0.25 μg/ml. Leiomyoma cells demonstrated significant growth inhibition 61±9.0% at 1 μg/ml, compared to controls. Myometrial cells at higher concentrations demonstrated 20% growth inhibition or 79±4.0% growth compared to the untreated cells. At the end of 120 h of exposure both the cell lines demonstrated a further 10% growth inhibition, compared to 72 h, at all concentrations. Control antibody did not affect proliferation of either myometrial or leiomyoma cells.
Fig. 2.
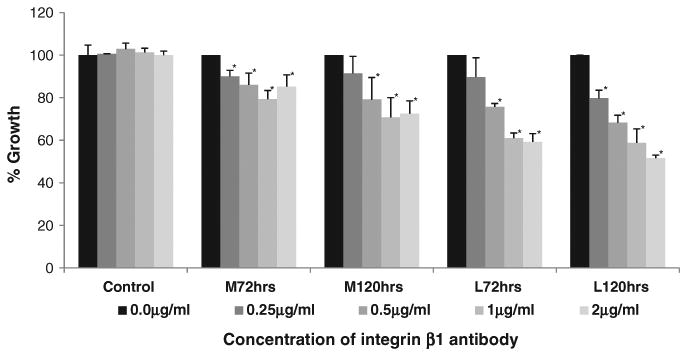
Inhibition of integrin β1 using function inhibiting antibody led to time and concentration dependent decrease in proliferation of myometrial (M) and leiomyoma (L) cells. Both cell lines demonstrated growth inhibition at concentration as low as 0.25 μg/ml. Compared to myometrial cells, leiomyoma cells demonstrated significantly higher inhibition at all concentrations. Control antibody did not inhibit growth of either M or L cells. Mean±SEM represented. Statistical analysis is to untreated controls (black bars)*=p<0.05.
2.3. Cytoskeletal reorganization of leiomyoma cells is dependent on integrin β1
Cytoskeletal filaments are reduced in cells cultured in the absence of serum (Hall, 1994; Machesky and Hall, 1997); therefore we used serum starvation of leiomyoma cells to study the effect of inhibition of integrin β1 on actin filament nucleation. Leiomyoma cells grown in complete media (DMEM/F12 containing 10% FBS) demonstrated the F-actin fibrils as shown by red fluorescence. Focal adhesion (FA) points were detected by staining of focal adhesion kinases (FAKs) in green fluorescence (Fig. 3A1 and A2). Serum starvation of the cells for 6 h (3B) and 24 h (3C) resulted in a continuous loss of FAKs, as well as F-actin, as indicated by decreased green and red fluorescence, respectively. Addition of complete media to the serum starved leiomyoma cells for 24 h demonstrated increased red (F-actin) and green (FAK) fluorescence indicating a recovery of the cytoskeleton organization in these cells. Serum starved leiomyoma cells that were pretreated for 6 h with the integrin β1 antibody before exposure to complete media (10% FBS) for 24 h demonstrated limited recovery of the cytoskeletal organization (3E).
Fig. 3.
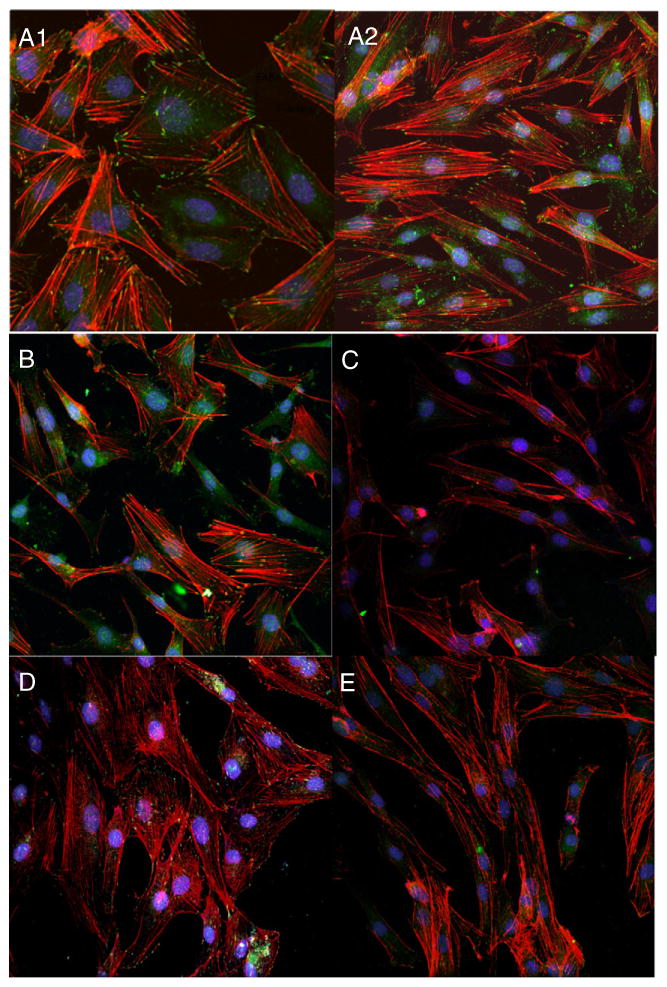
Cytoskeletal reorganization of leiomyoma cells is affected by serum and β1 antibody. A1 and A2: Leiomyoma cells grown in media containing 10% fetal bovine serum demonstrate F-actin fibrils (phalloidin; red fluorescence) and focal adhesion (FA) points as represented by presence of focal adhesion kinase (FAK; green fluorescence). B: Cells after 6 h of serum starvation. C: 24 h of serum starvation leads to loss of FAK as well actin filaments. D: Addition of complete media (10% FBS) for 24 h demonstrates reappearance of focal adhesions (FAK) and F-actin fibrils. E. Serum starved cells pre-exposed to 1 μg/ml of integrin β1 antibody for 24 h before exposure to complete media (10% FBS) show decreased recovery of actin stress fibers and FAKs.
2.4. RhoA activity and ERK pathway regulate cytoskeletal integrity in leiomyoma cells
Serum containing lysophosphatidic acid activates Rho GTPases resulting in assembly of a robust actin cytoskeleton (Machesky and Hall, 1997). Leiomyoma cells demonstrated 1.47±0.09 fold increased active Rho as compared to myometrial cells in culture in the presence of 10% FBS (Fig. 4a). After treatment with 1 μg/ml of anti-β1 antibody for 24 h, the total active Rho in treated leiomyoma cells was significantly decreased (0.81±0.02 fold) compared to the untreated leiomyoma cells cultured in complete media (1.54±0.01 fold). Serum starvation of leiomyoma cells resulted in a similar fold decrease (0.74±0.02 fold) of active Rho in leiomyoma cells compared to unexposed leiomyoma cells. After addition of complete media (10% FBS) to serum starved cells, an increase in active Rho was observed, to values similar to untreated control cells. Of note, an increase in active Rho upon addition of serum was inhibited by addition of 1 μg/ml of antibody directed against integrin β1.
Fig. 4.
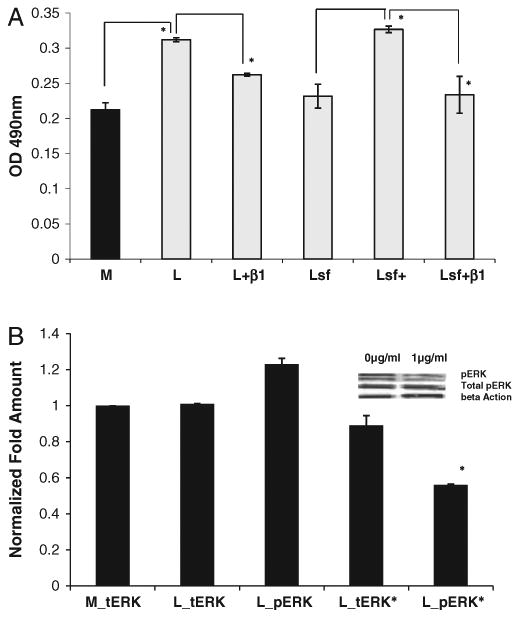
a. Levels of active Rho in leiomyoma cells were reduced by serum starvation and integrin β1 antibody. Leiomyoma (L) demonstrated a higher level of active Rho in serum rich complete media compared to myometrial cells (M). Addition of integrin β1 antibody (L+β1) or serum starvation for 24 h (Lsf) of leiomyoma cells result in decrease of active Rho. Cells exposed to complete media (10% FBS) for 24 h after serum starvation (Lsf+) show increase in active Rho, an effect that is inhibited on addition of 1 μg/ml of integrin β1 antibody (Lsf+β1). Sf = serum starvation; β1=1 μg/ml of integrin β1 antibody.*=p<0.01. Experiment repeated 4 times. Error bars = SEM. b. Leiomyoma cells treated with integrin β1 antibody(L_pERK*) at 1 μg/ml complete media demonstrated reduced pERK activity compared to control cells (L_pERK). No significant difference was observed in total ERK activity in myometrial (M_tERK) or leiomyoma (L_tERK) cells. No significant reduction in tERK was observed in leiomyoma cells treated with anti-β1 antibody (L_tERK). Experiment was repeated 2 times. Error bars = SEM.
As demonstrated in Fig. 4b, both myometrial and leiomyoma cells grown in complete media expressed similar total ERK concentration (42 and 44KDa bands). Concentration of phosphorylated-ERK (pERK) was 1.23±0.03 fold increased as compared to total ERK in leiomyoma cells. In the presence of 1 μg/ml of anti-β1 antibody for 24 h, the leiomyoma cells demonstrated a 1.78±0.06 fold lower amount of pERK and no significant change in total ERK as compared to the corresponding control cells.
2.5. Increased laminin expression may contribute to fibrotic nature of leiomyomata disease
Based on the flow cytometry results on laminin receptor specific integrin subunit α6 expression in leiomyoma cells, we analyzed the total laminins in leiomyoma cells. Increased laminins are also a hallmark of fibrotic diseases (Reilly, 1997; Neubauer et al., 2001; Chilosi et al., 2006). Compared to myometrial cells, we observed an elevated amount of total laminins in leiomyoma cells (2.04±0.03 fold, p<0.01). We further analyzed the gene expression of α, β and γ strands of laminin-5 to which the integrin α6 acts as a receptor. Using real time qRT-PCR we observed a significant increase in total RNA transcripts of laminin 5α (1.52±0.02), laminin 5β (3.06±0.92) and laminin 5γ (1.66±0.06) in leiomyoma cells. We also observed that the leiomyoma cells plated in laminin coated culture plates align themselves in parallel as compared to the same cells growing in either plastic or collagen-1 coated plates (Fig. 5).
Fig. 5.
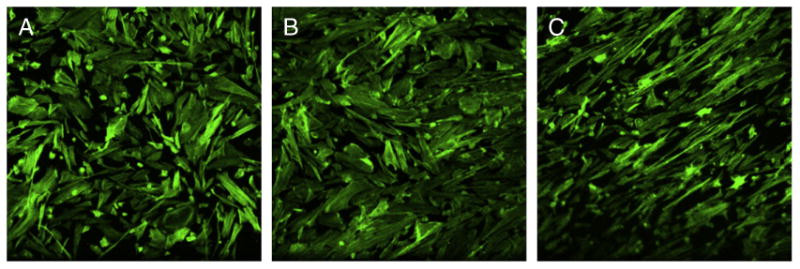
Leiomyoma cells aligned themselves and appear narrower when grown on laminin coated plates (C). Comparatively cells cultured on plastic (A) or collagen-1 (B) coated plates retained their spindle shape but appeared flattened. Experiment was repeated 3 times.
3. Discussion
Integrins are transmembrane receptors that integrate the intracellular cytoskeleton with the ECM. These adhesion receptors are directly or indirectly involved in organization of the cytoskeleton, cell proliferation, and detachment from the ECM resulting in programmed cell death (Schwartz and Ginsberg, 2002; Giancotti and Tarone, 2003). Integrin function is critical in the regulation of gene expression, tissue development, angiogenesis, tumor cell growth and metastasis (Kumar, 1998; Schwartz and Ginsberg, 2002). The presence of integrins in uterine leiomyomas has been observed by several investigators (Mechtersheimer et al., 1994; Taylor et al., 1996; Wolańska et al., 2001). Taylor and coworkers (Taylor et al., 1996) demonstrated no difference in integrin expression pattern between fixed leiomyoma and myometrium tissue sections by visual grading. Furthermore, in leiomyoma tissue, Wolańska et al. (2001) attributed increased weight of the leiomyomas to increased collagen secretion and consequently β1, which is a subunit to the most integrin receptors that bind to collagen. We went further, using leiomyoma and patient matched myometrial cell lines to demonstrate a significant upregulation of β1 (CD29) and α6 (CD49f) integrin subunits in leiomyoma cell line and the presence of integrin α2 in both cell lines with no significant difference (Table 1). We have previously demonstrated elevated COL1A, COL3A and COL4A expression in primary cultures and the immortalized leiomyoma cells similar to the tissue from which they are derived (Malik and Catherino, 2007; Malik et al., 2008), and now we demonstrate increased expression of integrin β1 in the immortalized cell lines.
Integrin β1 is a versatile subunit that binds to various α subunits to form receptors for most ECM proteins including collagen 1, fibronectin and laminins. In this study, the higher concentration of β1 subunit in leiomyoma cells correlated with the higher inhibition of proliferation compared to myometrial cells, when the action of integrin β1 subunit was inhibited. Our method of measuring cell proliferation demonstrated alterations in protein concentration as a surrogate for cell number. Due to this method, it is difficult to separate the impact of proliferation and apoptosis. We therefore cannot rule out that alterations in proliferation may be due to apoptosis as we have observed in 3D cultures (Malik and Catherino, 2012). In conclusion, inactivation of β1 subunit leads to loss of adherence between the cells and the matrix (Howlett et al., 1995; Wang et al., 2005; Lahlou and Muller, 2011; Malik and Catherino, 2012) which results in changes in cellular biochemical pathways and cell death (Stupack et al., 2001; Stupack, 2007).
In this study, actively growing leiomyoma cells demonstrated focal adhesions and activation of tyrosine kinase such as FAKs which are controlled by increased activation of Rho GTPases. Serum is known to contain lysophosphatidic acid which activates Rho and assembles stress actin filaments (Hall, 1994). We have demonstrated increased levels of active RhoA in leiomyoma tissues compared to patient matched myometrium (Rogers et al., 2008; Norian et al., 2012) consistent with the results in Fig. 4. Serum starvation can lead to dissolution of the actin cytoskeleton and disassembly of FA (Ridley and Hall, 1992; Hall, 1994, 1998) as we observed in leiomyoma cells. We further observed that re-exposure to serum results in increased Rho activity, assembly of FA complexes and repolymerization of actin stress fibers, as has been reported in other cell types (Chrzanowska-Wodnicka and Burridge, 1994; Hall, 1994, 1998). Importantly, in leiomyoma cells, integrin β1 activity was required for increase in RhoA in the serum-exposed cells following serum starvation, as well as formation of focal adhesions and actin repolymerization. Involvement of the β1 integrins in Rho-dependent activation of cytoskeletal actin fibrils has been well documented in many cell types (Wei et al., 2001; DeMali et al., 2003) and is involved in the overall cellular regulation (DeMali et al., 2003). The increased basal expression of integrin and laminin, coupled with the attenuated response of leiomyoma cells to mechanical cues, suggests that the impairment in mechanotransduction (Norian et al., 2012) may lie downstream of integrin signaling; a possibility that requires additional study.
One candidate biochemical pathway downstream of integrin aggregation and FAK activation is the MAPK/ERK pathway, a key pathway in gene expression and cell cycle/proliferation. ERK in turn can activate transcription factors such as serum response factor (SRF), a key player in cell growth and differentiation, wound healing and smooth muscle development (Chai and Tarnawski, 2002; Miano, 2003; Wang and Olson, 2004). Though no significant difference was observed in total ERK activity in myometrial and leiomyoma cells growing in culture, the phosphorylated ERK was increased in leiomyoma cells indicating activation of the ERK pathway. We observed that inhibition of the integrin β1 subunit activity led to overall inhibition of the integrin aggregation induced by the serum, and ultimately the inhibition of the ERK pathway. Inhibition of the ERK phosphorylation in the leiomyoma cells may trigger the apoptosis of cells as was observed in 3-dimensional cultures (Malik and Catherino, 2012). We interpret these findings to suggest that the impairment in mechanotransduction in leiomyoma cells is not related to reduction in ERK coupling to integrins.
An interesting observation was the upregulation of the CD49f (α6) subunit in the leiomyoma cells. For cellular adhesion, integrin α6 subunit along with the β1 subunit forms one of the major receptors for the laminins on surface of the cells (Enomoto-Iwamoto et al., 1993; Belkin and Stepp, 2000) and plays an important role in fibrotic diseases in both early and late stages (Magro et al., 1997; Wagrowska-Danilewicz and Danilewicz, 2004; Heng et al., 2006; Gressner et al., 2009; Nakanuma et al., 2010). We previously (Catherino et al., 2004) demonstrated the myofibroblastic phenotype of the leiomyomas and our results on increased laminins and its integrin receptors support the overall fibrotic nature of this disease. Increased laminin receptor integrins may also explain our findings of realignment of leiomyoma cells on laminin coated plates. Cultured cardiac myocytes when grown on laminin spread out more extensively and appear thinner due to altered surface/volume ratio (Delcarpio et al., 1989). Our cells grown on laminin appear narrower compared to cells grown either on rigid plastic or collagen coated plates.
Our previous studies emphasized the importance of the surrounding ECM microenvironment on the leiomyoma cells in-vivo and in-vitro (Rogers et al., 2008; Norian et al., 2012). Norian et al. (2012) noted the attenuated response of the leiomyoma cells, grown on a pronectin coated silicon substrate, to external stress. Our findings suggest that increased integrin β1 signaling may result in increased adhesion to the ECM, which may in turn impair the cellular response to ECM signals; thus resulting in an inability of the cell to modify cytoskeletal structure. This intriguing hypothesis is supported by the inability of leiomyoma cells to create a normal ECM structure (Leppert et al., 2004). However, inhibition of the Rho-kinase ROCK did not facilitate reorganization of the actin cytoskeleton (Norian et al., 2012). In addition, increased Rho activity is followed by phosphorylation of ERKs and subsequent increased production of ECM including laminins as shown by trabecular meshwork cells expressing a constitutively active form of RhoA (Pattabiraman and Rao, 2010). Exposure of cells to laminin can lead to loss of active Rho (Liu and Senger, 2004) and could explain why cells align due to an extracellular cue, since levels of active RhoA are elevated in leiomyoma cells grown on plastic or silicon substrate; however this explanation requires additional support. In conclusion, we demonstrated a state of altered mechanical signaling in leiomyoma cells, compared to normal myometrial cells. Additional studies are needed to unravel the paradox of the attenuated response to mechanical cues observed in leiomyoma cells.
4. Experimental procedures
4.1. Flow cytometry
Conjugated primary antibodies to integrins CD49a (α1), CD49b (α2), CD49c (α3), CD49d (α4), CD49e (α5), CD49f (α6), CD51 (αv), CD29 (β1), CD18 (β2), CD61 (β3), FIB504 (β7), and CD51/61 (αvβ3), as well as the isotype controls were purchased from BioLegend (San Diego, CA). Immortalized myometrial and leiomyoma cells (Malik et al., 2008) were analyzed for integrins. Briefly, cells were washed with 1X phosphate buffered saline (PBS) before trypsinization using TrypLE express (Invitrogen, Carlsbad, CA). The cells were counted and washed 2–3 times with cell staining buffer (CSB, 2% Fetal Bovine Serum and 0.1% sodium azide in 1X PBS; BioLegend). Aliquots of 1×106 cells were resuspended in 0.1 ml CSB and incubated with saturating amount of conjugated primary antibody and 1 μl of the reconstituted fluorescent reactive dye (Live/dead stain; Invitrogen) on ice for 30 min in dark. Following 2–3 washes with CSB the cells were fixed for 15 min on ice and in dark, using Cytofix (BD Biosciences, Oxnard, CA). After a final wash the cells were resuspended in 0.4 ml CSB and BD FACSDIva 6.1.3 software was used for acquisition (BD Biosciences). The data was analyzed by FlowJo 9.4.10 software. Positive and negative controls are indicated.
4.2. Cell proliferation
Immortalized myometrial and leiomyoma cells were plated in 48-well plates at the concentration of 1×103 cells/well in complete media containing DMEM-F12 (Dulbecco Modified Eagle's Medium: Nutrient Mixture F-12), 1X penicillin-streptomycin–neomycin (PSN), and amphotericin B (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, HyClone, ThermoFisher Scientific Inc., Rockford, IL). Once the cells reached 50% confluence they were exposed to integrin β1 function inhibiting antibody (MAB1959, Millipore) in a serial dilution of concentrations starting at 2 μg/ml, 1 μg/ml, 0.5 μg/ml and 0.25 μg/ml, 0.125 μg/ml and 0.025 μg/ml in DMEM-F12 media containing 10% FBS. Plates were collected at 24 h, 72 h and 120 h time points. The proliferation of the cells was measured using sulforhodamine-B method (Sigma-Aldrich) according to manufacturer's protocol. The experiment was repeated three times.
4.3. Cytoimmunofluorescence
To study the effect of serum starvation (complete media without the 10% FBS) as well as the presence of β1 antibody on the stress fibers (F-actin) and focal adhesion (FA) points, the leiomyoma cells were grown on 8-chambered glass slides (Nalgene Nunc Int., Rochester, NY). Previously published method was used with minor modifications (Malik and Catherino, 2007). Briefly, once the cells reached 50– 70% confluence they were either fixed for cytoimmunofluorescence (controls, 70% confluent) or underwent serum starvation (50% confluent) before exposure to complete media (10% FBS). The cells were first washed with 1X PBS (no Mg++ or Ca++) before fixation using 4% freshly made paraformaldehyde at room temperature (RT) for 10 min. After permeabilization with 0.2% Triton X-100 (10 min at RT), the cells were washed (3 times, 5 min with 1X PBS) and the non-specific sites were blocked using 1% BSA and 10% normal goat serum. The primary antibodies, Alexa 594 phalloidin (F-actin, 0.1 μM, Invitrogen) and focal adhesion kinase (FAK) (1:500, Invitrogen) were diluted in half-blocking buffer (blocking buffer 1:1 v/v with 1XPBS). The cells were exposed overnight at 4 °C in a moist chamber. After three gentle washes with 1X PBS, the Alexa 488 (Invitrogen) was used as conjugated secondary goat antibody (1 h, RT, moist chamber) to visualize the FAK expression. After the last wash the slide was dried and Prolong Gold with DAPI (Invitrogen) was used to fix the coverslip. The cells were examined under a confocal microscope (Axiovert 405 M epifluorescence inverted light microscope; Carl Zeiss, Oberkochen, Germany). Images were acquired with a CCD camera (Hamamatsu Orca, Shizuoka, Japan).
4.4. RNA and protein protocol
Immortalized myometrial and leiomyoma cells were plated in 6-well plates at concentration of 2×104 cells/well and maintained in complete media at 37 °C and 5% CO2. The monolayer cultures reaching approximately 70% confluence were exposed to serum-free media for 24 h and allowed to synchronize. This was followed by treatment with function inhibiting antibody to integrin β1 at concentrations of 0 μg/ml, 0.01 μg/ml, 0.1 μg/ml and 1 μg/ml in complete media for 24 h and 72 h. After the specified time points, the cells were either collected for RNA or protein for further analysis. The experiment was repeated three times with two replicates for each experiment.
4.5. Quantitative reverse transcriptase-polymerase chain reaction analysis
Real time reverse transcriptase-polymerase chain reaction (RT-PCR) method was used to evaluate expression of extracellular matrix gene laminin-5 as described previously (Malik et al., 2008). 18S ribosomal RNA gene was used as an internal control and each sample was analyzed in triplicate. Bio-Rad iCycler software, version 3.1 was used for data analysis.
4.6. ELISA and G-LISA
Quantification of total laminin proteins in the cells was done by enzyme linked immunosorbent assay (ELISA) kit (Millipore) according to manufacturers' protocol. Protein was collected from myometrial and leiomyoma cells grown in 6-well plates in complete media using RIPA buffer (Pierce Biotech., Rockford, IL) containing 1X Halt protease inhibitor (Pierce Biotech.) and quantitated using Precision Red Advanced Protein Assay Reagent (Cytoskeleton, Inc. Denver, CO).
For active Rho measurements, G-Lisa Rho activation assay kit (Cytoskeleton, Inc.) was used. Briefly, protein was collected following the manufacturers' protocol, from myometrial and leiomyoma cells grown in 6-well plates in complete media, as control. Protein was also collected from cells that underwent the following treatments: 1 μg/ml β1 antibody in complete media for 24 h, serum starvation for 24 h, re-exposure to complete media (10% FBS) for 30 min after serum starvation, and addition of integrin β1 antibody to serum starved cells before re-exposure to complete media. Absorbance was measured using spectrophotometric plate reader (BioRad) at 490 nm.
4.7. Western blot
Protein was isolated using RIPA lysis and extraction buffer as described previously (Malik et al., 2008). Briefly, aliquots of the proteins extracted from cultured cells treated with different concentrations of function inhibiting antibody to integrin β1 for different time points underwent electrophoreses on a SDS-PAGE under reducing conditions. For the detection of proteins, blots were incubated overnight at 4 °C, with primary antibody against ERK and pERK. Horseradish peroxidase (HRP)-conjugated secondary antibody (ImmunoPure, Pierce Biotech.) in combination with the SuperSignal West Pico (Pierce Biotech.) was used for the detection of the proteins. As an internal standard between the samples, HRP labeled anti-human beta-actin (sc-1616; 1:10,000) was used.
4.8. Statistical analysis
For real time RT-PCR data the results are reported as mean±SEM. For each result the average expression of three replicates was calculated before relative quantification using normalization against housekeeping gene (18S) was done. Relative expression was calculated based on Pfaffl Method (Pfaffl, 2001). Wilcoxon-Signed Rank test was used for nonparametric statistical evaluation. For proliferation data, statistical significance was calculated by ANOVA followed by student's t-test. Values below p<0.05 were considered significant. For western blot analysis, calculations were done using QualityOne software from Bio-Rad. Data is presented as fold difference between relative density units of treated and untreated samples, and was corrected for internal control, actin.
Acknowledgments
We would like to thank the following for their advice and suggestions; Dr. J Britten-Webb, Dr. D Jardine, Dr. JM Norian, Ms. CM Owen. We also acknowledge the help rendered by Mr. Tom Baginski, Dr. K Wolcott and Dr. K Lund from Biomedical Instrumentation Center, USUHS.
References
- Arao S, Masumoto A, Otsuki M. Beta1 integrins play an essential role in adhesion and invasion of pancreatic carcinoma cells. Pancreas. 2000;20:129–137. doi: 10.1097/00006676-200003000-00004. [DOI] [PubMed] [Google Scholar]
- Bagutti C, Wobus AM, Fassler R, Watt FM. Differentiation of embryonal stem cells into keratinocytes: comparison of wild-type and β1 integrin-deficient cells. Dev Biol. 1996;179:184–196. doi: 10.1006/dbio.1996.0250. [DOI] [PubMed] [Google Scholar]
- Belkin AM, Stepp MA. Integrins as receptors for laminins. Microsc Res Tech. 2000;51:280–301. doi: 10.1002/1097-0029(20001101)51:3<280::AID-JEMT7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Bouvard D, Brakebusch C, Gustafsson E, Aszodi A, Bengtsson T, Berna A, Fassler R. Functional consequences of integrin gene mutations in mice. Circ Res. 2001;89:211–223. doi: 10.1161/hh1501.094874. [DOI] [PubMed] [Google Scholar]
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206:211.e1–e9. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JM, Romero MR, Watt FM. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 1995;83:957–968. doi: 10.1016/0092-8674(95)90211-2. [DOI] [PubMed] [Google Scholar]
- Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK, Segars JH. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer. 2004;40:204–217. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J, Tarnawski AS. Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. J Physiol Pharmacol. 2002;53:147–157. [PubMed] [Google Scholar]
- Chilosi M, Zamò A, Doglioni C, Reghellin D, Lestani M, Montagna L, Pedron S, Ennas MG, Cancellieri A, Murer B, Poletti V. Migratory marker expression in fibroblast foci of idiopathic pulmonary fibrosis. Respir Res. 2006;7:95. doi: 10.1186/1465-9921-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Tyrosine phosphorylation is involved in reorganization of the actin cytoskeleton in response to serum or LPA stimulation. J Cell Sci. 1994;107:3643–3654. doi: 10.1242/jcs.107.12.3643. [DOI] [PubMed] [Google Scholar]
- Dekkers BG, Bos IS, Halayko AJ, Zaagsma J, Meurs H. The laminin β1-competing peptide YIGSR induces a hypercontractile, hypoproliferative airway smooth muscle phenotype in an animal model of allergic asthma. Respir Res. 2010;11:170. doi: 10.1186/1465-9921-11-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcarpio JB, Claycomb WC, Moses RL. Ultrastructural morphometric analysis of cultured neonatal and adult rat ventricular cardiac muscle cells. Am J Anat. 1989;186:335–345. doi: 10.1002/aja.1001860403. [DOI] [PubMed] [Google Scholar]
- DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol. 2003;15:572–582. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Menko AS, Philp N, Boettiger D. Evaluation of integrin molecules involved in substrate adhesion. Cell Adhes Commun. 1993;1:191–202. doi: 10.3109/15419069309097253. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Faraldo MM, Deugnier MA, Thiery JP, Glukhova MA. Development of mammary gland requires normal beta 1-integrin function. Adv Exp Med Biol. 2000;480:169–174. doi: 10.1007/0-306-46832-8_21. [DOI] [PubMed] [Google Scholar]
- Fassler R, Pfaff M, Murphy J, Noegel AA, Johansson S, Timpl R, Albrecht R. Lack of β1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J Cell Biol. 1995;128:979–988. doi: 10.1083/jcb.128.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M. Histological and biochemical studies on collagen in human uterine leiomyomas. Hokkaido Igaku Zasshi. 1985;60:602–615. [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- Gressner AM, Gao CF, Gressner OA. Non-invasive biomarkers for monitoring the fibrogenic process in liver: a short survey. World J Gastroenterol. 2009;15:2433–2440. doi: 10.3748/wjg.15.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Heng EC, Huang Y, Black SA, Jr, Trackman PC. CCN2, connective tissue growth factor, stimulates collagen deposition by gingival fibroblasts via module 3 and alpha6- and beta1 integrins. J Cell Biochem. 2006;98:409–420. doi: 10.1002/jcb.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K, Schulze E, Heckmann M, Schubert I, Meurer M, Ziegler V, Haustein UF, Mehlhorn J, Krieg T. Type III collagen aminopropeptide and laminin P1 levels in serum of patients with silicosis-associated and idiopathic systemic scleroderma. Br J Dermatol. 1990;123:1–7. doi: 10.1111/j.1365-2133.1990.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Howlett A, Bailey N, Damsky C, Petersen O, Bissell M. Cellular growth and survival are mediated by β1 integrins in normal human breast epithelium but not in breast carcinoma. J Cell Sci. 1995;108:1945–1957. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Johansson S, Svineng G, Wennerberg K, Armulik A, Lohikangas L. Fibronectin–integrin interactions. Front Biosci. 1997;2:d126–d146. doi: 10.2741/a178. [DOI] [PubMed] [Google Scholar]
- Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- Katayama M, Ishizaka A, Sakamoto M, Fujishima S, Sekiguchi K, Asano K, Betsuyaku T, Kotani T, Ware LB, Matthay MA, Hashimoto S. Laminin gamma2 fragments are increased in the circulation of patients with early phase acute lung injury. Intensive Care Med. 2010;36:479–486. doi: 10.1007/s00134-009-1719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar CC. Signaling by integrin receptors. Oncogene. 1998;17:1365–1373. doi: 10.1038/sj.onc.1202172. [DOI] [PubMed] [Google Scholar]
- Lahlou H, Muller WJ. β1-integrins signaling and mammary tumor progression in transgenic mouse models: implications for human breast cancer. Breast Cancer Res. 2011;13:229. doi: 10.1186/bcr2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau MT, So WK, Leung PC. Integrin β1 mediates epithelial growth factor-induced invasion in human ovarian cancer cells. Cancer Lett. 2012;320:198–204. doi: 10.1016/j.canlet.2012.02.028. [DOI] [PubMed] [Google Scholar]
- Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril. 2004;82:1182–1187. doi: 10.1016/j.fertnstert.2004.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Senger DR. Matrix-specific activation of Src and Rho initiates capillary morphogenesis of endothelial cells. FASEB J. 2004;18:457–468. doi: 10.1096/fj.03-0948com. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J Cell Biol. 1997;138:913–926. doi: 10.1083/jcb.138.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro G, Fraggetta F, Travali S, Lanzafame S. Immuno-histochemical expression and distribution of alpha2beta1, alpha6beta1, alpha5beta1 integrins and their extracellular ligands, type IV collagen, laminin and fibronectin in palmar fibromatosis. Gen Diagn Pathol. 1997;143:203–208. [PubMed] [Google Scholar]
- Malik M, Catherino WH. Novel method to characterize primary cultures of leiomyoma and myometrium with the use of confirmatory biomarker gene arrays. Fertil Steril. 2007;87:1166–1172. doi: 10.1016/j.fertnstert.2006.08.111. [DOI] [PubMed] [Google Scholar]
- Malik M, Catherino WH. Development and validation of a three-dimensional in vitro model for uterine leiomyoma and patient-matched myometrium. Fertil Steril. 2012;97:1287–1293. doi: 10.1016/j.fertnstert.2012.02.037. [DOI] [PubMed] [Google Scholar]
- Malik M, Webb J, Catherino WH. Retinoic acid treatment of human leiomyoma cells transformed the cell phenotype to one strongly resembling myometrial cells. Clin Endocrinol. 2008;69:462–470. doi: 10.1111/j.1365-2265.2008.03207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M, Norian J, McCarthy-Keith D, Britten J, Catherino WH. Why leiomyomas are called fibroids: the central role of extracellular matrix in symptomatic women. Semin Reprod Med. 2010;28:169–179. doi: 10.1055/s-0030-1251475. [DOI] [PubMed] [Google Scholar]
- Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol. 2011;23:607–614. doi: 10.1016/j.ceb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Mechtersheimer G, Barth T, Quentmeier A, Möller P. Differential expression of beta 1 integrins in nonneoplastic smooth and striated muscle cells and in tumors derived from these cells. Am J Pathol. 1994;144:1172–1182. [PMC free article] [PubMed] [Google Scholar]
- Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- Mitropoulou TN, Theocharis AD, Stagiannis KD, Karamanos NK. Identification, quantification and fine structural characterization of glycosaminoglycans from uterine leiomyoma and normal myometrium. Biochimie. 2001;83:529–536. doi: 10.1016/s0300-9084(01)01281-0. [DOI] [PubMed] [Google Scholar]
- Mizejewski G. Role of integrins in cancer: survey of expression patterns. Proc Soc Exp Biol Med. 1999;222:124–138. doi: 10.1177/153537029922200203. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay NK, Gilchrist D, Gordon GJ, Chen CJ, Bueno R, Lu ML, Salgia R, Sugarbaker DJ, Jaklitsch MT. Integrin-dependent protein tyrosine phosphorylation is a key regulatory event in collagen-IV-mediated adhesion and proliferation of human lung tumor cell line, Calu-1. Ann Thorac Surg. 2004;78:450–457. doi: 10.1016/j.athoracsur.2004.01.042. [DOI] [PubMed] [Google Scholar]
- Nakanuma Y, Harada K, Sato Y, Ikeda H. Recent progress in the etiopathogenesis of pediatric biliary disease, particularly Caroli's disease with congenital hepatic fibrosis and biliary atresia. Histol Histopathol. 2010;25:223–235. doi: 10.14670/HH-25.223. [DOI] [PubMed] [Google Scholar]
- Neubauer K, Saile B, Ramadori G. Liver fibrosis and altered matrix synthesis. Can J Gastroenterol. 2001;15:187–193. doi: 10.1155/2001/870205. [DOI] [PubMed] [Google Scholar]
- Norian JM, Owen CM, Taboas J, Korecki C, Tuan R, Malik M, Catherino WH, Segars JH. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31:57–65. doi: 10.1016/j.matbio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak RA. Identifications of new therapies: what in vitro studies can tell us. Clin Obstet Gynecol. 2001;44:327–334. doi: 10.1097/00003081-200106000-00019. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010;298:C749–C763. doi: 10.1152/ajpcell.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani M, Burlina A. Biochemical markers of hepatic fibrosis. Clin Biochem. 1991;24:219–239. doi: 10.1016/0009-9120(91)80013-s. [DOI] [PubMed] [Google Scholar]
- Reilly JT. Idiopathic myelofibrosis: pathogenesis, natural history and management. Blood Rev. 1997;11:233–242. doi: 10.1016/s0268-960x(97)90022-9. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rogers R, Norian J, Malik M, Christman G, Abu-Asab M, Chen F, Korecki C, Latridis J, Catherino WH, Tuan RS, Dhillon N, Leppert P, Segars JH. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198:474.e1–11. doi: 10.1016/j.ajog.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa H, Parise ER. Is there a place for serum laminin determination in patients with liver disease and cancer? World J Gastroenterol. 2008;14:3628–3632. doi: 10.3748/wjg.14.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabry M, Al-Hendy A. Medical treatment of uterine leiomyoma. Reprod Sci. 2012;19:339–353. doi: 10.1177/1933719111432867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai H, Okada Y, Funahashi H, Matsuo Y, Takahashi H, Takeyama H, Manabe T. Activation of focal adhesion kinase enhances the adhesion and invasion of pancreatic cancer cells via extracellular signal-regulated kinase-1/2 signaling pathway activation. Mol Cancer. 2005;4:37. doi: 10.1186/1476-4598-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65–E68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Shattil SJ. Signaling networks linking integrins and rho family GTPases. Trends Biochem Sci. 2000;25:388–391. doi: 10.1016/s0968-0004(00)01605-4. [DOI] [PubMed] [Google Scholar]
- Segarra A, Simó R, Masmiquel L, Segura RM, Fonollosa V, Huguet P, Majo J, Piera L, Schwartz S. Serum concentrations of laminin-P1 in thrombotic microangiopathy: usefulness as an index of activity and prognostic value. J Am Soc Nephrol. 2000;11:434–443. doi: 10.1681/ASN.V113434. [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Takagi J, Springer TA. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct. 2002;31:485–516. doi: 10.1146/annurev.biophys.31.101101.140922. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bissell MJ. Mammary epithelial cells, extracellular matrix, and gene expression. Cancer Treat Res. 1991;53:365–381. doi: 10.1007/978-1-4615-3940-7_17. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell–cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack DG. Integrins as a distinct subtype of dependence receptors. Cell Death Differ. 2005;12:1021–1030. doi: 10.1038/sj.cdd.4401658. [DOI] [PubMed] [Google Scholar]
- Stupack DG. The biology of integrins. Oncology. 2007;21:6–12. [PubMed] [Google Scholar]
- Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001;155:59–70. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CV, Letarte M, Lye SJ. The expression of integrins and cadherins in normal human uterus and uterine leiomyomas. Am J Obstet Gynecol. 1996;175:411–419. doi: 10.1016/s0002-9378(96)70155-2. [DOI] [PubMed] [Google Scholar]
- Tian B, Lessan K, Kahm J, Kleidon J, Henke C. beta 1 integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 3-kinase/Akt/protein kinase B signaling pathway. J Biol Chem. 2002;277:24667–24675. doi: 10.1074/jbc.M203565200. [DOI] [PubMed] [Google Scholar]
- Wagrowska-Danilewicz M, Danilewicz M. Expression of alpha5beta1 and alpha6beta1 integrins in IgA nephropathy (IgAN) with mild and severe proteinuria. An immunohistochemical study Int Urol Nephrol. 2004;36:81–87. doi: 10.1023/b:urol.0000032707.22306.d1. [DOI] [PubMed] [Google Scholar]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- Wang JC. Importance of plasma matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinase (TIMP) in development of fibrosis in agnogenic myeloid metaplasia. Leuk Lymphoma. 2005;46:1261–1268. doi: 10.1080/10428190500126463. [DOI] [PubMed] [Google Scholar]
- Wang DZ, Olson EN. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr Opin Genet Dev. 2004;14:558–566. doi: 10.1016/j.gde.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Wang R, Li J, Lyte K, Yashpal NK, Fellows F, Goodyer CG. Role for beta1 integrin and its associated alpha3, alpha5, and alpha6 subunits in development of the human fetal pancreas. Diabetes. 2005;54:2080–2089. doi: 10.2337/diabetes.54.7.2080. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Wang L, Carson JA, Agan JE, Imanaka-Yoshida K, Schwartz RJ. beta1 integrin and organized actin filaments facilitate cardiomyocyte-specific RhoA-dependent activation of the skeletal alpha-actin promoter. FASEB J. 2001;15:785–796. doi: 10.1096/fj.00-026com. [DOI] [PubMed] [Google Scholar]
- Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med. 2002;80:629–638. doi: 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- Whittard JD, Akiyama SK. Activation of beta1 integrins induces cell–cell adhesion. Exp Cell Res. 2001;263:65–76. doi: 10.1006/excr.2000.5099. [DOI] [PubMed] [Google Scholar]
- Wickström SA, Lange A, Montanez E, Fässler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J. 2010;29:281–291. doi: 10.1038/emboj.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolańska M, Sobolewski K, Drozdzewicz M, Bankowski E. Extracellular matrix components in uterine leiomyoma and their alteration during the tumour growth. Mol Cell Biochem. 1998;189:145–152. doi: 10.1023/a:1006914301565. [DOI] [PubMed] [Google Scholar]
- Wolańska M, Sobolewski K, Drozdzewicz M. Integrins and prolidase activity in uterine leiomyoma during tumor growth. Ginekol Pol. 2001;72:121–126. [PubMed] [Google Scholar]


