Abstract
Rainstorms, insect swarms, and galloping horses produce “sound textures” – the collective result of many similar acoustic events. Sound textures are distinguished by temporal homogeneity, suggesting they could be recognized with time-averaged statistics. To test this hypothesis, we processed real-world textures with an auditory model containing filters tuned for sound frequencies and their modulations, and measured statistics of the resulting decomposition. We then assessed the realism and recognizability of novel sounds synthesized to have matching statistics. Statistics of individual frequency channels, capturing spectral power and sparsity, generally failed to produce compelling synthetic textures. However, combining them with correlations between channels produced identifiable and natural-sounding textures. Synthesis quality declined if statistics were computed from biologically implausible auditory models. The results suggest that sound texture perception is mediated by relatively simple statistics of early auditory representations, presumably computed by downstream neural populations. The synthesis methodology offers a powerful tool for their further investigation.
Sensory receptors measure light, sound, skin pressure, and other forms of energy, from which organisms must recognize the events that occur in the world. Recognition is believed to occur via the transformation of sensory input into representations in which stimulus identity is explicit (for instance, via neurons responsive to one category but not others). In the auditory system, as well as other modalities, much is known about how this process begins, from transduction through the initial stages of neural processing. Something is also known about the system's output, reflected in the ability of human listeners to recognize sounds. Less is known about what happens in the middle – the stages between peripheral processing and perceptual decisions. The difficulty of studying these mid-level processing stages partly reflects a lack of appropriate stimuli, as the tones and noises that are staples of classical hearing research do not capture the richness of natural sounds.
Here we study “sound texture”, a category of sound that is well-suited for exploration of mid-level auditory perception. Sound textures are produced by a superposition of many similar acoustic events, such as arise from rain, fire, or a swamp full of insects, and are analogous to the visual textures that have been studied for decades (Julesz, 1962). Textures are a rich and varied set of sounds, and we show that listeners can readily recognize them. However, unlike the sound of an individual event, such as a footstep, or of the complex temporal sequences of speech or music, a texture is defined by properties that remain constant over time. Textures thus possess a simplicity relative to other natural sounds that makes them a useful starting point for studying auditory representation and sound recognition.
We explored sound texture perception using a model of biological texture representation. The model begins with known processing stages from the auditory periphery and culminates with the measurement of simple statistics of these stages. We hypothesize that such statistics are measured by subsequent stages of neural processing, where they are used to distinguish and recognize textures. We tested the model by conducting psychophysical experiments with synthetic sounds engineered to match the statistics of real-world textures. The logic of the approach, borrowed from vision research, is that if texture perception is based on a set of statistics, two textures with the same values of those statistics should sound the same (Julesz, 1962; Portilla and Simoncelli, 2000). In particular, our synthetic textures should sound like another example of the corresponding real-world texture if the statistics used for synthesis are similar to those measured by the auditory system.
Although the statistics we investigated are relatively simple, and were not hand-tuned to specific natural sounds, they produced compelling synthetic examples of many real-world textures. Listeners recognized the synthetic sounds nearly as well as their real-world counterparts. In contrast, sounds synthesized using representations distinct from those in biological auditory systems generally did not sound as compelling. Our results suggest that the recognition of sound textures is based on statistics of modest complexity computed from the responses of the peripheral auditory system. These statistics likely reflect sensitivities of downstream neural populations. Sound textures and their synthesis thus provide a substrate for studying mid-level audition.
Results
Our investigations of sound texture were constrained by three sources of information: auditory neuroscience, natural sound statistics, and perceptual experiments. We used the known structure of the early auditory system to construct the initial stages of our model and to constrain the choices of statistics. We then established the plausibility of different types of statistics by verifying that they vary across natural sounds and could thus be useful for their recognition. Finally, we tested the perceptual importance of different texture statistics with experiments using synthetic sounds.
Texture Model
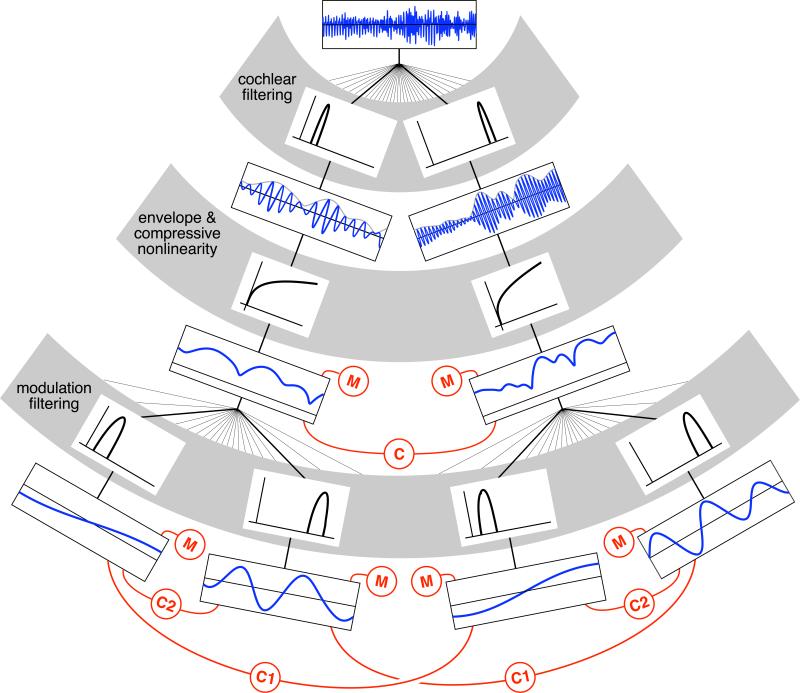
Our model is based on a cascade of two filter banks (Fig. 1) designed to replicate the tuning properties of neurons in early stages of the auditory system, from the cochlea through the thalamus. An incoming sound is first processed with a bank of 30 bandpass cochlear filters that decompose the sound waveform into acoustic frequency bands, mimicking the frequency selectivity of the cochlea. All subsequent processing is performed on the amplitude envelopes of these frequency bands. Amplitude envelopes can be extracted from cochlear responses with a low-pass filter and are believed to underlie many aspects of peripheral auditory responses (Joris et al., 2004). When the envelopes are plotted in grayscale and arranged vertically, they form a spectrogram, a two-dimensional (time vs. frequency) image commonly used for visual depiction of sound (e.g. Fig. 2a). Perceptually, envelopes carry much of the important information in natural sounds (Gygi et al., 2004; Shannon et al., 1995; Smith et al., 2002), and can be used to reconstruct signals that are perceptually indistinguishable from the original in which the envelopes were measured. Cochlear transduction of sound is also distinguished by amplitude compression (Ruggero, 1992) – the response to high intensity sounds is proportionally smaller than that to low intensity sounds, due to nonlinear, level-dependent amplification. To simulate this phenomenon, we apply a compressive nonlinearity to the envelopes.
Figure 1.
Model architecture. A sound waveform (top row) is filtered by a “cochlear” filterbank (gray stripe shows two example filters at different frequencies, on a log-frequency axis). Cochlear filter responses (i.e., subbands) are bandlimited versions of the original signal (third row), the envelopes of which (in gray) are passed through a compressive nonlinearity (gray stripe, fourth row), yielding compressed envelopes (fifth row), from which marginal statistics and cross band correlations are measured. Envelopes are filtered with a modulation filter bank (gray stripe, sixth row, showing two example filters for each of the two example cochlear channels, on a log-frequency axis), the responses of which (seventh row) are used to compute modulation marginals and correlations. Red icons denote statistical measurements: marginal moments of a single signal or correlations between two signals.
Figure 2.
Cochlear marginal statistics. (a) Spectrograms of three sound excerpts, generated by plotting the envelopes of a cochlear filter decomposition. Gray-level indicates the (compressed) envelope amplitude (same scale for all three sounds). (b) Envelopes of one cochlear channel for the three sounds from a. (c) Histograms (gathered over time) of the envelopes in b. Vertical line segments indicate the mean value of the envelope for each sound. (d-g) Envelope marginal moments for each cochlear channel of each of 168 natural sound textures. Moments of sounds in a-c are plotted with thick lines; dashed black line plots the mean value of each moment across all sounds.
Each compressed envelope is further decomposed using a bank of 20 bandpass modulation filters. Modulation filters are conceptually similar to cochlear filters, except that they operate on (compressed) envelopes rather than the sound pressure waveform, and are tuned to frequencies an order of magnitude lower, as envelopes fluctuate at relatively slow rates. A modulation filter bank is consistent with reports of modulation tuning in midbrain and thalamic neurons (Baumann et al., 2011; Joris et al., 2004; Miller et al., 2001; Rodriguez et al., 2010) and previous auditory models (Bacon and Grantham, 1989; Dau et al., 1997). Both the cochlear and modulation filters in our model had bandwidths that increased with their center frequency (such that they were approximately constant on a logarithmic scale), as is observed in biological auditory systems.
From cochlear envelopes and their modulation bands, we derive a representation of texture by computing statistics (red symbols in Fig. 1). The statistics are time-averages of nonlinear functions of either the envelopes or the modulation bands. Such statistics are in principle suited to summarizing stationary signals like textures, whose properties are constant over some moderate timescale. A priori, however, it is not obvious whether simple, biologically plausible statistics would have much explanatory power as descriptors of natural sounds, or of their perception. Previous attempts to model sound texture have come from the machine audio and sound rendering communities (Athineos and Ellis, 2003; Dubnov et al., 2002; Saint-Arnaud and Popat, 1995; Verron et al., 2009; Zhu and Wyse, 2004), and have involved representations unrelated to those in biological auditory systems.
Texture Statistics
Of all the statistics the brain might compute, which might be used by the auditory system? Natural sounds can provide clues: in order for a statistic to be useful for recognition, it must produce different values for different sounds. We considered a set of generic statistics, and verified that they varied substantially across a set of 168 natural sound textures.
We examined two general classes of statistic: marginal moments and pairwise correlations. Both types of statistic involve averages of simple nonlinear operations (e.g., squaring, products) that could plausibly be measured using neural circuitry (at a later stage of neural processing). Moments and correlations derive additional plausibility from their importance in the representation of visual texture (Heeger and Bergen, 1995; Portilla and Simoncelli, 2000), which provided inspiration for our work. Both types of statistic were computed on cochlear envelopes as well as their modulation bands (Fig. 1). Because modulation filters are applied to the output of a particular cochlear channel, they are tuned in both acoustic frequency and modulation frequency. We thus distinguished two types of modulation correlations: those between bands tuned to the same modulation frequency but different acoustic frequencies (C1), and those between bands tuned to the same acoustic frequency but different modulation frequencies (C2).
To provide some intuition for the variation in statistics that occurs across sounds, consider the cochlear marginal moments – statistics that describe the distribution of the envelope amplitude, collapsed across time, for a single cochlear channel. Figures 2a show the envelopes, displayed as spectrograms, for excerpts of three example sounds (pink (1/f) noise, a stream, and geese calls), and Fig. 2b plots the envelopes of one particular channel for each sound. It is visually apparent that the envelopes of the three sounds are distributed differently – those of the geese contain more high-amplitude and low-amplitude values than those of the stream or noise. Fig. 2c shows the envelope distributions for one cochlear channel. Although the mean envelope values are nearly equal in this example (because they have roughly the same average acoustic power in that channel), the envelope distributions differ in width, asymmetry about the mean, and the presence of a long positive tail. These properties can be captured by the marginal moments (mean, variance, skew, and kurtosis, respectively). Fig. 2d-g show these moments for our full set of sound textures. Marginal moments have previously been proposed to play a role in envelope discrimination (Lorenzi et al., 1999; Strickland and Viemeister, 1996), and often reflect the property of sparsity, which tends to characterize natural sounds and images. Intuitively, sparsity reflects the discrete events that generate natural signals – these events are infrequent, but produce a burst of energy when they occur, yielding high-variance amplitude distributions. Sparsity has been linked to sensory coding (Field, 1987; Olshausen and Field, 1996; Smith and Lewicki, 2006), but its role in the perception of real-world sounds has been unclear.
Each of the remaining statistics we explored (Fig. 1) captures distinct aspects of acoustic structure, and also exhibits large variation across sounds (Fig. 3). The moments of the modulation bands, particularly the variance, indicate the rates at which cochlear envelopes fluctuate, allowing distinction between rapidly modulated sounds (e.g., insect vocalizations) and slowly modulated sounds (e.g., ocean waves). The correlation statistics, in contrast, each reflect distinct aspects of coordination between envelopes of different channels, or between their modulation bands. The cochlear correlations (C) distinguish textures with broadband events that activate many channels simultaneously (e.g. applause), from those that produce nearly independent channel responses (many water sounds; see Experiment 1). The cross-channel modulation correlations (C1) are conceptually similar except that they are computed on a particular modulation band of each cochlear channel. In some sounds (e.g. wind, or waves) the C1 correlations are large only for low frequency modulation bands, whereas in others (e.g. fire) they are present across all modulation bands. The within-channel modulation correlations (C2) allow discrimination between sounds with sharp onsets or offsets (or both), by capturing the relative phase relationships between modulation bands within a cochlear channel. See Methods for detailed descriptions.
Figure 3.
Other statistics. (a) Modulation power in each band (normalized by the variance of the corresponding cochlear envelope) for insects, waves, and stream sounds of Fig. 4b. For ease of display and interpretation, this statistic is expressed in dB relative to the same statistic for pink noise. (b) Cross-band envelope correlations for fire, applause, and stream sounds of Fig. 4b. Each matrix cell displays the correlation coefficient between a pair of cochlear envelopes. (c) C1 correlations for waves and fire sounds of Fig. 4b. Each matrix contains correlations between modulation bands tuned to the same modulation frequency but to different acoustic frequencies, yielding matrices of the same format as a, but with a different matrix for each modulation frequency, indicated at the top of each matrix. (d) Spectrograms and C2 correlations for three sounds. Note asymmetric envelope shapes in first and second rows, and that abrupt onsets (top), offsets (middle), and impulses (bottom) produce distinct correlation patterns. In right panels, modulation channel labels indicate the center of low-frequency band contributing to the correlation. See also Fig. S6.
Sound Synthesis
Our goal in synthesizing sounds was not to render maximally realistic sounds per se, as in most sound synthesis applications (Dubnov et al., 2002; Verron et al., 2009), but rather to test hypotheses about how the brain represents sound texture, using realism as an indication of the hypothesis validity. Others have also noted the utility of synthesis for exploring biological auditory representations (Mesgarani et al., 2009; Slaney, 1995); our work is distinct for its use of statistical representations. Inspired by methods for visual texture synthesis (Heeger and Bergen, 1995; Portilla and Simoncelli, 2000), our method produced novel signals that matched some of the statistics of a real-world sound. If the statistics used to synthesize the sound are similar to those used by the brain for texture recognition, the synthetic signal should sound like another example of the original sound.
To synthesize a texture, we first obtained desired values of the statistics by measuring the model responses (Fig. 1) for a real-world sound. We then used an iterative procedure to modify a random noise signal (using variants of gradient descent) to force it to have these desired statistic values (Fig. 4a). By starting from noise, we hoped to generate a signal that was as random as possible, constrained only by the desired statistics.
Figure 4.
Synthesis algorithm and example results. (a) Schematic of synthesis procedure. Statistics are measured after a sound recording is passed through the auditory model of Fig. 1. Synthetic signal is initialized as noise, and the original sound's statistics are imposed on its cochlear envelopes. The modified envelopes are multiplied by their associated fine structure, and then recombined into a sound signal. The procedure is iterated until the synthesized signal has the desired statistics. (b) Spectrograms of original and synthetic versions of several sounds (same amplitude scale for all sounds). See also Figures S1, S2.
Figure 4b displays spectrograms of several naturally occurring sound textures along with synthetic examples generated from their statistics (see Fig. S1 for additional examples). It is visually apparent that the synthetic sounds share many structural properties of the originals, but also that the process has not simply regenerated the original sound – here and in every other example we examined, the synthetic signals were physically distinct from the originals (see also Experiment 1b, cond. 7). Moreover, running the synthesis procedure multiple times produced exemplars with the same statistics but whose spectrograms were easily discriminated visually (Fig. S2). The statistics we studied thus define a large set of sound signals (including the original in which the statistics are measured), from which one member is drawn each time the synthesis process is run.
To assess whether the synthetic results sound like the natural textures whose statistics they matched, we conducted several experiments. The results can also be appreciated by listening to example synthetic sounds, available online: http://www.cns.nyu.edu/~jhm/texture.html.
Experiment 1: Texture Identification
We first tested whether synthetic sounds could be identified as exemplars of the natural sound texture from which their statistics were obtained. Listeners were presented with example sounds, and chose an identifying name from a set of five. In Experiment 1a, sounds were synthesized using different subsets of statistics. Identification was poor when only the cochlear channel power was imposed (producing a sound with roughly the same power spectrum as the original), but improved as additional statistics were included as synthesis constraints (Fig. 5a; F(2.25, 20.25)=124.68, p<.0001; see figure for paired comparisons between conditions). Identification of textures synthesized using the full model approached that obtained for the original sound recordings.
Figure 5.
Experiment 1: Texture identification. (a) Identification improves as more statistics are included in the synthesis. Asterisks denote significant differences between conditions, p<.01 (paired t-tests, corrected for multiple comparisons). Here and elsewhere, error bars denote standard errors and dashed lines denote the chance level of performance. (b) The five categories correctly identified most often for conds.1 and 2, with mean percent correct in parentheses. (c) The five categories chosen incorrectly most often for conds.1 and 2, with mean percent trials chosen (of those where they were a choice) in parentheses. (d) Identification with alternative marginal statistics, and long synthetic signals. Horizontal lines indicate non-significant differences (p>.05). (e&f) The five most correctly identified (e) and most often incorrectly chosen (f) categories for conds.1-3. See also Figures S3, S4.
Inspection of listeners’ responses revealed several results of interest (Fig. 5b&c). In condition 1, when only the cochlear channel power was imposed, the sounds most commonly correctly identified were those that are often noise-like (wind, static etc.); such sounds were also the most common incorrect answers. This is unsurprising, because the synthesis was initialized with noise and in this condition simply altered its spectrum. A more interesting pattern emerged for condition 2, in which the cochlear marginal moments were imposed. In this condition, but not others, the sounds most often identified correctly, and chosen incorrectly, were water sounds. This is readily apparent from listening to the synthetic examples – water often sounds realistic when synthesized from its cochlear marginals, and most other sounds synthesized this way sound water-like.
Because the cochlear marginal statistics only constrain the distribution of amplitudes within individual frequency channels, this result suggests that the salient properties of water sounds are conveyed by sparsely distributed, independent, bandpass acoustic events. In Experiment 1b, we further explored this result – in conditions 1 and 2 we again imposed marginal statistics, but used filters that were either narrower or broader than the filters found in biological auditory systems. Synthesis with these alternative filters produced levels of performance similar to the auditory filter bank (condition 3; Fig. 5d), but in both cases, water sounds were no longer the most popular choices (Fig. 5e&f; the four water categories were all identified less well, and chosen incorrectly less often, in conds. 1 and 2 compared to cond. 3; p<.01, sign test). It thus seems that the bandwidths of biological auditory filters are comparable to those of the acoustic events produced by water (see also Fig. S3), and that water sounds often have remarkably simple structure in peripheral auditory representations.
Although cochlear marginal statistics are adequate to convey the sound of water, in general they are insufficient for recognition (Fig. 5a). One might expect that with a large enough set of filters, marginal statistics alone would produce better synthesis, since each filter provides an additional set of constraints on the sound signal. However, our experiments indicate otherwise. When we synthesized sounds using a filter bank with the bandwidths of our canonical model, but with four times as many filters (such that adjacent filters overlapped more than in the original filter bank), identification was not significantly improved (Fig. 5d, condition 4 vs. 3, t(9) = 1.27, p=.24). Similarly, one might suppose that constraining the full marginal distribution (as opposed to just matching the four moments in our model) might capture more structure, but we found that this also failed to produce improvements in identification (Fig. 5d, condition 5 vs. 3, t(9) = 1.84, p=.1; Fig. S4). These results suggest that cochlear marginal statistics alone, irrespective of how exhaustively they are measured, cannot account for our perception of texture.
Because the texture model is independent of the signal length, we could measure statistics from signals much shorter or longer than those being synthesized. In both cases the results generally sounded as compelling as if the synthetic and original signals were the same length. To verify this empirically, in condition 7 we used excerpts of 15-second signals synthesized from 7-second originals. Identification performance was unaffected (Figure 5d, condition 7 vs. 6; t(9) = .5, p=.63), indicating that these longer signals captured the texture qualities as well as signals more comparable to the original signals in length.
Experiment 2: Necessity of Each Class of Statistic
We found that each class of statistic was perceptually necessary, in that its omission from the model audibly impaired the quality of some synthetic sounds. To demonstrate this empirically, in Experiment 2a we presented listeners with excerpts of original texture recordings followed by two synthetic versions – one synthesized using the full set of model statistics, and the other synthesized with one class omitted – and asked them to judge which synthetic version sounded more like the original. Fig. 6a plots the percentage of trials on which the full set of statistics was preferred. In every condition, listeners preferred synthesis with the full set more than expected by chance (t-tests, p<.01 in all cases, Bonferroni corrected), though the preference was stronger for some statistic classes than others (F(4,36) = 15.39, p<.0001).
Figure 6.
Experiments 2&3: Omitting and manipulating statistics. (a) Experiment 2a: Synthesis with the full set of statistics is preferred over synthesis omitting any single class. In condition 1, the envelope mean was imposed, to ensure that the spectrum was approximately correct. Asterisks here and in (b) and (d) denote significant differences from chance or between conditions, p<.01. (b) Experiment 2b: Sounds with the correct cochlear marginal statistics were preferred over those with 1) the cochlear marginal moments of noise, 2) all cochlear marginals omitted (as in cond. 1 of (a)), or 3) the skew and kurtosis omitted. (c) Frequency responses of logarithmically and linearly spaced cochlear filter banks. (d) Experiment 3: Sounds synthesized with a biologically plausible auditory model were preferred over those synthesized with models deviating from biology (by omitting compression, or by using linearly spaced cochlear or modulation filter banks).
The effect of omitting a statistic class was not noticeable for every texture. A potential explanation is that the statistics of many textures are close to those of noise for some subset of statistics, such that omitting that subset does not cause the statistics of the synthetic result to deviate much from the correct values (because the synthesis is initialized with noise). To test this idea, we computed the difference between each sound's statistics and those of pink (1/f) noise, for each of the five statistic classes. When we reanalyzed the data including only the 30% of sounds whose statistics were most different from those of noise, the proportion of trials on which the full set of statistics was preferred was significantly higher in each case (t-tests, p<.05). Including a particular statistic in the synthesis process thus tends to improve realism when the value of that statistic deviates from that of noise. Because of this, not all statistics are necessary for the synthesis of every texture (although all statistics presumably contribute to the perception of every texture – if the values were actively perturbed from their correct values, whether noise-like or not, we found that listeners generally noticed).
We expected that the C2 correlation, which measures phase relations between modulation bands, would help capture the temporal asymmetry of abrupt onsets or offsets. To test this idea, we separately analyzed sounds that visually or audibly possessed such asymmetries (explosions, drum beats etc.). For this subset of sounds, and for other randomly selected subsets, we computed the average proportion of trials in which the full set of statistics was preferred. The preference for the full set of statistics was larger in the asymmetric sounds than in 99.96% of other subsets, confirming that the C2 correlations were particularly important for capturing asymmetric structure.
It is also notable that omitting the cochlear marginal moments produced a noticeable degradation in realism for a large fraction of sounds, indicating that the sparsity captured by these statistics is perceptually important. As a further test, we explicitly forced sounds to be nonsparse and examined the effect on perception. We synthesized sounds using a hybrid set of statistics in which the envelope variance, skew, and kurtosis were taken from pink noise, with all other statistics given the correct values for a particular real-world sound. Because noise is nonsparse (the marginals of noise lie at the lower extreme of the values for natural sounds; Fig. 2), this manipulation forced the resulting sounds to lack sparsity but to maintain the other statistical properties of the original sound. We found that the preference for signals with the correct marginals was enhanced in this condition (1 vs. 2, t(9) = 8.1, p<.0001; Fig. 6b), consistent with the idea that sparsity is perceptually important for most natural sound textures. This result is also an indication that the different classes of statistic are not completely independent: constraining the other statistics had some effect on the cochlear marginals, bringing them closer to the values of the original sound even if they themselves were not explicitly constrained. We also found that listeners preferred sounds synthesized with all four marginal moments to those with the skew and kurtosis omitted (t(8) = 4.1, p=.003). Although the variance alone contributes substantially to sparsity, the higher-order moments also play some role.
Experiment 3: Statistics of Non-Biological Sound Representations
How important are the biologically-inspired features of our model? As a test, we altered our model in three respects: 1) removing cochlear compression, 2) altering the bandwidths of the “cochlear” filters, and 3) altering bandwidths of the modulation filters (rows four, two and six of Fig 1). In the latter two cases, linearly spaced filter banks were substituted for the log-spaced filter banks found in biological auditory systems (Fig. 6c). We also included a condition with all three alterations. The altered models were treated identically to the biologically-inspired model, being used both to measure statistics in the original sound signal, and to impose them on synthetic sounds. In all cases, the number of filters was preserved, and thus all models had the same number of statistics.
We again performed an experiment in which listeners judged which of two synthetic sounds (one generated from our biologically-inspired model, the other from one of the non-biological models) more closely resembled the original from which their statistics were measured. In each condition, listeners preferred synthetic sounds produced by the biologically-inspired model (Fig. 6d; sign tests, p<.01 in all conditions), supporting the notion that the auditory system represents textures using statistics similar to those in this model. One might have expected that any large and varied set of statistics would produce signals that resemble the originals, but this is not the case.
Experiment 4: Realism Ratings
To illustrate the overall effectiveness of the synthesis, we measured the realism of synthetic versions of every sound in our set. Listeners were presented with an original recording followed by a synthetic signal matching its statistics. They rated the extent to which the synthetic signal was a realistic example of the original sound, on a scale of 1-7. Most sounds yielded average ratings above 4, suggesting successful synthesis of many types of sounds (Fig. 7a&b; Table S1). The sounds with low ratings, however, are of particular interest, as they are statistically matched to the original recordings and yet do not sound like them. Fig. 7c lists the sounds with average ratings below 2. They fall into three general classes - those involving pitch (railroad crossing, wind chimes, music, speech, bells; see also Fig. S5), rhythm (tapping, music, drum loops), and reverberation (drum beats, firecrackers). This suggests that the perception of these sound attributes involves measurements substantially different from those in our model.
Figure 7.
Experiment 4: Realism ratings. (a) Histogram of average realism ratings for each sound in our set. (b) List of 20 sound textures with high average ratings. Multiple examples of similar sounds are omitted for brevity. (c) List of all sounds with average realism ratings below 2, along with their average rating. See Table S1 for complete list. See also Figure S5.
Discussion
We have studied “sound textures”, a class of sounds produced by multiple superimposed acoustic events, as are common to many natural environments. Sound textures are notable for their temporal homogeneity, and we propose that they are represented with time-averaged statistics. We embody this hypothesis in a model based on statistics (moments and correlations) of a sound decomposition like that found in the subcortical auditory system. To test the role of these statistics in texture recognition, we conducted experiments with synthetic sounds matching the statistics of various real-world textures. We found that 1) such synthetic sounds could be accurately recognized, and at levels far better than if only the spectrum or sparsity was matched; 2) eliminating subsets of the statistics in the model reduced the realism of the synthetic results; 3) modifying the model to less faithfully mimic the mammalian auditory system also reduced the realism of the synthetic sounds; and 4) the synthetic results were often realistic, but failed markedly for a few particular sound classes.
Our results suggest that when listeners recognize the sound of rain, fire, insects and other such sounds, they are recognizing statistics of modest complexity computed from the output of the peripheral auditory system. These statistics are likely measured at downstream stages of neural processing, and thus provide clues to the nature of mid-level auditory computations.
Neural implementation
Because texture statistics are time-averages, their computation can be thought of as involving two steps: a nonlinear function applied to the relevant auditory response(s), and an average of this function over time. A moment, for instance, could be computed by a neuron that averages its input (e.g. a cochlear envelope) after raising it to a power (two for the variance, three for the skew etc.). We found that envelope moments were crucial for producing naturalistic synthetic sounds, indicating that tuning to moments is likely a prominent feature of auditory neural processing. Envelope moments convey sparsity, a quality long known to differentiate natural signals from noise (Field, 1987), and one that is central to many recent signal-processing algorithms (Asari et al., 2006; Bell and Sejnowski, 1996). Our results suggest that sparsity is represented in the auditory system and used to distinguish sounds. Although definitive characterization of the neural locus awaits, neural responses in the midbrain often adapt to particular amplitude distributions (Dean et al., 2005; Kvale and Schreiner, 2004), raising the possibility that envelope moments may be computed subcortically. The modulation power (also a marginal moment) at particular rates also seems to be reflected in the tuning of many thalamic and midbrain neurons (Joris et al., 2004).
The other statistics in our model are correlations. A correlation is the average of a normalized product (e.g. of two cochlear envelopes), and could be computed as such. However, a correlation can also be viewed as the proportion of variance in one variable that is shared by another, which is partly reflected in the variance of the sum of the variables. This formulation provides an alternative implementation (see Methods), and illustrates that correlations in one stage of representation (e.g. bandpass cochlear channels) can be reflected in the marginal statistics of the next (e.g. cortical neurons that sum input from multiple channels), assuming appropriate convergence. All of the texture statistics we have considered could thus reduce to marginal statistics at different stages of the auditory system.
Neuronal tuning to texture statistics could be probed using synthetic stimuli whose statistics are parametrically varied. Stationary artificial sounds have a long history of use in psychoacoustics and neurophysiology, with recent efforts to incorporate naturalistic statistical structure (Attias and Schreiner, 1998; Garcia-Lazaro et al., 2006; McDermott et al., 2011; Overath et al., 2008; Rieke et al., 1995; Singh and Theunissen, 2003). Stimuli synthesized from our model capture naturally occurring sound structure while being precisely characterized within an auditory model. They offer a middle ground between natural sounds and the tones and noises of classical hearing research.
Relation to visual texture
Visual textures, unlike their auditory counterparts, have been studied intensively for decades (Julesz, 1962), and our work was inspired by efforts to understand visual texture using synthesis (Heeger and Bergen, 1995; Portilla and Simoncelli, 2000; Zhu et al., 1997). How similar are visual and auditory texture representations? For ease of comparison, Figure 8 shows a model diagram of the most closely related visual texture model (Portilla and Simoncelli, 2000), analogous in format to our auditory model (Fig. 1) but with input signals and representational stages that vary spatially rather than temporally. The vision model has two stages of linear filtering (corresponding to LGN cells and V1 simple cells) followed by envelope extraction (corresponding to V1 complex cells), whereas the auditory model has the envelope operation sandwiched between linear filtering operations (corresponding to the cochlea and midbrain/thalamus), reflecting structural differences in the two systems. There are also notable differences in the stages at which statistics are computed in the two models: several types of visual texture statistics are computed directly on the initial linear filtering stages, whereas the auditory statistics all follow the envelope operation, reflecting the primary locus of structure in images vs. sounds. However, the statistical computations themselves – marginal moments and correlations – are conceptually similar in the two models. In both systems, relatively simple statistics capture texture structure, suggesting that texture, like filtering (Depireux et al., 2001), filling in (McDermott and Oxenham, 2008; Warren et al., 1972), and saliency (Cusack and Carlyon, 2003; Kayser et al., 2005), may involve analogous computations across modalities.
Figure 8.
Model of visual texture perception (variant of (Portilla and Simoncelli, 2000)) depicted in a format analogous to the auditory texture model of Figure 1. An image of beans (top row) is filtered into spatial frequency bands by center-surround filters (second row), as happens in the retina/LGN. The spatial frequency bands (third row) are filtered again by orientation selective filters (fourth row) analogous to V1 simple cells, yielding scale and orientation filtered bands (fifth row). The envelopes of these bands are extracted (sixth row) to produce analogues of V1 complex cell responses (seventh row). The linear function at the envelope extraction stage indicates the absence of the compressive nonlinearity present in the auditory model. As in Figure 1, red icons denote statistical measurements: marginal moments of a single signal (M) or correlations between two signals (AC, C1, or C2 for autocorrelation, cross-band correlation, or phase-adjusted correlation). C1 and C2 here and in Figure 1 denote conceptually similar statistics. The autocorrelation (AC) is identical to C1 except that it is computed within a channel.
It will be interesting to explore whether the similarities between modalities extend to inattention, to which visual texture is believed to be robust (Julesz, 1962). Under conditions of focused listening, we are often aware of individual events composing a sound texture, presumably in addition to registering time-averaged statistics that characterize the texture qualities. A classic example is the “cocktail party problem”, in which we attend to a single person talking in a room dense with conversations (Bee and Micheyl, 2008; McDermott, 2009). Without attention, individual voices or other sound sources are likely inaccessible, but we may retain access to texture statistics that characterize the combined effect of multiple sources, as is apparently the case in vision (Alvarez and Oliva, 2009). This possibility could be tested in divided attention experiments with synthetic textures.
Texture extensions
We explored the biological representation of sound texture using a set of generic statistics and a relatively simple auditory model, both of which could be augmented in interesting ways. The three sources of information that contributed to the present work – auditory neuroscience, natural sound analysis, and perceptual experiments – all provide directions for such extensions.
The auditory model of Fig. 1, from which statistics are computed, captures neuronal tuning characteristics of subcortical structures. Incorporating cortical tuning properties would likely extend the range of textures we can account for. For instance, cortical receptive fields have spectral tuning that is more complex and varied than that found subcortically (Barbour and Wang, 2003; Depireux et al., 2001), and statistics of filters modelled on their properties could capture higher-order structure that our current model does not. As discussed earlier, the correlations computed on subcortical representations could then potentially be replaced by marginal statistics of filters at a later stage.
It may also be possible to derive additional or alternative texture statistics from an analysis of natural sounds, similar in spirit to previous derivations of cochlear and V1 filters from natural sounds and images (Olshausen and Field, 1996; Smith and Lewicki, 2006), and consistent with other examples of congruence between properties of perceptual systems and natural environments (Attias and Schreiner, 1998; Garcia-Lazaro et al., 2006; Lesica and Grothe, 2008; Rieke et al., 1995; Rodriguez et al., 2010; Schwartz and Simoncelli, 2001; Woolley et al., 2005). We envision searching for statistics that vary maximally across sounds and would thus be optimal for recognition.
The sound classes for which the model failed to produce convincing synthetic examples (revealed by Experiment 4) also provide directions for exploration. Notable failures include textures involving pitched sounds, reverberation, and rhythmic structure (Fig. 7; Table S1; Fig. S5). It was not obvious a priori that these sounds would produce synthesis failures – they each contain spectral and temporal structures that are stationary (given a moderately long time window), and we anticipated that they might be adequately constrained by the model statistics. However, our results show that this is not the case, suggesting that the brain is measuring something that the model is not.
Rhythmic structure might be captured with another stage of envelope extraction and filtering, applied to the modulation bands. Such filters would measure “second-order” modulation of modulation (Lorenzi et al., 2001), as is common in rhythmic sounds. Alternatively, rhythm could involve a measure specifically of periodic modulation patterns. Pitch and reverberation may also implicate dedicated mechanisms. Pitch is largely conveyed by harmonically related frequencies, which are not made explicit by the pair-wise correlations across frequency found in our current model (see also Fig. S5). Accounting for pitch is thus likely to require a measure of local harmonic structure (de Cheveigne, 2004). Reverberation is also well understood from a physical generative standpoint, as linear filtering of a sound source by the environment (Gardner, 1998), and is used to judge source distance (Zahorik, 2002) and environment properties. However, a listener has access only to the result of environmental filtering, not to the source or the filter, implying that reverberation must be reflected in something measured from the sound signal (i.e. a statistic). Our synthesis method provides an unexplored avenue for testing theories of the perception of these sound properties.
One other class of failures involved mixtures of two sounds that overlap in peripheral channels but are acoustically distinct, such as broadband clicks and slow bandpass modulations. These failures likely result because the model statistics are averages over time, and combine measurements that should be segregated. This suggests a more sophisticated form of estimating statistics, in which averaging is performed after (or in alternation with) some sort of clustering operation, a key ingredient in recent models of stream segregation (Elhilali and Shamma, 2008).
Using texture to understand recognition
Recognition is challenging because the sensory input arising from different exemplars of a particular category in the world often varies substantially. Perceptual systems must process their input to obtain representations that are invariant to the variation within categories, while maintaining selectivity between categories (DiCarlo and Cox, 2007). Our texture model incorporates an explicit form of invariance by representing all possible exemplars of a given texture (Fig. S2) with a single set of statistical values. Selectivity across categories is less explicit. In particular, the model does not provide a validated measure of distance between texture classes, and thus cannot make predictions about perceptual discriminability. Moreover, texture properties are captured with a large number of simple statistics that are partially redundant. Humans, in contrast, categorize sounds into semantic classes, and seem to have conscious access to a fairly small set of perceptual dimensions. It should be possible to learn such lower-dimensional representations of categories from our sound statistics, combining the full set of statistics into a small number of “meta-statistics” that relate to perceptual dimensions. We have found, for instance, that most of the variance in statistics over our collection of sounds could be captured with a moderate number of their principal components, indicating that dimensionality reduction is feasible.
The temporal averaging through which our texture statistics achieve invariance is appropriate for stationary sounds, and it is worth considering how this might be relaxed to represent sounds that are less homogeneous. A simple possibility involves replacing the global time-averages with averages taken over a succession of short time windows. The resulting local statistical measures would preserve some of the invariance of the global statistics, but would follow a trajectory over time, allowing representation of the temporal evolution of a signal. By computing measurements averaged within windows of many durations, the auditory system could derive representations with varying degrees of selectivity and invariance, enabling the recognition of sounds spanning a continuum from homogeneous textures to singular events.
Methods
Auditory Model
Our synthesis algorithm utilized a classic “subband” decomposition in which a bank of “cochlear” filters were applied to a sound signal, splitting it into frequency channels. To simplify implementation, we used zero-phase filters, with Fourier amplitude shaped as the positive portion of a cosine function. We used a bank of 30 such filters, with center frequencies equally spaced on an ERBN scale (Glasberg and Moore, 1990), spanning 52-8844 Hz. Their (3-dB) bandwidths were comparable to those of the human ear (~5% larger than ERBs measured at 55 dB SPL; we presented sounds at 70 dB SPL, at which human auditory filters are somewhat wider). The filters did not replicate all aspects of biological auditory filters, but perfectly tiled the frequency spectrum – the summed squared frequency response of the filter bank was constant across frequency (to achieve this, the filter bank also included lowpass and highpass filters at the endpoints of the spectrum). The filter bank thus had the advantage of being invertible: each subband could be filtered again with the corresponding filter, and the results summed to reconstruct the original signal (as is standard in analysis-synthesis subband decompositions (Crochiere et al., 1976)).
The envelope of each subband was computed as the magnitude of its analytic signal, and the subband was divided by the envelope to yield the fine structure. The fine structure was ignored for the purposes of analysis (measuring statistics). Subband envelopes were raised to a power of 0.3 to simulate basilar membrane compression. For computational efficiency, statistics were measured and imposed on envelopes downsampled (following lowpass filtering) to a rate of 400 Hz. Although the envelopes of the high-frequency subbands contained modulations at frequencies above 200 Hz (because cochlear filters are broad at high frequencies), these were generally low in amplitude. In pilot experiments we found that using a higher envelope sampling rate did not produce noticeably better synthetic results, suggesting the high frequency modulations are not of great perceptual significance in this context.
The filters used to measure modulation power also had half-cosine frequency responses, with center frequencies equally spaced on a log scale (20 filters spanning .5 to 200 Hz), and a quality factor of 2 (for 3-dB bandwidths), consistent with those in previous models of human modulation filtering (Dau et al., 1997), and broadly consistent with animal neurophysiology data (Miller et al., 2001; Rodriguez et al., 2010). Although auditory neurons often exhibit a degree of tuning to spectral modulation as well (Depireux et al., 2001; Rodriguez et al., 2010; Schonwiesner and Zatorre, 2009), this is typically less pronounced than their temporal modulation tuning, particularly early in the auditory system (Miller et al., 2001), and we elected not to include it in our model. Because 200 Hz was the Nyquist frequency, the highest frequency filter consisted only of the lower half of the half-cosine frequency response.
We used a smaller set of modulation filters to compute the C1 and C2 correlations, in part because it was desirable to avoid large numbers of unnecessary statistics, and in part because the C2 correlations necessitated octave-spaced filters (see below). These filters also had frequency responses that were half-cosines on a log-scale, but were more broadly tuned (Q √2 ), with center frequencies in octave steps from 1.5625 to 100 Hz, yielding seven filters.
Boundary Handling
All filtering was performed in the discrete frequency domain, and thus assumed circular boundary conditions. To avoid boundary artifacts, the statistics measured in original recordings were computed as weighted time-averages. The weighting window fell from one to zero (half cycle of a raised cosine) over the one-second intervals at the beginning and end of the signal (typically a 7 sec segment), minimizing artifactual interactions. For the synthesis process, statistics were imposed with a uniform window, so that they would influence the entire signal. As a result, continuity was imposed between the beginning and end of the signal. This was not obvious from listening to the signal once, but it enabled synthesized signals to be played in a continuous loop without discontinuities.
Statistics
We denote the kth cochlear subband envelope by sk(t), and the windowing function by w(t), with the constraint that . The nth modulation band of cochlear envelope sk is denoted by bk,n(t), computed via convolution with filter fnn.
Cochlear Marginal Statistics
Our texture representation includes the first four normalized moments of the envelope:
The variance was normalized by the squared mean, so as to make it dimensionless like the skew and kurtosis.
The envelope variance, skew and kurtosis capture subband sparsity. Sparsity is often associated with the kurtosis of a subband (Field, 1987), and preliminary versions of our model were also based on this measurement (McDermott et al., 2009). However, the envelope's importance in hearing made its moments a more sensible choice, and we found them to capture similar sparsity behaviour.
Figure 2d-g shows the marginal moments for each cochlear envelope of each sound in our ensemble. All four statistics vary considerably across natural sound textures. Their values for noise are also informative. The envelope means, which provide a coarse measure of the power spectrum, do not have exceptional values for noise, lying in the middle of the set of natural sounds. However, the remaining envelope moments for noise all lie near the lower bound of the values obtained for natural textures, indicating that natural sounds tend to be sparser than noise (see also Expmt. 2b).
Cochlear Cross-Band Envelope Correlation
j, k ∈ [1...32] such that (k – j) ∈ [1,2,3,5,8,11,16,21] Our model included the correlation of each cochlear subband envelope with a subset of eight of its neighbors, a number that was typically sufficient to reproduce the qualitative form of the full correlation matrix (interactions between overlapping subsets of filters allow the correlations to propagate across subbands). This was also perceptually sufficient: we found informally that imposing fewer correlations sometimes produced perceptually weaker synthetic examples, and that incorporating additional correlations did not noticeably improve the results.
Figure 3b shows the cochlear correlations for recordings of fire, applause and a stream. The broadband events present in fire and applause, visible as vertical streaks in the spectrograms of Fig. 4b, produce substantial correlations between the envelopes of different cochlear subbands. Cross-band correlation, or “co-modulation”, is common in natural sounds (Nelken et al., 1999), and we found it to be to be a major source of variation among sound textures. The stream, for instance, contains much weaker co-modulation.
The mathematical form of the correlation does not uniquely specify the neural instantiation. It could be computed directly, by averaging a product as in the above equation. Alternatively, it could be computed with squared sums and differences, as are common in functional models of neural computation (Adelson and Bergen, 1985):
Modulation Power
For the modulation bands, the variance (power) was the principal marginal moment of interest. Collectively, these variances indicate the frequencies present in an envelope. Analogous quantities appear to be represented by the modulation-tuned neurons common to the early auditory system (whose responses code the power in their modulation passband). To make the modulation power statistics independent of the cochlear statistics, we normalized each by the variance of the corresponding cochlear envelope; the measured statistics thus represent the proportion of total envelope power captured by each modulation band:
k ∈ [1...32], n ∈ [1...20]
Note that the mean of the modulation bands is zero (since the filters fn are zero-mean). The other moments of the modulation bands were either uninformative or redundant (see SI), and were omitted from the model.
The modulation power implicitly captures envelope correlations across time, and is thus complementary to the cross-band correlations. Figure 3s shows the modulation power statistics for recordings of swamp insects, lakeshore waves and a stream.
Modulation Correlations
These correlations were computed using octave-spaced modulation filters (necessitated by the C2 correlations), the resulting bands of which are denoted by
The C1 correlation is computed between bands centered on the same modulation frequency but different acoustic frequencies:
j ∈ [1...32], (k – j) ∈ [1,2], n ∈ [2...7], and
We imposed correlations between each modulation filter and its two nearest neighbors along the cochlear axis, for six modulation bands spanning 3-100 Hz.
C1 correlations are shown in Fig. 3c for the sounds of waves and fire. The qualitative pattern of C1 correlations shown for waves is typical of a number of sounds in our set (e.g. wind). These sounds exhibit low-frequency modulations that are highly correlated across cochlear channels, but high-frequency modulations that are largely independent. This effect is not simply due to the absence of high-frequency modulation, as most such sounds had substantial power at high modulation frequencies (comparable to that in pink noise, evident from dB values close to zero in Fig. 3a). In contrast, for fire (and many other sounds), both high and low frequency modulations exhibit correlations across cochlear channels. Imposing the C1 correlations was essential to synthesizing realistic waves and wind, among other sounds. Without them, the cochlear correlations affected both high and low modulation frequencies equally, resulting in artificial sounding results for these sounds.
C1 correlations did not subsume cochlear correlations. Even when larger numbers of C1 correlations were imposed (i.e., across more offsets), we found informally that the cochlear correlations were necessary for high quality synthesis.
The second type of correlation, labeled C2, is computed between bands of different modulation frequencies derived from the same acoustic frequency channel. This correlation represents phase relations between modulation frequencies, important for representing abrupt onsets and other temporal asymmetries. Temporal asymmetry is common in natural sounds, but is not captured by conventional measures of temporal structure (e.g. the modulation spectrum), as they are invariant to time reversal (Irino and Patterson, 1996). Intuitively, an abrupt increase in amplitude (e.g., a step edge) is generated by a sum of sinusoidal envelope components (at different modulation frequencies) that are aligned at the beginning of their cycles (phase –π/2), whereas an abrupt decrease is generated by sinusoids that align at the cycle mid-point (phase π/2), and an impulse (e.g. a click) has frequency components that align at their peaks (phase 0). For sounds dominated by one of these feature types, adjacent modulation bands thus have consistent relative phase in places where their amplitudes are high. We captured this relationship with a complex-valued correlation measure (Portilla and Simoncelli, 2000).
We first define analytic extensions of the modulation bands:
The analytic signal comprises the responses of the filter and its quadrature twin, and is thus readily instantiated biologically (Adelson and Bergen, 1985). The correlation has the standard form, except it is computed between analytic modulation bands tuned to modulation frequencies an octave apart, with the frequency of the lower band doubled:
yielding
k ∈ [1...32], m ∈ [1...6], and (n – m) = 1, where * and ∥ ∥ denote the complex conjugate and modulus, respectively.
If the bands result from octave-spaced filters, the frequency doubling causes them to oscillate at the same rate, producing a consistent phase difference between adjacent bands in regions of large amplitude. Frequency doubling is essential because the frequency adjustment is achieved by raising the complex-valued analytic signal to a power, and this operation is only defined for integer powers, precluding the use of a smaller ratio. See Fig. S6 for further explanation.
C2k,mn is complex-valued, and the real and imaginary parts must be independently measured and imposed. Example sounds with onsets, offsets, and impulses are shown in Fig. 3d along with their C2 correlations.
All told, there are 128 cochlear marginal statistics, 189 cochlear cross-correlations, 640 modulation band variances, 366 C1 correlations, and 192 C2 correlations, for a total of 1515 statistics.
Imposition Algorithm
Synthesis was driven by a set of statistics measured for a sound signal of interest using the auditory model described above. The synthetic signal was initialized with a sample of Gaussian white noise, and was modified with an iterative process until it shared the measured statistics. Each cycle of the iterative process, as illustrated in Fig. 4a, consisted of the following steps:
The synthetic sound signal is decomposed into cochlear subbands.
Subband envelopes are computed using the Hilbert transform.
Envelopes are divided out of the subbands to yield the subband fine structure.
Envelopes are downsampled to reduce computation.
Envelope statistics are measured and compared to those of the original recording to generate an error signal.
Downsampled envelopes are modified using a variant of gradient descent, causing their statistics to move closer to those measured in the original recording.
Modified envelopes are upsampled and recombined with the unmodified fine structure to yield new subbands.
New subbands are combined to yield a new signal.
We performed conjugate gradient descent using Carl Rasmussen's “minimize” Matlab function (available online). The objective function was the total squared error between the synthetic signal's statistics and those of the original signal. The subband envelopes were modified one-byone, beginning with the subband with largest power, and working outwards from that. Correlations between pairs of subband envelopes were imposed when the second subband envelope contributing to the correlation was being adjusted.
Each episode of gradient descent resulted in modified subband envelopes that approached the target statistics. However, there was no constraint forcing the envelope adjustment to remain consistent with the subband fine structure (Ghitza, 2001), or to produce new subbands that were mutually consistent (in the sense that combining them would produce a signal that would yield the same subbands when decomposed again). It was thus generally the case that during the first few iterations, the envelopes measured at the beginning of cycle n+1 did not completely retain the adjustment imposed at cycle n, because combining envelope and fine structure, and summing up the subbands, tended to change the envelopes in ways that altered their statistics. However, we found that with iteration, the envelopes generally converged to a state with the desired statistics. The fine structure was not directly constrained, and relaxed to a state consistent with the envelope constraints.
Convergence was monitored by computing the error in each statistic at the start of each iteration, and measuring the SNR as the ratio of the squared error of a statistic class, summed across all statistics in the class, to the sum of the squared statistic values of that class. The procedure was halted once all classes of statistics were imposed with an SNR of 30 dB or higher, or when 60 iterations were reached. The procedure was considered to have converged if the average SNR of all statistic classes was 20 dB or higher. Occasionally the synthesis process failed to produce a signal matching the statistics of an original sound according to this criterion, but this was relatively rare. Such failures of convergence were not used in experiments.
Although the statistics in our model constrain the distribution of the sound signal, we have no explicit probabilistic formulation, and as such are not guaranteed to be drawing samples from an explicit distribution. Instead, we qualitatively mimic the effect of sampling by initializing the synthesis with different samples of noise (as in some visual texture synthesis methods; (Heeger and Bergen, 1995; Portilla and Simoncelli, 2000)). An explicit probabilistic model could be developed via maximum entropy formulations (Zhu et al., 1997), but sampling from such a distribution is generally computationally prohibitive.
Supplementary Material
Acknowledgments
We thank Dan Ellis for helpful discussions, and Mark Bee, Mike Landy, Gary Marcus, and Sam Norman-Haignere for comments on drafts of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adelson EH, Bergen JR. Spatiotemporal models for the perception of motion. Journal of the Optical Society of America A. 1985;2:284–299. doi: 10.1364/josaa.2.000284. [DOI] [PubMed] [Google Scholar]
- Alvarez G, Oliva A. Spatial ensemble statistics are efficient codes that can be represented with reduced attention. Proceedings of the National Academy of Sciences. 2009;106:7345–7350. doi: 10.1073/pnas.0808981106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asari H, Pearlmutter BA, Zador AM. Sparse representations for the cocktail party problem. Journal of Neuroscience. 2006;26:7477–7490. doi: 10.1523/JNEUROSCI.1563-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athineos M, Ellis D. ICASSP-03. Hong Kong; 2003. Sound texture modelling with linear prediction in both time and frequency domains. pp. 648–651. [Google Scholar]
- Attias H, Schreiner CE. Coding of naturalistic stimuli by auditory midbrain neurons. In: Jordan MI, Kearns MJ, Solla SA, editors. Advances in Neural Information Processing Systems. MIT Press; Cambridge, MA: 1998. pp. 103–109. [Google Scholar]
- Bacon SP, Grantham DW. Modulation masking: Effects of modulation frequency, depth, and phase. Journal of the Acoustical Society of America. 1989;85:2575–2580. doi: 10.1121/1.397751. [DOI] [PubMed] [Google Scholar]
- Barbour DL, Wang X. Contrast tuning in auditory cortex. Science. 2003;299:1073–1075. doi: 10.1126/science.1080425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann S, Griffiths TD, Sun L, Petkov CI, Thiele A, Rees A. Orthogonal representation of sound dimensions in the primate midbrain. Nature Neuroscience. 2011;14:423–425. doi: 10.1038/nn.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee MA, Micheyl C. The cocktail party problem: what is it? How can it be solved? And why should animal behaviorists study it? J Comp Psychol. 2008;122:235–251. doi: 10.1037/0735-7036.122.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. Learning the higher-order structure of a natural sound. Network: Computation in Neural Systems. 1996;7:261–266. doi: 10.1088/0954-898X/7/2/005. [DOI] [PubMed] [Google Scholar]
- Crochiere RE, Webber SA, Flanagan JL. Digital coding of speech in sub-bands. The Bell System Technical Journal. 1976;55:1069–1085. [Google Scholar]
- Cusack R, Carlyon RP. Perceptual asymmetries in audition. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:713–725. doi: 10.1037/0096-1523.29.3.713. [DOI] [PubMed] [Google Scholar]
- Dau T, Kollmeier B, Kohlrausch A. Modeling auditory processing of amplitude modulation. I. Detection and masking with narrow-band carriers. Journal of the Acoustical Society of America. 1997;102:2892–2905. doi: 10.1121/1.420344. [DOI] [PubMed] [Google Scholar]
- de Cheveigne A. In: Pitch perception models. Pitch CJ, Plack, Oxenham AJ, editors. Springer Verlag; New York: 2004. [Google Scholar]
- Dean I, Harper NS, McAlpine D. Neural population coding of sound level adapts to stimulus statistics. Nature Neuroscience. 2005;8:1684–1689. doi: 10.1038/nn1541. [DOI] [PubMed] [Google Scholar]
- Depireux DA, Simon JZ, Klein DJ, Shamma SA. Spectro-temporal response field characterization with dynamic ripples in ferret primary auditory cortex. Journal of Neurophysiology. 2001;85:1220–1234. doi: 10.1152/jn.2001.85.3.1220. [DOI] [PubMed] [Google Scholar]
- DiCarlo JJ, Cox DD. Untangling invariant object recognition. Trends in Cognitive Sciences. 2007;11:333–341. doi: 10.1016/j.tics.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Dubnov S, Bar-Joseph Z, El-Yaniv R, Lischinski D, Werman M. Synthesizing sound textures through wavelet tree learning. IEEE Computer Graphics and Applications. 2002;22:38–48. [Google Scholar]
- Elhilali M, Shamma SA. A cocktail party with a cortical twist: How cortical mechanisms contribute to sound segregation. Journal of the Acoustical Society of America. 2008;124:3751–3771. doi: 10.1121/1.3001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field DJ. Relations between the statistics of natural images and the response profiles of cortical cells. Journal of the Optical Society of America A. 1987;4 doi: 10.1364/josaa.4.002379. [DOI] [PubMed] [Google Scholar]
- Garcia-Lazaro JA, Ahmed B, Schnupp JW. Tuning to natural stimulus dynamics in primary auditory cortex. Current Biology. 2006;16:264–271. doi: 10.1016/j.cub.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Gardner WG. Reverberation algorithms. In: Kahrs M, Brandenburg K, editors. Applications of Digital Signal Processing to Audio and Acoustics. Kluwer Academic Publishers; Norwell, MA: 1998. [Google Scholar]
- Ghitza O. On the upper cutoff frequency of the auditory critical-band envelope detectors in the context of speech perception. Journal of the Acoustical Society of America. 2001;110:1628–1640. doi: 10.1121/1.1396325. [DOI] [PubMed] [Google Scholar]
- Glasberg BR, Moore BCJ. Derivation of auditory filter shapes from notched-noise data. Hearing Research. 1990;47:103–138. doi: 10.1016/0378-5955(90)90170-t. [DOI] [PubMed] [Google Scholar]
- Gygi B, Kidd GR, Watson CS. Spectral-temporal factors in the identification of environmental sounds. Journal of the Acoustical Society of America. 2004;115:1252–1265. doi: 10.1121/1.1635840. [DOI] [PubMed] [Google Scholar]
- Heeger DJ, Bergen J. Pyramid-based texture analysis/synthesis. Computer Graphics (ACM SIGGRAPH Proceedings) 1995:229–238. [Google Scholar]
- Irino T, Patterson RD. Temporal asymmetry in the auditory system. Journal of the Acoustical Society of America. 1996;99:2316–2331. doi: 10.1121/1.415419. [DOI] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiological Review. 2004;84:541–577. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Julesz B. Visual pattern discrimination. IRE Transactions on Information Theory. 1962;8:84–92. [Google Scholar]
- Kayser C, Petkov CI, Lippert M, Logothetis NK. Mechanisms for allocating auditory attention: An auditory saliency map. Current Biology. 2005;15:1943–1947. doi: 10.1016/j.cub.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Kvale M, Schreiner CE. Short-term adaptation of auditory receptive fields to dynamic stimuli. Journal of Neurophysiology. 2004;91:604–612. doi: 10.1152/jn.00484.2003. [DOI] [PubMed] [Google Scholar]
- Lesica NA, Grothe B. Efficient temporal processing of naturalistic sounds. PLoS One. 2008;3:e1655. doi: 10.1371/journal.pone.0001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi C, Berthommier F, Demany L. Discrimination of amplitude-modulation phase spectrum. Journal of the Acoustical Society of America. 1999;105:2987–2990. doi: 10.1121/1.426911. [DOI] [PubMed] [Google Scholar]
- Lorenzi C, Simpson MIG, Millman RE, Griffiths TD, Woods WP, Rees A, Green GGR. Second-order modulation detection thresholds for pure-tone and narrow-band noise carriers. Journal of the Acoustical Society of America. 2001;110:2470–2478. doi: 10.1121/1.1406160. [DOI] [PubMed] [Google Scholar]
- McDermott JH. The cocktail party problem. Current Biology. 2009;19:R1024–R1027. doi: 10.1016/j.cub.2009.09.005. [DOI] [PubMed] [Google Scholar]
- McDermott JH, Oxenham AJ. Spectral completion of partially masked sounds. Proceedings of the National Academy of Sciences. 2008;105:5939–5944. doi: 10.1073/pnas.0711291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JH, Oxenham AJ, Simoncelli EP. IEEE Workshop on Applications of Signal Processing to Audio and Acoustics. New Paltz, New York: 2009. Sound texture synthesis via filter statistics. pp. 297–300. [Google Scholar]
- McDermott JH, Wrobleski D, Oxenham AJ. Recovering sound sources from embedded repetition. Proceedings of the National Academy of Sciences. 2011;108:1188–1193. doi: 10.1073/pnas.1004765108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesgarani N, David SV, Fritz JB, Shamma SA. Influence of context and behavior on stimulus reconstruction from neural activity in primary auditory cortex. Journal of Neurophysiology. 2009;102:3329–3339. doi: 10.1152/jn.91128.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LM, Escabi MA, Read HL, Schreiner CE. Spectrotemporal receptive fields in the lemniscal auditory thalamus and cortex. Journal of Neurophysiology. 2001;87:516–527. doi: 10.1152/jn.00395.2001. [DOI] [PubMed] [Google Scholar]
- Nelken I, Rotman Y, Bar Yosef O. Responses of auditory-cortex neurons to structural features of natural sounds. Nature. 1999;397:154–157. doi: 10.1038/16456. [DOI] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Emergence of simple-cell receptive field properties by learning a sparse code for natural images. Nature. 1996;381:607–609. doi: 10.1038/381607a0. [DOI] [PubMed] [Google Scholar]
- Overath T, Kumar S, von Kriegstein K, Griffiths TD. Encoding of spectral correlation over time in auditory cortex. Journal of Neuroscience. 2008;28:13268–13273. doi: 10.1523/JNEUROSCI.4596-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portilla J, Simoncelli EP. A parametric texture model based on joint statistics of complex wavelet coefficients. International Journal of Computer Vision. 2000;40:49–71. [Google Scholar]
- Rieke F, Bodnar DA, Bialek W. Naturalistic stimuli increase the rate and efficiency of information transmission by primary auditory afferents. Proceedings of the Royal Society B. 1995;262:259–265. doi: 10.1098/rspb.1995.0204. [DOI] [PubMed] [Google Scholar]
- Rodriguez FA, Chen C, Read HL, Escabi MA. Neural modulation tuning characteristics scale to efficiently encode natural sound statistics. Journal of Neuroscience. 2010;30:15969–15980. doi: 10.1523/JNEUROSCI.0966-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero MA. Responses to sound of the basilar membrane of the mammalian cochlea. Current Opinion in Neurobiology. 1992;2:449–456. doi: 10.1016/0959-4388(92)90179-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Arnaud N, Popat K. Analysis and synthesis of sound texture. AJCAI workshop on Computational Auditory Scene Analysis. 1995:293–308. [Google Scholar]
- Schonwiesner M, Zatorre RJ. Spectro-temporal modulation transfer function of single voxels in the human auditory cortex measured with high-resolution fMRI. Proceedings of the National Academy of Sciences. 2009;106:14611–14616. doi: 10.1073/pnas.0907682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Simoncelli EP. Natural signal statistics and sensory gain control. Nature Neuroscience. 2001;4:819–825. doi: 10.1038/90526. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Singh NC, Theunissen FE. Modulation spectra of natural sounds and ethological theories of auditory processing. Journal of the Acoustical Society of America. 2003;114:3394–3411. doi: 10.1121/1.1624067. [DOI] [PubMed] [Google Scholar]
- Slaney M. Pattern playback in the 90's. In: Tesauro G, Touretsky D, Leen T, editors. Advances in Neural Information Processing Systems 7. MIT Press; Cambridge, MA: 1995. [Google Scholar]
- Smith EC, Lewicki MS. Efficient auditory coding. Nature. 2006;439:978–982. doi: 10.1038/nature04485. [DOI] [PubMed] [Google Scholar]
- Smith ZM, Delgutte B, Oxenham AJ. Chimaeric sounds reveal dichotomies in auditory perception. Nature. 2002;416:87–90. doi: 10.1038/416087a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland EA, Viemeister NF. Cues for discrimination of envelopes. Journal of the Acoustical Society of America. 1996;99:3638–3646. doi: 10.1121/1.414962. [DOI] [PubMed] [Google Scholar]
- Verron C, Pallone G, Aramaki M, Kronland-Martinet R. IEEE Workshop on Applications of Signal Processing to Audio and Acoustics. New Paltz, NY: 2009. Controlling a spatialized environmental sound synthesizer. pp. 321–324. [Google Scholar]
- Warren RM, Obusek CJ, Ackroff JM. Auditory induction: perceptual synthesis of absent sounds. Science. 1972;176:1149–1151. doi: 10.1126/science.176.4039.1149. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Fremouw TE, Hsu A, Theunissen FE. Tuning for spectro-temporal modulations as a mechanism for auditory discrimination of natural sounds. Nat Neurosci. 2005;8:1371–1379. doi: 10.1038/nn1536. [DOI] [PubMed] [Google Scholar]
- Zahorik P. Assessing auditory distance perception using virtual acoustics. Journal of the Acoustical Society of America. 2002;111:1832–1846. doi: 10.1121/1.1458027. [DOI] [PubMed] [Google Scholar]
- Zhu SC, Wu YN, Mumford DB. Minimax entropy principle and its applications to texture modeling. Neural Computation. 1997;9:1627–1660. [Google Scholar]
- Zhu XL, Wyse L. Conference on Digital Audio Effects. Naples, Italy: 2004. Sound texture modeling and time-frequency LPC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.