Abstract
Purpose
Whether and how to return individual genetic results to study participants is among the most contentious policy issues in contemporary genomic research.
Methods
We surveyed corresponding authors of genome-wide association studies (GWAS), identified through the National Human Genome Research Institute's Catalog of Published GWAS, to describe the experiences and attitudes of these stakeholders.
Results
Of 357 corresponding authors, 200 (56%) responded. One hundred twenty-six (63%) had been responsible for primary data and sample collection, whereas 74 (37%) had performed secondary analyses. Only 7 (4%) had returned individual results within their index GWAS. Most (69%) believed that return of results to individual participants was warranted under at least some circumstances. Most respondents identified a desire to benefit participants's health (63%) and respect for participants's; desires for information (57%) as major motivations for returning results. Most also identified uncertain clinical utility (76%), the possibility that participants will misunderstand results (74%), the potential for emotional harm (61%), the need to ensure access to trained clinicians (59%), and the potential for loss of confidentiality (51%) as major barriers to return.
Conclusion
Investigators have limited experience returning individual results from genome-scale research, yet most are motivated to do so in at least some circumstances.
Introduction
Through genome-wide association studies (GWAS), investigators have identified thousands of single nucleotide polymorphisms (SNPs) associated with a variety of diseases.1 Although most of these variants have modest relationship to disease risk, a subset of SNPs correspond to available clinical tests.2 In this context, a discussion has emerged about the ethics of returning individual genetic results to research participants.3-6 While participant preferences are not the only factor that should determine policies, data suggest that most participants desire results.7-9 Furthermore, guidelines support the return of non-genetic incidental findings, such as those encountered in neuroimaging research, at least when the findings have immediate clinical significance.10-12 Finally, although some commentators disagree13-15, guidelines recommend that a limited set of individual genetic results with clear clinical validity and utility should be returned to participants who have indicated during the consent process that they desire to receive such results.4,16,17
Genome investigators are critical stakeholders in this debate. However, evidence about their attitudes and practices is limited.18,19 We therefore surveyed corresponding authors of published GWAS in order to describe their perspectives and experiences with returning individual genetic research results.
Materials and Methods
Survey Instrument
We reviewed published work on the return of individual genetic results to research participants to develop a draft questionnaire. The draft survey consisted of four sections: demographics and work characteristics, study characteristics and roles, study practices, and views. When providing information about study characteristics, roles and practices, respondents were directed to consider the particular study on the basis of which they were identified for this survey. The survey was revised following face validity assessment by two genetic/genomic epidemiology researchers who were not eligible for participation in the study.
Study Population, Recruitment, and Survey Administration
Eligible investigators were identified through the National Human Genome Research Institute's (NHGRI) Catalog of Published GWAS (accessed April 15, 2010).20 When an individual was corresponding author on more than one study, he or she was contacted with reference to the most recent publication. Data collection occurred between October 2010 and February 2011.
Investigators were contacted up to five times to participate in the survey. An initial and two follow up invitations were sent via publicly available email addresses with a link to the online survey. The online survey was administered using a web-based survey self-administration system (Illume, DatStat Inc., Seattle, WA). The penultimate contact included a letter and paper version of the survey sent via US Postal Service or Federal Express to nonrespondents to the follow up emails. The final contact to nonresponders was via email with a link to the online survey.
The study was approved by the Institutional Review Boards (IRBs) at the Dana-Farber Cancer Institute and the Baylor College of Medicine.
Statistical Analysis
Stata 10 (Stata Corp., College Station, Texas) was used to perform appropriate descriptive statistical analyses and hypothesis tests. Chi-square tests were used to evaluate associations between categorical variables. Mann-Whitney U tests were used to assess the relationship between independent variables and ordinal outcome variables, such as the degree to which respondents perceived various factors as barriers to or motivations for return of results. In all analyses, statistical significance was declared if 2-sided p values were <0.05.
Results
Characteristics of respondents and of index GWAS
Of 360 unique corresponding authors eligible to take part in the survey, contact information was unavailable for three. Of the remaining 357, 200 responded (response rate 56%; 191 responses were complete). Respondents' demographic and professional characteristics are shown in Table 1; most were male and self-identified as white.
Table 1. Characteristics of respondents.
| Characteristic (n=200*) | Number (Percent) |
|---|---|
| Age, mean (standard deviation, range) | 47.5 (8.7, 26-74) |
| Female | 58 (29%) |
| Race | |
| Asian | 36 (18%) |
| Black or African American | 1 (1%) |
| White | 152 (77%) |
| Other | 9 (5%) |
| Hispanic or Latino/a | 5 (3%) |
| Prior service on an institutional review board or equivalent | 59 (30%) |
| Experience interacting directly with human subjects | 132 (66%) |
| Work setting | |
| University or academic medical center | 164 (83%) |
| Pharmaceutical or biotechnology | 13 (7%) |
| Government | 13 (7%) |
| Other | 8 (4%) |
| Academic rank among those in academic settings | |
| Professor | 88 (54%) |
| Associate professor | 33 (20%) |
| Assistant professor | 27 (16%) |
| Other | 16 (10%) |
| Degree† | |
| MD | 98 (49%) |
| PhD | 140 (70%) |
| MPH | 9 (5%) |
| Other | 8 (4%) |
| Location of employment | |
| United States | 95 (48%) |
| Canada | 6 (3%) |
| Europe | 71 (36%) |
| Asia | 22 (11%) |
| Other | 5 (3%) |
| Role in study† | |
| Overall study design | 184 (92%) |
| Participant recruitment or consent | 47 (24%) |
| Laboratory analysis | 67 (34%) |
| Collection of phenotypic information | 68 (34%) |
| Bioinformatic or statistical analysis | 128 (64%) |
| Other | 22 (11%) |
Denominators may not equal 200 due to missing data for individual questions
Total may exceed 100%, as respondents could choose more than one category
Among responding investigators, 62 (31%) had published their GWAS in Nature Genetics, 12 (6%) in the American Journal of Human Genetics, and 11 (5.5%) in PLoS Genetics. No other journal accounted for more than 5% of responses.
Respondents did not differ from non-respondents by journal in which the index GWAS was published or by region of origin; other characteristics were unavailable for non-respondents.
The characteristics of the index GWAS on the basis of which investigators were selected are shown in Table 2. Almost two-thirds of respondents reported that their study included primary collection of data and specimens, whereas 37% reported conducting secondary analyses using data and specimens obtained from another investigator or from a repository. Among those conducting secondary analyses, most had signed a data use agreement with the original collectors or repository, and most agreements forbade efforts at re-identifying participants. Regardless of whether the studies involved primary data and specimen collection or secondary analyses, most maintained links between the data and specimens and the identities of the individual participants using a code.
Table 2. Characteristics and return-of-results practices of index studies.
| Number (Percent) | ||
|---|---|---|
| Nature of data acquisition | ||
| Primary data/specimen collection | 126 (63%) | |
| Secondary data/specimen analysis | 74 (37%) | |
|
| ||
| Primary Collection | Secondary Use | |
|
| ||
| Mode of acquisition of data or specimens* | NA | |
| From those responsible for primary data collection | 57 (77%) | |
| From a public repository | 5 (7%) | |
| Other | 12 (16%) | |
| Signed data use agreement† | NA | |
| Yes | 49 (67%) | |
| No | 14 (19%) | |
| Unsure | 10 (14%) | |
| If present, data use agreement forbade participant re-identification‡ | NA | |
| Yes | 29 (60%) | |
| No | 9 (19%) | |
| Unsure | 10 (21%) | |
| Method to link data/specimens to individual identifiers§ | ||
| Directly labeled with identifiers | 3 (2%) | 0 (0%) |
| Linked to identifiers with code | 109 (87%) | 30 (67%) |
| No link to identifiers | 9 (7%) | 12 (27%) |
| Other | 3 (2%) | 3 (7%) |
| Unsure | 2 (2%) | 0 (0%) |
| Study has previously returned individual genetic test results to participant(s)** | ||
| Yes | 7 (6%) | 0 (0%) |
| No | 107 (91%) | 32 (97%) |
| Unsure | 3 (3%) | 1 (3%) |
| Study has plans to return individual genetic test results to participant(s) ††,‡‡ | ||
| Yes | 2 (2%) | 0 (0%) |
| No | 103 (94%) | 33 (100%) |
| Unsure | 5 (5%) | 0 (0%) |
NA, Not applicable
Secondary analysis, n=74
Secondary analysis, n=73
Secondary analysis, n=48
Primary analysis, n=126; secondary analysis, n=45
Primary analysis, n=117, secondary analysis, n=33
Primary analysis, n=110, secondary analysis, n=33
Among studies that had not previously returned results to individual participants
Only seven respondents (4%) indicated that they had returned results to individual participants in the context of their index GWAS. Of the remaining respondents, only two (1%) had plans to do so. Of the nine investigators who had returned results or had plans to do so, all had been responsible for primary collection of data and samples; none had performed secondary analyses on data or samples collected by others. Most who had returned results (N=5) did so directly to participants or their proxies, rather than through the participant's physician.
Investigators' attitudes regarding return of results
Most respondents (n=134, 69%) believed that the return of research results to individual GWAS participants was warranted under at least some circumstances. The belief that return of individual research results is warranted in at least some circumstances was generally unassociated with demographic and professional characteristics. There were no significant associations between believing that individual genetic results should be returned in at least some circumstances and holding an MD degree (p=0.48), having served on an IRB (p=0.84), having interacted directly with participants (p=0.31), or having been responsible for primary data or specimen collection (p=0.79).
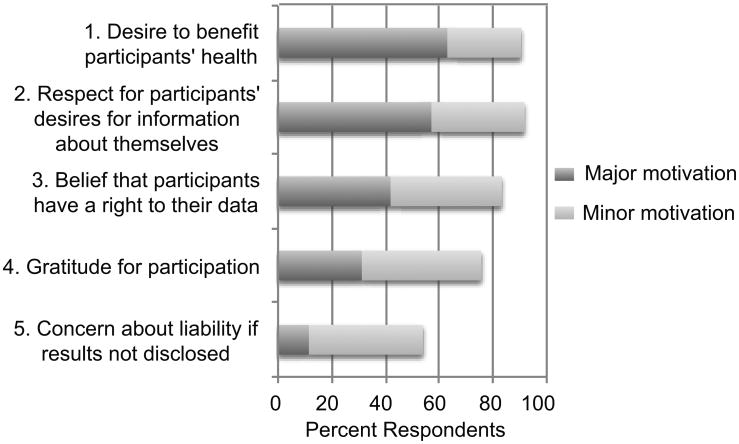
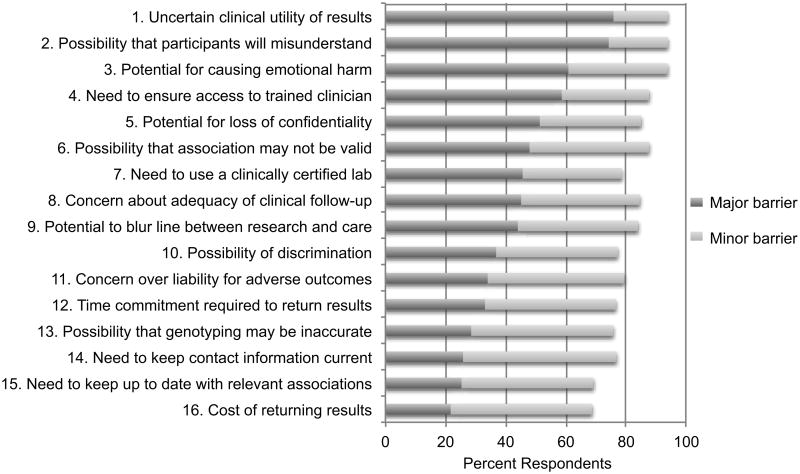
Respondents endorsed several factors as major motivations to return individual genetic research results (Figure 1). Most identified a desire to benefit participants' health (63%) and respect for participants' desire to have information about themselves (57%) as major motivations for returning individual results. Many (42%) also identified the belief that participants have a right to their data as a major motivation. However, respondents also reported a wide range of barriers to returning results (Figure 2). Most viewed the uncertain clinical utility of genetic research results (76%), the possibility that participants will misunderstand the information they are given (74%), the potential for emotional harm to participants (61%), the need to ensure access to an appropriately trained clinician (59%), and the potential for loss of confidentiality (51%) as major barriers to the return of results.
Figure 1. Motivations for the return of individual genetic research results among GWAS investigators.
Figure 2. Barriers to the return of individual genetic research results among GWAS investigators.
We sought to understand what attitudes and concerns distinguished investigators who believed that individual genetic results should be returned in at least some circumstances from those who believed they should generally not be returned. Investigators who believed that results should generally not be returned viewed the potential for loss of patient confidentiality (p=0.004), the possibility that participants will misunderstand the information that they are given (p=0.009), the potential for causing emotional harm to participants (p=0.011), and the potential to blur the boundary between research and clinical care (p=0.002) as greater barriers than those who believed that results should be returned under at least some circumstances. We also noted numerous differences between these two groups of investigators with respect to the motivations that they endorsed. Investigators who believed that results should be returned in some circumstances were more likely than those who did not share this belief to endorse the desire to benefit participants' health (p=0.003), respect for participants' desires to have information about themselves (p=0.002), the view that participants have a right to their data (p=0.0002), concern over legal liability if individual results are not disclosed (p=0.016), and gratitude to participants for taking part in the study (p=0.0008) as motivations for offering to return results.
Investigators who had been responsible for primary collection of data and samples and those who had performed secondary analyses of data or samples collected by others endorsed different barriers to and motivations for returning results. Those who had performed secondary data analyses perceived the time commitment required to return results (p=0.029), the need to keep contact information current (p=0.054), and the need to use a clinically certified lab (p=0.007) as greater barriers to the return of individual results. Those responsible for primary data collection, in contrast, viewed the desire to benefit participants' health as a greater motivation to return results (p=0.008).
Discussion
We surveyed corresponding authors of published GWAS to clarify their experiences with and attitudes towards return of individual results and incidental findings from genome-scale studies. Few respondents reported experience with returning individual results, at least within their index GWAS. This observation suggests that investigators may be unprepared for the challenges posed by whole-exome and whole-genome sequencing studies, which will identify clinically relevant incidental findings more frequently than do GWAS.21-23 In qualitative interviews, however, we observed that many genome investigators report having returned results identified through other study designs, including linkage and family studies.24 Although linkage and family designs differ in numerous ways from sequencing-based approaches, this experience may be helpful as investigators consider whether and how to return incidental findings uncovered by sequencing research.
Not with standing their lack of personal experience returning results from genome-scale studies, most respondents reported believing that return of individual genetic research results may be warranted under at least some circumstances. Major motivations cited by respondents for returning results included a desire to benefit participants' health and respect for participants' desires for information about themselves. Major barriers included the uncertain clinical utility of results, the possibility that participants might misunderstand information, the potential for emotional harm, the need to ensure access to appropriately trained clinicians, and the potential for loss of confidentiality.
Respondents who believed that individual results should generally not be offered to research participants differed from those who believed that results should at least sometimes be offered with respect to the motivations and barriers that they cited. In particular, they were more likely to identify beneficence-based concerns, such as loss of confidentiality, the possibility that participants would misunderstand the information, and the potential for emotional harm. Notably, respondents who believed that results should generally not be offered were not more likely than other respondents to identify logistical barriers to return, including the need to ensure access to a trained clinician, the need to use a clinically certified lab, the time and costs involved, or the need to keep contact information current.
Respondents who had been responsible for primary specimen and data collection identified different barriers and motivations as compared with those who had conducted secondary analyses of specimens and data collected by others. These differences may reflect closer interactions between investigators responsible for primary specimen and data collection and the participants in their studies, or may derive from these investigators' greater ability to provide results through established contact pathways.
Few previous studies have addressed researchers' perspectives on return of results. Among 19 GWAS investigators interviewed by Williams and colleagues, few had experience returning results. Investigators generally emphasized reasons not to return results, but felt compelled to disclose “when research resulted in genomic incidental findings with clear or probable medical significance.”19 Meacham et al presented 44 investigators who had received federal funding to conduct human subjects research, not limited to genomic research, with a hypothetical scenario involving identification of a genetic variant associated with a “high risk” of developing colorectal cancer.18 Most investigators said they would offer the results to participants, and identified ensuring high-quality information, the need to minimize harm to participants, and adherence to IRB and other rules as their principal concerns in deciding about return. Our study supports the observations of this previous work while extending the findings to a large, representative, international sample of genome investigators.
Although we cannot directly compare the views of investigators reported here to those of other stakeholder groups, some generalizations are possible. In most previous studies of participants or members of the public, a higher proportion of respondents than we observed among investigators—generally 80-90%—indicate a desire for access to individual results.8 Participants are also more likely to enroll in studies when they have access to results.7 In qualitative studies, most IRB representatives also indicate support for return of results with definite clinical utility while expressing concern about the possibility of returning unvalidated results.19,25 Our data suggest that most investigators share with IRB representatives both a willingness to return results in select circumstances and a recognition of the numerous concerns and caveats surrounding the move towards offering results.
The study reported here has limitations. First, the experiences and attitudes of non-respondents may differ from those of survey respondents. Although respondents and non-respondents were similar with respect to region of origin and journal in which their index GWAS was published, we lacked other data on non-respondents and therefore could not comprehensively compare the characteristics of respondents to those of non-respondents. Second, the issues addressed in this survey are complex, and the survey questions may not have fully captured investigators' perspectives. Follow-up qualitative interviews, reported separately, provide additional insights into the views of genome investigators regarding return of results.24
In conclusion, investigators have limited experience returning individual results identified in the course of genome-scale research, yet most are motivated to do so in at least some circumstances. Given the rapid transition of research from genotyping of common variants to sequencing for rare, potentially highly penetrant variants, and in light of policy guidance suggesting that offering results is sometimes ethically required4,16,17,26, the need to develop mechanisms to facilitate the return of results has become urgent. To be successful, such mechanisms must address the concerns identified by the genome researchers who will be responsible for implementing them in practice.
Supplementary Material
Acknowledgments
Dr. Ramoni's work on this study was supported by a grant from the National Institute of Dental and Craniofacial Research (K08DE016956). Dr. McGuire and Plon and Ms. Oliver Robinson wish to acknowledge research support from the Baylor College of Medicine Clinical and Translational Research Program and the Baylor Annual Fund. Dr. Plon wishes to acknowledge a grant from the National Human Genome Research Institute (U01HG006485-01). The investigators also wish to acknowledge the Survey and Data Management Core at the Dana-Farber Cancer Institute for assistance with fielding the investigator survey.
Footnotes
Disclosure Declaration: Dr. Ramoni reports that she has received funding from the National Institutes of Health and the Office of the National Coordinator for Health Information Technology. Drs. McGuire and Plon report that they have received funding from the National Institutes of Health and the Cancer Prevention Institute of Texas (CPRIT). Joffe reports that he receives funding from the National Institutes of Health and the Greenwall Foundation, that he was a paid member of a data monitoring committee for Genzyme/Sanofi, and that he is an expert witness for counsel representing Johns Hopkins University School of Medicine. Ms. Robinson and Dr. Morley have no financial relationships to report.
References
- 1.Johnson AD, O'Donnell CJ. An open access database of genome-wide association results. BMC Med Genet. 2009;10:6. doi: 10.1186/1471-2350-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson AD, Bhimavarapu A, Benjamin EJ, et al. CLIA-tested genetic variants on commercial SNP arrays: potential for incidental findings in genome-wide association studies. Genet Med. 2010 Jun;12(6):355–363. doi: 10.1097/GIM.0b013e3181e1e2a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bredenoord AL, Kroes HY, Cuppen E, Parker M, van Delden JJ. Disclosure of individual genetic data to research participants: the debate reconsidered. Trends Genet. 2011 Feb;27(2):41–47. doi: 10.1016/j.tig.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Fabsitz RR, McGuire A, Sharp RR, et al. Ethical and Practical Guidelines for Reporting Genetic Research Results to Study Participants: Updated Guidelines From a National Heart, Lung, and Blood Institute Working Group. Circ Cardiovasc Genet. 2010 Dec 1;3(6):574–580. doi: 10.1161/CIRCGENETICS.110.958827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meltzer LA. Undesirable implications of disclosing individual genetic results to research participants. Am J Bioeth. 2006 Nov-Dec;6(6):28–30. doi: 10.1080/15265160600935811. [DOI] [PubMed] [Google Scholar]
- 6.Bredenoord AL, Onland-Moret NC, Van Delden JJ. Feedback of individual genetic results to research participants: in favor of a qualified disclosure policy. Hum Mutat. 2011 Aug;32(8):861–867. doi: 10.1002/humu.21518. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman D, Murphy J, Scott J, Hudson K. Subjects matter: a survey of public opinions about a large genetic cohort study. Genet Med. 2008 Nov 1;10(11):831–839. doi: 10.1097/GIM.0b013e31818bb3ab. [DOI] [PubMed] [Google Scholar]
- 8.Shalowitz DI, Miller FG. Communicating the results of clinical research to participants: attitudes, practices, and future directions. PLoS Med. 2008 May 13;5(5):e91. doi: 10.1371/journal.pmed.0050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards MP, Ponder M, Pharoah P, Everest S, Mackay J. Issues of consent and feedback in a genetic epidemiological study of women with breast cancer. J Med Ethics. 2003 Apr;29(2):93–96. doi: 10.1136/jme.29.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf SM, Lawrenz FP, Nelson CA, et al. Managing incidental findings in human subjects research: analysis and recommendations. J Law Med Ethics. 2008 Summer;36(2):219–248. 211. doi: 10.1111/j.1748-720X.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Illes J, Kirschen MP, Edwards E, et al. Ethics. Incidental findings in brain imaging research. Science. 2006 Feb 10;311(5762):783–784. doi: 10.1126/science.1124665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute of Biomedical Imaging and Bioengineering. NIBIB Points to Consider for Investigators: Incidental Findings in Imaging Research. [Accessed February 22, 2013];2011 http://www.nibib.nih.gov/Research/Resources/PointsToConsider.
- 13.Bledsoe MJ, Clayton EW, McGuire AL, Grizzle WE, O'Rourke PP, Zeps N. Return of research results from genomic biobanks: cost matters. Genet Med. 2013 Feb;15(2):103–105. doi: 10.1038/gim.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ossorio P. Taking aims seriously: repository research and limits on the duty to return individual research findings. Genet Med. 2012 Apr;14(4):461–466. doi: 10.1038/gim.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beskow LM, Burke W. Offering individual genetic research results: context matters. Sci Transl Med. 2010 Jun 30;2(38):38cm20. doi: 10.1126/scitranslmed.3000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Bioethics Advisory Commission. Research Involving Human Biological Materials: Ethical Issues and Policy Guidance. Rockville, MD: National Bioethics Advisory Commission; 1999. [Google Scholar]
- 17.Beskow LM, Burke W, Merz JF, et al. Informed consent for population-based research involving genetics. JAMA. 2001 Nov 14;286(18):2315–2321. doi: 10.1001/jama.286.18.2315. [DOI] [PubMed] [Google Scholar]
- 18.Meacham MC, Starks H, Burke W, Edwards K. Researcher perspectives on disclosure of incidental findings in genetic research. J Empir Res Hum Res Ethics. 2010 Sep;5(3):31–41. doi: 10.1525/jer.2010.5.3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams JK, Daack-Hirsch S, Driessnack M, et al. Researcher and institutional review board chair perspectives on incidental findings in genomic research. Genet Test Mol Biomarkers. 2012 Jun;16(6):508–513. doi: 10.1089/gtmb.2011.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hindorff LA, Junkins HA, Manolio TA. A catalog of published genome-wide association studies. [Accessed February 22, 2013]; http://www.genome.gov/26525384.
- 21.Kohane IS, Hsing M, Kong SW. Taxonomizing, sizing, and overcoming the incidentalome. Genet Med. 2012 Apr;14(4):399–404. doi: 10.1038/gim.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston JJ, Rubinstein WS, Facio FM, et al. Secondary variants in individuals undergoing exome sequencing: screening of 572 individuals identifies high-penetrance mutations in cancer-susceptibility genes. Am J Hum Genet. 2012 Jul 13;91(1):97–108. doi: 10.1016/j.ajhg.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassa CA, Savage SK, Taylor PL, Green RC, McGuire AL, Mandl KD. Disclosing pathogenic genetic variants to research participants: quantifying an emerging ethical responsibility. Genome Res. 2012 Mar;22(3):421–428. doi: 10.1101/gr.127845.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuire AL, Robinson JO, Ramoni RB, Morley DS, Joffe S, Plon SE. Returning genetic research results: study type matters. Per Med. 2013 doi: 10.2217/pme.12.109. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dressler LG, Smolek S, Ponsaran R, et al. IRB perspectives on the return of individual results from genomic research. Genet Med. 2012 Feb;14(2):215–222. doi: 10.1038/gim.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knoppers BM, Joly Y, Simard J, Durocher F. The emergence of an ethical duty to disclose genetic research results: international perspectives. Eur J Hum Genet. 2006 Nov;14(11):1170–1178. doi: 10.1038/sj.ejhg.5201690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.