Abstract
Treatment in medical oncology is gradually shifting from the use of non-specific chemotherapeutic agents towards an era of novel targeted therapy in which drugs and their combinations target specific aspects of the biology of tumor cells. Multiple myeloma (MM) has become one of the best examples in this regard, reflected in the identification of new pathogenic mechanisms, together with the development of novel drugs that are being explored from the preclinical setting to the early phases of clinical development. We review the biological rationale for the use of the most important new agents for treating MM and summarize their clinical activity in an increasingly busy field. First, we discuss data from already approved and active agents (including second- and third-generation- proteasome inhibitors, immunomodulatory agents (IMIDs) and alkylators). Then we focus on agents with novel mechanisms of action, such as monoclonal antibodies (MoAb), cell cycle specific drugs, deacetylase inhibitors, agents acting on the unfolded protein response, signaling transduction pathway inhibitors, and kinase inhibitors.
Among this plethora of new agents or mechanisms some are specially promising: Anti-CD38 MoAb, such as daratumumab, are the first antibodies with clinical activity as single agents in MM. Also the kinesin spindle protein inhibitor Arry-520 is effective in monotherapy as well as in combination with dexamethasone in heavily pretreated patients. Immunotherapy against MM is also being explored, and probably the most attractive example of this approach is the combination of the anti-CS1 MoAb elotuzumab with lenalidomide and dexamethasone, that has produced exciting results in the relapsed/refractory setting.
Keywords: Multiple Myeloma, New Drugs, targeted agents, Phase I clinical trials
Introduction
Therapeutics in medical oncology has undergone a marked evolution in recent decades, moving from the chemotherapeutic era in which the drugs were non-specifically directed against highly proliferative cells, towards an era of novel targeted therapy in which drugs and their combinations target specific mechanisms of tumor cell growth and survival.1 Some targeted agents have changed the treatment paradigm in solid and hematological tumors, such as anti-erb2 monoclonal antibodies (MoAbs) in breast cancer, tyrosine kinase inhibitors (imatinib, dasatinib, nilotinib, ponatinib) in chronic myeloid leukemia, anti-CD20 MoAb in non-Hodgkin lymphoma, anti-VEGF-R MoAb in colon cancer and anti-BRAF in melanoma.
Multiple myeloma (MM) has followed a similar pattern in recent years: alkylators such as melphalan along with steroids have been the standard agents for the care of these patients for over 30 years. However, in the last decade, several agents (proteasome inhibitors and IMIDs) with singular mechanisms of action have been discovered, developed and approved.2, 3 These advances have resulted in a clear improvement in the outcome of MM patients,4 but despite this, MM remains incurable and patients who become refractory or ineligible to receive bortezomib and IMIDs have a dismal prognosis.5 This situation along with the pattern of subsequent responses/relapses that characterize the evolution of MM highlights the need for novel drugs. The investigation and discovery of these new drugs and, in particular, their use in combinations, should be based on a thorough knowledge and understanding of the pathogenesis of cancer6, specifically that of MM.7–9
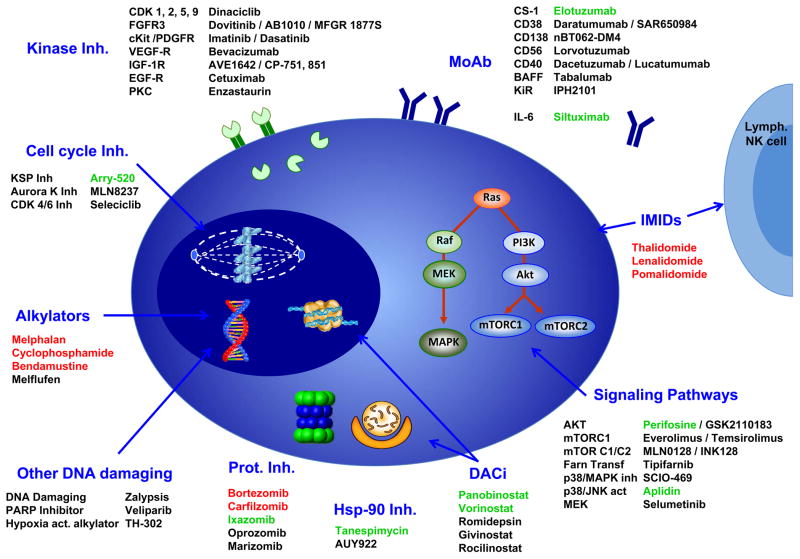
MM is probably one of the malignant diseases for which more active research into novel antitumoral agents has been carried out. However, only a few agents have successfully completed the early phases of clinical development. Moreover, the large number of novel agents under investigation has created some confusion in the clinical arena, whereby there is no consensus about which of them have clinically relevant antitumor activity. The purpose of this manuscript is to review and shed light on the rationale for the use and the clinical results obtained to date for the most promising novel agents currently under investigation. These agents have been divided into two main groups: first, those agents derived from the already approved and active agents (such as second- and third-generation proteasome inhibitors, immunomodulatory agents and alkylators) and second, (the main focus of this review), drugs with novel mechanisms of action, such as monoclonal antibodies, agents acting on the cell cycle, deacetylase inhibitors, agents acting on the unfolded protein response, signaling pathway inhibitors, and kinase inhibitors. Figure 1 illustrates a schematic representation of the main drugs that have been tested in MM and the mechanisms they target.
Figure 1. Schematic representation of the main targets in MM plasma cells and the drugs tested against them.
Approved drugs are presented in red and drugs that have reached phase III development in green.
For ease of reading, the mechanism of action is highlighted in italics, and the clinical results are detailed in the tables, with only the most relevant aspects discussed in the text. Once the mechanistic and clinical data has been presented, the discussion will analyze the future of this field of novel agents, emphasizing which of them seem more promising and how they should be developed.
Agents derived from those with proven clinical efficacy in MM
1. Novel proteasome inhibitors
One of the major advances in the treatment of MM patients in recent years has been the discovery of the catalytic activity of proteasomes,10 along with the synthesis of bortezomib (PS-341),11 the first-in-class proteasome inhibitor, which has demonstrated striking clinical12–14 efficacy in MM. The anti-MM activity of the inhibition of this pathway is the consequence of several biological effects,15–17 among which, the following are highlighted: 1) the accumulation of cyclin- or CDK-inhibitors and tumor suppressor proteins, 2) the inhibition of the clearance of misfolded proteins (inducing endoplasmic reticulum, stress and activation of the unfolded protein response),18,19 and 3) the blockade of the NF-κB transcription factor pathway through the prevention of IκB (Inhibitor of NF-κB) degradation after its polyubiquitination by IKK (IκB kinase).20 After bortezomib, several other proteasome inhibitors have been synthesized and are at different stages of clinical development. Some of them, as is the case of ixazomib (MLN-9708), are also boronate peptides, however, other structural families have been developed: the epoxyketones, including carfilzomib (PR-171) and oprozomib (ONX-0912 or PR-047), and the salinosporamides such as marizomib (NPI-0052). They differ in their biological properties as they target different catalytic subunits of the proteasome. Boronic acid containing PIs (bortezomib and ixazomib) inhibit both the chymotrypsin-like and the caspase-like activities of the proteasome, while carfilzomib and oprozomib are selective of chymotrypsin-like activity. Marizomib, by contrast, has a broader pattern of inhibition since it targets the three catalytic activities. The other major difference is the reversibility of the inhibition and, in this regard, carfilzomib, oprozomib and marizomib, unlike bortezomib and ixazomib, induce irreversible inhibition. Finally, some of these novel agents (such as ixazomib or oprozomib) are orally bioavailable. Table 1 summarizes the clinical data of these novel proteasome inhibitors used in monotherapy.
Table 1.
Summary of the most relevant clinical trials with novel proteasome inhibitors in monotherapy in relapsed/refractory MM
| Drug | Trial | Phase | n | prior lines | Dose | Schedule | ORR (≥ PR) | BR (≥ MR) | PFS (month) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Carfilzomib (PR-171) | PX-171-001 | 1 | 10 MM | - | MTD: 15 mg/m2 | 1–5/14d | 10% | 20% | - | O’Connor. CCR 2009175 |
| PX-171-002 | 1 | 28 | - | Recommended dose: 20 mg/m2 initially 27 mg/m2 from C1D8 |
1–2, 8–9, 15–16/28d | 19% | 27% | - | Alsina. CCR 2012176 |
|
| PX-171- 003A0 | 2 | 46 | 5 (2–16) | 20 mg/m2 | 1–2, 8–9, 15–16/28d | 17% | 24% | 3.5 | Jagannath. Clin Lymph Myeloma 2012177 |
|
| PX-171- 003A1 | 2 | 266 | 5 (1–20) | 20 mg/m2 in C1 27 mg/m2 from C2 |
1–2, 8–9, 15–16/28d | 24% | 37% | 3.7 | Siegel. Blood 2012178 |
|
| PX-171-004 | 2 | 129 Btz naïve patients | 2 (1–4) | C-1: 20 mg/m2 C-2: 20 mg/m2 in C1 27 mg/m2 from C2 |
1–2, 8–9, 15–16/28d | C-1: 42% C-2: 52% |
C-1: 59% C-2: 64% |
C-1: 8.2 C-2: NR |
Vij Blood 201221 | |
| 35 Btz treated patients | 3 (1–13) | 20 mg/m2 | 1–2, 8–9, 15–16/28d | 17% | 31% | 4.6 | Vij BJH 201223 | |||
| PX-171-005 | 2 | 50 (Renal impairment) | 5 (1–15) | 15 mg/m2 in C1 20 mg/m2 in C2 27 mg/m2 from C3 |
1–2, 8–9, 15–16/28d | 26% | 32% | - | Badros. Leukemia 201325 |
|
| Ixazomib (MLN-9708) | C16004 | 1 | 60 | 6 (2–18) | MTD: 2.97 mg/m2 | 1, 8, 15/28d | 15% | 17% | - | Kumar. ASCO 201343 |
| C16003 | 1 | 57 | 4 (1–28) | MTD: 2 mg/m2 | 1, 4, 8, 11/21d | 13% | 15% | - | Lonial. ASCO 201244 |
|
| Marizomib (NPI-0052) | NPI-0052-101 NPI-0052-102 |
1 | 34 | 6 | MTD: 0.4 mg/m2 in 1 h inf. & 0.5 mg/m2 in 2 h inf. | 1, 4, 8, 11/21d | 14% | 14% | - | Richardson. ASH 201147 |
MED: Minimum effective dose
MTD: Maximum tolerated dose
NR: Not reached
Carfilzomib is FDA-approved for the treatment of MM patients who have received at least two previous therapies, including bortezomib and an immunomodulatory agent, and are refractory to their last therapy. As a monotherapy, this drug induced an overall response rate (ORR) of 52% in bortezomib-naïve patients,21 and approximately 20% of patients refractory to bortezomib responded to carfilzomib.22, 23 Based on this, a phase 3 randomized trial (Focus) has compared carfilzomib with best supportive care in MM patients for whom no other therapeutic option is available.
With respect to safety, the most frequent grade 3 (G3) AEs were hematological with very mild peripheral neuropathy.24 However, other non-hematologic toxicities, albeit rare, have emerged, including cardiopulmonary or renal toxicity. Nevertheless, carfilzomib was also safe in patients with renal impairment in a trial specifically designed to evaluate this issue.25
Several drug combinations are currently being explored, including that of carfilzomib with lenalidomide and dexamethasone both in relapsed refractory patients,26 (basis for the phase 3 Aspire trial27) and in newly diagnosed patients. 28, 29 Also in newly diagnosed, carfilzomib + thalidomide + dexamethasone has been tested,30 even with the addition of cyclophosphamide.31 Moreover, carfilzomib plus steroids have also been combined in transplant ineligible newly diagnosed patients, with cyclophosphamide32 and with melphalan.33 Other innovative combinations are being explored with novel drugs such as histone deacetylase inhibitors,34–36 pomalidomide,37 and the kinase spindle protein inhibitor Arry-520,38, 39 in relapsed and refractory patients.
The second-generation compound oprozomib (ONX-0912; previously PR-047),40 is a structural analog of carfilzomib that is orally bioavailable. Oprozomib capsules administered in split doses demonstrated clinical activity in a phase 1 trial in patients with hematologic malignancies (MM & CLL).41 In order to improve gastrointestinal tolerability, a once-daily administered tablet was introduced in this phase 1b/2 trial with 16 MM and 5 Waldenström’s macroglobulinemia (WM) patients already enrolled with a good safety profile and promising preliminary response data.42
Ixazomib (MLN9708) is the first orally bioavailable proteasome inhibitor evaluated to date in clinical studies for the treatment of MM. Two studies are exploring its activity in monotherapy in relapsed/refractory MM patients previously exposed to proteasome inhibitors still with very preliminary results (table 1).43,44 With respect to toxicity, the most remarkable finding was the low rates of significant PN, although treatment related rash has been noted. Ixazomib is also being examined in combination with melphalan and prednisone45 and with lenalidomide and low-dose dexamethasone46 in newly diagnosed patients.
Marizomib (NPI-0052) is still in the early stages of development, showing minimal peripheral neuropathy with 15–20% ORR in heavily pretreated patients (table 1).47
2. Novel IMIDs
Since the discovery of the anti-MM activity of thalidomide,48, 49 several thalidomide analogs (lenalidomide-CC-5013 or pomalidomide-CC-4047) have been developed. Drugs in this group are called immunomodulatory drugs (IMIDs) due to their action on the immune system. Recent studies suggest that IMIDs exert their function by binding to cereblon, a molecule that forms an E3 ubiquitin ligase complex with damaged DNA binding protein 1 (DDB1) and Cul4A.50 In fact, the absence of cereblon is associated with resistance to IMIDs,51,52 and the teratogenic potential of this family of drugs has also been linked to the binding to this protein.50 Although their precise mode of action is not well established, three mechanisms have been implicated in their antimyeloma activity: tumoricidal, immunomodulatory and antiangiogenic. The tumoricidal activity of lenalidomide may be mediated by several mechanisms: 1) down regulation of IRF4 levels53, 54 that lead to an initial G1 cell cycle arrest, decreased cell proliferation, and cell death associated with a decrease in MYC levels and the induction of several CDK inhibitors (p15, p16, p21 and p27);55, 56 2) induction of p21 WAF-1 expression through an LSD1-mediated epigenetic mechanism;57 and 3) disruption of the interaction between tumor cells and their microenvironment.55, 58 The immunomodulatory effect is mediated through the augmentation of natural killer (NK) cytotoxicity,59, 60 the inhibition of regulatory T cells,61 or the restoration of the immune synapse formation.62 Thalidomide48, 49 and lenalidomide63–65 were approved in the last decade for the treatment of MM patients. However, pomalidomide has recently emerged as a very potent IMID, both alone and in several combinations (table 2). In this regard, similarly to lenalidomide and thalidomide, the addition of dexamethasone induces synergy, improving the response rate and the PFS,66 and this combination in the initial phase 2 study by Lacy and co-workers induced a 62% response rate with a PFS of 13 months (table 2),67 similar to that previously obtained with lenalidomide + dexamethasone.63–65 This is relevant considering that, in this trial, 62% of the patients had been previously exposed to IMIDs.
Table 2.
Summary of the most relevant clinical trials with pomalidomide in relapsed MM patients
| Phase | +/− Dex or other comb. | n | Prior lines | Dose | Schedule | ORR ≥ PR | CBR ≥ MR | PFS Months | OS Months | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | No | 24 | 3 (1–6) | MTD: 2 mg | 1–28 (daily) | 54% | 71% | 9.7 | 22.5 | Schey. JCO 2004 179 |
| 1 | No | 20 | 4 (1–7) | MTD: 5 mg | 1–28 (Every other day) | 50% | 55% | 10.5 | 33 | Streetly. BJH 2008 180 |
| 1b | Dex& | 38* | 6 (2–17) | MTD: 4 mg | 1–21 | Pom: 13% + Dex: 21% |
Pom: - + Dex: 42% |
4.6 | 18.3 | Richardson. Blood 201366 |
| 2 | No | 108* | 5 (1–13) | 4 mg | 1–21 | 15% | 31% | 2.6 | 13.6 | Richardson. ASH 2011181 & Siegel ASCO 2013182 |
| Dex | 113* | 4 mg | 1–21 | 34% | 45% | 4.6 | 16.5 | |||
| 2 | Dex | 60 | 2 (all ≤ 3) | 2 mg | 1–28 | 65% | - | 13 | 40 | Lacy. JCO 200967 & ASH 201269 |
| 2 | Dex | 34** | 4 (1–7+) | 2 mg | 1–28 | 32% | 47% | 5 | 33 | Lacy Leukemia 201068 & ASH 201269 |
| 2 | Dex | 60** | 2 (all ≤ 3) | 4 mg | 1–28 | 38% | - | 7.7 | 92%$ | Lacy ASH 201269 |
| 2 | Dex | 120** | - | 4 mg | 1–21 | 21% | - | 4.3 | 74%$ | Lacy ASH 201269 |
| 2 | Dex | 35*** | 6 (3–9) | 2 mg | 1–28 | 26% | 49% | 6.4 | 16 | Lacy Blood 201170 & ASH 201269 |
| Dex | 35*** | 6 (2–11) | 4 mg | 1–28 | 29% | 43% | 3.3 | 9.2 | ||
| 2 | Dex | 43*** | 5 (1–13) | 4 mg | 1–21 | 35% | - | 5.4 | 14.9 | Leleu Blood 201371 |
| Dex | 41*** | 4 mg | 1–28 | 34% | - | 3.7 | 14.8 | |||
| 3 | Dex | 302** | 5 (1–17) | 4 mg | 1–21 | 31% | - | 4 | NR | San Miguel ASCO 201372 |
| 2 | Clarithromycin/Dex | 100 | 5 (3–15) | 4 mg | 1–21 | 54% | 59% | 8.2 | NR | Mark ASH 2012183 |
| 1/2 | Carfilzomib/Dex | 32** | 6 (2–15)# | 4 mg | 1–21 | 33% | 56% | 70%$# | - | Shah ASH 201237 |
| 2 | PLD/Dex | 27 | 5 (1–18) | MTD: 3 mg | 1–21 | 22% | 39% | - | - | Hilger ASCO 2013184 |
| 1 | Bortezomib/Dex | 21** | 1–4 | MTD: 4 mg | 1–21 | 72% | - | - | - | Richardson ASCO 2013185 |
| 1 | Cyclophosphamide/Dex | 10** | 5 (3–10) | 4 mg | 1–21 | 40% | 50% | - | - | Baz ASH 2012186 |
| 1/2 | Cyclophosphamide/Prednisone | 55 | 3 (1–3) | MTD: 2.5 mg | - | 51% | - | 10.4 | - | Larocca Blood 2013187 |
Dex: Low Dose Dexamethasone (40 mg weekly) except for the trial with Cyclophosphamide + Dexamethasone that are high doses.
MTD: Maximum tolerated dose
PLD: Pegylated liposomal doxorubicin
Previous lenalidomide & bortezomib
Lenalidomide-refractory patients
Lenalidomide- & bortezomib-refractory
Dexamethasone added in 22 non-responding patients
OS/PFS at 6 months
Corresponds to the 12 patients enrolled in the phase 1
Several trials have explored the activity of pomalidomide + dexamethasone in lenalidomide-refractory patients68, 69 or in lenalidomide and bortezomib refractory patients.69–71 In these trials, approximately one-third of patients achieved at least PR and the PFS ranged from 3.3 to 7.7 months (table 2).
Regarding the optimal dose and schedule of administration (2 vs. 4 mg or 21/28 vs. 28/28 days), several schedules have been used and compared (see table 2).69–71 Based on these, although other possibilities may be acceptable, the dose of 4 mg on days 1–21 followed by a one-week rest period has been chosen as the standard for the subsequent randomized trials.
All these studies were the bases for the phase 3 trial (MM-003) in which MM patients that had failed both lenalidomide and bortezomib and were refractory to their last therapy, were randomized to receive pomalidomide + low dose dexamethasone vs high dose dexamethasone. There was a significant advantage for the pomalidomide arm over dexamethasone in terms of ORR (31% vs 10%), PFS (4 vs 1.9 months) and OS (NR vs 7.8 months).72 Also pomalidomide has been tested in genomically defined high risk relapsed MM patients with some activity in this setting.73
The safety profile of this agent is quite similar to that of lenalidomide, with hematological side effects being the main source of toxicity, with low rates of deep venous thrombosis, especially when using prophylactic measures.
As with carfilzomib, several trials in relapsed/refractory patients are already testing the activity of pomalidomide and dexamethasone in combination with several agents (Table 2).
3. Novel alkylators
Bendamustine has a quite unusual mechanism of action, since it combines an alkylator structure with a purine analog ring. In combination with prednisone it has already been approved in Europe for the treatment of newly diagnosed MM patients who are not candidates for ASCT and who are not eligible to receive proteasome inhibitors or thalidomide due to preexisting neuropathy. This was based on a phase III trial that compared bendamustine + prednisone with melphalan + prednisone in newly diagnosed patients, and showed a benefit especially in terms of TTP (14 vs. 10 months).74 Several pilot phase II studies have evaluated the activity of this agent in different combinations in relapsed refractory MM: with bortezomib (50%–75% ORR in combination with dexamethasone),75–79 thalidomide (26%–86% ORR),80–82 or, more recently, lenalidomide (52%–76% ORR with 24%–33% VGPR).83, 84 Results are quite variable, reflecting the heterogeneity of the patient population included in the different trials (mainly with regard to previous lines of therapy). Another novel alkylator undergoing with promising pre clinical testing is melphalan-flufenamide (mel-flufen), a novel dipeptide prodrug of melphalan. It consists of melphalan conjugated to an amino acid, phenylalanine, creating a dipeptide with higher antimyeloma potency than the parental drug based on a preferential delivery of melphalan to tumor cells due to the intracellular cleavage of melflufen by some peptidases overexpressed in malignant cells.85 Another alkylator with the peculiarity of being activated when in an hypoxic niche, TH-302, has been developed and tested but due to their particular mechanism, the clinical data is included in the last chapter of this review.
Agents with novel mechanisms of action
1. Immunotherapy/Monoclonal Antibodies
Activating the immune system against MM is one of the areas in which a more extensive investigation is being made. One of the agents included in this family are monoclonal antibodies (MoAbs) that are one of the paradigms of targeted therapy since they are specifically directed against antigens present in tumor cells. Once bound, they induce their antitumoral effect through several mechanisms:86, 87 1) direct cytotoxicity, which can be due to the direct induction of apoptosis or to the conjugation with radioisotopes or toxins; 2) to the enhancement of the immune function through antigen-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC). Rituximab (anti-CD20) was the first of these agents to be tested in MM, with discouraging results, as it was used as a debulking drug. whereas it might be more effective against immature CD20+ cells. Since then, several other MoAbs have been tested in MM (table 3).78,79
Table 3.
Summary of the most relevant clinical trials with monoclonal antibodies, alone and in combination with other agents in relapsed MM
| Drug | Target | Comb | Phase | n | Prior lines | ORR (>PR) | CBR (>MR) | Reference |
|---|---|---|---|---|---|---|---|---|
| Elotuzumab | CS1 | - | 1 | 35 | 4(2–10) | 0% | 0% | Zonder Blood 201288 |
| + Len-Dex | 1 | 29 | 3(1–10) | 82% | - | Lonial JCO 201289 | ||
| + Len-Dex | 2 | 73 | 55% ≥2 | 84% | - | Richardson ASH 201290, 91 | ||
| + Bort-Dex | 1 | 28 | 2 (1–3) | 40% | 60% | Jakubowiak JCO 2012188 | ||
| Daratumumab (HuMax-CD38, Ab005) | CD38 | - | 1 | 32 | 6(2–12) | 14% 42% in > 4 mg/kg |
28% 66% in > 4 mg/kg |
Plesner ASH 201292 & ASCO 201393 |
| nBT062-DM4 | CD138 | - | 1 | 32 | - | 4% | 52% | Jagannath ASH 201195 |
| - | 1/2a | 29 | - | 4% | 4% | Heffner ASH 201296 | ||
| Lorvotuzumab (IMGN901 – huN901-DM1) | CD56 | - | 1 | 37 CD56+ patients | Most of them ≥ 6 | 7% | 18% | Chanan-Khan ASH 201094 |
| + Len-Dex | 1 | 44 | 2 (1–11) | 59% | - | Berdeja ASH 2012189 | ||
| Dacetuzumab (SGN-40) | CD40 | - | 1 | 44 | 5(2–14) | 0% | 0% | Hussein Haemat 201098 |
| + Len-Dex | 1b | 36 | 4(2–14) | 39% | 81% | Agura ASH 2009190 | ||
| Lucatumumab | CD40 | - | 1 | 28 | 8(2–17) | 4% | 4% | Bensinger BJH 201297 |
| Tabalumab | BAFF | + Bort +/− Dex | 1 | 48 | 3 (1–10) | 46% | - | Raje ASH 201299 |
| Siltuximab | IL6 | + Dex | 2 | 49 | 4 (2–9) | 19% | 28% | Voorhes ASH 2011100 |
| + Bort-Dex | 2 | 21 Bort-naïve | 2 (1–3) | 57% | - | Rossi ASH 2008101 | ||
| IPH2101 | KIR | 1 | 32 | 2 (1–7) | 0% | 0% | Benson Blood 2012103 | |
| + Len | 1 | 13 | 4 (1–8) | 31% | 46% | Benson ASH 2012104 |
Elotuzumab is the best evaluated of these agents in MM. It is directed against CS1, a glycoprotein that is highly specific to plasma cells, although it may also be expressed in NK and CD8+ T cells. Although the results in monotherapy were modest (with stable disease as best response),88 the combination with lenalidomide and dexamethasone has given excellent results with more than 80% PR in relapsed patients and what is more important, prolonged PFS (33 months in the last update).89–91 The proposed mechanism of action of the synergy is an immune-mediated mechanism: lenalidomide would prepare the NK and lymphoid cells by, among other mechanisms, changing the conformation of their cytoskeleton, to favor the immune recognition, and elotuzumab would modify the plasma cells to be more prone to be targeted by the immune cells. A phase III registration enabling trial in relapsed myeloma comparing lenalidomide + dexamethasone with lenalidomide + dexamethasone + elotuzumab has just been completed.
CD38, CD138, CD56, and CD40 are other antigens of the plasma cells that have been targeted by MoAbs. Daratumumab is an anti-CD38 antibody designed to induce the killing of myeloma cells by the three proposed mechanisms. In the dose-escalation study with daratumumab monotherapy, in a very heavily pretreated population, 42% of them achieved at least PR at doses considered to reach therapeutic levels (≥ 4 mg/kg) (table 3).92, 93 These results are highly promising for a drug used in monotherapy in patients with a median of six previous treatments. This has prompted the development of other antiCD38 MoAbs, such as SAR650984, which has a similar profile and is already being tested in phase I clinical trials. Lorvotuzumab and nBT062 are two antibodies directed against CD56 and CD138, respectively. They have in common that they are conjugated with a cytotoxic agent (DM1 and DM4, respectively) that is released inside the plasma cell once bound to it. The results of the phase 1 trials in monotherapy showed some MRs and even PRs in very heavily pretreated patients (table 3).94–96 Two MoAbs against CD40, dacetuzumab and lucatumumab, have been designed, both of which have shown modest responses as monotherapy (table 3).97, 98 Some of these antibodies are currently being combined with other agents, several of them with lenalidomide and dexamethasone (table 3), in the search for a potential immune synergy.
BAFF (B-cell activating factor) is a member of the tumor necrosis factor superfamily that promotes the survival of malignant B cells, including those in MM. An anti-BAFF MoAb, tabalumab, has been combined with bortezomib with or without dexamethasone with 46% achieving PR or better (table 3).99 Siltuximab has a different mechanism as it is not directed against surface antigens but it targets soluble IL-6. Its purpose is to sequester this cytokine and prevent its binding to IL6-R. Two phase 2 trials in combination with dexamethasone or with bortezomib and dexamethasone have been carried out, yielding ORRs of 19% and 57%, respectively (table 3).100, 101 However, the results of the randomized trial that compared melfalan + Prednisone + bortezomib with or without siltuximab in newly diagnosed MM patients, were not positive, as there were no significant differences in terms of responses, PFS or OS.102
IPH2101 is an anti-KIR antibody that aims to block the immunotolerance induced by HLA class I molecules of MM cells when they bind to NK cell inhibitory killer immunoglobulin-like receptors (KIRs). No responses have been observed in monotherapy103 and only modest activity (31% ≥ PR) has been noted in combination with lenalidomide (table 6).104
Table 6.
Summary of the most relevant clinical trials with Hsp-90 inhibitors, agents interfering with signaling pathways, and agents with other mechanisms of action in MM
| Mechanism | Name | Combinations | Phase | n | Previous lines | ORR (≥ PR) | CBR (≥ MR) | Reference |
|---|---|---|---|---|---|---|---|---|
| Hsp-90 inhibitors | ||||||||
| Hsp-90 inhibitors | Tanespimycin | 1 | 29 | 4 (2–19) | 0% | 3% | Richardson BJH 2010200 | |
| + Bort-Dex | 1 | 22 | 5 (3–11) | 9% | 15% | Richardson BJH 2010145 | ||
| + Bort-Dex | 1/2 | 72 | 5 (1–15) | 15% | 27% | Richardson BJH 2011146 | ||
| Signaling pathways inhibitors | ||||||||
| AKT inhibitors | Perifosine | +/− Dex* | 2 | 64 | 4(1–11) | − Dex: 0% + Dex: 13% |
− Dex: 2% + Dex: 38% |
Richardson ASH 2007147 |
| + Bort +/− Dex** | 1/2 | 84 | 5 (1–13) | − Dex: 23% + Dex: 32% |
− Dex: 41% + Dex: 64% |
Richardson JCO 2011148 | ||
| + Len Dex | 1 | 32 | 2 (1–4) | 50% | 73% MR | Jakubobiak BJH 2012149 | ||
| GSK2110183 | 1 | 34 | 5 (2–8) | 9% | 19% | Spencer ASH 2011150 | ||
| mTORC1 inhibitors | Everolimus | 1/2 | 17 | - | 7% | 7% | Guenther ASCO 2010151 | |
| + Len | 1 | 26 | 4 | 21% | 58% | Mahindra ASH 2010154 & Yee ASH 2011155 | ||
| Temsirolimus | 2 | 16 | 2 (1–5) | 6% | 38% | Farag Leuk Research 2009152 | ||
| + Bort | 1/2 | 63 | 5 (1–14) | 28% | 42% | Ghobrial Lancet Oncol 2011153 | ||
| + Len | 1 | 21 | 3 (1–6) | 12% | 47% | Hofmeister JCO 2011156 | ||
| mTORC1/C2 inhibitors | MLN0128 INK128 | 1 | 30 | 2 (1–10) | 0% | 3% | Ghobrial ASH 2012157 | |
| Farnesyl transferase inhibitors | Tipifarnib | 2 | 43 | 4(1–6) | 0% | - | Alsina Blood 2004158 | |
| p38/MAPK inhibitors | SCIO-469 | +/− Bort*** | 2 | 62 | 5 | − Bort: 0% + Bort: 26% |
− Bort: 0% + Bort: 32% |
Siegel ASH 2006160 |
| p38/JNK activators | Plitidepsin (Aplidin) | +/− Dex** | 2 | 51 | 4 (1–8) | − Dex: 4% + Dex: 11% |
− Dex: 13% + Dex: 22% |
Mateos Clin Cancer Res 2010161 |
| MEK inhibitors | Selumetinib | 2 | 37 | 5 (2–11) | 8% | 8% | Holkova ASH 2011159 | |
| Other mechanisms | ||||||||
| TRAIL activators | Circularly permuted TR AIL (CPT) | 1b | 47 | - | 19% | 33% | Chen ASH 2012164 | |
| 2 | 27 | - | 33% | - | Chen ASH 2012165 | |||
| + Thal | 2 | 43 Thal- refractory | - | 22% | 34% | Chen ASH 2012166 | ||
| DNA damaging agents | Zalypsis | 1/2 | 22 | 3 (2–5) | 6% | 31% | Ocio ASH 2012167 | |
| PARP 1/2 inhibitors | Veliparib | + Bort | 1 | - | 3 (1–9) | 50% | 87% | Neri ASH 2012168 |
| Hypoxia-activated alkylator | TH-302 | + Dex | 1 | 11 | 6 (3–10) | 22% | 44% | Ghobrial ASCO 2013170 |
Dexamethasone added if PD
Dexamethasone added if < MR at cycle 4
Bortezomid added if < MR
2. DAC inhibitors
Deacetylases (DACs) are enzymes specialized in the removal of acetyl groups from several proteins. They have a role in oncogenesis through their epigenetic activity of targeting histones, but also through their regulation of non-histone proteins relevant to tumor progression, such as p53, E2F family members, Bcl-6, Hsp90, HIF-1α, and Nur77.105, 106 DACs are also overexpressed in several tumors, including MM, which has prompted the development of DAC inhibitors (DACis) for antitumoral purposes. There is a particular rationale for using these agents in MM in the search for some specific DACi mechanisms; the inhibition of the epigenetic inactivation of p53 and the blockade of the unfolded protein response, through the inhibition of the aggresome formation and autophagy (by targeting DAC6) and the inactivation of the chaperone system (by acetylating HSP-90).
Four classes of DACs have been described. Class I, II and IV DACs are known as classical DACs and are the ones that have been implicated in oncogenesis and are targets of DACis.105, 107. Class III DACs are called sirtuins, due to their homology with yeast Sir2, and display characteristic features.
Several DACis have been tested in MM. Despite their promising preclinical activity,108–113 their clinical efficacy in monotherapy in relapsed/refractory MM patients was very modest (table 5).114–117 This prompted the development of several combinations, among which, the one with the strongest scientific rationale is probably that of DACis and proteasome inhibitors. The basis is the simultaneous targeting of several mechanisms involved in the unfolded protein response: the inhibition of the proteasome blocks the degradation of the ubiquitinated misfolded proteins, and the use of DACis interferes with the activity of heat-shock proteins, which are necessary for the correct folding of proteins, and with aggresome formation and autophagy (through inhibition of DAC6), which is also important for the elimination of toxic misfolded proteins. Overall, this induces the accumulation of toxic misfolded proteins in the myelomatous cells with ineffective unfolded protein response, leading to apoptosis. The phase 1 trials with several of these DACis in combination with bortezomib have produced promising results (table 4),118–122 but the phase 3 randomized trial (Vantage 088) that compared bortezomib with bortezomib + vorinostat did not confirm them,123 since, although it showed an improved response rate (ORR 56% vs. 41%, P < 0.0001), this translated into only a minimal advantage in PFS (7.6 vs. 6.8 months. HR = 0.774 (0.64 – 0.94). p = 0.010) and no differences in OS (table 4). Another phase 3 randomized trial (Panorama 1) with the same rationale but with panobinostat instead of vorinostat and with the addition of dexamethasone in both arms has been recently completed, although results are not available yet. A question that remains unanswered is whether the addition of a DACi could revert bortezomib resistance. To address this, two trials, one with vorinostat and the other with panobinostat, are analyzing the activity of their combination with bortezomib (+/− dexamethasone) in bortezomib-refractory patients.124, 125 Results indicate that around 20–30% of these patients could be rescued by the addition of DACi to bortezomib (table 4).
Table 5.
Summary of the most relevant clinical trials with inhibitors of proteins acting in cell cycle and other kinase inhibitors in MM
| Mechanism | Name | Combinations | Phase | n | Previous lines | ORR (≥ PR) | CBR (≥ MR) | Reference |
|---|---|---|---|---|---|---|---|---|
| Agents acting on the cell cycle | ||||||||
| CDK 4/6 inhibitors | Seleciclib PD0332991 | + Bort–Dex | 2 | 30 | 2 (1–8) | 18% | 24% | Niesvitzky ASH 2010199 |
| Aurora kinase A inhibitors | MLN8237 | + Bort | 1 | 19 | - | 26% | 52% | Stewart ASH 2012129 |
| KSP inhibitors | ARRY-520 | 1 | 31 | 6 (1–16) | 10% | 13% | Shah ASH 2011130 | |
| 2 | 32 | 6 (2–19) | 16% | 19% | Shah ASH 2012131 | |||
| + Dex | 2 | 18 | 10 (5–13) | 22% | 28% | |||
| Kinase inhibitors | ||||||||
| CDK 1, 2, 5, 9 inhibitors | Dinaciclib | 1/2 | 29 | 4 (1–5) | 11% | 18% | Kumar ASH 2012133 | |
| FGFR3 inhibitors | Dovitinib (TKI-258) | 2 | 43 | 86% ≥ 3 | 0% | 0% | Scheid ASH 2012134 | |
| AB1010 | + Dex* | - | 24 t(4:14)+ | - | − Dex: 0% + Dex: 18% |
− Dex: 0% + Dex: 36% |
Arnulf ASH 2007136 | |
| MFGR1877S | 1 | 14 | 5 (1–10) | 0% | 0% | Trudel ASH 2012135 | ||
| cKIT/PDGFR inhibitors | Imatinib | 2 | 23 c-kit + | - | 0% | 0% | Dispenzieri Leuk Lymph 2006137 | |
| Dasatinib | 2 | 21 | 3 (1–14) | 5% | 5% | Wildes Leuk Lymph 2009138 | ||
| + Len–Dex | 1 | 16 | 3 (1–6) | 57% | 93% | Facon ASH 2009139 | ||
| VEGF-R inhibitors | Bevacizumab | + LD | 2 | 31 | 3 (1–7) | 71% | - | Callander ASH 2009140 |
| IGF1R inhibitors | AVE1642 | 1 | 15 | 4 | 0% | 7% | Moreau Leukemia 2011142 | |
| + Bort | 1 | 11 | 4 | 18% | 45% | |||
| CP-751,851 | +/− Dex** | 1 | 47 | 4 (0–8) | − Dex: 0% + Dex: 22% |
− Dex: 0% + Dex: 33% |
Lacy JCO 2008141 | |
| EGF-R inhibitors | Cetuximab | +/− Dex*** | 2 | 15 | - | − Dex: 0% + Dex: 7% |
− Dex: 0% + Dex: 27% |
Von Tresckow ASH 2011143 |
| PKC inhibitors | Enzastaurin | + Bort | 1 | 23 | 70% ≥ 3 | 17% | 26% | Ghobrial Am J Haem 2011144 |
Dexamethasone added if PD
Dexamethasone added if PD at cycle 2 or if < PR at cycle 4
Dexamethasone added if PD at week 5 or < PR at week 9
Table 4.
Summary of the most relevant clinical trials with deacetylase inhibitors in MM
| Drugs | Phase | n | Previous lines | ORR (≥ PR) | CBR (≥ MR) | Response in refractory patients** | Reference | |
|---|---|---|---|---|---|---|---|---|
| ORR (≥ PR) | CBR (≥ MR) | |||||||
| Monotherapy | ||||||||
| Vorinostat | 1 | 13 | 0% | 10% | - | - | Richardson Leuk Lymph 2008116 | |
| Panobinostat | 2 | 38 | 5 | 3% | 5% | - | - | Wolf Leuk Lymph 2012117 |
| Romidepsin | 2 | 13 | 3(2–4) | 0% | 0% | - | - | Niesvizky Cancer 2011115 |
| Givinostat +/− Dex | 2 | 19 | 3(1–8) | 0% | 0% | - | - | Galli Ann Hematol 2010114 |
| Rocilinostat | 1/2 | 13 | 88% ≥ 3 | 0% | 0% | - | - | Raje ASH 2012126 |
| + Bortezomib +/− Dexamethasone | ||||||||
| Vorinostat + Bort +/− Dex | 1 | 23 | 7(3–13) | 43% | 90% | 38% | 88% | Badros Clin Cancer Res 2009118 |
| Vorinostat + Bort +/− Dex | 1 | 34 | 4(1–14) | 27% | 32% | 14% | 14% | Weber Clin Lymph-M-L 2012121 |
| Vorinostat + Bortezomib* | 3 | 317 | 2 (1–3) | 56% | 71% | - | - | Dimopoulos ASH 2011123 |
| Panobinostat + Bort + Dex | 1b | 62 | 2 (1–10) | 68% | 82% | 43% | 71% | San Miguel IMW 2011120 |
| Romidepsin + Bort + Dex | 1/2 | 25 | 2(1–3) | 60% | 72% | - | - | Harrison Blood 2011119 |
| Quisinostat + Bort+Dex | 1b | 18 | 2 (1–3) | 88% | - | - | - | Leleu ASCO 2013122 |
| Vorinostat + Bortezomib$* | 2 | 143 Bort- refractory | 4 (2–17) | 18% | 33% | 18% | 33% | Siegel ASH 2011124 |
| Panobinostat + Bort + Dex$ | 2 | 55 Bort- refractory | 4(2–11) | 35% | 53% | 35% | 53% | Richardson ASH 2012125 |
| + Lenalidomide + Dexamethasone | ||||||||
| Vorinostat + Len + Dex | 1 | 31 | 4 (1–10) | 53% | 70% | 20% | 30% | Richardson ASH 2010191 |
| Vorinostat + Len + Dex$$ | 2 | 29 LD- refractory | 4 (2–13) | 24% | 51% | 24% | 51% | Richter ASH 2011192 |
| Panobinostat + Len + Dex | 1b | 46 | 2 (1–8) | 57% | - | - | - | Mateos ASCO 2010193 |
| Other combinations | ||||||||
| Vorinostat + PLD + Bort | 1 | 32 | 2 (1–9) | 65% | 74% | 45% in Bort-refractory | 64% in Bort- refractory | Voorhees ASH 2011194 |
| Vorinostat +Len + Bort + Dex in RR | 2 | 9 RVD- refractory | 5 (2–10) | 44% | 89% | 44% | 89% | Siegel IMW 2011195 |
| Vorinostat +Len + Bort + Dex in ND | 1 | 30 new diagnosis | 0 | 100% | 100% | - | - | Kaufman ASH 2012196 |
| Panobinostat + Melphalan | 1/2 | 25 | 4 (-17) | 16% | 60% | - | - | Berenson IMW 2011197 |
| Panobinostat + MPT | 1/2 | 24 | 21% ≥2 | 50% | - | - | - | Offidani IMW 2011198 |
| Panobinostat + Carfilzomib | 1/1b | 17 | 5 (2–15) | 35% | 41% | - | - | Shah ASH 201235 |
| Panobinostat + Carfilzomib | 1/2 | 10 | 3 (1–7) | 60% | 70% | - | - | Berdeja ASH 201234 |
Data obtained from the presentation at the ASH 2011 meeting
Indicates the response in patients previously refractory to the drugs administered in combination with the DAC inhibitors (bortezomib or lenalidomide in their respective combinations)
Bortezomib-refractory patients
Lenalidomide- and dexamethasone-refractory patients
All these DACis have a broad spectrum of inhibition of DACs, as they are either pan-DACi (inhibition of the classes of DAC) or class 1 inhibitors, and this has been associated with significant toxicity, which is mainly manifested as general or gastrointestinal symptoms. With the purpose of overcoming this, while maintaining efficacy, a novel HDAC-6-specific inhibitor (rocilinostat) has been developed. Although no responses were obtained as monotherapy, it showed good tolerability126 and is currently being combined with bortezomib and lenalidomide, with good preliminary results mainly in the combination with the IMID, with 5 out of 6 evaluable patients achieving PR or better.127
3. Agents acting on proteins and enzymes involved in the cell cycle
The only common oncogenic event found in MM patients to date is cyclin D deregulation.128 Therefore, efforts have been made to develop agents that can target the cell cycle abnormalities present in MM cells (table 5). The main focus has been the CDKs (cyclin-dependent kinases), which are the proteins that phosphorylate and activate these cyclins, in particular CDK 4/6, which is responsible for cyclin-D phosphorylation. Seleciclib (PD0332991) is a CDK 4/6 inhibitor that was combined with bortezomib using an attractive sequential approach that attempts to synchronize cells with the CDK inhibitor and make them more susceptible to the cytotoxic effect of the proteasome inhibitor. Nevertheless, results were discouraging and the development of this compound in MM was stopped. Other compounds evaluated in cell cycle have been those involved in the spindle formation and function: aurora kinase A inhibitors, such as the novel MLN8237, whose combination with bortezomib has been recently reported, with 52% of patients achieving at least MR and 26% PR or better (table 5).129
KSP (kinesin spindle protein) is a member of the kinesin superfamily of microtubule-based motors; it plays a critical role in mitosis as it mediates centrosome separation and bipolar spindle assembly and maintenance. Arry-520 is a KSP inhibitor that by blocking this protein, arrests cells in mitosis and subsequently induces apoptosis through the degradation of survival signals. The drug on its own has already shown up to 16% PR or better130, 131 and 22% in combination with dexamethasone131 in very refractory patients with a median of six and ten previous lines of therapy respectively (table 5). It is already being combined with proteasome inhibitors such as bortezomib and carfilzomib and is one of the most promising agents currently under exploration.
4. Kinase inhibitors
Several tyrosine or serine-threonine kinase inhibitors have been grouped within this section of the review. They have been clinically investigated in MM, yielding different outcomes (table 5). One of the most recent is the CDK inhibitor dinaciclib. It inhibits CDK 1, 2, 5 and 9 and is included in this rather than the previous section because it was selected on the basis of its CDK-5 inhibitory activity, which is not related to the cell cycle. CDK-5 inhibition was identified as one of the top bortezomib-sensitizing mechanisms in high-throughput RNAi screening.132 This inhibitor shows some activity as a single agent (18 ≥ MR and 11% ≥ PR; table 5),133 and may synergize with bortezomib. Among the tyrosine kinase inhibitors, those with the best rationale for use in MM are probably the FGFR3 inhibitors in patients with t(4;14). Two small molecules134, 135 and one MoAb136 have been explored in patients with this translocation, with disappointing results (table 5).
Inhibitors of cKit/PDGFR have also been tested: imatinib did not induce any response137 and dasatinib, demonstrating 5% response in monotherapy,138 has been tested with bortezomib and lenalidomide (table 5).139 This gave some responses but it was difficult to assess whether dasatinib added anything to the combination of agents. Other inhibitors are the anti-VEGF-R MoAb bevacizumab, which, in combination with lenalidomide, induced 71% of PR or better,140 and IGF1-R,141, 142 EGF-R143 and PKC144 inhibitors that did not respond in monotherapy, but may have some role in combination with other agents such as bortezomib (table 5).
5. Agents acting on the unfolded protein response (UPR) pathway
The chaperone system is responsible for the correct folding of proteins. Its malfunctioning therefore induces the accumulation of misfolded proteins and activates the unfolded protein response. Heat-shock 90 proteins (Hsp-90) are amongst the main members of this system, and represent a potential target for use in myeloma treatment. Similarly to DACis, there is a good rationale for combining Hsp-90 inhibitors with proteasome inhibitors in order to achieve synergistic activation of the unfolded protein response. In fact, one of these Hsp-90 inhibitors, tanespimycin, has been combined with bortezomib and dexamethasone in two phase 1 trials, giving an ORR of up to 15% in patients who had received five previous lines of therapy (table 6).145, 146 AUY922, another drug of this family, has also been combined with bortezomib +/− dexamethasone in relapsed/refractory patients, without reported clinical results yet.
Other agents that could have a role in this important pathway are the purine scaffold HSP90 inhibitors or the IRE1alpha inhibitors, but they are still in preclinical phases of development.
6. Signal transduction pathway inhibitors
Myeloma cells, like other tumor cells, are characterized by an abnormal activation of several of the most important signaling pathways, such as the PI3K/AKT/mTOR, RAF/MEK/ERK, JAK/STAT and NFkB pathways. This has prompted the development of several drugs aimed at blocking these routes at different levels. One of the main types is the group of proteasome inhibitors, which interfere with the NFkB pathway by hampering the degradation of the inhibition of NFkB (IkB) by the proteasome. Other more selective inhibitors of different components of these pathways are summarized in table 6.
The PI3K/AKT/mTOR pathway has been extensively studied and targeted, as it is probably one of the most important in MM pathogenesis. AKT inhibitors such as perifosine147 have been combined with bortezomib (in the search for the synergistic inhibition of AKT with perifosine and ERK with bortezomib)148 or with lenalidomide,149 with up to 32% and 50% with at least PR, respectively (table 6). GSK211083 is another novel AKT inhibitor that is active in monotherapy (9% ≥ PR. Table 6).150 The mTOR complexes lie downstream of this pathway. Two compounds targeting mTORC1, everolimus and temsirolimus, have been tested, with 6% and 7% PR in monotherapy, respectively.151, 152 These values improved when the compounds were combined with bortezomib153 or lenalidomide154–156 in more heavily pretreated patients (table 6). Recently, MLN1018, a new mTOR inhibitor targeting the mTOR-C1 and mTOR-C2 complexes, has been tested but no responses were observed in monotherapy (table 6).157
The RAS/RAF/MEK/ERK pathway was the second to be investigated, addressing not only the blockade of top upstream molecules of the pathway by the farnesyl-transferase inhibitor tipifarnib,158 which impedes the activation of RAS, to MEK inhibitors such as selumetinib (ARRY-6244),159 but also the p38/MAPK inhibitor SCIO-469, which has been combined with bortezomib.160 Another interesting drug, is the p38/JNK activator Plitidepsin, which after showing activity in heavily pretreated patients in the phase II trial (table 6), is currently in phase 3 evaluation.161 Of these, selumetinib is probably the most promising, since, as a single agent, it has given an 8% PR in patients with five previous lines of therapy (table 6). Recently, whole genome sequencing revealed activating mutations of the kinase BRAF in 4% MM patients.162 Vemurafenib, a small molecule inhibitor specifically targeting V600E-mutated BRAF, has been reported to induce a PR in a patient relapsing after several lines of therapy and harboring this mutation.163
7. Drugs with different mechanisms of action
The search for ligands of death receptors (FAS or TRAIL-R) that directly activate the extrinsic pathway of apoptosis has always been an area of interest in the field of novel antitumoral agents, although, to date, they have not shown significant efficacy and have been quite toxic. However, recent promising preliminary results from two trials in monotherapy with a circularly permuted TRAIL (CPT) have registered 19% and 33% PR or better.164, 165 This agent has also been combined with thalidomide, with 22% with at least PR and 34% with at least MR in thalidomide-refractory patients (table 6).166
Two novel agents share a common mechanism of DNA damage induction or DNA repair inhibition. Zalypsis is a marine-derived compound that binds to the minor groove of DNA and induces DNA double-strand breaks. As a single agent in patients with a median of three previous lines of therapy it has given 31% MR or better, including 6% PR. (table 6).167 The other agent is the PARP 1/2 inhibitor, velaparib, which has been combined with bortezomib in the search for a synergistic combination of DNA damage induction and DNA repair inhibition, and has resulted in 50% PR (table 6).168
The presence of a hypoxic niche in the bone marrow has been associated with MM pathogenesis.169 In this regard, TH-302, an alkylator designed to be activated by hypoxia has been developed and clinically tested in combination with dexamethasone, with some responses (22% PR and 22% MR) in heavily pretreated patients.170
Discussion
The incurable nature of MM makes it necessary to increase the treatment armamentarium against this disease. As it is shown in this review, the ongoing extensive research and the already positive clinical results with several agents, makes the future optimistic in the aim of transforming MM into a chronic disease. Although none of the agents with novel mechanisms of action (after proteasome inhibitors or IMIDs) are still approved, it is reasonable to think that several of them will be in the near future. The initial approval for most of them will be for patients refractory to proteasome inhibitors and IMIDs, but its use will be soon expanded to other settings and used in different combinations. Particularly valuable may be for newly diagnosed patients, where the disease is more sensitive, and probably the use of optimized multitargeted combinations in these patients could derive in the curability of some of them.
Nevertheless, this optimism should be balanced with the reality of the clinical results, since, many of the novel agents, despite having a good scientific rationale and promising activity in preclinical models of MM, have not demonstrated clinical activity. This discordance may be due to several reasons, one of them being the limitations of the preclinical models of MM to accurately reflect the patient’s setting. The other obvious issue is the heterogenetic and multigenetic nature of MM, and the pathogenesis of a complex malignancy, which seems to rely not only on one unique hit but on many of them. An example of this is that, although cyclin-D is deregulated in the vast majority of MM patients, agents targeting this mechanism have not produced the expected clinical results.
In fact, agents with a quite pleiotropic mechanism of action such as proteasome inhibitors, immunomodulatory agents or alkylators are those that have demonstrated to be effective in MM and therefore, along with steroids, have become the backbone of the treatment of MM patients. Nevertheless, not all agents with a broad spectrum of mechanisms have been effective in MM. As previously shown, DACi, which target several different proteins and mechanisms in the tumor cell, have not confirmed the expectations in the dual combination, based on the results of the phase 3 Vantage trial recently reported. However, data on a triple combination with corticosteroids is still pending (Panorama 1 trial); moreover, it could be that the use of more specific DACi such as the HDAC6 specific, rocilinostat may result in higher efficacy due to a more favorable toxicity profile that would translate into a prolonged drug exposure.
The results of the so-called targeted agents, that display quite specific mechanisms of action, when used in monotherapy, are usually not very optimistic, but we also have to consider that most of these trials have been performed in quite heavily pretreated patients. Accordingly, the lack of activity as single agents, should probably not preclude the future investigation of these drugs in MM in scientifically based combinations. A good example of this situation is the combination of the anti-CS1 MoAb elotuzumab with lenalidomide and dexamethasone; despite the lack of efficacy of elotuzumab as single agent, it has yielded remarkable results in terms of response rate, but particularly in terms of PFS (33 months) in the relapsed/refractory setting, based on the potentiation of an anti-MM immune response. This leads to an important point, as most of these novel agents in monotherapy does not induce long PFS, probably reflecting again the bad prognosis of the patients included in these trials, but also the fact that cells are able to rather quickly overcome the effects of these targeted drugs and develop mechanisms of resistance. Probably, the use of rationally based combinations as the one just mentioned, could avoid the development of this resistance and increase the durability of the responses.
One of the most promising strategies in the current arena is immunotherapy. This approach has been traditionally used in several cancers, and specifically in MM. In this regard we cannot forget the use of interferon, whose use was stopped due to the low tolerability but that showed benefit in the maintenance setting. Several decades later, a novel family of agents, IMIDs, appeared in the treatment armamentarium of MM, cooperating in the revolution of MM therapy and outcome. In this same line, immunotherapy with BCMA chimeric antigen receptors,171 dendritic cell/myeloma fusion cellular vaccine172 or the incorporation of the PD-1/PDL-1 axis antagonists 173, 174 may harness the body’s own immune system, generating an anti-tumor response have been preclinically explored. Quite recently, several drugs and combinations that are based on immunological mechanisms have appeared and are currently being tested in the clinics. This is the case of different MoAb that target surface molecules of the malignant plasma cell. In addition to the already mentioned elotuzumab, there are several other MoAb that by inducing direct cytotoxicity and, mainly, ADCC and CDC have raised quite interest. Probably the most exciting target is CD38, against which several antibodies have been developed. The most advanced of these antibodies, daratumumab, has demonstrated clear activity as monotherapy in heavily pretreated patients with 42% responses at therapeutic doses.
Several other of the currently tested agents have also already shown some activity in monotherapy. One of the most promising is the KSP inhibitor Arry-520, which alone or in combination with dexamethasone in very refractory patients, has produced 10–16% responses. This agent is now being investigated in several combinations with novel and conventional agents. The CDK5 inhibitor, identified in an RNAi screening of druggable targets, induced responses in 11% of cases, but, probably, the combination with bortezomib is expected to be more potent, based on the preclinical rationale. Other agents with some responses as single agents, although in more preliminary stages of development are agents targeting different signaling pathways such as PI3K/AKT/mTOR inhibitors and the novel MEK inhibitor selumetinib, all of which produce 5–10% PR. Also among these signaling pathways-specific agents we can emphasize aplidin, a p38, JNK activator with efficacy in the phase 2 trial, and that is being evaluated in a phase 3 trial in combination with dexamethasone.
Before the availability of the recently approved drugs, the limited availability of agents did not allow the selection of a particular therapy for a particular patient, and treatment was standard for all patients, with the only differentiation being based on age and transplant elegibility. The development of the novel agents has prompted the initiation of more personalized of therapy, in order to investigate the activity of new drugs/combinations in selected cohorts of patients, based on cytogenetic, molecular, or clinical (extramedullary disease). Moreover, biomarkers for sensitivity/resistance to particular drugs are under way. Examples of this situation is the use of CRBN to stratify patients sensitive or resistant to IMIDs or the measurement of serum AAG to also detect patients that will not respond to Arry-520.
*International Myeloma Working Group
Niels Abildgaard, Syddansk Universitet, Odense, Denmark
Rafat Abonour, Indiana University School of Medicine, Indianapolis, Indiana, USA
Ray Alexanian, MD Anderson, Houston, Texas, USA
Melissa Alsina, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA
Kenneth C. Anderson, DFCI, Boston, Massachusetts, USA
Michel Attal, Purpan Hospital, Toulouse, France
Hervé Avet-Loiseau, Institute de Biologie, Nantes, France
Ashraf Badros, University of Maryland, Baltimore, Maryland, USA
Dalsu Baris, National Cancer Institute, Bethesda, Maryland, USA
Bart Barlogie, M.I.R.T. UAMS Little Rock, Arkanas, USA
Régis Bataille, Institute de Biologie, Nantes, France
Meral Beksaç, Ankara University, Ankara, Turkey
Andrew Belch, Cross Cancer Institute, Alberta, Canada
Dina Ben-Yehuda, Hadassah University Hospital, Hadassah, Israel
Bill Bensinger, Fred Hutchinson Cancer Center, Seattle, Washington, USA
P. Leif Bergsagel, Mayo Clinic Scottsdale, Scottsdale, Arizona, USA
Jenny Bird, Bristol Haematology and Oncology Center, Bristol, UK
Joan Bladé, Hospital Clinica, Barcelona, Spain
Mario Boccadoro, University of Torino, Torino, Italy
Jo Caers, Centre Hospitalier Universitaire de Liège, Liège, Belgium
Michele Cavo, Universita di Bologna, Bologna, Italy
Asher Chanan-Khan, Mayo Clinic, Jacksonville, Florida, USA
Wen Ming Chen, MM Research Center of Beijing, Beijing, China
Marta Chesi, Mayo Clinic Scottsdale, Scottsdale, Arizona, USA
Tony Child, Leeds General Hospital, Leeds, United Kingdom
James Chim, Department of Medicine, Queen Mary Hospital, Hong Kong
Wee-Joo Chng, National University Health System, Singapore
Ray Comenzo, Tufts Medical School, Boston, Massachusetts, USA
John Crowley, Cancer Research and Biostatistics, Seattle, Washington, USA
William Dalton, H. Lee Moffitt, Tampa, Florida, USA
Faith Davies, Royal Marsden Hospital, London, England
Javier de la Rubia, Hospital Universitario La Fe, Valencia, Spain
Cármino de Souza, Univeridade de Campinas, Caminas, Brazil
Michel Delforge, University Hospital Gasthuisberg, Leuven, Belgium
Meletios Dimopoulos, University of Athens School of Medicine, Athens, Greece
Angela Dispenzieri, Mayo Clinic, Rochester, Minnesota, USA
Johannes Drach, University of Vienna, Vienna, Austria
Matthew Drake, Mayo Clinic Rochester, Rochester, Minnesota, USA
Brian G.M. Durie, Cedars-Sinai Samuel Oschin Cancer Center, Los Angeles, California, USA
Hermann Einsele, Universitätsklinik Würzburg, Würzburg, Germany
Theirry Facon, Centre Hospitalier Regional Universitaire de Lille, Lille, France
Dorotea Fantl, Socieded Argentinade Hematolgia, Buenos Aires, Argentina
Jean-Paul Fermand, Hopitaux de Paris, Paris, France
Carlos Fernández de Larrea, Hospital Clínic de Barcelona, Barcelona, Spain
Rafael Fonseca, Mayo Clinic Arizona, Scottsdale, Arizona, USA
Gösta Gahrton, Karolinska Institute for Medicine, Huddinge, Sweden
Ramón García-Sanz, University Hospital of Salamanca, Salamanca, Spain
Christina Gasparetto, Duke University Medical Center, Durham, North Carolina, USA
Morie Gertz, Mayo Clinic, Rochester, Minnesota, USA
Irene Ghobrial, Dana-Farber Cancer Institute, Boston, MA, USA
John Gibson, Royal Prince Alfred Hospital, Sydney, Australia
Peter Gimsing, University of Copenhagen, Copenhagen, Denmark
Sergio Giralt, Memorial Sloan-Kettering Cancer Center, New York, NY, USA
Hartmut Goldschmidt, University Hospital Heidelberg, Heidelberg, Germany
Philip Greipp, Mayo Clinic, Rochester, Minnesota, USA
Roman Hajek, Brno University, Brno, Czech Republic
Izhar Hardan, Tel Aviv University, Tel Aviv, Israel
Parameswaran Hari, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
Hiroyuki Hata, Kumamoto University Hospital, Kumamoto, Japan
Yutaka Hattori, Keio University School of Medicine, Tokyo, Japan
Tom Heffner, Emory University, Atlanta, Georgia, USA
Jens Hillengass, University of Heidelberg, Heidelberg, Germany
Joy Ho, Royal Prince Alfred Hospital, Sydney, Australia
Antje Hoering, Cancer Research and Biostatistics, Seattle, WA, USA
Jian Hou, Shanghai Chang Zheng Hospital, Shanghai, China
Vania Hungria, Clinica San Germano, Sao Paolo, Brazil
Shinsuke Ida, Nagoya City University Medical School, Nagoya, Japan
Andrzej J. Jakubowiak, University of Chicago, Chicago, Illinois, USA
Peter Jacobs, Constantiaberg Medi-Clinic, Plumstead, South Africa
Sundar Jagannath, Mt. Sinai Cancer Institute, New York, New York, USA
Hans Johnsen, Aalborg Hospital Science and Innovation Center, Aalborg, Denmark
Douglas Joshua, Royal Prince Alfred Hospital, Sydney, Australia
Artur Jurczyszyn, The Myeloma Treatment Foundation, Poland
Efstathios Kastritis, University of Athens, Athens, Greece
Jonathan Kaufman, Emory Clinic, Atlanta, Georgia, USA
Michio Kawano, Yamaguchi University, Ube, Japan
Eva Kovacs, Cancer Immunology Research-Life, Birsfelden, Switzerland
Amrita Krishnan, City of Hope, Duarte, California, USA
Sigurdur Kristinsson, Karolinska University Hospital and Karolinska Institutet, Stockholm, Sweden
Nicolaus Kröger, University Hospital Hamburg, Hamburg, Germany
Shaji Kumar, Department of Hematology, Mayo Clinic, Minnesota, USA
Robert A. Kyle, Department of Laboratory Med. and Pathology, Mayo Clinic, Minnesota, USA
Chara Kyriacou, Northwick Park Hospital, London, United Kingdom
Martha Lacy, Mayo Clinic Rochester, Rochester, Minnesota, USA
Juan José Lahuerta, Grupo Español di Mieloma, Hospital Universitario 12 de Octubre, Madrid, Spain
Ola Landgren, National Cancer Institute, Bethesda, Maryland, USA
Jacob Laubach, Dana-Farber Cancer Institute, Boston, Massachusetts, USA
Garderet Laurent, Hôpital Saint Antoine, Paris, France
Fernando Leal da Costa, Instituto Portugues De Oncologia, Lisbon, Portugal
Jae Hoon Lee, Gachon University Gil Hospital, Incheon, Korea
Merav Leiba, Sheba Medical Center, Tel Hashomer, Israel
Xavier LeLeu, Hospital Huriez, CHRU Lille, France
Suzanne Lentzsch, Columbia University, New York, New York, USA
Henk Lokhorst, University Medical CenterUtrecht, Utrecht, The Netherlands
Sagar Lonial, Emory University Medical School, Atlanta, Georgia, USA
Heinz Ludwig, Wilhelminenspital Der Stat Wien, Vienna, Austria
Anuj Mahindra, Dana-Farber Cancer Institute, Massachusetts General Hospital, Boston, MA, USA
Angelo Maiolino, Rua fonte da Saudade, Rio de Janeiro, Brazil
María-Marivi Mateos, University Hospital of Salamanca-IBSAL, IBMCC (USAL-CSIC), Salamanca, Spain.
Amitabha Mazumder, NYU Comprehensive Cancer Center, New York, New York, USA
Philip McCarthy, Roswell Park Cancer Institute, Buffalo, New York, USA
Jayesh Mehta, Northwestern University, Chicago, Illinois, USA
Ulf-Henrik Mellqvist, Sahlgrenska University Hospital, Gothenburg, Sweden
GiamPaolo Merlini, University of Pavia, Pavia, Italy
Joseph Mikhael, Mayo Clinic Arizona, Scottsdale, Arizona, USA
Philippe Moreau, University Hospital, Nantes, France
Gareth Morgan, Royal Marsden Hospital, London, England
Nikhil Munshi, Diane Farber Cancer Institute, Boston, Massachusetts, USA
Hareth Nahi, Karolinska University Hospital, Stockholm, Sweden
Ruben Niesvizky, Weill Cornell Medical College, New York, New York, USA
Amara Nouel, Hospital Rutz y Paez, Bolivar, Venezuela
Yana Novis, Hospital Sírio Libanês, Bela Vista, Brazil
Enrique Ocio, University Hospital of Salamanca-IBSAL, IBMCC (USAL-CSIC), Salamanca, Spain.
Robert Orlowski, MD Anderson Cancer Center, Houston, Texas, USA
Antonio Palumbo, Cathedra Ematologia, Torino, Italy
Santiago Pavlovsky, Fundaleu, Buenos Aires, Argentina
Linda Pilarski, University of Alberta, Alberta, Canada
Raymond Powles, Leukemia & Myeloma, Wimbledon, England
Noopur Raje, Massachusetts General Hospital, Boston, Massachusetts, USA
S. Vincent Rajkumar, Mayo Clinic, Rochester, Minnesota, USA
Donna Reece, Princess Margaret Hospital, Toronto, Canada
Tony Reiman, Saint John Regional Hospital, Saint John, New Brunswick, Canada
Paul G. Richardson, Dana Farber Cancer Institute, Boston, Massachusetts, USA
Angelina Rodríguez Morales, Bonco Metro Politano de Sangre, Caracas, Venezuela
Kenneth R. Romeril, Wellington Hospital, Wellington, New Zealand
David Roodman, Indiana University, Indianapolis, Indiana, USA
Laura Rosiñol, Hospital Clinic, Barcelona, Spain
Murielle Roussel, University of Toulouse, Toulouse, France
Stephen Russell, Mayo Clinic, Rochester, Minnesota, USA
Jesús San Miguel, University Hospital of Salamanca-IBSAL, IBMCC (USAL-CSIC), Salamanca, Spain.
Rik Schots, Universitair Ziekenhuis Brussel, Brussels, Belgium
Sabina Sevcikova, Masaryk University, Brno, Czech Republic
Orhan Sezer, Universität Hamburg, Hamburg, Germany
Jatin J. Shah, MD Anderson Cancer Institute, Houston, Texas, USA
John Shaughnessy, M.I.R.T. UAMS, Little Rock, Arkansas, USA
Kazuyuki Shimizu, Nagoya City Midori General Hospital, Nagoya, Japan
Chaim Shustik, McGill University, Montreal, Canada
David Siegel, Hackensack, Cancer Center, Hackensack, New Jersey, USA
Seema Singhal, Northwestern University, Chicago, Illinois, USA
Pieter Sonneveld, Erasmus MC, Rotterdam, The Netherlands
Andrew Spencer, The Alfred Hospital, Melbourne, Australia
Edward Stadtmauer, University of Pennsylvania, Philadelphia, Pennsylvania, USA
Keith Stewart, Mayo Clinic Arizona, Scottsdale, Arizona, USA
Evangelos Terpos, University of Athens School of Medicine, Athens, Greece
Patrizia Tosi, Italian Cooperative Group, Istituto di Ematologia Seragnoli, Bologna, Italy
Guido Tricot, Huntsman Cancer Institute, Salt Lake City, Utah, USA
Ingemar Turesson, SKANE University Hospital, Malmo, Sweden
Saad Usmani, M.I.R.T UAMS, Little Rock, Arkansas, USA
Ben Van Camp, Vrije Universiteit Brussels, Brussels, Belgium
Brian Van Ness, University of Minnesota, Minneapolis, Minnesota, USA
Ivan Van Riet, Brussels Vrija University, Brussels, Belgium
Isabelle Vande Broek, Vrije Universiteit Brussels, Brussels, Belgium
Karin Vanderkerken, Vrije University Brussels VUB, Brussels, Belgium
Robert Vescio, Cedars-Sinai Cancer Center, Los Angeles, California, USA
David Vesole, Hackensack Cancer Center, Hackensack, New Jersey, USA
Peter Voorhees, University of North Carolina, Chapel Hill, North Carolina, USA
Anders Waage, University Hospital, Trondheim, Norway NSMG
Michael Wang, MD Anderson, Houston, Texas, USA
Donna Weber, MD Anderson, Houston, Texas, USA
Jan Westin, Sahlgrenska University Hospital, Gothenburg, Sweden
Keith Wheatley, University of Birmingham, Birmingham, United Kingdom
Elena Zamagni, University of Bologna, Bologna, Italy
Jeffrey Zonder, Karmanos Cancer Institute, Detroit, Michigan, USA
Sonja Zweegman, VU University Medical Center, Amsterdam, The Netherlands
Footnotes
Conflicts of interest:EMO: Consultancy: Onyx; Bristol Myers Squibb; Array Pharmaceuticals. Research Funding: Celgene; Onyx; Pharmamar; Array Pharmaceuticals. PGR. Consultancy: Celgene; Millennium Takeda; Johnson & Johnson; Novartis; Bristol Myer Squibb. Research Funding: Celgene and Millenium. SVR: No conflicts to disclose. AP: Consultancy & Honoraria: Amgen; Bristol Myers Squibb; Celgene; Janssen-Cilag; Millennium; ONYX. MVM: Consultancy: Janssen-Cilag; Celgene; Millennium. RO: Consultancy: Abbott Laboratories; Centocor Ortho Biotech; Cephalon; Millennium; Novartis; Onyx. Research Funding: Celgene; Johnson and Johnson; Millennium; Onyx. SK: Consultancy: Millennium; Celgene; Onyx. Research Funding: Celgene; Millennium; Novartis; Celphalon; Sanofi; Onyx. SU: Consultancy: Celgene. Honoraria: Celgene; Onyx. Research Funding: Celgene; Onyx; Millennium. DR: Honoraria: Amgen. Research Funding: Eli Lilly. RN: Consultancy: Onyx; Millennium; Celgene. Honoraria: Onyx; Millennium; Celgene. Research Funding: Onyx; Millennium; Celgene. HE: Consultancy: Celgene; Janssen. Honoraria: Celgene; Janssen. Research Funding: Celgene; Janssen. KCA: Consultancy: Gilead; Sanofi-Aventis; Onyx; Celgene. Stock Ownership; Acetylon; Oncoprep. MAD: Consultancy: Celgene; Ortho Biotech. Honoraria: Celgene; Ortho Biotech. Research Funding: Celgene. HA: Honoraria: Celgene; Janssen; Onyx. UHM: Honoraria: Celgene; Janssen-Cilag. IT: No conflicts to disclose. GM: Consultancy: Millennium-Takeda; Neotype. Honoraria: Millennium-Takeda; Pfizer. RS: No conflicts to disclose. PM: Consultancy: Celgene; Janssen. Honoraria: Celgene; Janssen. PLB: Honoraria: Onyx. CSC: No conflicts to disclose. JJL: Honoraria: Celgene. Research Funding: Celgene; Janssen-Cilag. JS; Research Funding: Janssen-Cilag; Celgene; Onyx. AR: Consultancy: Celgene. Research Funding: Celgene; Bristol Myers Squibb; Millennium; Astra Zeneca; Onyx. JM: Research Funding: Celgene; Onyx; Sanofi. SZ: Research Funding: Celgene; Janssen-Cilag; Millennium. SL: Consultancy: Celgene; Millennium; Novartis; Bristol Myers Squibb; Onyx; Janssen-Cilag. RC: Consultancy: Millennium. Research Funding: Millennium; Prothena Biotech. WJC: Honoraria: Janssen; Celgene; Novartis. Research Funding: Celgene; Roche. PM: Consultancy: Celgene; Janssen; Millennium. Honoraria: Celgene; Janssen. PS: Research Funding: Janssen-Cilag; Celgene; Onyx. HL: Honoraria: Celgene; Mundi Pharma; Janssen-Cilag. Research Funding: Celgene; Mundi-Pharma; Janssen-Cilag. BD: Honoraria: Celgene Corporation; Onyx Pharmaceutical; Millennium Pharmaceutical, The Takeda Company. JFSM: Consultancy & Honoraria: Janssen-Cilag; Millennium; Celgene; Onyx; Novartis; Bristol Myers Squibb
References
- 1.Greene JA, Jones DS, Podolsky SH. Therapeutic evolution and the challenge of rational medicine. N Engl J Med. 2012;367(12):1077–82. doi: 10.1056/NEJMp1113570. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351(18):1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–60. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 4.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia. 2012;26(5):1153. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KC. New insights into therapeutic targets in myeloma. Hematology Am Soc Hematol Educ Program. 2011;2011:184–90. doi: 10.1182/asheducation-2011.1.184. [DOI] [PubMed] [Google Scholar]
- 8.Boyd KD, Davies FE, Morgan GJ. Novel drugs in myeloma: harnessing tumour biology to treat myeloma. Recent Results Cancer Res. 2011;183:151–87. doi: 10.1007/978-3-540-85772-3_8. [DOI] [PubMed] [Google Scholar]
- 9.Ocio EM, Mateos MV, Maiso P, Pandiella A, San-Miguel JF. New drugs in multiple myeloma: mechanisms of action and phase I/II clinical findings. Lancet Oncol. 2008;9(12):1157–1165. doi: 10.1016/S1470-2045(08)70304-8. [DOI] [PubMed] [Google Scholar]
- 10.Arrigo AP, Tanaka K, Goldberg AL, Welch WJ. Identity of the 19S ‘prosome’ particle with the large multifunctional protease complex of mammalian cells (the proteasome) Nature. 1988;331(6152):192–4. doi: 10.1038/331192a0. [DOI] [PubMed] [Google Scholar]
- 11.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59(11):2615–2622. [PubMed] [Google Scholar]
- 12.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 13.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 14.Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer E, Facon T, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110(10):3557–60. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 15.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Fanourakis G, Gu X, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci USA. 2002;99(22):14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hideshima T, Mitsiades C, Akiyama M, Hayashi T, Chauhan D, Richardson P, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101(4):1530–1534. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 17.Hideshima T, Richardson PG, Anderson KC. Targeting proteasome inhibition in hematologic malignancies. Rev Clin Exp Hematol. 2003;7(2):191–204. [PubMed] [Google Scholar]
- 18.Carvalho P, Goder V, Rapoport TA. Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell. 2006;126(2):361–73. doi: 10.1016/j.cell.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 19.Raasi S, Wolf DH. Ubiquitin receptors and ERAD: a network of pathways to the proteasome. Semin Cell Dev Biol. 2007;18(6):780–91. doi: 10.1016/j.semcdb.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18(49):6867–74. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 21.Vij R, Wang M, Kaufman JL, Lonial S, Jakubowiak AJ, Stewart AK, et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012;119(24):5661–70. doi: 10.1182/blood-2012-03-414359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817–25. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vij R, Siegel DS, Jagannath S, Jakubowiak AJ, Stewart AK, McDonagh K, et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. British journal of haematology. 2012;158(6):739–48. doi: 10.1111/j.1365-2141.2012.09232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singhal S, Siegel DS, Martin T, Vij R, Wang L, Jakubowiak AJ, et al. Integrated Safety From Phase 2 Studies of Monotherapy Carfilzomib in Patients with Relapsed and Refractory Multiple Myeloma (MM): An Updated Analysis. ASH Annual Meeting Abstracts. 2011;118(21):1876. [Google Scholar]
- 25.Badros AZ, Vij R, Martin T, Zonder JA, Kunkel L, Wang Z, et al. Carfilzomib in multiple myeloma patients with renal impairment: pharmacokinetics and safety. Leukemia. 2013 doi: 10.1038/leu.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Martin T, Bensinger W, Alsina M, Siegel DSD, Kavalerchik E, et al. Final results from the phase Ib/II study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone (CRd) in patients with relapsed or progressive multiple myeloma. ASCO Meeting Abstracts. 2013;31(15_suppl):8529. [Google Scholar]
- 27.Moreau P, Palumbo AP, Stewart AK, Rajkumar V, Jakubowiak AJ, Halka K, et al. A randomized, multicenter, phase (Ph) III study comparing carfilzomib (CFZ), lenalidomide (LEN), and dexamethasone (Dex) to LEN and Dex in patients (Pts) with relapsed multiple myeloma (MM) ASCO Meeting Abstracts. 2011;29(15_suppl):TPS225. [Google Scholar]
- 28.Jakubowiak AJ, Dytfeld D, Jagannath S, Vesole DH, Anderson TB, Nordgren BK, et al. Final Results of a Frontline Phase 1/2 Study of Carfilzomib, Lenalidomide, and Low-Dose Dexamethasone (CRd) in Multiple Myeloma (MM) ASH Annual Meeting Abstracts. 2011;118(21):631. [Google Scholar]
- 29.Korde N, Zingone A, Kwok M, Manasanch EE, Wu P, Tageja N, et al. Phase II Clinical And Correlative Study Of Carfilzomib, Lenalidomide, And Dexamethasone Followed By Lenalidomide Extended Dosing (CRD-R) In Newly Diagnosed Multiple Myeloma (MM) Patients. Haematologica. 2013;98(s1):Abstract 228. [Google Scholar]
- 30.Sonneveld P, Asselbergs E, Zweegman S, Van der Holt B, Kersten MJ, Vellenga E, et al. Carfilzomib Combined with Thalidomide and Dexamethasone (CTD) Is an Highly Effective Induction and Consolidation Treatment in Newly Diagnosed Patients with Multiple Myeloma (MM) Who Are Transplant Candidate. ASH Annual Meeting Abstracts. 2012;120(21):333. [Google Scholar]
- 31.Mikhael JR, Reeder CB, Libby EN, III, Costa LJ, Bergsagel PL, Buadi F, et al. Results From the Phase II Dose Expansion of Cyclophosphamide, Carfilzomib, Thalidomide and Dexamethasone (CYCLONE) in Patients with Newly Diagnosed Multiple Myeloma. ASH Annual Meeting Abstracts. 2012;120(21):445. [Google Scholar]
- 32.Bringhen S, Cavallo F, Petrucci MT, Gay F, Federico V, Conticello C, et al. Carfilzomib, Cyclophosphamide And Dexamethasone (CCD) For Newly Diagnosed Multiple Myeloma (MM) Patients: Initial Results Of A Multicenter, Open Label Phase II Study. Haematologica. 2013;98(S1):Abstract-S578. [Google Scholar]
- 33.Touzeau C, Kolb B, Hulin C, Caillot D, Benboubker L, Tiab M, et al. Effect of CMP, carfilzomib (CFZ) plus melphalan-prednisone (MP), on response rates in elderly patients (pts) with newly diagnosed multiple myeloma (NDMM): Results of a phase (Ph) I/II trial. ASCO Meeting Abstracts. 2013;31(15_suppl):8513. [Google Scholar]
- 34.Berdeja JG, Hart L, Lamar R, Murphy P, Morgan S, Flinn IW. Phase I/II Study of Panobinostat and Carfilzomib in Patients (pts) with Relapsed or Refractory Multiple Myeloma (MM), Interim Phase I Safety Analysis. ASH Annual Meeting Abstracts. 2012;120(21):4048. [Google Scholar]
- 35.Shah JJ, Thomas SK, Weber DM, Wang M, Alexanian R, Qazilbash MH, et al. Phase 1/1b Study of the Efficacy and Safety of the Combination of Panobinostat + Carfilzomib in Patients with Relapsed and/or Refractory Multiple Myeloma. ASH Annual Meeting Abstracts. 2012;120(21):4081. [Google Scholar]
- 36.Kauffman J, Zimmerman T, Jakubowiak A, Rosenbaum C, Lewis C, Harvey RD, et al. Phase I Study Of The Combination Of Carfilzomib And Panobinostat For Patients With Relapsed And Refractory Myeloma: A Multicenter MMRC Clinical Trial. Haematologica. 2013;98(S1):Abstract-P771. [Google Scholar]
- 37.Shah JJ, Stadtmauer EA, Abonour R, Cohen AD, Bensinger WI, Gasparetto C, et al. A Multi-Center Phase I/II Trial of Carfilzomib and Pomalidomide with Dexamethasone (Car-Pom-d) in Patients with Relapsed/Refractory Multiple Myeloma. ASH Annual Meeting Abstracts. 2012;120(21):74. [Google Scholar]
- 38.Shah JJ, Weber DM, Thomas SK, Alexanian R, Wang M, Qazilbash MH, et al. Phase 1 Study of the Novel Kinesin Spindle Protein Inhibitor ARRY-520 + Carfilzomib in Patients with Relapsed and/or Refractory Multiple Myeloma. ASH Annual Meeting Abstracts. 2012;120(21):4082. [Google Scholar]
- 39.Shah JJ, Thomas S, Weber DM, Wang M, Orlowski R. Phase 1 Study Of The Novel Kinesin Spindle Protein Inhibitor Arry–520 + Carfilzomib(Car) In Patients With Relapsed And/Or Refractory Multiple Myeloma (RRMM) Haematologica. 2013;98(S1):Abstract-S579. [Google Scholar]
- 40.Zhou HJ, Aujay MA, Bennett MK, Dajee M, Demo SD, Fang Y, et al. Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047) J Med Chem. 2009;52(9):3028–38. doi: 10.1021/jm801329v. [DOI] [PubMed] [Google Scholar]
- 41.Savona MR, Berdeja JG, Lee SJ, Wong H, Lee JR, Gillenwater HH, et al. A Phase 1b Dose-Escalation Study of Split-Dose Oprozomib (ONX0912) in Patients with Hematologic Malignancies. ASH Annual Meeting Abstracts. 2012;120(21):203. [Google Scholar]
- 42.Kaufman JL, Siegel D, Vij R, Ghobrial IM, Badros AZ, Neuman L, et al. Clinical Profile Of Once–Daily, Modified–Release Oprozomib Tablets In Patients With Hematologic Malignancies: Results Of A Phase 1 b/2 Trial. Haematologica. 2013;98(S1):Abstract-P233. [Google Scholar]
- 43.Kumar S, Bensinger W, Zimmerman TM, Reeder CB, Berenson JR, Berg D, et al. Weekly MLN9708, an investigational oral proteasome inhibitor (PI), in relapsed/refractory multiple myeloma (MM): Results from a phase I study after full enrollment. ASCO Meeting Abstracts. 2013;31(15_suppl):8514. [Google Scholar]
- 44.Lonial S, Baz RC, Wang M, Talpaz M, Liu G, Berg D, et al. Phase I study of twice-weekly dosing of the investigational oral proteasome inhibitor MLN9708 in patients (pts) with relapsed and/or refractory multiple myeloma (MM) ASCO Meeting Abstracts. 2012;30(15_suppl):8017. [Google Scholar]
- 45.San Miguel J, Hajek R, Spicka I, Chen C, Echeveste A, Schusterbauer C, et al. Oral MLN9708, An Investigational Proteasome Inhibitor, In Combination With Melphalan And Prednisone In Patients With Previously Untreated Multiple Myeloma: A Phase 1 Study. Haematologica. 2012;97(s1):Abstract-293. [Google Scholar]
- 46.Kumar SK, Berdeja JG, Niesvizky R, Lonial S, Hamadani M, Stewart AK, et al. A Phase 1/2 Study of Weekly MLN9708, an Investigational Oral Proteasome Inhibitor, in Combination with Lenalidomide and Dexamethasone in Patients with Previously Untreated Multiple Myeloma (MM) ASH Annual Meeting Abstracts. 2012;120(21):332. [Google Scholar]
- 47.Richardson PG, Spencer A, Cannell P, Harrison SJ, Catley L, Underhill C, et al. Phase 1 Clinical Evaluation of Twice-Weekly Marizomib (NPI-0052), a Novel Proteasome Inhibitor, in Patients with Relapsed/Refractory Multiple Myeloma (MM) ASH Annual Meeting Abstracts. 2011;118(21):302. [Google Scholar]
- 48.Barlogie B, Desikan R, Eddlemon P, Spencer T, Zeldis J, Munshi N, et al. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase 2 study of 169 patients. Blood. 2001;98(2):492–494. doi: 10.1182/blood.v98.2.492. [DOI] [PubMed] [Google Scholar]
- 49.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 50.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327(5971):1345–50. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 51.Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118(18):4771–9. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26(11):2326–35. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S, Pal R, Monaghan SA, Schafer P, Ouyang H, Mapara M, et al. IMiD immunomodulatory compounds block C/EBP{beta} translation through eIF4E down-regulation resulting in inhibition of MM. Blood. 2011;117(19):5157–65. doi: 10.1182/blood-2010-10-314278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez-Girona A, Heintel D, Zhang LH, Mendy D, Gaidarova S, Brady H, et al. Lenalidomide downregulates the cell survival factor, interferon regulatory factor-4, providing a potential mechanistic link for predicting response. Br J Haematol. 2011;154(3):325–36. doi: 10.1111/j.1365-2141.2011.08689.x. [DOI] [PubMed] [Google Scholar]
- 55.Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96(9):2943–2950. [PubMed] [Google Scholar]
- 56.Gandhi AK, Kang J, Capone L, Parton A, Wu L, Zhang LH, et al. Dexamethasone synergizes with lenalidomide to inhibit multiple myeloma tumor growth, but reduces lenalidomide-induced immunomodulation of T and NK cell function. Curr Cancer Drug Targets. 2010;10(2):155–67. doi: 10.2174/156800910791054239. [DOI] [PubMed] [Google Scholar]
- 57.Escoubet-Lozach L, Lin IL, Jensen-Pergakes K, Brady HA, Gandhi AK, Schafer PH, et al. Pomalidomide and lenalidomide induce p21 WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res. 2009;69(18):7347–56. doi: 10.1158/0008-5472.CAN-08-4898. [DOI] [PubMed] [Google Scholar]
- 58.Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15(12):1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 59.Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006;108(2):618–21. doi: 10.1182/blood-2005-10-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14(14):4650–7. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 61.Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol lmmunother. 2009;58(7):1033–45. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118(7):2427–37. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357(21):2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 64.Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 65.Dimopoulos MA, Chen C, Spencer A, Niesvizky R, Attal M, Stadtmauer EA, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23(11):2147–52. doi: 10.1038/leu.2009.147. [DOI] [PubMed] [Google Scholar]
- 66.Richardson PG, Siegel D, Baz R, Kelley SL, Munshi NC, Laubach J, et al. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121(11):1961–7. doi: 10.1182/blood-2012-08-450742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lacy MQ, Hayman SR, Gertz MA, Dispenzieri A, Buadi F, Kumar S, et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009;27(30):5008–14. doi: 10.1200/JCO.2009.23.6802. [DOI] [PubMed] [Google Scholar]
- 68.Lacy MQ, Hayman SR, Gertz MA, Short KD, Dispenzieri A, Kumar S, et al. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM) Leukemia. 2010;24(11):1934–9. doi: 10.1038/leu.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lacy MQ, Kumar SK, LaPlant BR, Laumann K, Gertz MA, Hayman SR, et al. Pomalidomide Plus Low-Dose Dexamethasone (Pom/Dex) in Relapsed Myeloma: Long Term Follow up and Factors Predicing Outcome in 345 Patients. ASH Annual Meeting Abstracts. 2012;120(21):201. [Google Scholar]
- 70.Lacy MQ, Allred JB, Gertz MA, Hayman SR, Short KD, Buadi F, et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dualrefractory disease. Blood. 2011;118(11):2970–5. doi: 10.1182/blood-2011-04-348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leleu X, Attal M, Arnulf B, Moreau P, Traulle C, Marit G, et al. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009–02. Blood. 2013;121(11):1968–75. doi: 10.1182/blood-2012-09-452375. [DOI] [PubMed] [Google Scholar]
- 72.San-Miguel JF, Weisel KC, Moreau P, Lacy M, Song KW, Delforge M, et al. MM-003: A phase III, multicenter, randomized, open-label study of pomalidomide (POM) plus low-dose dexamethasone (LoDEX) versus highdose dexamethasone (HiDEX) in relapsed/refractory multiple myeloma (RRMM) ASCO Meeting Abstracts. 2013;31(15_suppl):8510. [Google Scholar]
- 73.Usmani SZ, Hansen E, Steward D, Waheed S, Panozzo SB, Petty NM, et al. Phase II Study of Pomalidomide (Pom) in Genomically Defined High Risk Relapsed and Refractory Multiple Myeloma (RRMM) ASH Annual Meeting Abstracts. 2012;120(21):4083. [Google Scholar]
- 74.Ponisch W, Mitrou PS, Merkle K, Herold M, Assmann M, Wilhelm G, et al. Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone--a randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO) J Cancer Res Clin Oncol. 2006;132(4):205–12. doi: 10.1007/s00432-005-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berenson JR, Yellin O, Bessudo A, Boccia RV, Noga SJ, Gravenor DS, et al. Bendamustine Combined with Bortezomib Has Efficacy in Patients with Relapsed or Refractory Multiple Myeloma: A Phase 1/2 Study. ASH Annual Meeting Abstracts. 2011;118(21):1857. [Google Scholar]
- 76.Poenisch W, Bourgeois M, Wang S-Y, Heyn S, Jaekel N, Braunert L, et al. Bortezomib in Combination with Bendamustine and Prednisone in the Treatment of Patients with Refractory/Relapsed Multiple Myeloma. ASH Annual Meeting Abstracts. 2007;110(11):2723. [Google Scholar]
- 77.Ludwig H, Kasparu H, Linkesch W, Thaler J, Greil R, Leitgeb C, et al. Bortezomib-Bendamustine-Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma (MM) Shows Marked Efficacy and Is Well Tolerated, but Assessment of PNP Symptoms Shows Significant Discrepancies Between Patients and Physicians. ASH Annual Meeting Abstracts. 2011;118(21):2928. [Google Scholar]
- 78.Hrusovsky I, Heidtmann H-H. Combination Therapy of Bortezomib with Bendamustin in Elderly Patients with Advanced Multiple Myeloma. Clinical Observation. ASH Annual Meeting Abstracts. 2007;110(11):4851. [Google Scholar]
- 79.Rodon P, Hulin C, Pegourie B, Tiab M, Anglaret B, Ben-Boubker L, et al. Bendamustine, Bortezomib And Dexamethasone (BVD) In Elderly MM Progressive After 1st Line Therapy (IFM 2009–01 Trial): Predictive Factors Of Defavourable Outcome. Haematologica. 2013;98(S1):Abstract-P231. [Google Scholar]
- 80.Ponisch W, Rozanski M, Goldschmidt H, Hoffmann FA, Boldt T, Schwarzer A, et al. Combined bendamustine, prednisolone and thalidomide for refractory or relapsed multiple myeloma after autologous stem-cell transplantation or conventional chemotherapy: results of a Phase I clinical trial. Br J Haematol. 2008;143(2):191–200. doi: 10.1111/j.1365-2141.2008.07076.x. [DOI] [PubMed] [Google Scholar]
- 81.Ramasamy K, Hazel B, Mahmood S, Corderoy S, Schey S. Bendamustine in combination with thalidomide and dexamethasone is an effective therapy for myeloma patients with end stage renal disease. Br J Haematol. 2011;155(5):632–4. doi: 10.1111/j.1365-2141.2011.08754.x. [DOI] [PubMed] [Google Scholar]
- 82.Grey-Davies E, Bosworth JL, Boyd KD, Ebdon C, Saso R, Chitnavis D, et al. Bendamustine, Thalidomide and Dexamethasone is an effective salvage regimen for advanced stage multiple myeloma. Br J Haematol. 2012;156(4):552–5. doi: 10.1111/j.1365-2141.2011.08887.x. author reply 555. [DOI] [PubMed] [Google Scholar]
- 83.Lentzsch S, O’Sullivan A, Kennedy RC, Abbas M, Dai L, Pregja SL, et al. Combination of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory multiple myeloma is feasible and highly effective: results of phase 1/2 open-label, dose escalation study. Blood. 2012;119(20):4608–13. doi: 10.1182/blood-2011-12-395715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ponisch W, Heyn S, Beck J, Wagner I, Mohren M, Hoffmann FA, et al. Lenalidomide, Bendamustine and Prednisolone exhibits a favourable safety and efficacy profile in relapsed or refractory multiple myeloma: final results of a phase 1 clinical trial OSHO - #077. Br J Haematol. 2013 doi: 10.1111/bjh.12361. [DOI] [PubMed] [Google Scholar]
- 85.Chauhan D, Ray A, Viktorsson K, Spira J, Paba-Prada C, Munshi N, et al. In Vitro and In Vivo Antitumor Activity of a Novel Alkylating Agent, Melphalan-Flufenamide, against Multiple Myeloma Cells. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Richardson PG, Lonial S, Jakubowiak AJ, Harousseau JL, Anderson KC. Monoclonal antibodies in the treatment of multiple myeloma. Br J Haematol. 2011 doi: 10.1111/j.1365-2141.2011.08790.x. [DOI] [PubMed] [Google Scholar]
- 87.van de Donk NW, Kamps S, Mutis T, Lokhorst HM. Monoclonal antibody-based therapy as a new treatment strategy in multiple myeloma. Leukemia. 2012;26(2):199–213. doi: 10.1038/leu.2011.214. [DOI] [PubMed] [Google Scholar]
- 88.Zonder JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, et al. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood. 2012;120(3):552–9. doi: 10.1182/blood-2011-06-360552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lonial S, Vij R, Harousseau JL, Facon T, Moreau P, Mazumder A, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol. 2012;30(16):1953–9. doi: 10.1200/JCO.2011.37.2649. [DOI] [PubMed] [Google Scholar]
- 90.Richardson PG, Jagannath S, Moreau P, Jakubowiak A, Raab MS, Facon T, et al. A Phase 2 Study of Elotuzumab (Elo) in Combination with Lenalidomide and Low-Dose Dexamethasone (Ld) in Patients (pts) with Relapsed/Refractory Multiple Myeloma (R/R MM): Updated Results. ASH Annual Meeting Abstracts. 2012;120(21):202. [Google Scholar]
- 91.Facon T, Richardson PG, Jagannath S, Moreau P, Jakubowiak A, Raab M, et al. Phase I/II Study of ElotuzumabPlus Lenalidomide/Dexamethasone in Relapsed/Refractory Multiple Myeloma: Updated Phase II Results and Phase I/II Long Term Safety. Haematologica. 2013;98(s1):Abstract 228. [Google Scholar]
- 92.Plesner T, Lokhorst H, Gimsing P, Nahi H, Lisby S, Richardson PG. Daratumumab, a CD38 Monoclonal Antibody in Patients with Multiple Myeloma - Data From a Dose-Escalation Phase I/II Study. ASH Annual Meeting Abstracts. 2012;120(21):73. [Google Scholar]
- 93.Lokhorst HM, Plesner T, Gimsing P, Nahi H, Minnema M, Lassen UN, et al. Phase I/II dose-escalation study of daratumumab in patients with relapsed or refractory multiple myeloma. ASCO Meeting Abstracts. 2013;31(15_suppl):8512. [Google Scholar]
- 94.Chanan-Khan A, Wolf JL, Garcia J, Gharibo M, Jagannath S, Manfredi D, et al. Efficacy Analysis From Phase I Study of Lorvotuzumab Mertansine (IMGN901), Used as Monotherapy, In Patients with Heavily Pre-Treated CD56-Positive Multiple Myeloma - A Preliminary Efficacy Analysis. ASH Annual Meeting Abstracts. 2010;116(21):1962. [Google Scholar]
- 95.Jagannath S, Chanan-Khan A, Heffner LT, Avigan D, Zimmerman TM, Lonial S, et al. BT062, An Antibody-Drug Conjugate Directed Against CD138, Shows Clinical Activity in Patients with Relapsed or Relapsed/Refractory Multiple Myeloma. ASH Annual Meeting Abstracts. 2011;118(21):305. [Google Scholar]
- 96.Heffner LT, Jagannath S, Zimmerman TM, Lee KP, Rosenblatt J, Lonial S, et al. BT062, an Antibody-Drug Conjugate Directed Against CD138, Given Weekly for 3 Weeks in Each 4 Week Cycle: Safety and Further Evidence of Clinical Activity. ASH Annual Meeting Abstracts. 2012;120(21):4042. [Google Scholar]
- 97.Bensinger W, Maziarz RT, Jagannath S, Spencer A, Durrant S, Becker PS, et al. A phase 1 study of lucatumumab, a fully human anti-CD40 antagonist monoclonal antibody administered intravenously to patients with relapsed or refractory multiple myeloma. Br J Haematol. 2012;159(1):58–66. doi: 10.1111/j.1365-2141.2012.09251.x. [DOI] [PubMed] [Google Scholar]
- 98.Hussein M, Berenson JR, Niesvizky R, Munshi N, Matous J, Sobecks R, et al. A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica. 2010;95(5):845–8. doi: 10.3324/haematol.2009.008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raje N, Faber EA, Jr, Richardson PG, Schiller GJ, Hohl RJ, Cohen AD, et al. Phase 1 Study of Tabalumab, a Human Anti-BAFF Antibody and Bortezomib in Patients with Previously-Treated Multiple Myeloma. ASH Annual Meeting Abstracts. 2012;120(21):447. [Google Scholar]
- 100.Voorhees PM, Manges RF, Sonneveld P, Jagannath S, Somlo G, Krishnan A, et al. A Phase 2 Multicenter Study of Siltuximab, An Anti-IL-6 Monoclonal Antibody, in Patients with Relapsed or Refractory Multiple Myeloma. ASH Annual Meeting Abstracts. 2011;118(21):3971. doi: 10.1111/bjh.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rossi J-F, Manges RF, Sutherland HJ, Jagannath S, Voorhees P, Sonneveld P, et al. Preliminary Results of CNTO 328, An Anti-Interleukin-6 Monoclonal Antibody, in Combination with Bortezomib in the Treatment of Relapsed or Refractory Multiple Myeloma. ASH Annual Meeting Abstracts. 2008;112(11):867. [Google Scholar]
- 102.San Miguel J, Blade J, Samoilova OS, Novgorod N, Shpilberg O, Grosicki S, et al. Randomized, Open–Label, Phase 2 Study Of Siltuximab (An Anti–IL–6 Mab) And Bortezomib–Melphalan–Prednisone Versus Bortezomib–Melphalan–Prednisone In Patients With Previously Untreated Multiple Myeloma. Haematologica. 2013;98(S1):Abstract-P225. [Google Scholar]
- 103.Benson DM, Jr, Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagganath S, Abonour R, et al. A phase I trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012 doi: 10.1182/blood-2012-06-438028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Benson DM, Jr, Cohen AD, Munshi NC, Jagannath S, Spitzer G, Hofmeister CC, et al. A Phase I Trial of the Anti-Inhibitory KIR Antibody, IPH2101, and Lenalidomide in Multiple Myeloma: Interim Results. ASH Annual Meeting Abstracts. 2012;120(21):4058. [Google Scholar]
- 105.Ocio EM, San Miguel JF. The DAC system and associations with multiple myeloma. Invest New Drugs. 2010;28(Suppl 1):S28–35. doi: 10.1007/s10637-010-9589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5(10):981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 107.Witt O, Deubzer HE, Milde T, Oehme I. HDAC family: What are the cancer relevant targets? Cancer Lett. 2009;277(1):8–21. doi: 10.1016/j.canlet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 108.Catley L, Weisberg E, Kiziltepe T, Tai YT, Hideshima T, Neri P, et al. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood. 2006;108(10):3441–3449. doi: 10.1182/blood-2006-04-016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maiso P, Carvajal-Vergara X, Ocio EM, Lopez-Perez R, Mateo G, Gutierrez N, et al. The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res. 2006;66(11):5781–5789. doi: 10.1158/0008-5472.CAN-05-4186. [DOI] [PubMed] [Google Scholar]
- 110.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, et al. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc Natl Acad Sci USA. 2004;101(2):540–545. doi: 10.1073/pnas.2536759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khan SB, Maududi T, Barton K, Ayers J, Alkan S. Analysis of histone deacetylase inhibitor, depsipeptide (FR901228), effect on multiple myeloma. Br J Haematol. 2004;125(2):156–161. doi: 10.1111/j.1365-2141.2004.04882.x. [DOI] [PubMed] [Google Scholar]
- 112.Todoerti K, Barbui V, Pedrini O, Lionetti M, Fossati G, Mascagni P, et al. Pleiotropic anti-myeloma activity of ITF2357: inhibition of interleukin-6 receptor signaling and repression of miR-19a and miR-19b. Haematologica. 95(2):260–69. doi: 10.3324/haematol.2009.012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chesi M, Matthews GM, Garbitt VM, Palmer SE, Shortt J, Lefebure M, et al. Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy. Blood. 2012;120(2):376–85. doi: 10.1182/blood-2012-02-412783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Galli M, Salmoiraghi S, Golay J, Gozzini A, Crippa C, Pescosta N, et al. A phase II multiple dose clinical trial of histone deacetylase inhibitor ITF2357 in patients with relapsed or progressive multiple myeloma. Annals of hematology. 2010;89(2):185–90. doi: 10.1007/s00277-009-0793-8. [DOI] [PubMed] [Google Scholar]
- 115.Niesvizky R, Ely S, Mark T, Aggarwal S, Gabrilove JL, Wright JJ, et al. Phase 2 trial of the histone deacetylase inhibitor romidepsin for the treatment of refractory multiple myeloma. Cancer. 2011;117(2):336–42. doi: 10.1002/cncr.25584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Richardson P, Mitsiades C, Colson K, Reilly E, McBride L, Chiao J, et al. Phase I trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) in patients with advanced multiple myeloma. Leuk Lymphoma. 2008;49(3):502–507. doi: 10.1080/10428190701817258. [DOI] [PubMed] [Google Scholar]
- 117.Wolf JL, Siegel D, Goldschmidt H, Hazell K, Bourquelot PM, Bengoudifa BR, et al. Phase II trial of the pan-deacetylase inhibitor panobinostat as a single agent in advanced relapsed/refractory multiple myeloma. Leuk Lymphoma. 2012;53(9):1820–3. doi: 10.3109/10428194.2012.661175. [DOI] [PubMed] [Google Scholar]
- 118.Badros A, Burger AM, Philip S, Niesvizky R, Kolla SS, Goloubeva O, et al. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res. 2009;15(16):5250–5257. doi: 10.1158/1078-0432.CCR-08-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harrison SJ, Quach H, Link E, Seymour JF, Ritchie DS, Ruell S, et al. A high rate of durable responses with romidepsin, bortezomib, and dexamethasone in relapsed or refractory multiple myeloma. Blood. 2011;118(24):6274–83. doi: 10.1182/blood-2011-03-339879. [DOI] [PubMed] [Google Scholar]
- 120.San Miguel J, Sezer O, Gunther A, Siegel D, Blade J, Leblanc R, et al. Phase Ib Dose-Escalation Study Of Oral Panobinostat And iv Bortezomib In Patients With Relapsed Or Relapsed And Refractory Multiple Myeloma: Updated Results. Haematologica. IMW Meeting Abstracts. 2011;96(Supp-1):P-238. [Google Scholar]
- 121.Weber DM, Graef T, Hussein M, Sobecks RM, Schiller GJ, Lupinacci L, et al. Phase I Trial of Vorinostat Combined With Bortezomib for the Treatment of Relapsing and/or Refractory Multiple Myeloma. Clinical lymphoma, myeloma & leukemia. 2012;12(5):319–24. doi: 10.1016/j.clml.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 122.Leleu X, Touzeau C, Benboubker L, Facon T, Delain M, Fourneau N, et al. Phase Ib dose escalation study of oral quisinostat, a histone deacetylase inhibitor, in combination with bortezomib and dexamethasone for patients with relapsed multiple myeloma. ASCO Meeting Abstracts. 2013;31(15_suppl):8530. [Google Scholar]
- 123.Dimopoulos MA, Jagannath S, Yoon S-S, Siegel DS, Lonial S, Hajek R, et al. Vantage 088: Vorinostat in Combination with Bortezomib in Patients with Relapsed/Refractory Multiple Myeloma: Results of a Global, Randomized Phase 3 Trial. ASH Annual Meeting Abstracts. 2011;118(21):811. [Google Scholar]
- 124.Siegel DS, Dimopoulos MA, Yoon S-S, Laubach JP, Kaufman JL, Goldschmidt H, et al. Vantage 095: Vorinostat in Combination with Bortezomib in Salvage Multiple Myeloma Patients: Final Study Results of a Global Phase 2b Trial. ASH Annual Meeting Abstracts. 2011;118(21):480. [Google Scholar]
- 125.Richardson PG, Schlossman RL, Alsina M, Weber DM, Coutre SE, Gasparetto C, et al. PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood. 2013 doi: 10.1182/blood-2013-01-481325. [DOI] [PubMed] [Google Scholar]
- 126.Raje N, Hari PN, Vogl DT, Jagannath S, Orlowski RZ, Supko JG, et al. Rocilinostat (ACY-1215), a Selective HDAC6 Inhibitor, Alone and in Combination with Bortezomib in Multiple Myeloma: Preliminary Results From the First-in-Humans Phase I/II Study. ASH Annual Meeting Abstracts. 2012;120(21):4061. [Google Scholar]
- 127.Raje N, Mahindra A, Vogl D, Voorhees PM, Bensinger W, Parameswaran RV, et al. New Drug Partner For Combination Therapy In Multiple Myeloma (MM): Development Of ACY–1215, A Selective Histone Deacetylase 6 Inhibitor Alone And In Combination With Bortezomib Or Lenalidomide. Haematologica. 2013;98(S1):Abstract-P765. [Google Scholar]
- 128.Bergsagel PL, Kuehl WM, Zhan F, Sawyer J, Barlogie B, Shaughnessy J., Jr Cyclin D dysregulation: an early and unifying pathogenic event in multiple myeloma. Blood. 2005;106(1):296–303. doi: 10.1182/blood-2005-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stewart AK, Vij R, Laubach JP, Hofmeister CC, Hagerty R, Dueck AC, et al. Phase I Study of Aurora Kinase Inhibitor MLN8237 and Bortezomib in Relapsed or Refractory Multiple Myeloma. ASH Annual Meeting Abstracts. 2012;120(21):1859. [Google Scholar]
- 130.Shah JJ, Zonder J, Cohen A, Orlowski RZ, Alexanian R, Thomas SK, et al. ARRY-520 Shows Durable Responses in Patients with Relapsed/Refractory Multiple Myeloma in a Phase 1 Dose-Escalation Study. ASH Annual Meeting Abstracts. 2011;118(21):1860. [Google Scholar]
- 131.Shah JJ, Zonder JA, Cohen A, Bensinger W, Kaufman JL, Orlowski RZ, et al. The Novel KSP Inhibitor ARRY-520 Is Active Both with and without Low-Dose Dexamethasone in Patients with Multiple Myeloma Refractory to Bortezomib and Lenalidomide: Results From a Phase 2 Study. ASH Annual Meeting Abstracts. 2012;120(21):449. [Google Scholar]
- 132.Zhu YX, Tiedemann R, Shi CX, Yin H, Schmidt JE, Bruins LA, et al. RNAi screen of the druggable genome identifies modulators of proteasome inhibitor sensitivity in myeloma including CDK5. Blood. 2011;117(14):3847–57. doi: 10.1182/blood-2010-08-304022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kumar SK, LaPlant BR, Chng WJ, Zonder JA, Callander N, Roy V, et al. Phase 1/2 Trial of a Novel CDK Inhibitor Dinaciclib (SCH727965) in Patients with Relapsed Multiple Myeloma Demonstrates Encouraging Single Agent Activity. ASH Annual Meeting Abstracts. 2012;120(21):76. [Google Scholar]
- 134.Scheid C, Reece D, Beksac M, Spencer A, Callander N, Sonneveld P, et al. A Phase 2, Multicenter, Nonrandomized, Open-Label Study of Dovitinib (TKI258) in Patients with Relapsed or Refractory Multiple Myeloma with or without t(4;14) Translocation. ASH Annual Meeting Abstracts. 2012;120(21):4055. [Google Scholar]
- 135.Trudel S, Bergsagel PL, Singhal S, Niesvizky R, Comenzo RL, Bensinger WI, et al. A Phase I Study of the Safety and Pharmacokinetics of Escalating Doses of MFGR1877S, a Fibroblast Growth Factor Receptor 3 (FGFR3) Antibody, in Patients with Relapsed or Refractory t(4;14)-Positive Multiple Myeloma. ASH Annual Meeting Abstracts. 2012;120(21):4029. [Google Scholar]
- 136.Arnulf B, Ghez D, Leblond V, Choquet S, Belhadj K, Macro M, et al. FGFR3 Tyrosine Kinase Inhibitor AB1010 as Treatment of t(4;14) Multiple Myeloma. Blood. 2007;110(11):128a–Abstract 413. [Google Scholar]
- 137.Dispenzieri A, Gertz MA, Lacy MQ, Geyer SM, Greipp PR, Rajkumar SV, et al. A phase II trial of imatinib in patients with refractory/relapsed myeloma. Leuk Lymphoma. 2006;47(1):39–42. doi: 10.1080/10428190500271269. [DOI] [PubMed] [Google Scholar]
- 138.Wildes TM, Procknow E, Gao F, Dipersio JF, Vij R. Dasatinib in relapsed or plateau-phase multiple myeloma. Leuk Lymphoma. 2009;50(1):137–40. doi: 10.1080/10428190802563363. [DOI] [PubMed] [Google Scholar]
- 139.Facon T, Leleu X, Stewart AK, Spencer A, Rowlings P, Hulin C, et al. Dasatinib in Combination with Lenalidomide and Dexamethasone in Patients with Relapsed or Refractory Multiple Myeloma: Preliminary Results of a Phase I Study. ASH Annual Meeting Abstracts. 2009;114(22):1876. [Google Scholar]
- 140.Callander NS, Markovina S, Juckett MB, Wagner E, Kolesar J, Longo W, et al. The Addition of Bevacizumab (B) to Lenalidomide and Low-Dose Dexamethasone Does Not Significantly Increase Response in Relapsed or Refractory Multiple Myeloma (NCI#7317) ASH Annual Meeting Abstracts. 2009;114(22):3885. [Google Scholar]
- 141.Lacy MQ, Alsina M, Fonseca R, Paccagnella ML, Melvin CL, Yin D, et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 Receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(19):3196–203. doi: 10.1200/JCO.2007.15.9319. [DOI] [PubMed] [Google Scholar]
- 142.Moreau P, Cavallo F, Leleu X, Hulin C, Amiot M, Descamps G, et al. Phase I study of the anti insulin-like growth factor 1 receptor (IGF-1R) monoclonal antibody, AVE1642, as single agent and in combination with bortezomib in patients with relapsed multiple myeloma. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2011;25(5):872–4. doi: 10.1038/leu.2011.4. [DOI] [PubMed] [Google Scholar]
- 143.von Tresckow B, Boll B, Eichenauer DA, Peine D, Knop S, Goebeler M, et al. A Phase II Clinical Trial of the Anti-EGFR Antibody Cetuximab in Patients with Refractory or Relapsed Multiple Myeloma: Final Results. ASH Annual Meeting Abstracts. 2011;118(21):3965. [Google Scholar]
- 144.Ghobrial IM, Munshi NC, Harris BN, Shi P, Porter NM, Schlossman RL, et al. A phase I safety study of enzastaurin plus bortezomib in the treatment of relapsed or refractory multiple myeloma. Am J Hematol. 2011;86(7):573–8. doi: 10.1002/ajh.22048. [DOI] [PubMed] [Google Scholar]
- 145.Richardson PG, Badros AZ, Jagannath S, Tarantolo S, Wolf JL, Albitar M, et al. Tanespimycin with bortezomib: activity in relapsed/refractory patients with multiple myeloma. British journal of haematology. 2010;150(4):428–37. doi: 10.1111/j.1365-2141.2010.08264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Richardson PG, Chanan-Khan AA, Lonial S, Krishnan AY, Carroll MP, Alsina M, et al. Tanespimycin and bortezomib combination treatment in patients with relapsed or relapsed and refractory multiple myeloma: results of a phase 1/2 study. British journal of haematology. 2011;153(6):729–40. doi: 10.1111/j.1365-2141.2011.08664.x. [DOI] [PubMed] [Google Scholar]
- 147.Richardson P, Lonial S, Jakubowiak A, Krishnan A, Wolf J, Densmore J, et al. Multi-Center Phase II Study of Perifosine (KRX-0401) Alone and in Combination with Dexamethasone (dex) for Patients with Relapsed or Relapsed/Refractory Multiple Myeloma (MM): Promising Activity as Combination Therapy with Manageable Toxicity. Blood. 2007;110(11):353a–Abstract 1164. [Google Scholar]
- 148.Richardson PG, Wolf J, Jakubowiak A, Zonder J, Lonial S, Irwin D, et al. Perifosine plus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma previously treated with bortezomib: results of a multicenter phase I/II trial. J Clin Oncol. 2011;29(32):4243–9. doi: 10.1200/JCO.2010.33.9788. [DOI] [PubMed] [Google Scholar]
- 149.Jakubowiak AJ, Richardson PG, Zimmerman T, Alsina M, Kaufman JL, Kandarpa M, et al. Perifosine plus lenalidomide and dexamethasone in relapsed and relapsed/refractory multiple myeloma: a Phase I Multiple Myeloma Research Consortium study. British journal of haematology. 2012;158(4):472–80. doi: 10.1111/j.1365-2141.2012.09173.x. [DOI] [PubMed] [Google Scholar]
- 150.Spencer A, Yoon S-S, Harrison SJ, Morris S, Smith D, Freedman SJ, et al. Novel AKT Inhibitor GSK2110183 Shows Favorable Safety, Pharmacokinetics, and Clinical Activity in Multiple Myeloma. Preliminary Results From a Phase I First-Time-In-Human Study. ASH Annual Meeting Abstracts. 2011;118(21):1856. [Google Scholar]
- 151.Guenther A, Baumann P, Burger R, Klapper W, Schmidmaier R, Gramatzki M. Single-agent everolimus (RAD001) in patients with relapsed or refractory multiple myeloma: Final results of a phase I study. ASCO Meeting Abstracts. 2010;28(15_suppl):8137. [Google Scholar]
- 152.Farag SS, Zhang S, Jansak BS, Wang X, Kraut E, Chan K, et al. Phase II trial of temsirolimus in patients with relapsed or refractory multiple myeloma. Leuk Res. 2009;33(11):1475–80. doi: 10.1016/j.leukres.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ghobrial IM, Weller E, Vij R, Munshi NC, Banwait R, Bagshaw M, et al. Weekly bortezomib in combination with temsirolimus in relapsed or relapsed and refractory multiple myeloma: a multicentre, phase 1/2, open-label, dose-escalation study. Lancet Oncol. 2011;12(3):263–72. doi: 10.1016/S1470-2045(11)70028-6. [DOI] [PubMed] [Google Scholar]
- 154.Mahindra A, Richardson PG, Hari P, Sohani AR, Laubach JP, Burke J, et al. Updated Results of a Phase I Study of RAD001 In Combination with Lenalidomide In Patients with Relapsed or Refractory Multiple Myeloma with Pharmacodynamic and Pharmacokinetic Analysis. ASH Annual Meeting Abstracts. 2010;116(21):3051. [Google Scholar]
- 155.Yee AJ, Mahindra AK, Richardson PG, Cirstea DD, Scullen TA, Rodig SJ, et al. Biomarker Correlation with Outcomes in Patients with Relapsed or Refractory Multiple Myeloma on a Phase I Study of Everolimus in Combination with Lenalidomide. ASH Annual Meeting Abstracts. 2011;118(21):3966. [Google Scholar]
- 156.Hofmeister CC, Yang X, Pichiorri F, Chen P, Rozewski DM, Johnson AJ, et al. Phase I trial of lenalidomide and CCI-779 in patients with relapsed multiple myeloma: evidence for lenalidomide-CCI-779 interaction via P-glycoprotein. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(25):3427–34. doi: 10.1200/JCO.2010.32.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ghobrial IM, Siegel D, Vij R, Wolf JL, Berdeja JG, Matous JV, et al. MLN0128 (INK128), an Investigational Oral Dual TORC1/2 Inhibitor, in Patients (pts) with Relapsed or Refractory Multiple Myeloma (MM), Non-Hodgkin’s Lymphoma (NHL), or Waldenstrom Macroglobulinemia (WM): Preliminary Results From a Phase 1 Dose-Escalation Study. ASH Annual Meeting Abstracts. 2012;120(21):4038. [Google Scholar]
- 158.Alsina M, Fonseca R, Wilson EF, Belle AN, Gerbino E, Price-Troska T, et al. Farnesyltransferase inhibitor tipifarnib is well tolerated, induces stabilization of disease, and inhibits farnesylation and oncogenic/tumor survival pathways in patients with advanced multiple myeloma. Blood. 2004;103(9):3271–3277. doi: 10.1182/blood-2003-08-2764. [DOI] [PubMed] [Google Scholar]
- 159.Holkova B, Badros AZ, Geller R, Voorhees PM, Zingone A, Korde N, et al. A Phase II Study of the MEK 1/2 Inhibitor AZD6244 (Selumetinib, ARRY-142866) in Relapsed or Refractory Multiple Myeloma. ASH Annual Meeting Abstracts. 2011;118(21):2931. [Google Scholar]
- 160.Siegel DS, Krishnan A, Lonial S, Chatta G, Alsina M, Jagannath S, et al. Phase II Trial of SCIO-469 as Monotherapy (M) or in Combination with Bortezomib (MB) in Relapsed Refractory Multiple Myeloma (MM) Blood. 2006;108(11):Abstract 3580. [Google Scholar]
- 161.Mateos MV, Cibeira MT, Richardson PG, Prosper F, Oriol A, de la Rubia J, et al. Phase II clinical and pharmacokinetic study of plitidepsin 3-hour infusion every two weeks alone or with dexamethasone in relapsed and refractory multiple myeloma. Clin Cancer Res. 2010;16(12):3260–9. doi: 10.1158/1078-0432.CCR-10-0469. [DOI] [PubMed] [Google Scholar]
- 162.Chapman MA, Lawrence MS, Keats JJ, Cibulskis K, Sougnez C, Schinzel AC, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471(7339):467–72. doi: 10.1038/nature09837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lehners N, Andrulis M, Capper D, von Deimling A, Ho AD, Goldschmidt H, et al. BRAF V600E Mutations in Multiple Myeloma: Clinical and Therapeutic Implications. ASH Annual Meeting Abstracts. 2012;120(21):4040. [Google Scholar]
- 164.Chen W, Qiu L, Hou J, Zhang X, Ke X, Wang Z, et al. Phase Ib Study of Recombinant Circularly Permuted TRAIL (CPT) in Relapsed or Refractory multiple Myeloma Patients. ASH Annual Meeting Abstracts. 2012;120(21):1857. [Google Scholar]
- 165.Chen W, Qiu L, Hou J, Zhao Y, Pan L, Yang S, et al. Recombinant Circularly Permuted TRAIL (CPT) for the Treatment of Relapsed or Refractory Multiple Myeloma: An Open-Label, Multicenter Phase II Clinical Trial. ASH Annual Meeting Abstracts. 2012;120(21):78. [Google Scholar]
- 166.Chen W, Hou J, Zhao Y, Qiu L, Ke X, Wang Z, et al. Circularly Permuted TRAIL (CPT) combined with Thalidomide for the Treatment of Relapsed or Refractory Multiple Myeloma: An Open-Label, Multicenter Phase II Clinical Trial. ASH Annual Meeting Abstracts. 2012;120(21):2958. [Google Scholar]
- 167.Ocio EM, De La Rubia J, Oriol-Rocafiguera A, Blade J, Rodriguez J, Coronado C, et al. Phase II Optimization, Open-Label Clinical Trial of Zalypsis(R) (PM00104) in Relapsed/Refractory Multiple Myeloma Patients. ASH Annual Meeting Abstracts. 2012;120(21):4041. [Google Scholar]
- 168.Neri P, Duggan P, Gratton K, Ren L, Johnson J, Slaby J, et al. Phase I Study of the PARP1-2 Inhibitor Veliparib in Combination with Bortezomib in Patients with Relapsed or Refractory Multiple Myeloma. ASH Annual Meeting Abstracts. 2012;120(21):1862. [Google Scholar]
- 169.Martin SK, Diamond P, Gronthos S, Peet DJ, Zannettino AC. The emerging role of hypoxia, HIF-1 and HIF-2 in multiple myeloma. Leukemia. 2011;25(10):1533–42. doi: 10.1038/leu.2011.122. [DOI] [PubMed] [Google Scholar]
- 170.Ghobrial IM, Laubach J, Armand P, Boswell E, Hanlon C, Chuma S, et al. Phase I study of TH-302, an investigational hypoxia-targeted drug, and dexamethasone in patients with relapsed/refractory multiple myeloma. ASCO Meeting Abstracts. 2013;31(15_suppl):8602. [Google Scholar]
- 171.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19(8):2048–60. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rosenblatt J, Avivi I, Vasir B, Uhl L, Munshi NC, Katz T, et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res. 2013;19(13):3640–8. doi: 10.1158/1078-0432.CCR-13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34(5):409–18. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–94. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.O’Connor OA, Stewart AK, Vallone M, Molineaux CJ, Kunkel LA, Gerecitano JF, et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15(22):7085–91. doi: 10.1158/1078-0432.CCR-09-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Alsina M, Trudel S, Furman RR, Rosen PJ, O’Connor OA, Comenzo RL, et al. A phase I single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clin Cancer Res. 2012;18(17):4830–40. doi: 10.1158/1078-0432.CCR-11-3007. [DOI] [PubMed] [Google Scholar]
- 177.Jagannath S, Vij R, Stewart AK, Trudel S, Jakubowiak AJ, Reiman T, et al. An Open-Label Single-Arm Pilot Phase II Study (PX-171-003-A0) of Low-Dose, Single-Agent Carfilzomib in Patients With Relapsed and Refractory Multiple Myeloma. Clinical lymphoma, myeloma & leukemia. 2012;12(5):310–8. doi: 10.1016/j.clml.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 178.diCapua Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Jagannath S, et al. Results of PX-171-003-A1, An Open-Label, Single-Arm, Phase 2 (Ph 2) Study of Carfilzomib (CFZ) In Patients (pts) with Relapsed and Refractory Multiple Myeloma (MM) ASH Annual Meeting Abstracts. 2010;116(21):985. [Google Scholar]
- 179.Schey SA, Fields P, Bartlett JB, Clarke IA, Ashan G, Knight RD, et al. Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol. 2004;22(16):3269–76. doi: 10.1200/JCO.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 180.Streetly MJ, Gyertson K, Daniel Y, Zeldis JB, Kazmi M, Schey SA. Alternate day pomalidomide retains anti-myeloma effect with reduced adverse events and evidence of in vivo immunomodulation. Br J Haematol. 2008;141(1):41–51. doi: 10.1111/j.1365-2141.2008.07013.x. [DOI] [PubMed] [Google Scholar]
- 181.Richardson PG, Siegel DS, Vij R, Hofmeister CC, Jagannath S, Chen C, et al. Randomized, Open Label Phase 1/2 Study of Pomalidomide (POM) Alone or in Combination with Low-Dose Dexamethasone (LoDex) in Patients (Pts) with Relapsed and Refractory Multiple Myeloma Who Have Received Prior Treatment That Includes Lenalidomide (LEN) and Bortezomib (BORT): Phase 2 Results. ASH Annual Meeting Abstracts. 2011;118(21):634. [Google Scholar]
- 182.Siegel DSD, Richardson PGG, Vij R, Hofmeister CC, Baz RC, Jagannath S, et al. Long-term safety and efficacy of pomalidomide (POM) with or without low-dose dexamethasone (LoDEX) in relapsed and refractory multiple myeloma (RRMM) patients enrolled in the MM-002 phase II trial. ASCO Meeting Abstracts. 2013;31(15_suppl):8588. [Google Scholar]
- 183.Mark TM, Boyer A, Rossi AC, Shah M, Pearse RN, Zafar F, et al. ClaPD (Clarithromycin, Pomalidomide, Dexamethasone) Therapy in Relapsed or Refractory Multiple Myeloma. ASH Annual Meeting Abstracts. 2012;120(21):77. [Google Scholar]
- 184.Hilger JD, Berenson JR, Klein LM, Bessudo A, Rosen PJ, Eshaghian S, et al. A phase I/II study ( NCT01541332) of pomalidomide (POM), dexamethasone (DEX), and pegylated liposomal doxorubicin (PLD) for patients with relapsed/refractory (R/R) multiple myeloma (MM) ASCO Meeting Abstracts. 2013;31(15_suppl):8598. [Google Scholar]
- 185.Richardson PGG, Hofmeister CC, Siegel DSD, Lonial S, Laubach J, Efebera YA, et al. MM-005: A phase I trial of pomalidomide, bortezomib, and low-dose dexamethasone (PVD) in relapsed and/or refractory multiple myeloma (RRMM) ASCO Meeting Abstracts. 2013;31(15_suppl):8584. [Google Scholar]
- 186.Baz R, Shain KH, Alsina M, Nardelli LA, Nishihori T, Ochoa L, et al. Oral Weekly Cyclophosphamide in Combination with Pomalidomide and Dexamethasone for Relapsed and Refractory Myeloma: Report of the Dose Escalation Cohort. ASH Annual Meeting Abstracts. 2012;120(21):4062. [Google Scholar]
- 187.Larocca A, Montefusco V, Bringhen S, Rossi D, Crippa C, Mina R, et al. Pomalidomide, cyclophosphamide, and prednisone for relapsed/refractory multiple myeloma: a multicenter phase 1/2 open-label study. Blood. 2013;122(16):2799–806. doi: 10.1182/blood-2013-03-488676. [DOI] [PubMed] [Google Scholar]
- Jakubowiak AJ, Benson DM, Bensinger W, Siegel DS, Zimmerman TM, Mohrbacher A, et al. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol. 2012;30(16):1960–5. doi: 10.1200/JCO.2011.37.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Berdeja JG, Hernandez-Ilizaliturri F, Chanan-Khan A, Patel M, Kelly KR, Running KL, et al. Phase I Study of Lorvotuzumab Mertansine (LM, IMGN901) in Combination with Lenalidomide (Len) and Dexamethasone (Dex) in Patients with CD56-Positive Relapsed or Relapsed/Refractory Multiple Myeloma (MM) ASH Annual Meeting Abstracts. 2012;120(21):728. [Google Scholar]
- 190.Agura E, Niesvizky R, Matous J, Munshi N, Hussein M, Parameswaran RV, et al. Dacetuzumab (SGN-40), Lenalidomide, and Weekly Dexamethasone in Relapsed or Refractory Multiple Myeloma: Multiple Responses Observed in a Phase 1b Study. ASH Annual Meeting Abstracts. 2009;114(22):2870. [Google Scholar]
- 191.Richardson P, Weber D, Mitsiades CS, Dimopoulos MA, Harousseau J-L, Houp J, et al. A Phase I Study of Vorinostat, Lenalidomide, and Dexamethasone In Patients with Relapsed or Relapsed and Refractory Multiple Myeloma: Excellent Tolerability and Promising Activity In a Heavily Pretreated Population. ASH Annual Meeting Abstracts. 2010;116(21):1951. [Google Scholar]
- 192.Richter JR, Bilotti E, McBride L, Schmidt L, Gao Z, Tufail M, et al. Salvage Therapy with Vorinostat, Lenalidomide, and Dexamethasone (ZRD) in Lenalidomide/Dexamethasone Relapsed/Refractory Multiple Myeloma. ASH Annual Meeting Abstracts. 2011;118(21):3986. [Google Scholar]
- 193.Mateos M, Spencer A, Taylor K, Lonial S, De La Rubia J, Facon T, et al. Phase Ib study of oral panobinostat (LBH589) plus lenalidomide (LEN) plus dexamethasone (DEX) in patients (Pts) with relapsed (Rel) or Rel and refractory (Ref) multiple myeloma (MM) ASCO Meeting Abstracts. 2010;28(15_suppl):8030. [Google Scholar]
- 194.Voorhees PM, Gasparetto C, Osman K, Richards KL, Ferraro M, Garcia R, et al. Vorinostat in Combination with Pegylated Liposomal Doxorubicin (PLD) and Bortezomib (B) in Patients with Relapsed/Refractory Multiple Myeloma (R/R MM): Final Results of a Phase I Study. ASH Annual Meeting Abstracts. 2011;118(21):3985. [Google Scholar]
- 195.Siegel D, Bilotti E, McBride L, Richardson P, Schmidt L, Gao Z, et al. Vorinostat Overcomes Resistance In Patients With Multiple Myeloma Refractory To Bortezomib, Lenalidomide And Dexamethasone. Haematologica. IMW Meeting Abstracts. 2011;96(Supp-1):P-216. [Google Scholar]
- 196.Kaufman JL, Shah JJ, Laubach JP, Mitchell AR, Sharp C, Lewis C, et al. Lenalidomide, Bortezomib, and Dexamethasone (RVD) in Combination with Vorinostat As Front-Line Therapy for Patients with Multiple Myeloma (MM): Results of a Phase 1 Study. ASH Annual Meeting Abstracts. 2012;120(21):336. [Google Scholar]
- 197.Berenson JR, Yellin O, Kazamel T, Boccia RV, Matous J, Dressler K, et al. A Phase I/II Study Of Oral Melphalan (Mel) Combined With Panobinostat (Pan) For Patients With Relapsed Or Refractory (R/R) Multiple Myeloma (MM) Haematologica. IMW Meeting Abstracts. 2011;96(Supp-1):P-206. [Google Scholar]
- 198.Offidani M, Cavallo F, Polloni C, Liberati M, Ballanti S, Pulini S, et al. Phase I-II Study Of Melphalan, Thalidomide And Prednisone (MPT) Combined With Oral Panobinostat In Patients With Relapsed/Refractory MM. Haematologica. IMW Meeting Abstracts. 2011;96(Supp-1):P-191. [Google Scholar]
- 199.Niesvizky R, Lentzsch S, Badros AZ, Chanan-Khan AA, Singhal SB, Zonder JA, et al. A Phase I Study of PD 0332991: Complete CDK4/6 Inhibition and Tumor Response In Sequential Combination with Bortezomib and Dexamethasone for Relapsed and Refractory Multiple Myeloma. ASH Annual Meeting Abstracts. 2010;116(21):860. [Google Scholar]
- 200.Richardson PG, Chanan-Khan AA, Alsina M, Albitar M, Berman D, Messina M, et al. Tanespimycin monotherapy in relapsed multiple myeloma: results of a phase 1 dose-escalation study. British journal of haematology. 2010;150(4):438–45. doi: 10.1111/j.1365-2141.2010.08265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]