Abstract
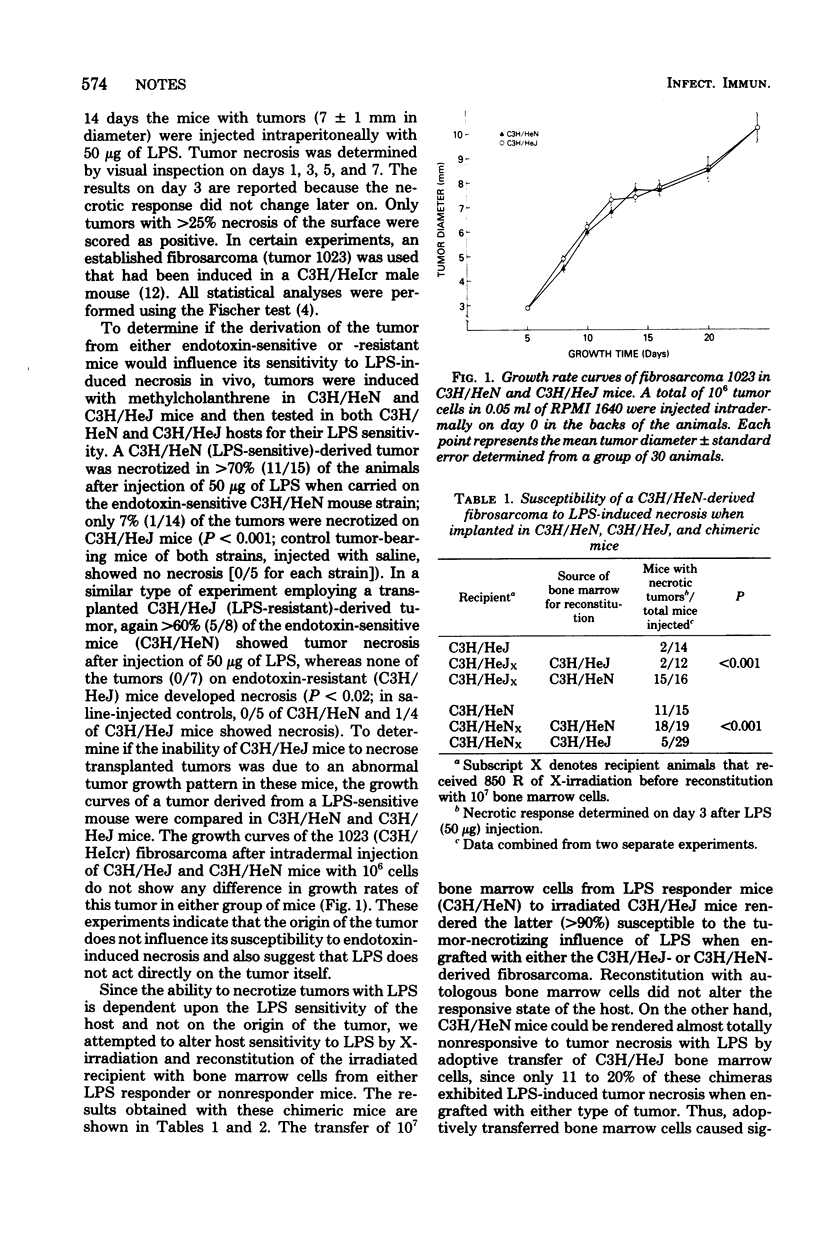
Lipopolysaccharide (LPS) induces rapid necrosis of intradermal fibrosarcomas in mice. The mechanism(s) by which LPS produces tumor necrosis has been investigated using histocompatible LPS-sensitive (C3H/HeN) and LPS-resistant (C3H/HeJ) mouse strains. C3H/HeN- or C3H/HeJ-derived fibrosarcomas were necrotized by LPS when they were grafted onto C3H/HeN mice but were not affected when growing on C3H/HeJ mice, indicating that LPS does not act directly on the tumor itself. In contrast, lethally X-irradiated C3H/HeJ mice exhibit necrosis of their tumors when reconstituted with C3H/HeN bone marrow cells, whereas C3H/HeN mice no longer exert LPS-induced tumor necrosis after the adoptive transfer of C3H/HeJ bone marrow cells. These findings clearly indicate that LPS produces necrosis of tumors by activating host lymphoreticular cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALGIRE G. H., LEGALLAIS F. Y., ANDERSON B. F. Vascular reactions of normal and malignant tissues in vivo. V. The rôle of hypotension in the action of a bacterial polysaccharide on tumors. J Natl Cancer Inst. 1952 Jun;12(6):1279–1295. [PubMed] [Google Scholar]
- Bartlett G. L., Zbar B., Rapp H. J. Suppression of murine tumor growth by immune reaction to the Bacillus Calmette-Guérin strain of Mycobacterium bovis. J Natl Cancer Inst. 1972 Jan;48(1):245–257. [PubMed] [Google Scholar]
- Berendt M. J., Mezrow G. F., Saluk P. H. Requirement for a radiosensitive lymphoid cell in the generation of lipopolysaccharide-induced rejection of a murine tumor allograft. Infect Immun. 1978 Sep;21(3):1033–1035. doi: 10.1128/iai.21.3.1033-1035.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun W. Immunologic and antineoplastic effects of endotoxin: role of membranes and mediation by cyclic adenosine-3',5'-monophosphate. J Infect Dis. 1973 Jul;128(Suppl):188–197. doi: 10.1093/infdis/128.supplement_1.s188. [DOI] [PubMed] [Google Scholar]
- Butler R. C., Abdelnoor A. M., Nowotny A. Bone marrow colony-stimulating factor and tumor resistance-enhancing activity of postendotoxin mouse sera. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2893–2896. doi: 10.1073/pnas.75.6.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode L. M., Mergenhagen S. E., Rosenstreich D. L. Significant contribution of spleen cells in mediating the lethal effects of endotoxin in vivo. Infect Immun. 1976 Sep;14(3):626–630. doi: 10.1128/iai.14.3.626-630.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Dobrjansky A., Carswell E. A., Kassel R. L., Old L. J., Fiore N., Schwartz M. K. Partial purification of a serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1976 Feb;73(2):381–385. doi: 10.1073/pnas.73.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Parr I., Wheeler E., Alexander P. Similarities of the anti-tumour actions of endotoxin, lipid A and double-stranded RNA. Br J Cancer. 1973 May;27(5):370–389. doi: 10.1038/bjc.1973.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., McAdam K. P. Lymphoid cells in endotoxin-induced production of the amyloid-related serum amyloid A protein. Infect Immun. 1979 Jan;23(1):181–183. doi: 10.1128/iai.23.1.181-183.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S., Rosenstreich D. L. Macrophage activation for tumor cytotoxicity: control of macrophage tumoricidal capacity by the LPS gene. J Immunol. 1978 Aug;121(2):543–548. [PubMed] [Google Scholar]



