Abstract
Hippocampus-dependent learning and memory are associated with trafficking of excitatory amino acid transporter type 3 (EAAT3) to the plasma membrane. To assess whether this trafficking is an intrinsic component of the biochemical responses underlying learning and memory, 7- to 9-week old male EAAT3 knockout mice and CD-1 wild-type mice were subjected to fear conditioning. Their hippocampal CA1 regions, amygdalae and entorhinal cortices were harvested before, or 30 min or 3 h after the fear conditioning stimulation. We found that EAAT3 knockout mice had worse contextual and tone-related learning and memory than did the wild-type mice. The expression of EAAT3, glutamate receptor (GluR)1 and GluR2 in the plasma membrane and of phospho-GluR1 (at Ser 831) and phospho-CaMKII in the hippocampus of the wild-type mice was increased at 30 min after the fear conditioning stimulation. Similar biochemical changes occurred in the amygdala. Fear conditioning also increased the expression of c-Fos and activity-regulated cytoskeleton-associated protein (Arc) in the CA1 regions and of Arc in the entorhinal cortices of the wild-type mice. These biochemical responses were attenuated in the EAAT3 knockout mice. These results suggest that EAAT3 plays a critical role in learning and memory. Our results also provide initial evidence that EAAT3 may have receptor-like functions to participate in the biochemical reactions underlying learning and memory.
Keywords: Glutamate transporters, Learning and memory, AMPA receptor
1. Introduction
The initiation of learning- and memory-related biochemical responses in neurons has been considered to start with a training stimulus that causes glutamate release from presynaptic termini. Glutamate then activates glutamate receptors (GluR) in the post-synaptic membrane. Activation of glutamate receptors increases intracellular Ca++, which then activates calcium/calmodulin-dependent protein kinase II (CaMKII) and other calcium-dependent protein kinases (Miyamoto, 2006). These kinases can phosphorylate a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, leading to their trafficking to the plasma membrane (Esteban et al., 2003; Man, Sekine-Aizawa, & Huganir, 2007) to increase synaptic strength (Man et al., 2007; Miyamoto, 2006). Additional responses required for learning and memory include increased expression of immediate–early gene family members, such as c-fos, and of the activity-regulated cytoskeleton-associated protein (Arc), which is necessary for memory formation (McIntyre et al., 2005; Miyamoto, 2006).
Learning and memory also can induce trafficking of the glutamate transporter (also named excitatory amino acid transporter, EAAT) type 3 (EAAT3) to the plasma membrane (Levenson et al., 2002; Pita-Almenar, Collado, Colbert, & Eskin, 2006). Five EAATs have been described. EAAT1 and EAAT2 are expressed in the glia. EAAT3 and EAAT4 are mainly in the neurons. EAAT5 is found in the retina. EAAT1, EAAT2 and EAAT3 are expressed in many regions of the brain; whereas EAAT4 is found mostly in the cerebellum (Danbolt, 2001). One of the major functions of EAATs is to transport glutamate from extracellular space into intracellular compartments under physiological conditions, thus regulating glutamate neurotransmission (Danbolt, 2001). It is not known yet whether the increased expression of EAAT3 in the plasma membrane after a learning stimulus is a feed-back response for the transient increase of synaptic glutamate caused by the learning stimulus or an intrinsic component of the biochemical responses underlying learning and memory. To address this issue, we used EAAT3 knockout mice and studied the learning- and memory-dependent biochemical responses of these mice and the corresponding wild-type mice.
2. Experimental methods
The animal protocol was approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80-23) revised in 2011.
2.1. Animals
The EAAT3 knockout mice were descendants of the mice established by Peghini, Janzen, and Stoffel (1997). A neomycin resistance cassette was inserted to disrupt exon 1 of the eaat3 gene in these mice. They were backcrossed with wild-type CD-1 mice for more than 10 generations to generate a strain of EAAT3 knockout mice before our study. We also backcrossed the EAAT3 knockout mice with wild-type CD-1 mice at least once every 8 generations to prevent genetic drift as recommended by the Banbury Conference (Silva et al., 1997). The CD-1 wild-type mice used for backcrossing were from Charles River Laboratories (Wilmington, MA). The wild-type mice used in this study were the littermates of the EAAT3 knockout mice obtained during the backcrossing. Our previous studies had shown that the EAAT3 knockout mice did not express EAAT3 proteins and that the expression of EAAT1 and EAAT2 in their brains was not affected (Lee, Li, & Zuo, 2010; Li & Zuo, 2011). A total of 20 wild-type CD-1 mice and 20 EAAT3 knockout mice were used in the fear conditioning behavioral test. Another 58 wild-type CD-1 mice and 58 EAAT3 knockout mice were used for harvesting brain tissues after fear conditioning stimulation for analyzing protein expression so that each experimental condition would have data from at least 5 animals. The number of animals for each experimental condition is stated in the figure legends.
2.2. Fear conditioning test
Seven- to nine-week old male wild-type or EAAT3 knockout mice were subjected to the fear conditioning test, a very sensitive and non-effort-dependent test of learning and memory. As we described before (Lin & Zuo, 2011), each animal was placed in a test chamber wiped with 70% alcohol and subjected to 3 tone-foot shock pairings (tone: 2000 Hz, 75 db, 30 s; foot shock: 0.3 mA, 2 s) with an intertrial interval 1 min in a relatively dark room (training sessions). The animal was removed from this test chamber 30 s after the conditioning training, then placed back in the chamber 24 h later for 5 min in the absence of tone and shock. The amount of time with freezing behavior was recorded in a 6 s interval. The animal was placed 2 h later in a test chamber that had different context and smell from the first test chamber (this second chamber was wiped with 1% acetic acid) in a relatively light room. After a 2-min acclimation time, the auditory stimulus then was turned on for 3 cycles, each cycle for 30 s followed by a 1-min inter-cycle interval (4.5 min in total). The freezing behavior in the 4.5 min was recorded. Freezing behavior was defined as absence of all movements except for respiration. Freezing behavior assessed from the video was scored by an observer who was blinded to the group assignment. A percentage of freezing behavior was calculated using the formula f/n, where f is the number of freezing events observed per mouse and n is the total number of observations of the mouse. These tests test hippocampus-dependent (context-related) and hippocampus-independent (tone-related) learning and memory functions (Kim & Fanselow, 1992).
2.3. Fear conditioning stimulation and brain tissue harvest
Seven- to nine-week old male wild-type or EAAT3 knockout mice were subjected to the fear conditioning stimuli (the 3 tone-foot shock pairings). Their brains were harvested at 30 min or 180 min after the last tone-foot shock pair. Brains also were harvested from a group of mice (time 0 or control mice) that did not receive the fear conditioning stimuli.
To harvest brain tissues, mice were anesthetized with 3% isoflurane and perfused transcardially with saline. Their brains were removed and immediately placed on ice. A 2-mm-thick coronal slice from Bregma –2 mm to Bregma –4 mm was taken from each mouse with the aid of a mouse brain matrix. The hippocampal CA1 region and the entorhinal cortex were dissected out from this slice for Western blotting. Similarly, a 2-mm-thick coronal slice from Bregma –1 mm to Bregma –3 mm was taken from each mouse and the amygdala was dissected from this slice for Western blotting.
2.4. Western blotting
Brain tissues were stored at −80 °C before they were used for Western blotting. To prepare total cellular protein extracts, brain tissues were homogenized in RIPA buffer (Cat. No. 89901; Thermo Scientific, Worcester, MA) containing protease inhibitor cocktail (Cat. No. P2714; Sigma, St. Louis, MO) and phosphatase inhibitor cocktail tablets (Cat. No. 04906845001; Roche Diagnostics Corporation, Mannheim, Germany). Homogenates were centrifuged at 16,060g at 4 °C for 15 min. The supernatant was saved and its protein concentration was determined by Bradford assay. To prepare the membrane protein fractions (for determining the expression of EAATs and some AMPA receptor subunits in the plasma membrane), brain tissues were placed in ice-cold buffer (80 mM HEPES, 200 mM mannitol, 1 mM ethylenediaminetetraacetic acid, 200 μM phenylmethylsulfonyl fluoride, 41 mM KOH, pH 7.4) containing protease inhibitor cocktail and Phosphatase Inhibitor Cocktail Tablets, and homogenized with 20 full strokes in glass homogenizers. The lysates were centrifuged for 10 min at 1700g at 4 °C. The super-natant was centrifuged again at 100,000g for 1 h at 4 °C. The pellet was resuspended in the lysis buffer and the protein concentrations of the samples were determined by Bradford assay.
Equal amounts of protein (50 μg per lane) were separated by electrophoresis through 10% sodium dodecyl sulfate–polyacrylamide gels and then electrotransferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were blocked with Protein-Free T20 Blocking Buffer (Cat. No. 37573, Thermo Scientific, Lot NC169569), then were incubated with the following primary antibodies: rabbit polyclonal anti-EAAT1 antibody (1:1,000 dilution; Cat. No. 4166S; Cell Signaling Technology, Beverly, MA), rabbit polyclonal anti-EAAT2 antibody (1:1000 dilution; Cat. No. 3838S; Cell Signaling Technology), rabbit polyclonal anti-phospho-CaMKII (Thr286) antibody (1:1000 dilution; Cat. No. 3361S; Cell Signaling Technology), rabbit polyclonal anti-CaMKII antibody (1:1000 dilution; Cat. No. 3362S; Cell Signaling Technology), rabbit polyclonal anti-c-Fos antibody (1:1000 dilution; Cat. No. 4384S; Cell Signaling Technology), goat polyclonal anti-GluR-1 (C-19) antibody (1:500 dilution; Cat. No. sc-7609; Santa Cruz Biotechnology, Santa Cruz, CA), goat polyclonal anti-GluR-2 (C-20) antibody (1:500 dilution; Cat. No. sc-7610; Santa Cruz Biotechnology), goat polyclonal anti-GluR-3 (C-20) antibody (1:500 dilution; Cat. No. sc-7612; Santa Cruz Biotechnology), goat polyclonal anti-GluR-4 (C-20) antibody (1:500 dilution; Cat. No. sc-7614; Santa Cruz Biotechnology), goat polyclonal anti-p-GluR-1 (Ser-831) antibody (1:500 dilution; Cat. No. sc-16313; Santa Cruz Biotechnology), goat polyclonal anti-p-GluR-1 (Ser-845) antibody (1:500 dilution; Cat. No. sc-16314; Santa Cruz Biotechnology), mouse monoclonal anti-Arc antibody (1:1000 dilution; Cat. No. sc-17839; Santa Cruz Biotechnology), rabbit polyclonal anti-EAAT3 antibody (1:500 dilution; Cat. No. EAAC11-A; Alpha Diagnostic International Inc., San Antonio, TX, USA) and rabbit polyclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (1:3000 dilution; Cat. No. G9545; Sigma–Aldrich; St. Louis, MO, USA). Appropriate secondary antibodies were used. Protein bands were visualized and quantified using the G:Box equipped with Gene tools analysis software (Syngene, Frederick, MD, USA). The densities of protein bands were normalized to those of GAPDH or GluR-1 (for the phospho-GluR1) to control for errors in protein sample loading and transferring during Western blotting. The results from EAAT3 knockout mice then were normalized to those from CD-1 wild-type mice.
2.5. Statistical analysis
Data are presented as means ± SEM (n ≥ 5). The results of fear conditioning behavior and Western blotting for the comparison between CD-1 wild-type mice and EAAT3 knockout mice under control condition were analyzed by Student's t-test. Comparison of Western blotting data from CA1 and entorhinal cortex of mice before vs. after fear conditioning stimuli was performed by oneway analysis of variance followed by Tukey test if the data were normally distributed or by one-way analysis of variance on ranks followed by Dunn's test if the data were not normally distributed. Western blotting data from the amygdala of mice before and after fear conditioning stimuli were analyzed by Student's t-test. The freezing behavior data during the training sessions for fear conditioning were analyzed by two-way repeated measures analysis of variance. These analyses were performed with SigmaStat software (SYSTAT Software, Inc., Point Richmond, CA, USA). P < 0.05 was accepted as significant.
3. Results
3.1. EAAT3 knockout mice had learning and memory impairment
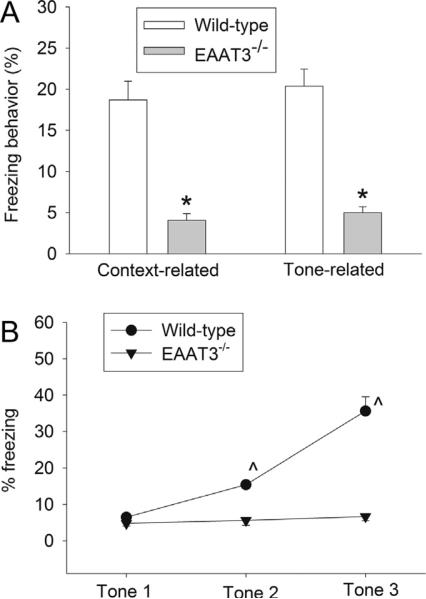
To assess a possible role of EAAT3 in learning and memory, we first determined whether EAAT3 knockout mice had learning and memory impairment. EAAT3 knockout mice had a reduced freezing behavior compared with the wild-type mice in the context- and tone-related fear conditioning tests (Fig. 1A). Since context- and tone-related fear conditioning tests hippocampus-dependent and hippocampus-independent learning and memory, respectively (Kim & Fanselow, 1992), our results suggest that EAAT3 knockout mice have impaired hippocampus-dependent and hippocampus-independent learning and memory. Also, there was a significant effect of EAAT3 knockout on the freezing behavior during the training sessions for fear conditioning [F(1,14) = 87.269, P < 0.001]. The freezing behavior of wild-type mice was increased with more training but this increase was not observed in the EAAT3 knockout mice (Fig. 1B), suggesting that EAAT3 knockout mice have a learning deficit or impairment in the induction of memory.
Fig. 1.
Performance in the fear conditioning test. Seven- to nine-week old CD-1 wild-type or EAAT3 knockout mice were subjected to the fear conditioning test and their results are presented in panel A (means ± SEM, n = 12). In another experiment, the freezing behavior of mice during the training sessions of fear conditioning was monitored and the data are presented in panel B (means ± SEM, n = 8). *P < 0.05 compared with wild-type mice; ^P < 0.05 compared with the corresponding value at tone 1. EAAT3 −/− : glutamate transporter type 3 knockout.
3.2. Fear conditioning stimuli increased EAAT2 and EAAT3 trafficking in the hippocampus and amygdala of wild-type mice but not in the EAAT3 knockout mice
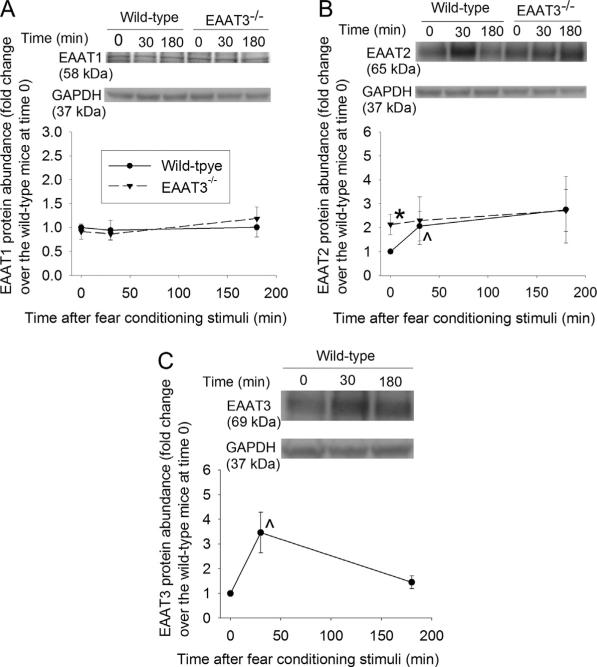
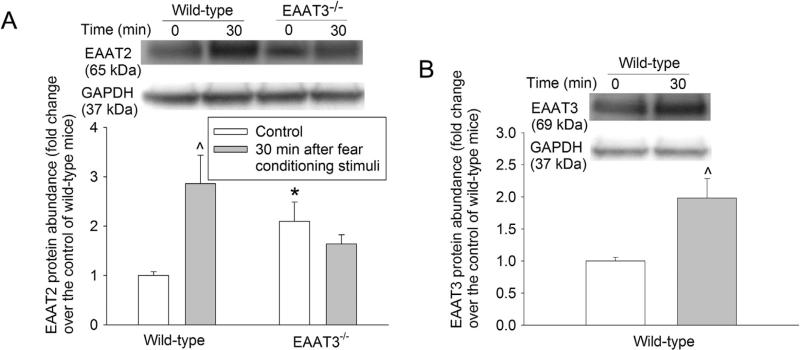
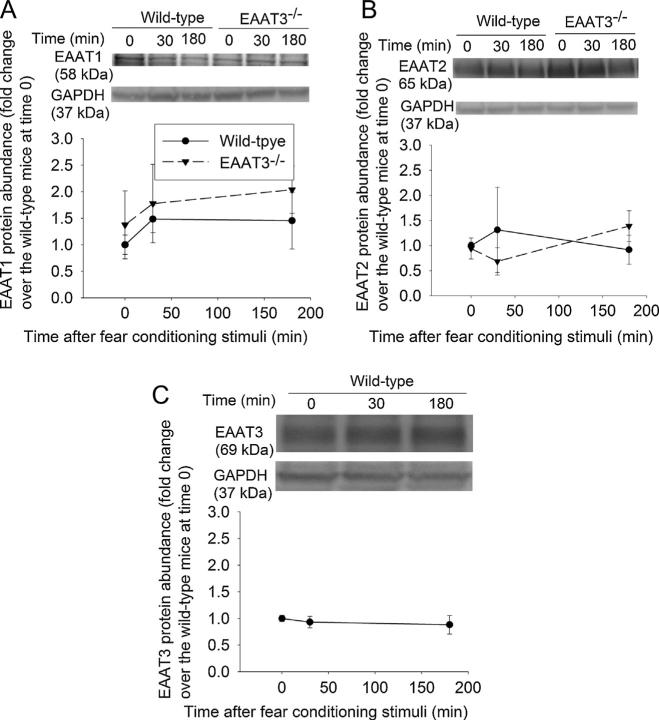
As the first step to determine the involvement of EAATs in the biochemical processes underlying learning and memory, we investigated whether fear-conditioning stimuli affected the trafficking of EAATs. These stimuli did not increase EAAT1 expression in the plasma membrane of the wild-type or EAAT3 knockout mice (Fig. 2A). EAAT2 and EAAT3 expression in the plasma membrane of the hippocampal CA1 region was significantly increased at 30 min after the fear conditioning stimulation in the wild-type mice. This increase disappeared at 3 h after the fear conditioning stimulation (Fig. 2B and C). EAAT3 knockout mice had more EAAT2 in the plasma membrane of the hippocampal CA1 region than did the wild-type mice. However, fear-conditioning stimuli did not change significantly the amount of EAAT2 in the plasma membrane of the CA1 region (Fig. 2B). Similarly, EAAT2 and EAAT3 expression in the plasma membrane of wild-type mouse amygdala was increased at 30 min after the fear conditioning stimuli (Fig. 3A and B). This EAAT2 increase did not occur in the EAAT3 knockout mouse amygdala, although EAAT2 in the plasma membrane of these mice under control condition was higher than that in the wild-type mice (Fig. 3A).
Fig. 2.
Expression of glutamate transporters (EAATs) in the plasma membrane of the CA1 region. Seven- to nine-week old CD-1 wild-type or EAAT3 knockout mice were subjected to the fear conditioning stimuli. Their CA1 regions were harvested before the stimuli, 30 min or 180 min after the stimuli for Western blotting of EAAT1, EAAT2 and EAAT3 (panels A–C). A representative Western blot is shown in the top of each panel. The bottom of each panel shows the EAAT3 protein abundance quantified by integrating the volume of the EAAT3 band in autoradiograms from 8 to 16 mice for each experimental condition. Values in graphs are the means ± SEM. *P < 0.05 compared with wild-type mice; ^P < 0.05 compared with the corresponding value at time 0. EAAT3 −/− : glutamate transporter type 3 knockout.
Fig. 3.
Expression of glutamate transporters (EAATs) in the plasma membrane of the amygdala region. Seven- to nine-week old CD-1 wild-type or EAAT3 knockout mice were subjected to the fear conditioning stimuli. Their amygdalae were harvested before the stimuli (time 0) and 30 min after the stimuli for Western blotting of EAAT2 and EAAT3 (panels A and B, respectively). A representative Western blot is shown in the top of each panel and a graphic presentation of the EAAT protein abundance quantified by integrating the volume of autoradiogram bands from 8 to 16 mice for each experimental condition is shown in the bottom of each panel. Values in graphs are the means ± SEM. *P < 0.05 compared with wild-type mice; ^P < 0.05 compared with the corresponding value at time 0 (control). EAAT3 −/− : glutamate transporter type 3 knockout.
3.3. Fear conditioning stimuli increased GluR1 trafficking in the hippocampus and amygdala of wild-type mice but not in the EAAT3 knockout mice
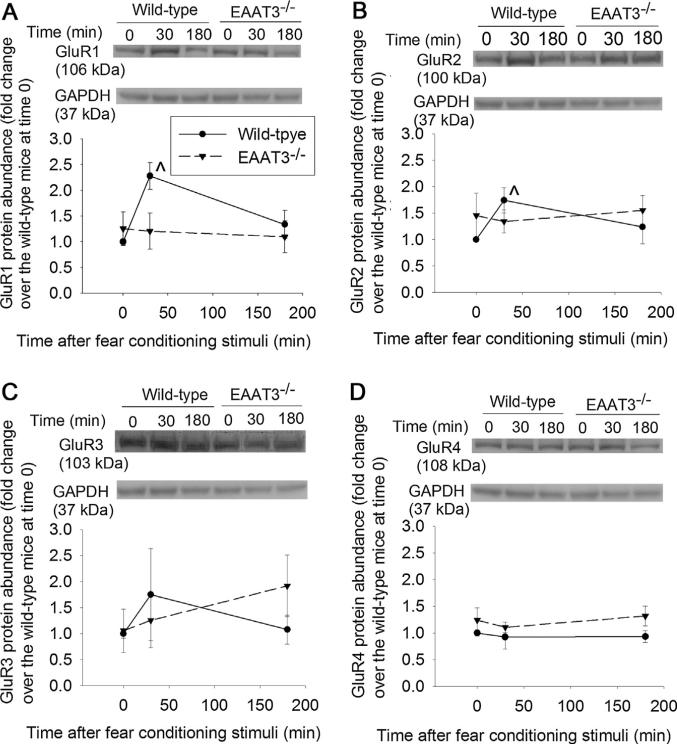
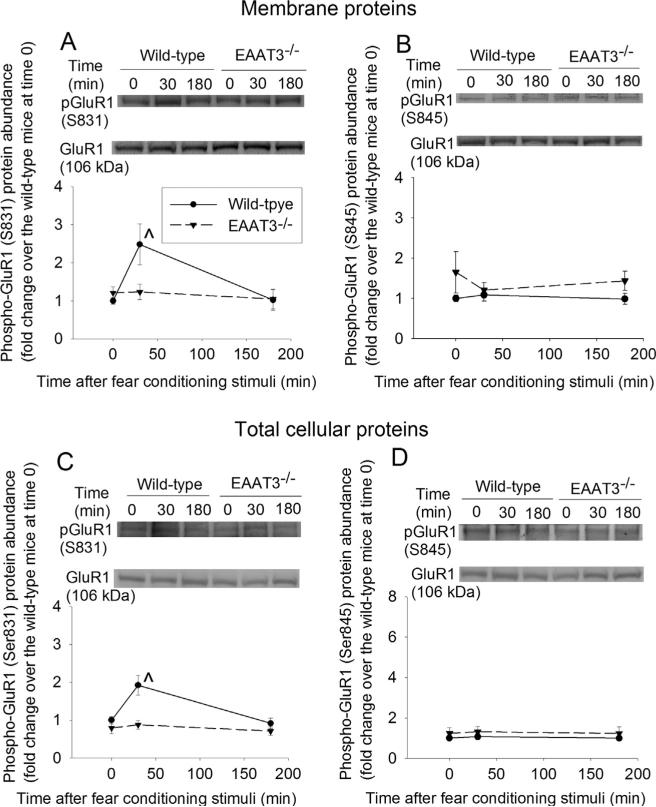
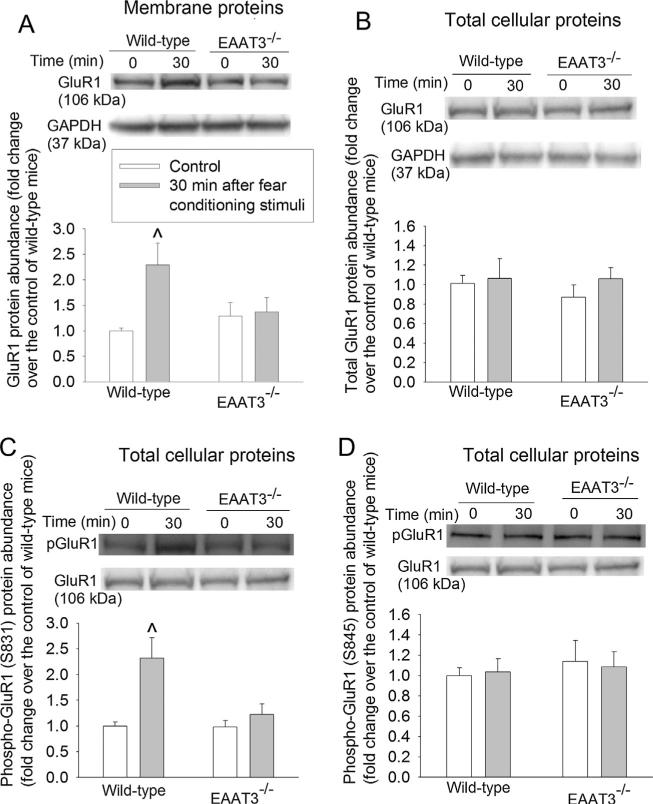
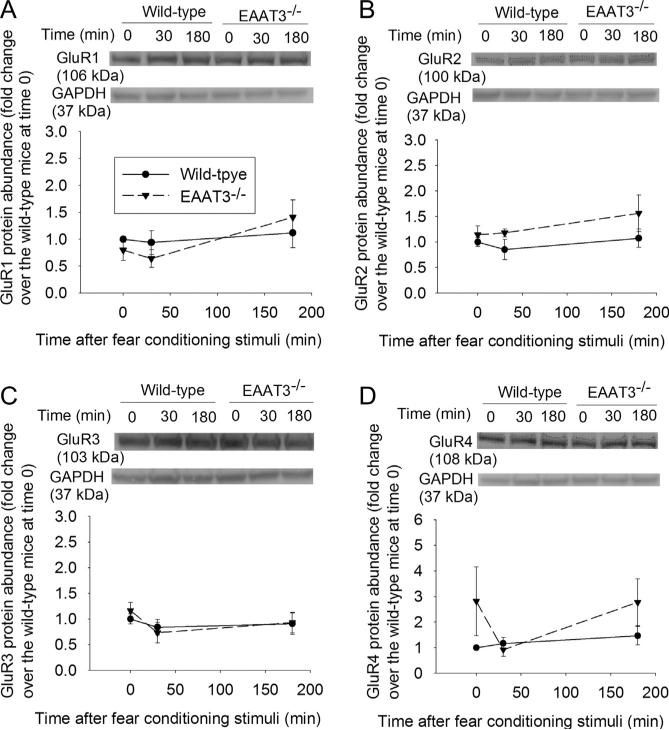
Since learning and memory often require trafficking of AMPA receptors to the plasma membrane (Miyamoto, 2006), we examined the expression of AMPA receptor subunits in the plasma membrane after fear conditioning stimulation. The expression of GluR1 and GluR2 in the plasma membrane of the CA1 region of wild-type mice increased at 30 min after the fear conditioning (Fig. 4A and B). This trafficking was not induced in the EAAT3 knockout mice. Fear conditioning stimuli did not affect the expression of GluR3 or GluR4 in the plasma membrane of the CA1 region in the wild-type or EAAT3 knockout mice (Fig. 4C and D). Consistent with the effects of fear conditioning stimuli on GluR1 expression in the plasma membrane, the stimuli also increased phosphorylation of GluR1 at S831, a CaMKII phosphorylation site (Hayashi et al., 2000), in the plasma membrane of the CA1 region from wild-type mouse (Fig. 5A). Fear conditioning stimuli did not affect the amount of phosphorylation of GluR1 at S845, a protein kinase A phosphorylation site (Esteban et al., 2003; Man et al., 2007) in the plasma membrane of the CA1 region in either wild-type or EAAT3 knockout mice (Fig. 5B). Similar results were observed when total cellular protein was used for the Western blotting (Fig. 5C and D). Similar to these results in the CA1 region, GluR1 in the plasma membrane of the amygdala from wild-type mouse was increased at 30 min after the fear conditioning stimulation. This increase did not occur in the EAAT3 knockout mice (Fig. 6A). Although total GluR1 and phospho-GluR1 at S845 in total cellular protein of wild-type mouse amygdala were not changed, phospho-GluR1 at S831 in total cellular protein of these mice was increased at 30 min after the fear conditioning stimulation. Fear conditioning stimuli did not change the expression of GluR1, phospho-GluR1 at S831 or phospho-GluR1 at S845 in the total cellular protein of the EAAT3 knockout mouse amygdala (Fig. 6B–D).
Fig. 4.
Expression of 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid (AMPA) receptor subunits in the plasma membrane of the CA1 region. Seven- to nine-week old CD-1 wild-type or EAAT3 knockout mice were subjected to the fear conditioning stimuli. Their CA1 regions were harvested before the stimuli, 30 min or 180 min after the stimuli for Western blotting of AMPA subunits (GluR1, GluR2, GluR3 and GluR4, panels A–D). A representative Western blot is shown in the top of each panel and a graphic presentation of the GluR protein abundance quantified by integrating the volume of autoradiogram bands from 8 to 16 mice for each experimental condition is shown in the bottom of each panel. Values in graphs are the means ± SEM. ^P < 0.05 compared with the corresponding value at time 0. EAAT3 −/− : glutamate transporter type 3 knockout.
Fig. 5.
Expression of phosphorylated 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid (AMPA) receptor subunit GluR 1 in the CA1 region. Seven- to nine-week old CD-1 wild-type or EAAT3 knockout mice were subjected to the fear conditioning stimuli. Their CA1 regions were harvested before the stimuli, 30 min or 180 min after the stimuli for Western blotting of phospho-GluR1 at S831 (panels A and C) or at S845 (panels B and D) in the plasma membrane (panels A and B) and in total cellular protein (panels C and D). A representative Western blot is shown in the top of each panel and a graphic presentation of the GluR1 protein abundance quantified by integrating the volume of autoradiogram bands from 6 to 16 mice for each experimental condition is shown in the bottom of each panel. Values in graphs are the means ± SEM. ^P < 0.05 compared with the corresponding value at time 0. EAAT3 −/− : glutamate transporter type 3 knockout.
Fig. 6.
Expression of the 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid (AMPA) receptor subunit GluR 1 and phosphorylated GluR1 in the amygdala. Seven-to nine-week old CD-1 wild-type or EAAT3 knockout mice were subjected to the fear conditioning stimuli. Their amygdalae were harvested before the stimuli (time 0) and 30 min after the stimuli for Western blotting of GluR1 in the plasma membrane (panel A), total GluR1 (panel B) and phospho-GluR1 (at S831, panel C and at S845, panel D) in total cellular protein. A representative Western blot is shown in the top of each panel and a graphic presentation of the GluR1 protein abundance quantified by integrating the volume of autoradiogram bands from 8 to 16 mice for each experimental condition is shown in the bottom of each panel. Values in graphs are the means ± SEM. ^P < 0.05 compared with the corresponding value at time 0. EAAT3 −/− : glutamate transporter type 3 knockout.
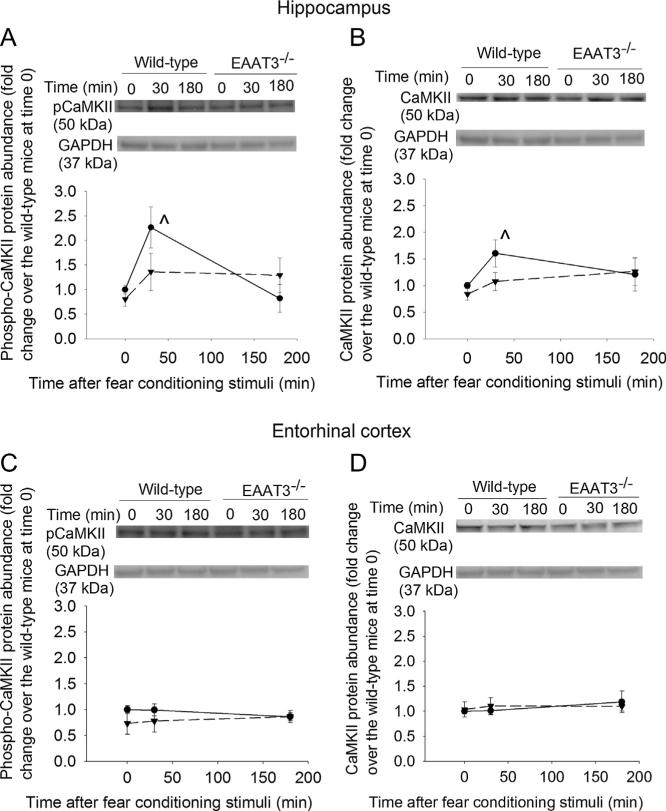
Total CaMKII and phosphorylated CaMKII in the CA1 region of wild-type mice were increased at 30 min after the fear conditioning stimulation (Fig. 7A and B). The fraction of phosphorylated CaMKII normalized to total CaMKII was increased 1.74 ± 0.43-fold after the fear conditioning stimulation (P = 0.033). Fear conditioning stimuli did not affect the expression of phospho-CaMKII or total CaMKII in the EAAT3 knockout mice (Fig 7A and B).
Fig. 7.
Expression of the calcium–calmodulin-dependent protein kinase II (CaMKII) in the CA1 region and entorhinal cortex. Seven- to nine-week old CD-1 wild-type or EAAT3 knockout mice were subjected to the fear conditioning stimuli. Their CA1 regions (panels A and B) and entorhinal cortices (panels C and D) were harvested before the stimuli, or 30 min or 180 min after the stimuli for Western blotting of phospho-CaMKII (panels A and C) and CaMKII (panels B and D). Total cellular protein was used for the assay. A representative Western blot is shown on the top of each panel and a graphic presentation of the CaMKII protein abundance quantified by integrating the volume of CaMKII autoradiogram bands from 8 to 16 mice for each experimental condition is shown in the bottom panel. Values in graphs are the means ± SEM. ^P < 0.05 compared with the corresponding value at time 0. EAAT3 −/− : glutamate transporter type 3 knockout.
3.4. Fear conditioning stimuli changed c-fos and Arc expression in the hippocampus and entorhinal cortex
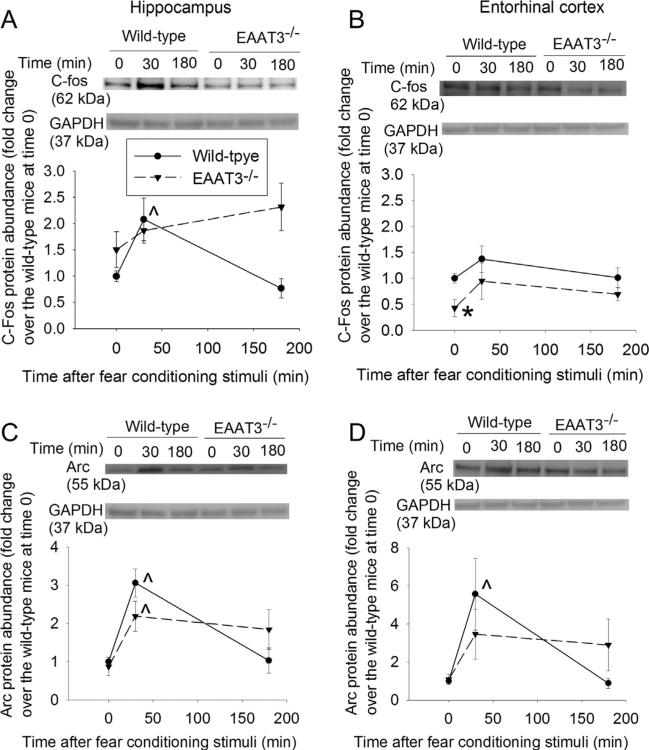
Learning and memory involve synthesis of proteins, such as c-fos and Arc (McIntyre et al., 2005; Miyamoto, 2006). Fear conditioning stimuli significantly increased c-fos and Arc in the CA1 region of wild-type mice and Arc in the CA1 region of EAAT3 knockout mice at 30 min after fear conditioning stimulation (Fig. 8A and C). The stimuli did not affect c-fos expression (Fig. 8B) but increased Arc expression in the entorhinal cortex, a brain structure involved in memory formation (Hafting, Fyhn, Molden, Moser, & Moser, 2005), of the wild-type mice at 30 min after the stimuli (Fig. 8D).
Fig. 8.
Expression of c-Fos and activity-regulated cytoskeleton-associated protein (Arc) in the CA1 region and entorhinal cortex. Seven- to nine-week old CD-1 wild-type or EAAT3 knockout mice were subjected to the fear conditioning stimuli. Their CA1 regions (panels A and C) and entorhinal cortices (panels B and D) were harvested before the stimuli, or 30 min or 180 min after the stimuli for Western blotting of c-Fos (panels A and B) and Arc (panels C and D). Total cellular protein was used for the assay. A representative Western blot is shown on the top of each panel and a graphic presentation of the c-Fos and Arc protein abundance quantified by integrating the volume of c-Fos autoradiogram bands from 5 to 16 mice for each experimental condition is shown in the bottom of each panel. Values in graphs are means ± SEM. *P < 0.05 compared with wild-type mice; ^P < 0.05 compared with the corresponding value at time 0. EAAT3 −/− : glutamate transporter type 3 knockout.
3.5. Fear conditioning stimuli did not affect EAAT2, EAAT3 or GluR1 trafficking in the entorhinal cortex of wild-type mice or EAAT3 knockout mice
To determine whether the changes in EAAT3 and GluR1 trafficking induced by fear conditioning stimuli were brain region-specific, we investigated potential changes in the entorhinal cortex. Fear conditioning stimuli did not affect the expression of EAAT1, EAAT2, EAAT3, GluR1, GluR2, GluR3 or GluR4 in plasma membrane of the entorhinal cortex from wild type or EAAT3 knockout mice. Expression of CaMKII and phospho-CaMKII in the entorhinal cortex of the wild-type and EAAT3 knockout mice also was not affected by the fear conditioning stimulation (Figs. 7C, D, 9 and 10).
Fig. 9.
Expression of the glutamate transporters (EAATs) in the plasma membrane of the entorhinal cortex. Seven- to nine-week old CD-1 wild-type or EAAT3 knockout mice were subjected to the fear conditioning stimuli. Their entorhinal cortices were harvested before the stimuli, or 30 min or 180 min after the stimuli for Western blotting of EAAT1 (panel A), EAAT2 (panel B) and EAAT3 (panel C). A representative Western blot is shown on the top of each panel and a graphic presentation of the EAAT protein abundance quantified by integrating the volume of EAAT autoradiogram bands from 5 to 12 mice for each experimental condition is shown in the bottom of each panel. Values in graphs are the means ± SEM. EAAT3 −/− : glutamate transporter type 3 knockout.
Fig. 10.
Expression of the 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid (AMPA) receptor subunits in the plasma membrane of the entorhinal cortex. Seven-to nine-week old CD-1 wild-type or EAAT3 knockout mice were subjected to the fear conditioning stimuli. Their entorhinal cortices were harvested before the stimuli, or 30 min or 180 min after the stimuli for Western blotting of AMPA subunits (GluR1, GluR2, GluR3 and GluR4, panels A–D, respectively). A representative Western blot is shown in the top of each panel and a graphic presentation of the GluR protein abundance quantified by integrating the volume of GluR autoradiogram bands from 5 to 10 mice for each experimental condition is shown in the bottom of each panel. Values in graphs are the means ± SEM. EAAT3 −/− : glutamate transporter type 3 knockout.
4. Discussion
Our results showed clearly that 7- to 9-week old EAAT3 knockout mice had learning and memory impairments as assessed with a fear-conditioning test. This finding is similar to our previous report (Lee, Park, & Zuo, 2012). We further showed here that EAAT3 knockout mice had impairments in learning or in the induction of memory because these mice did not increase their freezing behaviors with more training in the training sessions. However, a previous study showed that 7-week old EAAT3 knockout mice did not have learning and memory impairment as measured using the Morris water maze (Aoyama et al., 2006). Unlike the situation of 11-month old EAAT3 knockout mice that had both brain atrophy and impairment of learning and memory, 7-week old EAAT3 knockout mice had normal gross brain structures (Aoyama et al., 2006). The different findings in cognitive functions between our studies and the previous study may be due to the use of different modalities to measure learning and memory. Fear conditioning is a very sensitive method to assess hippocampus-dependent and hippocampus-independent learning and memory (Kim & Fanselow, 1992).
The mechanisms for the impaired learning and memory in the EAAT3 knockout mice are not known. The trafficking of AMPA receptors, especially GluR1 and GluR2, to the plasma membrane is a well-defined biochemical process in learning and memory (Man et al., 2007; Miyamoto, 2006; Whitlock, Heynen, Shuler, & Bear, 2006). We showed here that fear conditioning stimuli significantly increased the expression of GluR1 and GluR2 in the hippocampal plasma membrane prepared from the wild-type mice but did not affect the expression of AMPA receptors in the hippo-campal plasma membrane from the EAAT3 knockout mouse. Consistent with a previous study (Whitlock et al., 2006), the increase of GluR1 and GluR2 in the plasma membrane of wild-type mice occurred quickly (30 min) after the fear conditioning stimulation and this increase disappeared at 3 h after the stimulation. These results suggest that a basic biochemical process for learning and memory is interrupted in the EAAT3 knockout mice.
The trafficking of AMPA receptors to the plasma membrane often requires their phosphorylation (Esteban et al., 2003; Man et al., 2007). We showed that the phosphorylation of GluR1 at Ser831, a CaMKII phosphorylation site (Hayashi et al., 2000), but not at Ser845, a protein kinase A phosphorylation site (Esteban et al., 2003; Man et al., 2007), was significantly increased at 30 min after the fear conditioning stimulation in the hippocampus of the wild-type mice. Consistent with this result, the phosphorylation of CaMKII at Thr286 was increased. Phosphorylation of CaMKII at this site activates this enzyme (Barkai, Prigent-Tessier, Tessier, Gibori, & Gibori, 2000).
In addition to phosphorylating AMPA receptors, CaMKII can increase protein synthesis (Knapska et al., 2006; Miyamoto, 2006), which is necessary for memory formation. C-Fos and Arc are among the group of proteins whose expression often is increased in response to memory formation (McIntyre et al., 2005; Miyamoto, 2006). These two proteins are also immediate–early gene family members (Knapska et al., 2006; Miyamoto, 2006). Our results showed that fear conditioning stimuli significantly increased c-Fos and Arc in the hippocampus of the wild-type mice. Fear conditioning stimuli did not change the phosphorylation of GluR1 or CaMKII or the expression of c-Fos in the hippocampus of the EAAT3 knockout mice. These results suggest that EAAT3 knockout interrupts the biochemical responses/processes for learning and memory in the hippocampus. Consistent with this suggestion, fear conditioning stimuli induced EAAT3 trafficking to the plasma membrane of the wild-type rat hippocampus (Levenson et al., 2002).
Because our results also suggested impairment of auditory fear conditioning in the EAAT3 knockout mice, we also studied the amygdala, a brain region that is involved in auditory fear conditioning (Goosens & Maren, 2001). Similar to the pattern of changes in the hippocampus, fear conditioning stimuli increased the trafficking of EAAT2, EAAT3 and GluR1 to the plasma membrane and the phosphorylation of GluR1 at S831 in the amygdala of wild-type mice but not EAAT3 knockout mice, providing additional evidence for the involvement of EAAT3 in the biochemical processes underlying learning and memory.
The entorhinal cortex is the main interface between the hippocampus and the neocortex and it plays a critical role in memory formation (Hafting et al., 2005). Fear conditioning stimuli did not affect EAAT or AMPA receptor subunit trafficking, the phosphorylation of GluR1 or CaMKII, or the expression of c-Fos in the entorhinal cortex of the wild-type or EAAT3 knockout mice. However, fear conditioning stimuli significantly increased Arc in this cortex of the wild-type mice. This situation is similar to that in the hippocampus of the EAAT3 knockout mice where Arc increase was the only positive finding after fear conditioning stimuli. These results suggest that the increased Arc after fear conditioning stimulation does not require CaMKII. Multiple other protein kinases, such as mitogen-activated protein kinases, are known to regulate Arc expression (Waltereit et al., 2001). These signaling molecules may be involved in the increased expression of Arc after fear conditioning stimulation.
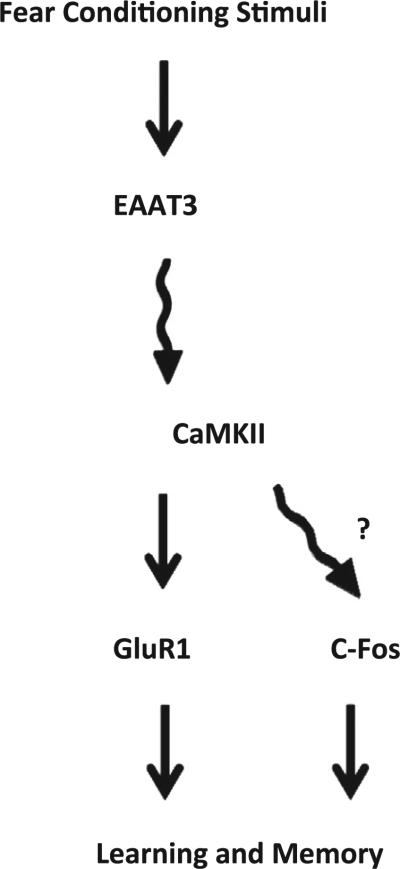
In summary, our study showed (a) that EAAT3 knockout mice had learning and memory impairment, (b) that fear conditioning stimuli induced EAAT3 trafficking to the plasma membrane and a series of biochemical reactions for learning and memory in the wild-type mice, and (c) that fear conditioning stimuli failed to activate CaMKII and related biochemical changes for learning and memory in the EAAT3 knockout mouse hippocampus. Fear conditioning stimuli have been shown to increase EAAT3 trafficking to the plasma membrane of mouse hippocampus (Levenson et al., 2002). However, it was not known whether this increase was causal or merely an associated phenomenon. Our results provide initial evidence that this increased EAAT3 trafficking may be an important process for learning and memory (see schematic in Fig. 11).
Fig. 11.
Diagram of a possible role for glutamate transporter type 3 (EAAT3) in the biochemical responses underlying learning and memory. The wavy line from EAAT3 to calcium–calmodulin-dependent protein kinase II (CaMKII) means that it is not clear whether the effects are direct or indirect via other molecules. The question mark and wavy line from CaMKII to C-Fos mean that the relationship has not been determined in this study but from the evidence in the literature.
EAATs are important in maintaining proper synaptic transmission (Tzingounis & Wadiche, 2007) and glutamate neurotransmission is necessary for learning and memory (Man et al., 2007; Miyamoto, 2006). Since EAATs can bind glutamate and transport it via an electrogenic process (Do, Kamatchi, Washington, & Zuo, 2002), it is possible that their function in learning and memory is mediated via aiding glutamate neurotransmission. Since EAAT3 is the major neuronal EAAT in the brain (Danbolt, 2001), it may be the EAAT involved in this function. EAAT3 also can transport cysteine, which provides a substrate for the synthesis of glutathione in mature neurons (Aoyama et al., 2006; Cao, Li, & Zuo, 2012). Thus, the impaired learning and memory in the EAAT3 knockout mice also may be a consequence of reduced glutathione and subsequent increase in oxidative stress in the neurons of these mice. However, this possibility seems unlikely because it would be difficult to explain why CaMKII-dependent and learning and memory-related biochemical processes are affected whereas the pathway leading to increased Arc in the EAAT3 knockout mice is not inhibited.
In addition to inducing EAAT3 trafficking to the plasma membrane, fear-conditioning stimuli also increased EAAT2 in the plasma membrane of the wild-type mouse hippocampus and amygdala. The delayed phase of long-term potentiation is associated with increased EAAT activity due to EAAT2 (Pita-Almenar et al., 2006). Thus, the increased EAAT2 expression in the plasma membrane observed in our study may contribute to the delayed phase of long-term potentiation. The role of increased EAAT2 expression in learning and memory is not known, but EAAT2 may be downstream of EAAT3 because fear-conditioning stimuli did not increase EAAT2 trafficking to the plasma membrane of the hippocampus and amygdala in the EAAT3 knockout mice.
Our study has limitations. We used constitutive EAAT3 knockout mice in the study. A significant issue with constitutive gene knockout animals is that compensatory changes may occur. Our previous studies did not identify significant compensatory changes in the expression of other EAATs in the brain (Deng et al., 2013; Lee et al., 2010; Li & Zuo, 2011). However, we found here that the EAAT3 knockout mice had an increased expression of EAAT2 in the plasma membrane of the hippocampus and amygdala at baseline. This increase may be a compensatory effect due to EAAT3 knockout. The EAAT3 knockout mice also had a decreased baseline c-Fos in the entorhinal cortex. The reason for this effect is not clear.
In conclusion, our results showed clearly that 7- to 9-week old EAAT3 knockout mice had learning and memory impairment and that fear conditioning stimuli did not induce the activation of learning and memory-related CaMKII signaling in these mice. These findings extend the biological functions of EAAT3 to learning and memory.
Acknowledgments
This study was supported by Grants (R01 GM065211 and R01 GM098308 to Z. Zuo) from the National Institutes of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z. Zuo), Cleveland, Ohio, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z. Zuo), Baltimore, Maryland, and the Robert M. Epstein Professorship endowment, University of Virginia.
Abbreviations
- Arc
activity-regulated cytoskeleton-associated protein
- AMPA
a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- Arc
activity-regulated cytoskeleton-associated protein
- CaMKII
calcium/calmodulin-dependent protein kinase II
- EAAT3
excitatory amino acid transporter type 3
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GluR
glutamate receptor
Footnotes
Conflict of interest
None.
References
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nature Neuroscience. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Barkai U, Prigent-Tessier A, Tessier C, Gibori GB, Gibori G. Involvement of SOCS-1, the suppressor of cytokine signaling, in the prevention of prolactin-responsive gene expression in decidual cells. Molecular Endocrinology. 2000;14:554–563. doi: 10.1210/mend.14.4.0437. [DOI] [PubMed] [Google Scholar]
- Cao L, Li L, Zuo Z. N-acetylcysteine reverses existing cognitive impairment and increased oxidative stress in glutamate transporter type 3 deficient mice. Neuroscience. 2012;220:85–89. doi: 10.1016/j.neuroscience.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in Neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Deng J, Li J, Li L, Feng C, Xiong L, Zuo Z. Glutamate transporter type 3 knockout leads to decreased heart rate possibly via parasympathetic mechanism. Transgenic Research. 2013;22:757–766. doi: 10.1007/s11248-012-9680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do S-H, Kamatchi GL, Washington JM, Zuo Z. Effects of volatile anesthetics on glutamate transporter, excitatory amino acid transporter type 3. Anesthesiology. 2002;96:1492–1497. doi: 10.1097/00000542-200206000-00032. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nature Neuroscience. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learning and Memory. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: Requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Knapska E, Walasek G, Nikolaev E, Neuhausser-Wespy F, Lipp HP, Kaczmarek L, et al. Differential involvement of the central amygdala in appetitive versus aversive learning. Learning and Memory. 2006;13:192–200. doi: 10.1101/lm.54706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SN, Li L, Zuo Z. Glutamate transporter type 3 knockout mice have a decreased isoflurane requirement to induce loss of righting reflex. Neuroscience. 2010;171:788–793. doi: 10.1016/j.neuroscience.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Park SH, Zuo Z. Effects of isoflurane on learning and memory functions of wild-type and glutamate transporter type 3 knockout mice. Journal of Pharmacy and Pharmacology. 2012;64:302–307. doi: 10.1111/j.2042-7158.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nature Neuroscience. 2002;5:155–161. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. Journal of Cerebral Blood Flow and Metabolism. 2011;31:1283–1292. doi: 10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology. 2011;61:1354–1359. doi: 10.1016/j.neuropharm.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, et al. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto E. Molecular mechanism of neuronal plasticity: Induction and maintenance of long-term potentiation in the hippocampus. Journal of Pharmacological Sciences. 2006;100:433–442. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO Journal. 1997;16:3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pita-Almenar JD, Collado MS, Colbert CM, Eskin A. Different mechanisms exist for the plasticity of glutamate reuptake during early long-term potentiation (LTP) and late LTP. Journal of Neuroscience. 2006;26:10461–10471. doi: 10.1523/JNEUROSCI.2579-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Simpson EM, Takahashi JS, Lipp H-P, Nakanishi S, Wehner JM, et al. Mutant mice and neuroscience: Recommendations concerning genetic background. Banbury conference on genetic background in mice. Neuron. 1997;19:755–759. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: Confining runaway excitation by shaping synaptic transmission. National Review of Neuroscience. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, et al. Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. Journal of Neuroscience. 2001;21:5484–5493. doi: 10.1523/JNEUROSCI.21-15-05484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]