Abstract
Background
Previous studies have shown that a western diet impairs, whereas physical exercise enhances hippocampus-dependent learning and memory. Both diet and exercise influence expression of hippocampal brain-derived neurotrophic factor (BDNF), which is associated with improved cognition. We hypothesized that exercise reverses diet-induced cognitive decline while increasing hippocampal BDNF.
Methods
To test the effects of exercise on hippocampal-dependent memory, we compared cognitive scores of Sprague-Dawley rats exercised by voluntary running wheel (RW) access or forced treadmill (TM) to sedentary (Sed) animals. Memory was tested by two-way active avoidance test (TWAA), in which animals are exposed to a brief shock in a specific chamber area. When an animal avoids, escapes or has reduced latency to do either, this is considered a measure of memory. In a second experiment, rats were fed either a high-fat diet or control diet for 16 weeks, then randomly assigned to running wheel access or sedentary condition, and TWAA memory was tested once a week for seven weeks of exercise intervention.
Results
Both groups of exercised animals had improved memory as indicated by reduced latency to avoid and escape shock, and increased avoid and escape episodes (p<0.05). Exposure to a high-fat diet resulted in poor performance during both the acquisition and retrieval phases of the memory test as compared to controls. Exercise reversed high-fat diet-induced memory impairment, and increased brain-derived neurotrophic factor (BDNF) in neurons of the hippocampal CA3 region.
Conclusions
These data suggest that exercise improves memory retrieval, particularly with respect to avoiding aversive stimuli, and may be beneficial in protecting against diet induced cognitive decline, likely via elevated BDNF in neurons of the CA3 region.
Keywords: BDNF, hippocampus, two-way active avoidance, treadmill, running wheel, high-fat diet, memory
Introduction
Several lines of evidence illustrate that physical activity is beneficial toward maintaining and improving cognitive function in humans and animals. Physical exercise enhances learning, memory retention, and cognitive task performance [1, 2]. In addition, physical activity enhances both acquisition rate and performance on spatial learning task [3]. The cognition enhancing effect of exercise is observed both in young and aged animals [3, 4]. Similarly, in humans, regular aerobic exercise prevents brain tissue loss and decline in hippocampal volume during aging, ameliorates age-related cognitive decline [5, 6], and improves spatial memory, [7] [3, 8, 9].
The hippocampus is an important area for spatial, contextual, and avoidance learning as well as memory processing. In particular, the dorsal hippocampus has long been considered the site of temporal integration of the sequence of events that ultimately forms a memory, including spatial and contextual learning (reviewed in [10]). Hippocampal neurons play a critical role in the retrieval of information related to memory tasks [11]. Within the dorsal hippocampus, the CA3 region is important for memory retention, which likely involves both neurogenesis and long-term potentiation. For example, atrophy of apical dendrites of the CA3 neurons leads to memory impairments in the rat [12] and smaller hippocampal CA3/dentate volumes in humans is associated with learning and memory problems [13].
Evidence shows that neurogenesis, maturation and synaptogenesis in the hippocampal formation is critical for learning and memory [14]. High fat diet impairs neurogenesis, whereas, voluntary exercise in the form of wheel running promotes neurogenesis in the dentate gyrus of adult mice [3, 15]. Furthermore, exercise induced hippocampal neurogenesis is shown to be rapid and robust in nature [16]. In addition, voluntary exercise increases the length and complexity of dendritic spines of the hippocampal neurons [17].
Brain derived neurotrophic factor (BDNF) plays a key role in regulating adult hippocampal neurogenesis and synaptogenesis, both of which are critical to learning and memory. Dense expression of BDNF is observed in the hippocampus [18]. BDNF increases occur in the dorsal hippocampus during TWAA memory consolidation [19], and during contextual fear conditioning [20]. Genetic manipulation studies show that decreased levels of BDNF impairs hippocampus-dependent behavioral tasks such as spatial learning in the Morris water maze in rats and mice [21–23]. Additionally, central lenti-virus mediated infusions and acute injections of BDNF enhance learning in animals [24, 25]. Similarly, higher levels of BDNF were associated with enhanced memory, cognition and increased hippocampal volumes in human subjects [26]. Taken together, these studies indicate that elevated hippocampal BDNF is essential to memory [27].
Similar to physical exercise enhancing cognition, physical exercise is known to robustly enhance BDNF signaling, and hence neural cell proliferation, neurogenesis, and synaptic plasticity in rodents [16, 28–31]. These changes result in enhanced long-term potentiation, learning and retention, and enhance performance in cognitive tasks [2, 32, 33]. A recent study confirmed these findings, showing direct correlation between increased hippocampal volume and memory performance improvements in people who exercised [34]. Additionally, human exercisers have been shown to outperform non-exercisers on tasks such as reasoning, working memory, Stroop, Trails-B, Symbol digit, vigilance monitoring, and fluid intelligence tests [3]. These beneficial effects of exercise are attributed to increased BDNF signaling [26, 35].
While exercise is reportedly beneficial for memory function, a high-energy, high-saturated fat diet is associated with cognitive impairment, with a specific effect on learning and memory functions that are dependent on the integrity of the hippocampus [36]. Similarly, saturated fat intake is associated with impaired memory [37] and greater cognitive decline with aging [38, 39]. As mentioned before, hippocampal BDNF is increased with exercise [35, 40, 41], it is reduced by a high-fat diet [1, 42]. An elegant study by Molteni et al. showed that physical exercise initiated simultaneously with 2 months of high-fat diet prevents the spatial memory impairing effects of high-fat diet [1]. Whether exercise can reverse cognitive impairments associated with prior exposure to high-fat diet remains unanswered
The aim of this study was to test whether exercise enhances performance on a hippocampus dependent memory test and reverses hippocampus dependent memory impairment produced by high-fat diet exposure, and if exercise-induced cognitive improvement is associated with increased hippocampal BDNF.
Results
Experiment 1
At baseline, animals were randomized to groups such that there were no differences in fat, % fat, weight, lean mass, or % lean mass between groups prior to the initiation of exercise (Table 1A) to avoid potential differences in physical abilities between groups. After the intervention animals with voluntary wheel access weighed less and had lower body fat, % body fat, and elevated lean mass compared with sedentary controls (p<0.05) (Table 1B).
Table 1.
Comparison of body compositions at baseline and post-five weeks of exercise intervention (Experiment One). Characteristics of treadmill (TM), running wheel (RW) and sedentary (Sed) animals were not different from each other prior to exercise intervention (A). After five weeks of exercise RW animals had significantly improved body composition compared with Sed animals (B); N=9–10.
| A | |||||||
|---|---|---|---|---|---|---|---|
| TM | RW | Sed | |||||
| mean | SEM | mean | SEM | mean | SEM | p value | |
|
| |||||||
| Fat (g) | 102.2 | 8.0 | 101.5 | 7.3 | 100.9 | 7.2 | 0.99 |
| Lean (g) | 444.1 | 9.0 | 436.4 | 12.1 | 443.3 | 13.6 | 0.88 |
| Weight (g) | 622.9 | 11.9 | 619.9 | 15.5 | 619.6 | 18.1 | 0.99 |
| %Fat | 16.3 | 1.1 | 16.3 | 1.0 | 16.2 | 1.0 | 0.99 |
| %Lean | 71.4 | 1.2 | 70.5 | 1.3 | 71.6 | 0.9 | 0.98 |
| B | |||||||
|---|---|---|---|---|---|---|---|
| TM | RW | Sed | |||||
| mean | SEM | mean | SEM | mean | SEM | p value | |
|
| |||||||
| Fat (g) | 105.9 | 10.0 | 81.6* | 8.4 | 130.7 | 11.3 | 0.01 |
| Lean (g) | 455.2 | 11.3 | 435.4 | 10.4 | 445.5 | 12.6 | 0.49 |
| Weight (g) | 637.9 | 11.8 | 588.5* | 14.9 | 648.9 | 18.6 | 0.02 |
| %Fat | 16.5 | 1.4 | 13.8* | 1.2 | 20.0 | 1.5 | 0.01 |
| %Lean | 71.4 | 1.4 | 74.1* | 1.2 | 68.8 | 1.3 | 0.03 |
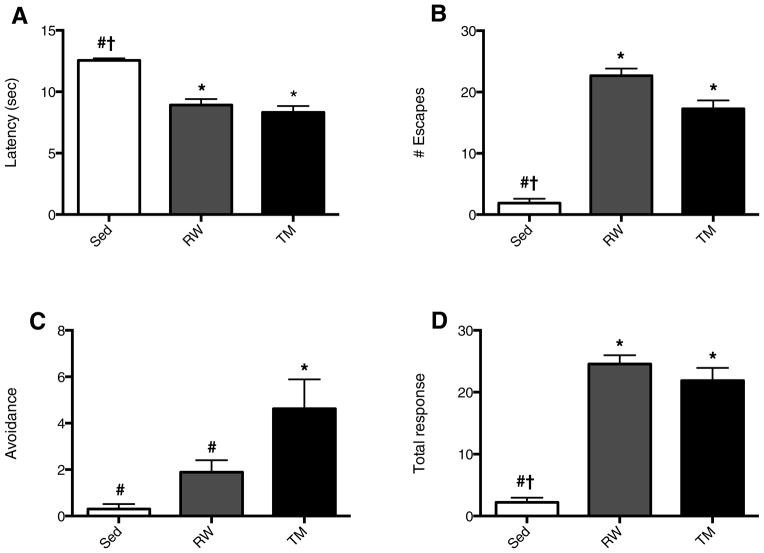
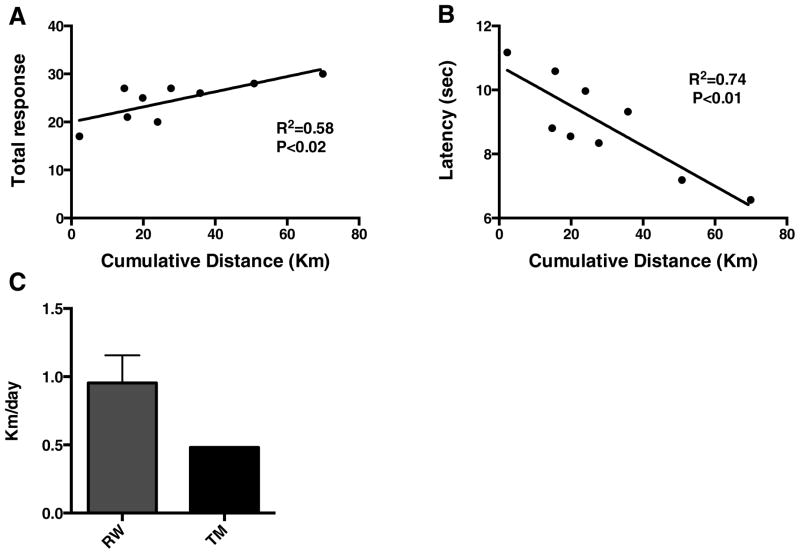
After 5 weeks of either running wheel exercise, treadmill exercise or being sedentary, there was a significant effect of exercise on latency (F2, 24 = 33.10, p<0.0001) (A) number of escapes (F2, 24 = 105.3, p<0.0001) (B), avoidances (F2, 24 = 8.9, p=0.001) (C) and the total response (escape plus avoidance) (F2, 24= 77.8, p<0.0001) (D). Five weeks of exercise significantly increased retention of contextual memory, as indicated by decreased latency (p<0.05) (Fig. 1A), increased number of escapes (p<0.05) (Fig. 1B) and avoidance episodes, as well as total response (p<0.05) (Fig. 1D). Treadmill exercised animals had a significantly greater number of shock avoidances (p<0.05) compared with running wheel and sedentary animals, indicating enhanced capacity to pair the conditioned stimulus of sound and light to the impending shock. The number of avoidances in animals exercising on running wheels tended to be positively related to running distance, although the correlation was not significant (data not shown, R2= 0.42, p=0.057). After five weeks, running distance was positively correlated with total responses (R2=0.58, p<0.02) (Fig. 2A) and negatively with response latency (R2=0.74, p<0.01) (Fig. 2B) in the TWAA test.
Fig. 1. Five weeks of exercise enhances memory.
There was a significant effect of exercise on latency (F (2, 24) = 33.10, p<0.0001) (A) number of escapes (F (2, 24) = 105.3, p<0.0001) (B), avoidances (F (2, 24) = 8.9, p=0.001) (C) and total response (escape plus avoidance) (F (2, 24)= 77.8, p<0.0001) (D). Compared with sedentary (Sed) animals, both running wheel (RW) and treadmill (TM) exercise reduced latency (A), increased the number of escapes (B), and total response (D), (escape plus avoidance). TM and RW animals did not differ from each other except in the number of avoidances (C), where TM exercise resulted in a significantly greater number of total avoidances. * Indicates significantly different compared with Sed; # indicates significantly different compared with TM; † indicates significantly different compared with RW; p<0.05, N=9–10.
Fig. 2.
Cognitive performance is linearly associated with magnitude of exercise. The total response (A) is positively, and latency (B) is negatively correlated with cumulative running distance. Daily running wheel (RW) and treadmill (TM) average distances run (C).
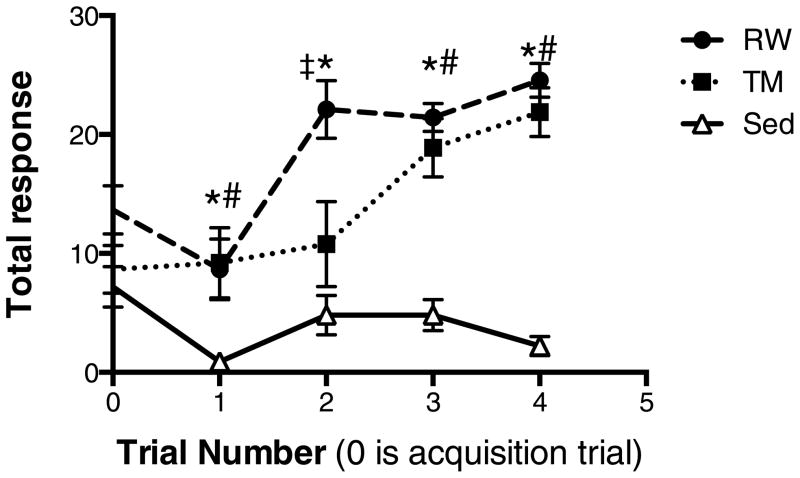
Animals were six months old at the start of the study and those in voluntary running wheels ran an average of 0.95 ± 0.20 (SEM) kilometers/day (Fig. 2C), which is similar to what has been reported previously for rats five months of age fed standard chow [43]. Cumulatively, animals on running wheels ran an average distance of 29.0 ± 6.9 kilometers, and had an average total response of 24.56 ± 1.42 and latency of 8.94 ± 0.5 sec. Rats exercised on treadmills ran 16.9 kilometers; their mean number of total responses was 21.88 ± 2.06 (SEM) and mean latency was 8.32 ± 0.53 sec. The mean number of total responses in sedentary animals was 2.2 ± 0.76 and the latency was 12.56 ± 0.17 sec. There was a significant interaction (exercise treatment group × trial number) in the outcome of total response (F8, 104= 5.1, p<0.0001). Running wheel access improved total response beginning at two weeks after the initiation of exercise, and treadmill exercise improved total response after three weeks compared with sedentary controls (p<0.05) (Fig. 3).
Fig. 3. Exercise-induced memory improvements begin after two weeks.
There was a significant interaction (exercise treatment group × trial number) in the outcome of total response (F8, 104= 5.1, p<0.0001). After two weeks of running wheel (RW) access, animals had significantly improved total responses in the two-way active avoidance test. By three weeks treadmill (TM) exercised animals also had improved performance compared with sedentary (Sed) animals. * p<0.05 for RW vs. Sed; # p<0.05 for TM vs. Sed; ‡ p<0.05 for TM vs. RW; N=9–10. RW had improved performance from baseline at weeks two and four (p<0.01), TM animals improved from baseline at weeks 3 and 4 (p<0.01) and Sed animals had reduced performance compared with baseline at weeks one and four (p<0.05).
Experiment Two
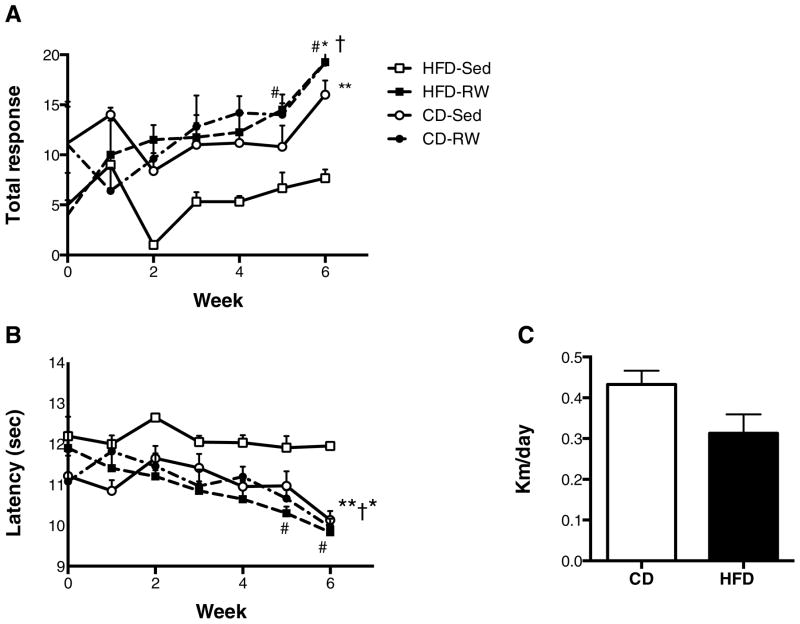
Animals were assigned to experimental groups such that there were no differences in fat mass, lean mass, %fat, %lean or weight between groups prior to initiation of exercise (Table 2A). Animals ran an average of 0.43 ± 0.03 kilometers/day on the control diet (Fig. 5C), only slightly higher than high-fat diet-fed animals (0.31 ± 0.05). These animals were 11 months old at the beginning of the exercise intervention. Distances of around 0.3 km/day have previously been reported for rats maintained on high-fat diet [43]. After the exercise intervention, animals on high-fat diet that were allowed voluntary running wheel access had significantly reduced body fat %, and a higher TWAA score compared with high-fat diet fed sedentary animals (p<0.05) (Table 2B).
Table 2.
Baseline (A) and post-exercise intervention (B) characteristics after 16 weeks of dietary intervention. Baseline characteristics were not significantly different between treatment groups within the same dietary category (A). Baseline measurements were taken post dietary and prior to exercise intervention. Post-exercise intervention % body fat and TWAA score were both significantly lower in exercised compared with sedentary animals on high fat diet (B). TWAA score is the number of total responses. p values indicate significant differences within dietary groups; N=5 (CD Sed and RW), N=4 (HFD RW), N=3 (HFD Sed).
| A | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control diet | High-fat diet | |||||||||
| Sed | RW | p value | Sed | RW | p value | |||||
| mean | SEM | mean | SEM | mean | SEM | mean | SEM | |||
|
| ||||||||||
| Fat (g) | 211 | 25.4 | 215.6 | 37.6 | 0.92 | 282.6 | 31.1 | 268.6 | 40.5 | 0.81 |
| Lean (g) | 461.3 | 19 | 477.5 | 23.9 | 0.61 | 463.2 | 30.3 | 490.8 | 25.4 | 0.51 |
| Weight (g) | 748.6 | 47.4 | 773.2 | 61.2 | 0.76 | 819.7 | 58.3 | 845.8 | 68.1 | 0.79 |
| %Fat | 27.8 | 1.8 | 27.3 | 2.5 | 0.85 | 34.4 | 2 | 31.3 | 2.5 | 0.42 |
| TWAA score | 11.2 | 4.1 | 11 | 3.8 | 0.97 | 5 | 3.2 | 4 | 1.5 | 0.77 |
| B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control diet | High-fat diet | |||||||||
| Sed | RW | p value | Sed | RW | p value | |||||
| mean | SEM | mean | SEM | mean | SEM | mean | SEM | |||
|
| ||||||||||
| Fat (g) | 229.6 | 31.6 | 175.7 | 30.3 | 0.25 | 300.9 | 41.1 | 199.2 | 34.4 | 0.12 |
| Lean (g) | 473.7 | 18.8 | 477.6 | 22.8 | 0.90 | 473.9 | 31.9 | 493.2 | 24.5 | 0.65 |
| Weight (g) | 787.8 | 55.4 | 736.8 | 48.8 | 0.51 | 853.0 | 75.4 | 778.0 | 55.8 | 0.45 |
| %Fat | 28.6 | 2.1 | 23.4 | 2.6 | 0.20 | 35.0 | 2.3 | 25.2 | 2.7 | 0.05 |
| TWAA score | 16 | 1.4 | 19.2 | 1.1 | 0.25 | 7.7 | 0.9 | 19.3 | 1.3 | 0.004 |
Fig. 5. Exercise improves high-fat diet-induced cognitive decline.
Exercise, increases total response (A) and reduces latency (B) in high-fat diet rats. There was a significant effect of time on total response (F3,6=4.0; p=0.01) and latency (F3,6=3.8; p=0.01) for high-fat diet fed running wheel animals. High-fat diet-RW had improved total response and reduced latency at weeks five and six compared with baseline. There was a significant effect of treatment group on total response (F3, 13=7.3; p=0.004) and latency (F3, 13=8.0; p=0.003) at week 6. Daily running wheel distances in animals fed high-fat diet (HFD) (C) were not significantly different from animals fed the control diet (CD). *p≤0.05 for high-fat diet-RW vs. high-fat diet-Sed; **p≤0.05 for control diet-Sed vs. high-fat diet-Sed. †p≤0.05 for control-diet running wheel vs. high-fat diet-Sed. # p ≤ 0.05 for high-fat diet-RW vs. their baseline N=5 for control diet-Sed, N=5 for control diet-RW, N= 4 for high-fat diet-RW, and N=3 for high-fat diet-Sed.
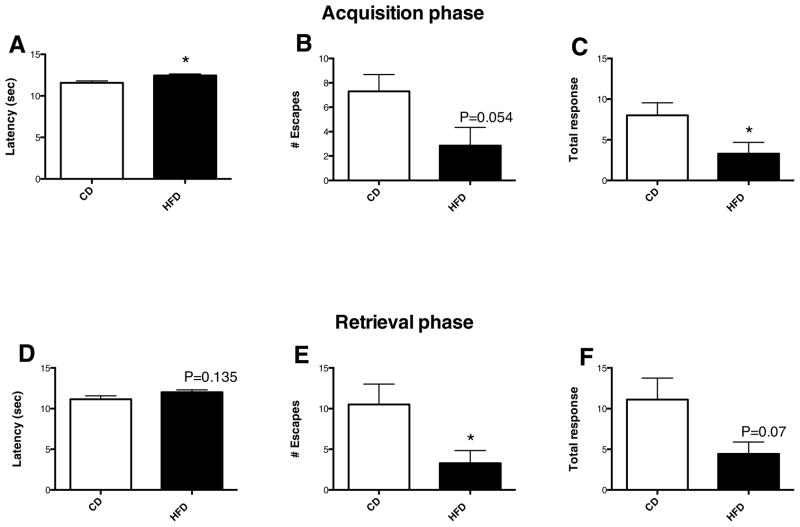
High-fat diet consumption for 16 weeks resulted in significant cognitive decline, indicated by increased latency (Fig. 4A), decreased number of escapes (Fig. 4B) and total responses (Fig. 4C) during the acquisition phase (p<0.05). Similarly, high-fat diet consumption increased latency (Fig. 4D), decreased number of escapes (Fig. 4E) and total responses (Fig. 4F) during the retrieval testing (p<0.05). The number of avoidances was low in both high-fat and control-fed animals prior to exercise (data not shown), and remained low throughout the study. However rats on high-fat diet had a reduced number of escapes, which contributed to an overall reduced total response and increased latency compared to control diet-fed animals. There was robust improvement in total response in the high-fat diet-fed animals after only two weeks of running, which persisted for the remainder of the study (Fig. 5A). There was a significant effect of time on total response (F3,6=4.0; p=0.01) and latency (F3,6=3.8; p=0.01) for high-fat diet fed running wheel animals. At week 5 and 6 high fat diet fed animals that had running access had significantly improved total responses and latency scores compared with baseline. Control diet fed animals that had access to running wheels had improvements in total response, however the effect of time was not significant (F4,6=2.4; p=0.056). During week 6, the number of total responses was elevated in both exercised and sedentary control diet groups and high fat diet exercised animals compared with high-fat diet fed sedentary controls (p<0.05) (Fig 5A). Similarly, the latency was reduced in these three groups compared with high-fat diet sedentary controls (p<0.05) (Fig. 5B). Taking into account the within group improvements observed in the high-fat diet sedentary group relative to baseline, together these data indicate that exercise reverses high-fat diet induced cognitive impairments.
Figure 4. High-fat diet consumption impairs contextual memory.
High-fat diet increases latency (A), and decreases numbers of escapes (B) and total response (C) during memory acquisition testing, and decreases number of escapes (E) and total response (F) in memory retrieval testing. *p < 0.05. N=10 for control diet, N = 7 for high-fat diet.
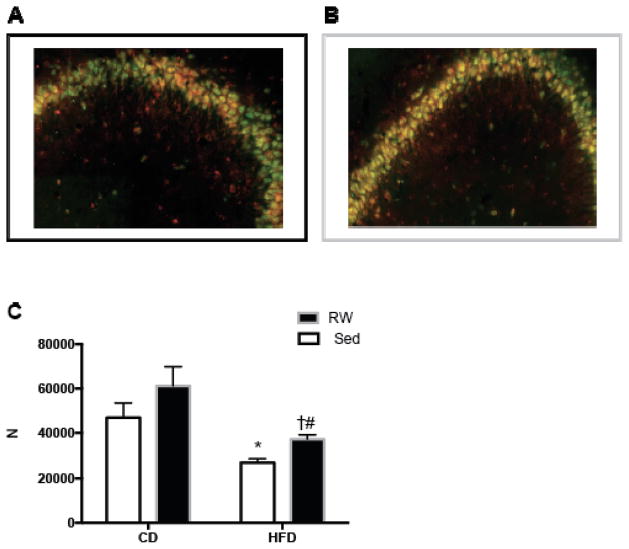
Without exercise, high-fat diet reduces memory improvements over time, as indicated by the reduction in total response compared with control diet-fed animals at some of the later time-points. Similarly, latency increased in sedentary animals given high-fat diet compared with those on control diet at some of the later time-points. Thus, our results indicate that running wheel exercise completely reversed the TWAA impairments resulting from the consumption of HFD. Corresponding to the behavior change, our stereological results indicated significantly increased co-localization of BDNF and NeuN in the pyramidal layer of CA3 in high-fat diet-fed exercised vs. high-fat diet-fed sedentary animals (Fig. 6A–C). Both sedentary and running wheel exercised animals had reduced CA3 neuronal BDNF when animals were maintained on a high-fat diet compared with animals fed control chow (Fig. 6C). Though exercise increased BDNF in both control diet and high-fat diet groups, significant differences in BDNF levels were only observed in the high-fat diet fed group. Our data demonstrates that HFD reduces the expression of BDNF in the dorsal CA3, and exercise partially reverses this trend.
Fig. 6.
In high-fat diet fed rats, seven weeks of exercise increased BDNF in neurons of the hippocampal CA3. Representative images of CA3 region stained for BDNF (red) NeuN (green) and BDNF+NeuN (yellow) in sedentary (Sed) (A) and running wheel (RW) (B) rats. There was a significant effect of diet (F1,8=14.1, p=0.001) and activity level (F1,8=4.4, p=0.02) but no significant interaction (diet × activity) (C). The effect of diet was significant within both Sed and RW exercised animals. Within high-fat diet (HFD)-fed animals, the RW exercised group had significantly more neuronal BDNF. * p=0.005 compared with Sed control diet (CD); # p=0.01 compared with RW CD. † p=0.03 compared with Sed HFD; N=2–4 per group.
Discussion
Our experiments indicate that exercise reverses cognitive deficits related to diet, at least in the context of active avoidance learning and memory. While previous studies have shown that exercise can prevent cognitive declines from high fat diet, this is the first demonstration of exercise reversal of prior declines induced by high fat diet. The reversal of cognitive deficit was associated with enhanced expression of BDNF in the CA3 region of the dorsal hippocampus. To our knowledge, this is the first demonstration that exercise induced enhancement of TWAA performance is associated with increased BDNF expression in a specific subfield (CA3 region) of the dorsal hippocampus.
Our findings that exercise improves performance on the hippocampus dependent two-way active avoidance (TWAA) task (Experiment One) are in agreement with previous animal and human studies. In one such study, running wheel exercise improved memory retrieval in both young and old mice, when tested with the TWAA task [44]. In addition, a number of studies have shown enhanced spatial memory in mice and rats, following physical exercise [45, 15, 49, 50]. Furthermore, physical activity is demonstrated to enhance learning in the radial-arm maze task [51] and passive avoidance tasks in rats [4]. Similarly, a study in mice showed that environmental enrichment, involving exposure to running wheels, enhanced spatial memory and reduced anxiety-like behavior [46]. Interestingly, a study demonstrated that wheel running improves contextual memory, but not cue-conditioned performance in rats, [47]. The enhancement of contextual fear conditioning following exposure to running wheel provides further evidence that exercise alters hippocampus dependent learning and memory [48]. Along the same lines, human studies also demonstrated that aerobic exercise improves learning and memory in healthy individuals, as well as during aging [5, 52]. This improvement in learning and memory was associated with increased hippocampus volume. Our results, together with the above findings, support the idea that exercise enhances learning in a number of hippocampus dependent tasks.
We found that after 5 weeks of exercise, animals that ran on a treadmill had a greater number of shock avoidances compared with animals with running wheel access, however both running wheel and treadmill improved total response and latency, and both total response and latency correlated with distance run (Fig. 2). Treadmill exercise is a forced paradigm in which animals were exposed to moderately aversive stimuli to enforce running (puff of air, wire brush poke, mild foot shock). Thus the greater number of avoidances in treadmill animals may be due to neuronal changes specific to forced exercise [53]. Although treadmill exercise was associated with more avoidances during the TWAA task, we chose to use the voluntary running paradigm for our second experiment on TWAA performance in animals fed high-fat and control diet due to the potential confound of using aversive stimuli that would potentially be required to motivate larger animals with greater percentage of body fat. Forced exercise produces physiological adaptations indicative of chronic stress, which could potentially impact cognitive measures [54]. In addition, forced exercise was found to increase anxiety like behaviors as tested in the open field, even though neurogenesis was higher with forced exercise [55].
In the present study, animals maintained on a high-fat diet had reduced performance in the TWAA test compared with animals on control chow (Experiment Two). From our current data it is not possible to determine whether cognitive deficits associated with high-fat diet can be attributed to the components of the diet, or to obesity consequent to the diet, since all animals gained weight after 16 weeks of high fat vs control diet consumption. Nevertheless, we found it remarkable that with voluntary running wheel exercise, cognitive performance in animals maintained on high-fat diet was more similar to those fed control diet. This suggests that aerobic exercise may improve cognitive deficits even when dietary interventions are unsuccessful, and that voluntary exercise reverses the HFD induced cognitive decline. Previous studies have reported cognitive deficits in diet-induced obese rats and not obesity resistant rats who consumed less of an energy dense diet; however the question of whether the cognitive deficits are related to obesity itself or higher doses of some specific or grouping of dietary components persists [36]. The diet used in this study contained 45% kcal from fat, mainly comprised of lard; thus, saturated fat comprised 16.2% of the total kcal. This diet contains 35% kcal carbohydrate, about half of which is comprised of sucrose (50% kcal of sucrose). This diet slightly exceeds the average American diet in percentages of saturated fat (16.2%, the average American diet contains 11%) and sucrose (17%, the American diet contains about 16% calories from added sugars) [56]. Recent reports suggest that the amount of saturated fat in the diet might contribute to cognitive impairment. For example, rodents fed a 2% cholesterol and 10% saturated fat diet had more memory errors in the water radial arm maze than animals fed a diet with isocaloric amounts of control diet (12% soybean oil) [57]. Similarly, a diet high in saturated fat was associated with reduced performance on Olton’s radial arm maze, a variable-interval delayed alternation task and the Hebb-Williams maze series compared with standard chow fed, or high-polyunsaturated fat diets [58].
The magnitude of total response number was lower for animals in experiment two compared with experiment one, and, though there was a trend, we did not observe significant differences in total response scores between sedentary and running wheel animals in the control diet-fed group. The rats in experiment two were older (11 months compared with 6 months at the initiation of exercise), and the voluntary dose of exercise was smaller, which might explain the discrepancies in the effect of running between experiments. The animal number was also smaller in experiment two compared with experiment one, thus we may not have had sufficient power to detect a significant effect. Nevertheless, in experiment two we found that exercise rescued cognitive impairments induced by high-fat diet in aging (>1 year by the end of the study) rats.
Herein we provide evidence consistent with a BDNF related mechanism by which exercise improves high-fat diet-induced cognitive impairments. BDNF via its receptor tropomyosin-receptor kinase B (TrkB) increases synaptic plasticity and promotes neurogenesis and neuronal survival (for review see [59]). Voluntary wheel exercise has been extensively shown to elevate hippocampal BDNF [35, 40, 41, 60, 61], and promote neurogenesis [62], synaptic plasticity [1, 42, 63], and cognition [1]. Conversely, consumption of a palatable high-fat and refined sugar diet reduces BDNF and associated proteins essential to synaptic function, resulting in impaired spatial learning [1, 42]. The present study is in agreement with an earlier study showing that exercise attenuates the high fat diet induced learning impairments in the spatial version of Morris water maze [1]. That study found that exercise also prevented the high-fat diet-induced deficits in BDNF in whole hippocampal extracts. However, our current results indicate the involvement of hippocampus CA3 region BDNF in exercise reversal of high fat diet induced impairments in TWAA task performance. In addition, Molteni et al., started the diet and exercise interventions simultaneously, which lasted for two months, and the cognition test was performed once every month. By starting the diet and exercise interventions simultaneously, the Molteni et al., study showed that exercise blocks or prevents the memory impairing effects of HFD. In the current study, exercise intervention began after sixteen weeks of dietary intervention, and lasted for seven weeks. During these seven weeks rats were maintained on the respective diets and were tested for TWAA performance once every week. We demonstrated a true reversal of HFD induced cognitive decline by voluntary exercise. Results of the first experiment also showed a linear correlation between the running distance and task performance.
We chose the CA3 region specifically because TWAA performance is critically dependent on the integrity of dorsal hippocampal CA3 sub-field. Several studies have demonstrated that lesions in the CA3 subfield prevent retention, but not acquisition, of TWAA memory [10, 64–67]. There is increased activation of the immediate and early gene cFos in the CA3 region following both TWAA training and intracranial self-stimulation of the lateral hypothalamus, each of which improved memory retention during TWAA and together their actions had an additive on both CA3 cFos expression and TWAA performance [68]. Taken together these data implicate the importance of CA3 to TWAA memory retention.
Though we observed elevated BDNF in the CA3 of control diet fed animals compared with the matched group (running wheel, sedentary) on the high-fat diet (Fig. 6C), the TWAA scores were not significantly different between the control diet and high fat diet running wheel exercised animals (Fig. 5A, B). Together these data suggest that exercise rescues HFD-induced deficits in performance associated with CA3 neuronal BDNF, but that there may be a ceiling effect, where further increases in BDNF do not translate to elevated performance passed a certain level. Thus it may be that CA3 BDNF is important to normal functioning, but does not confer extraordinary performance.
Exercise may increase cognitive performance via CA3 BDNF increases in neuronal plasticity. In support of this idea, running increases dendritic length and spine density in CA3 pyramidal neurons [69], while chronic stress-induced atrophy in the apical dendrites of the CA3 neurons leads to memory impairments in the rat [70]. Lin et al observed that both treadmill and running wheel exercise augmented the dendritic field of CA3 neurons, and that contextual fear learning required TrkB signaling in the dorsal hippocampus [47]. It has been reported that exercise increases BDNF expression in whole hippocampus after three, seven and 28 days and there is an associated up-regulation of genes involved in machinery for synaptic vesicle trafficking as well as genes for second messengers involved in synaptic function [63]. Taken together, these data suggest that high fat diet reduces, but exercise increases hippocampal BDNF, leading to improved cognition, possibly via neuronal plasticity.
The CA3 region plays an important role in regulating adult neurogenesis in the dentate gyrus (a process involved in memory formation) [71]. In addition, exercise increases the number of new neurons produced in the dentate gyrus of the hippocampus [52, 72]. Previous reports have demonstrated neuroprotective effects of exercise in the CA3 region following either excitotoxic injury or restraint stress [73]. Thus increased BDNF in the CA3 with exercise could have a role in promoting neurogenesis or neuroprotection. It is well known that BDNF and exercise increase adult neurogenesis [74, 75]. Newly generated neurons are rapidly integrated into the hippocampal network and are particularly excitable and sensitive to incoming input [76]. Studies suggest that new neurons display unique electrophysiological properties that may increase likelihood of action potential firing in response to environmental stimuli, and hence enhance information processing aiding in higher cognitive abilities [77]. Thus, these neurons contribute and facilitate hippocampal-dependent task learning [76], and the resulting structural changes may also help to keep the brain fit for future learning [78]. Taken together with observations from recent literature, results of this study provide an important foundation for the hypothesis that exercise mediated reversal of high-fat diet-induced memory impairment might be resulting from higher BDNF expression in the CA3 region and associated neural activation, structural and functional changes. Future studies are needed to determine whether high-fat diet-reduced cognitive impairments are related to hippocampal BDNF and its role in neurogenesis, neuroprotection and/or synaptic plasticity.
Overall, similar to previous animal studies [36, 79], we found that high-fat diet impaired cognitive performance on a hippocampal-dependent learning task in rodents. These studies are in agreement with human studies that show impaired cognitive performance as a result of dietary factors. For example, diets high in saturated fat and sugar are associated with poorer performance on neuropsychological memory task [80]. When controlling for sex, age, energy intake and education, processed food consumption was associated with poorer performance on cognitive tests of executive functions [81], and higher saturated fatty acid intake is associated with worse global cognitive and verbal memory trajectories [38]. Saturated fat intake is associated with impaired memory in middle-aged people [37] and in women with type-2 diabetes [82] and is associated with age-related cognitive decline and mild cognitive impairment [83]. Additionally, over a 6-year period, a diet high in saturated fat was associated with declining cognitive test scores [36, 84]. Conversely, similar to the present results, a recent study reported improvements in memory performance in people who exercised [7]. That study also reported a direct correlation between increased hippocampal volume and improvements in memory performance [7]. Similarities between these human data and our findings, suggest that the mechanisms by which diet and exercise affect brain function in rats may be similarly applicable to humans.
In summary, high fat diet contributes to impaired hippocampus-dependent cognitive processing. Aerobic exercise reverses cognitive impairments induced by diet, and increases hippocampal neuronal BDNF. Future studies are needed to elucidate how high fat diets contribute to cognitive deficits, however the current data suggest that exercise may be an appropriate intervention to improve brain health in populations where access to healthy foods meets resistance or is cost prohibitive.
Materials and Methods
Animals
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were caged singly with a 12 hour light/dark cycle (lights on at 0600) in a temperature controlled room at 21–22°C. Teklad lab Chow and water were provided ad libitum except where noted. This protocol was approved by the Institutional Animal Care and Use Committee at the Minneapolis Veterans Affairs Health Care System.
Exercise protocols
Animals were either singly housed in standard cages, or, where noted, were singly housed in standard cages with ad libitum access to running wheels (RW). Running wheels, which animals could access at will, were externally fixed to standard shoebox cages via a short tunnel. Running was monitored using Activity Wheel Monitoring Software (Lafayette Instrument, Lafayette, IN). Animals given forced treadmill exercise (TM) were acclimated gradually for 1 week using interval training, and performed treadmill exercise 5 days per week at a moderate pace (~15 m/min) for 45 min/day. The treadmill consists of a single belt containing 5 individual lanes with foot shock available at the end of the belt (Harvard Apparatus, Holliston, MA). Motivators were used to keep the animals running on the treadmill including a puff of air, foot shock (0.2 mA), or a wire bristle brush. Use of the brush, the air puff or the foot shock was animal dependent. Only when the rats were reluctant to run, these stimuli were used. A puff of compressed air was used as an initial resort to motivate animals to run when they were reluctant to move on the treadmill. When this method failed, a wire brush was placed at the back of the treadmill belt and the sensation of something behind them would motivate them to keep running. When both the air puff and the wire brush method failed to motivate the animals, the electric foot shock wiring at the end of the belt was activated and a mild (0.2 mA) electric foot shock was used to keep the animals moving. The use of the foot shock was a rare event (< 2× per week).
Two-way active avoidance task
We used an automated two-way shuttle box (size, 45.7 × 20.3 × 30.5 cm; model 75-FSFX-Fusion; AccuScan Instruments, Columbus, OH). Sides of this shuttle box are made of high-grade acrylic and floors are made of stainless steel bars suitable for application of the foot shock. A vertical partition bisects the box with an opening in the middle, which permits the animal to travel freely from one side of the shuttle box to the other. The box contains a front and a rear sensor with a linear array of sixteen infrared light beams, which allow for locational detection of the animal. Each compartment of the shuttle box contains a light bulb (adjustable intensity, 6 W at 115 V AC) and a speaker capable of generating a pure Sine Wave tone of any frequency (10–20,000 Hz; adjustable 0.00–85 dB at 30 cm) as sound stimuli. The interface unit, known as Fusion Node, supplies power and permits interconnections between the computer and the shuttle box. A personal computer using remote monitoring system software (Fusion software) controls experimental protocols and data collection.
Procedure
Rats were placed in one compartment of the apparatus. After 5 min of acclimation, training trials were begun. During acclimatization and the training trials, the rats could move freely from one compartment to the other within the shuttle box. Rats were trained on a massed 30-trial shuttle box two-way active avoidance task. The procedures for the conditioned stimulus and unconditioned stimulus were as follows. A tone (5000 Hz; 45 dB) and a pulsatile light (2.5 Hz) were presented as a conditioned stimulus in the compartment with the animal, paired 5 sec later with a 0.5 mA scrambled foot shock (unconditioned stimulus) delivered through the floor grid (steel rods 0.5 cm in diameter, spaced 1.5 cm between centers). The “scrambled” nature of the shock prevents the animals from finding a “no-shock” position on the floor. To avoid receiving a foot shock the rat had 5 sec to move to the opposite compartment. If the animal did not move to the other compartment, the unconditioned stimulus was delivered for a maximum of 5 sec, and the conditioned stimulus ended with the unconditioned stimulus. While receiving the unconditioned stimulus, if the animal moved to the other compartment, both conditioned and unconditioned stimuli ended immediately. The inter-trial interval was variable with a mean of 60 sec.
Body composition
An EchoMRI-700 (Houston, TX) was used to measure fat mass, lean mass, free water and total water in the rats. Each animal was weighed and then placed into a plastic holders based on their body weight with limited restraint. Then the holder was placed in the EchoMRI machine for scanning. Each scan took 1–2 minutes. Earlier, we have shown that the fat mass and lean mass measured with the MRI is consistent with those chemically analyzed, with an almost 1:1 correlation [85, 86].
Immunofluorescence and unbiased stereology
Animals were perfused transcardially using 4% paraformaldehyde. Brains were collected and post-fixed for 12 h in 4% paraformaldehyde, then transferred through a gradient to 30% sucrose and finally stored in cryoprotectant at −20C until ready for use. Brain tissue was transferred to cold PBS for 48 h before sectioning to 40 μm using a freezing stage microtome (American Optical Co. model 860, Buffalo, NY). A brain from one sedentary animal in high-fat diet was accidently sectioned at 25 μm, was thus removed from the stereology analysis. Systematic random sampling was used for tissue collection and a total of six series were collected. Section collection began roughly at 2.1 mm posterior to bregma and ended at 3.2 mm posterior, according the Paxinos and Watson rat brain atlas [87]. Approximately 3–4 sections per animal were stained using the following procedure: free-floating sections were blocked using 5% normal donkey serum (NDS; Jackson Laboratories, West Grove, PA) in 0.01 M PBS with 0.3% Triton X-100. Tissue was then incubated in primary antibodies chicken anti-BDNF (Promega, Madison, WI; 1:100) and rabbit anti-NeuN (Millipore, Billerica, MA; 1:500) for 72 h at 4°C with gentle agitation. Sections were then washed in .01 M PBS and incubated in secondary antibodies: donkey anti-chicken Cy3 (Jackson Laboratories, West Grove, PA; 1:200) and donkey anti-rabbit Alexafluor 647 (Jackson Laboratories, West Grove, PA; 1:150) for two h at 22°C in the dark. Sections were mounted onto slides and coverslipped using VECTASHIELD hardset mounting medium with DAPI (Vector Laboratories, Burlingame, CA.). An image at 5× magnification was collected from each hemisphere containing the pyramidal layer of CA3 or CA1 using a Zeiss Axio Imager M2 (Gottingen, DE). The region of interest (ROI) was outlined and a random offset grid drawn onto the image using imageJ software (NIH, Bethesda, MD). At each grid crossing within the ROI an image was collected at 63× magnification. Optical sectioning was performed through 21 μm of the tissue using the Z stack function of Axiovision Software (Carl Zeiss Vision, version 4.8). Unbiased stereological methods were performed in order to count BDNF, NeuN and BDNF+NeuN positive cells [88]. Briefly, images were uploaded into image J where cells were counted using an unbiased counting frame (27×27μm). In order to avoid counting cells more than once, cells were counted only when “tops” of nuclei first came into focus. The estimated number of cells containing respective staining in each reference space (N) was calculated using the fractionator method [89, 90]:
where ssf = the number of sections analyzed/total number of sections, asf = the area of the dissector frame/area of the xy step in the random offset grid and tsf = the dissector height/section thickness.
Experimental protocol
Experiment 1
Six-month old Rats were given free access to a running wheel (RW, 24h/d and 7d/wk) (n=9), exercised on a treadmill (TM, speed of 15m/min, 45min/d and 5d/wk) (n=10), or left undisturbed (Sedentary, Sed) (n=10) for 5 weeks. One week after the beginning of exercise, rats underwent a session (30 trials) of TWAA acquisition training. During training sessions, rats were placed in the shuttle box. After 5 min of acclimation, condition stimulus-unconditioned stimulus trials began. After 30 trials, rats were transferred to their respective home cages. They were tested 48 h later and thereafter once every week for retention of the task, a procedure similar to that of acquisition training.
Experiment 2
Seven-month old Naïve rats were maintained on either a high-fat (n=7) or control diet (n=10) for 16 weeks (Research Diets D12451 and D12450B, respectively). The rats were trained using the TWAA test and assessed for retention 24 h later. Rats in each dietary category were then given access to a running wheel (24h/7d) or were allowed to remain sedentary (n=5 for control diet Sed and RW, n=4 for high-fat diet RW, n=3 for high-fat diet Sed), and memory was tested once a week during seven weeks of exercise as described under experiment one. At the end of study, rats were perfused transcardially and brains were analyzed for BDNF and NeuN using immunofluorescent procedures.
Data Analysis
To measure performance on the TWAA task, each trial was analyzed for successful avoidances, escapes and escape latency. Experiment one data were analyzed using two-factor (time and treatment) ANOVA, followed by the Holm-Sidak post-hoc analysis for multiple comparisons. For experiment two, data were analyzed using three-factor (diet, time, and activity) ANOVA. The three-factor ANOVA, after identifying main effect of factors, did not provide further information between treatments within same diet at each time point, between diets within same treatment at each time point, or between pre- and post-intervention within same treatment, and thus a one-way ANOVA was used for further analysis, followed by the Holm-Sidak post-hoc test. Acquisition and retrieval data for experiment 2 prior to treatment were analyzed using unpaired t-tests. For both experiments, within group comparisons were performed using one-way repeated measures ANOVA to compare each time point with baseline, in order to determine whether improvements in memory occurred over time. Significance was considered to be achieved where p<0.05. Analyses were performed to determine, a) the differences in acquisition between different treatment groups (following a week of exercise in experiment one, and following 16 weeks of high-fat diet exposure in experiment two), and b) differences in retention between treatment groups. The improvement of performance between training trials (first session) and test trials (all sessions thereafter) were analyzed for avoidances (crossing after conditioned stimulus, but before shock delivery) escapes (crossing to opposite side during shock delivery), total responses (avoidances + escapes) and for escape latency (interval between conditioned stimulus and a response). These parameters were also compared during test sessions, between groups, to see if there was any specific effect of exercise (RW, or TM in experiment one and RW in experiment two) on memory retention. For analysis of BDNF neuronal counts in the CA3 region, data were transformed using reciprocal transformation. Two-factor ANOVA was performed (diet, activity) followed by the Holm-Sidak post-hoc test. Statistics were performed using Sigma Plot 11.0 (Systat Software, Inc,) and Prism 5.0 (Graphpad Software, Inc.).
Highlights.
Exercise improves performance on two-way active avoidance task.
High fat diet is associated with reduced CA3 BDNF and reduced performance on an active avoidance test.
Exercise rescues two-way active avoidance performance deficits induced by high fat diet.
Diet-induced reductions in CA3 BDNF are elevated in running wheel- exercised rats.
Acknowledgments
This work was supported by the National Institutes of Health, and the Department of Veterans Affairs. Additional support was provided by Award Number T32DK083250 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the National Institutes of Health. We would like to thank Martha Grace for her laboratory assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Molteni R, et al. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123(2):429–40. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Vaynman S, Ying Z, Gomez-Pinilla F. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience. 2007;144(3):825–33. doi: 10.1016/j.neuroscience.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Churchill JD, et al. Exercise, experience and the aging brain. Neurobiology of aging. 2002;23(5):941–55. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 4.Radak Z, et al. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochemistry international. 2001;38(1):17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- 5.Colcombe SJ, et al. Aerobic exercise training increases brain volume in aging humans. The journals of gerontology Series A Biological sciences and medical sciences. 2006;61(11):1166–70. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 6.Zhao G, et al. Leisure-time aerobic physical activity, muscle-strengthening activity and mortality risks among US adults: the NHANES linked mortality study. British journal of sports medicine. 2014;48(3):244–9. doi: 10.1136/bjsports-2013-092731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer AF, Erickson KI. Effects of physical activity on cognition, well-being, and brain: human interventions. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2007;3(2 Suppl):S45–51. doi: 10.1016/j.jalz.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. Journal of applied physiology. 2006;101(4):1237–42. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 10.Datta S, et al. Pontine-wave generator activation-dependent memory processing of avoidance learning involves the dorsal hippocampus in the rat. Journal of neuroscience research. 2005;80(5):727–37. doi: 10.1002/jnr.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deadwyler SA, Bunn T, Hampson RE. Hippocampal ensemble activity during spatial delayed-nonmatch-to-sample performance in rats. J Neurosci. 1996;16(1):354–72. doi: 10.1523/JNEUROSCI.16-01-00354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 13.Yassa MA, et al. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010;51(3):1242–52. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng W, et al. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(43):13532–42. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Praag H, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(23):13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr AL, Swain RA. Rapid cellular genesis and apoptosis: effects of exercise in the adult rat. Behavioral neuroscience. 2011;125(1):1–9. doi: 10.1037/a0022332. [DOI] [PubMed] [Google Scholar]
- 17.Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137(4):1299–307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 18.Noble EE, et al. The lighter side of BDNF. American journal of physiology. Regulatory, integrative and comparative physiology. 2011 doi: 10.1152/ajpregu.00776.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulloor J, Datta S. Spatio-temporal activation of cyclic AMP response element-binding protein, activity-regulated cytoskeletal-associated protein and brain-derived nerve growth factor: a mechanism for pontine-wave generator activation-dependent two-way active-avoidance memory processing in the rat. Journal of neurochemistry. 2005;95(2):418–28. doi: 10.1111/j.1471-4159.2005.03378.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, et al. Contextual learning induces an increase in the number of hippocampal CA1 neurons expressing high levels of BDNF. Neurobiology of learning and memory. 2007;88(4):409–15. doi: 10.1016/j.nlm.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9(12):2581–7. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno M, et al. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20(18):7116–21. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorski JA, et al. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121(2):341–54. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- 24.Nagahara AH, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15(3):331–7. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moguel-Gonzalez M, Gomez-Palacio-Schjetnan A, Escobar ML. BDNF reverses the CTA memory deficits produced by inhibition of protein synthesis. Neurobiology of learning and memory. 2008;90(3):584–7. doi: 10.1016/j.nlm.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Erickson KI, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30(15):5368–75. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Datta S, Siwek DF, Huang MP. Improvement of two-way active avoidance memory requires protein kinase a activation and brain-derived neurotrophic factor expression in the dorsal hippocampus. Journal of molecular neuroscience : MN. 2009;38(3):257–64. doi: 10.1007/s12031-009-9206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eadie BD, V, Redila A, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- 30.Farmer J, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124(1):71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 31.Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1017–22. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 33.Stranahan AM, et al. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol Aging. 2010;31(11):1937–49. doi: 10.1016/j.neurobiolaging.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neeper SA, et al. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain research. 1996;726(1–2):49–56. [PubMed] [Google Scholar]
- 36.Davidson TL, et al. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiology & behavior. 2012;107(1):26–33. doi: 10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalmijn S, et al. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62(2):275–80. doi: 10.1212/01.wnl.0000103860.75218.a5. [DOI] [PubMed] [Google Scholar]
- 38.Okereke OI, et al. Dietary fat types and 4-year cognitive change in community-dwelling older women. Annals of neurology. 2012;72(1):124–34. doi: 10.1002/ana.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortega RM, et al. Dietary intake and cognitive function in a group of elderly people. The American journal of clinical nutrition. 1997;66(4):803–9. doi: 10.1093/ajcn/66.4.803. [DOI] [PubMed] [Google Scholar]
- 40.Adlard PA, et al. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neuroscience letters. 2004;363(1):43–8. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 41.Oliff HS, et al. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain research Molecular brain research. 1998;61(1–2):147–53. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- 42.Molteni R, et al. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112(4):803–14. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 43.Judge MK, et al. Prolonged hyperphagia with high-fat feeding contributes to exacerbated weight gain in rats with adult-onset obesity. American journal of physiology Regulatory integrative and comparative physiology. 2008;295(3):R773–80. doi: 10.1152/ajpregu.00727.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adlard PA, Engesser-Cesar C, Cotman CW. Mild stress facilitates learning and exercise improves retention in aged mice. Experimental gerontology. 2011;46(1):53–9. doi: 10.1016/j.exger.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Clark PJ, et al. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155(4):1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 46.Meshi D, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nature neuroscience. 2006;9(6):729–31. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 47.Lin TW, et al. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiol Learn Mem. 2012;97(1):140–7. doi: 10.1016/j.nlm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behavioral neuroscience. 2004;118(5):1123–7. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- 49.Fordyce DE, Farrar RP. Physical activity effects on hippocampal and parietal cortical cholinergic function and spatial learning in F344 rats. Behavioural brain research. 1991;43(2):115–23. doi: 10.1016/s0166-4328(05)80061-0. [DOI] [PubMed] [Google Scholar]
- 50.Fordyce DE, Wehner JM. Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase C activity in C57BL/6 and DBA/2 mice. Brain research. 1993;619(1–2):111–9. doi: 10.1016/0006-8993(93)91602-o. [DOI] [PubMed] [Google Scholar]
- 51.Anderson BJ, et al. Exercise influences spatial learning in the radial arm maze. Physiology & behavior. 2000;70(5):425–9. doi: 10.1016/s0031-9384(00)00282-1. [DOI] [PubMed] [Google Scholar]
- 52.Curlik DM, 2nd, Shors TJ. Training your brain: Do mental and physical (MAP) training enhance cognition through the process of neurogenesis in the hippocampus? Neuropharmacology. 2013;64:506–14. doi: 10.1016/j.neuropharm.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayes K, et al. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta neuropathologica. 2008;115(3):289–96. doi: 10.1007/s00401-008-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moraska A, et al. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. American journal of physiology Regulatory integrative and comparative physiology. 2000;279(4):R1321–9. doi: 10.1152/ajpregu.2000.279.4.R1321. [DOI] [PubMed] [Google Scholar]
- 55.Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156(3):456–65. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 56.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. U.S. Government Printing Office; Washington DC: 2011. Foods and Food Components to Reduce; pp. 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Granholm AC, et al. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis. 2008;14(2):133–45. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenwood CE, Winocur G. Learning and memory impairment in rats fed a high saturated fat diet. Behav Neural Biol. 1990;53(1):74–87. doi: 10.1016/0163-1047(90)90831-p. [DOI] [PubMed] [Google Scholar]
- 59.Noble EE, et al. The lighter side of BDNF. American journal of physiology Regulatory integrative and comparative physiology. 2011;300(5):R1053–69. doi: 10.1152/ajpregu.00776.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124(4):985–92. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 61.Berchtold NC, et al. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. The European journal of neuroscience. 2001;14(12):1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- 62.Lafenetre P, et al. Exercise can rescue recognition memory impairment in a model with reduced adult hippocampal neurogenesis. Frontiers in behavioral neuroscience. 2010;3:34. doi: 10.3389/neuro.08.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16(6):1107–16. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 64.Calderazzo Filho LS, Cavalheiro EA., II Effect of lesions in hippocampal subareas on rat shuttle behavior. Physiol Behav. 1979 Dec;23(6):989–93. doi: 10.1016/0031-9384(79)90286-5. [DOI] [PubMed] [Google Scholar]
- 65.Calderazzo Filho LS, Moschovakis A., II Effect of hippocampal lesions on rat shuttle responses in four different behavioral tests. Physiol Behav. 1977;19(4):569–72. doi: 10.1016/0031-9384(77)90236-0. [DOI] [PubMed] [Google Scholar]
- 66.Bailey EL, Overstreet DH, CAD Effects of intrahippocampal injections of the cholinergic neurotoxin AF64A on open-field activity and avoidance learning in the rat. Behav Neural Biol. 1986 May;45(3):263–74. doi: 10.1016/s0163-1047(86)80015-2. [DOI] [PubMed] [Google Scholar]
- 67.Lermontova N, et al. Effects of tacrine on deficits in active avoidance performance induced by AF64A in rats. Molecular and chemical neuropathology/sponsored by the International Society for Neurochemistry and the World Federation of Neurology and research groups on neurochemistry and cerebrospinal fluid. 1998;33(1):51–61. doi: 10.1007/BF02815859. [DOI] [PubMed] [Google Scholar]
- 68.Aldavert-Vera L, et al. Intracranial self-stimulation facilitates active-avoidance retention and induces expression of c-Fos and Nurr1 in rat brain memory systems. Behav Brain Res. 2013;250:46–57. doi: 10.1016/j.bbr.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 69.Yau SY, et al. Hippocampal neurogenesis and dendritic plasticity support running-improved spatial learning and depression-like behaviour in stressed rats. PLoS One. 2011;6(9):e24263. doi: 10.1371/journal.pone.0024263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McEwen BS. Stress and hippocampal plasticity. Annual review of neuroscience. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 71.Liu JX, Pinnock SB, Herbert J. Novel control by the CA3 region of the hippocampus on neurogenesis in the dentate gyrus of the adult rat. PloS one. 2011;6(3):e17562. doi: 10.1371/journal.pone.0017562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eadie BD, V, Redila A, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. The Journal of comparative neurology. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- 73.Kim BS, Kim MY, Leem YH. Hippocampal neuronal death induced by kainic acid and restraint stress is suppressed by exercise. Neuroscience. 2011;194:291–301. doi: 10.1016/j.neuroscience.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 74.Kobilo T, et al. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18(9):605–9. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pencea V, et al. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21(17):6706–17. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Couillard-Despres S, Iglseder B, Aigner L. Neurogenesis, cellular plasticity and cognition: the impact of stem cells in the adult and aging brain - a mini-review. Gerontology. 2011;57(6):559–64. doi: 10.1159/000323481. [DOI] [PubMed] [Google Scholar]
- 77.Clark PJ, et al. New neurons generated from running are broadly recruited into neuronal activation associated with three different hippocampus-involved tasks. Hippocampus. 2012;22(9):1860–7. doi: 10.1002/hipo.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shors TJ, et al. Use it or lose it: how neurogenesis keeps the brain fit for learning. Behavioural brain research. 2012;227(2):450–8. doi: 10.1016/j.bbr.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiology & behavior. 2011;103(1):59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Francis HM, Stevenson RJ. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behavioral neuroscience. 2011;125(6):943–55. doi: 10.1037/a0025998. [DOI] [PubMed] [Google Scholar]
- 81.Akbaraly TN, et al. Education attenuates the association between dietary patterns and cognition. Dement Geriatr Cogn Disord. 2009;27(2):147–54. doi: 10.1159/000199235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Devore EE, et al. Dietary fat intake and cognitive decline in women with type 2 diabetes. Diabetes care. 2009;32(4):635–40. doi: 10.2337/dc08-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Solfrizzi V, et al. Metabolic syndrome, mild cognitive impairment, and progression to dementia. The Italian Longitudinal Study on Aging. Neurobiology of aging. 2011;32(11):1932–41. doi: 10.1016/j.neurobiolaging.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 84.Morris MC, et al. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology. 2004;62(9):1573–9. doi: 10.1212/01.wnl.0000123250.82849.b6. [DOI] [PubMed] [Google Scholar]
- 85.Nixon JP, et al. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity. 2010;18(8):1652–9. doi: 10.1038/oby.2009.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Godar R, et al. Reduction of high-fat diet-induced obesity after chronic administration of brain-derived neurotrophic factor in the hypothalamic ventromedial nucleus. Neuroscience. 2011;194:36–52. doi: 10.1016/j.neuroscience.2011.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. San Diego, CA: Academic Press; 2007. [Google Scholar]
- 88.West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. The Journal of comparative neurology. 1990;296(1):1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- 89.Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. Journal of microscopy. 1986;143(Pt 1):3–45. [PubMed] [Google Scholar]
- 90.Mouton PR. Unbiased stereology : a concise guide. Baltimore: Johns Hopkins University Press; 2011. p. xiii.p. 177. [Google Scholar]