Abstract
Recognition of pathogens by insect pattern recognition receptors is critical to mount effective immune responses. In this study, we reported a new member (βGRP3) of the β-1, 3-glucan recognition protein (βGRP) family from the tobacco hornworm Manduca sexta. Unlike other members of the M. sexta βGRP family proteins, which contain an N-terminal small glucan binding domain and a C-terminal large glucanase-like domain, βGRP3 is 40–45 residues shorter at the N-terminus and lacks the small glucan binding domain. The glucanase-like domain of β GRP3 is most similar to that of M. sexta microbe binding protein (MBP) with 78% identity. βGRP3 transcript was mainly expressed in the fat body, and both its mRNA and protein levels were not induced by microorganisms in larvae. Recombinant βGRP3 purified from Drosophila S2 cells could bind to several Gram-negative and Gram-positive bacteria and yeast, as well as to laminarin (β-1, 3-glucan), mannan, lipopolysaccharide (LPS), lipoteichoic acid (LTA), and meso-diaminopimelic acid (DAP)-type peptidoglycan (PG), but did not bind to Lysine-type PG. Binding of βGRP3 to laminarin could be competed well by free laminarin, mannan, LPS and LTA, but almost not competed by free PGs. Recombinant βGRP3 could agglutinate Bacillus cereus and Escherichia coli in a calcium-dependent manner and showed antibacterial (bacteriostatic) activity against B. cereus, novel functions that have not been reported for the βGRP family proteins before. M. sexta βGRP3 may serve as an immune surveillance receptor with multiple functions.
Keywords: βGRP, microbial cell walls, antibacterial, agglutination, innate immunity
1. Introduction
The innate immune system is the first line of defense against pathogenic invaders in animals (Mogensen, 2009; Sukhithasri et al., 2013) and relies on surveillance molecules, named pattern recognition receptors (PRRs), to recognize pathogen-associated molecular patterns (PAMPs) present in pathogens but not in the hosts (Charroux et al., 2009; Charroux and Royet, 2010; Kanost et al., 2004; Lemaitre and Hoffmann, 2007). PRRs include C-type lectins, β-1, 3-glucan recognition/binding proteins (βGRPs/BGRPs) and Gram-negative bacteria binding proteins (GNBPs), peptidoglycan recognition proteins (PGRPs), Toll-like receptors (TLRs), retinoic acid-inducible gene 1 (RIG-I)-like receptors, nucleotide-binding oligomerization domain (NOD) receptors, and Dectin receptors (Pal and Wu, 2009; Takeuchi and Akira, 2010). Examples of PAMPs include lipopolysaccharide (LPS), lipoteichoic acid (LTA) and peptidoglycans (PG) from bacteria and β-1, 3-glucan from fungi (Rao and Yu, 2010). Upon binding to PAMPs, PRRs can stimulate humoral and cellular immune responses, such as phagocytosis, nodule formation, encapsulation and melanization, synthesis of antimicrobial peptides and activation of the prophenoloxidase (proPO) system (Jiang et al., 2010; Kanost et al., 2004; Thompson et al., 2011). In insect hemolymph, activation of serine proteinase cascade leads to the proteolytic activation of proPO to active phenoloxidase (PO) (Gupta et al., 2005; Jiang et al., 2010). PRRs such as C-type lectins, βGRPs/BGRPs and PGRPs can stimulate proPO activation in hemolymph when binding to various PAMPs (Jiang et al., 2004; Lee et al., 2004; Ma and Kanost, 2000; Rao and Yu, 2010; Wang et al., 2011).
Gram-negative bacteria binding protein (GNBP) was first characterized as a 50-kDa hemolymph protein from the silkworm Bombyx mori that can bind to Escherichia coli (Lee et al., 1996). GNBPs actually belong to the β-1, 3-glucan recognition protein (βGRP/BGRP) family, which is one of the major pattern recognition receptors that can bind to β-1, 3-glucans on bacteria and fungi (Hughes, 2012; Jiang et al., 2004; Lee et al., 1996; Ma and Kanost, 2000; Wang et al., 2011). Members of the βGRP family proteins contain a small (~100 residues) N-terminal glucan binding domain and a large (~350 residues) C-terminal glucanase-like domain that lacks key residues in the active sites for glucanase activity (Ma and Kanost, 2000; Ochiai and Ashida, 2000). The small glucan binding domains of βGRPs bind to β-1, 3-glucan with a mechanism different from that of glucanase-like domains (Dai et al., 2013; Kanagawa et al., 2011; Mishima et al., 2009; Takahasi et al., 2009). βGRPs have been identified in invertebrates, including insects and crustaceans, and they can bind to microbial cell wall components, leading to activation of proPO (Cerenius et al., 1994; Duvic and Soderhall, 1990; Vargas-Albores et al., 1996; Vargas-Albores et al., 1997; Zheng and Xia, 2012). In the tobacco hornworm Manduca sexta, βGRP1 and βGRP2 have been identified and they can greatly stimulate proPO activation after binding to laminarin (β-1, 3-glucan) (Jiang et al., 2004; Ma and Kanost, 2000). M. sexta microbe binding protein (MBP), a β-1, 3-glucanase related protein, binds to bacteria and fungi, and MBP alone weakly stimulates proPO activation, but can significantly activate proPO when combined with different microbial elicitors (Wang et al., 2011). An inducible GNBP was purified from the silkworm, B. mori (Hughes, 2012; Lee et al., 1996), and silkworm βGRP can bind to β-1, 3-glucan to initiate activation of the proPO cascade (Ochiai and Ashida, 2000). Drosophila DGNBP-1 can bind to LPS and β-1, 3-glucan and enhance immune gene expression induced by LPS and β-1, 3-glucan (Kim et al., 2000). Anopheles gambiae GNBPs are involved in anti-Plasmodium responses (Warr et al., 2008).
In this paper, we reported the characterization and functional analysis of βGRP3, a new member of the M. sexta βGRP family. We investigated tissue distribution of βGRP3 transcript and induced expression of βGRP3 mRNA in fat body, hemocytes and midgut as well as βGRP3 protein in hemolymph by immune challenge. We also expressed and purified recombinant βGRP3 from Drosophila S2 cells and studied binding of βGRP3 to microorganisms and to various microbial cell wall components, including laminarin (β-1, 3-glucan), mannan, LPS, LTA, meso-diaminopimelic acid (DAP)-type and Lysine-type PGs. Interestingly, we found that βGRP3, which is 40–45 residues shorter at the N-terminus and lacks the small glucan binding domain, possessed novel properties with calcium-dependent agglutinating activity against Bacillus cereus and E. coli and strong antibacterial (bacteriostatic) activity against B. cereus.
2. Materials and methods
2.1 Insect rearing and Drosophila S2 cell line
M. sexta eggs were purchased from Carolina Biological Supply (Burlington, NC). Larvae were reared on artificial diet at 25°C (Dunn and Drake, 1983), and the fifth instar larvae were used for the experiments. D. melanogaster Schneider S2 cells were purchased from American Type Culture Collection (ATCC).
2.2 cDNA cloning of βGRP3 and sequence analysis
An M. sexta EST sequence was predicted to encode a partial BGRP-like protein (Accession number: GR922389.1). We then designed primers based on the EST sequence to clone the full-length cDNA. Briefly, total RNA was prepared from the fat body of day 3 naïve larvae using TRIzol® Reagent (T9424, Sigma–Aldrich). For reverse transcription, total RNA was treated with RQ1 RNase-free DNase I (Promega) at 37°C for 30 min to remove contaminated genomic DNA, and DNase was inactivated by heating to 65°C for 20 min. Reverse transcription was performed using oligo(dT) primer (Promega) and ImProm-II reverse transcriptase (Promega) following the manufacturer’s instructions. M. sexta βGRP3 full-length cDNA was cloned using the forward primer βGRP3-F1: 5′-ACG ACT CGA TCA CAA GCA AC-3′ and the reverse primer βGRP3-R1: 5′-CAG AAC TTG AGC ATG GCT TT-3′. 5′and 3′ RACE reactions were performed using smarter race kit (Clontech). The opening reading frame (ORF) of βGRP3 was predicted from the nucleotide sequence using DNAMAN (Lynnon Corporation, Quebec, Canada). BLASTP (http://www.ncbi.nlm.nih.gov/) was used to search homologous βGRP, BGRP or GNBP protein sequences. Multiple sequence alignment was performed by ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/) with protein sequences retrieved from the NCBI database using default settings, and the phylogenetic tree was generated from the conserved regions of 18 proteins (not including the N-terminal regions) by Neighbor-Joining method with bootstrap of 1,000 replications using MEGA5 (Tamura et al., 2011). Figures were made from one representative set of data with the GraphPad Prism software (GraphPad, CA). Significance of difference was determined by one way ANOVA followed by a Tukey’s multiple comparison test using the same software (GraphPad, CA).
2.3 Expression and purification of recombinant βGRP3 and GFP
Recombinant βGRP3 was expressed in E. coli and purified for production of polyclonal antibody in rabbit. The cDNA sequence encoding mature βGRP3 (residues 24–441) was obtained by PCR using primers βGRP3-F2 (5′-CAT GCC ATG GTT TAT CGG TCC CGT TCC ACG TCT TTG-3′) and βGRP3-R2 (5′-CCC AAG CTT TTA CAG CGC GAC TAC TTT GAC ATA GTC-3′). PCR fragment was purified by agarose gel electrophoresis, digested with Nco I and Hind III enzymes, ligated into the Nco I/Hind III-digested expression vector H6pQE-60 (Lee et al., 1994) and then transformed into competent E. coli XL1-Blue cells. Recombinant plasmids were prepared from positive clones and confirmed by restriction enzyme digestion and DNA sequencing. Single bacterial colony on petri dish plates containing recombinant plasmid DNA was inoculated into LB medium containing ampicillin (100 μg/ml) and incubated at 37°C overnight. The overnight culture was diluted 1:100 in LB medium and incubated at 37°C to OD600=0.8 and then isopropyl-D-thiogalactoside (IPTG) (0.5 mM final concentration) was added to induce protein expression for another 6 h at 37°C. Bacterial cells were harvested by centrifugation and lyzed with the lysis buffer (50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 0.5% Triton X-100, 2 mg/ml lysozyme), and recombinant protein was purified using Ni-NTA agarose beads (Qiagen) following the manufacturer’s instructions. The purified protein was further separated on 12% SDS-PAGE and the gel slice containing recombinant βGRP3 was used as an antigen to produce rabbit polyclonal antiserum at Cocalico Biologicals, Inc (Pennsylvania, USA).
Recombinant βGRP3 was also expressed in Drosophila S2 cells and purified. The cDNA sequence encoding mature βGRP3 (residues 24-441) was amplified by PCR using primers βGRP3-F3 (5′-GGA AGA TCT TAT CGG TCC CGT TCC ACG TCT TTG A-3′) and βGRP3-R3 (5′-CCG CTC GAG CAG CGC GAC TAC TTT GAC ATA GTC-3′). The PCR product was recovered by agarose gel electrophoresis-Wizard® SV Gel and PCR Clean-Up System (A9285, Promega), subcloned into T-Easy vector (A1360, Promega), and recombinant plasmid was isolated. After digested with Bgl II/Xho I, cDNA fragment was recovered and inserted into Bgl II/Xho I digested expression vector pMT/BiP/V5-His A (V413020, Invitrogen) using T4 DNA ligase (M0202L, NEB). Green fluorescent protein (GFP) in the expression vector pMT/Bip/V5-His/GFP (V413020, Invitrogen) was used as a control protein. Recombinant expression vectors were purified using PureYield™ Plasmid Miniprep System (A1222, Promega) according to the manufacturer’s instruction and the insert sequences were confirmed by DNA sequencing. These plasmids were used to transfect S2 cells and generate stable S2 cell lines following the manufacturer’s instructions.
To purify recombinant βGRP3 and GFP, copper sulfate (final concentration of 250 μM) was added to stable S2 cells to induce expression of βGRP3 or GFP. Cell culture medium was collected starting at 24 h after protein expression for several days by collecting culture medium every day and re-suspending the cells with fresh medium, and recombinant βGRP3 and GFP were purified by affinity chromatography using anti-V5 agarose beads (A7345, Sigma–Aldrich) as described previously (Xu et al., 2012; Zhong et al., 2012). Fractions were analyzed by 10% SDS-PAGE, and those containing recombinant βGRP3or GFP were combined and desalted using D-salt™ Excellulose™ GF-5 desalting column (#1851850, Pierce) pre-equilibrated with H2O. Recombinant βGRP3 or GFP was eluted with water, and fractions containing βGRP3 or GFP were pooled and concentrated for the following assays.
2.4 Tissue distribution and expression of βGRP3 mRNA in response to immune challenge
To determine tissue distribution of βGRP3 mRNA, day 2 fifth instar M. sexta naïve larvae were used in the following experiments. Larvae were injected with 50 μl of heated-killed E. coli DH5a, Serratia marcescens, Staphylococcus aureus, Bacillus subtilis (5 × 107 cells/larva) or yeast (Saccharomyces cerevisiae) (107 cells/larva), or H2O (as a control), hemocytes, fat body, midgut, epidermis and testis were collected at 24 h post-injection and washed 3 times in anti-coagulant (AC) saline (4 mM NaCl, 40 mM KCl, 8 mM EDTA, 9.5 mM citric acid monohydrate, 27 mM sodium citrate, 5% sucrose, 0.1% polyvinyl pyrollidone, 1.7 mM PIPES). Total RNAs were isolated from these tissues using TRIzol® Reagent, and cDNAs were prepared from total RNAs (1 μg from each tissue) in a 25-μl reaction as described above. Real-time PCR was performed with SYBR Premix (Takara) on a 7500 system (Applied Biosystems) using primers βGRP3-F4 (5′-AAG AAG CGA CGG CTC TGC TTG G-3′) and βGRP3-R4 (5′-TGG ACA GCC AGG AGT TGG TGC-3′) for βGRP3 gene, and RPS3-F (5′-GTT GCG AGG TGG TGG TTT C-3′) and RPS3-R (5′-CCG TTC TTG CCC TGT TGG TC-3′) for the control ribosomal protein S3 (rpS3) gene, and the reaction conditions were: 95°C for 10 min, followed by 40 cycles of 95° C for 15 s and 60° C for 1 min. Then dissociation curve analysis was performed. Data from three replicates of each sample were analyzed with SDS software (ABI) using a comparative method (2−ΔΔ Ct) using rpS3 gene as an internal control gene. Expression in naïve or water-injected larvae was set to 1.
2.5 Time-course induction of βGRP3 protein in hemolymph
To determine time-course induction of βGRP3 protein in hemolymph by Western blot analysis, day 2 fifth instar naïve larvae were injected with E. coli, Micrococcus luteus or S. cerevisiae as described above. Hemolymph (~50 μl) was collected every 2 h after injection (at least 4 larvae in each group). Hemolymph samples were centrifuged at 3000 g for 10 min and cell-free plasma samples were collected. Equal volumes of plasma samples (from at least 4 larvae) were mixed and 0.5 μl mixed plasma samples were analyzed by 10% SDS-PAGE. Western blot was performed using rabbit anti-βGRP3 antiserum (1:2000 dilution) and goat anti-rabbit IgG conjugated to alkaline phosphatase (1:10,000 dilution) (Sigma-Aldrich).
To determine the concentration of βGRP3 protein in hemolymph, cell-free plasma samples from fifth instar naïve or E. coli-injected larvae were collected and pooled (from at least 30 larvae in each group). The amounts of βGRP3 in plasma samples were estimated by comparing the intensity of endogenous βGRP3 in the plasma samples with that of recombinant βGRP3 purified from Drosophila S2 cells by Western blot analysis using myImage Analysis V1.1 (Thermo Scientific).
2.6 Binding of βGRP3 to microbial cell wall components
To test binding of βGRP3 to microbial cell wall components, plate ELISA assays were performed. Ultrapure TLR grade lipopolysaccharide (LPS) and peptidoglycan (PG) from E. coli K12 (LPS-K12 and PG-K12), lipoteichoic acid (LTA) and PG from B. subtilis (LTA-BS and PG-BS) and S. aureus (LTA-SA and PG-SA) were from Invivogen. Mannan and laminarin (β-1, 3-glucan) were from Sigma-Aldrich. Briefly, wells of a flat bottom 96-well plate (Costar, Fisher) were coated with different microbial components (2 μg/well) as described previously (Yu and Kanost, 2000; Yu et al., 2005). The plates were placed overnight at room temperature until the water evaporated completely, heated to 60°C for 30 min, and then blocked with 200 μl/well of 1 mg/ml BSA in Tris buffer (TB) (50 mM Tris-HCl, 50 mM NaCl, pH 8.0) for 2 h at 37°C. The plates were rinsed and increasing concentrations of purified recombinant βGRP3 or GFP (a control protein) in TB containing 0.1 mg/ml BSA were added to the coated plates (50 μl/well). For competitive binding assay, recombinant βGRP3 (diluted to 2 μg/ml) was pre-incubated with increasing concentrations of free microbial components for 1 h at room temperature as described previously (Yu et al., 2005) and then added to the laminarin-coated plates. Binding of recombinant proteins to microbial components was allowed to occur for 3 h at room temperature. Then, the plates were incubated with monoclonal anti-polyhistidine antibody (Sigma-Aldrich) (1:2,000 in TB containing 0.1 mg/ml BSA) (100 μl/well) overnight at 4°C, followed by incubation with goat anti-mouse IgG conjugated to alkaline phosphatase (Sigma–Aldrich) (1:3,000 in TB containing 0.1 mg/ml BSA) (100 μl/well) for 2 h at 37°C. The plates were rinsed, p-nitro-phenyl phosphate (1 mg/ml in 10 mM diethanolamine, 0.5 mM MgCl2) was added (50 μl/well), and absorbance at 405 nm of each well was determined every minute for 30 min using a microtiter plate reader (Bio-Tek Instrument, Inc.). The data were analyzed by the GraphPad Prism software.
2.7 Binding of βGRP3 to microorganisms
Binding of recombinant βGRP3 to microorganisms was performed using a published method (Du et al., 2009) with slight modifications. Briefly, Gram-negative E. coli, Pseudomonas aeruginosa, Serratia marcescens, Gram-positive S. aureus, B. subtilis, M. luteus and Bacillus cereus, as well as S. cerevisiae were cultured overnight. The microorganisms were pelleted, washed twice with Tris-buffered saline (TBS) (25 mM Tris-HCl, 2.7 mM KCl, 137 mM NaCl, pH 7.5), then thoroughly resuspended in TBS containing 2 mM CaCl2 and 1 mM MgCl2. Purified recombinant βGRP3 or GFP (as a control) (each at 2 μg in 50 μl) were added to 50 μl bacteria (4 × 108 cells/ml) or yeast (4 × 107 cells/ml) with rotation for 1 h at room temperature. The microorganisms were pelleted and the supernatants were collected as unbound proteins. These microorganisms were washed four times with TBS, subjected to elution with 7% SDS (100 μl) for 10 min, washed in 1 ml TBS four times, and finally lyzed in lysis buffer. Samples of the unbound proteins, TBS wash, 7% SDS elution and microbial lysates (to detect tightly bound proteins) were subjected to immunoblotting analysis using monoclonal anti-V5 antibody (V8012, Sigma-Aldrich).
2.8 Agglutination of microorganisms by βGRP3
E. coli, S. marcescens, P. aeruginosa, S. aureus, B. subtilis, B. cereus, M. luteus, and S. cerevisiae were used for the agglutination assays. Aliquots (3.5 μl) of bacteria (3 × 109 cells/ml) and yeast (2.5 × 108 cells/ml) suspensions were mixed with purified recombinant βGRP3 or GFP protein (final concentration of 8 μg/ml) in a total of 25 μl TBS containing 2 mM CaCl2 and 1 mM MgCl2 or 5mM EDTA. The mixtures were incubated at room temperature for 1 h and then observed by microscopy as described previously (Yu et al., 1999; Yu et al., 2006).
2.9 Antimicrobial activity assay
The antimicrobial inhibition zone assay was performed according to a published protocol (Hultmark, 1998). A single colony of E. coli, S. marcescens, B. cereus, B. subtilis, or S. cerevisiae was grown overnight in LB broth (for bacteria) or YPD medium (for S. cerevisiae) at 37°C. Overnight bacterial cultures were then sub-cultured in LB medium and S. cerevisiae culture was sub-cultured in YPD medium until mid-log phase (OD600=~0.6). Fifteen microliters (15 μl) of log-phase cultures were diluted into 20 ml LB or YPD medium containing 1.5% agarose (kept in a 45°C heating block), mixed well and spread immediately on a petri dish. After the medium was solidified, 5-mm wells were punched in the agarose. Buffer, ampicillin, or purified βGRP3 was added into each well. The plates were incubated at 37°C overnight, and the diameters of inhibition zones were measured and recorded.
B. cereus growth curves were also determined in the presence of recombinant βGRP3 and various microbial components as described previously (Rao et al., 2012). Briefly, overnight B. cereus culture was sub-cultured in LB medium until mid-log phase, centrifuged at 1000 g for 10 min at 4°C, washed once in 10 mM Tris-HCl, pH 7.5, 0.1 mM EDTA, and diluted to OD600 = 10−3 with LB medium. The diluted B. cereus cultures (75 μl/well) was mixed with purified βGRP3 alone (final concentration of 2 μg/ml), βGRP3 mixed with different microbial components (final concentration of 20 μg/ml), or water (Control) in 96-well plates, and the total volume was adjusted to 100 μl with LB medium. B. cereus cultures were incubated at 37°C with 220 rpm shaking. OD600 was measured every hour by PowerWave XS plate reader (Bio-Tek, USA). Bacterial growth curves were generated using the Graphpad Prism software (GraphPad, CA).
3. Results
3.1 cDNA cloning and sequence analysis of M. sexta βGRP3
An M. sexta EST sequence was found to encode a partial BGRP-like protein (Accession number: GR922389.1), which is different from M. sexta βGRP1 and βGRP2 proteins reported previously (Jiang et al., 2004; Ma and Kanost, 2000). To clone the new βGRP homologue, we designed gene-specific primers based on the EST sequence. The full-length cDNA sequence of M. sexta βGRP3 was obtained by 5′ and 3′ RACE. The complete ORF of βGRP3 is 1323 nucleotides long encoding a protein of 441 amino acids with a putative signal peptide of 23 residues. The calculated molecular mass of mature βGRP3 is 48.9 kDa with an isoelectric point (pI) of 8.33. There are four potential N-linked glycosylation sites in βGRP3 (Asn46, Asn79, Asn124 and Asn177), and the glycosyl hydrolase like domain is located close to the C-terminus of βGRP3 (Fig. S1). βGRP3 is ~40–45 residues shorter at the N-terminus compared to M. sexta βGRP1 (487 residues), βGRP2 (482 residues) and microbe binding protein (MBP) (482 residues), and it lacks the small glucan binding domain (Fig. S2). The N-terminal region (~57 residues, not including the 23-residues putative signal peptide) of βGRP3 shows no similarity to any sequence in the NCBI database (data not shown).
BLASTP search showed that the glucanase-like domain of M. sexta βGRP3 is most similar to that of M. sexta MBP (78% identity), a β-1, 3-glucanase related protein (Wang et al., 2011), Hyphantria cunea β-1, 3-glucan binding protein (63% identity) (Shin et al., 1998) and B. mori BGRP-2 (63% identity) (Lee et al., 1996), followed by Helicoverpa armigera BGRP-3 (61% identity) (Pauchet et al., 2009), Ostrinia furnacalis BGRP (60% identity), Papilio xuthus Gram-negative bacteria binding protein-3 (GNBP-3) (58% identity). M. sexta βGRP3 also shows similarity to M. sexta βGRP1 (36% identity) (Ma and Kanost, 2000), βGRP2 (35% identity) (Jiang et al., 2004) and β-1, 3-glucanase (36% identity), but lacks the four key residues (W, E, D, E) required for glucanase activity (Fig. 1A) (Ma and Kanost, 2000). Therefore, βGRP3 may serve as a pattern recognition receptor in the immune system of M. sexta.
Fig. 1. Multiple sequence alignment of the active sites of glucanases and some βGRP family proteins and phylogenetic tree of the βGRP family proteins.
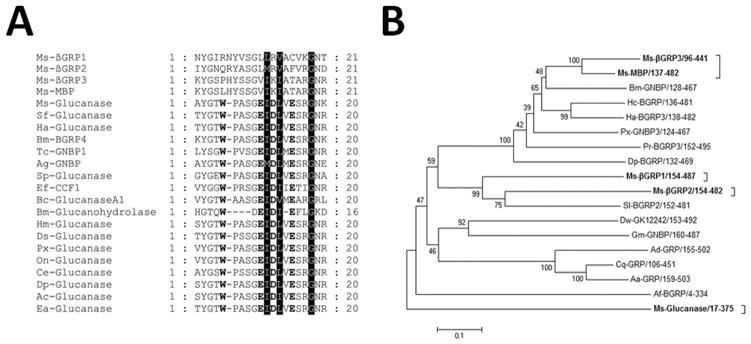
Glucanase-related proteins and the βGRP family proteins were retrieved from the NCBI database, multiple sequence alignment was performed by ClustalW2, and only residues around the active site of glucanase were shown (A). The Glycine residue conserved in all sequences and the two highly conserved hydrophobic residues are shaded. Residues corresponding to the active site (W, E, D and E) of the Bacillus macerans glucanase are shown in bold. A phylogenetic tree of 17 members of the βGRP family proteins and M. sexta beta-1, 3-glucanase (based on the large glucanase-like domains, see Fig. S3) was constructed by MEGA5 using the Neighbor-Joining method with bootstraps of 1,000. Ms-βGRP1, M. sexta beta-1, 3-glucan recognition protein 1 (AF177982); Ms-βGRP2, M. sexta beta-1, 3-glucan recognition protein 2 (AAN10151); Ms-βGRP3, M. sexta beta-1, 3-glucan recognition protein 3 (ADK39022); Ms-MBP, M. sexta microbe binding protein (GNBP-like) (ADT82662); Ms-Glucanase, M. sexta beta-1, 3-glucanase (AEV66276); Af-BGRP, Apis florea beta-1, 3-glucan-binding protein (XP_003694585); Hc-BGRP, Hyphantria cunea beta-1, 3-glucan-binding protein (O96363.1); Bm-GNBP, Bombyx mori beta-1, 3-glucan recognition protein 2 precursor (NP_001037450); Ha-BGRP3, Helicoverpa armigera beta-1, 3-glucan recognition protein 3 (ACI32828.1); Px-GNBP3, Papilio xuthus Gram-negative bacteria binding protein 3 (BAM19646.1); Dp-BGRP, Danaus plexippus beta-1, 3-glucan-binding protein (EHJ65186.1); Pr-BGRP3, Pieris rapae beta-1, 3-glucan recognition protein 3 (ACI32823.1); Sl-BGRP2, Spodoptera littoralis beta-1, 3-glucan recognition protein 2 (ACI32819.1); Cq-GRP, Culex quinquefasciatus beta-1, 3-glucan recognition protein (XP_001850624.1); Dw-GK12242, Drosophila willistoni GK12242 (XP_002068068.1); Gm-GNBP, Glossina morsitans Gram-negative binding protein 1-like protein (ABC25063.1); Aa-GRP, Aedes aegypti AAEL007626-PA (EAT40654.2); Ad-GRP, Anopheles darling hypothetical protein (EFR19153.1).
The βGRP family proteins have been identified in different invertebrate species. The phylogenetic tree constructed based on the glucanase-like domains of 17 βGRP related proteins and M. sexta beta-1, 3-glucanase showed that M. sexta βGRP3 and microbe binding protein (Ms-MBP) are clustered in the same clade, βGRP1 and βGRP2 are in a different clade, while M. sexta β-1, 3-glucanase is in a distant clade (Fig. 1B). These results suggest that the common ancestor of M. sexta βGRP family proteins diverged into three clades with possible functional variances.
3.2 Expression profiles of βGRP3 mRNA and protein
We expressed M. sexta βGRP3 in E. coli and purified the recombinant protein for production of rabbit polyclonal antibody. We also expressed and purified V5-tagged βGRP3 and GFP from Drosophila S2 cells for functional study. Recombinant βGRP3 purified from S2 cells migrated close to 58 kDa, which is larger than the calculated mass of 48.9 kDa, probably due to the addition of V5-His tag and post-translational modifications (Fig. 2). Recombinant βGRP3 could be recognized by both monoclonal anti-V5 and polyclonal anti-βGRP3 antibodies (Fig. 2B and C).
Fig. 2. Analysis of recombinant GFP and βGRP3 purified from Drosophila S2 cells.
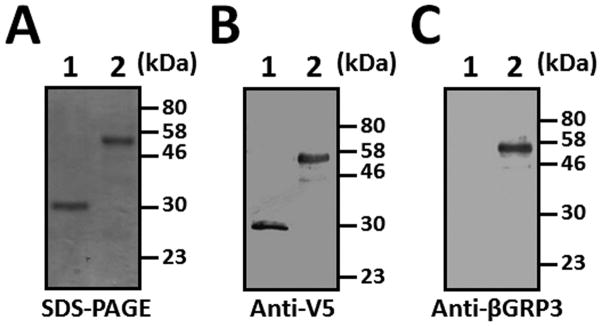
V5-tagged recombinant GFP and βGRP3 proteins were purified from Drosophila S2 cells and analyzed by SDS-PAGE (1.5 μg each protein) (A) and Western blot (0.2 μg each protein) using monoclonal anti-V5 (B) or polyclonal anti-βGRP3 (C) antibody. Lane 1, GFP; lane 2, βGRP3.
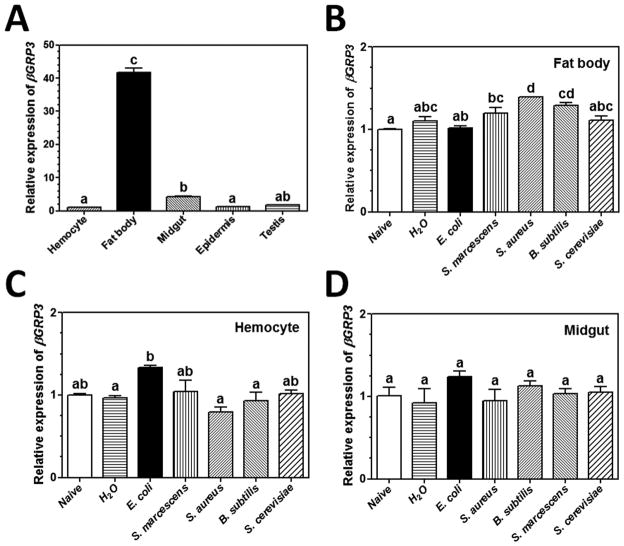
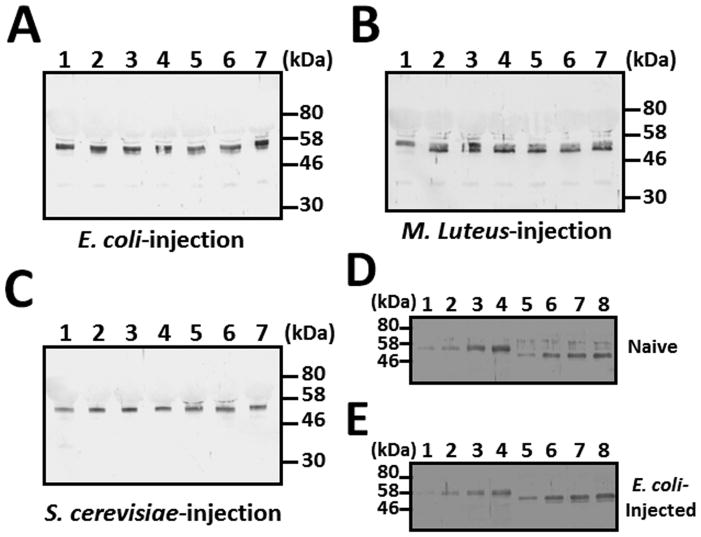
The mRNA level of βGRP3 in different tissues of M. sexta naïve larvae was determined by real-time PCR, and the results showed that βGRP3 transcript was mainly expressed in fat body at a significantly higher level than in hemocytes, midgut and other tissues (Fig. 3A). Expression of βGRP3 mRNA in fat body, hemocytes and midgut was not significantly up-regulated in response to microbial challenges (Fig. 3B-D), which is similar to that of M. sexta βGRP1 (Ma and Kanost, 2000). We also determined βGRP3 protein concentration in hemolymph by Western blot. The results showed that βGRP3 protein concentration in the hemolymph of E. coli, M. luteus and S. cerevisiae injected larvae did not change significantly from 0 to 12 h post-injection (Fig. 4A–C). To estimate the concentration of endogenous βGRP3 protein in hemolymph, Western blot was performed with increasing volumes of hemolymph from naïve and E. coli-injected larvae along with increasing amounts of purified recombinant βGRP3, and endogenous βGRP3 protein was estimated at ~ 260 μg/ml in the hemolymph of naïve larvae and ~255 μg/ml in the hemolymph of E. coli-injected larvae (Fig. 4D and E). These results suggest that both βGRP3 transcript and protein levels were not induced by immune challenges.
Fig. 3. Tissue distribution and expression of βGRP3 mRNA in response to immune challenge.
(A) Tissue distribution of βGRP3 mRNA. Expression of βGRP3 mRNA in hemocytes, fat body, midgut, epidermis and testis of the 5th instar M. sexta naïve larvae was determined by real-time PCR and normalized to rpS3 gene. (B–D): Expression of βGRP3 mRNA in fat body (B), hemocytes (C), and midgut (D) after immune challenge. Expression of βGRP3 mRNA in hemocytes, fat body and midgut of the 5th instar M. sexta naïve larvae or larvae injected with H2O, E. coli, S. marcescens, S. aureus, B. subtilis, or S. cerevisiae at 24 h post-injection was determined by real-time PCR. The bars represent the mean of three individual measurements ± SEM. Relative expression of βGRP3 mRNA in naïve larval hemocytes (A), or in tissues of naïve larvae (B–D) was set as 1. Comparing expression of βGRP3 mRNA in different tissues (A) or after microbial injections, identical letters among tissues or treatments indicate not significant difference (p>0.05), while different letters indicate significant difference (p<0.05) determined by one way ANOVA followed by a Tukey’s multiple comparison test.
Fig. 4. Expression of βGRP3 protein in hemolymph in response to immune challenge.
The 5th instar M. sexta naïve larvae were injected with E. coli (A), M. luteus (B), or S. cerevisiae (C), and hemolymph samples were collected at 0, 2, 4, 6, 8, 10 and 12 h post-injection as described in the Materials and Methods. Cell-free mixed plasma samples (0.5 μl each) were subjected to Western blot analysis using rabbit anti-βGRP3 antibody. (D) and (E): Concentration of endogenous βGRP3 in hemolymph. Increasing amounts of purified recombinant βGRP3 (lanes 1–4) or increasing volumes of mixed plasma samples (lanes 5–8) from naïve larvae (D) or E. coli-injected larvae (E) were analyzed by Western blot using rabbit anti-βGRP3 antibody. Lanes 1–4: 10, 25, 50 and 75 ng purified recombinant βGRP3, respectively; lanes 5–8: 0.1, 0.2, 0.3 and 0.4 μl cell-free mixed plasma samples, respectively.
3.3 Binding of βGRP3 to microbial cell wall components and microorganisms
To determine whether recombinant βGRP3 can bind to different microbial cell wall components, plate ELISA assay was performed. Compared to the control GFP protein, βGRP3 could bind to LPS-K12 and PG-K12 from E. coli K12, LTA-BS and PG-BS from B. subtilis, mannan and laminarin from fungi, and LTA-SA from S. aureus (Fig. 5), but did not bind to PG-SA from S. aureus (Fig. 5D). To confirm binding of βGRP3 to microbial components is specific, a competitive binding assay was performed. Binding of βGRP3 to laminarin was well competed by free laminarin, mannan, LPS-K12, LTA-SA and LTA-BS, but only slightly competed by PG-K12 and PG-BS (Fig. 5I), indicating that βGRP3 has broad binding spectra to microbial cell wall components with different affinities.
Fig. 5. Binding of βGRP3 to microbial cell wall components.
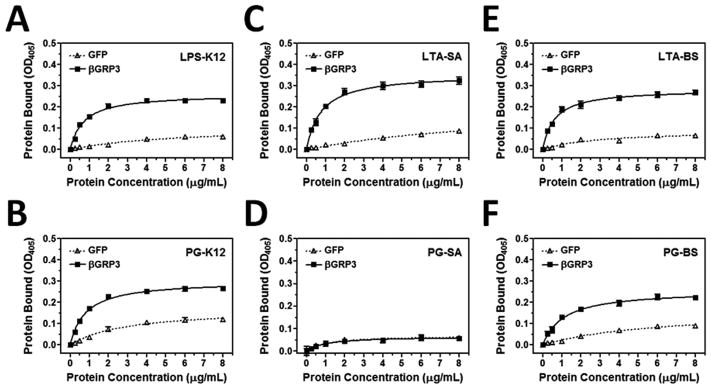
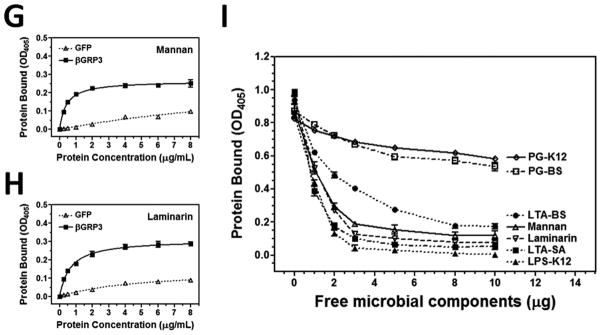
Increasing concentrations of recombinant βGRP3 or GFP purified from Drosophila S2 cells were added to 96-well plates coated with LPS-K12 (A), PG-K12 (B), LTA-SA (C), PG-SA (D), LTA-BS (E), PG-BS (F), mannan (G) or laminarin (H), and binding of recombinant proteins to microbial components was determined by plate ELISA using anti-V5 antibody. Recombinant βGRP3 (2 μg/ml) was pre-incubated with increasing amounts of free microbial components and then added to the laminarin-coated 96-well plates (I), and binding of βGRP3 to laminarin was determined by plate ELISA assay. The figures showed total binding of recombinant proteins to microbial components. Each point represents the mean of three individual measurements ± SEM, and the lines in (A–H) represent nonlinear regression calculation of one-site binding curves for βGRP3 (solid line) and GFP (dotted line).
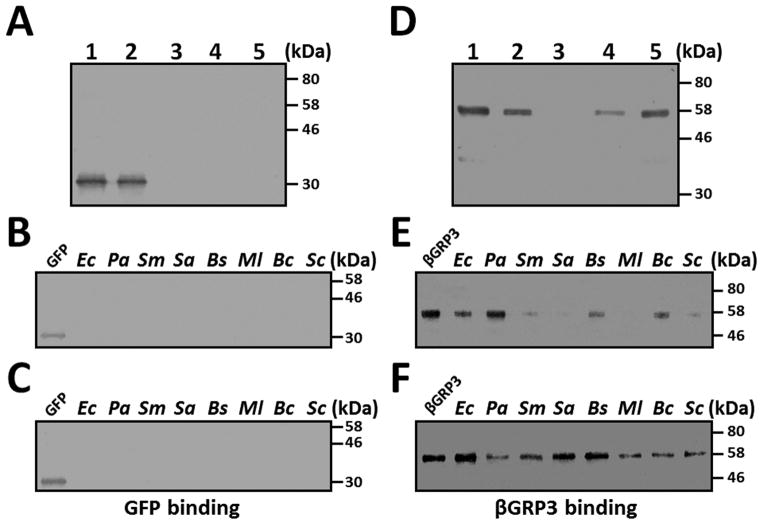
To further confirm broad binding spectra of βGRP3, a direct binding of βGRP3 to different microorganisms was performed. We first tested binding of GFP and βGRP3 to E. coli and found that GFP did not bind to E. coli and remained in the unbound fraction (Fig. 6A), but βGRP3 bound tightly to E. coli since only a small fraction of βGRP3 was eluted by 7% SDS and more βGRP3 remained in the E. coli lysates (Fig. 6D). We then tested binding of GFP and βGRP3 to several Gram-negative and Gram-positive bacteria as well as yeast. GFP did not bind to any of the microorganisms tested (Fig. 6B and C), but βGRP3 bound to all eight microorganisms tested (Fig. 6E and F). More βGRP3 protein bound to E. coli (Ec), P. aeruginosa (Pa), S. aureus (Sa) and B. subtilis (Bs) than to the other four microorganisms (Fig. 6E and F). Binding of βGRP3 to S. marcescens (Sm), S. aureus (Sa), B. subtilis (Bs), M. luteus (Ml), and S. cerevisiae (Sc) seems to be stronger since little or no βGRP3 was eluted by 7% SDS and more β GRP3 remained in microbial lysates (comparing Fig. 6E and F). These results suggest that β GRP3 is able to bind to multiple types of PAMPs on various microorganisms.
Fig. 6. Binding of βGRP3 to microorganisms.
Microorganisms were incubated with recombinant GFP (A–C) or βGRP3 (D–F) at room temperature for 1 h, washed with TBS, and bound proteins were eluted with 7% SDS. The remaining microorganisms were lyzed in lysis buffer. Recombinant proteins in each fraction were detected by Western blot using anti-V5 antibody. (A) and (D): Binding of recombinant GFP (A) and βGRP3 (D) to E. coli. Lane 1: purified recombinant proteins (0.2 μg each); lane 2: unbound proteins; lane 3: the fourth TBS wash; lane 4: 7% SDS elution; lane 5: E. coli lysates. In lanes 2–5, equivalent volumes to lane 1 were loaded in each lane. (B, C, E and F): Binding of GFP and βGRP3 to various microorganisms. Only SDS elution fractions (B and E) and microbial lysates after SDS eluting (C and F) were analyzed by Western blot using anti-V5 antibody. Ec: E. coli, Pa: P. aeruginosa, Sm: S. marcescens, Sa: S. aureus, Bs: B. subtilis, Ml: M. luteus, Bc: B. cereus, Sc: S. cerevisiae.
3.4 Agglutination of E. coli and B. cereus by βGRP3
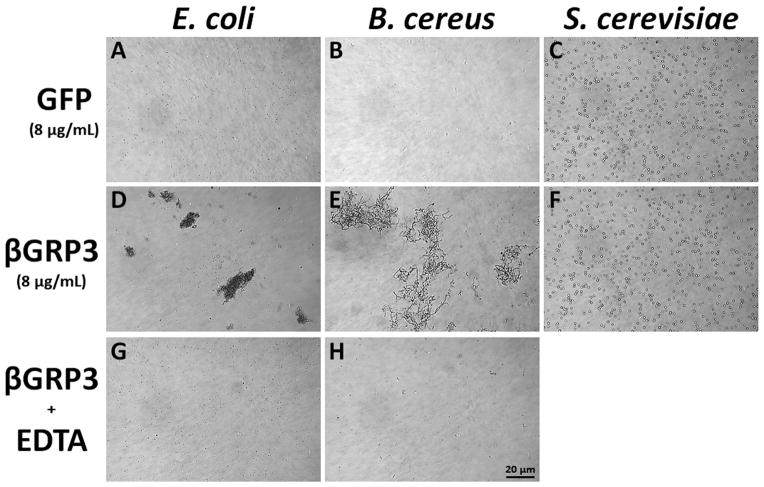
To test whether βGRP3 also has agglutinating activity against microorganisms, purified recombinant βGRP3 or GFP was incubated with eight microorganisms, and agglutination of microbial cells was observed under microscope. GFP did not agglutinate any of the eight microorganisms tested (Fig. 7A–C and data not shown), but βGRP3 could agglutinate E. coli and B. cereus (Fig. 7D and E), but not S. cerevisiae (Fig. 7F) and the other five bacteria (data not shown), and agglutination of E. coli and B. cereus was calcium-dependent (Fig. 7G and H). In addition, E. coli cells formed aggregates in the presence of βGRP3 (Fig. 7D), while B. cereus cells were cross-linked by βGRP3 to form large networks (Fig. 7E).
Fig. 7. Agglutination of microorganisms by βGRP3.
Purified recombinant GFP or βGRP3 was incubated with E. coli, B. cereus or S. cervisiae in TBS containing 2 mM CaCl2 and 1 mM MgCl2 (A–F) or 5 mM EDTA (G and H) at room temperature for 60 min, samples of microbial cells were examined by microscopy. Bar = 20 μm.
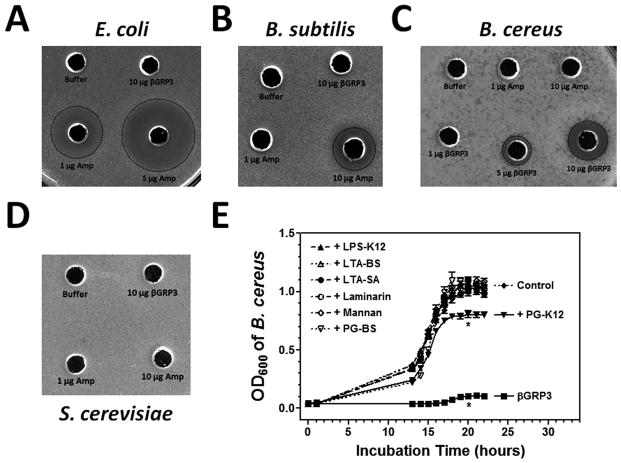
3.5 Antibacterial activity of βGRP3 against B. cereus
Since βGRP3 could bind to microorganisms (Fig. 6) and agglutinate E. coli and B. cereus (Fig. 7), it may have antimicrobial activity and an inhibition zone assay was performed. Ampicillin and buffer were used as positive and negative controls. Ampicillin showed high activity against E. coli and relatively high activity against B. subtilis, but recombinant βGRP3 did not show activity against E. coli or B. subtilis (Fig. 8A and B). Interestingly, ampicillin was not active against B. cereus, but βGRP3 showed strong activity against B. cereus in a dose-dependent manner (Fig. 8C). Both ampicillin and βGRP3 did not have activity against S. cerevisiae (Fig. 8D). To test whether binding of microbial components to βGRP3 may inhibit its activity against B. cereus, bacterial growth curves were determined in presence of βGRP3 alone or βGRP3 mixed with microbial components. The results showed that βGRP3 alone almost completely inhibited B. cereus growth (OD600 = 0.104 compared to OD600 = 1.013 in the control group (water) at 20 h after incubation). LPS-K12, LTA-BS, LTA-SA, laminarin, mannan and PG-BS could all completely block the antibacterial activity of βGRP3 against B. cereus (OD600 = 0.998 – 1.087 compared to OD600 = 1.013 in the control group) (Fig. 8E). PG-K12 also significantly blocked the activity of βGRP3 against B. cereus growth (OD600 = 0.805 vs. OD600 = 0.104) (pitalic>0.001), but B. cereus growth was still significantly inhibited compared to the control group (OD600 = 0.805 vs. OD600 = 1.013) (pbold>0.001) (Fig. 8E).
Fig. 8. Antibacterial activity of βGRP3 against B. cereus.
Antibacterial activity of recombinant βGRP3 was determined by inhibition zone assay (A–D) and broth micro-dilution assay (E) as described in the Materials and Methods. Buffer and different amounts of ampicillin (Amp) were used as controls in the inhibition zone assay, the plates were incubated at 37°C overnight, and the diameters of inhibition zones were measured and recorded (A–D). Diluted B. cereus culture was incubated with recombinant βGRP3 alone (2 μg/ml) or βGRP3 (2 μg/ml) mixed with different free microbial components (20 μg/ml each) in 96-well plates at 37°C, and OD600 was recorded every hour up to 22 h. The points represent the mean of four individual measurements ± SEM. Asterisks indicate significant difference in OD600 values at 20 h among the control, βGRP3, and βGRP3+PG-K12 groups (p<0.001) determined by one way ANOVA followed by a Tukey’s multiple comparison test.
4. Discussion
Invertebrates lack the adaptive immune system and rely on pattern recognition receptors (PRRs) as surveillance molecules to detect microorganisms and mount immune responses. These surveillance molecules circulate in the animal body to recognize pathogen-associated molecular patterns (PAMPs) (Mogensen, 2009; Pal and Wu, 2009; Sukhithasri et al., 2013). β-1, 3-glucan recognition proteins (βGRPs/BGRPs) are a family of proteins containing a small N-terminal glucan binding domain and a large C-terminal glucanase-like domain, which lacks key residues for glucanase activity (Fig. 1A) but still can bind to β-1, 3-glucan. In this study, we have cloned a new member (βGRP3) of the M. sexta βGRP family, and investigated functions of βGRP3 in innate immunity.
M. sexta βGRP1 and βGRP2 transcripts are mainly expressed in fat body. βGRP1 mRNA is expressed in both feeding and wandering stages of larvae, while βGRP2 transcript is only expressed in the wandering stage larvae (Jiang et al., 2004; Ma and Kanost, 2000). We found that β GRP3 mRNA was predominantly expressed in fat body of naïve larvae (Fig. 3A). Expression of βGRP3 transcript in fat body, hemocytes and midgut as well as βGRP3 protein in hemolymph was not induced by different immune challenges (Figs. 3B–D and 4), which is similar to that of M. sexta βGRP1 but different from that of βGRP2 (Jiang et al., 2004; Ma and Kanost, 2000). M. sexta MBP mRNA is mainly expressed in fat body and is significantly induced by immune challenge, and MBP protein concentration in hemolymph also increases after immune stimulations (Wang et al., 2011). These results indicate that the βGRP family proteins are differentially regulated by development and immune challenge.
Recombinant βGRP3 had a broad binding spectrum to microbial components (Fig. 5) and microorganisms (Fig. 6). Broad binding spectra of insect PRRs, including members of the βGRP family, PGRPs and C-type lectins, have been reported. For example, two beetle 1, 3-β-D-glucan-binding proteins can recognize β-1, 3-D-glucan and peptidoglycan (Lee et al., 2004). M. sexta C-type lectins can bind to several different microbial components, including LPS, LTA and PG (Yu et al., 2005; Yu et al., 1999; Yu and Kanost, 2000; Yu et al., 2006). Drosophila DGNBP-1 can bind to LPS and β-1, 3-glucan (Kim et al., 2000). M. sexta βGRP1 can bind to E. coli, M. luteus and S. cerevisiae (Ma and Kanost, 2000), βGRP2 can bind to laminarin and LTA but not LPS (Jiang et al., 2004), and MBP can bind to LPS, LTA and DAP-type PG (Wang et al., 2011). Thus, the βGRP family proteins may have broad binding spectra for various pathogens but differ in binding affinities. Less M. sexta βGRP3 bound to DAP-type PGs than to LPS, LTA and laminarin (Fig. 5), which is consistent with the results of competitive binding assay that binding of βGRP3 to laminarin was only slightly competed by PGs but completely competed by LPS, LTA and laminarin (Fig. 5I).
The βGRP family proteins are mainly involved in activation of the proPO system (Fabrick et al., 2003; Jiang et al., 2004; Ma and Kanost, 2000; Ochiai and Ashida, 2000), and some βGRPs can also aggregate microorganisms (Beschin et al., 1998; Fabrick et al., 2003; Jiang et al., 2004). Like most βGRP proteins, M. sexta βGRP3 could activate proPO in plasma (Zhong X and Yu X, unpublished results), more importantly, it possess some unique properties that have not been reported for the βGRP family proteins: 1) calcium-dependent agglutination of E. coli and B. cereus (Fig. 7), which is similar to that of C-type lectins (Yu et al., 2005; Yu et al., 1999; Yu and Kanost, 2000; Yu et al., 2006), and 2) direct antibacterial (bacteriostatic) activity against B. cereus that is resistant to ampicillin (Fig. 8C). Compared to M. sexta βGRP1, βGRP2 and MBP, βGRP3 is ~40–45 residues shorter at the N-terminus and lacks the small glucan binding domain (Fig. S2). BlastP search showed that the shorter N-terminal domain (~57 residues) of M. sexta βGRP3 does not have a hit in the NCBI database (data not shown), indicating that the N-terminus of βGRP3 is not a truncated form of the small glucan binding domain but a new sequence that may have unknown functions. We speculate that the shorter N-terminal domain of βGRP3 may be involved in protein-protein interactions for formation of dimers or oligomers, which are required for agglutination of bacterial cells, since βGRP3 only contains a glucanase domain. Such a protein-protein interaction may also be calcium-dependent. The coelomic cytolytic factor 1 (CCF-1) of earthworm Eisenia fetida, which is a member of the βGRP family, also lacks the small N-terminal glucan binding domain, but it can still agglutinate Gram-negative bacteria, indicating that the N-terminal glucan binding domain is not absolutely required for agglutination (Beschin et al., 1998). Antibacterial activity of βGRP3 is due to bacteriostatic effect but not bactericidal effect as the activity of βGRP3 was completely blocked by free laminarin, mannan, LPS, LTA and PG-BS, and significantly blocked by PG-K12 (Fig. 8E). Future work is to understand how βGRP3 agglutinates bacterial cells and exerts antibacterial activity.
Supplementary Material
M. sexta β-1, 3-glucan recognition protein 3 (βGRP3) was mainly expressed in fat body of naïve larvae.
Expression of both βGRP3 mRNA and protein was not induced by microbial challenge.
Recombinant βGRP3 bound to laminarin, LPS, LTA and DAP-type PG, as well as to several bacteria and yeast.
βGRP3 could agglutinate E. coli and Bacillus cereus in a calcium-dependent manner.
βGRP3 showed strong bacteriostatic activity against B. cereus which is resistant to ampicillin.
Acknowledgments
This work was supported by National Institutes of Health Grant GM066356
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beschin A, Bilej M, Hanssens F, Raymakers J, Van Dyck E, Revets H, Brys L, Gomez J, De Baetselier P, Timmermans M. Identification and cloning of a glucan- and lipopolysaccharide-binding protein from Eisenia foetida earthworm involved in the activation of prophenoloxidase cascade. J Biol Chem. 1998;273:24948–24954. doi: 10.1074/jbc.273.38.24948. [DOI] [PubMed] [Google Scholar]
- Cerenius L, Liang Z, Duvic B, Keyser P, Hellman U, Palva ET, Iwanaga S, Soderhall K. Structure and biological activity of a 1,3-beta-D-glucan-binding protein in crustacean blood. J Biol Chem. 1994;269:29462–29467. [PubMed] [Google Scholar]
- Charroux B, Rival T, Narbonne-Reveau K, Royet J. Bacterial detection by Drosophila peptidoglycan recognition proteins. Microbes Infect. 2009;11:631–636. doi: 10.1016/j.micinf.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Charroux B, Royet J. Drosophila immune response: From systemic antimicrobial peptide production in fat body cells to local defense in the intestinal tract. Fly. 2010;4:40–47. doi: 10.4161/fly.4.1.10810. [DOI] [PubMed] [Google Scholar]
- Dai H, Hiromasa Y, Takahashi D, VanderVelde D, Fabrick JA, Kanost MR, Krishnamoorthi R. An initial event in the insect innate immune response: structural and biological studies of interactions between beta-1,3-glucan and the N-terminal domain of beta-1,3-glucan recognition protein. Biochemistry. 2013;52:161–170. doi: 10.1021/bi301440p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du ZQ, Ren Q, Zhao XF, Wang JX. A double WAP domain (DWD)-containing protein with proteinase inhibitory activity in Chinese white shrimp, Fenneropenaeus chinensis. Comp Biochem Physiol B Biochem Mol Biol. 2009;154:203–210. doi: 10.1016/j.cbpb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Dunn PE, Drake D. Fate of bacteria injected into naive and immunized larvae of the tobacco hornworm, Manduca sexta. J Invertebr Pathol. 1983;41:77–85. [Google Scholar]
- Duvic B, Soderhall K. Purification and characterization of a beta-1,3-glucan binding protein from plasma of the crayfish Pacifastacus leniusculus. J Biol Chem. 1990;265:9327–9332. [PubMed] [Google Scholar]
- Fabrick JA, Baker JE, Kanost MR. cDNA cloning, purification, properties, and function of a beta-1,3-glucan recognition protein from a pyralid moth, Plodia interpunctella. Insect Biochem Mol Biol. 2003;33:579–594. doi: 10.1016/s0965-1748(03)00029-8. [DOI] [PubMed] [Google Scholar]
- Gupta S, Wang Y, Jiang H. Manduca sexta prophenoloxidase (proPO) activation requires proPO-activating proteinase (PAP) and serine proteinase homologs (SPHs) simultaneously. Insect Biochem Mol Biol. 2005;35:241–248. doi: 10.1016/j.ibmb.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AL. Evolution of the betaGRP/GNBP/beta-1,3-glucanase family of insects. Immunogenetics. 2012;64:549–558. doi: 10.1007/s00251-012-0610-8. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Quantification of antimicrobial activity, using the inhibition-zone assay 1998 [Google Scholar]
- Jiang H, Ma C, Lu ZQ, Kanost MR. Beta-1,3-glucan recognition protein-2 (betaGRP-2)from Manduca sexta; an acute-phase protein that binds beta-1,3-glucan and lipoteichoic acid to aggregate fungi and bacteria and stimulate prophenoloxidase activation. Insect Biochem Mol Biol. 2004;34:89–100. doi: 10.1016/j.ibmb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Jiang H, Vilcinskas A, Kanost MR. Immunity in lepidopteran insects. Adv Exp Med Biol. 2010;708:181–204. doi: 10.1007/978-1-4419-8059-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa M, Satoh T, Ikeda A, Adachi Y, Ohno N, Yamaguchi Y. Structural insights into recognition of triple-helical beta-glucans by an insect fungal receptor. J Biol Chem. 2011;286:29158–29165. doi: 10.1074/jbc.M111.256701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanost MR, Jiang H, Yu XQ. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- Kim YS, Ryu JH, Han SJ, Choi KH, Nam KB, Jang IH, Lemaitre B, Brey PT, Lee WJ. Gram-negative bacteria-binding protein, a pattern recognition receptor for lipopolysaccharide and beta-1,3-glucan that mediates the signaling for the induction of innate immune genes in Drosophila melanogaster cells. J Biol Chem. 2000;275:32721–32727. doi: 10.1074/jbc.M003934200. [DOI] [PubMed] [Google Scholar]
- Lee E, Linder ME, Gilman AG. Expression of G-protein alpha subunits in Escherichia coli. Methods Enzymol. 1994;237:146–164. doi: 10.1016/s0076-6879(94)37059-1. [DOI] [PubMed] [Google Scholar]
- Lee MH, Osaki T, Lee JY, Baek MJ, Zhang R, Park JW, Kawabata S, Soderhall K, Lee BL. Peptidoglycan recognition proteins involved in 1,3-beta-D-glucan-dependent prophenoloxidase activation system of insect. J Biol Chem. 2004;279:3218–3227. doi: 10.1074/jbc.M309821200. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Lee JD, Kravchenko VV, Ulevitch RJ, Brey PT. Purification and molecular cloning of an inducible gram-negative bacteria-binding protein from the silkworm, Bombyx mori. Proc Natl Acad Sci USA. 1996;93:7888–7893. doi: 10.1073/pnas.93.15.7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The Host Defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Ma C, Kanost MR. A beta1,3-glucan recognition protein from an insect, Manduca sexta, agglutinates microorganisms and activates the phenoloxidase cascade. J Biol Chem. 2000;275:7505–7514. doi: 10.1074/jbc.275.11.7505. [DOI] [PubMed] [Google Scholar]
- Mishima Y, Quintin J, Aimanianda V, Kellenberger C, Coste F, Clavaud C, Hetru C, Hoffmann JA, Latge JP, Ferrandon D, Roussel A. The N-terminal domain of Drosophila Gram-negative binding protein 3 (GNBP3) defines a novel family of fungal pattern recognition receptors. J Biol Chem. 2009;284:28687–28697. doi: 10.1074/jbc.M109.034587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai M, Ashida M. A pattern-recognition protein for beta-1,3-glucan. The binding domain and the cDNA cloning of beta-1,3-glucan recognition protein from the silkworm, Bombyx mori. J Biol Chem. 2000;275:4995–5002. doi: 10.1074/jbc.275.7.4995. [DOI] [PubMed] [Google Scholar]
- Pal S, Wu LP. Pattern recognition receptors in the fly: lessons we can learn from the Drosophila melanogaster immune system. Fly (Austin) 2009;3:121–129. doi: 10.4161/fly.8827. [DOI] [PubMed] [Google Scholar]
- Pauchet Y, Freitak D, Heidel-Fischer HM, Heckel DG, Vogel H. Immunity or digestion: glucanase activity in a glucan-binding protein family from Lepidoptera. J Biol Chem. 2009;284:2214–2224. doi: 10.1074/jbc.M806204200. [DOI] [PubMed] [Google Scholar]
- Rao XJ, Yu XQ. Lipoteichoic acid and lipopolysaccharide can activate antimicrobial peptide expression in the tobacco hornworm Manduca sexta. Dev Comp Immunol. 2010;34:1119–1128. doi: 10.1016/j.dci.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao XJ, Xu XX, Yu XQ. Functional analysis of two lebocin-related proteins from Manduca sexta. Insect Biochem Mol Biol. 2012;42:231–239. doi: 10.1016/j.ibmb.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SW, Park SS, Park DS, Kim MG, Kim SC, Brey PT, Park HY. Isolation and characterization of immune-related genes from the fall webworm, Hyphantria cunea, using PCR-based differential display and subtractive cloning. Insect Biochem Mol Biol. 1998;28:827–837. doi: 10.1016/s0965-1748(98)00077-0. [DOI] [PubMed] [Google Scholar]
- Sukhithasri V, Nisha N, Biswas L, Anil Kumar V, Biswas R. Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microbiol Res. 2013;168:396–406. doi: 10.1016/j.micres.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Takahasi K, Ochiai M, Horiuchi M, Kumeta H, Ogura K, Ashida M, Inagaki F. Solution structure of the silkworm betaGRP/GNBP3 N-terminal domain reveals the mechanism for beta-1,3-glucan-specific recognition. Proc Natl Acad Sci USA. 2009;106:11679–11684. doi: 10.1073/pnas.0901671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MR, Kaminski JJ, Kurt-Jones EA, Fitzgerald KA. Pattern recognition receptors and the innate immune response to viral infection. Viruses. 2011;3:920–940. doi: 10.3390/v3060920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Albores F, Jimenez-Vega F, Soderhall K. A plasma protein isolated from brown shrimp (Penaeus californiensis) which enhances the activation of prophenoloxidase system by beta-1,3-glucan. Dev Comp Immunol. 1996;20:299–306. doi: 10.1016/s0145-305x(96)00007-9. [DOI] [PubMed] [Google Scholar]
- Vargas-Albores F, Jimenez-Vega F, Yepiz-Plascencia GM. Purification and comparison of beta-1,3-glucan binding protein from white shrimp (Penaeus vannamei) Comp Biochem Physiol B: Biochem Mol Biol. 1997;116:453–458. doi: 10.1016/s0305-0491(96)00268-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sumathipala N, Rayaprolu S, Jiang H. Recognition of microbial molecular patterns and stimulation of prophenoloxidase activation by a beta-1,3-glucanase-related protein in Manduca sexta larval plasma. Insect Biochem Mol Biol. 2011;41:322–331. doi: 10.1016/j.ibmb.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr E, Das S, Dong Y, Dimopoulos G. The Gram-negative bacteria-binding protein gene family: its role in the innate immune system of anopheles gambiae and in anti-Plasmodium defence. Insect Mol Biol. 2008;17:39–51. doi: 10.1111/j.1365-2583.2008.00778.x. [DOI] [PubMed] [Google Scholar]
- Xu XX, Zhong X, Yi HY, Yu XQ. Manduca sexta gloverin binds microbial components and is active against bacteria and fungi. Dev Comp Immunol. 2012;38:275–284. doi: 10.1016/j.dci.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XQ, Tracy ME, Ling E, Scholz FR, Trenczek T. A novel C-type immulectin-3 from Manduca sexta is translocated from hemolymph into the cytoplasm of hemocytes. Insect Biochem Mol Biol. 2005;35:285–295. doi: 10.1016/j.ibmb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Gan H, Kanost MR. Immulectin, an inducible C-type lectin from an insect, Manduca sexta, stimulates activation of plasma prophenol oxidase. Insect Biochem Mol Biol. 1999;29:585–597. doi: 10.1016/s0965-1748(99)00036-3. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Kanost MR. Immulectin-2, a lipopolysaccharide-specific lectin from an insect, Manduca sexta, is induced in response to gram-negative bacteria. J Biol Chem. 2000;275:37373–37381. doi: 10.1074/jbc.M003021200. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Ling E, Tracy ME, Zhu Y. Immulectin-4 from the tobacco hornworm Manduca sexta binds to lipopolysaccharide and lipoteichoic acid. Insect Mol Biol. 2006;15:119–128. doi: 10.1111/j.1365-2583.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- Zheng X, Xia Y. beta-1,3-Glucan recognition protein (betaGRP) is essential for resistance against fungal pathogen and opportunistic pathogenic gut bacteria in Locusta migratoria manilensis. Dev Comp Immunol. 2012;36:602–609. doi: 10.1016/j.dci.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Zhong X, Xu XX, Yi HY, Lin C, Yu XQ. A Toll-Spatzle pathway in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 2012;42:514–524. doi: 10.1016/j.ibmb.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.