Abstract
Background
Pneumonectomy for benign disease is often complicated by inflammatory processes that obscure operative planes. We reviewed our experience to evaluate the impact of requiring urgent or emergent pneumonectomy on outcomes.
Study Design
All pneumonectomies for benign conditions from 1997–2012 at a single institution were retrospectively reviewed. Mortality was assessed using multivariable logistic regression that included laterality, age, and surgery status, which was emergent if performed within 24 hours of initial evaluation, urgent if performed after 24 hours but within the same hospital stay, and otherwise elective.
Results
Of 42 pneumonectomies, completion pneumonectomy after previous ipsilateral lung resection was performed in 14 patients (33%). Resection was elective in 22 patients (52%), urgent in 12 (28%), and emergent in 8 (19%). The most common indication was for necrotic lung (n=12; 29%). Muscle flaps were used in 26 patients (62%). Perioperative mortality for the entire cohort was 29% (n=12) and was significantly higher when surgery was urgent (5/13; 38%) or emergent (5/8; 62.5%) compared to elective (2/21; 9.5%) p=0.03). Requiring urgent or emergent surgery remained a significant predictor of mortality in multivariable analysis (odds ratio 10.4, p=0.01).
Conclusions
Pneumonectomy for benign disease has significant risk for mortality, particularly when not performed electively. Although surgery cannot be planned in the setting of trauma or some situations of acute infection, patients known to have conditions that are likely to require pneumonectomy should be considered for surgery earlier in their disease course before developing an acute problem that requires urgent or emergent resection.
Keywords: Pneumonectomy, Outcomes
Introduction
Despite improvements in surgical technique, anesthetic management, and overall perioperative care, pneumonectomy for either benign or malignant lung disease is associated with significant morbidity and mortality. A recent review of 1,267 pneumonectomy patients in the Society of Thoracic Surgeons (STS) database demonstrated an overall perioperative morbidity of 30.4% and mortality of 5.6%. While only 56 patients in this cohort had a benign condition as the indication for surgery, it was demonstrated to be a risk factor for postoperative complications and death with morbidity and mortality in this subgroup being 53.5% and 7.1%, respectively (1). This mortality rate is similar to other relatively small single institution case series of pneumonectomies for benign conditions (2,3).
The increased morbidity and mortality associated with pneumonectomy for benign conditions may be explained by a variety of factors. Patients that require a pneumonectomy in the benign setting are often suffering from other significant conditions, such as acute trauma that has led to devastating injuries or medical diseases that have resulted in immunocompromised states (3). In addition, surgery for benign disease can be technically challenging due to the acute and chronic inflammation that arises from previous or persistent pulmonary infection (4). The risks are potentially even more substantial when a patient has had previous ipsilateral partial lung resection and is undergoing completion pneumonectomy (5).
Although surgery often cannot be delayed in the setting of trauma, sepsis, or massive hemoptysis, some patients present with progressive conditions that result in destroyed lung, secondary to previous tuberculosis infection or bronchiectasis. These patients are often known to have markedly abnormal imaging studies, but can have long periods of times where they have minimal respiratory symptoms. When faced with the risks of pneumonectomy, physicians may be hesitant to refer for surgical evaluation, surgeons may be disinclined to offer surgery, and patients may be reluctant to agree to surgery. In this study, we reviewed our experience with benign pneumonectomy in order to quantify the impact of performing non-elective surgery on outcomes. Our goal was to better understand perioperative risk in order to improve patient selection and the preoperative counseling process in both the elective and non-elective settings.
Methods
After local Institutional Review Board approval was granted, all patients who had a pneumonectomy for a benign condition between 1997 and 2012 at Duke University Medical Center were identified. Retrospective review of an institutional, prospective database maintained on all thoracic surgery patients was performed. Data collected included demographics, presentation, preoperative functional status, smoking history, significant comorbidities, intraoperative details, and postoperative course. Chart review was utilized as necessary to complete data collection. Any postoperative event prolonging or otherwise altering the postoperative course was recorded along with all operative deaths, which were defined as deaths that occurred within 30 days after operation or those that occurred later but during the same hospitalization. Deaths were captured both by chart review and use of the Social Security Death Index Database. Survival was calculated from the time of surgery.
If the patient had had surgery performed within 24 hours of initial evaluation the procedure was considered emergent. An operation done after 24 hours but during the same hospitalization for an acute presentation was considered urgent. All other patients were considered to have had their operations performed on an elective basis. Multivariable analysis was performed with mortality as the outcome and surgery status (emergent/urgent versus elective), laterality (right versus left), and age as predictors. The number of variables included in this logistic regression model was limited to these three due to the small sample size and overall small number of events. Although other patient factors may also be important predictors of mortality, such as comorbid conditions, previous ipsilateral resection, and quantitative pulmonary function measurements, these variables were selected a priori because our literature review suggested that they were most strongly associated with the outcome of interest. The significance level for all tests was set at 0.05 and all tests were two-sided. Data are presented as mean ± standard deviation unless otherwise noted. The SAS 9.0 statistical package (SAS Institute, Cary, North Carolina) was used for statistical analyses.
Results
A total of 42 patients underwent a benign pneumonectomy between 1997 and 2012 at our institution. Patient characteristics are listed in Table 1 for both the entire cohort and also stratified by whether the procedure was a primary or completion pneumonectomy. Patients were evenly distributed by gender and had a median age of 51 (10–77). Roughly two-thirds of the patients carried a history of chronic obstructive pulmonary disease (COPD), smoking, or malignancy. Slightly more than half (24/42 patients) of the cohort had preoperative pulmonary function tests (PFTs) recorded (mean 47±18, range 21–95). Many patients were not tested due to the emergent or urgent nature of their clinical presentation. Of these 24 patients, 79% (19/24) had a forced expiratory volume in 1 second (FEV1) of less than 60% predicted and 45% (11/24) had an FEV1 of less than 40% predicted, demonstrating the high operative risk associated with this patient population. In addition to pulmonary function tests, preoperative evaluation included a quantitative differential ventilation perfusion scan (V/Q scan), routine echocardiogram, or stress echocardiogram. While 18 patients had none of these additional tests performed, 20 patients had a V/Q scan either alone or in conjunction with a routine echocardiogram (n=5) or stress echocardiogram (n=2). Routine echocardiogram was performed alone in three patients and one patient had a stress echocardiogram only. Incidentally, only 15 patients had both PFTs and one of these additional studies performed prior to surgery and nine had neither PFTs nor the aformentioned studies completed.
Table 1.
Patient Characteristics
| Patient characteristics | Overall (n=42) | Primary (n=28) | Completion (n=14) |
|---|---|---|---|
|
| |||
| Disease category | |||
| Necrotic lung (infection/infarct) | 12 (29%) | 6(21%) | 6(43%) |
| Hemoptysis | 8 (19%) | 6 (21%) | 2(14%) |
| Bronchopleural fistula/empyema | 6 (14%) | 0(0%) | 6(43%) |
| Bronchiectasis | 5 (12%) | 5(18%) | 0(0%) |
| Congenital | 4 (10%) | 4(14%) | 0(0%) |
| Benign mass | 4 (10%) | 4(14%) | 0(0%) |
| Trauma | 3 (7%) | 3(11%) | 0(0%) |
|
| |||
| Median age, y | 51 (10,77) | 41(10,77) | 60(26,73) |
|
| |||
| Sex | |||
| Male | 22 (52%) | 14 (50%) | 8(57%) |
| Female | 20 (48%) | 14 (50%) | 6(43%) |
|
| |||
| Comorbidities | |||
| Heart disease | 8 (19%) | 5(18%) | 3(21%) |
| Renal insufficiency | 1 (2%) | 1(4%) | 0(0%) |
| Diabetes | 2 (5%) | 2(7%) | 0(0%) |
| COPD/ smoking | 15 (36%) | 8(29%) | 7(50%) |
| Malignancy/other cancers | 14 (33%) | 4(14%) | 10(71%) |
| Immunosuppression | 7 (17%) | 5(18%) | 2(14%) |
|
| |||
| FEV1, % predicted | |||
| >80 | 2 (5%) | 1 (4%) | 1 (7%) |
| 60–79 | 3 (7%) | 2(7%) | 1(7%) |
| 40–59 | 8 (19%) | 4 (14%) | 4(29%) |
| <40 | 11 (26%) | 6(21%) | 5(36%) |
| Unknown | 18 (42%) | 15(54%) | 3(21%) |
|
| |||
| Laterality | |||
| Right | 28 (67%) | 15 (54%) | 12(86%) |
| Left | 14 (33%) | 13(46%) | 2(14%) |
|
| |||
| Procedure | |||
| Primary pneumonectomy | 28 (67%) | N/A | N/A |
| Completion pneumonectomy | 14 (33%) | ||
|
| |||
| Nature of Procedure | |||
| Elective | 22 (52%) | 14(50%) | 8(57%) |
| Urgent | 12 (29%) | 6(21%) | 6(43%) |
| Emergent | 8 (19%) | 8(29%) | 0(0%) |
|
| |||
| Median operative time, min | 248(83, 560) | 244 (123,560) | 278 (83,480) |
|
| |||
| Median blood loss, L | 1.05 (0.06, 20.8) | 0.98 (0.06,20.8) | 0.85 (0.4,4.5) |
The most common indication for benign pneumonectomy was necrotic lung. As an example of this group, many of these patients had either persistently infected or sterile necrotic lung following radiation for malignancy. Overall, five patients developed necrotic lung after previous treatment for malignancy with surgical resection combined with either induction chemoradiation (three patients) or adjuvant chemotherapy (two patients). The final pathology in all of these cases was benign without any evidence of residual malignancy. The second most common indication was for hemoptysis, while three of the patients in the study underwent pneumonectomy secondary to traumatic injury. Overall, roughly half the patients had their operations performed in an elective manner, a third had their surgeries done urgently and 19% were taken to the operating room emergently. In most patients, CT was performed prior to intervention. Figure 1 shows examples of preoperative imaging for two patients; one who went to the operating room emergently for hemoptysis versus one who went electively. As these scans demonstrate, one should not place undo emphasis on an otherwise unremarkable CT scan when evaluating these patients for surgery as the need for acute intervention is not always readily apparent from imaging.
Figure 1.
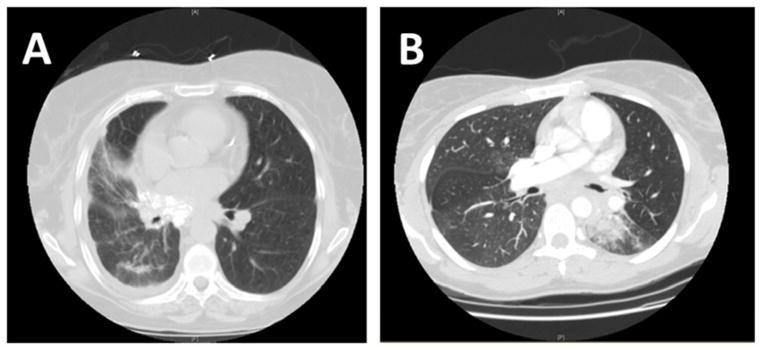
CT images of patients undergoing (A) elective vs (B) emergent pneumonectomy for a benign indication.
Intraoperatively, two-thirds of the procedures were performed as primary pneumonectomies and two-thirds were right-sided. Median operative time was 248 minutes and was slightly longer in the completion pneumonectomy subset but this was not statistically significant (244 minutes for primary pneumonectomy vs. 278 minutes for completion pneumonectomy, p=0.5). In addition, blood loss was, on average, 1 liter with no significant difference depending on completion or primary pneumonectomy (1.2 L for primary pneumonectomy vs. 0.85 L for completion pneumonectomy, p=0.4). Bronchial stump coverage was utilized variably, based on the operative indication and surgeon preference, with a higher percentage of patients who underwent completion pneumonectomy having a buttress placed: 78% (11/14) versus 53% (15/28) in the primary pneumonectomy group (Table 2). Muscle flap coverage was more commonly utilized on the right side regardless of completion or primary pneumonectomy. The most common muscle utilized for both right and left-sided pneumonectomies was the serratus muscle in 38% of patients. Postoperatively, one patient developed a bronchopleural fistula (BPF). This individual had had previous chemoradiation for a left upper lobe malignancy complicated by a persistent infection requiring an Eloesser flap. The eventual development of persistent hemoptysis necessitated completion pneumonectomy following which the patient developed the BPF. Multiple attempts to repair this defect were unsuccessful and the patient eventually succumbed secondary to this complication 15 months after his initial operation.
Table 2.
Utilization of Muscle Flaps
| Type of pneumonectomy
| ||||
|---|---|---|---|---|
| Right | Left | |||
|
| ||||
| Flap | Primary | Completion | Primary | Completion |
|
|
||||
| Latissimus | 1 | 0 | 0 | 0 |
| Pectoralis | 0 | 0 | 0 | 1 |
| Intercostal | 3 | 2 | 1 | 0 |
| Serratus | 5 | 6 | 5 | 0 |
| Pericardial | 0 | 2 | 0 | 0 |
| None | 7 | 2 | 6 | 1 |
|
| ||||
| Totals | 16 | 12 | 12 | 2 |
Operative mortality was 29% for the entire cohort. Table 3 shows the characteristics of the patients who died in the perioperative period. Patients who had their operations performed on an emergent or urgent basis accounted for 83% of the deaths in this study. Likewise, 83% of these patients had an American Society of Anesthesia score of 4 or higher. The median operative time was shorter than for the group as a whole, but the median age of the patients who died was higher as was the intraoperative blood loss. Mortality was no different depending on whether the operation was a primary or completion pneumonectomy. Perhaps most notable, was that 75% of the deaths following surgery occurred after right pneumonectomy. Nevertheless, the median survival of those patients who survived and were eventually discharged was 6 years and 19 of these patients remain alive at last follow-up (45%). Operative morbidity was also high with 79% of patients experiencing at least one complication. The most common complication was the need for a postoperative blood transfusion, in 76% of patients. Other notable complications included 24% of the patients developing pneumonia in the remaining lung, a third requiring reintubation and 24% needing eventual tracheostomy. Again, the differences in overall complication rates between the primary and completion pneumonectomies were not significant (20/28; 71%) versus (13/14; 93%, p=0.23). There was, however, a significant difference in the rate of reintubation, 5/28 (18%) in the primary pneumonectomy group versus 8/14 in the completion pneumonectomy subset (57%; p=0.01).
Table 3.
Mortalities
| Mortalities | (n=12) |
|---|---|
|
| |
| Sex | |
| Male | 8 (67%) |
| Female | 4 (33%) |
|
| |
| Median operative time, min | 214 (83, 560) |
|
| |
| Median EBL, L | 1.7 (0.40, 20.8) |
|
| |
| Median age, y | 60 (26, 68) |
|
| |
| ASA 4 (or higher) | 10 (83%) |
|
| |
| Completion pneumonectomy | 4 (33%) |
|
| |
| Procedure | |
|
| |
| Elective | 2 (17%) |
| Urgent | 5 (42%) |
| Emergent | 5 (42%) |
|
| |
| Laterality | |
| Right | 9 (75%) |
| Left | 3 (25%) |
|
| |
| Indication | |
| Hemoptysis | 4 (33%) |
| BPF/Empyema | 2 (17%) |
| Trauma | 2 (17%) |
| Necrotic Lung | 2 (17%) |
| Bronchiectasis | 1 (8%) |
| Non-Malignant Mass | 1 (8%) |
Multivariable analysis was performed to assess predictors of mortality in this patient cohort (Table 4). In this analysis, requiring urgent or emergent surgery remained a significant predictor of mortality, while age and laterality were not statistically significant.
Table 4.
Multivariate Risk Model for Mortality
| Odds Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|
| Age (per 1-y increase) | 1.05 | 0.99–1.10 | 0.08 |
| Urgent/elective surgery | 10.42 | 1.65–66.67 | 0.01 |
| Laterality (right versus left) | 3.51 | 0.55–22.2 | 0.2 |
Discussion
Pneumonectomy for benign conditions is associated with significant morbidity and mortality, even in an era associated with improvements in surgical technique, anesthesia, and postoperative care. In this series of 42 patients who required pneumonectomy for a variety of benign conditions, the perioperative morbidity was 79% and the mortality was 29%. Although the majority of complications might be considered relatively minor, such as the need for blood transfusion, the rates of pneumonia, re-intubation, and ventilator dependence still ranged from 24 to 31%. Although muscle flaps were used selectively, only one patient developed a bronchopleural fistula. The overall mortality was high at 29%, though mortality for the 21 patients who had surgery in an elective setting was less than 10%. Consequently, what is unique to this study is its ability to demonstrate that, for patients requiring benign pneumonectomy, the most significant predictor of mortality is urgent or emergent surgery.
Although this series is relatively small, its size is on par with many studies from institutions in the United States, where conditions associated with the need for pneumonectomy such as mycobacterial infections are less common than in other parts of the world. Some of the best results reported for benign pneumonectomy have come from areas where pulmonary tuberculosis is endemic and thus experience with the operative management of these patients is vast. Conlan and colleagues (6) reported the results of benign pneumonectomy in 124 patients over a 14 year period in South Africa and Canada demonstrating an impressive hospital mortality of only 2.4%. Unlike the current study, the patients in this report all underwent pneumonectomy in the elective setting and the authors acknowledge that they did not choose to include the results of 47 patients who underwent emergent pneumonectomy over that same time period. Likewise, Blythe (7) reported a 1.2% mortality in the treatment of 155 patients, all but two of which were done in the elective setting. In both these series, the authors emphasize a detailed approach for identifying these patients early on and optimizing their overall performance status thus avoiding the consequences of emergent operations. Similarly, in the United States, investigators at the University of Colorado have developed an algorithm to treating resistant mycobacterial tuberculosis as well as other mycobacterial pulmonary infections that emphasizes a proactive surgical approach and advocates operative intervention before the extent of parenchymal destruction requires a pneumonectomy (8).
Given the complexities of these operations, it is appealing to attempt to identify risk factors for morbidity and mortality. A few authors have suggested that patients undergoing completion pneumonectomy are not at an increased risk of complications (2,3). Conversely, a number of authors have recently reported sobering results related to completion pneumonectomy (5,9,10). For example, Miller, Fujimoto, and Sherwood published mortality rates of 20.9%, 23%, and 23% respectively for completion pneumonectomy. In addition, a more recent review of outcomes after completion pneumonectomy from Washington University in St. Louis noted that patients undergoing completion pneumonectomy for benign conditions were more likely to have major complications and lower median survival than those with malignancy (11). Our study did not find a higher mortality in the completion pneumonectomy subset but this was a small group in an already small series of individuals.
As with laterality, our regression model did not find age to be a risk factor for increased mortality. Unfortunately, our study was likely underpowered to demonstrate an impact of these variables due to our sample size, which also did not allow us to include additional variables for the assessment of perioperative risk. Other authors, however, have identified risk factors for morbidity and mortality. For example, Mansour et al retrospectively reviewed 323 patients undergoing pneumonectomy, mostly for malignancy, and concluded via multivariable analysis that the only consistent risk factor for mortality was right pneumonectomy (12). Similarly, in a recent review by Hu, right pneumonectomy, operative duration of greater than 4 hours, and more than a liter of blood loss was associated with increased risk of complications (13). Likewise, Miller pointed to increased age, corticosteroid use, decreased preoperative DLCO, intraoperative blood transfusion and excess crystalloid infusion as risk factors for mortality following completion pneumonectomy. Admittedly, while two-thirds our pneumonectomies were right-sided, these cases did account for 75% of our mortalities. With regard to the traditional predictors of postoperative morbidity and mortality such as FEV1 and DLCO, many of our patients did not undergo PFT testing (50% of the entire cohort) presumably due to their high acuity. Notably, of those that did, 45% had an FEV1 of less than 40%, which in and of itself is not surprising and illustrates how debilitated these patients tend to be leading up to surgery. Finally, as Table 3 demonstrates, the postoperative deaths were well distributed across the variety of indications and cannot be simply explained by a preponderance of a single disease process; nor can mortality be attributed to one infectious organism. For instance, in our study, aspergillus was the most common isolate with seven patients having invasive aspergillus in the surgical specimen. Historically, invasive aspergillus has been associated with a 10% mortality and comparably the current study demonstrated a mortality rate of 14% (1/7)(14).
In an attempt to further explain the high mortality rate amongst those treated urgently or emergently, the clinical history of each patient who died was more closely reviewed. We surmised that some of these patients may have been candidates for elective surgery and that they were simply not referred in a timely fashion and that this delay contributed to their eventual outcome. Of the five deaths in the urgently treated cohort, one patient’s history fit this profile, a 65 year old woman with a lengthy stay in the medical intensive care unit for a complicated pneumonia who was severely debilitated by the time of her operation. In comparison, of the emergently treated patients there were two individuals who might have benefited from earlier surgical involvement. The first was a 65 year old gentleman with a history of nocardia infection that had been unsuccessfully treated as an outpatient and subsequently presented with sepsis and pulmonary necrosis. The second was a 44 year old woman who had undergone multiple bronchoscopic interventions for bronchial stenosis only to develop acute hemoptysis secondary to an aortobronchial fistula. In these cases, earlier referral for surgical consideration may have allowed resection to be attempted in an elective and presumably better controlled situation, which could have resulted in improved outcomes for these patients.
The limitations of this study include its retrospective nature and the small size that makes drawing definitive conclusions difficult. That said, the size of this series compares favorably with other published series and is notable for the spectrum of disease processes that it includes. The study demonstrates that pneumonectomy in the presence of benign disease continues to be associated with significant morbidity and mortality. Unfortunately, viable alternative operative or non-operative management strategies do not exist for the benign conditions that require pneumonectomy. Avoiding emergent or urgent pneumonectomies is advisable, although surgery cannot be electively planned in the setting of trauma or some situations of acute infection. Completion pneumonectomies are theoretically more daunting but may not enhance morbidity or mortality. In sum, the care of patients with chronic pulmonary disorders resulting in destroyed and devitalized lung with its attendant sequelae deserves careful scrutiny by all caregivers. Patients known to have conditions that are likely to require pneumonectomy should be considered for surgery earlier in their disease course before developing an acute problem that requires non-elective resection. Earlier surgical consultation and involvement may not only allow lesser resection than pneumonectomy, but can also allow aggressive nutritional support and optimal preoperative antibiotic administration thus potentially preventing the suboptimal situation of emergent pneumonectomy.
Acknowledgments
Support: Dr Berry has received support from the National Institute of Health (NIH) funded Cardiothoracic Surgical Trials Network.
Footnotes
Disclosure Information: Dr D’Amico received an honorarium from Scanlan. All other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shapiro M, Swanson S, Wright C, et al. Predictors of major morbidity and mortality after pneumonectomy utilizing the society for thoracic surgeons general thoracic surgery database. Ann Thorac Surg. 2010;90:927–935. doi: 10.1016/j.athoracsur.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 2.Owen R, Force S, Pickens A, et al. Pneumonectomy for benign disease: analysis of the early and late outcomes. Eur J Cardiothorac Surg. 2012:1–6. doi: 10.1093/ejcts/ezs284. [DOI] [PubMed] [Google Scholar]
- 3.Reed C. Pneumonectomy for chronic infection: fraught with danger. Ann Thorac Surg. 1995;59:408–411. doi: 10.1016/0003-4975(94)00867-7. [DOI] [PubMed] [Google Scholar]
- 4.Massard G, Dabbagh A, Wihlm J, et al. Pneumonectomy for chronic infection is a high-risk procedure. Ann Thorac Surg. 1996;62:1033–1038. doi: 10.1016/0003-4975(96)00596-6. [DOI] [PubMed] [Google Scholar]
- 5.Miller D, Deschamps C, Jenkins G, et al. Completion pneumonectomy: factors affecting operative mortality and cardiopulmonary morbidity. Ann Thorac Surg. 2002;74:876–884. doi: 10.1016/s0003-4975(02)03855-9. [DOI] [PubMed] [Google Scholar]
- 6.Conlan A, Lukanich J, Shutz J, et al. Elective pneumonectomy for benign lung disease: modern-day mortality and morbidity. J Thorac Cardiovasc Surg. 1995;110:1118–1124. doi: 10.1016/s0022-5223(05)80181-3. [DOI] [PubMed] [Google Scholar]
- 7.Blyth D. Pneumonectomy for inflammatory lung disease. Eur J Cardiothorac Surg. 2000;18:429–434. doi: 10.1016/s1010-7940(00)00526-1. [DOI] [PubMed] [Google Scholar]
- 8.Pomerantz M, Madsen L, Goble M, et al. Surgical management of resistant mycobacterial tuberculosis and other mycobacterial pulmonary infections. Ann Thorac Surg. 1991;52:1108–1112. doi: 10.1016/0003-4975(91)91289-8. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto T, Zaboura G, Fechner S, et al. Completion pneumonectomy: current indications, complications and results. J Thorac Cardiovasc Surg. 2001;121:484–490. doi: 10.1067/mtc.2001.112471. [DOI] [PubMed] [Google Scholar]
- 10.Sherwood J, Mitchell J, Pomerantz M. Completion pneumonectomy for chronic mycobacterial disease. J Thorac Cardiovasc Surg. 2005;129:1258–1265. doi: 10.1016/j.jtcvs.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 11.Puri V, Tran A, Bell J, et al. Completion Pneumonectomy: Outcomes for benign and malignant indications. Ann Thorac Surg. 2013;95:1885–1891. doi: 10.1016/j.athoracsur.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansour Z, Kocketkova E, Santelmo N, et al. Risk factors for early mortality and morbidity and mortality after pneumonectomy: a reappraisal. Ann Thorac Surg. 2009;88:1737–1744. doi: 10.1016/j.athoracsur.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Hu X, Duan L, Jiang G, et al. Risk factors for early postoperative complications after pneumonectomy for benign lung disease. Ann Thorac Surg. 2013;95:1899–1904. doi: 10.1016/j.athoracsur.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 14.Battaglini J, Murray G, Keagy B, et al. Surgical management of symptomatic pulmonary aspergilloma. Ann Thorac Surg. 1985;39:512–516. doi: 10.1016/s0003-4975(10)61986-8. [DOI] [PubMed] [Google Scholar]


