Abstract
Background
Hypogonadism in men (total testosterone level < 350 ng/dL) is associated with higher risk of cardiovascular disease and mortality in men on dialysis. We evaluated the association of hypogonadism with all-cause mortality in men with non–dialysis-dependent CKD.
Study Design
Retrospective, cohort study.
Setting & Participants
2419 men with CKD stages 3–4 (estimated glomerular filtration rate [eGFR], 15–59 mL/min/1.73 m2) who had total testosterone measured for-cause between January 1, 2005 and October 31, 2011 at a tertiary care center in Cleveland, Ohio.
Predictors
Total testosterone measured using an immunoassay measurement in 3 forms: a) categorized as low or testosterone replacement therapy versus normal, b) continuous log testosterone, and c) quintiles (100–226, 227–305, 306–392, 393–511, 512–3153 ng/dL).
Outcomes
Factors associated with low total testosterone, and association between low total testosterone and all-cause mortality were evaluated using logistic regression, Cox proportional hazard models, and Kaplan-Meier survival curves.
Results
Hypogonadism was found in 1288/2419 (53%) of men. In a multivariable logistic regression analysis, African American ethnicity and higher eGFR were associated with lower odds of having hypogonadism. Diabetes and higher body mass index were associated with higher odds of having hypogonadism. 357/2419 (15%) patients died during a median follow up of 2.3 years. In the multivariate Cox model, testosterone <350 ng/dL or testosterone replacement therapy were not associated with mortality. In a multivariable model also adjusted for testosterone supplementation, higher log testosterone was associated with significantly lower mortality (HR per 1 log unit, 0.70; 95% CI, 0.55–0.89). When compared to the highest quintile, the second lowest quintile of testosterone was associated with higher mortality (HR, 1.53; 95% CI, 1.09–2.16).
Limitations
Single center study, timing of testosterone testing, lack of adjustment for proteinuria, and sampling bias.
Conclusions
Low total testosterone may be associated with higher mortality in men with CKD stages 3–4 but more studies are needed.
Index words: hypogonadism, low testosterone, chronic kidney disease (CKD), mortality risk, testosterone replacement therapy (TRT), CKD registry
Diagnosis of low testosterone (“hypogonadism”) and the indications, risks and benefits of testosterone replacement therapy (TRT) are controversial. While the greatest focus has been on men with sexually related symptoms such as low libido and erectile dysfunction (ED)1, there are clear associations between hypogonadism and systemic conditions such as HIV/AIDS, type II diabetes mellitus, metabolic syndrome, and osteoporosis and chronic use of steroids or opioids2–4. Some of these associations may be coincidental while others may have direct cause-and-effect relationships in either direction. Several studies have shown a high incidence of hypogonadism in male dialysis patients.
In addition, low total testosterone levels have been associated with higher all-cause mortality in these men5–8. A study including 1822 men found that men with chronic kidney disease (CKD) and low total testosterone had significantly increased all-cause mortality9. Cigarran et al10 reported lower muscle mass in those with low testosterone levels but did not evaluate mortality. Causality has not been shown in prior studies, but they are consistent in their finding of a strong association between low total testosterone and death in this cohort. The scope of the problem in men with non–dialysis-dependent CKD is less studied.
The most important issue is whether total testosterone represents a therapeutic target for early intervention in men with CKD stages 3–4, and whether there is a window of opportunity for affecting outcomes. Thus, the objective of our study was to investigate the association between total testosterone and all-cause mortality in men with CKD stages 3–4.
METHODS
Study Cohort
We extracted data from our electronic health record–based CKD registry from a tertiary care center in Cleveland, Ohio, which has been developed and validated at our institution7. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)39A statement guidelines in conducting analyses. Females were excluded. Men with the following inclusion criteria starting January 1, 2005 and ending October 31, 2011 were selected: (1) men with two eGFR values < 60 mL/min/1.73m2 (using the CKD-EPI [CKD Epidemiology Collaboration] creatinine equation39B) more than 90 days apart, and (2) men with measurement of outpatient total testosterone level. Men with eGFR < 15 mL/min/1.73m2, receiving renal replacement therapy, and with total testosterone < 100 ng/dL were excluded (group we excluded the last group because there was high likelihood that these men were on androgen ablation therapy for advanced prostate cancer). We also excluded men who had improved kidney function with eGFR >60 mL/min/1.73m2 at the time of first testosterone measurement. In the current study inception time was the start of time for survival analysis which corresponded to second eGFR <60 mL/min/1.73m2 or first testosterone value, whichever was last. Information in the registry includes demographics, laboratory data, eGFR, and comorbidities. ICD-9 codes for comorbid conditions such as diabetes mellitus, hypertension, coronary artery disease, cerebrovascular disease, congestive heart failure, and hyperlipidemia have been validated using prespecified criteria. Our CKD registry has been linked with the Social Security Death Index where information regarding deaths was gathered.
Definitions and Outcome Measures
Exposure of Interest
Kidney function
Men with CKD stages 3–4 qualified for the study. Creatinine was measured using a Hitachi D 2400 Modular Chemistry Analyzer (Roche Diagnostics, www.roche.com). We verified that patients were not on dialysis by merging our data with data from the US Renal Data System. However, those data were only available until September 15, 2009, and not available from September 15, 2009 to October 31, 2011, so we excluded patients with second or study inception (see below) eGFR < 15 mL/min/1.73m2 to avoid including patients with the highest risk of being on dialysis without our knowledge.
Total testosterone
Total testosterone was measured using Siemens ADVIA Centaur CP immunoassay system (Siemens Healthcare Diagnostics, www.usa.healthcare.siemens.com). Only outpatient laboratory values between January 1, 2005 and October 31, 2011 and prior to end-stage renal disease were considered for analysis. For uniformity, we only used total testosterone results from assays with reference values of 220-1000 ng/dL. Greater than 90% of our cohort had testing within this reference range. Since we lacked the time of specimen collection, we selected a random sample of 40 patients and all their total testosterone results for chart review to ensure that most values were obtained in the morning before 12:00 PM (testosterone should be measured in the morning for the most accurate values, as it declines as the day passes). Overall, 54% of test results were collected between 7:00 AM and noon. We defined low testosterone as any value <350 ng/dL11–13 or presence of testosterone supplementation. Testosterone was only measured in patients with a clinical indication; however, it is unknown whether testing was done due to presence of symptoms or another cause. Possible symptoms that may prompt testosterone testing include decreased libido, erectile dysfunction, generalized fatigue, weakness, and others. Information regarding testosterone supplements was extracted from our electronic medical record and included the following supplements: testosterone patch/gel/intramuscular/buccal/implanted pellet, oral methyltestosterone, fluoxymesterone, intramuscular nandrolone decanoate or nandrolone phenpropionate, clomiphene, anastrozole, and stanozolol. Forty random charts were reviewed to confirm the use of testosterone supplementation.
Outcome of Interest
Mortality
The primary endpoint was all-cause mortality, which was obtained from our electronic medical record and by linking our registry with the Social Security Death Index. Mortality data up to October 31, 2011 were obtained.
Additional Covariates
Demographic information and laboratory results included age, race/ethnicity, BMI, and albumin level, which were extracted from electronic health records. Comorbidities, including diabetes mellitus, hypertension, coronary artery disease, cerebrovascular disease, congestive heart failure, hyperlipidemia, malignancy, and smoking, were defined using prespecified criteria and validated previously8. The validation was done as of March 2010 by using ICD-9 codes.
Statistical Analysis
To evaluate the type of patients having testosterone measured in our CKD registry and their representativeness, we compared characteristics of patients with and without testosterone data using t-tests for continuous variables and Chi-square tests for categorical variables. We also compared characteristics of patients with low and normal testosterone levels using t-tests for continuous variables and Chi-square tests for categorical variables. We used a logistic regression analysis to evaluate whether age, ethnicity, eGFR, BMI group, smoking and comorbidities were associated with low testosterone level.
We used Kaplan-Meier plots and log-rank tests to evaluate the association between low testosterone level and survival. Cox proportional hazards models were used to evaluate whether low testosterone was associated with survival while adjusting for age, eGFR, race, BMI group, albumin and comorbidities (diabetes mellitus, hypertension, coronary artery disease, cerebrovascular disease, congestive heart failure, hyperlipidemia, malignancy, and smoking). Albumin was missing in 8% of the patients, and, in order to include all patients in the analysis, we used mean value imputation along with a dummy indicator for missing data. We evaluated the relationship of total testosterone and allcause mortality in three separate models. The first model compared low (<350 ng/dL or presence of testosterone supplementation) versus normal total testosterone as a time-dependent variable. Patients with low testosterone or testosterone supplementation remained in the low group for the entire follow up whereas patients who had normal testosterone level at the start of the study but had a low value or started testosterone supplementation during follow up were switched to the low group at that time. The second survival model used continuous log testosterone, and the third model used testosterone quintiles while adjusting for the previously mentioned covariates plus testosterone supplementation. The continuous and quintile models used the lowest testosterone result before study inception. We chose to model testosterone in a binary manner because this is most clinically useful to primary care physicians as well as nephrologists, urologists, and endocrinologists. The other two models (continuous and categorical [quintiles]) were used as exploratory analyses to evaluate for underlying association. Binary14 and categorical5, 6 models have been used in the literature. In the continuous and quintile models study, inception was the date of first testosterone measurement or date of second eGFR<60 mL/min/1.73m2, whichever was last. In the binary model, inception was the first date of testosterone measurement or testosterone supplement, or date of second eGFR<60 mL/min/1.73m2, whichever was last.
RESULTS
Patient Characteristics
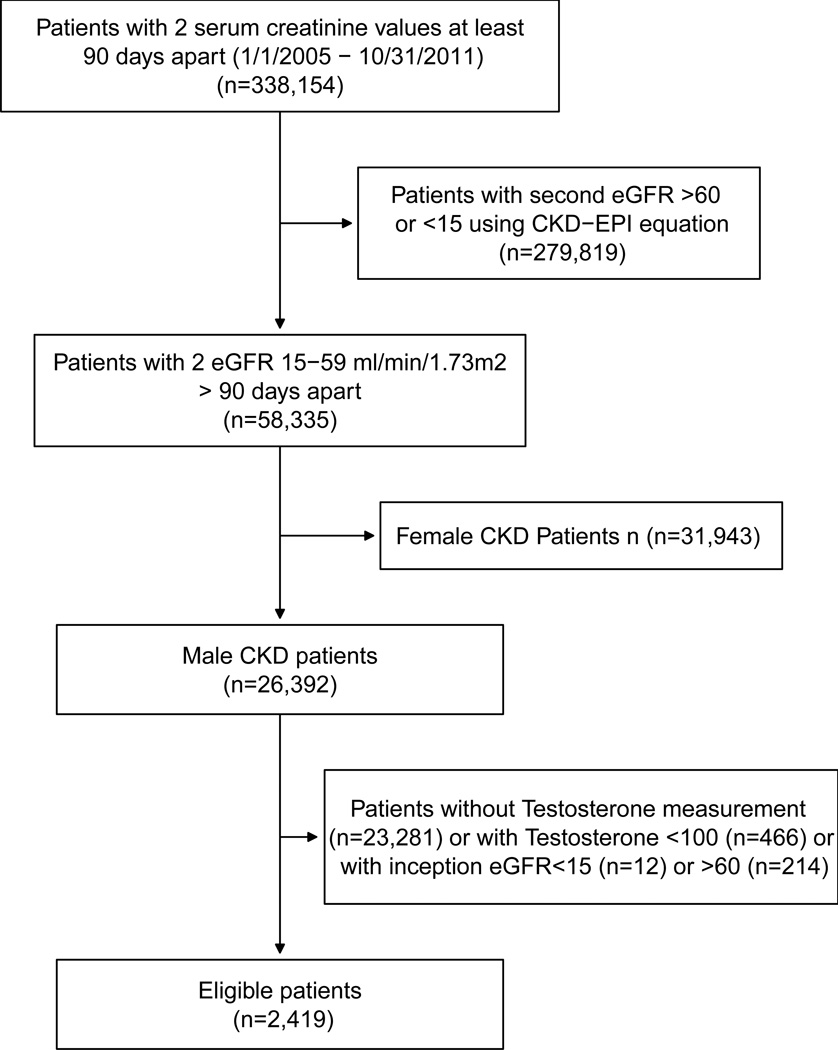
There were 338,154 patients with 2 eGFR values 90 days apart between January 1, 2005 and October 31, 2011. Then 279,819 patients were excluded because their second eGFR value was either <15 mL/min/1.73m2 or >60 mL/min/1.73m2. There were 58,335 patients left who had two eGFR values between 15–59 mL/min/1.73 m2 at least 90 days apart, but 31,943 were female and thus excluded. Of the remaining 26,392 male patients in our registry during the study period, 23,973 were excluded as follows: 23,281, lack of testosterone measurement; 466, testosterone <100 ng/dL; 12, eGFR value at inception <15 mL/min/1.73m2; and 214, eGFR value at inception >60 mL/min/1.73m2.
Therefore, 2,419 patients met inclusion criteria for the current study (Figure 1). Mean ± standard deviation age of patients included in the study was 67.3 ±11.3 (range, 22–95) years, eGFR at study inception was 49.1 ± 9.5 (range, 15.3–59.95) mL/min/1.73m2, and BMI was 29.4 ± 5.4 (range, 14.4–64.3) kg/m2. 11% were African American. In this cohort whose testosterone was measured for clinical indications, 1288 of 2419 (53%) men had low total testosterone levels or were receiving testosterone medication. An additional 97 patients who had a normal testosterone at study inception had a low value during follow-up or started to receive testosterone medication. Low total testosterone was found in approximately half the men in each decade of life from 20–90 years of age. At study inception, 301 patients had a prescription for testosterone medication. By the end of the study, 447 patients had a prescription for testosterone medication.
Figure 1.
Flowchart of patient selection for study inclusion
(CKD: chronic kidney disease, CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration equation, eGFR: estimated glomerular filtration rate)
Table S1 (provided as online supplementary material) shows the comparison of clinical characteristics for patients with versus without testosterone measurement. Patients without total testosterone measurement were older, had lower eGFR, lower BMI, and had less diabetes, hypertension, congestive heart failure, and hyperlipidemia. Table 1 compares patients with low total testosterone and normal total testosterone. Patients with low versus normal total testosterone were significantly different in their eGFR, CKD stage, BMI, diabetes and cerebrovascular disease.
Table 1.
Characteristics of patients with low and normal total testosterone levels
| Factor | Low (n=1288) |
Normal (n=1131) |
|---|---|---|
| Age (y) | 67.5±11.3 | 67.0±11.3 |
| African American | 128(9.9) | 140(12.4) |
| eGFR at inception (mL/min/1.73m2)* | 48.4±9.8 | 49.8±9.1 |
| eGFR category* | ||
| 45–59 mL/min/1.73m2 | 906(70.3) | 870(76.9) |
| 30–44 mL/min/1.73m2 | 303(23.5) | 208(18.4) |
| 15–29 mL/min/1.73m2 | 79(6.1) | 53(4.7) |
| BMI (kg/m2)* | 30.3±5.7 | 28.4±5.0 |
| BMI category* | ||
| <18.5 kg/m2 | 3(0.23) | 7(0.62) |
| 18.5–24.9 kg/m2 | 189(14.7) | 268(23.7) |
| 25–29.9 kg/m2 | 496(38.5) | 501(44.3) |
| ≥30 kg/m2 | 588(45.7) | 343(30.3) |
| Missing | 12(0.93) | 12(1.1) |
| Smoking Status | ||
| No | 1144(88.8) | 979(86.6) |
| Yes | 81(6.3) | 95(8.4) |
| Missing | 63(4.9) | 57(5.0) |
| Diabetes * | 459(35.6) | 285(25.2) |
| Hypertension | 1206(93.6) | 1037(91.7) |
| Cerebrovascular disease * | 150(11.6) | 102(9.0) |
| Malignancy | 367(28.5) | 306(27.1) |
| Coronary artery disease | 390(30.3) | 324(28.6) |
| Chronic heart failure | 178(13.8) | 152(13.4) |
| Hyperlipidemia | 1102(85.6) | 953(84.3) |
| Lowest testosterone before inception (ng/dL) | 253.5[199.0–303.0] | 478.0[405.0–562.0] |
| Albumin (g/dL)^ | 4.2±0.40 | 4.2±0.41 |
Note: Values for categorical variables are given as number (percentage) with chi-square test; values for continuous variables are given as mean ± standard deviation with t-test or median [interquartile range] with Kruskal-Wallis test.
BMI: body mass index, eGFR, estimated glomerular filtration rate
Albumin data missing for 207 (9%) patients
Significantly different between patients with low and normal testosterone.
Predictors of Low Testosterone
In a multivariable logistic regression analysis, African American ethnicity and higher eGFR were associated with lower odds of having low total testosterone. Diabetes mellitus and higher BMI were associated with significantly higher odds of having lower total testosterone (Table 2).
Table 2.
Multivariable logistic regression of factors associated with low testosteronea
| Factor | OR (95% CI) |
|---|---|
| Age, per 10-y increase | 1.06 (0.98, 1.15) |
| African American | 0.65 (0.50, 0.85)b |
| eGFR, per 5-ml/min/1.73 m2 greater | 0.92 (0.88, 0.96) b |
| Diabetes | 1.47 (1.21, 1.78) b |
| Hypertension | 1.07 (0.77, 1.49) |
| Cerebrovascular disease | 1.32 (0.996, 1.74) |
| Malignancy | 1.10 (0.91, 1.33) |
| Coronary artery disease | 0.96 (0.79, 1.17) |
| Chronic heart failure | 1.00 (0.77, 1.30) |
| Hyperlipidemia | 0.89 (0.70, 1.14) |
| BMI | |
| <18.5 vs 18.5–24.9 kg/m2 | 0.52 (0.13, 2.08) |
| 25–29.9 vs 18.5–24.9 kg/m2 | 1.41 (1.12, 1.77)b |
| ≥30 vs 18.5–24.9 kg/m2 | 2.40 (1.89, 3.06) b |
| Missing vs 18.5–24.9 kg/m2 | 1.53 (0.66, 3.56) |
| Smoking | |
| Yes vs. no | 0.77 (0.56, 1.06) |
| Missing vs. no | 1.01 (0.68, 1.51) |
eGFR: estimated glomerular filtration rate, BMI: body mass index; OR, odds ratio; CI, confidence interval.
Model was also adjusted for year of inclusion in study
Statistically significant.
Associations With Death
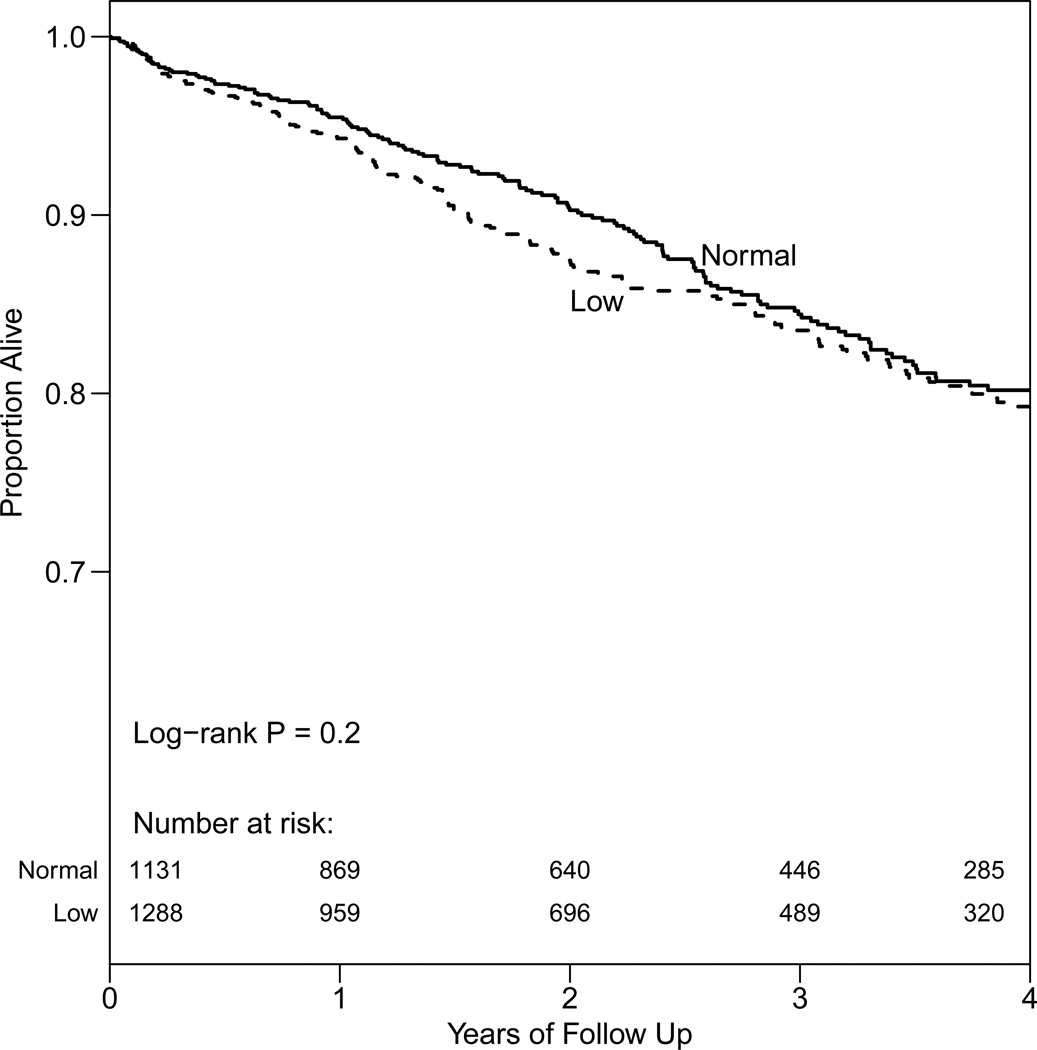
There were 357 of 2419 (14.8%) patients who died during a median follow-up of 2.3 (range, 0.003–6.5) years. Figure 2 shows a Kaplan-Meier survival curve of men with normal testosterone level compared with those having low testosterone or testosterone supplementation. The two groups did not have significantly different survival. In a multivariable Cox model adjusted for age, eGFR, BMI, comorbidities, and albumin, time-dependent low testosterone (<350 ng/dL or presence of testosterone supplementation) was not significantly associated with mortality (hazard ratio [HR], 1.16; 95% confidence interval [CI], 0.93–1.64). In a model adjusted for the previously mentioned variables plus testosterone medication, higher log testosterone was associated with significantly lower mortality (HR per 1 log unit, 0.70; 95% CI, 0.55–0.89). In the quintile model, the two lowest quintiles of total testosterone (100–226 and 227–305 ng/dL) were associated with higher mortality when compared to the highest quintile (512–3153 ng/dL [the reference range]), but the association for the lowest quintile did not reach statistical significance (Table 3). When using a Bonferroni correction to adjust for the 3 ways we evaluated testosterone (P=0.05/3=0.017), the log testosterone analysis (P=0.003) and the comparison between the second lowest and the highest quintiles (P=0.01) were the only results suggesting a significant association between testosterone level and mortality.
Figure 2.
Kaplan-Meier survival curve of men with chronic kidney disease stages 3–4 categorized as normal testosterone vs. low (testosterone <350 ng/dL or testosterone supplementation)
Table 3.
Cox proportional models of total testosterone vs. all-cause mortality
| Unadjusted | Adjusted for age, African American race, and eGFR |
Multivariable adjusted model |
|
|---|---|---|---|
| Low(<350 mg/dL or medication) vs. normal | 1.14 (0.92, 1.41) | 1.10 (0.89, 1.36) | 1.16 (0.93, 1.44)* |
| Continuous Log Testosterone | 0.65 (0.52, 0.82) | 0.71 (0.56, 0.89) | 0.70 (0.55, 0.89)** |
| Quintiles of testosterone | |||
| 100–226 ng/dL | 1.58 (1.13, 2.21) | 1.36 (0.97, 1.91) | 1.42 (0.995, 2.02) ** |
| 227–305 ng/dL | 1.52 (1.09, 2.13) | 1.43 (1.02, 2.00) | 1.53 (1.09, 2.16) ** |
| 306–392 ng/dL | 1.18 (0.84, 1.67) | 1.17 (0.83, 1.65) | 1.22 (0.86, 1.73) ** |
| 393–511 ng/dL | 1.01 (0.71, 1.45) | 0.93 (0.65, 1.33) | 1.01 (0.70, 1.45) ** |
| 512–3153 ng/dL | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
Note: Associations given as hazard ratio (95% confidence interval).
eGFR, estimated glomerular filtration rate
Adjusted for age, African American race, eGFR, diabetes, hypertension, cerebrovascular disease, coronary artery disease, congestive heart failure, hyperlipidemia, malignancy, body mass index category, smoking status and albumin
Adjusted for age, African American race, eGFR, diabetes, hypertension, cerebrovascular disease, coronary artery disease, congestive heart failure, hyperlipidemia, malignancy, body mass index category, smoking status, albumin and testosterone medication
DISCUSSION
The popularity of testosterone replacement therapy is rapidly growing, with huge increases in utilization and costs over the past 10 years15. As a panacea for erectile dysfunction, general malaise and “lost youth”, testosterone is almost certainly being overprescribed in ways that do not follow best practice guidelines. On the other hand, men with serious systemic health conditions often associated with low testosterone and its effects are seldom screened and treated. In addition, early evidence suggests an association between low testosterone and mortality in some patient populations. The objective of this study was to investigate whether low total testosterone was associated with increased all-cause mortality in men with kidney disease who were not receiving dialysis. We found that higher testosterone level may be associated with significantly lower mortality when examined as a continuous variable or comparing second lowest quintile to highest quintile [the reference range], but not when using a cutoff of <350 ng/dL.
Studies investigating testosterone levels in men with non–dialysis-dependent CKD are limited. Previous studies have been done mainly in the hemodialysis population, and have found that low testosterone is associated with increased cardiovascular disease and all-cause mortality7. One study of 239 men assessed the association of testosterone level and cardiovascular disease, and found that an increase of 1 nmol/L (or 28.8 ng/dL) decreased the likelihood of cardiovascular event by 22%16. A recent study of 126 dialysis-dependent males with total testosterone measured prospectively and followed up for a mean of 41 months found that the lowest tertile of total testosterone (≤ 233 ng/dL) was associated with significantly increased all-cause mortality (HR, 2.03; 95% CI, 1.24–3.31) as well as death due to cardiovascular disease (HR, 3.19; 95% CI, 1.49–6.83)5. Another study of 111 hemodialysis-dependent males had similar findings6. In this study, 54 men with low total testosterone (< 369 ng/dL [12.8 nmol/L]) were compared to 57 men with normal total testosterone. There were 49 deaths during a median follow-up of 37 months. Low total testosterone was associated with increased cardiovascular disease and all-cause mortality (HRs of 3.14 [95% CI, 1.21–8.16] and 3.09 [95% CI, 1.53–6.25], respectively). This study also found that men with total testosterone below 231 ng/dL [8 nmol/L] were at higher risk of cardiovascular disease and all-cause mortality, and the lowest tertile of total testosterone (<150 ng/dL [5.2 nmol/L]) had the highest risk.
These studies reflect a strong association between total testosterone level, cardiovascular disease, and all-cause mortality in men with CKD. The risk for cardiovascular disease and/or death seems to increase for decreasing levels of total testosterone. The evidence is stronger for dialysis-dependent men than non–dialysis-dependent men; however, the study by Yilmaz et al16 and our study point to a similarly strong association between testosterone and death in men who are not yet on dialysis.
Some of the potential mechanisms that may be responsible for the association between low testosterone and mortality include the effect on the cardiovascular system, and cognitive function, and other less well studied phenomena. Several studies have investigated the pathophysiological effects of testosterone on the cardiovascular system that may be contributing to decreased overall survival. Testosterone has been associated with heart failure, and a double-blind randomized controlled trial showed that TRT increased exercise capacity and symptoms in men with moderately severe heart failure17. Another randomized controlled trial showed that TRT improved exercise capacity, muscle strength, and insulin resistance in men with moderately severe chronic heart failure18. A recent meta-analysis supported these findings19. These effects have been linked to a vasodilatory property of testosterone20 resulting in increase in cardiac output and decrease in systemic vascular resistance21. Similarly, greater endothelial dysfunction was seen in castrated rats in one study, and administration of testosterone resulted in lower apoptosis, fibrosis, and angiotensin II receptor expression22. Several studies have shown a strong association between depression, cognitive function and mortality in CKD patients23, and testosterone has been known to be associated with depression24
In our study, there was an association of increased BMI and diabetes mellitus with lower total testosterone. This association has been seen in the other studies of men with CKD16 as well as in men with hypogonadism without CKD25, 26. In a study of 1059 men, total testosterone decreased with increasing quartiles of BMI (<25, 25–29.9, 30–34.9, and ≥35 kg/m2). The cause is still unclear, but sex hormone binding globulin has been suggested26. Similarly, diabetes mellitus has been shown to be associated with low testosterone in the normal population. A study of 1413 men showed that the lowest tertile of free testosterone was highly associated with diabetes mellitus (HR, 4.12; 95% CI, 1.25–13.55) after adjusting for age, ethnicity, and BMI27. Alterations in glucose metabolism and insulin resistance have been discussed as possible mechanisms27.
Our study also found that higher eGFR was associated with higher total testosterone. This is in agreement with another study with a similar finding16. Progression to more severe CKD stages is well known to be associated with worse cardiovascular and allcause mortality28. However, in our study, greater all-cause mortality was found even after adjusting for eGFR.
African American ethnicity was associated with improved total testosterone level in our study. Previous studies assessing the relationship between ethnicity and total testosterone levels provided conflicting results. In a study of 1475 community-dwelling men in Boston, equal proportions of Caucasian, Hispanic, and African-American men were interviewed and had blood samples collected for total and free testosterone measurements29. The prevalence of symptomatic hypogonadism was 5.6%, and there was equal distribution among all three ethnic groups. A study of 1413 African-American, Caucasian, and Hispanic-American men found that total testosterone did not differ between African-American and Caucasian men, but was higher in Hispanic-American men30. Since these papers did not adjust for comorbidities and other confounding factors, it is difficult to discern the true relationship of ethnicity with total testosterone levels.
The benefits of testosterone replacement in men with CKD are severely understudied. One study found that testosterone replacement restored sexual function in 11% of men, with partial response in 70%, but the effects were not long lasting31. Hypogonadism has been shown to be associated with anemia and hyporesponsiveness to erythropoiesis-stimulating agents32. In a study of 84 patients, nandrolone decanoate, 200 mg, was given intramuscularly weekly for six months33. Hematocrit increased from 6.9 to 8.7 g/dL, with the greatest increase in patients older than 55 years. A follow-up study by the same group showed that the increase in hemoglobin was similar with erythropoietin as compared to nandrolone decanoate34. Another benefit of androgen therapy was seen in a randomized controlled trial that reported significant increase in lean body mass, functional status, and quality of life in dialysis patients treated with nandrolone decanoate35. Though these effects may not be causal, there is evidence of an association between androgen therapy and improvement in sexual function, anemia, muscle mass, and functional status. However, the effect of TRT on cardiovascular status and mortality are yet to be proven. An important finding in our study is that among men found to have low total testosterone, only 31.6% were ever on TRT. The low usage of therapy in this cohort may be due to the unproven benefit of TRT in CKD36, 37.
Our study has several strengths. This is one of the few studies investigating the role of low total testosterone in men with CKD stages 3–4. Our registry is validated, and we were able to study a large cohort of patients. We adjusted for variety of comorbidities in addition to age, race, and eGFR.
The study is not without limitations. The study was done at a single tertiary care center, and may not be applicable in other clinical settings. We also restricted analysis to men with eGFR less than 60 ml/min/1.73m2, and are unable to state whether these findings hold true in men with less severe stages of CKD. Additionally, though our data shows an association between low total testosterone and all-cause mortality, we cannot infer causality. Other limitations include not adjusting for inflammatory markers such as C-reactive protein as these data were not available for majority of our study population, for the time of total testosterone blood draw, or for other medications that may affect testosterone level. We were unable to adjust for proteinuria since majority of study participants did not have proteinuria assessed around the time of serum testosterone measurements. It is quite possible that testosterone may be a marker of illness rather than on the pathogenetic pathway to mortality. One study showed that over half of all patients admitted to the hospital had hypogonadism38. Another paper showed that testosterone was associated with metabolic syndrome in elderly men, but lost its association when adjusted for lipids, C-reactive protein, BMI and insulin level39. Further investigation of these factors may be important for future studies. Moreover, since not all men were tested, our results might not be generalizable to entire CKD population, and we are unable to comment on the prevalence of symptomatic hypogonadism in men with non–dialysis-dependent CKD. We found that only 11.8% of men in our registry were tested for clinical indications, and 54% of those men (6.4% of the entire cohort) were found to have low total testosterone. It is unknown whether these men were symptomatic or if total testosterone levels were checked for another reason. Despite selective testing, it is important to note that in our cohort a clinically significant number of men tested had hypogonadism.
In summary, this study shows that low total testosterone may be associated with all-cause mortality in men with CKD stages 3–4. The findings of this study should be confirmed in other cohorts. It remains to be seen whether replacing testosterone will improve survival.
Supplementary Material
Acknowledgements
Support: None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Supplementary Material
Table S1: Characteristics of patients with and without testosterone measurement.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Descriptive Text for Online Delivery of Supplementary Material
Supplementary Table S1 (PDF)
Characteristics of patients with and without testosterone measurement.
References
- 1.Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 2.De Ryck I, Van Laeken D, Apers L, Colebunders R. Erectile dysfunction, testosterone deficiency, and risk of coronary heart disease in a cohort of men living with HIV in belgium. J Sex Med. 2013;10(7):1816–1822. doi: 10.1111/jsm.12175. [DOI] [PubMed] [Google Scholar]
- 3.Smith HS, Elliott JA. Opioid-induced androgen deficiency (OPIAD) Pain Physician. 2012;15(3 Suppl):ES145–ES156. [PubMed] [Google Scholar]
- 4.Ghazi S, Zohdy W, Elkhiat Y, Shamloul R. Serum testosterone levels in diabetic men with and without erectile dysfunction. Andrologia. 2012;44(6):373–380. doi: 10.1111/j.1439-0272.2012.01292.x. [DOI] [PubMed] [Google Scholar]
- 5.Carrero JJ, Qureshi AR, Parini P, et al. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol. 2009;20(3):613–620. doi: 10.1681/ASN.2008060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyriazis J, Tzanakis I, Stylianou K, et al. Low serum testosterone, arterial stiffness and mortality in male haemodialysis patients. Nephrol Dial Transplant. 2011;26(9):2971–2977. doi: 10.1093/ndt/gfq847. [DOI] [PubMed] [Google Scholar]
- 7.Bello AK, Stenvinkel P, Lin M, et al. Serum testosterone levels and clinical outcomes in male hemodialysis patients. Am J Kidney Dis. 2013 doi: 10.1053/j.ajkd.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Navaneethan SD, Jolly SE, Schold JD, et al. Development and validation of an electronic health record-based chronic kidney disease registry. Clin J Am Soc Nephrol. 2011;6(1):40–49. doi: 10.2215/CJN.04230510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haring R, Nauck M, Volzke H, et al. Low serum testosterone is associated with increased mortality in men with stage 3 or greater nephropathy. Am J Nephrol. 2011;33(3):209–217. doi: 10.1159/000324562. [DOI] [PubMed] [Google Scholar]
- 10.Cigarran S, Pousa M, Castro MJ, et al. Endogenous testosterone, muscle strength, and fat-free mass in men with chronic kidney disease. J Ren Nutr. 2013;23(5):e89–e95. doi: 10.1053/j.jrn.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Kang S, Park HJ, Park NC. Serum total testosterone level and identification of late-onset hypogonadism: A community-based study. Korean J Urol. 2013;54(9):619–623. doi: 10.4111/kju.2013.54.9.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mechlin CW, Frankel J, McCullough A. Coadministration of anastrozole sustains therapeutic testosterone levels in hypogonadal men undergoing testosterone pellet insertion. J Sex Med. 2013 doi: 10.1111/jsm.12320. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan C, Goldstein FC, Tenover JL. Exogenous testosterone alone or with finasteride does not improve measurements of cognition in healthy older men with low serum testosterone. J Androl. 2007;28(6):875–882. doi: 10.2164/jandrol.107.002931. [DOI] [PubMed] [Google Scholar]
- 14.Carrero JJ, Qureshi AR, Nakashima A, et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant. 2011;26(1):184–190. doi: 10.1093/ndt/gfq397. [DOI] [PubMed] [Google Scholar]
- 15.Gan EH, Pattman S, H S Pearce S, Quinton R. A UK epidemic of testosterone prescribing, 2001–2010. Clin Endocrinol (Oxf) 2013;79(4):564–570. doi: 10.1111/cen.12178. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz MI, Sonmez A, Qureshi AR, et al. Endogenous testosterone, endothelial dysfunction, and cardiovascular events in men with nondialysis chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(7):1617–1625. doi: 10.2215/CJN.10681210. [DOI] [PubMed] [Google Scholar]
- 17.Malkin CJ, Pugh PJ, West JN, van Beek EJ, Jones TH, Channer KS. Testosterone therapy in men with moderate severity heart failure: A double-blind randomized placebo controlled trial. Eur Heart J. 2006;27(1):57–64. doi: 10.1093/eurheartj/ehi443. [DOI] [PubMed] [Google Scholar]
- 18.Caminiti G, Volterrani M, Iellamo F, et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009;54(10):919–927. doi: 10.1016/j.jacc.2009.04.078. [DOI] [PubMed] [Google Scholar]
- 19.Toma M, McAlister FA, Coglianese EE, et al. Testosterone supplementation in heart failure: A meta-analysis. Circ Heart Fail. 2012;5(3):315–321. doi: 10.1161/CIRCHEARTFAILURE.111.965632. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor EK, Ivey JR, Bowles DK. Differential effects of androgens on coronary blood flow regulation and arteriolar diameter in intact and castrated swine. Biol Sex Differ. 2012;3(1) doi: 10.1186/2042-6410-3-10. 10-6410-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pugh PJ, Jones TH, Channer KS. Acute haemodynamic effects of testosterone in men with chronic heart failure. Eur Heart J. 2003;24(10):909–915. doi: 10.1016/s0195-668x(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 22.Kang NN, Fu L, Xu J, et al. Testosterone improves cardiac function and alters angiotensin II receptors in isoproterenol-induced heart failure. Arch Cardiovasc Dis. 2012;105(2):68–76. doi: 10.1016/j.acvd.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Palmer SC, Vecchio M, Craig JC, et al. Association between depression and death in people with CKD: A meta-analysis of cohort studies. Am J Kidney Dis. 2013;62(3):493–505. doi: 10.1053/j.ajkd.2013.02.369. [DOI] [PubMed] [Google Scholar]
- 24.Afsar B. Relationship between total testosterone, cognitive function, depressive behavior, and sleep quality in chronic kidney disease patients not on dialysis. Clin Exp Nephrol. 2013;17(1):59–65. doi: 10.1007/s10157-012-0652-0. [DOI] [PubMed] [Google Scholar]
- 25.Corona G, Mannucci E, Fisher AD, et al. Low levels of androgens in men with erectile dysfunction and obesity. J Sex Med. 2008;5(10):2454–2463. doi: 10.1111/j.1743-6109.2008.00856.x. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26(6):833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 27.Selvin E, Feinleib M, Zhang L, et al. Androgens and diabetes in men: Results from the third national health and nutrition examination survey (NHANES III) Diabetes Care. 2007;30(2):234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 28.Chronic Kidney Disease Prognosis Consortium. Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- 30.Rohrmann S, Nelson WG, Rifai N, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of americans. J Clin Endocrinol Metab. 2007;92(7):2519–2525. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence IG, Price DE, Howlett TA, Harris KP, Feehally J, Walls J. Correcting impotence in the male dialysis patient: Experience with testosterone replacement and vacuum tumescence therapy. Am J Kidney Dis. 1998;31(2):313–319. doi: 10.1053/ajkd.1998.v31.pm9469503. [DOI] [PubMed] [Google Scholar]
- 32.Carrero JJ, Barany P, Yilmaz MI, et al. Testosterone deficiency is a cause of anaemia and reduced responsiveness to erythropoiesis-stimulating agents in men with chronic kidney disease. Nephrol Dial Transplant. 2012;27(2):709–715. doi: 10.1093/ndt/gfr288. [DOI] [PubMed] [Google Scholar]
- 33.Teruel JL, Aguilera A, Marcen R, Navarro Antolin J, Garcia Otero G, Ortuno J. Androgen therapy for anaemia of chronic renal failure, indications in the erythropoietin era. Scand J Urol Nephrol. 1996;30(5):403–408. doi: 10.3109/00365599609181318. [DOI] [PubMed] [Google Scholar]
- 34.Teruel JL, Marcen R, Navarro-Antolin J, Aguilera A, Fernandez-Juarez G, Ortuno J. Androgen versus erythropoietin for the treatment of anemia in hemodialyzed patients: A prospective study. J Am Soc Nephrol. 1996;7(1):140–144. doi: 10.1681/ASN.V71140. [DOI] [PubMed] [Google Scholar]
- 35.Johansen KL, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: A randomized controlled trial. JAMA. 1999;281(14):1275–1281. doi: 10.1001/jama.281.14.1275. [DOI] [PubMed] [Google Scholar]
- 36.Brockenbrough AT, Dittrich MO, Page ST, Smith T, Stivelman JC, Bremner WJ. Transdermal androgen therapy to augment EPO in the treatment of anemia of chronic renal disease. Am J Kidney Dis. 2006;47(2):251–262. doi: 10.1053/j.ajkd.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Iglesias P, Carrero JJ, Diez JJ. Gonadal dysfunction in men with chronic kidney disease: Clinical features, prognostic implications and therapeutic options. J Nephrol. 2012;25(1):31–42. doi: 10.5301/JN.2011.8481. [DOI] [PubMed] [Google Scholar]
- 38.Iglesias P, Prado F, Macias MC, et al. Hypogonadism in aged hospitalized male patients: Prevalence and clinical outcome. J Endocrinol Invest. 2014;37(2):135–141. doi: 10.1007/s40618-013-0009-x. [DOI] [PubMed] [Google Scholar]
- 39.Yeap BB, Knuiman MW, Divitini ML, et al. Differential associations of testosterone, dihydrotestosterone and estradiol with physical, metabolic and health-related factors in community-dwelling men aged 17–97 years from the busselton health survey. Clin Endocrinol (Oxf) 2014 doi: 10.1111/cen.12407. [DOI] [PubMed] [Google Scholar]
- 39A.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE initiative: the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 39B.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.