Abstract
Objective
Previous research has linked complex or formed visual hallucinations (VH) to Lewy-type alpha-synucleinopathy (LTS) in neocortical and limbic areas. As Alzheimer’s disease pathology often co-occurs with LTS, we questioned whether this pathology - amyloid plaques and neurofibrillary tangles - might also be linked to VH.
Methods
We performed a semi-quantitative neuropathological study across brainstem, limbic, and cortical structures in subjects with a documented clinical history of VH and a clinicopathological diagnosis of Parkinson’s disease (PD), Alzheimer’s disease (AD), or dementia with Lewy bodies (DLB). 173 subjects – including 50 with VH and 123 without VH – were selected from the Arizona Study of Aging and Neurodegenerative Disorders. Clinical variables examined included the Mini-mental State Exam, Hoehn & Yahr stage, and total dopaminergic medication dose. Neuropathological variables examined included total and regional LTS and plaque and tangle densities.
Results
A significant relationship was found between the density of LTS and the presence of VH in PD, AD, and DLB. Plaque and tangle densities also were associated with VH in PD (p=.003 for plaque and p=.004 for tangles) but not in AD, where densities were high regardless of the presence of hallucinations. Furthermore, with DLB cases excluded, comorbidity of PD and AD was significantly more prevalent among subjects +VH than subjects −VH (p<.001).
Conclusion
These findings suggest that both AD and PD neuropathology contribute to the pathogenesis of VH. Incident VH could be predictive of concomitant AD/PD pathology even when criteria are not met for a second diagnosis.
Keywords: visual hallucinations, hallucinations, psychosis, Alzheimer’s disease, Parkinson’s disease
Introduction
Complex or “formed” visual hallucinations (VH) are a core feature of dementia with Lewy bodies (DLB) [1], and occur in an estimated 22% of patients with Parkinson’s disease (PD) [2]. Visual hallucinations also occur in up 25% of patients with Alzheimer’s disease (AD), usually in later stages of dementia [2].
VH in PD and DLB frequently are reported as non-threatening images of people, animals, or objects that persist for seconds to minutes [3]. The images may be highly detailed; for example, the people may be dressed in colorful costumes although only a few inches tall (“Lilliputian”). The hallucinations are experienced in a clear sensorium with eyes open. In the absence of dementia, insight into the hallucinatory nature of the images usually is retained [2]. The phenomenology of hallucinations in AD is somewhat different, in that images tend to be less well elaborated and less persistent, and insight generally is not retained [4]. Even so, VH in these different conditions may have a common pathological substrate
Given the frequent occurrence of VH in DLB and PD, it is not surprising that clinicopathological studies have implicated Lewy-type alpha-synucleinopathy (LTS) as the pathological culprit, both in neocortex [5–7] and limbic structures [5, 7, 8]. Less well investigated has been the contribution of plaque and tangle pathology to VH in PD, DLB, or AD. In one study, tangle density in frontal cortex was found to correlate with psychosis in patients with AD, but that study included patients with delusions as well as hallucinations [9]. Another study found that, even in AD, VH were associated with cortical LTS, although neuropathology was evaluated only qualitatively (present or absent), and regional differences were not studied [10].
Just as LTS can be present in neuropathologically-confirmed AD [11, 12], plaque and tangle pathology often are present in neuropathologically-confirmed PD [13]. The presence of LTS in AD does not necessarily warrant a concomitant diagnosis of DLB or PD; the diagnosis depends upon the amount and location of LTS [14, 15].
The question arises whether AD and LTS pathologies might both contribute to the genesis of VH, or whether in fact only LTS is implicated. To address this question, we performed a semi-quantitative neuropathological study across brainstem, limbic, and cortical structures in patients with a documented clinical history of VH and a clinicopathological diagnosis of AD, PD, or DLB. Overlapping diagnoses of AD+PD and AD+DLB also were included.
Subjects and Methods
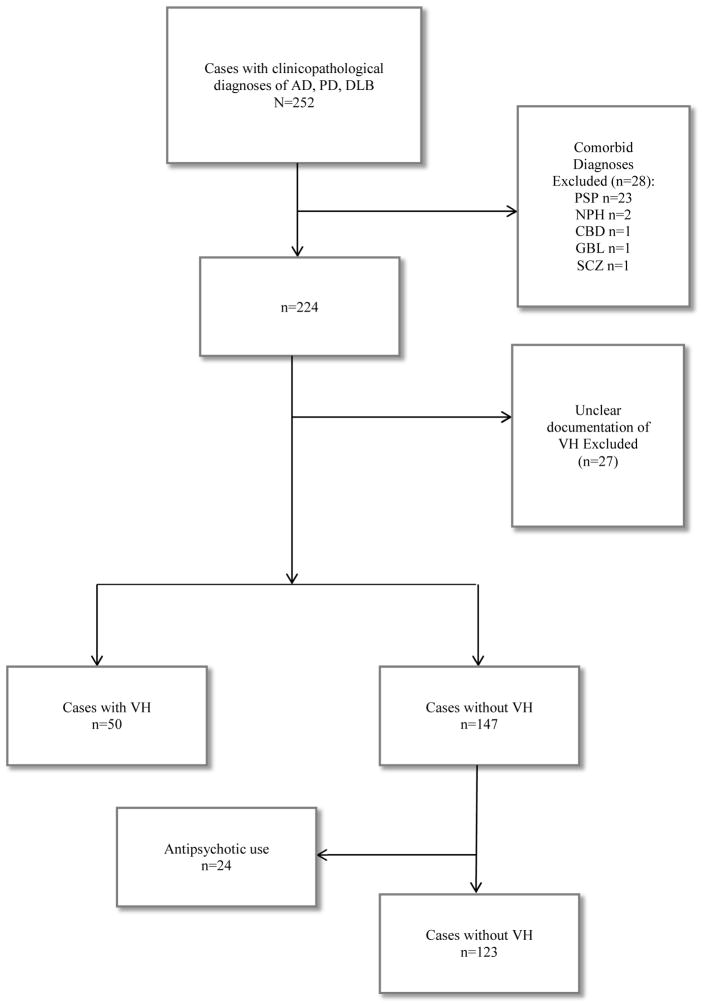
The research was in full compliance with the ethical rules for human experimentation stated in the Declaration of Helsinki. All subjects were enrolled in an ongoing longitudinal clinicopathological study, the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND) within the Banner Sun Health Research Institute Brain and Body Donation Program (BBDP) [16]. All subjects or their legally authorized representatives signed an Institutional Review Board-approved informed consent form at the time of enrollment for clinical assessments as well as for brain and/or body donation after death. For this study, data from all subjects with a pathologicallyconfirmed clinical diagnosis of AD, PD, or DLB were identified from the AZSAND database. Subjects with potentially confounding conditions were excluded (Figure 1) to maintain homogeneity of pathology related to disease. Also excluded were subjects with unclear documentation regarding VH and those without documentation of VH who had used antipsychotic medication, in most cases for delusions. The latter were excluded because antipsychotic medication effectively treats VH, so that group assignment (VH versus no VH) for treated patients would be uncertain.
Figure 1.
Flow diagram showing case exclusions.
AD=Alzheimer’s disease
PD=Parkinson’s disease
DLB=dementia with Lewy bodies
PSP=progressive supranuclear palsy
NPH=normal pressure hydrocephalus
CBD=corticobasal degeneration
GBL=glioblastoma
SCZ=schizophrenia
VH=visual hallucinations
Clinical Evaluation. Annually, each subject underwent a standardized physical, neurological, and movement disorder examination, as well as comprehensive neuropsychological testing, performed in accordance with the National Alzheimer’s Coordinating Center Uniform Data Set guidelines (NACC UDS). Clinical scales completed at each visit included the Clinical Dementia Rating Scale [17] and the Neuropsychiatric Inventory-Q [18]. Clinical diagnoses were made in accord with published guidelines for dementia [19], Alzheimer’s disease [20], Parkinson’s disease [21], and dementia with Lewy bodies [1]. [22][23][24]
For the purpose of this study, visual hallucinations were defined as recognizable images – usually people, animals, or objects – reported at one or more evaluations [2]. The presence of VH was first ascertained using the caregiver-based structured interview of the Neuropsychiatric Inventory-Q. After the patient interview and examination, the study physician completed a form (NACC UDS Form B9) that required documentation of hallucinations as visual, and also whether or not they were “formed” – indicated by recognizable images, as noted above. Other clinical variables examined included the Minimental State Exam or MMSE [25] and Hoehn & Yahr stage [26] at the index visit, when VH were noted. Total dopaminergic medication dose at the index visit was calculated [27]. Neuropathological examination.
Specific neuropathological criteria were used in the post-mortem evaluation of Alzheimer’s disease [22], Parkinson’s disease [23], and dementia with Lewy bodies [24]. Neuropathological assessment was performed blinded to clinical categorization, and diagnosis was assigned by an experienced neuropathologist (TGB).
Neuropathological variables examined for all subjects included total and regional Lewy body/neurite scores, plaque and tangle density scores, and Braak neurofibrillary stages. Tissue processing methods have been described in detail elsewhere [16]. In brief, the cerebrum was cut in the coronal plane at the time of brain removal into 1-cm thick slices and then divided into right and left halves. Slices from the right half were frozen between slabs of dry ice, and slices from the left half were fixed by immersion in neutral-buffered 4% formaldehyde for 48 hours at 4° C. Following cryoprotection in ethylene glycol and glycerol with 0.1 M pH 7.4 phosphate buffer, selected 3 × 4 cm blocks were sectioned at 40 or 80 μm thickness on a sliding freezing microtome. Sections were stained with Hematoxylin and Eosin, Thioflavin S, and enhanced Silver methods. For detection of amyloid plaques and neurofibrillary tangles, Campbell-Switzer and Gallyas methods were used, respectively; these are the original methods used to develop Thal-Braak amyloid staging [28] and Braak neurofibrillary staging [29]. Thioflavin S is one of two methods recommended and validated for neuritic plaque density grading by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) [22].
To detect LTS, formalin-fixed, 5μm paraffin-embedded sections were used for immunohistochemistry with an antibody against phosphorylated α-synuclein peptide (1:10,000; rabbit polyclonal anti-human phosphoserine 129, gift of Dr. Akiyama) [30]. Density of LTS was graded according to the criteria of McKeith and colleagues [24] on a scale of 0–4, with 0=none, 1=mild, 2=moderate, 3=severe, 4=very severe. This grading system includes intra-cytoplasmic inclusions as well as those contained in neuritic processes. LTS density scores in the middle frontal gyrus, middle temporal gyrus, inferior parietal gyrus, cingulate cortex, transentorhinal region including the hippocampus, amygdala, substantia nigra, anterior medulla including the nuclei of the IX and X cranial nerves, and the olfactory bulb and tract were determined. In addition, each case was classified according to the Unified Staging System for Lewy Body Disorders, with scores ranging from 1 (olfactory bulb only) to 4 (cortical) [12].
Amyloid plaque and neurofibrillary tangle (NFT) density were graded and staged using large-format, 40–80 μm-thick formaldehyde-fixed sections of the superior frontal gyrus at the level of the genu, parietal cortex at the level of the splenium, two temporal cortex sections - one at the level of the anterior amygdala including the body of the hippocampus and substantia nigra, and the other at the level of the posterior half of the amygdala including the anterior thalamus and lenticular nucleus - and occipital lobe including the line of Gennari. Neurofibrillary tangles were detected with Gallyas silver stains and Campbell-Switzer silver stains were used to detect amyloid plaques. Thioflavin-S was used as a secondary stain to detect LTS, neurofibrillary tangles and amyloid plaques. Plaque and tangle density scores were assigned according to the published CERAD templates [22], and tangle distribution also was staged according to the original Braak protocol [29]. Scores for plaque density were derived from a consideration of all types of plaques - cored, neuritic and diffuse - together, while cored and neuritic plaques also were estimated separately to obtain a CERAD neuritic plaque density score. Plaque and tangle density scores were obtained separately by assigning values of none, sparse, moderate, and frequent, according to the published CERAD templates [22]. Conversion of the descriptive terms to numerical values resulted in scores of 0–3 (0=none, 1=sparse, 2=moderate, 3=frequent) for each area (frontal, temporal, and parietal cortex as well as entorhinal cortex and CA1 area of hippocampus), with a maximum total score of 15 for all five cortical areas combined (“total plaque score” and “total tangle score”). A neuropathological diagnosis of AD was made on the basis of the NIA-Reagan criteria indicating “intermediate” or “high” probability that the dementia was due to AD; for this determination, any combination of Braak NFT stages III–VI and moderate or frequent CERAD neuritic plaque density was used. A neuropathological diagnosis of PD was made on the basis of Lewy bodies and pigmented neuron loss in the substantia nigra [23, 31]. Subjects received a diagnosis of AD if they had a clinical history of dementia and were classified as “intermediate” or “high” probability that dementia was due to AD according to the NIA-Reagan criteria [22]. DLB was diagnosed according to consensus criteria published by the Dementia with Lewy Bodies Consortium [24]. Cases were classified as AD/DLB or PD/AD if criteria were met for both diagnoses. The diagnoses of PD and DLB are mutually exclusive.
Data Analysis and Statistics. For the first analysis, the sample was divided into two groups for comparison: those with VH and those without VH. For the second analysis, AD and PD groups each were divided into two sets for comparison: those with VH and those without VH. Data were analyzed using SAS software Version 9.2. Mean values were compared using the two-sample t test. Frequencies of dichotomous characteristics were compared using the Pearson Chi-square test. The Fisher exact test was used instead if the minimum expected cell count was less than five. The Bonferroni correction was made to adjust for nine primary multiple comparisons (p-value of .05/9=.006): LTS, tangle, and plaque totals in the PD, AD, and combined groups. Regions were tested if the total score was significant.
Results
There were 252 cases identified with a clinicopathological diagnosis of PD, AD, and/or DLB. A flow diagram of cases excluded is shown in Figure 1. Fifty cases with VH (+VH) and 147 cases without visual hallucinations (−VH) were culled. In 20 +VH cases and 24 −VH cases, subjects had used atypical antipsychotics; the 24 −VH cases were excluded, as noted above. The final study group comprised 173 cases: 50 with VH and 123 without VH.
Mean and standard deviation values for selected clinical and clinicopathological variables for the entire sample are shown in Table 1, which compares +VH to −VH groups and displays p-values. In the sample as a whole, subjects +VH expired at a significantly younger age than subjects −VH. Using the pre-established p-value of <.006, no difference was found in sex ratio (72% male in +VH group versus 53% male in −VH group; p=.02), years of education (15.3±2.3 in +VH group, 14.9±2.3 in −VH group; p=.37), presence of cognitive impairment (see Table 1), or Mini-mental State Exam Score. On the other hand, subjects +VH were more likely to have neuropathologically-confirmed PD, either alone or comorbid with AD, a higher Hoehn & Yahr stage, and to be treated with dopaminergic medication, and at a higher mean dosage (Table 1). On neuropathological examination, all brain areas examined had significantly more LTS in +VH subjects compared to −VH subjects (p<.001 for all areas). In contrast, neither plaque nor tangle densities distinguished +VH from −VH subjects when AD, PD, and DLB groups were combined for analysis.
Table 1.
All Subjects Selected Clinical and Clinicopathological1 Data (N=173)
| Hallucinators (n=50) | Non-hallucinators (n=123) | p-value | |
|---|---|---|---|
| Expired age | 80.8 (5.9) | 85.0 (7.3) | <.001 |
| Dementia or mild cognitive impairment | 47/50 (94%) | 108/123 (88%) | .23 |
| MMSE2 | 18.6 (8.7) | 20.0 (7.8) | .48 |
| Hoehn & Yahr stage | 3.5 (1.2) | 2.0 (1.9) | <.001 |
| Duration of Parkinson’s disease | 16.4 ± 7.3 | 11.9 ± 6.6 | .009 |
| Levodopa equivalent doses | 506 (486) | 256 (550) | 0.001 |
| Clinicopathological diagnoses1: | |||
| Alzheimer’s disease | 34/50 (68%) | 97/123 (79%) | .13 |
| Parkinson’s disease | 38/50 (76%) | 33/123 (27%) | <.001 |
| Alzheimer’s disease + Parkinson’s disease | 22/50 (44%) | 7/123 (6%) | <.001 |
| Dementia with Lewy bodies | 6/50 (12%) | 13/123 (11%) | .79 |
| Alzheimer’s disease + dementia with Lewy bodies | 6/50 (12%) | 13/123 (11%) | .79 |
| Alzheimer’s disease with Lewy bodies | 3/50 (6%) | 28/123 (23%) | .009 |
Clinicopathological diagnoses were established by pathological confirmation of clinical diagnoses made using published criteria for each disorder, as described in the text.
MMSE is the Mini-mental State Exam.
Mean values were compared using the two-sample t test. Frequencies of dichotomous characteristics were compared using the Pearson Chi-square test. The Fisher exact test was used instead if the minimum expected cell count was less than five.
Selected clinical data and clinicopathological breakdown are shown separately for AD and PD groups in Table 2. in. Among subjects with AD, +VH subjects expired at a younger age and were more likely to have comorbid PD. Consistent with this comorbidity, AD subjects +VH had a higher Hoehn & Yahr stage score, were more likely treated with dopaminergic medications, and were treated with a higher average dose of dopaminergic medications than −VH subjects. Among subjects with PD, +VH subjects were more cognitively impaired and more likely to have comorbid AD than −VH subjects. Some degree of AD pathology was present in most PD subjects +VH, even those who did not meet neuropathological criteria for AD or clinicopathological criteria for DLB.
Table 2.
| AD Hallucinators (n=34) | AD Non-Hallucinators (n=97) | p-value | PD Hallucinators (n=38) | PD Non-Hallucinators (n=33) | p-value | |
|---|---|---|---|---|---|---|
| Expired age | 81.8 (5.7) | 86.4 (6.7) | <.001 | 80.2 (6.1) | 80.5 (6.6) | .82 |
| MMSE3 | 15.6 (8.8) | 18.9 (7.5) | .11 | 22.0 (6.4) | 26.3 (3.8) | .03 |
| Hoehn & Yahr | 3.4 (1.2) | 1.7 (2.0) | <.001 | 3.62 (0.92) | 3.03 (1.15) | .02 |
| Levodopa equivalents | 411 (376) | 101 (334) | <.001 | 665 (468) | 884 (766) | .21 |
| Clinicopathological Diagnoses:1 | ||||||
| Alzheimer’s disease | 34/34 (100%) | 97/97 (100%) | >.99 | 22/38 (58%) | 7/33 (21%) | .002 |
| Parkinson’s disease | 22/34 (65%) | 7/97 (7%) | <.001 | 38/38 (100%) | 33/33 (100%) | >.99 |
| Dementia with Lewy bodies | 6/34 (18%) | 13/97 (13%) | .58 | -- | -- | -- |
Clinicopathological diagnoses were established by pathological confirmation of clinical diagnoses made using published criteria for each disorder, as described in the text.
The diagnoses of AD and PD are not mutually exclusive. Patients with AD + PD would have a clinical diagnosis of AD with parkinsonism, and would meet neuropathological criteria for both conditions.
MMSE is the Mini-mental State Exam.
Mean values were compared using the two-sample t test. Frequencies of dichotomous characteristics were compared using the Pearson Chi-square test. The Fisher exact test was used instead if the minimum expected cell count was less than five.
AD subjects +VH had significantly more Lewy-type synucleinopathy (LTS) in all brain areas examined, as shown in Table 3. Neither plaque nor tangle densities distinguished the AD subjects +VH from −VH; plaque and tangle values were high for both groups. PD subjects +VH had significantly more LTS in olfactory bulb/tract and cingulate, temporal, and parietal cortex. In addition, PD subjects +VH as a group had significantly greater total plaque density, neuritic plaque density, and plaque density in frontal, parietal, and hippocampal areas; and significantly greater total tangle density and tangle density in parietal and transentorhinal areas than PD subjects −VH.
Table 3.
Neuropathological Data AD (N=131) and PD (N=71) Groups
| AD Hallucinators (n=34) | AD Non-Hallucinators (n=97) | p-value | PD Hallucinators (n=38) | PD Non-Hallucinators (n=33) | p-value | |
|---|---|---|---|---|---|---|
| Lewy-Type α-Synucleinopathy or LTS (Lewy bodies + Lewy neurites) | ||||||
| Unified Lewy Body Stage | 3.2 (1.3) | 1.3 (1.5) | <.001 | 3.50 (0.56) | 2.97 (0.65) | <.001 |
| olfactory bulb/tract | 3.1 (1.4) | 1.3 (1.6) | <.001 | 2.9 (1.2) | 2.0 (1.1) | .002 |
| IX, X | 2.7 (1.6) | 0.9 (1.4) | <.001 | 3.22 (0.80) | 2.87 (1.02) | .12 |
| locus coeruleus | 2.5 (1.5) | 0.7 (1.4) | <.001 | 3.03 (0.83) | 2.88 (0.91) | .47 |
| substantia nigra | 2.4 (1.5) | 0.6 (1.2) | <.001 | 2.67 (0.99) | 2.41 (1.13) | .31 |
| amygdala | 3.3 (1.4) | 1.2 (1.7) | <.001 | 3.61 (0.55) | 3.03 (1.12) | .007 |
| transentorhinal cortex | 2.7 (1.5) | 0.9 (1.5) | <.001 | 3.03 (0.94) | 2.34 (1.17) | .01 |
| cingulate cortex | 2.6 (1.5) | 0.7 (1.2) | <.001 | 2.8 (1.0) | 2.0 (1.1) | .002 |
| temporal cortex | 1.9 (1.4) | 0.5 (1.0) | <.001 | 1.9 (1.1) | 1.1 (1.1) | .003 |
| frontal cortex | 1.71 (1.29) | 0.33 (0.68) | <.001 | 1.50 (1.08) | 0.97 (0.64) | .02 |
| parietal cortex | 1.62 (1.26) | 0.37 (0.78) | <.001 | 1.45 (1.03) | 0.81 (0.74) | .005 |
| Total | 25 (13) | 6 (10) | <.001 | 26.8 (7.3) | 19.9 (7.3) | .001 |
| Plaques: | ||||||
| Total | 12.1 (2.4) | 12.5 (2.5) | .44 | 8.0 (5.6) | 4.2 (4.5) | .003 |
| frontal | 2.75 (0.44) | 2.73 (0.54) | .85 | 1.9 (1.2) | 1.0 (1.1) | .003 |
| temporal | 2.65 (0.53) | 2.72 (0.45) | .48 | 1.8 (1.2) | 1.1 (1.1) | .01 |
| parietal | 2.69 (0.51) | 2.76 (0.45) | .45 | 1.9 (1.2) | 1.0 (1.1) | .004 |
| hippocampus | 1.55 (0.84) | 1.82 (0.95) | .15 | 0.91 (0.92) | 0.29 (0.54) | .001 |
| transentorhinal cortex | 2.46 (0.64) | 2.43 (0.67) | .83 | 1.6 (1.2) | 0.9 (1.0) | .008 |
| Plaque density (neuritic plaque) | 2.62 (0.55) | 2.71 (0.46) | .33 | 1.8 (1.1) | 1.0 (1.0) | .003 |
| Tangles: | ||||||
| Total | 7.9 (4.3) | 10.0 (4.1) | .01 | 5.3 (2.7) | 3.6 (2.0) | .004 |
| frontal | 0.8 (1.1) | 1.4 (1.2) | .01 | 0.24 (0.39) | 0.06 (0.15) | .02 |
| temporal | 1.4 (1.1) | 2.0 (1.1) | .01 | 0.68 (0.71) | 0.29 (0.41) | .008 |
| parietal | 0.9 (1.1) | 1.3 (1.2) | .11 | 0.30 (0.53) | 0.03 (0.12) | .005 |
| hippocampus | 2.18 (0.98) | 2.56 (0.81) | .03 | 1.75 (0.02) | 1.41 (0.93) | .15 |
| transentorhinal cortex | 2.57 (0.66) | 2.80 (0.50) | .04 | 2.36 (0.68) | 1.80 (0.87) | .004 |
The diagnoses of AD and PD are not mutually exclusive. Patients with AD + PD would have a clinical diagnosis of AD with parkinsonism, and would meet neuropathological criteria for both conditions.
Mean values were compared using the two-sample t test.
Discussion
We examined neuropathological findings and selected clinical characteristics of 173 cases with clinicopathological diagnoses of Parkinson’s disease (PD), Alzheimer’s disease (AD), and/or dementia with Lewy bodies (DLB), and compared the findings of those with visual hallucinations (+VH) to those without VH (−VH). In accord with previously published work [5–8], a significant relationship was found between the extent of Lewy-type alpha-synucleinopathy (LTS) and the presence of VH in both PD and AD. A novel finding was the relationship between plaque and neurofibrillary tangle burden and the presence of VH in patients with PD. In addition, with DLB cases excluded, comorbidity of PD and AD was significantly more prevalent among subjects +VH than subjects −VH. In fact, some degree of AD pathology was present in most PD subjects +VH, even those who did not meet neuropathological criteria for AD or clinical/neuropathological criteria for DLB. These findings are consistent with the idea that both AD and PD neuropathology contribute to the pathogenesis of VH.
For LTS, findings in some cases were localized to olfactory bulb and tract, while in others had spread to involve the brainstem, cingulate cortex, temporal cortex, and parietal cortex. For plaques, findings were localized to hippocampus, frontal cortex, and parietal cortex. For tangles, findings were localized to transentorhinal and parietal cortex. In previous studies of patients with PD with VH, higher Lewy body densities were found in neocortex [5–7], but also in limbic structures [5–8].
This is the first study using semi-quantitative neuropathological methods to confirm the observation that LTS is implicated when VH arise in AD subjects, as suggested by McShane and colleagues [10]. Although it is known that limbic Lewy body pathology often is found at autopsy in patients with sporadic AD [11, 14], past studies of VH generally have not included subjects with AD. This is also the first study to demonstrate that AD pathology is associated with VH in PD subjects, possibly consistent with an additive pathological effect analogous to that proposed for dementia in this population [32].
With respect to clinical findings, the presence of VH was associated with more advanced PD (as reflected by higher Hoehn & Yahr stages) and with higher levodopa equivalent medication dosages. These findings confirm those of earlier investigations [6, 33]. It has previously been noted that, although these factors are associated with VH, they are not sufficient to explain their occurrence [34].
One clinical implication of our findings is that incident VH may be an indicator of underlying pathological milestones. For example, when a patient with AD develops VH, it may be that LTS has developed, and when a patient with PD develops VH, it may be that plaques and/or neurofibrillary tangles have developed. Thus, the occurrence of VH could be predictive of concomitant AD/PD pathology even when criteria are not met for a second diagnosis.
The findings also help to address the question of why it would be that VH become more prevalent as PD progresses. This observation has been difficult to reconcile with the dopamine excess hypothesis of hallucinations – if indeed it applies to hallucinations in neurodegenerative disease – because dopamine is progressively depleted in the course of PD. As noted above, it is now understood that increasing doses of dopaminergic medications do not fully explain this observation [34]. Supervening AD pathology could provide the explanation.
Strengths of this study include the large sample size, the comprehensive and standardized protocol for clinical and neuropathological evaluation, and the fact that only subjects with clearly documented visual hallucinations were included. Both delusions and other types of visual hallucinations – “presence” or “passage” hallucinations, for example – might arise from different pathological substrates. Weaknesses of the study included the small sample of subjects with DLB and of hallucinators with AD-only for adequate investigation. In addition, information was not available to examine temporal aspects of VH in the context of PD or AD.
In summary, our findings suggest that both LTS and AD pathology - plaques and tangles – may contribute to the pathogenesis of VH. Incident VH may be an indicator of particular neuropathological milestones: the development of LTS in the AD patient, and of plaques and/or tangles in the PD patient.
Highlights.
In subjects with PD, plaques and tangles as well as Lewy-type synucleinopathy were associated with visual hallucinations.
With DLB excluded, PD/AD comorbidity was more prevalent in subjects with visual hallucinations than those without.
Findings suggest that both AD and PD neuropathology contribute to the pathogenesis of visual hallucinations.
Incident VH could be predictive of concomitant AD/PD pathology, even when criteria are not met for a second diagnosis.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9:417–23. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- 2.Fenelon G, Mahieux F, Huon R, Ziegler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123 ( Pt 4):733–45. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- 3.Barnes J, David AS. Visual hallucinations in Parkinson’s disease: a review and phenomenological survey. J Neurol Neurosurg Psychiatry. 2001;70:727–33. doi: 10.1136/jnnp.70.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballard C, McKeith I, Harrison R, O’Brien J, Thompson P, Lowery K, et al. A detailed phenomenological comparison of complex visual hallucinations in dementia with Lewy bodies and Alzheimer’s disease. Int Psychogeriatr. 1997;9:381–8. doi: 10.1017/s1041610297004523. [DOI] [PubMed] [Google Scholar]
- 5.Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002;125:391–403. doi: 10.1093/brain/awf033. [DOI] [PubMed] [Google Scholar]
- 6.Papapetropoulos S, McCorquodale DS, Gonzalez J, Jean-Gilles L, Mash DC. Cortical and amygdalar Lewy body burden in Parkinson’s disease patients with visual hallucinations. Parkinsonism & related disorders. 2006;12:253–6. doi: 10.1016/j.parkreldis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher DA, Parkkinen L, O’Sullivan SS, Spratt A, Shah A, Davey CC, et al. Testing an aetiological model of visual hallucinations in Parkinson’s disease. Brain. 2011;134:3299–309. doi: 10.1093/brain/awr225. [DOI] [PubMed] [Google Scholar]
- 8.Kalaitzakis ME, Christian LM, Moran LB, Graeber MB, Pearce RK, Gentleman SM. Dementia and visual hallucinations associated with limbic pathology in Parkinson’s disease. Parkinsonism & related disorders. 2009;15:196–204. doi: 10.1016/j.parkreldis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Zubenko GS, Moossy J, Martinez AJ, Rao G, Claassen D, Rosen J, et al. Neuropathologic and neurochemical correlates of psychosis in primary dementia. Archives of neurology. 1991;48:619–24. doi: 10.1001/archneur.1991.00530180075020. [DOI] [PubMed] [Google Scholar]
- 10.McShane R, Gedling K, Reading M, McDonald B, Esiri MM, Hope T. Prospective study of relations between cortical Lewy bodies, poor eyesight, and hallucinations in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1995;59:185–8. doi: 10.1136/jnnp.59.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jellinger KA. Alpha-synuclein pathology in Parkinson’s and Alzheimer’s disease brain: incidence and topographic distribution--a pilot study. Acta neuropathologica. 2003;106:191–201. doi: 10.1007/s00401-003-0725-y. [DOI] [PubMed] [Google Scholar]
- 12.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta neuropathologica. 2009;117:613–34. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braak H, Braak E. Cognitive impairment in Parkinson’s disease: amyloid plaques, neurofibrillary tangles, and neuropil threads in the cerebral cortex. Journal of neural transmission Parkinson’s disease and dementia section. 1990;2:45–57. doi: 10.1007/BF02251245. [DOI] [PubMed] [Google Scholar]
- 14.Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–97. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frigerio R, Fujishiro H, Ahn TB, Josephs KA, Maraganore DM, DelleDonne A, et al. Incidental Lewy body disease: do some cases represent a preclinical stage of dementia with Lewy bodies? Neurobiology of aging. 2011;32:857–63. doi: 10.1016/j.neurobiolaging.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–45. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 18.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 19.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4. 4. Washington, D.C: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Massano J, Bhatia KP. Clinical approach to Parkinson’s disease: features, diagnosis, and principles of management. Cold Spring Harbor perspectives in medicine. 2012;2:a008870. doi: 10.1101/cshperspect.a008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 23.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Archives of neurology. 1999;56:33–9. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 24.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 27.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2010;25:2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 28.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 29.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 30.Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, et al. Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol. 2008;210:409–20. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiology of aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- 32.Kempster PA, O’Sullivan SS, Holton JL, Revesz T, Lees AJ. Relationships between age and late progression of Parkinson’s disease: a clinico-pathological study. Brain. 2010;133:1755–62. doi: 10.1093/brain/awq059. [DOI] [PubMed] [Google Scholar]
- 33.Saint-Cyr JA, Taylor AE, Lang AE. Neuropsychological and psychiatric side effects in the treatment of Parkinson’s disease. Neurology. 1993;43:S47–52. [PubMed] [Google Scholar]
- 34.Holroyd S, Currie L, Wooten GF. Prospective study of hallucinations and delusions in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2001;70:734–8. doi: 10.1136/jnnp.70.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]