Abstract
Background and Purpose
Smoking greatly increases the risk of atherosclerotic plaque and the effect may vary from individual to individual. A genome-wide scan was performed for smoking×single nucleotide polymorphism (SNP) interactions on carotid plaque burden (CPB) to identify the potential genetic moderators in Hispanics.
Methods
Carotid B-mode ultrasonography and genotyping by the Affymetrix 6.0 chip were performed in a discovery sample of 665 Caribbean Hispanics, followed by replication analyses in 264 Caribbean Hispanics. CPB was expressed as the sum of plaque areas over the segments in common and internal carotid arteries and bifurcation. Smoking was classified as 0, <20, and ≥20 cigarette pack-years. Assuming an additive genetic model, regression analysis was conducted to test for smoking×SNP interaction on the cube root transformed CPB while controlling for age, sex, and the top 3 principal components of ancestry.
Results
Two SNPs showed a significant interaction with smoking on CPB with the similar effects in both discovery (P<1.0e−5) and replication (P<0.05) populations. Specifically, for SNP rs10205487 within MXD1, more smoking was significantly associated with greater CPB in A allele carriers (beta±SE: 0.24±0.08, P=0.005 in AG carriers; beta±SE: 0.48±0.12, P=0.0002 in AA carriers) but not in GG (P=0.06). For SNP rs7001413 within LY96 and JPH1, more smoking was significantly associated with greater CPB in GG carriers (beta±SE: 0.24±0.06, P=6.8e−5) but not in T carriers (P=0.06).
Conclusions
Our study suggests that genetic variants may modulate the effect of smoking on CPB and highlights several genes for further investigation of their role in atherosclerosis, especially in smoking population.
Keywords: Smoking, Carotid plaque, Smoking-gene Interaction, Atherosclerosis, Hispanics, Carotid ultrasonography
1. Introduction
Atherosclerosis is a chronic and multifactorial process underlying most ischemic strokes (IS) and myocardial infarctions (MI), the leading causes of disability and death in the Western countries [1,2]. Numerous studies have clearly established that intermediate markers of subclinical atherosclerosis may be useful in risk prediction of clinical vascular events [3–5]. These markers reflecting biological and genetic different phenotypes of atherosclerosis [6] include artery flow-mediated dilation, arterial stiffness, carotid intima-media thickness (cIMT), coronary calcification, carotid plaque, and stenosis. Recently, the Tromsø Study, in a research conducted in 3,240 men and 3,344 women, demonstrated as total plaque area appears to be a stronger predictor than cIMT for IS [7]. Moreover, compared to other methods such as measurement of cIMT, carotid plaque burden (CPB), as the sum of plaque area, has been indicated as the strongest cross-sectional predictor of coronary artery calcium score suggesting its clinical utility as predictor of future cardiovascular events [8]. Given that CPB reflects distinct biological and genetic aspects of atherogenesis comparing to other markers of atherosclerosis [9], evaluation of these individuals may reduce heterogeneity and facilitate discovery of novel genetic variants that influence susceptibility to atherosclerosis.
Despite several decades of efforts, there were still 21.2% of men and 17.5% of women who continued to be cigarette smokers among US adults in 2010; and more importantly, overall, 26% of students in grades 9 through 12 reported current tobacco use [2]. Globally, the number of smokers even continues to increase and is estimated to be 1.7 billion by 2025 [10]. Experimental, epidemiological and clinical data strongly implicate that cigarette smoking is one of the major modifiable risk factors for cardiovascular disease (CVD) and IS, and impacts all phases of atherosclerosis [2,11]. Smoking can initiate and accelerate atherosclerosis either directly or indirectly by multiple mechanisms such as causing endothelial dysfunction and vascular smooth muscle cell (VSMC) proliferation in the artery wall, increasing lipid peroxidation and free-radical oxidation stress (ROS) and altering the procoagulant status [11].
Population, family and twin studies have demonstrated that atherosclerosis and stroke are under substantial genetic control [6]. However, the associated genetic variants found in candidate gene or genome-wide association studies (GWAS) just account for a small proportion of variation in the atherosclerosis phenotypes [6]. Part of the difficulty in identifying the associated genes could reflect biological interaction between risk alleles and exposure to environmental risk factors. Recently, in the Northern Manhattan Study (NOMAS) cohort population we demonstrated BTB (POZ) domain containing protein 1 (RCBTB1) gene as a modifier for smoking effect on cIMT, further supporting the hypothesis that including gene-environment interaction can help identify genes that may be missed in genome-wide association studies [12].
Given that cigarette smoking is one of well-established risk factors, the degree of the cigarette smoking-induced damage varies from individual to individual [13], and carotid plaque is considered a genetic and biologic distinct subclinical phenotype of atherosclerosis compared to cIMT [14], we conducted a genome-wide interaction study (GWIS) to scan for smoking × single nucleotide polymorphism (SNP) interactions on CPB and identify the potential genetic moderators.
2. Materials and Methods
2.1. Study population
Subjects used in this study are nested with the population-based NOMAS, which has been described extensively before [15,16]. In brief, NOMAS participants had never been diagnosed with a stroke, were at least 40 years of age, and resided for at least 3 months in a household with a telephone in Northern Manhattan. Within NOMAS, all Hispanic subjects who had high resolution B-mode ultrasound measurement of CPB and genotype available were used for the study (N=929) (Table 1). Demographic, socioeconomic and risk factor data were collected through direct interview based on the NOMAS instruments. All subjects provided informed consent and the study was approved by the Institutional Review Boards of Columbia University and University of Miami.
Table 1.
Characteristics of Discovery and Replication Hispanic Samples
| Hispanic Discovery (N=665) | Hispanic Replication (N=264) | P | |
|---|---|---|---|
| Age, years, mean±SD | 68 ± 8 | 69 ± 8 | 0.032 |
| Sex, n (%) | 0.217 | ||
| Male | 248 (37.3) | 110 (41.7) | |
| Female | 417 (62.7) | 154 (58.3) | |
| Smoking pack-years, n (%) | 0.089 | ||
| 0 | 342 (51.4) | 144 (54.5) | |
| 1-<20 | 217 (32.6) | 68 (25.8) | |
| ≥20 | 106 (15.9) | 52 (19.7) | |
| Carotid plaque area, mm3, median (IQR) | 2.9 (0–12.0) | 3.3 (0–14.1) | 0.519* |
| Carotid plaque area, cubic root transformed, mean±SD | 1.3 ± 1.3 | 1.3 ± 1.4 | 0.552 |
Based on Wilcoxon–Mann–Whitney test.
2.2. Carotid Plaque Phenotypes
High-resolution B-mode 2-dimensional ultrasound was performed for the examination of carotid plaque according to the standard scanning and reading protocols [3]. Carotid bifurcation and internal and common carotid arteries were examined for plaque defined as an area of focal wall thickening >50% greater than surrounding wall thickness in millimeters. Once plaques were detected, in-depth imaging of plaques was performed in long axes and multiple angles. The optimized and normalized images were analyzed offline by automated computerized edge detection system M’Ath (Intelligence in Medical Technologies, Inc, Paris, France) and area of each plaque was measured. The sum of all plaque areas (mm2) within each subject was calculated and expressed as a total carotid plaque area (CPB) [17].
2.3. Genotyping and Quality Control
Through the two waves of whole-genome genotyping (665 in the first wave and 264 in the second wave), all subjects included in the current study were genotyped using the Human SNP Array 6.0 chip (AffyMetrix) at the Genotyping Core of the Hussman Institute for Human Genomics (HIHG) at University of Miami following manufacturer’s instruction. Extensive quality control at both sample and SNP levels were carried out to ensure the integrity of the genotype data. Samples were excluded if they had call rates below 95%, relatedness, gender discrepancies, or were outliers beyond six standard deviations from the mean based on Eigenstrat analysis [18]. SNPs with severe deviation from Hardy-Weinberg equilibrium (p<10−6) or a genotyping call rate less than 95% were also removed using PLINK 1.05 [19].
2.4. Data Analysis
For sample characteristics, continuous variables were summarized as means with standard deviations and compared with t tests or with Mann–Whitney–Wilcoxon test if not normally distributed, whereas categorical variables were presented as percentages and compared with Chi-squared tests. In the genome-wide analysis of discovery Hispanic sample, the interaction between each SNP and smoking cigarette pack-years was evaluated using a linear regression model given in the following equation: . Here, y is the level of CPB (after cubic root transformation), G (G=0, 1 or 2) is the genotype of a SNP assuming an additive genetic effect, S (S=0, 1, 2 for never smoking, cigarette pack-years <20 and ≥20, respectively) is the status of cigarette smoking pack-years, GS is the product of the genotype and category of smoking, and Ck is the kth covariate (including age, sex and the top 3 eigenvectors of ancestry derived from principal component analysis with EIGENSTRAT) [18]. In the model, we tested if βgs=0 against a two-sided alternative hypothesis and performed the analysis using PLINK [19]. In the replication analysis, the interaction between each SNP showing an interaction with a P value less than 1×10−5 and smoking cigarette pack-years was evaluated using the same linear regression model as in the discovery analysis and was performed in Hispanic replication sample. To show the modification effects of the SNPs of interest in the combined Hispanic sample, linear regression model was used to estimate smoking effect on CPB stratified by the genotype and adjusted for the same covariates using SAS version 9.3 (SAS Institute Inc., Cary, NC).
3. Results
3.1. GWIS in the Discovery Sample
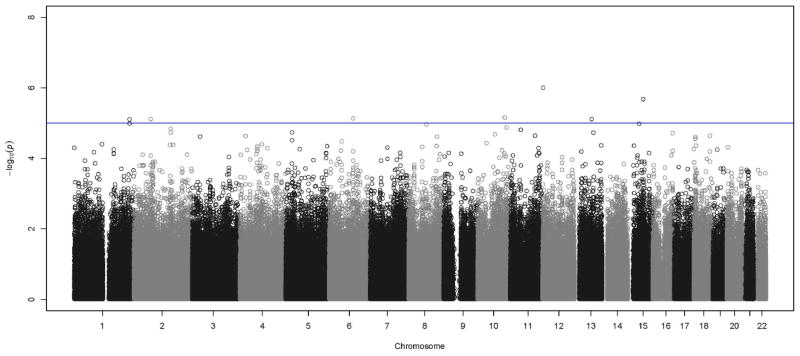
After QC, 722,379 SNPs were available in the discovery stage. The characteristics for discovery and replication Hispanic samples are reported in Table 1. Compared with the discovery sample, the replication sample had similar distribution of sex, smoking status and CPB, but was, on average, one year older. Figure 1 is the Manhattan plot displaying the p values for the interaction effect between each SNP and cigarette smoking on CPB. The quantile-quantile (QQ) plot of the expected vs. observed genome-wide interaction P values suggests no inflated type I (Supplementary Figure 1). The most significant interaction was found at SNPs on chromosome 12 in the CACNA1C (calcium channel, voltage-dependent, L type, alpha 1C subunit) gene (P<1.0×10−6). We carried forward 11 SNPs that have an interaction p value less than 1×10−5 (Table 2) in the discovery stage.
Figure 1.
Manhattan plot showing genome-wide interaction P values (−log10) of single nucleotide polymorphisms (SNPs) with smoking on carotid plaque burden (CPB) in Hispanic discovery sample. Horizontal line indicates the threshold for suggestive significance (P=1.0e−5) for replication.
Table 2.
Top SNPs Showing an Interaction with Smoking on Carotid Plaque Area In Discovery Sample
| SNP | Chr | BP | Minor/Major Allele | Discovery
|
Replication
|
Combined
|
Nearby Gene | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAF | Beta ± SE | P | MAF | Beta ± SE | P | MAF | Beta±SE | P | |||||
| rs6700792 | 1 | 231170013 | C/T | 0.43 | 0.41±0.09 | 1.0E-05 | 0.46 | 0.22±0.15 | 0.149 | 0.44 | 0.36±0.08 | 6.9E-06 | FAM89A |
| rs4074493 | 1 | 231172281 | T/C | 0.43 | 0.42±0.09 | 7.9E-06 | 0.45 | 0.20±0.15 | 0.182 | 0.43 | 0.36±0.08 | 6.6E-06 | FAM89A |
| rs10205487 | 2 | 70151471 | A/G | 0.45 | 0.43±0.10 | 7.7E-06 | 0.45 | 0.29±0.14 | 0.041 | 0.45 | 0.38±0.08 | 2.3E-06 | MXD1 |
| rs2894889 | 6 | 100161940 | T/A | 0.41 | −0.41±0.09 | 7.4E-06 | 0.46 | −0.05±0.14 | 0.736 | 0.42 | −0.28±0.08 | 2.2E-04 | PRDM13||MCHR2 |
| rs7001413 | 8 | 75010233 | T/G | 0.07 | −0.74±0.17 | 1.1E-05 | 0.08 | −0.48±0.24 | 0.046 | 0.08 | −0.63±0.14 | 5.0E-06 | LY96||JPH1 |
| rs7901717 | 10 | 113593918 | T/C | 0.38 | −0.48±0.11 | 7.0E-06 | 0.35 | −0.07±0.15 | 0.649 | 0.37 | −0.34±0.09 | 1.1E-04 | GPAM |
| rs215986 | 12 | 2709880 | T/C | 0.21 | 0.54±0.11 | 1.0E-06 | 0.26 | 0.04±0.19 | 0.819 | 0.22 | 0.39±0.09 | 4.2E-05 | CACNA1C |
| rs216008 | 12 | 2721137 | T/C | 0.20 | 0.53±0.11 | 9.8E-07 | 0.25 | 0.06±0.19 | 0.761 | 0.22 | 0.40±0.09 | 2.7E-05 | CACNA1C |
| rs17087142 | 13 | 71014985 | A/G | 0.08 | −0.64±0.14 | 7.7E-06 | 0.09 | 0.17±0.27 | 0.517 | 0.09 | −0.46±0.13 | 3.3E-04 | ATXN8OS||DACH1 |
| rs7182209 | 15 | 46132501 | C/T | 0.12 | −0.58±0.13 | 1.0E-05 | 0.11 | −0.27±0.22 | 0.224 | 0.11 | −0.50±0.11 | 9.2E-06 | SQRDL||SEMA6D |
| rs16945167 | 15 | 62790756 | T/A | 0.07 | −0.80±0.17 | 2.1E-06 | 0.05 | 0.35±0.36 | 0.337 | 0.07 | −0.57±0.15 | 2.0E-04 | MGC15885 |
3.2. Replication in an Independent Sample
Table 2 reports interactions between the top 11 SNPs identified in the discovery sample in the replication Hispanic data set. Among those, two SNPs have nominal interaction P values less than 0.05. Specifically, interaction was found for SNP rs10205487 within MXD1 (MAX dimerization protein 1) gene (P<8.0×10−6 in Hispanic discovery sample; P=0.041 in the replication Hispanic dataset); and for SNP rs7001413 located within LY96 (lymphocyte antigen 96) gene and JPH1 (junctophilin 1) gene (P<1.0×10−5 in Hispanic discovery sample; P=0.046 in replication Hispanic dataset).
3.3. Stratified Analysis in the Combined Sample
To explore the modification effects of the two replicated SNPs, we examined the relationship between smoking and CPB in the combined sample stratified by the genotype of the two SNPs. As shown in Table 3, for SNP rs10205487 within MXD1, more smoking was significantly associated with greater CPB in A allele carriers (beta±SE: 0.24±0.08, P=0.005 in AG carriers; beta±SE: 0.48±0.12, P=0.0002 in AA carriers) but tended to show no effect in GG carriers (beta±SE: −0.21±0.11, P=0.06). For SNP rs7001413 within LY96 and JPH1, more smoking was significantly associated with greater CPB in GG carriers (beta±SE: 0.24±0.06, P=6.8e-5) but tended to show no effect in TG carriers (beta±SE: −0.21±0.11, P=0.06).
Table 3.
Smoking Effect on CPB, Stratified by the Genotypes at rs10205487 and rs7001413
| SNP | Genotype | N | Beta ± SE* | P |
|---|---|---|---|---|
| rs10205487 | GG | 261 | −0.21 ± 0.11 | 0.06 |
| AG | 452 | 0.24 ± 0.08 | 0.005 | |
| AA | 164 | 0.48 ± 0.12 | 0.0002 | |
| rs7001413 | GG | 791 | 0.24 ± 0.06 | 6.8E-05 |
| TG | 131 | −0.33 ± 0.17 | 0.06 | |
| TT | 5 | N/A | --- |
Estimated effect for smoking pack-years adjusted for age, sex and the top 3 PCAs.
4. Discussion
Atherosclerosis is a complex and multifactorial disease caused by the combination of vascular risk factors, environment and genetic factors. Experimental researches using both linkage and candidate gene association studies identified several genes that potentially play a role in the processes leading to atherosclerosis in human [6,14]. Currently, however, genetic studies on atherosclerosis have not reached consistent results. In the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) study, which consists of four prospective epidemiological cohorts of nearly 19,600 subjects, just two independent loci achieved the genome-wide significance threshold in the combined meta-analysis of carotid plaque [20]. Different issues may account for the inconsistent findings, among those one of the main limitations is represented by genetic–environment (vascular risk factors) interaction that may influence the impact of genetics on different atherosclerotic phenotypes.
Cigarette smoking is an important risk factor for atherosclerosis [11]. However, recently using NOMAS we demonstrated that the variation in CPB is largely unexplained by traditional and less traditional vascular risk factors, including smoking, suggesting that other unaccounted environmental and genetic factors play an important role in the determination of atherosclerotic plaque [17]. By performing genome-wide gene-smoking interaction study, here we identified novel genetic variants that modify the association between smoking and atherosclerosis. Using a multi-stage design, we found MXD1, LY96, and JPH1 as potential modifier genes for smoking’s effect on inter-individual variance on CPB, which were replicated in an independent Hispanic dataset.
MXD1 encodes a member of the MYC/MAX/MAD network of basic helix-loop-helix leucine zipper transcription factors. This network mediates cellular proliferation, differentiation and apoptosis through different mechanisms including telomerase silencing and activation [21]. Its deregulation is an important aspect of Myc’s ability to stimulate tumor formation such as leukemia [22]. In fact, MXD1 is designated as antagonizer of myc transcriptional activity. The advanced stages of atherosclerosis, similarly to cancer, are characterized by a local increase in tissue mass that may be hard to control. Myc has been involved in both endothelial dysfunction and atherogenesis as well [23]. Therefore, different MXD1 genetic variants may have diverse ability in inhibit myc-dependent signaling by playing opposed roles in mechanisms leading to atherosclerosis such as carotid plaque. Moreover, regulation of myc has been strongly associated in experimental studies with carcinogenesis induced by cigarette smoke [24].
LY96 encodes a protein which provides a link between the toll-like receptor (TLR) 4 and lipopolysaccyaride (LPS) signaling. TLR4 and LPS have been strongly associated with tissue inflammatory mechanisms related to smoking [25]. Endothelial cells are activated through TLRs to express inflammatory mediators implicating in both acute and chronic inflammatory states such atherosclerosis. TLR4 is not only located intracellularly but also functions intracellularly and internalization of LPS is required for activation. In human coronary artery endothelial cells it has been demonstrated as LPS uptake may happen only in the presence of LPS binding proteinencoded by LY96 [26]. Therefore, LY96 polymorphisms may be relevant to initiating inflammatory responses in the vasculature finally leading to atherosclerosis. SNP rs7001413 is also located near to JPH gene which is a member of the junctophilin gene family and encodes for junctional complexes located between the plasma membrane and endoplasmic/sarcoplasmic reticulum which mediates cross talk between cell surface and intracellular ion channels. Junctophilin proteolysis has been shown to contribute to skeletal muscle weakness and cardiac dysfunction in a range of circumstances such as ischemia-reperfusion [27]. In fact, JPH1 knockout mice show no milk suckling and die shortly after birth [28]. Interesting, a study investigating gene-disease association and gene-environment interaction, after GWAS conducted in 479 smokers, included JPH1 in a group of genomic markers associated with successful quitting from cigarette smoking [29].
Since today, unfortunately no information is available regarding function of FAM89A gene. We just know that its homologous in rat plays important roles in maintaining normal eye function and disease [30]. Further studies are needed to clarify the role of the protein encoded by this gene in human physiology and pathology.
In a recent study conducted in the same NOMAS samples by using GWIS analysis we demonstrated a strong interaction between RCBTB1 gene and smoking in modifying risk for cIMT [12]. In the present study, which is aimed to investigate risk in carotid plaque, the lack in the replication data regarding genetic interactions with smoking previously found in cIMT study may further support the hypothesis suggesting as plaque and cIMT are two distinct phenotypes of atherosclerosis under different genetic and environmental control. Genome-wide gene-smoking interaction study allows us to catch up interesting genes associated with subclinical markers of atherosclerosis, although the gene-environment investigation further increases inter-subject variability to the disease. So far the studies that analyzed the interactions between cigarette smoking and gene variants in relation to risks for vascular disease did not reach consistent results. Cigarettes can modify genes quantitatively by changing expression levels and qualitatively by causing DNA mutations [31]. Mainly, Wang XL et al. [31] summarized these results as: “although relationships between increased exposure to cigarette smoking or genetic risk and vascular disease can be expected to be linear, the relationships are significantly modified when cigarette and genes interact in real life”. In fact, various animal models have been exposed to cigarette smoking to observe smoking effects on atherogenesis and most of them have shown accelerated atherogenesis under smoke influence. However, none of the studied genes in these animals are specific and direct targets of cigarette smoking [31]. A possible reason could be just the difficulty in reproducing the real-life human effect of cigarette smoking in which lungs process smoke toxins before they get to the target via the blood stream. Of particular interest may be to analyze the interaction between smoking effect and genetic variants associated with endothelial dysfunction [32] as precursor of overt atherosclerosis that may allow anticipating the possible biases present when the disease already exist in the vessel, such as the carotid plaque. Moreover, further studies could be important to understand the impact of smoking/genetics interactions in different circulatory systems such as coronary microcirculation with a specific blood flow hemodynamic and diverse genetic variants associated with risk for atherosclerosis [33,34]. Therefore, to continue to explore gene-smoking interaction in relationship with vascular disease such as atherosclerosis will help us in better understanding not just the harmful effect of this noxious vicious but also it would serve as a good model system in understanding gene-environment interaction and its implications to different health problems.
We acknowledge several limitations in the current study. First, only common tagging SNPs were used for the genome-wide interaction study. Such approach is cost-efficient for screening large numbers of genes and individuals by capturing the majority of common variants in the population. Additional follow up studies, however, are often required to identify the functional variants responsible for the detected interaction. Second, the replication data sets are small. Lack of significance in replication sample might be due to small sample size. Additional validation in larger data sets including samples with different race-ethnicities other than Hispanics are imperative. Moreover, further analyses including risk factors such as hypertension, diabetes mellitus, and dyslipidemia should be pursued to evaluate whether the observed interactions are independent of these risk factors.
In conclusion, using a non-biased genome-wide approach, we have identified novel genetic variants that may act as modifier for smoking effect on inter-individual variance on carotid plaque burden. Further studies are required to confirm and validate our results and to explore the biological effects of these intriguing interactions.
Supplementary Material
Quantile-quantile plot of genome-wide interaction results in Hispanic discovery sample.
Highlights.
Smoking increases risk of atherosclerotic and the effect vary among individuals.
Genome-wide scan was performed for smoking× SNP interactions on carotid plaque.
SNPs showed significant interaction with smoking on plaque burden in Hispanics.
Genetic variants may modulate the effect of smoking on atherosclerosis.
Acknowledgments
Sources of funding
This research was supported by grants from James & Esther King Biomedical Research Program (2KN01), the National Institute of Neurological Disorders and Stroke grants (R01NS065114, K24NS062737, R37NS029993), and Evelyn F. McKnight Brain Institute.
The authors are grateful to the study participants for their collaboration and to all staff of the Northern Manhattan Study for their energetic efforts to this study.
Footnotes
Author Contributions
David Della-Morte and Chuanhui Dong: conception and design of the study, analysis of data, drafting the article, and revising the manuscript.
Chuanhui Dong and Ashley Beecham: statistical analysis of data.
Liyong Wang, Susan H. Blanton, Hongyu Zhao, Ralph L. Sacco, Tatjana Rundek: conception and design of the study, analysis of data, and revising the manuscript.
Disclosure
The authors have no conflicts of interest to disclose regarding this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep. 2009;11(1):21–27. doi: 10.1007/s11886-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics--2013 Update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology. 2008;70(14):1200–1207. doi: 10.1212/01.wnl.0000303969.63165.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Touboul PJ, Labreuche J, Vicaut E, Amarenco P. Carotid intima-media thickness, plaques, and Framingham risk score as independent determinants of stroke risk. Stroke. 2005;36(8):1741–1745. doi: 10.1161/01.STR.0000174490.23495.57. [DOI] [PubMed] [Google Scholar]
- 5.Prabhakaran S, Singh R, Zhou X, Ramas R, Sacco RL, Rundek T. Presence of calcified carotid plaque predicts vascular events: the Northern Manhattan Study. Atherosclerosis. 2007;195(1):e197–201. doi: 10.1016/j.atherosclerosis.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Della-Morte D, Guadagni F, Palmirotta R, Testa G, Caso V, Paciaroni M, et al. Genetics of ischemic stroke, stroke-related risk factors, stroke precursors and treatments. Pharmacogenomics. 2012;13(5):595–613. doi: 10.2217/pgs.12.14. [DOI] [PubMed] [Google Scholar]
- 7.Mathiesen EB, Johnsen SH, Wilsgaard T, Bonaa KH, Lochen ML, Njolstad I. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the Tromso Study. Stroke. 2011;42(4):972–978. doi: 10.1161/STROKEAHA.110.589754. [DOI] [PubMed] [Google Scholar]
- 8.Sillesen H, Muntendam P, Adourian A, Entrekin R, Garcia M, Falk E, et al. Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc Imaging. 2012;5(7):681–689. doi: 10.1016/j.jcmg.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Spence JD. Measurement of intima-media thickness vs. carotid plaque: uses in patient care, genetic research and evaluation of new therapies. Int J Stroke. 2006;1(4):216–221. doi: 10.1111/j.1747-4949.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 10.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Rundek T, Beecham A, Hudson B, Blanton SH, Zhao H, et al. Genome-Wide Interaction Study Identifies RCBTB1 as a Modifier for Smoking Effect on Carotid Intima-Media Thickness. Arterioscler Thromb Vasc Biol. 2014;34(1):219–25. doi: 10.1161/ATVBAHA.113.302706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis. 2003;46(1):91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 14.Della-Morte D, Guadagni F, Palmirotta R, Testa G, Caso V, Paciaroni M, et al. Genetics of ischemic stroke, stroke-related risk factors, stroke precursors and treatments. Pharmacogenomics. 2012;13(5):595–613. doi: 10.2217/pgs.12.14. [DOI] [PubMed] [Google Scholar]
- 15.Sacco RL, Boden-Albala B, Abel G, Lin IF, Elkind M, Hauser WA, et al. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001;32(8):1725–1731. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 16.Dong C, Beecham A, Wang L, Slifer S, Wright CB, Blanton SH, et al. Genetic loci for blood lipid levels identified by linkage and association analyses in Caribbean Hispanics. J Lipid Res. 2011;52(7):1411–1419. doi: 10.1194/jlr.P013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo F, Gardener H, Dong C, Cabral D, Della-Morte D, Blanton SH, et al. Traditional cardiovascular risk factors explain the minority of the variability in carotid plaque. Stroke. 2012;43(7):1755–1760. doi: 10.1161/STROKEAHA.112.651059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 19.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bis JC, Kavousi M, Franceschini N, Isaacs A, Abecasis GR, Schminke U, et al. Meta-analysis of genome-wide association studies from the CHARGE consortium identifies common variants associated with carotid intima media thickness and plaque. Nat Genet. 2011;43(10):940–947. doi: 10.1038/ng.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge Z, Li W, Wang N, Liu C, Zhu Q, Bjorkholm M, et al. Chromatin remodeling: recruitment of histone demethylase RBP2 by Mad1 for transcriptional repression of a Myc target gene, telomerase reverse transcriptase. FASEB J. 2010;24(2):579–586. doi: 10.1096/fj.09-140087. [DOI] [PubMed] [Google Scholar]
- 22.Luscher B. Function and regulation of the transcription factors of the Myc/Max/Mad network. Gene. 2001;277(1–2):1–14. doi: 10.1016/s0378-1119(01)00697-7. [DOI] [PubMed] [Google Scholar]
- 23.de Nigris F, Sica V, Herrmann J, Condorelli G, Chade AR, Tajana G, et al. c-Myc oncoprotein: cell cycle-related events and new therapeutic challenges in cancer and cardiovascular diseases. Cell Cycle. 2003;2(4):325–328. [PubMed] [Google Scholar]
- 24.Jin Z, Gao F, Flagg T, Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through phosphorylation in regulating cell survival and proliferation. J Biol Chem. 2004;279(38):40209–40219. doi: 10.1074/jbc.M404056200. [DOI] [PubMed] [Google Scholar]
- 25.Comer DM, Kidney JC, Ennis M, Elborn JS. Airway epithelial cell apoptosis and inflammation in COPD, smokers and nonsmokers. Eur Respir J. 2013;41(5):1058–1067. doi: 10.1183/09031936.00063112. [DOI] [PubMed] [Google Scholar]
- 26.Dunzendorfer S, Lee HK, Soldau K, Tobias PS. Toll-like receptor 4 functions intracellularly in human coronary artery endothelial cells: roles of LBP and sCD14 in mediating LPS responses. FASEB J. 2004;18(10):1117–1119. doi: 10.1096/fj.03-1263fje. [DOI] [PubMed] [Google Scholar]
- 27.Murphy RM, Dutka TL, Horvath D, Bell JR, Delbridge LM, Lamb GD. Ca2+-dependent proteolysis of junctophilin-1 and junctophilin-2 in skeletal and cardiac muscle. J Physiol. 2013;591(Pt3):719–729. doi: 10.1113/jphysiol.2012.243279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito K, Komazaki S, Sasamoto K, Yoshida M, Nishi M, Kitamura K, et al. Deficiency of triad junction and contraction in mutant skeletal muscle lacking junctophilin type 1. J Cell Biol. 2001;154(5):1059–1067. doi: 10.1083/jcb.200105040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rose JE, Behm FM, Drgon T, Johnson C, Uhl GR. Personalized smoking cessation: interactions between nicotine dose, dependence and quit-success genotype score. Mol Med. 2010;16(7–8):247–253. doi: 10.2119/molmed.2009.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed F, Torrado M, Zinovieva RD, Senatorov VV, Wistow G, Tomarev SI. Gene expression profile of the rat eye iridocorneal angle: NEIBank expressed sequence tag analysis. Invest Ophthalmol Vis Sci. 2004;45(9):3081–3090. doi: 10.1167/iovs.04-0302. [DOI] [PubMed] [Google Scholar]
- 31.Wang XL, Raveendran M, Wang J. Genetic influence on cigarette-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;45(5):361–382. doi: 10.1053/pcad.2003.11. [DOI] [PubMed] [Google Scholar]
- 32.Fedele F, Mancone M, Chilian WM, Severino P, Canali E, Logan S, et al. Role of genetic polymorphisms of ion channels in the pathophysiology of coronary microvascular dysfunction and ischemic heart disease. Basic Res Cardiol. 2013;108(6):387. doi: 10.1007/s00395-013-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fedele F, Severino P, Bruno N, Stio R, Caira C, D’Ambrosi A, et al. Role of ion channels in coronary microcirculation: a review of the literature. Future Cardiol. 2013;9(6):897–905. doi: 10.2217/fca.13.65. [DOI] [PubMed] [Google Scholar]
- 34.Davies RW, Wells GA, Stewart AF, Erdmann J, Shah SH, Ferguson JF, et al. A genome-wide association study for coronary artery disease identifies a novel susceptibility locus in the major histocompatibility complex. Circ Cardiovasc Genet. 2012;5(2):217–25. doi: 10.1161/CIRCGENETICS.111.961243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantile-quantile plot of genome-wide interaction results in Hispanic discovery sample.