Abstract
Mosquitoes possess an innate immune system that is capable of limiting infection by a variety of pathogens, including the Plasmodium spp. parasites responsible for human malaria. The Anopheles immune deficiency (IMD) innate immune signaling pathway confers resistance to Plasmodium falciparum. While some previously identified Anopheles anti-Plasmodium effectors are regulated through signaling by Rel2, the transcription factor of the IMD pathway, many components of this defense system remain uncharacterized. To begin to better understand the regulation of immune effector proteins by the IMD pathway, we used oligonucleotide microarrays and iTRAQ to analyze differences in mRNA and protein expression, respectively, between transgenic An. stephensi mosquitoes exhibiting blood meal-inducible overexpression of an active recombinant Rel2 and their wild-type conspecifics. Numerous genes were differentially regulated at both the mRNA and protein levels following induction of Rel2. While multiple immune genes were up-regulated, a majority of the differentially expressed genes have no known immune function in mosquitoes. Selected up-regulated genes from multiple functional categories were tested for both anti-Plasmodium and anti-bacterial action using RNA interference (RNAi). Based on our experimental findings, we conclude that increased expression of the IMD immune pathway-controlled transcription factor Rel2 affects the expression of numerous genes with diverse functions, suggesting a broader physiological impact of immune activation and possible functional versatility of Rel2. Our study has also identified multiple novel genes implicated in anti-Plasmodium defense.
Keywords: Mosquitoes, transgenesis, Rel2, transcriptome, proteome, innate immunity, Plasmodium
1. Introduction
Mosquitoes are vectors for many important human pathogens, including viruses, filarial worms, and apicomplexan parasites. A number of Plasmodium spp. parasites, vectored exclusively by Anopheles spp. mosquitoes, cause human malaria. Because of difficulties in the distribution of anti-malarial chemotherapeutics and the rise of drug resistance in the parasite, vector control remains at the forefront of malaria control efforts. However, after decades of insecticide spraying, bed net distribution, and habitat remodeling, the disease remains established, so novel vector-control methods must be developed. Recently, methods have been developed to generate genetically modified mosquitoes (Ito et al., 2002), and various strategies based on their release are being investigated for malaria control. Conversion of a natural mosquito population to a transgenic population that overexpresses anti-Plasmodium immune system activators or effector molecules could represent one such method, and multiple mosquito lines expressing such transgenes in different tissues have already been developed (Dong et al., 2011; Dong et al., 2012).
Mosquitoes possess an innate immune system that is capable of responding to, and controlling, infection by diverse pathogens, including bacteria, viruses, and apicomplexan parasites (Cirimotich et al., 2009; Garver et al., 2009). Two immune pathways, the Toll and immune deficiency (IMD) pathways, have been extensively studied in both Drosophila and mosquitoes. Both pathways recognize invading pathogens through the association of host pattern recognition receptors (PRR) with pathogen associated molecular patterns (PAMPs), leading to a signaling cascade, nuclear localization of transcription factors, and subsequent induction of the expression of numerous immune effector molecules and anti-microbial peptides. Invading pathogens are then killed by various mechanisms, such as phagocytosis and complement-like killing.
The nuclear translocation of the NF-κB transcription factor Rel2 leads to an induction of immune gene expression that constitutes the IMD pathway-mediated immune response (Meister et al., 2005). The IMD pathway has been shown to regulate the mosquito’s resistance to P. falciparum infection (Garver et al., 2009), and numerous mosquito lines with inducible overexpression of the constitutively active short form of Rel2 have been created (Dong et al., 2011). One such line (henceforth referred to as the CP15 line) uses the carboxypeptidase gene promoter to limit Rel2 overexpression to the midgut following a blood meal, while another line (the VG1 line) overexpresses the same Rel2 transgene under the control of the vitellogenin gene promoter, leading to fat body-specific expression after a blood meal (Dong et al., 2011). Both these lines exhibit a greatly reduced susceptibility to Plasmodium infection following an infected blood meal and may represent viable tools for future release as part of a malaria control program. However, immune pathways and their downstream transcription factors can regulate a large variety of both immunity and non-immunity related processes (Dong et al., 2006; Xi et al., 2008). Hence, the overexpression of the Rel2 transcription factor affects the immune system, but it is also likely to regulate other physiological processes entailing genes of diverse functions.
A majority of studies on the insect immune system have relied on infection with a pathogen and observation of the insect’s response to the insult (Imroze and Prasad, 2012; McKean et al., 2008; Zerofsky et al., 2005). Using transgenic mosquitoes that overexpress Rel2 in an inducible fashion provides a pathogen-independent system to study IMD pathway-regulated immune response and eliminates any confounding factors brought about by the presence of the infecting organism. Zou and colleagues used a Rel2-overexpressing Aedes aegypti to study the IMD pathway-regulated transcriptome (Zou et al., 2013).
We used whole-genome oligonucleotide microarrays to study recombinant Rel2-induced changes in mRNA abundance, as well as isobaric tags for relative and absolute quantitation (iTRAQ) to study changes in protein abundance after Rel2 overexpression in transgenic mosquitoes. Measuring the expression levels of both mRNA and protein allowed us to look for correlations between transcript and protein abundance following up-regulation of a transcription factor. We then used RNA interference (RNAi) assays to investigate a subset of genes, both with and without known immune function, for anti-Plasmodium and anti-bacterial activity, leading to the identification of multiple novel anti-Plasmodium effectors.
2. Materials and Methods
2.1 Mosquito rearing
A. stephensi Liston strain wild-type, CP, and VG transgenic Rel2-overexpressing lines (Dong et al., 2011) were maintained according to standard insectary procedures. In brief, larvae were reared at low densities in trays and fed a combination of ground fish flakes (Tetra) and cat food pellets (Purina). Upon emergence, adults were maintained on a 12 h/12 h light/dark cycle at 27° C with 80% humidity and constant access to a 10% solution of sucrose in water. In order to stimulate egg production, adults were fed on ketamine-anesthetized mice according to IACUC-approved protocols.
2.2 RNA extraction and microarrays
One-week-old adult female mosquitoes were given a human blood meal from water-jacketed membrane feeders maintained at 37° C. Mosquito tissues were dissected in sterile PBS as follows: midguts were collected at 6 and 12 h after blood feeding, while fat bodies were collected at 12 and 18 h after the blood meal. Total mosquito RNA from dissected tissues was extracted using RNeasy kits (Qiagen) according to the manufacturer’s protocols and quantified on a NanoDrop spectrophotometer before quality assessment on an Agilent Bioanalyzer 2100. Probes were synthesized using 200 ng of RNA and the Low-Input RNA Labeling Kit (Agilent) according to the manufacturer’s protocol. These probes were hybridized to a custom-designed Agilent microarray slide, which was scanned with an Axon GenePix 4200AL scanner at 2-μm resolution. After scanning, statistical analysis was performed using the TIGR, MIDAS, and TMEV software packages (Dudoit et al., 2003), following standard laboratory protocol (Dong et al., 2006), and analysis was performed using a t-test, with a significance level of α=0.05. Changes in gene expression were considered significant if the absolute value of the gene regulation was >=0.75 on a log2 scale. For each treatment, three biological replicates and one psuedoreplicate were performed. The array was designed using Array Designer software (Premier Biosoft, www.premierbiosoft.com) and based on an early version of the A. stephensi transcriptome obtained from Dr. Jake Tu of Virginia Polytechnic Institute and State University, and putative function and gene ontology (GO) terms were assigned to transcript sequences based on homology to previously annotated A. gambiae genes discovered by a blastn search (Altschul et al., 1990). The blast search was performed against gene set AgamP3.7, downloaded from vectorbase.org; for each gene, the most significant hit was used for annotation, with a maximum e-value of 0.0001 used as a cutoff. Any genes that did not have significant homology to any previously annotated An. gambiae genes were used for a blastn (Altschul et al., 1990) search against the non-redundant nucleotide database from NCBI to assign putative function if similar genes or conserved sequences were identified in other species. While the gene with the highest blast homology between An. stephensi and An. gambiae may not represent a true orthologue, this is our best prediction given the early state of the annotation of the An. stephensi genome. Seven genes were analyzed by qRT-PCR to verify the results of the microarray (Figure S4).
2.3 Protein extraction and iTRAQ
One-week-old adult female mosquitoes from the WT, CP, and VG lines were given a human blood meal from membrane feeders at 37° C. Prior to the blood meal and 24 h afterward, mosquitoes were dissected in sterile PBS and their midguts and fat bodies collected. Three replicates of 10 midguts or fat bodies were resuspended in lysis buffer (10 mM HEPES, 0.1 mM MgCl2, 0.1% Triton, and protease inhibitors [Roche]) and left on ice for 30 min. Tissues were homogenized, freeze-thawed twice, and centrifuged at 14,000 rpm for 30 min at 4°C. The supernatant fraction was collected and used as the total protein extract. Total protein concentration was measured by Bradford assay. Consistency among the three replicates was assessed by running 0.1 μg of total extract on a 4–12% Tris gel and silver staining. Replicates were then combined and used for iTRAQ analysis: 50 μg of protein was reduced using 2 μL of 50 mM TCEP for 1 h at 60° C, cooled to room temperature, and incubated with 1 μL of 100 mM MMTS for 15 min in the dark. Samples were then TCA-precipitated, and air-dried pellets were submitted to the Johns Hopkins School of Medicine Mass Spectrometry and Proteomics Facility for identification and relative quantitation using iTRAQ. The samples were trypsin-digested, labeled with 8plex iTRAQ reagents, fractionated by both strong cation exchange and reverse phase chromatography, and identified by mass spectrometry using a LTQ Orbitrap Velos mass spectrometer (Thermo Scientific) as described in detail (Guo et al., 2007; Martin et al., 2008; Pierce et al., 2008; Ross et al., 2004). MS/MS spectra were searched against a custom A. stephensi database generated from sequences provided by Dr. Jake Tu (Virginia Polytechnic Institute and State University) using Mascot (Matrix Science) and Proteome Discoverer (v1.2 Thermo Scientific) with the high peptide confidence filter. The resulting data were considered significant if the ratio of GM:WT protein levels was >0.75 on a log2 scale. Sequences obtained from the mass spectrometer were compared to previously annotated A. gambiae gene sequences using blastp (Altschul et al., 1990) in order to assign them putative functions and GO terms.
2.4 dsRNA-mediated gene silencing
Double-stranded RNAs (dsRNAs) targeting selected genes were synthesized from PCR products using the HiScribe T7 in vitro transcription kit (NEB). Adult female mosquitoes (3–4 days old) were anesthetized on ice and injected with 69 nl of 3 μg/μl dsRNA targeting a gene of interest or GFP as a control, then maintained under normal mosquito rearing conditions. At 3 days post-injection, groups of 10 mosquitoes were collected for silencing efficiency measurement using qRT-PCR. Primers used for the PCR amplification of oligos were designed using the Primer3 program (http://frodo.wi.mit.edu) and are listed in table S1. Silencing of the genes was verified by qRT-PCR 3 days post-injection (Figure S5).
2.5 Quantitative Real-Time PCR (qRT-PCR)
Total RNA used to verify gene knockdown was extracted from whole mosquitoes with their heads and legs removed, while total RNA used to verify the microarray results was collected from dissected midguts or fat bodies using a Qiagen RNEasy kit according to the manufacturer’s instructions. qRT-PCR was carried out using Sybr Green PCR Master Mix (ABI) on an ABI StepOnePlus Real-Time PCR System with the ABI StepOne Software. Transcript abundance was normalized to mosquito ribosomal protein S7 gene levels and the -fold change of each gene was calculated using the ΔΔct method. Primers were designed using the Primer3 program (http://frodo.wi.mit.edu) and are listed in table S1.
2.6 P. falciparum infections
P. falciparum challenges were performed according to a standard laboratory protocol: Three days after dsRNA injection, mosquitoes were fed on human blood containing NF54W strain P. falciparum gametocytes through a membrane feeder at 37° C. Unfed mosquitoes were discarded and mosquitoes were maintained as usual for 7 days, at which point their midguts were dissected and stained with 0.1% mercurochrome for oocyst enumeration by manual counting under a light microscope (Olympus).
2.7 Enumeration of midgut bacteria
The number of colony forming units (CFUs) of midgut bacteria for gene-silenced sugar or blood-fed mosquitoes was counted as previously described: Mosquitoes were surface-sterilized with ethanol and rinsed in PBS before their midguts were dissected into PBS. Midguts were then homogenized in PBS, and serial dilutions were plated onto LB agar plates and incubated at room temperature. Three days after plating, the number of colonies per plate was counted, and the total number of culturable bacteria per midgut was calculated. Samples were collected for sugar-fed mosquitoes 3 days after gene silencing, while other mosquitoes were provided a blood meal 3 days after gene silencing, with dissections being performed 24 h later.
2.8 Statistical Analyses
Statistical analyses were performed using the GraphPad Prism 5 software (Graphpad Software). The tests used are indicated in the Results section and figure captions.
3. Results and Discussion
3.1 Expression of active Rel2 leads to broad transcriptome and proteome changes
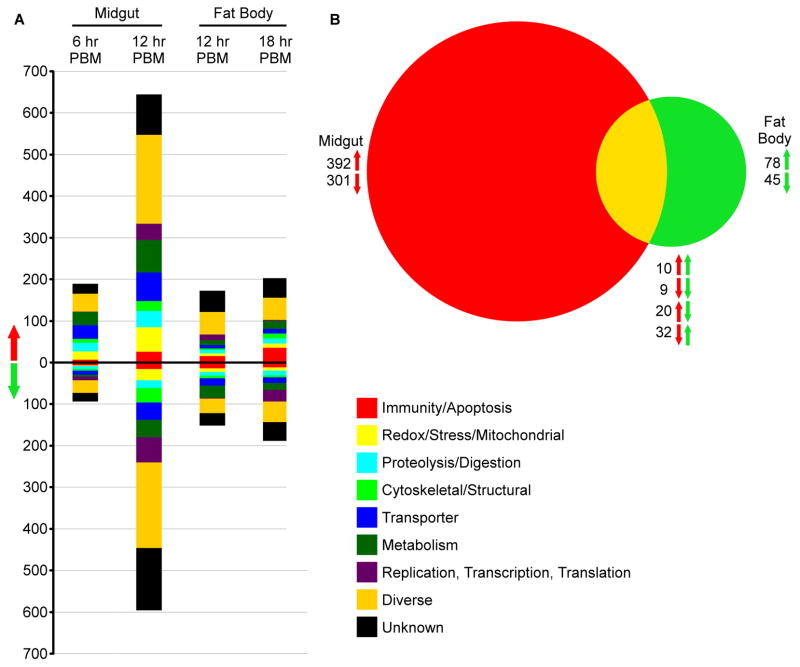
Using whole-genome microarrays, we compared the mRNA abundance of all predicted An. stephensi genes in transgenic Rel2-overexpressing (Dong et al., 2011) and WT mosquito lines at two time points following a blood meal in order to identify the Rel2-driven changes in the midgut and fat body transcriptomes. Mosquitoes with Rel2 expression driven by the midgut-specific carboxypeptidase promoter (CP line) were assayed at 6 and 12 h post-blood meal (PBM)), and samples with Rel2 expression driven by the fat body-specific vitellogenin promoter (VG line) at 12 and 18 h PBM. Selection of these time points was based on the recombinant Rel2 induction profile in the two tissues (Dong et al., 2011). At 6 h PBM, there were 190 up-regulated and 94 down-regulated genes in the midguts of CP line mosquitoes (Fig 1A), and these totals increased to 645 up-regulated and 596 down-regulated genes at 12 h (Fig 1A). In VG line mosquitoes, there were 173 up-regulated and 152 down-regulated genes in the fat body at 12 h PBM and 203 up-regulated and 189 down-regulated genes at 18 h (Fig 1A). This magnitude of gene regulation is similar to that observed in the fat bodies of Rel2 overexpressing Ae. aegypti mosquitoes, which upregulated 123 and downregulated 176 genes following Rel2 activation (Zou et al., 2011). A total of 71 transcripts were regulated by recombinant Rel2 activation in both the CP midgut and VG fat body, with 19 being regulated in the same direction at 12 h PBM (10 up-regulated in both tissues and 9 down-regulated in both tissues) and 20 being up-regulated in the midgut and down-regulated in the fat body; 32 displayed the opposite mRNA abundance pattern (Fig 1B). It is unlikely that a transcription factor would up-regulate and down-regulate the same gene in different tissues, so it is much more likely that the differences in expression pattern are related to interactions with other transcription factors, feedback loops, or mRNA or protein degradation rates. Because we were looking at global changes following Rel2 induction, we could not differentiate between an mRNA directly up-regulated by Rel2 and another that was down-regulated because of a silencing factor, or an mRNA with a long half-life compared to another that was degraded by a micro-RNA that was up-regulated by Rel2.
Figure 1. Global changes in transcript levels in transgenic A. stephensi following Rel2 induction.
A) The total number of genes significantly up- or down-regulated that are predicted to be in each GO category. Genes were considered significantly differentially regulated if the -fold change was>= 0.75 on a log2 scale. B) Venn diagram comparing the total number of regulated transcripts between the midgut of CP line mosquitoes and fat body of VG line mosquitoes at 12 h PBM. Red arrows correspond to midgut samples, and green arrows correspond to fat body samples; the arrow direction indicates significant up- or down-regulation.
Predicted gene ontology (GO) categories were assigned to genes based on homology to previously annotated An. gambiae genes (vectorbase.org). In both tissues and at all time points, the predicted GO category with the greatest number of significantly regulated genes was the diverse functional category (a total of 508 differentially regulated genes at 12 h PBM) followed by the unknown category (328 total differentially regulated genes at 12 hours PBM). This is to be expected, since these two GO categories represent over half of the annotated genes in the An. gambiae genome (vectorbase.org), and therefore the predicted GO terms for our An. stephensi genes. The broad functional spectrum of genes assigned to the diverse category reflects the far-reaching effects of Rel2 activation. Because Rel2 is the major transcription factor of the IMD pathway, we conjectured that numerous genes assigned to the immune/apoptosis GO category would be up-regulated following Rel2 expression. There were 26 genes from this category that were up-regulated in the midgut of CP mosquitoes at 12 h PBM, representing 4% of the total up-regulated genes at that time point. There were also 16 down-regulated genes in this category at the same time. In the VG line fat body, there were 36 up-regulated immune genes at 18 h PBM, representing 17.7% of up-regulated fat body genes at 18 h PBM, with 12 down-regulated at the same time. Highly up-regulated immune genes included both known anti-Plasmodium effectors, such as TEP1 and LRIM1 (Blandin et al., 2004; Povelones et al., 2009), and genes that have not yet been associated with Plasmodium resistance but that have shared domains with known anti-Plasmodium effectors, such as multiple leucine-rich repeat and fibrinogen domain-encoding genes and two MD-like genes (Garver et al., 2009; Povelones et al., 2009; Riehle et al., 2008). While a smaller proportion of immune genes were found to be differentially regulated in the An. stephensi genome following Rel2 expression than in Ae. aegypti (Zou et al., 2011), many of the same functional classes of immune genes are represented in the upregulated group, including thio-ester proteins and leucine rich repeat containing proteins. Representatives from other GO categories, such as serine proteases in the Proteolysis/Digestion group, redox responsive genes in the Redox/Stress/Mitochondrial category, and many others, are likely to be relevant for reciprocal interactions between Plasmodium and the mosquito. Other up-regulated genes such as cytochrome P450s may play a role in the mosquito’s resistance to insecticides (David et al., 2013) and thereby affect the ability of these transgenic mosquitoes to survive and compete in the wild.
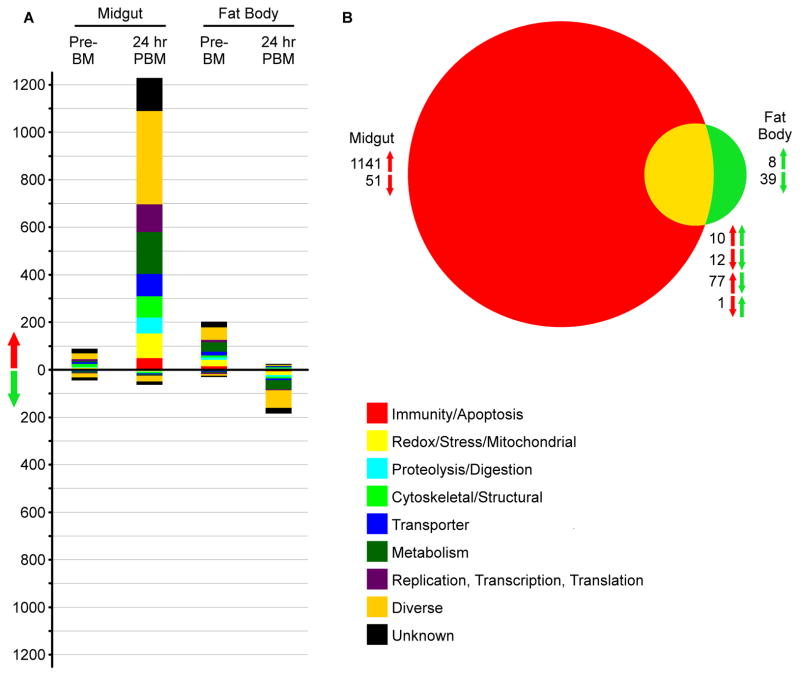
To determine changes in midgut and fat body proteomes following Rel2 activation in these tissues, we used iTRAQ to quantify the relative amounts of all proteins in Rel2-overexpressing transgenic mosquitoes relative to their wild-type conspecifics both before and 24 h after a blood meal. Filtering for only high confidence peptides, we identified 31,392 peptide spectrum matches (PSMs) corresponding to 8,574 peptides that mapped to 2,244 unique protein contigs previously annotated by the Jake Tu Lab (Virginia Polytechnic Institute and State University). These proteins were assigned putative names and functions through a BLASTp search against the An. gambiae genome, yielding 2041 unique proteins, 2024 of which had significant similarity (BLASTp e-value < 0.01) to An. gambiae genes. Prior to a blood meal, the transgenic mosquito midgut displayed significantly higher levels of 89 proteins and lower levels of 45 proteins. The transgenic mosquito fat body displayed higher levels of 204 proteins and lower levels of 31 proteins prior to the blood meal (Fig. 2A). One reason for the differential expression of these proteins in the absence of Rel2 induction may be that they represent genes with permanently altered expression patterns, either as a result of position effects of transgenesis or adaptation during the numerous generations since the insertion of the transgene. Alternatively, there may be leaky expression from the CP and VG promoters, as has been observed before for the VG promoter for some autogenous mosquito species (Provost-Javier et al., 2010), though not in Anopheles spp. mosquitoes. The reason for this leakiness could be further explained by examining the expression profiles of multiple lines with different transgene insertion locations. However, due to the labor intensive maintainence of transgenic lines, only one line of each strain, possessing the most potent anti-Plasmodium activity, has been kept and therefore limiting the analysis to a single line for each tissue. However, since these lines were selected based on their most potent anti-P. falciparum activity they represent the most relevant lines for this study (Dong et al., 2011). As expected, at 24 h PBM, there were many more proteins displaying significantly altered abundances. Specifically, after a blood meal there were 1,230 up-regulated and 64 down-regulated proteins in the midgut and 26 up-regulated and 185 down-regulated proteins in the fat body (Fig. 2A). Of these, only 22 were significantly regulated in the same direction (10 up and 12 down) in both the midgut and the fat body, while 78 were significantly regulated in opposite directions (77 up in the midgut and down in the fat body, and 1 with the reverse) (Fig. 2B). As at the transcript level, the GO category with the greatest number of differentially regulated proteins assigned to it was the diverse category (493 total differentially regulated proteins at 24 h PBM), although all the GO categories were represented, especially in the list of proteins up-regulated in the midgut at 24 h PBM. In the midgut, there were 50 proteins, or 4% of the total up-regulated proteins, belonging to the immune GO category that were significantly up-regulated at 24 h PBM, but there were only 2 significantly down-regulated immune proteins, or 3.1%, at that time. In the fat body at 24 h PBM, there were only 2 significantly up-regulated immune proteins, but these proteins represent 7.7% of the total significantly up-regulated proteins, and there were 7 significantly down-regulated proteins representing 3.7%. As for the transcriptome, there was a functionally diverse set of up-regulated immune proteins, including some corresponding to genes found to be regulated in the microarray–based transcriptome analyses, such as An. gambiae MD2-like protein 6 (AgMDL6) and neuronal leucine-rich repeat protein 3 (NLRR3). Other significantly up-regulated proteins were not found to be significantly up-regulated in the same tissue at the transcript level, but the regulated transcriptome and proteome sets contained many genes belonging to the same families, such as leucine-rich repeat and fibrinogen domain-containing proteins. Interestingly, the levels of both Toll and Rel1, which are Toll pathway-associated genes, were significantly up-regulated at the protein level following Rel2 activation, possibly indicating an interplay between the IMD and Toll pathways.
Figure 2. Global changes in protein levels in transgenic A. stephensi following Rel2 induction.
A) The total number of proteins significantly up- or down-regulated that are predicted to be in each GO category. Genes were considered significantly differentially regulated if the ratio of transgenic to wild type was>= 0.75 on a log2 scale. B) Venn diagram comparing the total number of regulated proteins between the midgut of CP line mosquitoes and fat body of VG line mosquitoes at 24 h PBM. Red arrows correspond to midgut samples, and green arrows correspond to fat body samples; the arrow direction indicates significant up- or down-regulation.
In both the transcriptomic and proteomic analyses, we observed differential expression of genes belonging to various functional classes (Figs. 1 and 2). Here, we present a brief overview of the several classes of genes that we believe are relevant to the Rel2 pathway or provide interesting insights on the physiological influence of this pathway.
Immune-related genes
In total, 102 separate immune-related genes were up-regulated under at least one experimental condition, while 44 were down-regulated. These genes represent a large number of different families of immune genes, including 8 leucine-rich repeat containing proteins, 5 tep proteins, 14 CLIP domain serine proteases, and 7 serine protease inhibitors, as well as many anti-microbial peptides and other genes. Interestingly, in addition to IMD pathway-associated genes such as Rel2, a number of Toll pathway-associated genes, including Toll and Rel1, were also significantly up-regulated, indicating that there may be interaction between the two major immune pathways. Similarly, Hop and JNK, components of the Jak/Stat and JNK pathways, were also up-regulated. These data indicate that the various immune pathways do not act in isolation and may instead act together to attain a broader immune response. The JNK pathway has recently been shown to play a role in Plasmodium resistance (Garver et al., 2013), and interaction between the JNK and IMD pathways may lead to greater Plasmodium immunity. Up-regulation of AMP genes such as defensin and cecropins likely plays a direct role in the control of bacteria and other pathogens (Meister et al., 2005), and similar upregulation of AMP expression has been seen following Rel2 induction in Ae. aegypti (Zou et al., 2011), while other genes such as the serine proteases likely trigger cascades to amplify and diversify the response (An et al., 2010).
Digestive
485 digestion-related genes were up-regulated across the experimental conditions, and 280 were down-regulated. Among these were genes involved in both protein and sugar digestion, as well as many Ras family proteins. Numerous proteolysis related genes were also found to be differentially regulated in the Ae. aegypti Rel2 regulated transcriptome (Zou et al., 2011). Up-regulated protein digestion-related genes, such as 26s proteosome subunits and serine/threonine phosphatases, work as part of the system for digesting blood meals following ingestion and may aid in the control of bacterial proliferation in the midgut following a blood meal. Ras family proteins are GTPases involved in cell proliferation and signaling, some of which have been implicated in Drosophila immunity (Ye and Zhang, 2013). While evidence from Drosophila implicates the Ran subfamily of Ras genes in the phagocytosis of virus-infected cells, other Ras genes may play a role in phagocytosis of other infectious organisms. Alternatively, GTPases, such as Ras family proteins, and kinases may act in a similar fashion to serine proteases and related genes to amplify and diversify the signal from the IMD pathway.
Cell structure genes
There were also 82 up-regulated and 48 down-regulated genes in the Cell Structure GO category. These included genes vital for muscle function or cell motility, such as 7 up-regulated and 3 down-regulated actins and 13 up-regulated and 8 down-regulated myosins; genes used for cell division, such as 3 up-regulated tubulins; and numerous cuticular proteins, actin- and chitin-binding proteins, and various other proteins. Previous research has shown that the anti-Plasmodium immune response involves remodeling of the midgut epithelium (Han and Barillas-Mury, 2002), as does bacterial resistance in the Drosophila midgut (Buchon et al., 2013). In Drosophila, infected cells are expelled into the midgut lumen and must be regenerated, and both processes require action by the cell structure and motility system. Other studies have also shown that remodeling of the cytoskeleton is necessary for successful Plasmodium infection and traversal of the midgut (Han and Barillas-Mury, 2002), so changes in the cytoskeleton by the immune system may be important for resistance to infection.
Redox genes
Finally, many redox and stress-related genes showed differential regulation. Components of the ROS system have been implicated in resistance to both bacteria (Oliveira et al., 2011) and Plasmodium, and multiple NOS and mitochondrial carrier (Goncalves et al., 2012) genes are up-regulated following IMD pathway induction. The up-regulation of ROS-related genes by the IMD pathway may be a link between the various ways of fighting off midgut infection by both Plasmodium and bacteria. By up-regulating these genes, the mosquito can attack pathogens with both ROS and AMPs at the same time, increasing the potential for clearance. Many components of the oxidative phosphorylation system, including various reductases and oxidases, were also up-regulated under at least one condition, indicating that the mosquito may increase ATP production in order to compensate for energy use by the immune system.
Genes with few representatives
In addition to the gene families with large numbers of representatives listed above, there were also gene families with very few differentially regulated representatives. For instance, odorant receptors and other sensory proteins were almost completely absent from the differentially regulated datasets, despite having many representatives in the genome (vectorbase.org). Thus, the mosquito IMD pathway likely does not greatly affect the ability of mosquitoes to sense their environment.
While we have identified a large number of transcripts and proteins that displayed altered expression in our genetically modified mosquitoes following up-regulation of Rel2, not all of these genes are likely to be controlled directly by Rel2, and there are many Rel2-regulated genes that were not discovered through our approach. We tested only a limited number of time points, and there may be short-lived transcripts and proteins that were degraded before we collected our samples. Similarly, some genes may take longer to transcribe and translate than others, and our time points may have been too early to observe the changes in expression. Other genes may require the binding of different transcription factors not present in this study in addition to Rel2, and thus they would not be differentially regulated following the up-regulation of Rel2 alone. Conversely, some genes having promoter sequences with low affinity for Rel2 may be differentially regulated in our transgenic mosquitoes, likely because of the overabundance of an active Rel2 form in the system. The differential regulation of many genes upon activation of recombinant Rel2 is also likely to represent a secondary effect and a general physiological response to immune activation. However, we believe that the time points we have chosen are well chosen to capture both the timing of Rel2 up-regulation in our mosquitoes and the physiologically relevant times for P. falciparum invasion of the midgut, in the under-studied mosquito vector An. stephensi.
3.2 Correlation between mosquito transcript and protein expression levels
Because we measured both transcript abundance and protein abundance, we were able to compare the expression of genes at the two levels in order to assess a correlation between transcript levels and protein abundance. Previous studies in other organisms have shown only weak concordance between transcripts and proteins (de Sousa Abreu et al., 2009; Vogel and Marcotte, 2012), but such studies are usually restricted to unicellular organisms or cell lines, and this study provided an opportunity to expand this knowledge to multicellular eukaryotes.
When we looked at all the genes that displayed a significant difference at the protein abundance level at 24 h PBM and a significant difference at the transcript abundance level at one of the two time points, we saw no significant correlation between protein and transcript levels in the midgut when we used a linear model (Fig. S6 A). However, we saw a significant correlation for the fat body (fig. S6 B). Our assays generated data for 1,273 genes at both the transcript and protein levels in the midgut, 240 of which were significantly regulated in the same direction at both the protein and transcript levels, while 119 were significantly regulated in opposite directions. In the fat body, we obtained data for 1,538 genes at both the transcript and protein levels, with 33 significantly being regulated in the same direction at both protein and transcript levels and only 8 significantly regulated in opposite directions. The lack of significant correlation between mRNA and protein levels in the midgut is likely due to the wide variety of genes that are differentially expressed in that tissue and the various post-transcriptional modifications and regulatory mechanisms involved. The lack of correlation is also indicative of the large proportion of genes that are not directly regulated by the Rel2 transcription factor. Furthermore, factors such as transcript and protein degradation rates, miRNAs, mRNA secondary structure, the presence of other transcription reguators, and the availability of ribosomes and amino acids to build proteins could all contribute to differences between the abundance of mRNA and proteins (Maier et al., 2009; Vogel and Marcotte, 2012). For instance, if the mRNA of a particular transcript is quickly degraded but the protein is long-lived, or vice-versa, then an increase in the transcription of that gene will not necessarily lead to a measurable change in the gene expression at both levels. Similarly, other transcriptional and translational regulators, including transcription factors, miRNAs, and feedback loops, may promote or inhibit transcription and translation differentially, leading to discordance between mRNA and protein levels. Finally, it is possible that the time points at which we measured expression levels did not adequately capture the timing of gene expression. Our previous work indicates that Rel2 expression levels should be high at 12 h and remain elevated for many hours afterward in both the midgut and fat body, and protein levels would, if directly governed by transcription, follow soon after. An increase in transcripts, even if not immediately translated, could allow mosquitoes to increase the abundance of a protein quickly if other signals necessary for the translation of that gene were present. Similarly, differential degradation of transcripts and proteins may allow mosquitoes to remove unnecessary gene products, even if transcription is initiated by a promiscuous promoter. Other studies have also shown a lack of correlation between mRNA and protein levels (Gedeon and Bokes, 2012; You and Yin, 2000). Our results show that a lack of correlation between mRNA and protein expression levels is also prominent in mosquitoes, likely providing mosquitoes a fine level of control over the proteins expressed in their cells.
3.3 Identification of novel anti-Plasmodium immune genes
The analyses of transcript and protein abundance following Rel2 induction in transgenic mosquitoes allowed us to select a variety of genes to investigate further for involvement in the mosquito’s immune defense. Because Rel2 is an IMD pathway-associated transcription factor, we expected a large number of significantly up-regulated immune genes. Similarly, because this immune pathway is responsible for the mosquito’s resistance to Plasmodium infection (Garver et al., 2009), we were interested in identifying IMD pathway-regulated genes that control resistance to P. falciparum. We began by selecting five of the immune genes with the greatest up-regulation at the protein level at 24 h PBM in the midgut. We chose to consider only the protein level for these genes because it takes approximately 24 h for P. falciparum to exit the midgut lumen, so proteins with increased expression at this time will be available to act against the parasite.
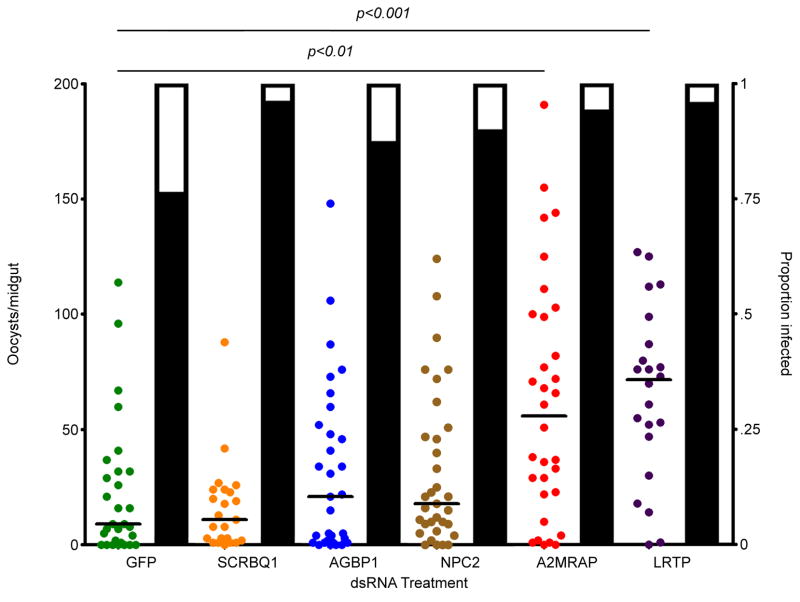
From the 20 most abundant immune proteins, we chose 5 that may have anti-Plasmodium activity for further testing based on the level of up-regulation and predicted functions based on sequence homology. Specifically, we chose a class B scavenger receptor containing a SCRBQ1 domain (SCRBQ1), bacteria response protein 1 (AGBP1), Neimann-Pick type C-2 (NPC2), alpha-2-macroglobulin receptor-associated protein (A2MRAP), and a leucine-rich transmembrane protein (LRTP). The selected genes were knocked down by RNAi prior to parasite exposure, and the resulting impact on P. falciparum infection of the mosquito midgut was assayed by oocyst counting (Fig. 3). RNAi-based depletion of SCRBQ1, AGBP1, and NPC2 had no significant effect on Plasmodium infection levels; however, depletion of A2MRAP and LRTP led to significant increases in the number of oocysts per midgut (Kruskal-Wallis test, p<0.0001; Dunn’s post-hoc test: A2MRAP, p<0.01; LRTP, p<0.001). While the median number of oocysts was decreased following both A2MRAP and LRTP knockdown, none of the dsRNA treatments had a significant effect on oocyst prevalence, even though previous studies have shown that Rel2 knockdown by RNAi leads to an increase in oocyst prevalence. This difference in result may stem from the fact that the genes we knocked down are only a part of the whole immune response to Plasmodium infection, and their knockdown may not be efficient enough to affect the prevalence, whereas the full complement of IMD pathway-regulated immune effectors together cause a greater effect.
Figure 3. P. falciparum infection intensity following RNAi knockdown of immune genes.
The number of oocysts per midgut of wild-type A. stephensi following RNAi-mediated depletion of GFP, SCRBQ1, bacterial response protein (AGBP1), Niemann-Pick type-C (NPC2), alpha-2-macroglobulin (A2MRAP), or leucine-rich transmembrane protein (LRTP). Depletion of both A2MRAP and LRTP led to a significant increase in the number of oocysts per mosquito midgut. Each circle represents a single midgut, and horizontal black bars represent the median of the sample. Significance was determined by a Kruskal-Wallis test followed by Dunn’s post-hoc test to compare immune-depleted mosquitoes to GFP controls. Significance was assessed at α=0.05. Supplementary data for this figure is given in table S2.
SCRBQ1 (ASTE009112/AGAP010132) is homologous to the Croquemort (CRQ) gene in D. melanogaster, which is essential for efficient phagocytosis of apoptotic cells in Drosophila embryos (Franc et al., 1996; Franc et al., 1999), and may play a role in anti-Plasmodium defense through apoptosis or phagocytosis (Blandin and Levashina, 2007; Hurd et al., 2006). AGBP1 (STE009712/AGAP008061) is one of a number of bacterial infection-responsive proteins that have been identified in An. gambiae, and is up-regulated following infection with P. berghei (Dong et al., 2006). However, P. berghei infection is principally controlled by the Toll pathway (Garver et al., 2009), so the relevance of this protein in defending against P. falciparum infection may be minimal. NPC2 (ASTE004995/AGAP002851) is a small, highly conserved, secreted protein that plays an important role in regulating sterol homeostasis in Drosophila (Ioannou, 2007). Plasmodium parasites are unable to synthesize their own sterols and must scavenge these molecules from their host (Bano et al., 2007), so changes in the abundance of sterols may affect the ability of Plasmodium to infect mosquitoes. NPC2 also plays an important role in dengue resistance of Ae. aegypti mosquitoes and may play a role in a variety of infection systems (Jupatanakul et al., 2013). A2MRAP (ASTE011001/AGAP003521) is a protein associated with the receptor for alpha-2-macroglobulins. Alpha-2-macroglobulin is an abundant protein that binds to a variety of ligands and is involved with the lectin-dependant cytolytic pathway in arthropods (Armstrong and Quigley, 1999). The diversity of ligands to which alpha-2-macroglobulin can bind and its importance for the lysis of cells indicate that it, and its receptors, could be important for the lysis of Plasmodium infected cells and help mosquitoes fight off the parasite. Finally, LRTP (ASTE008359/AGAP007061) bears structural similarity to other proteins containing leucine-rich repeats. Two such proteins, LRIM1 and APL1C, have been shown to be important for the anti-Plasmodium defense in An. gambiae (Dong et al., 2006; Povelones et al., 2011), suggesting that similar proteins may also act against Plasmodium in An. stephensi.
The lack of an increase in Plasmodium oocysts per midgut following knockdown of SCRBQ1 is likely a result of gene redundancy, with alternative proteins also controlling apoptosisCRQ is largely expressed in the Drosophila embryo and not in adults, and it is possible that SCRBQ1 is similarly more active during mosquitoes’ immature stages than in adults. Both AGBP1 and NPC2 are known to be immune modulators for other parasites and may simply display specificity for bacteria and viruses and not act against Plasmodium. Knockdown of both A2MRAP and LRTP led to significant increases in Plasmodium infection, indicating that these proteins are important in modulating P. falciparum infection in the mosquito.
3.4 Serine proteases affect mosquito anti-Plasmodium defenses
A large number of proteases and digestive enzymes were highly up-regulated at both the protein and transcript levels. Serine proteases and other proteolytic enzymes are often part of proteolytic cascades that can lead to the amplification of signals that control downstream effector mechanisms (An et al., 2010). In mosquitoes, some serine proteases have been implicated in blood digestion (Yang and Davies, 1971), the anti-Plasmodium defense (Blumberg et al., 2013; Volz et al., 2005), signal transduction, and many other diverse functions. The TOLL immune pathway, for instance, is activated through a serine protease-dependent signaling cascade (Ligoxygakis et al., 2002; Weber et al., 2003). A number of serpins are involved in the prophenoloxidase (PPO) activation cascade, an important part of the innate immune system (Christophides et al., 2002; Ligoxygakis et al., 2003; Weber et al., 2003), but the functions of many other serpins have yet to be elucidated. The presence of many proteolytic regulators, including serine proteases and their inhibitors, in the highly up-regulated gene group at both the transcript and protein levels indicates that serine proteases play a role in the IMD pathway-based immune response and the mosquito anti-Plasmodium defense. It may also help to explain the broad diversity of genes affected by increased expression of Rel2, since serine protease-dependent signaling cascades can both amplify and diversify the signal, causing changes in the regulation of many different genes.
Given the significant up-regulation of numerous proteases and related enzymes at both the protein and transcript levels, we decided to investigate a number of them further. Serine protease inhibitor 10 (SRPN10), Rel2-responsive serine protease 1 (R2RSP1), Rel2-responsive serine protease 2 (R2RSP2), serine protease precursor 1 (SEPRP1), trypsin precursor (TRYPP), angiotensin converting enzyme precursor (ACEP), and serine protease precursor 2 (SEPRP2) were all chosen because they showed at least a 2-fold induction at the transcript level at either 6 or 12 h PBM and at the protein level 24 h PBM in the midgut. Silencing of R2RSP2, ACEP, and SEPRP2 significantly increased oocyst loads, suggesting that these factors are P. falciparum antagonists (Kruskal-Wallis test, p<0.0001; Dunn’s post-hoc test for R2RSP2, p<0.01, ACEP p<0.05, SEPRP2 p<0.001). However, as with the immune genes, none of the silenced genes had a significant effect on oocyst prevalence. Again, this may be due to the limited activity of only a few genes, when compared to the overall effects of the full IMD pathway-based response. Serine proteases act in diverse processes in mosquitoes and other dipterans, including activating signaling cascades (such as the Toll and PPO cascades) and regulating development. SRPN10 (ASTE007248/AGAP005246) is one of many serine protease inhibitors (serpins) found in mosquitoes. Studies in An. gambiae have shown that some isoforms of SRPN10 are up-regulated during the parasite’s traversal of the midgut and may be involved in anti-Plasmodium defense (Danielli et al., 2005). R2RSP1 (ASTE006240/AGAP007142) and R2RSP2 (ASTE014104/AGAP007165) are both examples of trypsin-like serine proteases. SEPRP1 (ASTE009202/AGAP005065) and SEPRP2 (ASTE010540/AGAP005310) are both precursors to serine proteases and may be involved in many of the same functions as R2RSP1 and R2RSP2. TRYPP (ASTE010330/AGAP006709) is a precursor for a chymotrypsin which are proteolytic enzymes and form a subset of serine proteases that play a role in the digestion of mosquito blood meals (Yang and Davies, 1971). Serine proteases similar to those identified in this study cleave a variety of targets and may have many different functions, including the regulation of anti-Plasmodium activity. Finally, ACEP (ASTE004060/AGAP004563) is a precursor for angiotensin converting enzyme (ACE), a family of proteins found in the hemolymph of insects and cleave a broad range of substrates (Burnham et al., 2005; Riordan, 2003). While various ACE-like proteins have been studied in An. gambiae and an immune function has been suggested, their potential effect on Plasmodium has not been investigated.
Two serpins, SRPN6 and SRPN7, have previously been shown to play roles in the anti-Plasmodium defense (Abraham et al., 2005; Blumberg et al., 2013), and we have identified a series of new serine proteases and precursors that also play a role in this defense. R2RSP2 is one of many mosquito serine proteases and may play a role in amplifying the immune signal. Other serine proteases have also been shown to be vital for the melanization response in mosquitoes (Christophides et al., 2002), which can help to clear parasites; therefore, it is possible the R2RSRP2 increases melanization, although we have not measured this specific immune action. While ACEs have not been shown to have a direct effect on Plasmodium infection in mosquitoes, it has been suggested that they can cleave immune-related substrates and thereby alter immune activity. Our results demonstrate that ACEP has anti-Plasmodium activity in An. stephensi mosquitoes. Further investigation is needed to determine whether this activity is a direct or indirect effect. Similarly, since SEPRP2 was identified as a precursor for an unknown serine protease, it is difficult to accurately predict all effects of altering the expression patterns of this gene, but, given the ability of serine proteases to act against Plasmodium discussed above, a precursor of any one of a number of serine proteases has the potential to be important for insect immunity.
3.5 Effect of anti-Plasmodium effector genes on midgut bacterial load
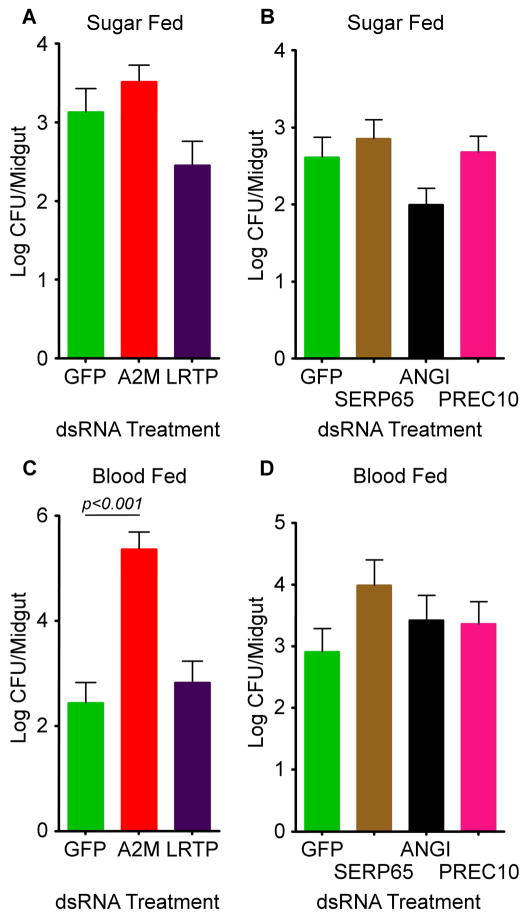
In order to determine whether the genes identified previously as having an anti-Plasmodium effect act as general immune factors or are Plasmodium-specific, we tested them for involvement in controlling the mosquito midgut microbiota. The mosquito midgut is colonized by a variety of bacteria that need to be tightly controlled to prevent overproliferation and damage to the insect host (Pumpuni et al., 1996; Straif et al., 1998). Many different bacterial strains can be present in the mosquito gut, and the community varies from mosquito to mosquito and species to species; however, Gram-negative bacteria are considered to make up the majority of the species (Straif et al., 1998). Previous studies have implicated the IMD pathway as the main pathway involved in controlling the levels of bacteria in the mosquito midgut (Dong et al., 2009). Similarly, other studies have shown that the midgut microbiota is necessary to stimulate and prime the mosquito immune system and prepare it for future challenge (Clayton et al., 2012; Dong et al., 2009). Some anti-Plasmodium factors also act against the midgut microbiota, while others do not. Therefore, we tested our newly identified anti-Plasmodium effectors for an effect on the levels of bacteria in the mosquito midgut. The number of culturable bacteria per mosquito midgut was quantified by CFU assays following RNAi knockdown of potential novel anti-Plasmodium effector genes. Thus, we tested A2MRAP, LRTP, R2RSP2, ACEP, and SEPRP2 for antibacterial effect in both sugar-fed and blood-fed mosquitoes. In sugar-fed mosquitoes, one-way ANOVA showed a significant change in the log transformed number of colonies per midgut following knockdown of A2MRAP and LRTP (ANOVA p<0.05); however, a Dunnett’s multiple comparison test showed no difference between either A2MRAP or LRTP and the GFP control, indicating that neither gene significantly affects midgut bacterial load (Fig 5A). The ANOVA revealed no significant differences between GFP, R2RSP2, ACEP, and SEPRP2 in sugar-fed mosquitoes (Fig 5B). Following a blood meal, ANOVA revealed a highly significant difference in bacterial load between GFP, A2MRAP, and LRTP (ANOVA p<0.0001), and a Dunnett’s multiple comparison test showed a significant increase in bacterial load following knockdown of A2MRAP (Dunnett’s p<0.001) (Fig 5C). As with the sugar meal, there was no significant difference in bacterial loads after knockdown of GFP, R2RSP2, ACEP, or SEPRP2 after a blood meal (Fig 5D).
Figure 5. Influence of novel anti-Plasmodium genes on midgut microbiota.
The number of colony forming units of culturable bacteria in the midguts of females following RNAi-mediated knockdown of genes shown above to have anti-Plasmodium effects. A) Sugar-fed mosquitoes following depletion of GFP, A2MRAP, and LRTP. B) Sugar-fed mosquitoes following depletion of GFP, R2RSP2, ACEP, and SEPRP2. C) Blood-fed mosquitoes following depletion of GFP, A2MRAP, and LRTP showed that A2MRAP depletion leads to a significant increase in CFUs per midgut. D) Blood-fed mosquitoes following depletion of GFP, R2RSP2, ACEP, and SEPRP2. Significance was determined by a one-way ANOVA, followed by Dunnett’s multiple comparison test, with significance assessed at α=0.05. Bars represent the mean of three biological replicates, and error bars represent the standard error of the mean.
The fact that none of the five genes tested had any effect on culturable bacteria levels in the sugar-fed mosquito midgut may indicate that the midgut microbiota is somewhat stable at this point. The midgut microbiota is adapted to the midgut environment and may therefore be able to evade action by various mosquito immune effectors. Alternatively, while multiple immune genes are able to affect the midgut microbiota, the genes we tested may display specificity for Plasmodium parasites and therefore not affect bacteria to a considerable degree. Specificity in mosquito immune response is common, since even pathogens in the same genus, such as P. falciparum and P. berghei, elicit strikingly different immune responses (Garver et al., 2009), and infection by the two is controlled by separate immune pathways. Thus, it is likely that highly divergent pathogens such as Plasmodium and bacteria would also be affected differently. Similarly, we saw no effect of AGBP1 on Plasmodium infection, although it has been shown to have an effect on Staphylococcus aureus infection in the mosquito (Dong et al., 2006), adding credence to the theory of divergent immune action.
Following a blood meal, only A2MRAP had an effect on bacterial load. Blood meals have been shown in the past to have a large effect on the midgut microbiota of mosquitoes, leading to a large increase in the number of bacteria (Oliveira et al., 2011). This disturbance and proliferation may allow more opportunity for anti-bacterial genes to take effect. Alternatively, the expansion in bacterial numbers following a blood meal may allow for dangerous levels of bacteria in the midgut that need to be controlled by the immune system in order to prevent damage to the mosquito. Alpha-2-macroglobulins have relatively broad binding specificities (Riordan, 2003), which may explain why this protein can act against divergent pathogens such as Plasmodium and bacteria. Alternatively, since A2MRAP is an alpha-2-macroglobulin receptor-associated protein, it may be able to bind to multiple different alpha-2-macroglobulin receptors and therefore have a broad specificity based on the different combinations of alpha-2-macroglobulins and their receptors.
Our findings support the hypothesis that the mosquito’s immune system is able to react in a specific manner to different pathogens, especially when they are distantly related. In addition, the fact that many more genes are up-regulated following Rel2 induction in the midgut than in the fat body implies that mosquito immune pathways exhibit tissue specificity and that the IMD pathway may be more important for immune defense in the midgut than in the fat body. The IMD pathway acts specifically against Gram-negative bacteria, which make up the majority of the mosquito midgut microbiota (Straif et al., 1998), and this pathway has been shown to be the major pathway involved in controlling the midgut microbiota in both mosquitoes and Drosophila. The greater expression of IMD pathway-responsive genes in the midgut than in the fat body may allow better control and a faster response to perturbations in the midgut microbiota.
Conclusions
In this work, we have measured the changes in expression, at both the transcript and protein level, in the midguts and fat bodies of P. falciparum-resistant, genetically modified mosquitoes that are transiently overexpressing the IMD pathway-regulated transcription factor Rel2 (Dong et al., 2011). Through a combination of full-genome microarray-based expression analyses and iTRAQ proteomic analyses, we were able to see that specific up-regulation of Rel2 leads to differential regulation of a large number of both immune-related and other genes, including general immune genes and a large number of serine proteases cascade-related genes. We measured the effect of knockdown of multiple immune genes including serine protease cascade-related genes on P. falciparum infection in mosquitoes, thereby identifying a number of novel genes implicated in anti-Plasmodium defense. The presence of multiple serine proteases and their observed effect on Plasmodium infection indicate that these genes may be responsible for expanding and amplifying the IMD pathway signal and support the importance of serine proteases in the mosquito’s immune defense. Of the five newly identified anti-Plasmodium genes, only one, A2MRAP, had an effect on the mosquito microbiota, and only after a blood meal. Finally, by observing significant changes at both the transcript and protein levels, we were able to look for any correlation between transcript and protein levels. In the midguts of our GM mosquitoes, we observed no correlation between the two, while in the fat body we saw a significant correlation, although many fewer genes were included in the fat body analysis. The lack of a strong correlation between transcripts and proteins concurs with the results of other studies that have observed a similar lack of correlation, and it likely indicates a large role for post-transcriptional processing and control of translation, allowing mosquitoes to have a finer level of control over protein expression. Overall, our results indicate that Rel2 has a significant impact on both the mosquito transcriptome and proteome. The patterns of differential gene expression in the fat body were similar to those seen in transgenic Ae. aegypti overexpressing Rel2 under the VG promoter, indicating that these pathways may be well conserved across mosquito species (Zou et al., 2011). Also, as expected, we found that a number of genes controlled by the IMD pathway have an effect on anti-Plasmodium defenses, while others have no known immune function. This result indicates that the IMD pathway, and Rel2 specifically, controls or affects the expression of many non-immune processes. By means of these effects, the mosquito’s immune response may be influenced by many factors that have not previously been considered to be part of canonical immune pathways. Similarly, induction of the mosquito immune system may alter many non-immune processes that can have far-reaching implications for mosquito fecundity and fitness. Overall, these results indicate that mosquito immune pathways act on a global level to produce complex changes in gene expression that will require further investigation to unravel fully.
Supplementary Material
PCR primers used in this study. “Gene name” displays the name of the gene targeted by the primer, “AGAPID” displays the vectorbase identifier for the targeted gene, “Primer name” is the name given to the primer, and “Primer sequence” displays the sequence of the primer.
Supplementary data for figures 3 and 4. Includes number of mosquitoes assayed, range, prevalence median, and % change in the number of oocysts.
Microarray and proteomic data analyzed in this study. “An. stephensi contig id” is a unique id to each sequence in the A. stephensi transcriptome, “An. gambiae gene id” is the vectorbase ID of the closest corresponding An. gambiae gene, “Predicted GO category” is the GO category of the closest corresponding An. gambiae gene, “An. gambiae gene name” is the name of the closes corresponding An. gambiae gene, and the rest of the columns are log2 transformed values of the transcript or protein abundance ratios for the different experimental groups. GO abbreviations are; I: immunity and apoptosis; Redox/Stress/Mit: oxidoreductive, stress-related and mitochondrial; CS: cytoskeletal, structural; M: metabolism; RTT: replication, transcription, translation; P/D: proteolysis, digestion; TRP: transport; D: diverse; U: unknown functions.
qRT-PCR verification of microarray data. 7 genes upregulated in the 12 hours PBM midguts samples of the microarray were chosen and their fold-change measured by qRT-PCR to verify their regulation. Of the 7, 4 were similarly regulated as measured by qRT-PCR and all 7 were upregulated in at least one experimental condition.
qRT-PCR verification of dsRNA mediated gene knockdown. mRNA was collected from dsRNA injected mosquitoes and used for qRT-PCR to measure the percent knockdown of the gene. All genes showed greater than 50% knockdown.
Correlation between mRNA and protein levels in the mosquito midgut (A, B) and fat body (C, D) following a blood meal. In the midgut there was no significant correlation between mRNA and protein levels whether looking at all genes (A, r2=0.0003, F-statistic = 0.4276, p-value = 0.5133) or only significantly regulated genes (B, r2=.0005, F-statistic = 0.1766, p-value = 0.6746), while in the fat body there was a significantly correlation both when looking at all genes (C, r2=0.02183, F-statistic = 34.28, p-value < 0.001) and when looking at only significantly regulated genes (D, r2=0.3213, F-statistic = 18.47, p-value < 0.001).
Figure 4. P. falciparum infection intensity following RNAi knockdown of protease genes.
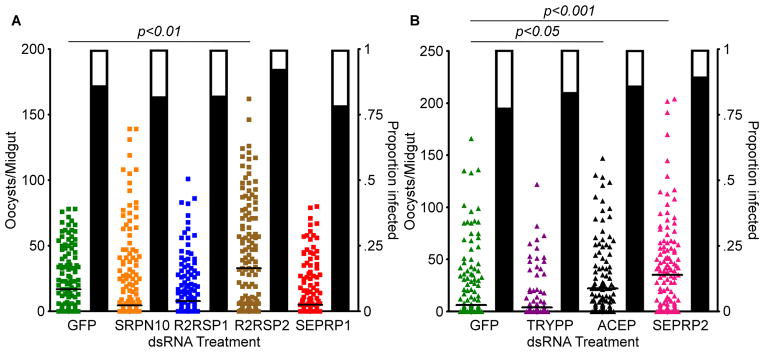
The number of oocysts per midgut of wild-type A. stephensi following RNAi-mediated depletion of: A) green fluorescent protein (GFP), serpin 10 (SRPN10), Rel2-responsive serine protease 1 (R2RSP1), Rel2-responsive serine protease 2 (R2RSP2), or serine protease precursor 1 (SEPRP1); and B) trypsin precursor (TRYPP), anigiotensin-converting enzyme precurser (ACEP), or serine protease precursor 2 (SEPRP2). Silencing of R2RSP2, ACEP, and SEPRP2 all led to significant increases in the number of oocysts per midgut. Each circle represents a single midgut, and horizontal black bars represent the median of the sample. Significance was determined by a Kruskal-Wallis test followed by Dunn’s post-hoc test to compare immune-depleted mosquitoes to GFP controls. Significance was assessed at α=0.05. Supplementary data for this figure is given in table S2.
Highlights.
Transgenic over-expression of the IMD immune pathway-controlled transcription factor Rel2 affects the expression of numerous genes with diverse functions, suggesting a broader physiological role of this immune pathway.
Our study has identified multiple novel A.stephensi anti-Plasmodium effectors.
Acknowledgments
We would like to thank the Johns Hopkins Malaria Research Institute Insectary Core and Parasite Core for supplying us with mosquitoes and gametocyte cultures, respectively, as well as Dr. Christopher Cirimotich for his helpful comments when writing this manuscript. We would also like to thank Linda Orzolek and the Johns Hopkins School of Medicine Microarray facility for scanning our arrays. Similarly, we would like to thank the Dr. Bob Cole and the Johns Hopkins School of Medicine Mass Spectrometry and Proteomics facility for running the iTRAQ. We also thank Jake Tu from Virginia Polytechnic Institute and State University for providing us with draft versions of the An. stephensi transcriptome, without which we could not have created our microarrays or annotated our proteins. Finally, we thank Dr. Deborah McClellan for editing the manuscript. AP was supported by a Johns Hopkins Malaria Research Institute Predoctoral Fellowship. This work was funded by R01AI061576.
Abbreviations
- IMD
Immune Deficiency
- PRR
pattern recognition receptors
- PAMPs
pathogen associated molecular patterns
- CP15
carboxypeptidase gene promoter –driven Rel2 line 15
- VG
vitellogenin gene promoter –driven Rel2 line
- GO
gene ontology
- RNAi
RNA interference
- iTRAQ
isobaric tags for relative and absolute quantitation
- dsRNA
double-stranded RNA
- qRT-PCR
quantitative Real-Time PCR
- CFUs
colony forming units
- PBM
post-blood meal
- PSMs
peptide spectrum matches
- AgMDL6
An. gambiae MD2-like protein 6
- NLRR3
neuronal leucine-rich repeat protein 3
- SCRBQ1
scavenger receptor BQ1 domain
- AGBP1
bacteria response protein 1
- NPC2
Neimann-Pick type C-2
- A2MRAP
alpha-2-macroglobulin receptor-associated protein
- LRTP
leucine-rich transmembrane protein
- CRQ
Croquemort
- SRPN10
serine protease inhibitor 10
- R2RSP1
Rel2-responsive serine protease 1
- R2RSP2
Rel2-responsive serine protease 2
- SEPRP1
serine protease precursor 1
- TRYPP
trypsin precursor
- ACEP
angiotensin converting enzyme precursor
- SEPRP2
serine protease precursor 2
- GFP
green fluorescent protein
- SRPN10
serpin 10
- R2RSP1
Rel2-responsive serine protease 1
- R2RSP2
Rel2-responsive serine protease 2
- SEPRP1
serine protease precursor 1
- TRYPP
trypsin precursor
- ACEP
anigiotensin-converting enzyme precurser
- SEPRP2
serine protease precursor 2
Footnotes
Competing Interests
The authors have declared that no competing interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew Pike, Email: apike@jhsph.edu.
Alekhya Vadlamani, Email: alvadlam@jhsph.edu.
Simone L. Sandiford, Email: ssandifo@jhsph.edu.
Anthony Gacita, Email: agacita1@jhu.edu.
George Dimopoulos, Email: gdimopou@jhsph.edu.
References
- Abraham EG, Pinto SB, Ghosh A, Vanlandingham DL, Budd A, Higgs S, Kafatos FC, Jacobs-Lorena M, Michel K. An immune-responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proc Natl Acad Sci U S A. 2005;102:16327–16332. doi: 10.1073/pnas.0508335102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- An C, Jiang H, Kanost MR. Proteolytic activation and function of the cytokine Spatzle in the innate immune response of a lepidopteran insect, Manduca sexta. FEBS J. 2010;277:148–162. doi: 10.1111/j.1742-4658.2009.07465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong PB, Quigley JP. Alpha2-macroglobulin: an evolutionarily conserved arm of the innate immune system. Dev Comp Immunol. 1999;23:375–390. doi: 10.1016/s0145-305x(99)00018-x. [DOI] [PubMed] [Google Scholar]
- Bano N, Romano JD, Jayabalasingham B, Coppens I. Cellular interactions of Plasmodium liver stage with its host mammalian cell. Int J Parasitol. 2007;37:1329–1341. doi: 10.1016/j.ijpara.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- Blandin SA, Levashina EA. Phagocytosis in mosquito immune responses. Immunol Rev. 2007;219:8–16. doi: 10.1111/j.1600-065X.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- Blumberg BJ, Trop S, Das S, Dimopoulos G. Bacteria- and IMD pathway-independent immune defenses against Plasmodium falciparum in Anopheles gambiae. PLoS One. 2013;8:e72130. doi: 10.1371/journal.pone.0072130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol. 2013;11:615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- Burnham S, Smith JA, Lee AJ, Isaac RE, Shirras AD. The angiotensin-converting enzyme (ACE) gene family of Anopheles gambiae. BMC Genomics. 2005;6:172. doi: 10.1186/1471-2164-6-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Muller HM, Osta MA, Paskewitz SM, Reichhart JM, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, von Mering C, Xu J, Zheng L, Bork P, Kafatos FC. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Cirimotich CM, Dong Y, Garver LS, Sim S, Dimopoulos G. Mosquito immune defenses against Plasmodium infection. Dev Comp Immunol. 2009;34:387–395. doi: 10.1016/j.dci.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton AM, Cirimotich CM, Dong Y, Dimopoulos G. Caudal is a negative regulator of the Anopheles IMD pathway that controls resistance to Plasmodium falciparum infection. Dev Comp Immunol. 2012;39:323–332. doi: 10.1016/j.dci.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielli A, Barillas-Mury C, Kumar S, Kafatos FC, Loukeris TG. Overexpression and altered nucleocytoplasmic distribution of Anopheles ovalbumin-like SRPN10 serpins in Plasmodium-infected midgut cells. Cell Microbiol. 2005;7:181–190. doi: 10.1111/j.1462-5822.2004.00445.x. [DOI] [PubMed] [Google Scholar]
- David JP, Ismail HM, Chandor-Proust A, Paine MJ. Role of cytochrome P450s in insecticide resistance: impact on the control of mosquito-borne diseases and use of insecticides on Earth. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120429. doi: 10.1098/rstb.2012.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5:1512–1526. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Das S, Cirimotich C, Souza-Neto JA, McLean KJ, Dimopoulos G. Engineered anopheles immunity to Plasmodium infection. PLoS Pathog. 2011;7:e1002458. doi: 10.1371/journal.ppat.1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Cirimotich C, Pike A, Chandra R, Dimopoulos G. Anopheles NF-κB -regulated splicing factors direct pathogen-specific repertoires of the hypervariable pattern recognition receptor AgDscam. Cell Host & Microbe. 2012;12:521–530. doi: 10.1016/j.chom.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudoit S, Gentleman RC, Quackenbush J. Open source software for the analysis of microarray data. Biotechniques. 2003;(Suppl):45–51. [PubMed] [Google Scholar]
- Franc NC, Dimarcq JL, Lagueux M, Hoffmann J, Ezekowitz RA. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity. 1996;4:431–443. doi: 10.1016/s1074-7613(00)80410-0. [DOI] [PubMed] [Google Scholar]
- Franc NC, Heitzler P, Ezekowitz RA, White K. Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila. Science. 1999;284:1991–1994. doi: 10.1126/science.284.5422.1991. [DOI] [PubMed] [Google Scholar]
- Garver LS, de Almeida Oliveira G, Barillas-Mury C. The JNK pathway is a key mediator of Anopheles gambiae antiplasmodial immunity. PLoS Pathog. 2013;9:e1003622. doi: 10.1371/journal.ppat.1003622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver LS, Dong Y, Dimopoulos G. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 2009;5:e1000335. doi: 10.1371/journal.ppat.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedeon T, Bokes P. Delayed protein synthesis reduces the correlation between mRNA and protein fluctuations. Biophys J. 2012;103:377–385. doi: 10.1016/j.bpj.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves RL, Oliveira JH, Oliveira GA, Andersen JF, Oliveira MF, Oliveira PL, Barillas-Mury C. Mitochondrial reactive oxygen species modulate mosquito susceptibility to Plasmodium infection. PLoS One. 2012;7:e41083. doi: 10.1371/journal.pone.0041083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Singleton PA, Rowshan A, Gucek M, Cole RN, Graham DR, Van Eyk JE, Garcia JG. Quantitative proteomics analysis of human endothelial cell membrane rafts: evidence of MARCKS and MRP regulation in the sphingosine 1-phosphate-induced barrier enhancement. Mol Cell Proteomics. 2007;6:689–696. doi: 10.1074/mcp.M600398-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YS, Barillas-Mury C. Implications of Time Bomb model of ookinete invasion of midgut cells. Insect Biochem Mol Biol. 2002;32:1311–1316. doi: 10.1016/s0965-1748(02)00093-0. [DOI] [PubMed] [Google Scholar]
- Hurd H, Grant KM, Arambage SC. Apoptosis-like death as a feature of malaria infection in mosquitoes. Parasitology. 2006;132(Suppl):S33–47. doi: 10.1017/S0031182006000849. [DOI] [PubMed] [Google Scholar]
- Imroze K, Prasad NG. Mating with large males decreases the immune defence of females in Drosophila melanogaster. J Genet. 2012;90:427–434. doi: 10.1007/s12041-011-0105-7. [DOI] [PubMed] [Google Scholar]
- Ioannou YA. Niemann-Pick C proteins in sterol transport and absorption: flies in the ointment. Dev Cell. 2007;12:481–483. doi: 10.1016/j.devcel.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- Jupatanakul N, Sim S, Dimopoulos G. Aedes aegypti ML and Niemann-Pick type C family members are agonists of dengue virus infection. Dev Comp Immunol. 2013;43:1–9. doi: 10.1016/j.dci.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart JM. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science. 2002;297:114–116. doi: 10.1126/science.1072391. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Roth S, Reichhart JM. A serpin regulates dorsal-ventral axis formation in the Drosophila embryo. Curr Biol. 2003;13:2097–2102. doi: 10.1016/j.cub.2003.10.062. [DOI] [PubMed] [Google Scholar]
- Maier T, Guell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583:3966–3973. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Martin B, Brenneman R, Becker KG, Gucek M, Cole RN, Maudsley S. iTRAQ analysis of complex proteome alterations in 3xTgAD Alzheimer’s mice: understanding the interface between physiology and disease. PLoS One. 2008;3:e2750. doi: 10.1371/journal.pone.0002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean KA, Yourth CP, Lazzaro BP, Clark AG. The evolutionary costs of immunological maintenance and deployment. BMC Evol Biol. 2008;8:76. doi: 10.1186/1471-2148-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister S, Kanzok SM, Zheng XL, Luna C, Li TR, Hoa NT, Clayton JR, White KP, Kafatos FC, Christophides GK, Zheng L. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. Proc Natl Acad Sci U S A. 2005;102:11420–11425. doi: 10.1073/pnas.0504950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JH, Goncalves RL, Lara FA, Dias FA, Gandara AC, Menna-Barreto RF, Edwards MC, Laurindo FR, Silva-Neto MA, Sorgine MH, Oliveira PL. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011;7:e1001320. doi: 10.1371/journal.ppat.1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A, Unwin RD, Evans CA, Griffiths S, Carney L, Zhang L, Jaworska E, Lee CF, Blinco D, Okoniewski MJ, Miller CJ, Bitton DA, Spooncer E, Whetton AD. Eight-channel iTRAQ enables comparison of the activity of six leukemogenic tyrosine kinases. Mol Cell Proteomics. 2008;7:853–863. doi: 10.1074/mcp.M700251-MCP200. [DOI] [PubMed] [Google Scholar]
- Povelones M, Upton LM, Sala KA, Christophides GK. Structure-function analysis of the Anopheles gambiae LRIM1/APL1C complex and its interaction with complement C3-like protein TEP1. PLoS Pathog. 2011;7:e1002023. doi: 10.1371/journal.ppat.1002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M, Waterhouse RM, Kafatos FC, Christophides GK. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science. 2009;324:258–261. doi: 10.1126/science.1171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost-Javier KN, Chen S, Rasgon JL. Vitellogenin gene expression in autogenous Culex tarsalis. Insect Mol Biol. 2010;19:423–429. doi: 10.1111/j.1365-2583.2010.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumpuni CB, Demaio J, Kent M, Davis JR, Beier JC. Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am J Trop Med Hyg. 1996;54:214–218. doi: 10.4269/ajtmh.1996.54.214. [DOI] [PubMed] [Google Scholar]
- Riehle MM, Xu J, Lazzaro BP, Rottschaefer SM, Coulibaly B, Sacko M, Niare O, Morlais I, Traore SF, Vernick KD. Anopheles gambiae APL1 is a family of variable LRR proteins required for Rel1-mediated protection from the malaria parasite, Plasmodium berghei. PLoS One. 2008;3:e3672. doi: 10.1371/journal.pone.0003672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JF. Angiotensin-I-converting enzyme and its relatives. Genome Biol. 2003;4:225. doi: 10.1186/gb-2003-4-8-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- Straif SC, Mbogo CN, Toure AM, Walker ED, Kaufman M, Toure YT, Beier JC. Midgut bacteria in Anopheles gambiae and An. funestus (Diptera: Culicidae) from Kenya and Mali. J Med Entomol. 1998;35:222–226. doi: 10.1093/jmedent/35.3.222. [DOI] [PubMed] [Google Scholar]
- Valanne S, Wang JH, Ramet M. The Drosophila Toll signaling pathway. J Immunol. 2011;186:649–656. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]
- Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz J, Osta MA, Kafatos FC, Muller HM. The roles of two clip domain serine proteases in innate immune responses of the malaria vector Anopheles gambiae. J Biol Chem. 2005;280:40161–40168. doi: 10.1074/jbc.M506191200. [DOI] [PubMed] [Google Scholar]
- Weber AN, Tauszig-Delamasure S, Hoffmann JA, Lelievre E, Gascan H, Ray KP, Morse MA, Imler JL, Gay NJ. Binding of the Drosophila cytokine Spatzle to Toll is direct and establishes signaling. Nat Immunol. 2003;4:794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YJ, Davies DM. Trypsin and chymotrypsin during metamorphosis in Aedes aegypti and properties of the chymotrypsin. J Insect Physiol. 1971;17:117–131. doi: 10.1016/0022-1910(71)90048-5. [DOI] [PubMed] [Google Scholar]
- Ye T, Zhang X. Involvement of Ran in the regulation of phagocytosis against virus infection in S2 cells. Dev Comp Immunol. 2013;41:491–497. doi: 10.1016/j.dci.2013.07.015. [DOI] [PubMed] [Google Scholar]
- You L, Yin J. Patterns of regulation from mRNA and protein time series. Metab Eng. 2000;2:210–217. doi: 10.1006/mben.1999.0139. [DOI] [PubMed] [Google Scholar]
- Zerofsky M, Harel E, Silverman N, Tatar M. Aging of the innate immune response in Drosophila melanogaster. Aging Cell. 2005;4:103–108. doi: 10.1111/j.1474-9728.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- Zou Z, Souza-Neto J, Xi Z, Kokoza V, Shin SW, Dimopoulos G, Raikhel A. Transcriptome analysis of Aedes aegypti transgenic mosquitoes with altered immunity. PLoS Pathog. 2011;7:e1002394. doi: 10.1371/journal.ppat.1002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR primers used in this study. “Gene name” displays the name of the gene targeted by the primer, “AGAPID” displays the vectorbase identifier for the targeted gene, “Primer name” is the name given to the primer, and “Primer sequence” displays the sequence of the primer.
Supplementary data for figures 3 and 4. Includes number of mosquitoes assayed, range, prevalence median, and % change in the number of oocysts.
Microarray and proteomic data analyzed in this study. “An. stephensi contig id” is a unique id to each sequence in the A. stephensi transcriptome, “An. gambiae gene id” is the vectorbase ID of the closest corresponding An. gambiae gene, “Predicted GO category” is the GO category of the closest corresponding An. gambiae gene, “An. gambiae gene name” is the name of the closes corresponding An. gambiae gene, and the rest of the columns are log2 transformed values of the transcript or protein abundance ratios for the different experimental groups. GO abbreviations are; I: immunity and apoptosis; Redox/Stress/Mit: oxidoreductive, stress-related and mitochondrial; CS: cytoskeletal, structural; M: metabolism; RTT: replication, transcription, translation; P/D: proteolysis, digestion; TRP: transport; D: diverse; U: unknown functions.
qRT-PCR verification of microarray data. 7 genes upregulated in the 12 hours PBM midguts samples of the microarray were chosen and their fold-change measured by qRT-PCR to verify their regulation. Of the 7, 4 were similarly regulated as measured by qRT-PCR and all 7 were upregulated in at least one experimental condition.
qRT-PCR verification of dsRNA mediated gene knockdown. mRNA was collected from dsRNA injected mosquitoes and used for qRT-PCR to measure the percent knockdown of the gene. All genes showed greater than 50% knockdown.
Correlation between mRNA and protein levels in the mosquito midgut (A, B) and fat body (C, D) following a blood meal. In the midgut there was no significant correlation between mRNA and protein levels whether looking at all genes (A, r2=0.0003, F-statistic = 0.4276, p-value = 0.5133) or only significantly regulated genes (B, r2=.0005, F-statistic = 0.1766, p-value = 0.6746), while in the fat body there was a significantly correlation both when looking at all genes (C, r2=0.02183, F-statistic = 34.28, p-value < 0.001) and when looking at only significantly regulated genes (D, r2=0.3213, F-statistic = 18.47, p-value < 0.001).