Abstract
Background & Aims
Aberrant activation of βcatenin and Yes-associated protein 1 (Yap1) signaling pathways have been associated with development of multiple tumor types. Yap functions as a transcriptional co-activator by interacting with TEAD DNA binding proteins. We investigated the interactions among these pathways during hepatic tumorigenesis.
Methods
We used immunohistochemical analysis to determine expression of β-catenin and Yap1 in liver cancer specimens collected from patients in Europe and the US, consisting of 104 hepatocellular carcinoma (HCC), 62 intrahepatic cholangiocarcinoma (ICC), and 94 hepatoblastoma samples. We assessed βcatenin and Yap1 signaling and interactions in hepatoblastoma cell lines ((HuH6, HepG2, HepT1, HC-AFW1, HepG2, and HC-AFW1); proteins were knocked down with small interfering (si)RNAs and effects on proliferation and cell death were measured. Sleeping beauty-mediated hydrodynamic transfection was used to overexpress constitutively active forms of β catenin ( N90-βcatenin) and Yap1 (YapS127A) in livers of mice; tissues were collected and histologic and immunohistochemical analyses were performed.
Results
We observed nuclear localization of βcatenin and Yap1 in 79% of hepatoblastoma samples, but not in most HCC or ICC tissues. Yap1 and β catenin co-precipitated in hepatoblastoma but not HCC cells. siRNA-mediated knockdown of Yap1 or β catenin in hepatoblastoma cells reduced proliferation in an additive manner. Knockdown of Yap1 reduced its ability to co-activate transcription with βcatenin; βcatenin inhibitors inactivated Yap1. Overexpression of constitutively active forms of Yap1 and βcatenin in mouse liver led to rapid tumorigenesis, with 100% mortality by 11 weeks. Tumors cells expressed both proteins, and human hepatoblastoma cells expressed common targets of their 2 signaling pathways. Yap1 binding of TEAD factors was required for tumorigenesis in mice.
Conclusions
β catenin and the transcriptional regulator Yap1 interact physically and are activated in most human hepatoblastoma tissues; overexpression of activated forms of these proteins in livers of mice leads to rapid tumor development. Further analysis of these mice will allow further studies of these pathways in hepatoblastoma pathogenesis and could lead to the identification of new therapeutic targets.
Keywords: liver cancer, gene regulation, oncogene, tumor formation
INTRODUCTION
The role of developmental pathways in oncogenesis is indisputable. Often these pathways cooperate in the process of tumorigenesis where a single pathway alone is insufficient. Among them, the Wnt/β-catenin signaling cascade has been implicated as the driver oncogenic event in various tumor types including liver cancer, where its aberrant activation is evident in a significant subset of hepatocellular carcinomas (HCC) and hepatoblastomas (HB). β-Catenin activation in 20-30% of HCC and 25% of HB is due to point mutations in exon 3 of CTNNB1, while additional 50% of HB show βcatenin activation due to monoallelic deletions affecting exon 3 (Reviewed in1, 2). As a consequence of these genetic alterations, β-catenin is stabilized and translocates to the nucleus, where it acts as a co-factor to dictate expression of target genes implicated in cell cycle progression, survival, angiogenesis, and metabolism. Interestingly, over-expression of full-length, point-mutant or deletion-mutant (ΔN90) β-catenin alone in mouse hepatocytes is insufficient for oncogenesis. Instead, the presence of a second hit, including concomitant monoallelic LKB loss, Ha-ras activation, or exposure to diethylnitrosamine led to enhanced HCC development in mice.2 Recently, interactions of Yap and Wnt/β-catenin signaling pathways have become the focus of much research. Intriguingly, the crosstalk between the two pathways is context-dependent and can result in synergism or antagonism.3,4
The Hippo tumor suppressor cascade is an evolutionally conserved developmental pathway involved in the control of organ size, tissue regeneration, stem cell self-renewal, and tumor development (Reviewed in 5). Yes-associated protein (Yap) is the major downstream effector of the Hippo pathway.6 Hippo cascade phosphorylates Yap, leading to its cytoplasmic localization and proteolysis. Yap functions as a transcriptional co-activator and interacts with TEA Domain (TEAD) DNA binding proteins to initiate the expression of target genes, such as Survivin, CTGF, Jag1, and Cyr61.7,8 Yap activation has been described in hepatic and biliary regeneration9 and also detected in multiple tumor types, including HCC, where Yap nuclear localization has been observed in ~60% of cases, and in HB, where its nuclear localization is evident in ~70% of cases.10-12 Here, we show that concomitant activation of Yap and β-catenin occurs in the majority of human HB specimens but not in HCC or intrahepatic cholangiocarcinomas (ICC) samples. The cooperation between these two pathways is evident in HB cell lines, whose in vitro growth is strongly restrained by combined suppression of β-catenin and Yap protooncogenes. Furthermore, we demonstrate that overexpression of active Yap or β-catenin gene alone does not lead to any liver tumor development in mice, whereas co-expression of the two genes results in rapid hepatocarcinogenesis. Intriguingly, most tumors that developed in Yap/β-catenin mice display histologic features reminiscent of human HB and expression of markers such as delta-like protein/preadipocyte factor 1/fetal antigen 1 (Dlk1), α-fetoprotein (AFP), glypican-3 (GPC), cyclin D1 (Cnnd1) and c-Myc.13 Altogether, the presented data supports a crucial role of the Yap and β-catenin pathways in human HB development. The Yap/β-catenin mouse model might be useful both to elucidate the biology of HB and to test novel therapies.
MATERIALS AND METHODS
Human HB, HCC, and ICC Samples
Ninety-four HB samples collected at the University of Basel (Switzerland), University of Greifswald (Germany), University of Tuebingen (Germany), and University of Pittsburgh (U.S.A.) were assessed for Yap and β-catenin expression by immunohistochemistry (Online Supplementary Table 1). A collection of 103 HCC specimens from Europe recently described (Online Supplementary Table 2)14 and a collection of 62 ICC samples from the Universities of Basel and Greifswald were also assessed for Yap and β-catenin immunostaining (Online Supplementary Table 3). Institutional Review Board approval was obtained at participating hospitals.
Additional methods can be found in supplementary information
RESULTS
Concurrent nuclear localization of YAP and β-catenin is evident in human HB but not HCC or ICC specimens
To investigate any possible cooperation between Yap and β-catenin pathways in human liver tumors, first we evaluated the activation of the two cascades in a large collection of human HCC, ICC, and HB specimens. Nuclear accumulation of β-catenin and Yap proteins via immunohistochemistry is indicative of activation of respective pathways. In HCC samples (n=103), 28% of HCC cases showed nuclear β-catenin immunoreactivity, whereas nuclear Yap was observed in 65% of tumors (Table 1 and Figure 1). Interestingly, only 4 of 103 HCC cases (<4%) showed concomitant nuclear β-catenin and Yap localization. In ICC, nuclear accumulation of β-catenin was extremely rare (2/62; 3.2%), whereas Yap protein was almost ubiquitously detected in the nucleus (98.4%, Table 1 and Figure 1). In HB, nuclear accumulation of β-catenin and Yap was detected in ~88% and ~85% cases (n=94), respectively (Table 1). Intriguingly, consecutive sections show a notable overlap in nuclear staining of β-catenin and Yap in most tumor cells in ~79% of HB cases (Figure 1 and Table 1). Altogether, this novel observation suggests a possible cooperation between β-catenin and Yap signaling pathways especially in HB.
Table 1.
Immunolocalization of YAP and β-catenin proteins by immunohistochemistry in human hepatocellular carcinoma (HCC), hepatoblastoma (HB) and intrahepatic cholangiocarcinoma (ICC) specimens.
| HCC |
β-Catenin nuclear
staining |
Total
(YAP) |
||
| Positive | Negative | |||
|
YAP nuclear
staining |
Positive | 4 | 63 | 67 |
| Negative | 25 | 11 | 36 | |
| Total (β-Catenin) | 29 | 74 | 103 | |
| HB |
β-Catenin nuclear
staining |
Total
(YAP) |
||
| Positive | Negative | |||
|
YAP nuclear
staining |
Positive | 74 | 6 | 80 |
| Negative | 9 | 5 | 14 | |
| Total (β-Catenin) | 83 | 11 | 94 | |
| ICC |
β-Catenin nuclear
staining |
Total
(YAP) |
||
| Positive | Negative | |||
|
YAP nuclear
staining |
Positive | 2 | 59 | 61 |
| Negative | 0 | 1 | 1 | |
| Total (β-Catenin) | 2 | 60 | 62 | |
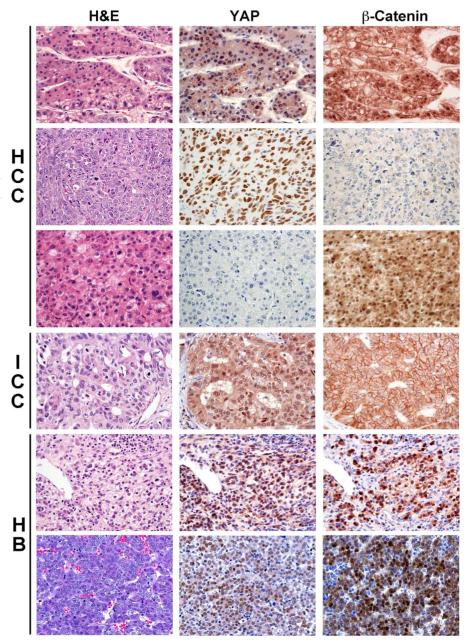
Figure 1. Representative immunohistochemical staining for Yap and β-catenin in human liver tumor samples.
Consecutive sections from various liver tumors including hepatocellular carcinomas (HCC), intrahepatic cholangiocarcinomas (ICC) and hepatoblastomas (HB) were stained for H&E, Yap and β-catenin. Representative HCC samples in top 3 rows show the three types of staining patterns, i.e. a small subset with moderate nuclear Yap and nuclear β-catenin (top) staining, and predominant subsets of either nuclear Yap (middle) or nuclear β-catenin (lower) only. The fourth row shows a representative ICC with the predominant pattern of only nuclear Yap and lack of any nuclear β-catenin in the tumor cells. The bottom two rows show two representative HB, both displaying the predominant pattern of simultaneous nuclear Yap and β-catenin immunoreactivity in several tumor cells.
Combined β-catenin and Yap silencing impairs the growth of human HB cells
To unravel the role of β-catenin and Yap cascades and their possible crosstalk in HB, we investigated the two pathways in various pediatric liver tumor cell lines (HuH6, HepG2, HepT1, HC-AFW1). β-Catenin and Yap proteins were strongly expressed in all tumor cell lines tested (Supplementary Figure 1A). Subsequently, HepG2 and HCAFW1 cells were further assessed for functional interaction of Yap and β-catenin, since both cell lines, like most HB, harbor significant deletions in β-catenin exon-3. In fact HepG2 cells show deletion of 116 and HC-AFW1 a deletion of 49 amino acids.15,16 Successful silencing of β-catenin or Yap gene in these two cell lines (Figure 2A) led to a notable decrease in cell proliferation and a significant increase in apoptosis as well (Figure 2B-C). However, more pronounced and synergistic anti-growth effect was observed following simultaneous silencing of β-catenin and Yap genes in HepG2 and HC-AFW1 cells (Figure 2B-C). Additionally, a significant decrease in HepG2 cell viability was observed after concurrent knockdown of Yap and β-catenin as compared to each on its own (Figure 2D). HepG2 and HC-AFW1 cells were also treated with inhibitors of Wnt/β-catenin (IWR-1-endo and PNU74654) and/or Yap/TEAD (Verteporfin) cascades. While treatment with one inhibitor alone decreased proliferation and increased apoptosis, the combined treatment of IWR-1-endo or PNU74654 with Verteporfin led to strong decrease in proliferation and massive apoptosis in HepG2 and HC-AFW1 cells (Supplementary Figure 1B-E).
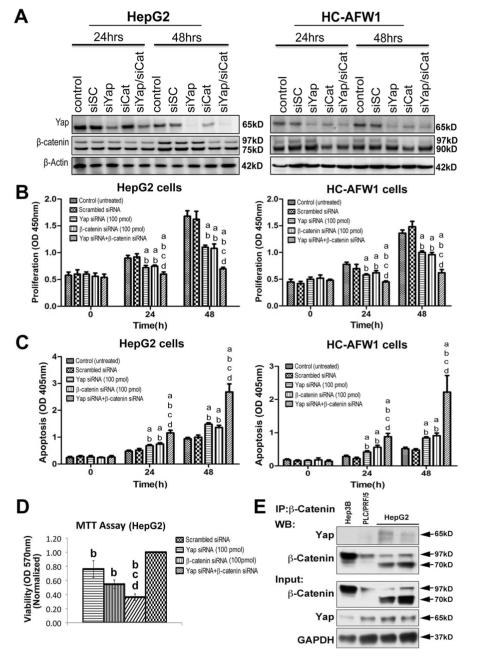
Figure 2. Combined β-catenin and Yap silencing impairs growth of human HB cells.
A. Western blotting shows a notable decrease in β-catenin and Yap levels after βcatenin siRNA transfection in HepG2 and HC-AFW1, whereas Yap siRNA transfection led to decrease in Yap but not β-catenin protein levels. Combination of the two siRNAs against β-catenin and Yap led to decrease in both proteins at 24 and 48 hours. β-Actin was used as loading control. A Western blot shows full length (97kD) and truncated (75kD) forms of β-catenin in HepG2 cells on the left and full length (97kD) and truncated form (~90kD) of β-catenin in HC-AFW1 cells on the right due to respective deletions in exon-3.
B. Single knockdown of β-catenin and Yap affects cell proliferation of HepG2 cells (left) and HC-AFW1 (right) as assessed by BrdU proliferation assay at both 24 and 48 hours. However, double knockdown has an even greater effect on proliferation of both cell lines.
C. An increase in HepG2 (left) and HC-AFW1 (right) cell death is evident by knockdown of either Yap or β-catenin at 24 and 48 hours as assessed by the Cell Death detection Elisa assay. Nevertheless, combined knockdown of both further augmented cell death in the two cell lines.
D. Cell death was also confirmed by MTT assay in HepG2 cells after knockdown of either Yap, β-catenin or combination. While silencing of these genes singly affected viability significantly, a more pronounced effect was evident after double knockdown.
E. Immunoprecipitation done with β-catenin-preconjugated A/G agarose beads shows association of β-catenin to Yap in two different batches of HepG2 cells and not in HCC cell lines (Hep3B and PLC/PRF/5). Pull down of β-catenin in each cell line was verified by Western blotting after immunoprecipitation for βcatenin. Western blot analysis using whole cell lysates from all cell lines show expression of β-catenin and Yap as input along with GAPDH as a loading control.
All experiments were conducted at least 3 times in triplicate. Statistical analysis: a-different from Control; b- different from Scrambled siRNA; c- different from Yap siRNA; d- different from β-catenin. All p values < 0.001.Abbreviations: si, β-catenin siRNA; siSC, scrambled siRNA.
Yap and β-catenin associate in HB but not HCC cells
Since Wnt and Hippo signaling can crosstalk at many different levels,3,4 we first investigated whether β-catenin and Yap physically interact in liver tumor cells. We assayed whole cell lysates from two HCC cell lines (Hep3B and PLC/PRF/5) and HepG2 cells for any association of the two proteins. While β-catenin was pulled down in all cell lines, its association with Yap was evident only in HepG2 cells as shown in co-precipitation studies (Figure 2E). Reverse immunoprecipitation also verified the association between β-catenin and Yap in HepG2 cells only (data not shown). β-Catenin and Yap protein expression was evident in all three-cell lines examined (Figure 2E).
Mutual and synergistic effect on β-catenin and Yap signaling by silencing the two genes individually or dually in human HB cells
Next, to address the interplay between Yap and β-catenin signaling in HB, we examined the impact of Yap and/or β-catenin gene silencing in HepG2 (Figure 3) and HC-AWF1 (Supplementary Figure 2) cells. We found that knockdown of Yap led to decreased expression of Yap target genes, including CTGF, Cyr61, Jag1, and Survivin in both HB cell lines (Figure 3A and Supplementary Figure 2A). Although β-catenin expression levels were unchanged, some of the Wnt/β-catenin target genes, such as c-Myc and Cyclin D1, were significantly downregulated, whereas Axin2 and DKK1 levels remained unmodified, in Yap-depleted HepG2 (Figure 3A) and HC-AFW1 (Supplementary Figure 2A) cells. In addition, Yap knockdown strongly reduced β-catenin-TCF reporter activity as reflected by a significant decrease in TopFlash reporter assay in both HepG2 (Figure 3B) and HC-AFW1 (Supplementary Figure 2B) cells. Next, we wanted to address if decrease of β-catenin signaling after Yap knockdown is unique to HB. We chose the Hep3B cells, a human HCC cell line, for transfection with siRNA against Yap. Intriguingly, Yap knockdown led to an increase in TopFlash reporter activity in Hep3B cells, suggesting that Yap-β-catenin cooperation is unique to HB cells (Figure 3C).
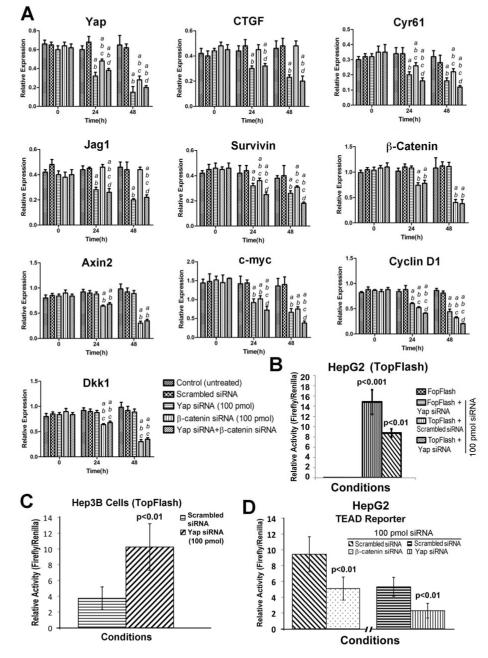
Figure 3. Impact of Yap and β-catenin knockdown in HB cells, singly or dually, on β-catenin and Yap target gene expression.
A. A decrease in expression of Yap and its targets like CTGF, Cyr61, Jag1 and survivin, after silencing Yap in HepG2 cells at 24 and 48 hours after transfection. Transfection of β-catenin siRNA also led to decrease in the expression of Yap and some of its targets like Cyr61 and survivin, although no change in expression of CTGF or Jag1 was observed. Dual knockdown of β-catenin and Yap led to a more pronounced impact on Yap targets Cyr61 and survivin. A decrease in expression of β-catenin and its target genes such as Axin2, c-Myc, Cyclin D1 and DKK1 was evident upon β-catenin silencing in HepG2 cells at both 24 and 48 hours after transfection. While, Yap suppression had no impact on expression of β-catenin, it reduced expression of c-Myc and Cyclin D1 but not Axin2 or Dkk1. Dual knockdown of Yap and β-catenin led to a more pronounced decrease in c-Myc and Cyclin D1 expression. All experiments were conducted at least 3 times and in triplicates. Statistical analysis: a- different from Control; b- different from Scrambled siRNA; c- different from Yap siRNA; d- different from β-catenin siRNA. All p values < 0.001.
B. A significant decrease in β-catenin transactivation was observed in a TopFlash reporter assay at 48 hours after silencing of Yap in HepG2 cells (p<0.01). FopFlash –transfected cells showed no luciferase activity.
C. A significant increase in β-catenin transactivation was observed in a TopFlash reporter assay at 48 hours of Yap silencing in Hep3B cells, a human HCC cell (p<0.01) as compared to negative control siRNA transfected cells.
D. A significant decrease in TEAD-luciferase activity was observed in HepG2 cells that were transfected with siRNA against YAP (p<0.01) or β-catenin (p<0.01) for 48 hours as compared to control siRNA.
To determine the impact of β-catenin modulation on Yap signaling, we next examined the effect of β-catenin knockdown in HB cells. As expected, β-catenin knockdown decreased the levels of β-catenin and its targets, including c-Myc, Cyclin D1, Axin2, and Dkk1 in both cell lines (Figure 3A and Supplementary Figure 2A). Intriguingly though, levels of Yap and its downstream targets, Cyr61 and Survivin, were also suppressed in the two cell lines depleted of β-catenin, whereas expression of CTGF and Jag1 remained unaltered (Figure 3A and Supplementary Figure 2A). As expected, knockdown of Yap in HepG2 cells strongly decreased TEAD reporter activity. A significant decline in TEAD transcriptional activity was also detected following β-catenin knockdown in the same cells (Figure 3D).
More importantly, concomitant knockdown of both β-catenin and Yap genes in the two HB cell lines was accompanied by a synergistic effect leading to further decreases in expression of c-Myc, Cyclin D1, Cyr61, and Survivin when compared to knockdown of β-catenin or Yap alone (Figure 3A and Supplementary Figure 2A). Altogether, these data suggests an important cooperative role of Yap and β-catenin pathways in HB through cross-regulation of specific downstream targets.
Constitutively active Yap and β-catenin together trigger liver tumor development in mice
To further ascertain the effect of concomitant activation of Yap and β-catenin cascades on hepatocarcinogenesis in vivo, we hydrodynamically delivered to the mouse liver constitutively active Yap (YapS127A)11 with a FLAG tag, and constitutively active β-catenin (ΔN90-β-catenin)17 with a Myc tag, either alone or in combination. As we reported previously, overexpression of ΔN90-β-catenin did not lead to any tumor formation in mice even 1-year post injection17 (Supplementary Figure 3A). Similarly, overexpression of YapS127A alone did not result in any liver anomaly even when harvested at 22.5 weeks post injection (Supplementary Figure 3B). In striking contrast, co-expression of YapS127A and ΔN90-β-catenin, which will be here referred henceforth as Yap/β-catenin, led to lethal burden of liver tumors by 9-11 weeks post injection (Figure 4A). Macroscopically, livers of Yap/β-catenin mice harbored white nodules as early as 3 weeks post injection and these lesions rapidly expanded and occupied most of the liver surface at all later stages (Figure 4B and Supplementary Figure 3C).
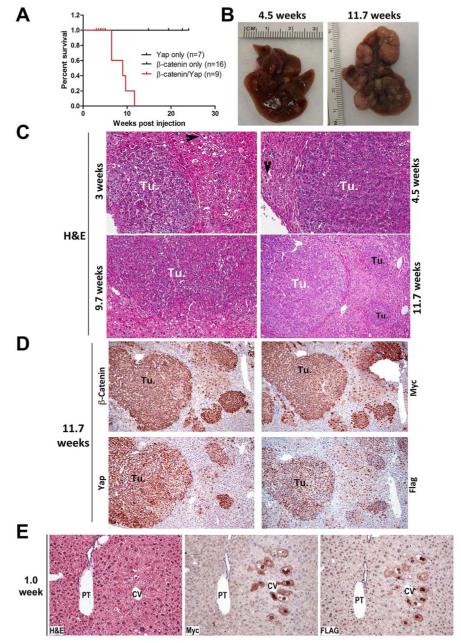
Figure 4. Constitutively active Yap1 and β-catenin co-expression leads to HB in mice.
A. Kaplan-Meier curve shows increased mortality in mice co-expressing constitutively active Yap and β-catenin in the liver of Yap/β-catenin mice.
B. Gross specimen of livers from Yap/β-catenin mice at 4.5 and 11.7 weeks after injection exhibit large tumors on the surface.
C. H&E sections of livers from different times in Yap/β-catenin mice showing preneoplastic lesions and tumors at 3, 4.5, 9.7 (200x), and 11.7 (100x) weeks after injection. Tumors (Tu.) are composed of small hepatocytes with prominent nuclei and histology is reminiscent of fetal or crowded fetal HB. Arrowheads indicate preneoplastic single or clusters of transfected clear-cell hepatocytes.
D. IHC performed on contiguous liver sections from 11.7 week-injected Yap/βcatenin mice show tumors (Tu.) at 100x to be composed of cells over-expressing β-catenin and Myc-tag as well as Yap and Flag-tag. A corresponding H&E of these sections is shown in the right lower panel of C.
E. A representative H&E and IHC on adjacent sections, shows large hepatocytes with clear cytoplasm in the pericentral area to be positive for Myc-tag and Flag-tag at 1 week after Yap/β-catenin injection.
Histological examination of the nodules in Yap/β-catenin mouse livers proved them to be indeed hepatocellular tumors having developed as early as 3 weeks after injection. In addition to these tumors, preneoplastic single or clusters of clear-cell hepatocytes were also seen (arrowheads in Figure 4C and Supplementary Figure 4A). Despite an occasional hepatocellular adenoma found 11.7 weeks after injection, the vast majority of the tumors were reminiscent of human HB (Figure 4C). Characteristic for HB, the tumor cells in all investigated time-points showed the morphology of fetal or intermediate hepatoblasts, being smaller than normal hepatocytes with small round or oval nuclei. Mitoses were frequently seen but the tumors lacked severe nuclear pleomorphism, a fibrous capsule, inflammation, necrosis or fibrosis. Thus, they morphologically resembled the fetal or crowded fetal subtype of HB (Figure 4C). A more detailed histology of these representative HB is shown in supplementary figure 4B.
To demonstrate that the observed tumors were indeed derived from cells expressing Yap and β-catenin transgenes, immunohistochemistry (IHC) was performed for Flag and Myc tags that were indicative of expression of Yap and β-catenin, respectively. As shown in representative tumors at 11.7 weeks, the lesions were concomitantly positive for β-catenin and Myc as well as Yap and Flag (Figure 4D).
Hepatocytes in pericentral area of liver are the source of HB in the current model
To address the cell of origin of the observed HB, we performed ultrastructural analysis of the Yap/β-catenin livers by electron microscopy (EM) after 1, 3, and 7 days of hydrodynamic injection. In semi-thin section, transfected hepatocytes exhibited enlarged nuclei with prominent nucleoli and were located in acinar zone 3 of the liver acinus close to the hepatic vein, but were absent near the portal tracts in acinar zone 1, reflecting the transfection pattern of hydrodynamic gene delivery (Supplementary Figure 5A-C). In addition, EM showed that transfected hepatocytes contained glycogen, formed tight cell junctions with neighbouring normal hepatocytes and showed small bile canaliculi, further underscoring the hepatocellular origin of the tumors (Supplementary Figure 5D-5G).
We next examined the expression of Myc-tag and Flag-tag in adjacent sections as early as 1 week after Yap/β-catenin injection. A common subset of hepatocytes in pericentral area exhibited simultaneous positivity for Myc and Flag indicating expression of the injected β-catenin and Yap genes, respectively (Figure 4E). The hepatocytes showed an altered morphology, as these were larger, with enlarged clear cytoplasm and atypical nuclei (Supplementary Figure 5H-5J). Subsequently, small hepatocellular tumors began to emerge in the hepatic parenchyma as early as 3 weeks post injection (Supplementary Figure 5K-5M). During evolution from preneoplastic lesions to small tumors, altered hepatocytes remodeled towards a smaller size with more rounded nuclei within the tumor core, whereas enlarged hepatocytes continued to persist singly either in the periphery of tumors or in the neighboring parenchyma (Supplementary Figure 5K-5M).
To further verify the cell of origin of HB in the current mouse model, we used lineage tracing in Alb-CreERT2;R26R EYFP mice. A week after four daily injections of 1 mg Tamoxifen, these animals were given a hydrodynamic injection of Yap127A/ΔN90βcatenin and followed for 11 weeks. β-Catenin and EYFP double immunofluorescence shows clear co-localization of the two proteins in the tumors, further validating the hepatocytes to be the cell of origin of HB in the current mouse model (Supplementary Figure 5N-5P).
Immunohistochemical characterization of HB in Yap/β-catenin mice
To further characterize the observed tumors in Yap/β-catenin mice, IHC and RNA analysis was performed for various downstream targets of the Wnt and Yap signaling pathways. Besides being strongly immunoreactive to β-catenin, the observed tumors were also strongly positive for Cyclin D1 and c-Myc (non-c-Myc-tag reactive antibody) (Figure 5A and Supplementary Figure 6A). Intriguingly, the tumors were GS-negative despite histological resemblance to fetal HB (Figure 5A).
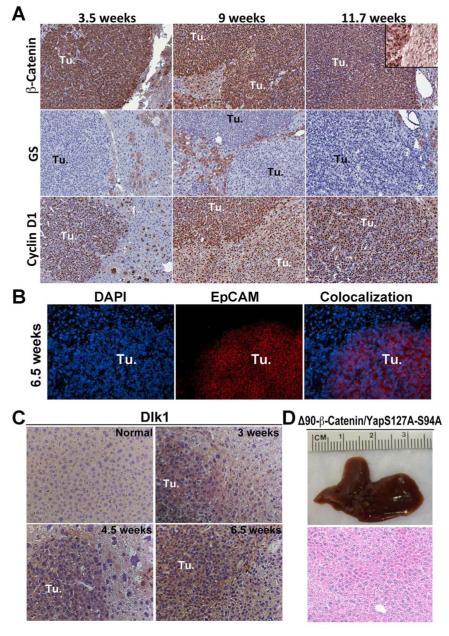
Figure 5. Molecular characterization of liver tumors induced by activated Yap1 and β-catenin in mice.
A. IHC on liver sections from Yap/β-catenin at 3.5, 9 and 11.7 weeks shows that tumors (Tu.) express high levels of cytoplasmic and nuclear β-catenin (top row). Inset in the top right panel shows β-catenin staining in the hepatocyte membrane in non-tumor area of liver of Yap/β-catenin mouse at 11.7 weeks. Tumors were positive for Cyclin D1 (bottom row) but negative for GS staining (middle row) at all examined time points. (100x)
B. Representative immunofluorescence staining on liver sections of Yap/β-catenin-injected mice after 6.5 weeks shows EpCAM immunoreactivity (red) limited to tumors, while DAPI (blue) stained nuclei of all cells in the section (100x).
C. IHC on livers sections from a normal liver of a Yap only mouse or Yap/β-catenin mice showing increased Dlk1 expression in the tumors (Tu.) at all times as shown in representative images from 3, 4.5 and 6.5 weeks after injection (200x).
D. A representative gross and H&E image (200x) of liver section showing complete lack of any hepatic tumor in mice co-injected with Δ90-β-catenin and a Yap mutant, lacking TEAD binding activity (YapS127AS94A) even after 12 weeks of injection.
Yap targets including Cyr61, Survivin, CTGF and Jag1 were concomitantly expressed at high levels in Yap/β-catenin tumors (Supplementary Figure 6B). Also, expression of genes encoding for markers of hepatoblasts and undifferentiated hepatocytes, such as Glypican 3 (Gpc3), EpCAM (Epcam) and α-fetoprotein (Afp), were upregulated in the tumors (Supplementary Figure 6B). Strong EpCAM immunoreactivity was also confirmed by immunofluorescence (Figure 5B).
Since markers of HB such as Glypican 3 and α-fetoprotein are not specific and can be expressed by a high percentage of HCC, we next assessed the liver tumors in Yap/βcatenin mice for a more specific marker of HB. Dlk-1 has been recently characterized to be specifically expressed in HB in patients13. Notably, all the tumors observed in Yap/βcatenin mice were notably positive for Dlk-1, further validating diagnosis of HB while normal adult liver is negative for this marker (Figure 5B)
As a result of increased expression of β-catenin and Yap targets involved in cell cycle progression such as Cyclin D1 and c-Myc, the tumors were also positive for proliferating cell nuclear antigen (PCNA; not shown), and Ki67 staining (Supplementary Fig 6A). Consequently, high proliferation in preneoplastic and neoplastic lesions from Yap/β-catenin mice was detected at all examined time points (Supplementary Fig 6A). Intriguingly, Yap/β-catenin preneoplastic and neoplastic lesions also showed elevated apoptosis (Supplementary Fig 6B).
Transcriptionally active Yap is required for hepatocarcinogenesis in association with β-catenin
Yap mainly functions as a transcriptional co-activator by interacting with TEAD DNA binding proteins.18 However, recent studies indicate that Yap can also act independent of TEAD by binding to other transcription factors, including Runx, Erb4, and Smad1.19 To investigate whether Yap/β-catenin induced tumor formation requires interaction with TEAD, the binding site for TEAD transcription factors was mutated in the constitutively active Yap (YapS94AS127A). Strikingly, combined injection of YapS94AS127A and ΔN90-β-catenin led to complete abrogation of tumorigenesis in mice (n=8) even at 12 weeks after injections (Figure 5C). This observation highlights the importance of Yap transcriptional activity via TEAD-binding domain to be critical in cooperation with βcatenin to induce HB in the current model.
DISCUSSION
HB is the most common primary malignant neoplasm of the liver in childhood1. The cellular and molecular basis of the disease is poorly understood. Aberrant Wnt/β-catenin signaling has been long considered a hallmark of HB with rate of point mutations and genetic deletions in β-catenin gene evident in almost 90% of the cases. However, overexpression of oncogenic forms of β-catenin have never led to HB in mice, which has strongly suggested its cooperation with another pathway in HB pathogenesis.2 Yap signaling has gained importance in recent years as a major regulator of organ size in various organs, including the liver. Alterations in various members of the pathway, such as Lats1 downregulation and Yap amplification, have been associated with HCC development in mice and men.12,20,21 Recently, Yap activation was shown to induce Notch signaling in HCC, which was independent of Yap-β-catenin interaction.14 In accordance with the latter finding, our current analysis showed lack of concomitant nuclear Yap and β-catenin in ~96% of HCC, while Yap and β-catenin were independently nuclear in around 65% and 28% of the patients, respectively. While 11 of 103 HCC patients in our dataset showed progenitor cell features based on positive EpCAM immunoreactivity, only 2 showed simultaneous nuclear Yap and β-catenin. Because of limited numbers of cases, it is difficult to infer if these signaling cascades could be playing a pathogenetic role in HCC with progenitor cell features. Intriguingly, this was in stark contrast to HB, where 79% of the cases showed concurrent nuclear localization of Yap and β-catenin, suggesting simultaneous activation of these two pathways in this tumor type.
The biochemical interplay between Yap and β-catenin cascades was confirmed next in liver tumor cell lines that are either characterized as HB or harbor exon-3 deletions in CTNNB1 gene, similar to what is observed in predominant subsets of HB in patients. Firstly, an association of β-catenin and Yap was evident in HB cells. In addition, Yap and β-catenin pathways robustly regulated each other’s activity in HepG2 and HCAFW1 cells. Yap depletion reduced β-catenin transcriptional activity, albeit without affecting its gene expression. Interestingly, β-catenin silencing was accompanied by downregulation of Yap levels, similar to that observed in colorectal cancer.4 Intriguingly, Yap silencing led to downregulation of some (c-Myc and Cyclin D1) but not all (Axin2 and DKK1) β-catenin targets, while TopFlash activity was consistently decreased. This was unique to HB cells as in HCC cells Yap silencing led to increased TopFlash reporter activity, which has also been reported in other models.3 This was evident despite lack of association between Yap and β-catenin in Hep3B cells and may be due to interactions of other components of the two cascades. This suggests cell intrinsic differences between HB and HCC. Similarly, TEAD reporter activity along with expression of Yap targets such as Cyr61 and Survivin were decreased following βcatenin knockdown in HB cells. Suffice to say that inhibition of β-catenin and Yap simultaneously and hence of the common four targets led to a synergistic effect on dampening HB cell proliferation and survival. Thus, the present data imply the existence of a complex relationship between Yap and β-catenin, which plays a unique role in growth and development of HB. Although specific nodes of such interactions remain to be determined in the future, various recent reports have identified diverse crosstalks between Yap and β-catenin signaling. Recently, β-catenin activation in HCC has been shown to induce the upregulation of tribbles homolog 2 (TRIB2), which in turn promotes Yap stabilization by inhibiting its proteolysis via the proteasome.22 In contrast, we found that β-catenin knockdown resulted in downregulation of Yap mRNA levels in HB cell lines, implying regulation of Yap levels by β-catenin at transcriptional level. Similarly, Yap has been shown to activate the Wnt/β-catenin cascade in various cell lines by promoting the nuclear translocation of the tyrosine phosphatase SHP2.23 Additional studies will be necessary to determine the molecular basis of β-catenin-Yap cross-regulation in HB cells.
The synergistic impact of β-catenin and Yap in HB pathogenesis was strongly substantiated by our in vivo findings. While overexpression of either activated Yap or βcatenin genes in the mouse liver was not oncogenic, simultaneous expression of the two protooncogenes led to significant morbidity and mortality in mice due to liver tumor development. Intriguingly, Yap/β-catenin mice developed malignant lesions resembling histological features of human HB. The HB observed in the mouse model were reminiscent of human fetal or crowded fetal morphology. The histological findings were validated by molecular analysis including presence of nuclear β-catenin and Yap in tumors along with increased expression of c-Myc, cyclin-D1 and EpCAM. High expression of Afp and Gpc3 was evident in livers of Yap/β-catenin mice as well. The most striking finding was that the tumors were positive for Dlk1, a more specific marker in human HB.13 Intriguingly, despite showing a more fetal morphology, the HB in Yap/βcatenin mice were GS-negative. This is in contrast to fetal HB in patients, which are known to be strongly GS-positive.1 This discrepancy could be due to innate differences in mouse and human HB or to other obscure reasons requiring additional studies.
It should be emphasized that Yap and β-catenin were overexpressed in an adult liver and that the cell of origin of the disease is a mature adult hepatocyte in pericentral area of the liver and not a hepatoblast or a hepatic progenitor, which is typically observed in the periportal area.24 Ultrastructural analysis, time course of Myc-tag and Flag-tag expression after Yap/β-catenin injection as well as lineage tracing studies affirm that HB in our model are derived from pericentral adult hepatocytes. These observations suggest that forced expression of Yap and β-catenin in adult hepatocyte may reprogram it to eventually give rise to HB. Further, constitutively active β-catenin overexpression in mice by itself has never yielded HB,25-28 despite being frequently activated in human HB.1 Therefore, activation of Yap signaling in addition to β-catenin activation may be the initiation event in mouse and human HB, at least in the fetal/crowded fetal subtype. With the current model, we may be able address mechanisms of HB disease initiation and progression with emphasis on Yap and β-catenin signaling cascades. Current analysis already reveals an important role of Yap-β-catenin-TEAD interaction in HB pathogenesis since mutation in TEAD binding domain of Yap abrogated tumorigenesis. Whether TCF binding of β-catenin is essential or there are alternate transcription factors at play remains an open-ended question. This is of utmost relevance since the molecular basis of HB pathogenesis continues to remain obscure. Overall, our observations for the first time support a critical cooperative role of Yap and β-catenin signaling pathways in the development of HB. Future studies using this model will be critical in elucidating the mechanisms of such cooperation and may yield new understanding and therapeutics for this disease.
Supplementary Material
A. Supplemental Table 1: Summary of nuclear localization of Yap and β-catenin in 94 human hepatoblastoma cases.
B. Supplemental Table 2: Summary of nuclear localization of Yap and β-catenin in 103 human hepatocellular carcinoma cases.
C. Supplemental Table 3: Summary of nuclear localization of Yap and β-catenin in 62 human intrahepatic cholangiocarcinoma cases.
D. Supplementary Table 4: Information on primers used for QRTPCR analysis in the current study.
E. Supplementary Table 5: Summary of all mice used in the current study for hydrodynamic injections.
Supplementary Figure 1: A. Western blot using whole cell lysates from four liver tumor cell lines showing β-catenin expression and truncations due to deletions in exon-3. Yap protein expression is also show in the same four cell lines. Protein loading for each cell line is verified by a blot for β-actin.
B-E. Pharmacological inhibition of β-catenin by small molecules PNU74654 and IWR1 endo and Yap-TEAD pathway by small molecule Verteporfin or their combination led to significantly decreased cell proliferation and increased apoptosis in both HepG2 and HC-AFW1 cells when compared to no treatment or treatment of cells with DMSO (solvent for inhibitors) or IWR1 exo (negative control for IWR1 endo). Experiments were conducted at least 3 times in triplicate. Statistical analysis: a- different from Control; b- different from DMSO (solvent); c- different from IWR1 exo; d- different from IWR1 endo; e- different from PNU74654; f- different from Verteporfin; g- different from IWR1 endo + Verteporfin. All p values in the current study were less than 0.0005.
Supplementary Figure 2: A. Decreased expression of Yap and its targets such as CTGF, Cyr61, Jag1 and survivin, after Yap knockdown in HC-AFW1 cells at 24 and 48 hours after transfection. β-Catenin knockdown led to suppression of Yap expression and of its targets Cyr61 and survivin, while no demonstrable effect was evident on CTGF or Jag1. Combined knockdown of both Yap and β-catenin, led to a more pronounced effect on Cyr61 and survivin expression. β-Catenin silencing in HC-AFW1 cells after 24 and 48 hours of transfection causes reduced expression of β-catenin and its targets, including Axin2, c-Myc, Cyclin D1 and DKK1. Yap silencing did not impact β-catenin expression but affected expression of c-Myc and Cyclin D1. Dual knockdown of β-catenin and Yap had a more robust impact on expression of c-Myc and Cyclin D1. All experiments were conducted at least 3 times and in triplicate. Statistical analysis: a- different from Control; b- different from Scrambled siRNA; c- different from Yap siRNA; d-different from β-catenin siRNA. All p values < 0.001.
B. A significant decrease in β-catenin transactivation was observed in a TopFlash reporter assay 48 hours after silencing of Yap in HC-AFW1 cells (p<0.01). Transfection of the same cells with FopFlash containing TCF-mutant showed no luciferase activity.
Supplementary Figure 3: A. H&E staining of a representative liver section showing absence of any microscopic tumor foci at one year after hydrodynamic injection of Δ90βcatenin only.
B. A representative gross specimen and H&E on a liver section show absence of macroscopic or microscopic tumor foci at 22.5 weeks after hydrodynamic injection of Yap127A alone.
C. Gross morphology of livers after co-injection of Yap and β-catenin genes showing multiple tumors at 3, 3.5, 6.5, 9 and 9.7 weeks post injection.
D. IHC for Glutamine Synthetase (GS) shows preneoplastic (PN) lesions to be positive at 3 weeks after Yap/β-catenin injection. In contrast, all HB observed at 3.5, 6.5 and 11.7 weeks after Yap/β-catenin injection were GS-negative as shown in representative liver sections.
Supplementary Figure 4: A. Representative histology from 3 week old Yap/β-catenin livers showing single or clusters of preneoplastic clear-cell hepatocytes (arrowhead) (200x).
B. Representative histology from additional Yap/β-catenin livers showing HB occurrence. A tumor at 6.5 weeks post injection shows the least maturation, being composed of cells with relatively uniform nuclei that are ovoid to angulated with inconspicuous nucleoli arranged in a vague trabecular and glandular pattern with suggestion of rosettes/canaliculi in an occasional focus. There is an increase in nucleo:cytoplasmic ratio in these cells. These areas resemble those that can be seen in embryonal hepatoblastomas though classic rosettes and clear-cut blastemal cells are not identified. Another photomicrograph showing a tumor from 9 weeks with no significant pleomorphism with tumor cells arranged in a trabecular pattern with one to two cell plate thick trabeculae and sinusoids in between. Mitoses are easily noted. The nuclei are rounded with inconspicuous nucleoli. These areas resemble a crowded fetal pattern of hepatoblastoma. The left tumor from 11.7 weeks is a good example of a matured HB. Tumor cells are bigger with uniform round nuclei with a small nucleolus, the cytoplasm is either eosinophilic or vacuolated. A trabecular arrangement is noted and mitoses are easily recognized. This tumor corresponds to human fetal subtype of HB. Another tumor also from 11.7 weeks shows a more immature appearance of cells with nucleo:cytoplasmic ratio and angulated to ovoid nuclei arranged in a trabecular pattern, 2-3 cell thick. Again note the lack of pleomorphism.
Supplementary Figure 5: A-C. Semi-thin sections of liver parenchyma seven days after hydrodynamic injection of Yap and β-Catenin plasmids. Single (A, inset B shows magnified area of A) or groups (C) of transfected/altered hepatocytes (arrows) with irregular cell borders, enlarged nuclei and prominent nucleoli are distributed within the acinus, whereas the portal tract (asterisk) is surrounded by unaltered hepatocytes with round/oval nuclei and regular cell shapes. Richardson staining. Length of the lower edge A- 0.25 mm, B- 83 μm, C- 62 μm.
D-G. Ultrastructural analysis of liver parenchyma. Transfected/altered hepatocytes reveal atypical, irregularly shaped nuclei and several prominent nucleoli, notched nuclear membranes, and nuclear inclusions (arrowheads). Note the loss and irregular distribution of glycogen deposits in the cytoplasm (D, boxed magnified area in E). Hepatocytes are still in close contact to neighboring hepatocytes, forming tight cell junctions and small bile canaliculi (F, boxed magnified area in G).
H-M. Kinetics of HB development after Yap/β-catenin injection in mice. First row: Histological examination of Yap/β-catenin mouse livers one week post injection show several altered hepatocytes with an enlarged clear cytoplasm and atypical nuclear morphology. These altered hepatocytes expressed the injected genes, as indicated by the positivity to FLAG-tag and MYC-tag staining. Second row: Subsequently, small hepatocellular tumors begin to emerge in the liver parenchyma as early as 3 weeks post injection. Of note, in the transition from preneoplastic lesions to small tumors, altered hepatocytes remodeled towards a smaller size and rounded nuclear shape, especially in the tumor core, whereas enlarged hepatocytes were still present either in the periphery of tumors or in the neighboring parenchyma (arrowheads). Original magnification: x 400 first row; x 200 second row.
N-P. Livers from tamoxifen-injected Alb-CreERT2;R26R EYFP mice administered hydrodynamically Yap127A/ΔN90β-catenin at 11 weeks showing tumor (Tu.) that is strongly positive for β-catenin (green) and EYFP (red) showing co-localization indicating the hepatocyte origin of HB in the current mouse model.
Supplementary Figure 6: A. Immunohistochemistry using antibody that detects endogenous protein only, identifies c-Myc expression localized to HB, which were observed at various time points after Yap/β-catenin injection (200x).
B. Real-time PCR displays increased expression of several Yap1 targets, including Jag1, Cyr61, survivin and CTGF and of immature hepatocyte markers such as Glypican-3 (GPC3), α-fetoprotein (Afp) and EpCAM in Yap/β-catenin livers versus livers of wild-type (WT) mice.
C. Enhanced cell proliferation as indicated by greater numbers of cells in S-phase of cell cycle detected by IHC for Ki-67 was observed in Yap/βcatenin mice as compared to wild-type mice (WT). The differences from WT mice were statistically significant at all times examined (a: p<0.001). A significant increase in the number of apoptotic nuclei were also observed in Yap/β-catenin injected livers at different times as compared to WT mice (a: p<0.001). 3-5 samples per each group per each time point were analyzed. (200x)
Acknowledgments
Grant Support: The work in the study was supported by 1R01DK62277, 1R01DK100287 to SPSM. It was also supported by 1R01CA136606 to XC. It was also supported in part by EV 168/2-1 to ME.
Abbreviations
- HB
hepatoblastoma
- HCC
hepatocellular carcinoma
- ICC
intrahepatic cholangiocarcinoma
- YAP
Yes-associated protein
- TEAD
TEA domain
- CTGF
connective tissue growth factor
- Jag1
gene encoding Jagged-1
- LKB
gene encoding liver kinase B
- Cyr61
gene encoding Cysteine rich 61
- Dkk1
gene encoding Dickkopf-1
- IHC
immunohistochemistry
- EM
electron microscopy
Footnotes
Disclosure: None of the authors have any disclosures for the work presented in the current manuscript
Author contributions: Junyan Tao: acquisition of data; analysis and interpretation of data
Diego F. Calvisi: analysis and interpretation of data, drafting of the manuscript
Sarangarajan Ranganathan: acquisition of data; analysis and interpretation of data
Antonio Cigliano: acquisition of data; analysis and interpretation of data
Lili Zhou: acquisition of data; analysis and interpretation of data
Sucha Singh: acquisition of data
Lijie Jiang: acquisition of data
Biao Fan: acquisition of data
Luigi Terracciano: acquisition of data
Sorin Armeanu-Ebinger: acquisition of data
Silvia Ribback: acquisition of data
Frank Dombrowski: acquisition of data, analysis and interpretation of data
Matthias Evert: acquisition of data, analysis and interpretation of data, obtained funding
Xin Chen: study concept and design, analysis and interpretation of data, drafting of the manuscript; obtained funding
Satdarshan P. S. Monga: study concept and design, analysis and interpretation of data, drafting of the manuscript; obtained funding
IRB Approvals: For use of patient tissue, IRB (or Exempt) protocols approvals were obtained from all participating institutes and are as follows:
University of Pittsburgh: PRO09030166 (Exempt)
Univeristätsklinikum Tübingen: 565/2012B02
University Hospital of Basel (EKKB): 310/12
Ernst-Moritz-Arndt Universität Greifswald: BB 67/10
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Armengol C, Cairo S, Fabre M, et al. Wnt signaling and hepatocarcinogenesis: the hepatoblastoma model. Int J Biochem Cell Biol. 2011;43:265–70. doi: 10.1016/j.biocel.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imajo M, Miyatake K, Iimura A, et al. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J. 2012;31:1109–22. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konsavage WM, Jr., Kyler SL, Rennoll SA, et al. Wnt/beta-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730–9. doi: 10.1074/jbc.M111.327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizuno T, Murakami H, Fujii M, et al. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene. 2012;31:5117–22. doi: 10.1038/onc.2012.5. [DOI] [PubMed] [Google Scholar]
- 7.Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEADYAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–5. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pobbati AV, Hong W. Emerging roles of TEAD transcription factors and its coactivators in cancers. Cancer Biol Ther. 2013;14 doi: 10.4161/cbt.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai H, Zhang N, Xu Y, et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012;56:1097–107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu MZ, Yao TJ, Lee NP, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–85. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou D, Conrad C, Xia F, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–38. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Wolfe A, Septer S, et al. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. 2012;32:38–47. doi: 10.1111/j.1478-3231.2011.02646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Terrada D, Gunaratne PH, Adesina AM, et al. Histologic subtypes of hepatoblastoma are characterized by differential canonical Wnt and Notch pathway activation in DLK+ precursors. Hum Pathol. 2009;40:783–94. doi: 10.1016/j.humpath.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Tschaharganeh DF, Chen X, Latzko P, et al. Yes-Associated Protein Up-regulates Jagged-1 and Activates the NOTCH Pathway in Human Hepatocellular Carcinoma. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armeanu-Ebinger S, Wenz J, Seitz G, et al. Characterisation of the cell line HC-AFW1 derived from a pediatric hepatocellular carcinoma. PLoS One. 2012;7:e38223. doi: 10.1371/journal.pone.0038223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch A, Denkhaus D, Albrecht S, et al. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res. 1999;59:269–73. [PubMed] [Google Scholar]
- 17.Tward AD, Jones KD, Yant S, et al. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci U S A. 2007;104:14771–6. doi: 10.1073/pnas.0706578104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–71. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao B, Li L, Lei Q, et al. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–74. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avruch J, Zhou D, Fitamant J, et al. Mst1/2 signalling to Yap: gatekeeper for liver size and tumour development. Br J Cancer. 2011;104:24–32. doi: 10.1038/sj.bjc.6606011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huo X, Zhang Q, Liu AM, et al. Overexpression of Yes-associated protein confers doxorubicin resistance in hepatocellullar carcinoma. Oncol Rep. 2013;29:840–6. doi: 10.3892/or.2012.2176. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Park JS, Wei Y, et al. TRIB2 acts downstream of Wnt/TCF in liver cancer cells to regulate YAP and C/EBPalpha function. Mol Cell. 2013;51:211–25. doi: 10.1016/j.molcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsutsumi R, Masoudi M, Takahashi A, et al. YAP and TAZ, Hippo Signaling Targets, Act as a Rheostat for Nuclear SHP2 Function. Dev Cell. 2013;26:658–665. doi: 10.1016/j.devcel.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Turner R, Lozoya O, Wang Y, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53:1035–45. doi: 10.1002/hep.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cadoret A, Ovejero C, Saadi-Kheddouci S, et al. Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin. Cancer Res. 2001;61:3245–9. [PubMed] [Google Scholar]
- 26.Harada N, Miyoshi H, Murai N, et al. Lack of tumorigenesis in the mouse liver after adenovirus-mediated expression of a dominant stable mutant of beta-catenin. Cancer Res. 2002;62:1971–7. [PubMed] [Google Scholar]
- 27.Harada N, Oshima H, Katoh M, et al. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res. 2004;64:48–54. doi: 10.1158/0008-5472.can-03-2123. [DOI] [PubMed] [Google Scholar]
- 28.Nejak-Bowen KN, Thompson MD, Singh S, et al. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology. 2010;51:1603–13. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Supplemental Table 1: Summary of nuclear localization of Yap and β-catenin in 94 human hepatoblastoma cases.
B. Supplemental Table 2: Summary of nuclear localization of Yap and β-catenin in 103 human hepatocellular carcinoma cases.
C. Supplemental Table 3: Summary of nuclear localization of Yap and β-catenin in 62 human intrahepatic cholangiocarcinoma cases.
D. Supplementary Table 4: Information on primers used for QRTPCR analysis in the current study.
E. Supplementary Table 5: Summary of all mice used in the current study for hydrodynamic injections.
Supplementary Figure 1: A. Western blot using whole cell lysates from four liver tumor cell lines showing β-catenin expression and truncations due to deletions in exon-3. Yap protein expression is also show in the same four cell lines. Protein loading for each cell line is verified by a blot for β-actin.
B-E. Pharmacological inhibition of β-catenin by small molecules PNU74654 and IWR1 endo and Yap-TEAD pathway by small molecule Verteporfin or their combination led to significantly decreased cell proliferation and increased apoptosis in both HepG2 and HC-AFW1 cells when compared to no treatment or treatment of cells with DMSO (solvent for inhibitors) or IWR1 exo (negative control for IWR1 endo). Experiments were conducted at least 3 times in triplicate. Statistical analysis: a- different from Control; b- different from DMSO (solvent); c- different from IWR1 exo; d- different from IWR1 endo; e- different from PNU74654; f- different from Verteporfin; g- different from IWR1 endo + Verteporfin. All p values in the current study were less than 0.0005.
Supplementary Figure 2: A. Decreased expression of Yap and its targets such as CTGF, Cyr61, Jag1 and survivin, after Yap knockdown in HC-AFW1 cells at 24 and 48 hours after transfection. β-Catenin knockdown led to suppression of Yap expression and of its targets Cyr61 and survivin, while no demonstrable effect was evident on CTGF or Jag1. Combined knockdown of both Yap and β-catenin, led to a more pronounced effect on Cyr61 and survivin expression. β-Catenin silencing in HC-AFW1 cells after 24 and 48 hours of transfection causes reduced expression of β-catenin and its targets, including Axin2, c-Myc, Cyclin D1 and DKK1. Yap silencing did not impact β-catenin expression but affected expression of c-Myc and Cyclin D1. Dual knockdown of β-catenin and Yap had a more robust impact on expression of c-Myc and Cyclin D1. All experiments were conducted at least 3 times and in triplicate. Statistical analysis: a- different from Control; b- different from Scrambled siRNA; c- different from Yap siRNA; d-different from β-catenin siRNA. All p values < 0.001.
B. A significant decrease in β-catenin transactivation was observed in a TopFlash reporter assay 48 hours after silencing of Yap in HC-AFW1 cells (p<0.01). Transfection of the same cells with FopFlash containing TCF-mutant showed no luciferase activity.
Supplementary Figure 3: A. H&E staining of a representative liver section showing absence of any microscopic tumor foci at one year after hydrodynamic injection of Δ90βcatenin only.
B. A representative gross specimen and H&E on a liver section show absence of macroscopic or microscopic tumor foci at 22.5 weeks after hydrodynamic injection of Yap127A alone.
C. Gross morphology of livers after co-injection of Yap and β-catenin genes showing multiple tumors at 3, 3.5, 6.5, 9 and 9.7 weeks post injection.
D. IHC for Glutamine Synthetase (GS) shows preneoplastic (PN) lesions to be positive at 3 weeks after Yap/β-catenin injection. In contrast, all HB observed at 3.5, 6.5 and 11.7 weeks after Yap/β-catenin injection were GS-negative as shown in representative liver sections.
Supplementary Figure 4: A. Representative histology from 3 week old Yap/β-catenin livers showing single or clusters of preneoplastic clear-cell hepatocytes (arrowhead) (200x).
B. Representative histology from additional Yap/β-catenin livers showing HB occurrence. A tumor at 6.5 weeks post injection shows the least maturation, being composed of cells with relatively uniform nuclei that are ovoid to angulated with inconspicuous nucleoli arranged in a vague trabecular and glandular pattern with suggestion of rosettes/canaliculi in an occasional focus. There is an increase in nucleo:cytoplasmic ratio in these cells. These areas resemble those that can be seen in embryonal hepatoblastomas though classic rosettes and clear-cut blastemal cells are not identified. Another photomicrograph showing a tumor from 9 weeks with no significant pleomorphism with tumor cells arranged in a trabecular pattern with one to two cell plate thick trabeculae and sinusoids in between. Mitoses are easily noted. The nuclei are rounded with inconspicuous nucleoli. These areas resemble a crowded fetal pattern of hepatoblastoma. The left tumor from 11.7 weeks is a good example of a matured HB. Tumor cells are bigger with uniform round nuclei with a small nucleolus, the cytoplasm is either eosinophilic or vacuolated. A trabecular arrangement is noted and mitoses are easily recognized. This tumor corresponds to human fetal subtype of HB. Another tumor also from 11.7 weeks shows a more immature appearance of cells with nucleo:cytoplasmic ratio and angulated to ovoid nuclei arranged in a trabecular pattern, 2-3 cell thick. Again note the lack of pleomorphism.
Supplementary Figure 5: A-C. Semi-thin sections of liver parenchyma seven days after hydrodynamic injection of Yap and β-Catenin plasmids. Single (A, inset B shows magnified area of A) or groups (C) of transfected/altered hepatocytes (arrows) with irregular cell borders, enlarged nuclei and prominent nucleoli are distributed within the acinus, whereas the portal tract (asterisk) is surrounded by unaltered hepatocytes with round/oval nuclei and regular cell shapes. Richardson staining. Length of the lower edge A- 0.25 mm, B- 83 μm, C- 62 μm.
D-G. Ultrastructural analysis of liver parenchyma. Transfected/altered hepatocytes reveal atypical, irregularly shaped nuclei and several prominent nucleoli, notched nuclear membranes, and nuclear inclusions (arrowheads). Note the loss and irregular distribution of glycogen deposits in the cytoplasm (D, boxed magnified area in E). Hepatocytes are still in close contact to neighboring hepatocytes, forming tight cell junctions and small bile canaliculi (F, boxed magnified area in G).
H-M. Kinetics of HB development after Yap/β-catenin injection in mice. First row: Histological examination of Yap/β-catenin mouse livers one week post injection show several altered hepatocytes with an enlarged clear cytoplasm and atypical nuclear morphology. These altered hepatocytes expressed the injected genes, as indicated by the positivity to FLAG-tag and MYC-tag staining. Second row: Subsequently, small hepatocellular tumors begin to emerge in the liver parenchyma as early as 3 weeks post injection. Of note, in the transition from preneoplastic lesions to small tumors, altered hepatocytes remodeled towards a smaller size and rounded nuclear shape, especially in the tumor core, whereas enlarged hepatocytes were still present either in the periphery of tumors or in the neighboring parenchyma (arrowheads). Original magnification: x 400 first row; x 200 second row.
N-P. Livers from tamoxifen-injected Alb-CreERT2;R26R EYFP mice administered hydrodynamically Yap127A/ΔN90β-catenin at 11 weeks showing tumor (Tu.) that is strongly positive for β-catenin (green) and EYFP (red) showing co-localization indicating the hepatocyte origin of HB in the current mouse model.
Supplementary Figure 6: A. Immunohistochemistry using antibody that detects endogenous protein only, identifies c-Myc expression localized to HB, which were observed at various time points after Yap/β-catenin injection (200x).
B. Real-time PCR displays increased expression of several Yap1 targets, including Jag1, Cyr61, survivin and CTGF and of immature hepatocyte markers such as Glypican-3 (GPC3), α-fetoprotein (Afp) and EpCAM in Yap/β-catenin livers versus livers of wild-type (WT) mice.
C. Enhanced cell proliferation as indicated by greater numbers of cells in S-phase of cell cycle detected by IHC for Ki-67 was observed in Yap/βcatenin mice as compared to wild-type mice (WT). The differences from WT mice were statistically significant at all times examined (a: p<0.001). A significant increase in the number of apoptotic nuclei were also observed in Yap/β-catenin injected livers at different times as compared to WT mice (a: p<0.001). 3-5 samples per each group per each time point were analyzed. (200x)