Abstract
BACKGROUND & AIMS
Early-onset ulcerative colitis, severe colonic inflammation that develops in infants and young children, can be caused by alterations in interleukin 10 (IL10) signaling, although other factors are involved in its pathogenesis. We investigated whether loss of phosphatase and tensin homologue (PTEN), which regulates many important cell functions including cell proliferation, cell survival, and toll-like receptor (TLR) signaling pathways, contributes to development of colitis in Il10−/− mice.
METHODS
We generated Il10−/− mice (in C57BL/6 and C3H/HeJBir background strains) with disruption of Pten in the intestinal epithelium (IntsΔPten/ΔPten; Il10−/− mice), and IntsΔCont;Il10−/− (control) mice. Colon tissues were collected and histologic, transmission electron microscopy, and gene expression analyses were performed. Fecal microbiota samples were analyzed by sequencing of 16S ribosomal RNA genes. We disrupted Tlr4 in IntsΔPten/ΔPten;Il10−/− mice. Lipopolysaccharide signaling via TLR4 was blocked by feeding mice polymyxin B.
RESULTS
Il10−/− mice developed colitis when they were 6–7 months old, whereas IntsΔPten/ΔPten;Il10−/− mice developed severe colitis and colon tumors by the time they were 36 days old. Within 3 months of birth, 80% of IntsΔPten/ΔPten;Il10−/− mice developed severe colitis and colonic malignancy, whereas none of IntsΔ Cont;Il10−/− mice had these phenotypes. IntsΔPten/ΔPten;Il10−/− mice had alterations in fecal microbiota compared with controls, such as increased proportions of Bacteroides species, which are Gram negative. Disruption of Tlr4 or feeding IntsΔPten/ΔPten;Il10−/− mice polymyxin B delayed the development of colitis and reduced disease severity.
CONCLUSIONS
Disruption of Pten in the intestinal epithelium of Il10−/− mice speeds the onset and increases the severity of colitis. Fecal microbiota from IntsΔPten/ΔPten;Il10−/− mice have increased proportions of Bacteroides species. Development of colitis is delayed and reduced by blocking TLR4 signaling. IntsΔPten/ΔPten;Il10−/− mice might be studied as a model for early-onset ulcerative colitis and used to identify new therapeutic targets.
Keywords: inflammatory bowel disease, innate immunity, intestinal epithelial cells, pediatric
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic inflammatory condition occurring in the gastrointestinal tract and classified into Crohn’s disease (CD), ulcerative colitis (UC), and indeterminate colitis1, 2. Although IBD is able to occur at any age, intriguingly, a subset of the patients develops severe colonic inflammation at infants and young children, termed early-onset ulcerative colitis3, accompanied with an increased risk for primary sclerosing cholangitis4. In addition to the involvement in both CD and UC, defective mutation at the gene encoding interleukin10 (Il10) or IL10 receptor (Il10r) was identified in the patients of early-onset ulcerative colitis1, 5, 6, indicating that impaired IL10 signaling should be involved in colitis development at early ages.
Similarly, Il10−/− mice in specific pathogen free (SPF) or conventional housing environment are predisposed to developing spontaneous colitis7. Il10−/− mice, however, do not develop colitis under germ-free conditions, indicating that the flare-up of colitis in these mice is driven by antigens in gut microflora8. Thus, enteric microbes influence the development of colitis. The timing of developing colitis, however, depends on each housing condition and/or genetic background. Indeed, an elegant study demonstrated that both the severity of colitis and the frequency of colitis development in Il10−/− mice are greatly influenced by the genetic background9, indicating that another genetic factor would participate in eliciting colitis in an Il10 deficient environment.
Pten is a phosphoinositide-3-phosphatase converting phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] to PI(4,5)P2. With an antagonistic effect against the phosphatidylinositol 3-kinase signaling regulating cell proliferation and apoptosis, Pten plays an important role in suppressing tumorigenesis in multiple organs. In keeping with this notion, conventional Pten+/− mice exhibited hyperplastic-dysplastic changes in the prostate and skin10. In contrast to the embryonic lethality of conventional Pten knockout mice10, global Pten knockout induced by interferon-α/β-inducible Cre-expression system using Pten-floxed (PtenloxP/loxP) mice resulted in polyposis in the intestine11. On the other hand, Langlois et al12 demonstrated that intestinal epithelial cell specific-Pten knockout mice using Villin promoter-driven Cre expression system were healthy and normal without any gross abnormalities such as tumorigenesis. Indeed, we confirmed that those intestinal epithelial cell specific Pten knockout mice (IntsΔPten/ΔPten) generated in our laboratory did not exhibit any gross abnormalities in the intestine (Supplementary Figure 1 and Supplementary Figure 2). Accordingly, the impact of Pten in the intestine still remains to be elucidated.
Although Pten plays a critical role in regulating cell proliferation, growth, and apoptosis13 which are the propensity of intestinal epithelial cells, we recently demonstrated that Pten could be an important factor regulating TLR5-midiated signaling pathways, indicating the involvement of Pten in host-microbial interaction in the gut14. With this in mind, we hypothesized that Pten would be involved in developing and perpetuating colitis on which enteric microflora has profound impacts. To test this hypothesis, we investigated whether Pten in the intestinal epithelial cells could affect the development of colitis in an Il10 deficient environment.
MATERIALS AND METHODS
Animals
Pten floxed (PtenloxP/loxP)15, Villin promoter-driven Cre expression mouse (VilCre/+)16, and Il10−/− (C3H/HeJBir or C57BL/6 background) mouse7 were obtained from the Jackson Laboratory (Bar Harbor, ME). As Langlois et al12 did, intestinal epithelial cell specific Pten knockout mouse (IntsΔPten/ΔPten) was generated by crossing PtenloxP/loxP mouse with VilCre/+ mouse. Then, IntsΔPten/ΔPten mouse was crossed with Il10−/− mouse to generate IntsΔPten/ΔPten; Il10−/− mouse in which Il10 was conventionally deleted and simultaneously, Pten was deleted in the intestinal epithelial cells. IntsΔPten/ΔPten;Il10−/− mouse was backcrossed into C57BL/6 or C3H/HeJBir background for at least 6 generations prior to performing the experiments. Mice were bred and maintained under standard SPF conditions with normal drinking water ad libitum at the animal facility of the Division of Laboratory Animal Medicine, University of California, Los Angeles, under the approval by the Institutional Animal Care and Use Committee of the UCLA. All animal experiments were approved by the Animal Research Committee of UCLA.
All other protocols for materials and experiments are detailed in Supplementary Materials and Methods.
RESULTS
Intestinal epithelial Pten deletion in Il10−/− mice elicits spontaneous colitis at early ages
We generated IntsΔPten/ΔPten;Il10−/− (C57BL/6 background) mice having both global Il10 gene knockout and intestinal epithelial cell specific Pten deletion. IntsΔPten/ΔPten;Il10−/− mice grew smaller than age- and gender-matched littermate control IntsCont;Il10−/− (Figure 1A). Subsequently, we identified that IntsΔPten/ΔPten;Il10−/− mice had severe inflammation occurring along the colon and cecum characterized with pale and enlarged colon and reduced colonic contents, while IntsCont;Il10−/− mice had normal intestine (Figure 1B). More than 60% of IntsΔPten/Δ;Il10Pten−/− mice failed to survive 3 months after their birth due to the complication of severe colitis, whereas IntsCont;Il10−/− mice remained healthy during the experimental period (Figure 1C). It is of interest to note that IntsΔPten/ΔPten;Il10−/− mice died as early as 36 days after their birth due to severe colitis. Notably, we confirmed that Il10−/− mice on C57BL/6 or C3H/HeJBir background took at least 6~7 months after their birth to show signs of severe colitis in our animal facility, as evaluated by mortality (Figure 1D). Considering both the onset timing of colitis in Il10−/− mice and the baseline life span for laboratory mice which is approximately more than 2 years, spontaneous colitis in IntsΔPten/ΔPten;Il10−/− mice occurred at very early ages. With this in mind, the onset of colitis in young IntsΔPten/ΔPten;Il10−/− mice seems to be similar to the development of early-onset ulcerative colitis in humans with which loss-of-function mutations at Il10 or Il10r gene are associated.
Figure 1.
Development of spontaneous colitis in IntsΔPten/ΔPten;Il10−/− mice at early ages. (A) Gender (female)- and age-matched (3 months old) IntsΔPten/ΔPten;Il10−/− and littermate IntsCont;Il10−/− mice on C57BL/6 background were presented. (B) Gross appearance of the colon and cecum from these mice was compared. (C) Survival was monitored until the mouse reached to the age of 3 months. IntsΔPten/ΔPten;Il10−/− (n=22), IntsCont;Il10−/− (n=15). (P < 0.0001). (D) Survival of Il10−/− mice on C3H/HeJBir (HeJBir) (n=9) and C57BL/6 (BL/6) (n=19) background were monitored. P<0.0001. (E) Body weight change was monitored every other day, starting at the age of 32 days. Results are means ± SD. IntsΔPten/ΔPten;Il10−/− (n=8), IntsCont;Il10−/− (n=7). *P<0.05, **P<0.01, ***P<0.001, #P<0.0001. (F) Full-length of the colon was prepared in a ‘swiss-roll’ method and subjected to H&E staining. Low and high magnifications of representative photograph were presented at the upper and lower position, respectively (scale bars, 100 μm). The yellow inset areas were enlarged in the lower panel. Note, gross image (scale bar, 1 mm) of the colon swiss-roll of IntsΔPten/ΔPten;Il10−/− mouse was positioned at ‘F-i’. The red-inset area of ‘F-i’ was enlarged at ‘F-ii’. Data (C,D,E) were analyzed with the results accumulated by 6 independent experiments.
In keeping with the incidence of spontaneous colitis, IntsΔPten/ΔPten;Il10−/− mice were characterized with slow and limited weight-gaining compared to the gradual and consistent pattern in IntsCont;Il10−/− mice (Figure 1E). While IntsCont;Il10−/− mice exhibited normal colonic mucosa, IntsΔPten/ΔPten;Il10−/− mice revealed dramatic changes in colonic histology caused by inflammation (Figure 1F). Together, these data demonstrate that IntsΔPten/ΔPten;Il10−/− mice develop spontaneous colitis which can be incurred at very early ages.
Colonic malignancies are found in IntsΔPten/ΔPten; Il10−/− mice
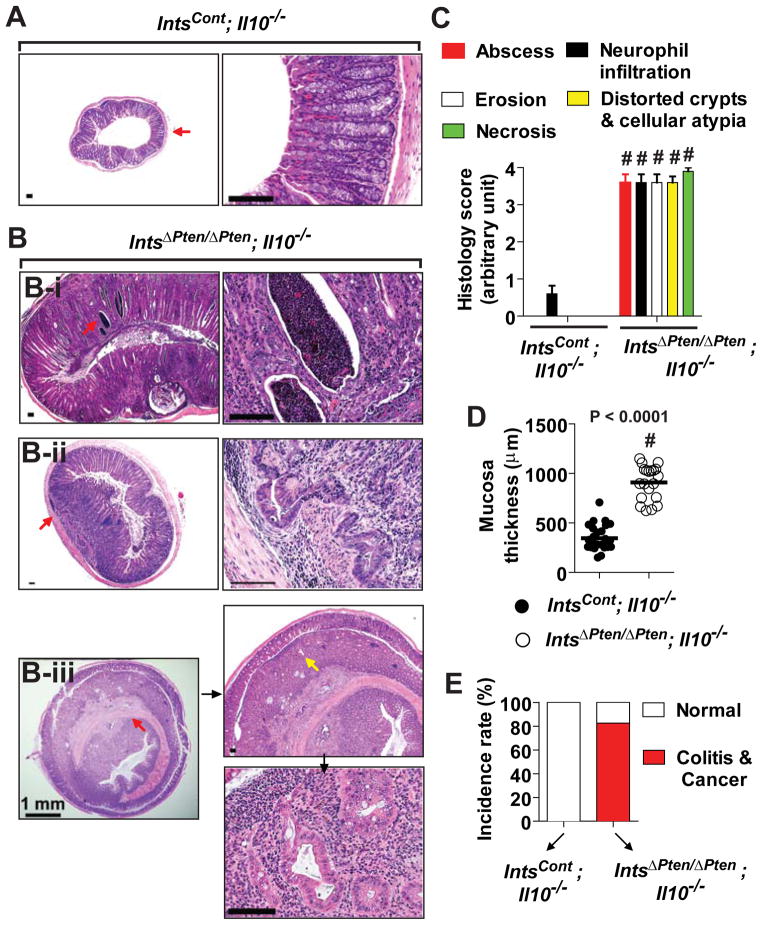
IntsCont;Il10−/− mice at 3 months old age had healthy colon (Figure 2A). In contrast, age-matched IntsΔPten/ΔPten;Il10−/− mice diagnosed with colitis were characterized with various pathological and histological abnormalities in the colon. The colon of IntsΔPten/ΔPten;Il10−/− mice were featured with hyperplastic mucosa with diffused leukocyte infiltrations causing colon obstruction (Figure 2B-i). Surprisingly, sessile adenocarcinoma attached directly by its base without a stalk was also found along with the penetration of cancerous cells into the submucosa (Figure 2B-ii). Moreover, in IntsΔPten/ΔPten;Il10−/− mice, a large tumor mass was developed in the colonic epithelium and grown up to the extent of causing bowel obstruction (Figure 2B-iii). These observations suggested that the colon of IntsΔPten/ΔPten;Il10−/− mice is not only highly susceptible to early-onset spontaneous colitis, but also prone to colonic malignancy. Accordingly, microscopic examination demonstrated that the colonic mucosa of IntsΔPten/ΔPten;Il10−/− mice were characterized with prominent abscesses, massive neutrophil infiltration, increased epithelial erosion, marked architectural distortion of crypts with epithelial cell atypia, and enhanced necrosis, while these clinical parameters were almost negligible in the colon of IntsCont;Il10−/− mice (Figure 2C). Due to the inflammation and malignancy, colonic mucosal thickness of IntsΔPten/ΔPten;Il10−/− mice was excessively increased, compared to that of IntsCont;Il10−/− mice (Figure 2D). With the histological analysis, we determined that more than 80% of IntsΔPten/ΔPten;Il10−/− mice had a diagnosis of colitis and malignancies in the colon by 3 months after their birth, whereas none of the age-matched IntsCont;Il10−/− mice showed such phenotypes (Figure 2E).
Figure 2.
Development of colonic malignancy in IntsΔPten/ΔPten;Il10−/− mice. (A) Microscopic examination revealed normal colonic mucosa in IntsCont;Il10−/− mice. (B) IntsΔPten/ΔPten;Il10−/− mouse colon had diffused hyperplasia with marked inflammation (B-i), sessile adenocarcinoma (B-ii), and large tumor mass in the colon with typical granuloma (B-iii). Area of red arrow in the left panel was magnified in the right. In ‘B-iii’, area of yellow arrow was enlarged in the lower. Scale bars, 100 μm. (C) Histological parameters were quantified with H&E sections (n=10/group). (D) The thickness of colonic mucosa was measured using Axio Imager Z1 microscope (Carl Zeiss). (n=20–27/group). (E) Percentage of the mice diagnosed with colitis and colonic malignancy until the mice became 3 months old. IntsΔPten/ΔPten;Il10−/− (n=22), IntsCont;Il10−/− (n=15). # P<0.0001 (Mann-Whitney U test). Representative images are presented.
Electron microscopy demonstrates microvilli degeneration and epithelial lesions in the colon of IntsΔPten/ΔPten;Il10−/− mice
We examined the colonic epithelium from IntsΔPten/ΔPten;Il10−/− and IntsCont;Il10−/− mice under transmission electron microscopy. In the colonic epithelium of IntsCont;Il10−/− mice, columnar epithelial cells were held well one another along the lateral surface by cell-to-cell junctions and identified with well-developed microvilli and intact intracellular organelles including mitochondria, vacuoles, and lysosomes (Figure 3A–C). In contrast, in the colonic epithelium of IntsΔPten/ΔPten;Il10−/− mice, epithelial microvilli were effaced, cell-to-cell junctions were disrupted, and the cytoplasm became electron lucent due to necrosis (Figure 3D). Well differentiated adenocarcinoma cells harboring atypical nuclei with excessive mitosis and chromosomal irregularities were observed (Figure 3E). We also observed many apoptotic cells displaying distinctive features of apoptosis such as cell shrinkage, apoptotic body, condensation and fragmentation of chromatin, and cellular disintegration (Figure 3F). Electron micrographs demonstrated that IntsΔPten/ΔPten;Il10−/− mice had morphological alterations, tissue degeneration, and epithelial cell differentiation occurred in the colon, reflecting the pathophysiology of colonic inflammation and malignancy.
Figure 3.
Electron micrographs of colonic epithelium. (A to C) IntsCont;Il10−/− mice showed normal columnar epithelium in which microvilli (MV), cell-to-cell adhesion, and intracellular organelles such as mitochondria (M), lysosome (L), vacuole (V), goblet cells possessing mucin droplets (Md), and solid nucleus (N) and nucleolus (Nc) are intact. (D to F) The colonic epithelium of IntsΔPten/ΔPten;Il10−/− mouse exhibited effaced microvilli, necrosis, damaged cell-to-cell adhesion (D), adenocarcinoma cells with atypical and irregular nuclei (open arrowhead) (E), and apoptosis (closed arrowhead) (F). Inset areas in ‘A-i’ and ‘D-i’ were enlarged in ‘A-ii’ and ‘D-ii’, respectively. Representative images are presented.
The development of inflammation in IntsΔPten/ΔPten;Il10−/− mice is limited to the colon
In this study, Pten deletion in the intestinal epithelium of IntsΔPten/ΔPten;Il10−/− mice was accomplished by Villin promoter-driven Cre expression. Villin promoter activity is specific to both the small intestine and the colon. Thus, we next investigated whether IntsΔPten/ΔPten;Il10−/− mice would also develop inflammation in the small intestine. The small intestine of IntsΔPten/ΔPten;Il10−/− mice, however, did not show any signs of abnormalities. Just like the normal epithelial mucosa from IntsCont;Il10−/− mice, the small intestine of IntsΔPten/ΔPten;Il10−/− mice exhibited normal epithelial mucosa (Supplementary Figure 3A and B). Of importance is the fact that both IntsΔPten/ΔPten;Il10−/− and IntsCont;Il10−/− mice did not have any abnormalities in the liver, kidney, and spleen (Supplementary Figure 3C), indicating that the incidence of inflammation and tumorigenesis in IntsΔPten/ΔPten;Il10−/− mice is specific to the colon.
Blood serum chemistry and hematologic analysis identified that albumin, total protein, alkaline phosphatase (ALP), and glucose levels were substantially decreased in the blood serum of IntsΔPten/ΔPten;Il10−/− mice, compared to the levels in IntsCont;Il10−/− mice (Figure 4A). The level of ALT and AST signifying liver-related diseases and the total amount of fatty substances (triglyceride and cholesterol) indicative of metabolic defects were similar between IntsΔPten/ΔPten;Il10−/− and IntsCont;Il10−/− mice (Supplementary Figure 4A). Malnutrition is related to lower ALP level, while elevated ALP is associated with bile duct obstruction or liver diseases including hepatitis and liver cancer. Having found that the liver, kidney, and spleen were normal in IntsΔPten/ΔPten;Il10−/− and IntsCont;Il10−/− mice, however, these data excluded a possibility of inflammation or tumorigenesis in the liver. Thus, reduced levels of ALP, albumin, total protein, and glucose in IntsΔPten/ΔPten;Il10−/− mice are likely due to the complication of colitis and colon malignancy and consequent malnutrition.
Figure 4.
Serum chemistry and hematologic analysis using blood samples. (A) Serum chemistry data with significant difference were presented. IntsΔPten/ΔPten;Il10−/− (n=13); IntsCont;Il10−/− (n=14). (B) Complete blood count was performed for hematologic analysis. IntsΔPten/ΔPten;Il10−/− (n=12), IntsCont;Il10−/− (n=7–9). *P<0.05, **P<0.01, ***P<0.001 (Mann-Whitney U test). Complementary results of this analysis were presented in Supplementary Figure 4. HB, hemoglobin; HCT, hematocrit; NE, neutrophil; RBC, red blood cell.
In addition, neutrophil level in IntsΔPten/ΔPten;Il10−/− mice (35.15 %) was substantially increased compared to IntsCont;Il10−/− mice (17.98 %) (Figure 4B), which was in line with the diagnosis of severe inflammation in the colon. Conversely, the level of lymphocytes in IntsΔPten/ΔPten;Il10−/− mice (56.57 %) was slightly lower than IntsCont;Il10−/− mice (74.73 %), while the levels of monocytes, eosinophils, and platelets were not altered in these mice (Supplementary Figure 4B). Since the neutrophil count rose in IntsΔPten/ΔPten;Il10−/− mice, its lymphocyte count appeared to be lowered proportionally. In contrast, there were no differences in the level of red blood cell, hematocrit, and hemoglobin counts between these animal groups, indicating that the mice did not suffer from anemia or internal bleeding. Taken together, these results support that the diagnosis of severe inflammation and malignancy in IntsΔPten/ΔPten;Il10−/− mice are limited to the colon.
The genes regulating inflammation and tumorigenesis are differentially expressed in the colon of IntsΔPten/ΔPten; Il10−/− mice
We next analyzed the inflammation- or tumorigenesis-associated gene expression in the colon tissues using inflammation (Supplementary Figure 5A) and cancer (Supplementary Figure 5B) pathway-focused PCR array assay (SABiosciences), followed by individual quantitative real time PCR (qPCR). We found that various pro-inflammatory genes including Cxcl5, Cxcr2, Nos2, Ifnγ, Il-1β, Tnfα, Il6, and Il17a were markedly up-regulated, and simultaneously, potent anti-inflammatory genes such as Nr3c1 and Il5 were substantially down-regulated in the colon of IntsΔPten/ΔPten;Il10−/− mice, compared to the levels in IntsCont;Il10−/− mouse colon (Supplementary Figure 5 and Supplementary Figure 6).
Similarly, Hmox1 and Ccl2 promoting tumorigenesis were significantly up-regulated in the colon of IntsΔPten/ΔPten;Il10−/− mice, compared to IntsCont;Il10−/− mice. In parallel, Il18 and Sirt1 capable of inducing potent anti-tumor activity were significantly down-regulated in the colon of IntsΔPten/ΔPten;Il10−/− mice relative to IntsCont;Il10−/− mice. It is worth noting that Wee1 expression was reduced in IntsΔPten/ΔPten;Il10−/− mouse colon, compared to IntsCont;Il10−/− mouse colon. Since Wee1 was under-expressed in human colon cancer patients’ tissues and several colon cancer cell lines17, we believe that reduced Wee1 expression in IntsΔPten/ΔPten;Il10−/− mouse colon is indicative of the malignancy. In accordance with the induction of colitis and colonic malignancy, together, our data show that the colon tissues of IntsΔPten/ΔPten;Il10−/− and IntsCont;Il10−/− mice have the opposing expression patterns of pro- vs. anti-inflammatory or tumor promoting- vs. tumor suppressing genes.
Anti-bacterial responses are altered in the colon of IntsΔPten/ΔPten; Il10−/− mice
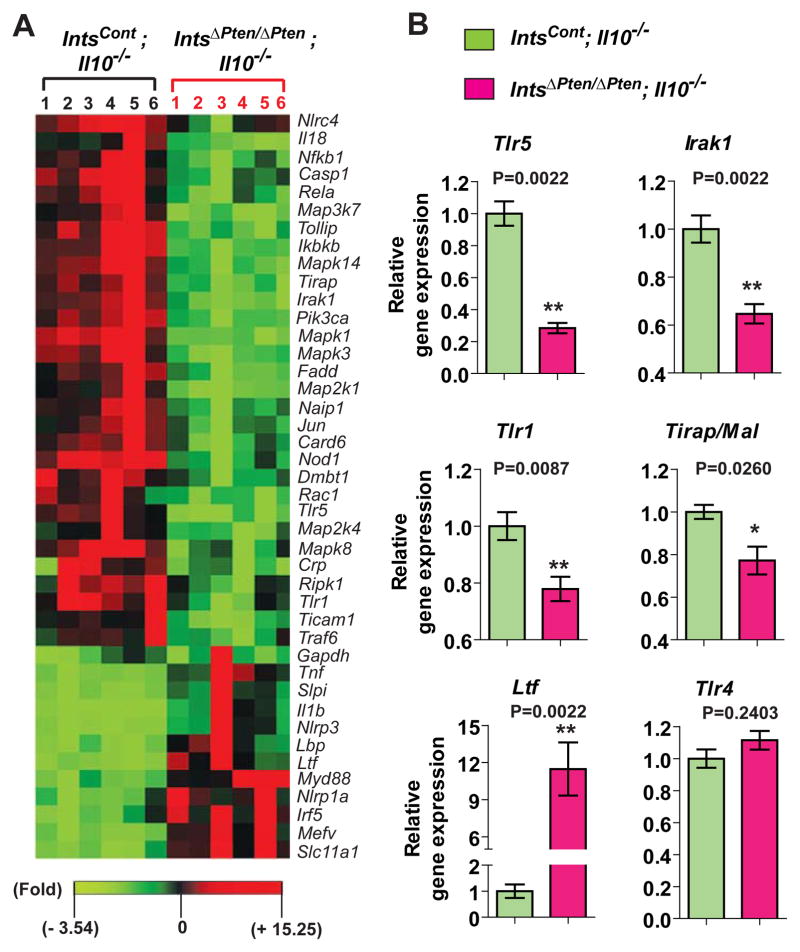
Considerable evidence suggested that impaired Pten alters TLR-induced signaling pathways14, 18 which play a key role in activating the innate immunity including the expression of antimicrobial peptides. Having this in mind, we tested whether anti-bacterial responses would be altered in the colon of IntsΔPten/ΔPten;Il10−/− mice using an anti-bacterial response-focused PCR array (SABiosciences), followed by individual qPCR. Various genes associated with anti-bacterial responses were differentially expressed in the colon tissues (Figure 5A). Among them, Tlr5, a specific receptor recognizing bacterial flagellin was prominently down-regulated in the colon of IntsΔPten/ΔPten;Il10−/− mice compared to Ints;Il10Cont−/− mice (Figure 5B). Moreover, the levels of Tlr1 and TLR-associated signaling molecules, Irak1 and Tirap/Mal, were substantially lower in IntsΔPten/ΔPten;Il10−/− colon than IntsCont;Il10−/− colon, while Tlr4 expression was similar in these colon tissues. Notably, lactotransferrin (Ltf), a potent anti-bacterial protein for a variety of different microorganisms, was dramatically up-regulated in the colon of IntsΔPten/ΔPten;Il10−/− mice compared to IntsCont;Il10−/− mice. Together, these data indicate that anti-bacterial responses are altered in the colon of IntsΔPten/ΔPten;Il10−/− mice.
Figure 5.
Altered anti-bacterial responses in the colon of IntsΔPten/ΔPten;Il10−/− mouse relative to IntsCont;Il10−/− mouse. (A) The expression of anti-bacterial responses-focused genes was analyzed using mouse colon tissues. Gene expression profiles with significant difference were visualized in the heat map. (B) Representative gene expression having significant difference was separately determined by qPCR. Error bars indicate ± SEM. n=6/group. *P<0.05, **P<0.01 (Mann-Whitney U test). Data are the representative of three independent experiments.
Fecal microbiota of IntsΔPten/ΔPten;Il10−/− mice is distinguished from that of IntsCont;Il10−/− mice
In IntsΔPten/ΔPten;Il10−/− mice, spontaneous inflammation was developed only in the colon (Figure 2 and Supplementary Figure 3). Moreover, in early-onset ulcerative colitis patients having a defective IL10 signaling, inflammation develops primarily in the colon. Given that the colon is the primary reservoir of gut microbiota and anti-bacterial responses are altered in the colon of IntsΔPten/ΔPten;Il10−/− mice (Figure 5), we speculated that the development of spontaneous colitis in IntsΔPten/ΔPten;Il10−/− mice would be associated with altered microbiota in the colon. Furthermore, emerging evidence suggests that the pathogenesis of human IBD19, 20 and colon tumorigenesis21 are profoundly influenced by altered gut microbiota. Those considerations prompted us to examine whether IntsΔPten/ΔPten;Il10−/− mice predisposed to developing early-onset colitis would have altered colonic microbiota, as compared to IntsCont;Il10−/− mice. To test this, fecal samples were harvested from age (6 weeks old)- and gender matched Ints ΔPten/ΔPten;Il10−/− and littermate IntsCont;Il10−/− mice. 16S ribosomal RNA (rRNA) gene in the fecal samples was sequenced. 100% of analyzed sequences belonged to the kingdom bacteria, and assigned to 7 phyla including Bacteroidetes, Firmicutes, and Proteobacteria encompassing the majority of sequences (> 95%) (Supplementary Figure 7A and B).
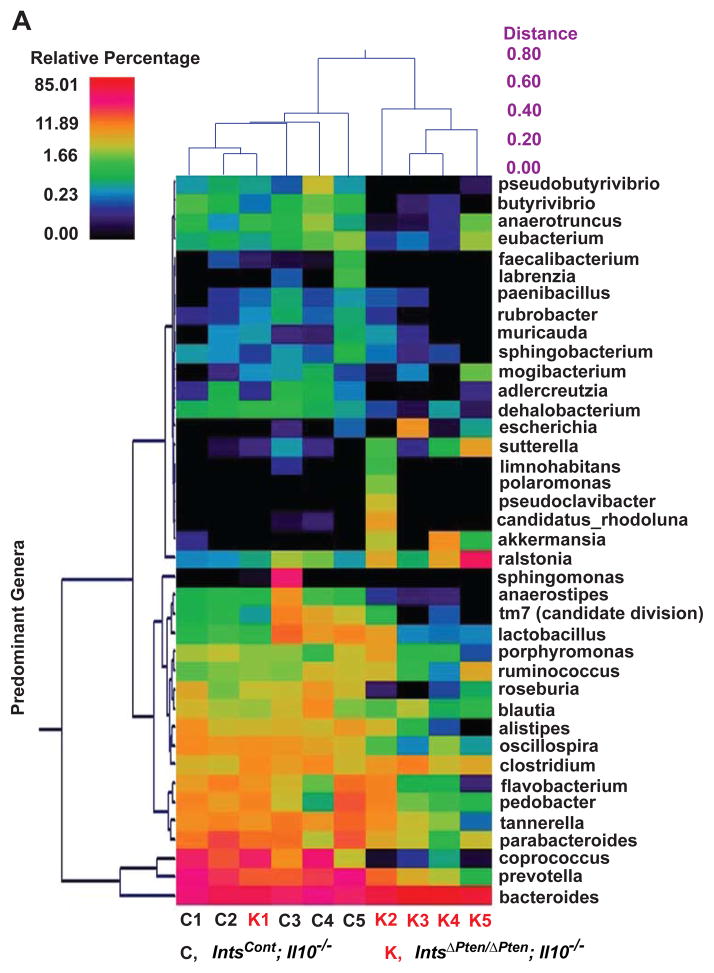
From dual hierarchal dendrogram (Figure 6A) and principal coordinates analysis (PCoA) plot (Figure 6B) based upon the predominant genera, we identified that IntsΔPten/ΔPten;Il10−/− and IntsCont;Il10−/− mice formed its own strong single cluster. From the PCoA plot, we noted that the fecal microbiota from IntsΔPten/ΔPten;Il10−/− and IntsCont;Il10−/− mice were easily distinguished within the primary 3 axis which together accounted for 92.3% of the sample variability. It is notable that one of IntsΔPten/ΔPten;Il10−/− samples harvested from the mouse exhibiting no signs of colitis did cluster away from the rest of its group and instead, close to IntsCont;Il10−/− group. Nevertheless, these data demonstrate that the fecal microbiota communities are indeed different in IntsΔPten/ΔPten;Il10−/− and IntsCont;Il10−/− mice.
Figure 6.
Changed fecal microflora in IntsΔPten/ΔPten;Il10−/− mice compared to IntsCont;Il10−/− mice. (A) Based upon the predominant genera using Ward’s minimum variance clustering and Manhattan distances, a dual hierarchal dendrogram was constructed. Samples were clustered along the x-axis. The heatmap describes the relative percentage in each sample of the associated genera with a legend provided in the upper left of the figure. Predominant genera were clustered along the Y-axis. (B) PCoA plot of weighted unifrac metrics was generated. Samples were separated along principal coordinate (PC) 3 (92.3% of the sample variability). Each dot represents individual microbiota samples from the mouse. (C to E) The abundance of major bacteria at the genus (C), family (D), and species (E) level were analyzed. Horizontal bar indicates a mean value. *P<0.05; **P<0.01. (Mann-Whitney U test). (n=6/group).
The abundance of Bacteroides is increased in IntsΔPten/Δ;Il10−/−Pten mice
At the genus level, we identified that Bacteroides (Mean: 22.75 % in IntsCont;Il10−/− vs. 60.31 % in IntsΔPten/ΔPten;Il10−/−) was the most abundant, followed by Prevotella (16.36 % vs. 3.92 %) in the mouse feces (Figure 6C). It was of importance that the abundance of Bacteroides was dramatically increased in IntsΔPten/ΔPten;Il10−/− mice compared to IntsCont;Il10−/− mice. In contrast, the abundance of Prevotella, Tanerella, Roseburia, Butyrivibrio, and Adlercreutzia were reduced in IntsΔPten/ΔPten;Il10−/− mice compared to IntsCont;Il10−/− mice. Similarly, the family level of Bacteroidaceae was markedly increased in the feces of IntsΔPten/ΔPten;Il10−/− mice compared to IntsCont;Il10−/− mice (21.21 vs. 59.91%) (Figure 6D). The level of Prevotellaceae was substantially reduced in IntsΔPten/ΔPten;Il10−/− mice relative to IntsCont;Il10−/− mice (16.36 vs. 3.92%). Among the genus of Bacteroides which were dramatically increased in the fecal sample of IntsΔPten/ΔPten;Il10−/− mice, Bacteroides acidifaciens species surged in the samples (5.50 vs. 46.97 %), while other Bacteroides species were reduced in the fecal sample of IntsΔPten/ΔPten;Il10−/− mice compared to IntsCont;Il10−/− mice (Figure 6E). Taken together, these results suggest that compared to healthy IntsCont;Il10−/− mice, the family of Bacteroidaceae, the genus of Bacteroides, and the species of Bacteroides acidifaciens are greatly increased in the feces of IntsΔPten/ΔPten;Il10−/− mice which are prone to spontaneous colitis at early ages.
Defective TLR4 inhibits colitis development in IntsΔPten/ΔPten;Il10−/− mice
In the fecal samples of IntsΔPten/ΔPten;Il10−/− mice, Bacteroides were by far the biggest genus (60.31%), followed by Prevotella (3.92%) and Tannerella (2.10%). Simultaneously, the abundance of Bacteroides was greatly increased in the fecal samples of IntsΔPten/ΔPten;Il10−/− mice, compared to IntsCont;Il10−/− mice. Given that Bacteroides are classified as Gram-negative bacteria, we hypothesized that lipopolysaccharide (LPS) from these bacteria would be associated with the pathogenesis of colitis in IntsΔPten/ΔPten;Il10−/− mice. To test this, we generated IntsΔPten/ΔPten;Il10−/− mice on C3H/HeJBir background which has a missense mutation (P712H) at Tlr4 gene, rendering the mouse non-responsive to LPS from Gram-negative bacteria22, 23. Surprisingly, we did not observe gross abnormalities in the appearance of age (3 months old)- and gender-matched IntsΔPten/ΔPten;Il10−/− (HeJBir) and littermate control IntsCont;Il10−/− (HeJBir) mice (Figure 7A). These mice showed no difference in mortality over 3 months after their birth, as virtually most of IntsΔPten/ΔPten;Il10−/− (HeJBir) (93.3%) and IntsCont;Il10−/− (HeJBir) (100%) mice survived during this experimental period (Figure 7B). In line with these results, there was no difference in the weight gaining until the age of 80 days (Figure 7C). However, the weight gaining pattern of IntsΔPten/ΔPten;Il10−/− (HeJBir) mice was a little slower than that of control mice as they aged. Indeed, from the age of 82 days, IntsΔPten/ΔPten;Il10−/− (HeJBir) mice began to exhibit significantly reduced body weight compared to IntsCont;Il10−/− (HeJBir) mice. Importantly, there was no difference in hematologic data of these mice (Supplementary Figure 8A). Instead, the levels of albumin, total protein, and ALP were reduced in IntsΔPten/ΔPten;Il10−/− (HeJBir) mice compared to IntsCont;Il10−/− (HeJBir) mice (Supplementary Figure 8B). Therefore, these data indicated that enhanced susceptibility of colitis in IntsΔPten/ΔPten;Il10−/− mice could still remain on the C3H/HeJBir background. But the development of colitis was substantially delayed, and the severity of the colitis was greatly ameliorated on the C3H/HeJBir background having non-functional TLR4.
Figure 7.
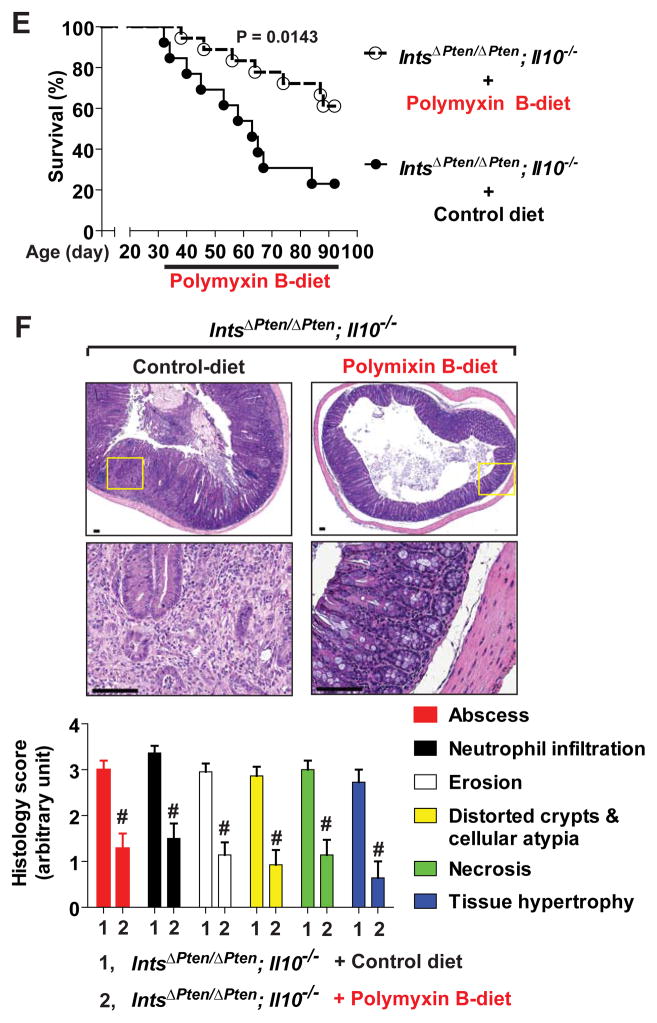
Reduced colitis development in IntsΔPten/ΔPten;Il10−/− mice by blocking LPS-TLR4 engagement. (A) Gender (female)- and age (3 months)-matched IntsΔPten/ΔPten;Il10−/− (HeJBir) and littermate IntsCont;Il10−/− (HeJBir) mice were presented. (B) No difference in survival rate was observed over 3 months after their birth. IntsΔPten/ΔPten;Il10−/− (HeJBir), n=15; IntsCont;Il10−/− (HeJBir), n=22. (C) Body weight change was monitored, starting at the age of 32 days. Results are means ± SD. *P<0.05, **P<0.01. IntsΔPten/ΔPten;Il10−/− (HeJBir) (n=10), IntsCont;Il10−/− (HeJBir) (n=32). Data (B, C) were analyzed with the results accumulated by 6 independent experiments. (D) Representative images of colon H&E sections were presented. Inset areas at low magnification photographs were enlarged at the lower (scale bars, 100 μm). Histological parameters were quantified with H&E sections. IntsΔPten/ΔPten;Il10+/− (n=7), IntsCont;Il10−/− (n=30), IntsΔPten/ΔPten;Il10−/− (n=20). (E and F) IntsΔPten/ΔPten;Il10−/− mice on C57BL/6 background at the age of 32 days started to be under polymyxin B (100 ppm)-mixed or control diet until the age of 3 months. Data were analyzed with the results accumulated by 7 independent experiments. Polymyxin B-treated (n=18), control group (n=13), P=0.0143 (E). H&E sections were prepared from the colon of the mouse survived until the age of 3 months. Representative micrographs were presented. Inset areas inside the upper panel were magnified at the lower. Scale bars, 100 μm. Histological parameters were evaluated. Polymyxin B-treated (n=14), control (n=22). # P≤0.0001 (Mann-Whitney U test) (F).
Il10−/− mice on C3H/HeJBir background are more vulnerable to spontaneous colitis than C57BL/6 background Il10−/− mouse. In agreement with this, none of IntsCont;Il10−/− on C57BL/6 background exhibited any signs of colitis over 3 months after their birth (Figure 1 and Figure 2). However, IntsCont;Il10−/− (HeJBir) mice at the same age revealed a patchy mild to moderate inflammation in the colon, in contrast to normal colonic mucosa of age-matched littermate IntsΔPten/ΔPten;Il10+/− (HeJBir) mice (Figure 7D). By histologic evaluation, we confirmed that IntsCont;Il10−/− (HeJBir) and IntsΔPten/ΔPten;Il10−/−(HeJBir) mice had similar degree of mild colitis. Since we suggest that the severity of colitis in IntsΔPten/ΔPten;Il10−/− mice is dramatically exacerbated on C57BL/6 background, our data from C3H/HeJBir background mice suggest that the absence of functional TLR4 is associated with less recognizable severity of colitis in IntsΔPten/ΔPten;Il10−/− (HeJBir) mice.
LPS scavenger, polymyxin B, ameliorates colitis in IntsΔPten/ΔPten; Il10−/− mice
We next tested whether blocking LPS-TLR4 engagement would reduce the pathogenesis of colitis in IntsΔPten/ΔPten;Il10−/− mice on C57BL/6 background. Polymyxin B detoxifies the endotoxicity of LPS by specifically interacting with LPS and disrupting the supramolecular structure of LPS24. Thus, IntsΔPten/ΔPten;Il10−/− mice at 32 days old were fed with polymyxin B (100 ppm)- or vehicle mixed chow for 2 months. IntsΔPten/ΔPten;Il10−/− mice with polymyxin B-mixed diet revealed substantially improved survival rate compared to the mice with control diet (Figure 7E). At the age of 3 months old, mice fed with control diet had severely inflamed colon. In contrast, age matched mice treated with polymyxin B preserved the integrity of colonic mucosa (Figure 7F). Compared to enhanced degree of colitis in IntsΔPten/ΔPten;Il10−/− mice under control diet, the histological parameters of colitis were greatly reduced in polymyxin B-treated IntsΔPten/ΔPten;Il10−/− mice. Taken together, these data indicate that inhibiting endogenous LPS-TLR4 engagement not only delays the onset of colitis, but also ameliorates the severity of colitis in IntsΔPten/ΔPten;Il10−/− mice.
DISCUSSION
Emerging evidence indicated that Pten could be an important signaling molecule regulating the inflammation and innate immunity in response to commensal and pathogenic microbes14, 18. Commensal microbes mainly reside in the luminal side of the intestine. Therefore, it is of interest to investigate the impact of Pten in gut homeostasis controlled by the coherence of pro- and anti-inflammatory responses upon enteric microbiota. Our study demonstrated that loss of Pten in the intestinal epithelium induced severe colitis and colon malignancy at very early ages in Il10−/− mice, indicating that together with defective IL10 signaling, impaired Pten could be a polygenic factor to colitis occurring at very early ages.
In addition to colitis, IntsΔPten/ΔPten;Il10−/− mice had tumor development in the colon. Inflamed colon of IntsΔPten/ΔPten;Il10−/− mice had dysplastic lesions that can be localized, flat, or polypoid characteristics of colitis-associated colon cancer (CAC). As chronic UC precedes CAC development in humans, we believe that colon malignancy in IntsΔPten/ΔPten;Il10−/− mice could result from severe colitis developed at early ages.
Our data showed that only Bacteroides were greatly increased in the fecal sample of IntsΔPten/ΔPten;Il10−/− mice compared to IntsCont;Il10−/− mouse samples, implying that Bacteroides could be responsible for eliciting spontaneous colitis in IntsΔPten/ΔPten;Il10−/− mice. This notion is supported by the following evidence: The abundance of Bacteroides is much higher in the colon of UC patients than healthy control subjects25. Oral gavage of intestinal Bacteroides induces colitis in IBD-susceptible mice26. Helicobacter species was known to affect the pathogenesis of colitis in Il10−/− mice27, 28. However, the presence of Helicobacter was not detected from the fecal samples of the mice (Supplementary Table 1 and Supplementary Table 2). Accordingly, it is reasonable to believe that increased Bacteroides should be associated with inducing colitis in IntsΔPten/ΔPten;Il10−/− mice.
Gram-negative bacterial LPS is one of the most potent microbial factors in terms of proinflammatory properties. Despite the protective effect from LPS in a mouse epithelial injury model29, numerous studies indicated that LPS in the intestine promoted the intestinal inflammation30, 31. Gram-negative bacteria, moreover, are remarkably enhanced in the intestine of IBD patients32–34 and accumulated at high concentrations in an inflamed lesion of IBD patients35. These studies implied that LPS might be the microbial antigen eliciting intestinal inflammatory diseases. In agreement with this notion, our data showed that genetic or pharmacologic intervention to LPS-TLR4 engagement greatly delayed the onset of colitis and markedly reduced the severity of colitis in IntsΔPten/ΔPten;Il10−/− mice (Figure 7). Given that some antibiotic regimens improve clinical outcomes in IBD patients36, polymyxin B-driven effects in IntsΔPten/ΔPten;Il10−/− mice might be interpreted as a general effect of an antibiotic treatment. But, loss of the LPS specific receptor Tlr4 in IntsΔPten/ΔPten;Il10−/− mice dramatically inhibited the incidence of colitis. These data, therefore, substantiate that endogenous LPS in the colon plays a critical role in developing spontaneous colitis in IntsΔPten/ΔPten;Il10−/− mice.
In summary, intestinal epithelial Pten deletion in an Il10 deficient environment triggers spontaneous colitis and colon malignancy at very early ages by inducing gut dysbiosis with increased Gram-negative Bacteroides, in which endogenous LPS from altered gut microflora plays a critical role in colitis development. Our study, therefore, suggests that in addition to defective IL10 signaling, impaired Pten in the intestine may participate in the pathogenesis of early-onset ulcerative colitis, as a polygenic component.
Supplementary Material
Acknowledgments
Funding: This research was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIP) (No. 2009-0083538 & 2012R1A1A1042566, E.I.) and the National Institutes of Health (DK079015, S.H.R).
We thank Alyssa Leiva and Luis Papa at DLAM Diagnostic Laboratory for blood analyses and Dr. Sirus A. Kohan for supervising electron microscopy at the Electron Microscopy Services Center of Brain Research Institute at UCLA.
Abbreviations
- Ccl2
Chemokine (C-C motif) ligand 2
- Cxcl5
chemokine (C-X-C motif) ligand 5
- Cxcr2
chemokine (C-X-C motif) receptor 2
- Hmox1
heme oxygenase (decycling) 1
- Il 5
interleukin 5
- Il 18
interleukin 18
- Irak 1
interleukin 1 receptor-associated kinase 1
- Nos2
nitric oxide synthase 2
- Nr3c1
nuclear receptor subfamily 3, group C, member 1
- Sirt1
sirtuin 1
- Tirap/Mal
toll-interleukin 1 receptor (TIR) domain containing adaptor protein/ Myeloid differentiation factor 88 (MyD88)-adapter-like
Footnotes
Disclosures:
The authors declare no competing financial interests.
Author Contribution:
S.H.R. and E.I. carried out experiments, analyzed the data, and wrote the manuscript. S.H.R. conceived and supervised the study. J.J. did experiments. C.P. helped data interpretation and manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–45. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–21. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotlarz D, Beier R, Murugan D, et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143:347–55. doi: 10.1053/j.gastro.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 4.Lindberg J, Stenling R, Palmqvist R, et al. Early onset of ulcerative colitis: long-term follow-up with special reference to colorectal cancer and primary sclerosing cholangitis. J Pediatr Gastroenterol Nutr. 2008;46:534–8. doi: 10.1097/MPG.0b013e31815a98ef. [DOI] [PubMed] [Google Scholar]
- 5.Begue B, Verdier J, Rieux-Laucat F, et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Am J Gastroenterol. 2011;106:1544–55. doi: 10.1038/ajg.2011.112. [DOI] [PubMed] [Google Scholar]
- 6.Shah N, Kammermeier J, Elawad M, et al. Interleukin-10 and interleukin-10-receptor defects in inflammatory bowel disease. Curr Allergy Asthma Rep. 2012;12:373–9. doi: 10.1007/s11882-012-0286-z. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 8.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg DJ, Davidson N, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–20. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Cristofano A, Pesce B, Cordon-Cardo C, et al. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–55. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 11.He XC, Yin T, Grindley JC, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–98. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langlois MJ, Roy SA, Auclair BA, et al. Epithelial phosphatase and tensin homolog regulates intestinal architecture and secretory cell commitment and acts as a modifier gene in neoplasia. FASEB J. 2009;23:1835–44. doi: 10.1096/fj.08-123125. [DOI] [PubMed] [Google Scholar]
- 13.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and crosstalks. Oncogene. 2008;27:5527–41. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 14.Choi YJ, Jung J, Chung HK, et al. PTEN regulates TLR5-induced intestinal inflammation by controlling Mal/TIRAP recruitment. FASEB J. 2013;27:243–54. doi: 10.1096/fj.12-217596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groszer M, Erickson R, Scripture-Adams DD, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–9. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 16.Madison BB, Dunbar L, Qiao XT, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–83. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 17.De Witt Hamer PC, Mir SE, Noske D, et al. WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res. 2011;17:4200–7. doi: 10.1158/1078-0432.CCR-10-2537. [DOI] [PubMed] [Google Scholar]
- 18.Schabbauer G, Matt U, Gunzl P, et al. Myeloid PTEN promotes inflammation but impairs bactericidal activities during murine pneumococcal pneumonia. J Immunol. 2010;185:468–76. doi: 10.4049/jimmunol.0902221. [DOI] [PubMed] [Google Scholar]
- 19.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strober W. Impact of the gut microbiome on mucosal inflammation. Trends Immunol. 2013;34:423–30. doi: 10.1016/j.it.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arthur JC, Perez-Chanona E, Muhlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–3. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 23.Rhee SH, Hwang D. Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappa B and expression of the inducible cyclooxygenase. J Biol Chem. 2000;275:34035–40. doi: 10.1074/jbc.M007386200. [DOI] [PubMed] [Google Scholar]
- 24.Velkov T, Thompson PE, Nation RL, et al. Structure--activity relationships of polymyxin antibiotics. J Med Chem. 2010;53:1898–916. doi: 10.1021/jm900999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucke K, Miehlke S, Jacobs E, et al. Prevalence of Bacteroides and Prevotella spp. in ulcerative colitis. J Med Microbiol. 2006;55:617–24. doi: 10.1099/jmm.0.46198-0. [DOI] [PubMed] [Google Scholar]
- 26.Bloom SM, Bijanki VN, Nava GM, et al. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011;9:390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chichlowski M, Sharp JM, Vanderford DA, et al. Helicobacter typhlonius and Helicobacter rodentium differentially affect the severity of colon inflammation and inflammation-associated neoplasia in IL10-deficient mice. Comp Med. 2008;58:534–41. [PMC free article] [PubMed] [Google Scholar]
- 28.Kullberg MC, Ward JM, Gorelick PL, et al. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–66. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Im E, Riegler FM, Pothoulakis C, et al. Elevated lipopolysaccharide in the colon evokes intestinal inflammation, aggravated in immune modulator-impaired mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G490–7. doi: 10.1152/ajpgi.00120.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrance IC, Wu F, Leite AZ, et al. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750–61. doi: 10.1053/j.gastro.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 32.Martin HM, Campbell BJ, Hart CA, et al. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology. 2004;127:80–93. doi: 10.1053/j.gastro.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 33.Seksik P, Rigottier-Gois L, Gramet G, et al. Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut. 2003;52:237–42. doi: 10.1136/gut.52.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swidsinski A, Weber J, Loening-Baucke V, et al. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43:3380–9. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heimesaat MM, Bereswill S, Fischer A, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006;177:8785–95. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 36.Taylor KM, Irving PM. Optimization of conventional therapy in patients with IBD. Nat Rev Gastroenterol Hepatol. 2011;8:646–56. doi: 10.1038/nrgastro.2011.172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.