Abstract
There is increasing awareness of the role of sleep disturbance as an important factor in health and disease. Although subclinical sleep disturbances (insufficient sleep duration or inadequate sleep quality) may be difficult to assess with conceptual and/or methodological clarity, this review attempts to summarize and synthesize these findings. First, the concept of sleep disturbance in a public health context is introduced, to provide context and rationale. Second, operational definitions of ‘cardiometabolic disease’ and ‘sleep disturbance’ are offered, to address many unclear operationalizations. Third, the extant literature is summarized regarding short or long sleep duration and/or insufficient sleep, insomnia and insomnia symptoms, general (non-specific sleep disturbances), circadian rhythm abnormalities that result in sleep disturbances, and, briefly, sleep-disordered breathing. Fourth, the review highlights the social/behavioural context of sleep, including discussions of sleep and race/ethnicity, socio-economic position, and other social/environmental factors, in order to place these findings in a social-environmental context relevant to public health. Fifth, the review highlights the issue of sleep as a domain of health behaviour and addresses issues regarding development of healthy sleep interventions. Finally, a research agenda of future directions is proposed.
Introduction
Sleep disorders, such as insomnia, sleep apnoea, and others, have been researched extensively regarding aetiology, pathophysiology, and effects on health and functioning. Still, much work needs to be done to achieve a comprehensive understanding of these conditions. Less attention has focused on unique problems associated with sleep disturbances that may or may not be indicative of a sleep disorder. Many epidemiological studies, for example, assess sleep disturbance broadly. These assessments can be problematic, in that it is often unclear whether these measurements capture disorders, symptoms of disorders, or transient/non-significant complaints. With the relatively recent recognition of the significant public health implications of insufficient sleep duration and/or inadequate sleep quality (sometimes referred to as ‘sleep deficiency’), increased attention has focused on sleep as a domain of health behaviour over and above specific disorders. This recognition has come in several forms; for example, healthy sleep has been the focus of two Institute of Medicine reports (Colten et al., 2006; Ulmer et al., 2009), adequate sleep has been included as a federal health and funding priority in Healthy People 2020 (Office of Disease Prevention and Health Promotion, 2011), and the public health impact of ‘sleep deficiency’ was a major focus of the recent research agenda published by the joint taskforce of the Sleep Research Society and the American Academy of Sleep Medicine (Zee et al., 2014).
Thus, healthy sleep, in a general sense, has been implicated as a major research and public health issue. This is largely because of the large number of studies that have implicated generally unhealthy sleep as a risk factor for many chronic diseases. Some of these relationships will be discussed in this review. The focus of this review is on ‘sleep disturbance’ which (as described below) can refer to several types of sleep complaints. The review also focuses on ‘chronic disease’ which has also been variously defined in the literature and is clarified below. The structure of this paper is intended to (1) introduce the concepts of chronic disease and sleep disturbance as they are used in this review, (2) summarize the extant literature linking general sleep disturbances to chronic cardiometabolic disease, (3) discuss the issue of sleep disturbance in a public health context, (4) address the possibility that intervening on sleep disturbance represents an opportunity to prevent chronic cardiometabolic disease, and (5) propose a research agenda for the future.
What is meant by ‘cardiometabolic disease?’
Approximately 100 years ago, the leading causes of death in the USA were more likely to be infectious diseases (tuberculosis and pneumonia were both in the top three). Currently, the leading causes of death include heart disease, cancer, chronic lower respiratory diseases, and other diseases that are not infectious (Kung et al., 2008). These conditions typically arise insidiously over years and represent outcomes of complex pathways of illness. Many of these leading causes of death in the USA are likely caused by lifestyle behavioural risk factors (Mokdad et al., 2004). Understanding these risk factors is crucial to understanding how to ameliorate the effects of (and possibly prevent) these diseases. Many of the health conditions that account for the most deaths have related pathways that include obesity, diabetes, and cardiovascular disease. A global term for these related conditions is ‘cardiometabolic disease’.
There are other important chronic diseases as well. For example, cancer is a chronic disease that is a major cause of death. It should be noted that there are many studies documenting sleep disturbances in cancer (Sharma et al., 2012), and several studies showing the benefits of treating sleep disorders in the context of cancer (Garland et al., 2011, 2014; Roscoe et al., 2007; Sharma et al., 2012). Also, sleep disturbances have been described in other chronic diseases as well, such as pain syndromes (Besteiro Gonzalez et al., 2011; Landis, 2011; Lindstrom et al., 2012; Perlis et al., 1997; Wagner et al., 2012), dementias (Jaussent et al., 2012; Jirong et al., 2013; Zhou et al., 2012), human immunodeficiency virus (HIV) (Gamaldo et al., 2013b; Saberi et al., 2013; Taibi, 2013), and others (Cengic et al., 2012; Chakravorty et al., 2013; Jensen et al., 2013; Parhami et al., 2012; Wallhausser-Franke et al., 2013). This review focuses primarily on cardiometabolic disease, since the goal is to understand the potential role of sleep disturbance in preventing chronic disease. Unfortunately, there is no available evidence that addressing sleep disturbances may aid in the prevention of pain, cancer or HIV infections, for example. There are studies showing that improving sleep can potentially improve outcomes in these disorders, though (Jungquist et al., 2010; Woodward, 2011). Since cardiometabolic disease can be postponed or prevented using lifestyle behavioural approaches (that would likely include healthy sleep), these are the focus of this review.
What do we mean by ‘sleep disturbances?’
The term ‘sleep disturbance’ is problematic in that it is non-specific. The term may refer to diagnosed or undiagnosed sleep disorders including insomnia and/or sleep apnoea, subclinical sleep continuity disturbances, non-restorative sleep, insufficient sleep, or even abnormally short or long sleep duration in the absence of overt symptoms. (A similar criticism applies to the recently adopted term ‘sleep deficiency’ (Zee et al., 2014).) The major limitation of this term is that because it is non-specific, it is difficult to gain conceptual or methodological clarity regarding its meaning and role in causal pathways. On the other hand, the use of general sleep disturbance can capture sleep problems in a global way. For epidemiological data collections, this has generally been the only way to assess sleep at a population level. Although there is a lack of precision in these assessments, this is balanced by an increase in generalizability and sample size. For example, typical polysomnographic studies have up to dozens of subjects, whereas population-level surveys can study thousands.
In some cases the ‘sleep disturbance’ was measured in terms of a specific symptom (e.g. difficulty initiating sleep at least 15 days/month (Grandner et al., 2013d)) and in some cases it was compounded (e.g. difficulty falling asleep, staying asleep, or sleeping too much, for at least 6 of 14 days (Grandner et al., 2012a)). In some cases, there was a chronicity component (as in the previous examples), and sometimes it only involved a subjective measure of severity. In some cases (particularly smaller studies), previously validated measures were used, whereas in most larger studies, these were simple survey questions. For the purposes of this review, the term ‘sleep disturbances’ is meant broadly, and throughout this review, the specific operationalization of the term will be described.
Cardiometabolic disease risk associated with sleep disturbance
Since sleep disturbances are variously defined, this section will deal separately with short and/or insufficient sleep duration, general (non-specific) sleep disturbance, and insomnia, with mentions of issues related to circadian rhythm disorders and sleep-disordered breathing. Many laboratory studies have demonstrated adverse effects of sleep deprivation that suggest mechanistic links between sleep loss and chronic disease (Grandner et al., 2010a; Knutson, 2010, 2012, 2013). However, these are beyond the scope of the present review. Rather, this review will focus on studies of individuals who experience sleep disturbances in a naturalistic setting, rather than studies of individuals for whom sleep disturbances are artificially and acutely induced.
Short or long sleep duration and insufficient sleep
For approximately 50 years and across approximately 50 studies, both short and long sleep duration have been associated with increased mortality risk (Cappuccio et al., 2010; Gallicchio & Kalesan, 2009; Grandner et al., 2010c). These studies used a variety of methods and designs, studied a variety of different populations, and had varying levels of statistical controls. Taken together, though, it seems that a robust finding linking both short and long sleep duration with mortality emerges. The mechanisms for this relationship are unclear, though several studies have shown that adverse outcomes are associated with both short and long sleep.
Among long sleepers, results have been mixed. This may be due to the fact that long sleepers tend to be individuals who have excessive sleep opportunity and frequently include individuals who are retired, unemployed, or disabled (Grandner & Drummond, 2007; Patel et al., 2012). That said, several studies have shown adverse outcomes associated with long sleep duration, including depression (Patel et al., 2006), obesity (Buxton & Marcelli, 2010), diabetes (Buxton & Marcelli, 2010; Kachi et al., 2012), carotid intima-media thickness (a measure of atherosclerosis risk) (Abe et al., 2011), and presence of cardiovascular disease in general (Buxton & Marcelli, 2010; Cappuccio et al., 2011) and heart attack (Altman et al., 2012; Magee et al., 2009) and stroke (Altman et al., 2012; Kakizaki et al., 2013) in specific.
Among short sleepers, dozens of studies have been conducted showing associations to chronic disease and/or associated risk factors. These have been summarized in several recent reviews (Faraut et al., 2012a; Grandner et al., 2010a, 2010c, 2013e; Knutson, 2010, 2012, 2013; Knutson et al., 2007; Knutson & Van Cauter, 2008; Liu et al., 2012a; Lucassen et al., 2012; Nielsen et al., 2011; Patel, 2009; Patel & Hu, 2008; Solarz et al., 2012; Wang et al., 2012; Zimberg et al., 2012). Briefly, short sleep duration has been associated with obesity-related outcomes, diabetes-related outcomes, and cardiovascular-related outcomes. Regarding obesity-related outcomes, short sleep duration has been associated with incident weight gain (Kobayashi et al., 2012; Nagai et al., 2013), incident obesity (Watanabe et al., 2010), current body mass index (Patel, 2009), and current adiposity(Chaput et al., 2007; Liu et al., 2012b; Moraes et al., 2013; Taveras et al., 2011; Yu et al., 2007). Regarding diabetes-related outcomes, short sleep duration has been associated with diabetes (Buxton & Marcelli, 2010; Grandner et al., 2014a; Hsieh et al., 2011; Kachi et al., 2012), insulin and/or glucose dysregulation (Beihl et al., 2009; Byberg et al., 2012; Chang et al., 2012b; Chaput et al., 2009; Javaheri et al., 2011; Liu et al., 2011; Lucassen et al., 2012; Zuo et al., 2012), and dysregulation of metabolic hormones (Chaput et al., 2007; Gangwisch, 2009; Hayes et al., 2011; Martinez-Gomez et al., 2011). Regarding cardiovascular-related outcomes, short sleep duration has been associated with incident hypertension (Cappuccio et al., 2011; Meng et al., 2013), current hypertension (Altman et al., 2012; Fang et al., 2012; Faraut et al., 2012b; Grandner et al., 2014a; Wang et al., 2012), dyslipidemia (Altman et al., 2012; Gangwisch et al., 2010a; Grandner et al., 2014a), atherosclerosis risk factors (King et al., 2008; Nakazaki et al., 2012; Sands et al., 2012), inflammation (Grandner et al., 2013e), and cardiovascular disease in general (Buxton & Marcelli, 2010), as well as history of heart attack (Altman et al., 2012) and stroke (Altman et al., 2012; Cappuccio et al., 2011; Eguchi et al., 2010; Hamazaki et al., 2011). More information about these and other associations can be found in recent reviews (Adenekan et al., 2013; Cappuccio et al., 2008, 2010, 2011; Cappuccio & Miller, 2011; Faraut et al., 2012a; Gallicchio & Kalesan, 2009; Gangwisch, 2009; Grandner et al., 2010a, 2010c, 2013e; Knutson, 2010, 2012, 2013; Knutson et al., 2007; Knutson & Van Cauter, 2008; Patel, 2009; Patel & Hu, 2008; Solarz et al., 2012; Van Cauter & Knutson, 2008).
One of the reasons that short sleep duration may be considered a form of sleep disturbance despite the fact that many people engage in this behaviour volitionally is because short sleep duration may confer risk for, or serve as a proxy for, sleep disturbances. For example, short sleep duration overlaps significantly with daytime dysfunction due to sleepiness, tiredness, and/or fatigue (Grandner & Kripke, 2004). In addition, individuals who report short sleep duration are also more likely to report difficulty initiating sleep, difficulty maintaining sleep, early morning awakenings, and non-restorative sleep (Grandner & Kripke, 2004).
If this is the case, especially if to the degree to which short sleep duration confers risk for sleepiness and/or fatigue, short sleep duration may be a proxy for insufficient sleep. Insufficient sleep has been variously defined in the literature and has rarely been explored as a construct separate from short sleep duration (which may include individuals with naturally reduced sleep need)(Altman et al., 2012). However, several studies have looked at self-reported insufficient sleep as a health risk factor. For example, Shankar and colleagues found a negative association between unmet sleep need and cardiometabolic health (2010). Earlier studies also found a significant positive correlation between perceived sleep insufficiency and reported poor general health, functional deficits and increased cortisol secretion and abnormal growth hormone metabolism (Copinschi, 2005).
Although the concept of insufficient sleep may overlap with short sleep duration, few studies have attempted to clarify these constructs. In an attempt at this, Altman and colleagues (2012) used data from the 2009 Behavioral Risk Factor Surveillance System (BRFSS) to compare sleep duration and sleep insufficiency in the prediction of BMI, obesity, diabetes, hypertension, hypercholesterolaemia, diabetes, heart attack, and stroke. In this study, both short sleep and insufficient sleep were related to outcomes. After adjusting for 18 sociodemographic and health covariates, both short sleep duration and insufficient sleep were associated with elevated BMI, obesity, hypertension and hypercholesterolaemia; only short and long sleep duration were associated with heart attack and stroke. When sleep duration and insufficiency were combined in the same model, most of the effects of insufficiency were subsumed by sleep duration, except for hypertension (which demonstrated unique effects for both variables) and hypercholesterolaemia (which was no longer significantly explained by sleep duration). This demonstrates that for obesity, much of the relationship with insufficiency is due to sleep duration, but this is not the case for high blood pressure and cholesterol. Further research is needed to better clarify the independent and/or interdependent roles of insufficiency versus duration.
Mechanisms of this relationship are not fully known. Several reviews have attempted to schematically represent potential pathways linking short sleep and cardiometabolic disease (Knutson et al., 2007; Patel, 2009). In general, these schematics have several potential pathways in common. One of the most commonly discussed pathways includes the metabolic hormones leptin and ghrelin. Leptin is a hormone secreted by fat cells that signals satiety via the hypothalamus. Laboratory sleep deprivation studies have shown that sleep deprivation is associated with decreased leptin levels, suggesting decreased satiety (Spiegel et al., 2004a, 2004b). Ghrelin is a hormone secreted by the stomach that stimulates hunger. Studies of sleep deprivation in the laboratory show increased ghrelin associated with sleep deprivation (Copinschi, 2005; Knutson & Van Cauter, 2008; Spiegel et al., 2004b; Van Cauter et al., 2008). This decrease in leptin and increase in ghrelin may lead to an orexigenic eating pattern that may predispose to weight gain and cardiometabolic disease, and is reflected in some of the changes in energy balance (Markwald et al., 2013; St-Onge et al., 2011) and hunger ratings (Spiegel et al., 2004b) observed in laboratory studies. Another potential pathway is via alterations in insulin and glucose functioning. Sleep deprivation studies have shown insulin and glucose dysregulation to be associated with sleep deprivation (Broussard et al., 2012; Buxton et al., 2010; Darukhanavala et al., 2011; Knutson & Van Cauter, 2008; Padilha et al., 2011; Reynolds et al., 2012). As insulin also plays an important role in leptin and other metabolic hormones, this pathway may intersect with the previous one as well. A third potential pathway involves the role of sleep deprivation as a proinflammatory state. Many studies have shown that sleep deprivation is associated with increased levels of inflammatory cytokines and other markers (for review see Grandner et al., 2013e). Inflammation is itself an important risk factor for cardiometabolic disease, as it is related to endothelial function, oxidative stress, and chronic stress. Interestingly, certain inflammatory pathways known to be associated with sleep deprivation also play a role in insulin regulation. A fourth potential pathway involves neurocognitive processing. Sleep deprivation has been frequently shown to impair cognitive function (Banks & Dinges, 2007; Durmer & Dinges, 2005; Lal et al., 2012). In particular, sleep deprivation impairs decision-making (Killgore, 2010; Killgore et al., 2007, 2008a, 2008b, 2009a, 2009b, 2012). In addition to the humoral pathways described above, sleep deprivation may lead to an impaired ability to make healthy food choices (St-Onge et al., 2012). This, coupled with the propensity of sleep-deprived individuals to indulge in late-night snacks (Markwald et al., 2013; Nedeltcheva et al., 2009; Weiss et al., 2010), may represent another pathway. Other mechanistic links have been suggested, such as the role of protein kinase B (Broussard et al., 2012), adiponectin (Simpson et al., 2010), and others.
Insomnia and insomnia symptoms
In the epidemiologic literature, insomnia is rarely assessed using gold-standard measures. Usually, insomnia symptoms are asked individually (e.g. Do you have difficulty falling asleep at the beginning of the night?), usually with Likert scales. Since these assessments are not standardized, they should be interpreted with some level of caution. Still, these studies allow for assessments of relationships with initial insomnia (difficulty initiating sleep), middle insomnia (difficulty maintaining sleep), and late insomnia (early morning awakenings), since these symptoms are often asked separately.
There is an emerging literature documenting adverse cardiometabolic risk factors associated with insomnia. Several studies have shown that insomnia symptoms are associated with hypertension in the population duration (Gangwisch et al., 2006; Gangwisch et al., 2010b; Knutson et al., 2009; Phillips & Mannino, 2007; Suka et al., 2003), especially when the insomnia is accompanied by short sleep duration (Fernandez-Mendoza et al., 2012b; Vgontzas et al., 2009a). It is currently unclear whether insomnia itself poses a risk, or whether insomnia with short sleep duration is required.
Meng and colleagues (2013) reviewed studies of insomnia and incident hypertension in a meta-analysis, showing that overall insomnia is associated with a small but significant (5%) increased risk of incident hypertension. Separately, although initial insomnia was not significantly associated with incident hypertension, middle insomnia was associated with a 20% increased risk and late insomnia was associated with a 14% increased risk. In addition to hypertension, several studies have shown insomnia to be associated with inflammation (Jain et al., 2012; Laugsand et al., 2012; Motivala, 2011; Okun et al., 2011), impaired heart rate variability (De Zambotti et al., 2011; Israel et al., 2012; Spiegelhalder et al., 2011; Stein & Pu, 2012), and other risk factors (Balbo et al., 2010; Lattova et al., 2011; Laugsand et al., 2013; Strand et al., 2013).
In a recent study, Sands-Lincoln and colleagues evaluated the relationship of insomnia to coronary heart disease and cardiovascular events among > 85,000 women enrolled in the Women’s Health Initiative (Sands-Lincoln et al., 2013b). They found that in fully adjusted models, insomnia symptoms were associated with a 19% increased risk of coronary heart disease and an 11% increased risk of cardiovascular events.
In addition to these studies, there is a literature that has suggested that the cardiometabolic risks associated with insomnia are particularly evident in the subset of sufferers that also experience short sleep duration (Vgontzas et al., 2013). In this way, there is an interaction between sleep duration and insomnia such that those with insomnia and short sleep duration demonstrate greater risks than those with either condition alone. Several studies have explored this phenomenon. For example, individuals with both short sleep and insomnia have been shown to be at increased risk for hypertension (Fernandez-Mendoza et al., 2012b; Vgontzas et al., 2009a), diabetes (Vgontzas et al., 2009b), depression (Fernandez-Mendoza et al., 2012a), and mortality (Vgontzas et al., 2010), relative to short sleepers without insomnia and individuals with insomnia without short sleep. These results may be moderated by unknown factors. For example, Sands-Lincoln and colleagues found that among postmenopausal women in a national sample, although there was a sleep duration by insomnia interaction in the prediction of coronary heart disease, they found that it was insomnia coupled with long sleep that was associated with the highest risk (Sands-Lincoln et al., 2013b).
General sleep disturbance (non-specific)
Although the use of individual insomnia symptoms presents methodological and interpretive challenges due to non-standardized measures, these challenges become even greater with assessments of general sleep quality. That said, there are several studies which only had access to general sleep quality data for analyses. Grandner and colleagues found that sleep disturbance, assessed as difficulty falling asleep, staying asleep, or sleeping too much in at least 7 of the past 14 days (assessed using the 2006 BRFSS), was associated with a 35% increased risk of obesity, a 54% increased risk of diabetes, an 80% increased risk of heart attack, a 102% increased risk of stroke, and a 98% increased risk of any coronary artery disease (Grandner et al., 2012a). All of these relationships remained significant (though attenuated) after adjustment for demographics, socio-economics, mental health, healthcare access, smoking, alcohol use and BMI (for outcomes other than obesity). When overall physical health was entered into the model, the relationships with diabetes and stroke were no longer significant. Still, this study showed that poor sleep quality in general is an important health risk factor. A separate analysis of the 2006 BRFSS data asked a different question. Rather than using sleep disturbance to predict outcomes of interest, is it the case that those with obesity and diabetes sleep poorly overall, and is this mediated by access to exercise? In this separate study (Grandner et al., 2011), those with obesity and diabetes were more likely to report both night-time sleep disturbance and daytime tiredness. When exercise (assessed as any exercise in the past 30 days) was entered into the model, the relationships did not change (no mediation). Presumably, the amount of exercise measured by this variable includes small amounts of activity, which likely do not impact obesity or diabetes. However, simply reporting any activity was associated with a decreased likelihood of reporting sleep disturbance (around 30% decrease) and daytime tiredness (around 50% decrease). This suggests that general sleep disturbance is strongly associated with other general lifestyle health factors, such as physical activity.
Other studies have also assessed links between sleep disturbance and cardiometabolic disease risk. For example, Troxel and colleagues found that sleep complaints were associated with greater risk of metabolic syndrome (Troxel et al., 2010a). Also, Sands-Lincoln and colleagues found that self-reported non-restorative sleep was associated with hypertension in a nationally representative sample of US adults (Sands-Lincoln et al., 2013a).
Circadian rhythm disturbances
Circadian rhythm disturbances frequently interfere with sleep. Circadian rhythms refer to the ~ 24-h rhythms in the body responsible for regulation of a large host of physiological processes. When these rhythms are disturbed, sleep is also frequently disturbed as well. For example, advanced sleep phase refers to the condition where an individual’s natural bed and rise times are much earlier than they prefer; similarly, delayed sleep phase refers to natural bed and rise times that are later than preferred. There is evidence that preferred sleep phase is, to some degree, driven by development (Sadeh et al., 2009; Tarokh et al., 2012) and genetic predisposition (Kerman et al., 2012; Lamont et al., 2007; Saini et al., 2011), but it can also be entrained by behaviours and exposure to light at inappropriate times (Chang et al., 2012a; Corbett et al., 2012; Kavcic et al., 2011; Sharkey et al., 2011). Another example of a circadian disturbance occurs with shift work. In this case, individuals are tasked with maintaining wakefulness and performance during hours for which this is not biologically optimal. This leads to several situations, including consequences of repeatedly maintaining wakefulness when biologically predisposed to sleep, consequences of the mismatch between internal and external rhythms, and consequences of the inability for the internal rhythm to adjust, especially in the case of rotating schedules or weekends off (Bannai & Tamakoshi, 2014; Cheng et al., 2014; Darwent et al., 2012; Guyette et al., 2013; Lin et al., 2012; Machi et al., 2012; Roenneberg et al., 2013; Ruggiero et al., 2012; Walia et al., 2012).
Most studies of circadian rhythm disturbances (including circadian rhythm sleep disorders) tend to focus on either basic physiological processes or, when they examine outcomes, these outcomes tend to be in the psychological domain. For example, several studies have shown that advanced and delayed sleep phase are both associated with insomnia (Kohyama, 2011; Martinez & Lenz Mdo, 2010; Reid & Zee, 2009), affective disorders (Kripke et al., 2008; Mendlewicz, 2009; Okawa, 2011; Robillard et al., 2013; Saxvig et al., 2012) and functional impairments (Bijlenga et al., 2013; Dowling & Mastick, 2010; Hida et al., 2012; Miller et al., 2012; Saxvig et al., 2012; Zhou et al., 2011).
Studies of shift work, on the other hand, have focused attention on health consequences of shift work. This is relevant to the current discussion since shift work is very frequently associated with abbreviated or otherwise disturbed sleep (Ferguson et al., 2012; Lin et al., 2012; Machi et al., 2012; Mahan et al., 1990; Roach et al., 2012). For example, Cheng and colleagues found that men working longer than 60 h/week were at approximately double the risk for coronary heart disease (Cheng et al., 2014). Notably, short sleep duration in this group was found to be a significant moderator of the effect, with those sleeping < 6 h on average being at the highest risk. This is consistent with a recent review by Bannai and Tamakoshi, which found that long working hours is detrimental to health and functioning (Bannai & Tamakoshi, 2014). In addition to the longer hours usually associated with shift work, a recent review by Szosland summarizes data that implicates shiftwork in diabetes, ischaemic heart disease, and metabolic syndrome (Szosland, 2010). Successful acclimation to the night work may alleviate many of these risks. For example, Boudreau and colleagues found that circadian adaptation to night work resulted in improved sleep, improved performance, and improved heart rate variability (Boudreau et al., 2013).
Sleep-disordered breathing
Many reviews have been written on the cardiometabolic risk factors associated with sleep-disordered breathing and sleep apnoea (Butt et al., 2010; Fava et al., 2011; Golbidi et al., 2012; Kent et al., 2011; Loke et al., 2012; Lurie, 2011; Monahan & Redline, 2011; Morselli et al., 2012; Pak et al., 2014; Phillips et al., 2013; Primhak & Kingshott, 2012). Thus, this issue is largely outside the scope of this paper. But it should be noted that sleep apnoea often presents as daytime sleepiness or non-restorative sleep (Alam et al., 2012; Cruz et al., 2012; Lal et al., 2012; Lee et al., 2012; Sunwoo et al., 2012) and sometimes as insomnia (Al-Jawder & Bahammam, 2012; Glidewell et al., 2012). Sleep apnoea is associated with significant sleep fragmentation (Bianchi et al., 2012) which may be one of the causal pathways linking this disorder to poor sleep, though the risks are probably largely due to the intermittent hypoxia and co-morbid obesity (Pak et al., 2014).
In summary, sleep disturbances, which could be defined as short or long sleep duration, perceived insufficient sleep, inadequate sleep quality, insomnia, circadian rhythm disturbance, and/or sleep-disordered breathing, are related to cardiometabolic disease risk factors. These relationships depend on the sleep variable studied. For example, short sleep duration has been associated with many adverse in mostly cross-sectional (but some longitudinal) designs. Long sleep duration and perceived insufficient sleep, however, have not been studied as thoroughly. Insomnia and its symptoms are strongly related to mental health outcomes, but cardiometabolic relationships have just recently been explored. It is possible that much of the relationship between insomnia and cardiometabolic disease involves an interaction with short sleep duration. Circadian rhythm problems can also predispose individuals to adverse outcomes, especially when accompanied by long work hours. Sleep-disordered breathing, especially (untreated) obstructive sleep apnoea, is a well-known cardiometabolic risk factor, associated with cardiac and vascular outcomes, diabetes, and mortality.
Given that these sleep disturbances are prevalent and are associated with important clinical outcomes, it is important to consider the broader public health implications. If the above outcomes represent the downstream effects, attention must also be paid to the upstream determinants of sleep in the population. Understanding the contextual factors that give rise to sleep disturbances may better aid in the identification of high-risk groups, the understanding of mechanisms leading to adverse health outcomes, and the conceptualization of effective interventions. The next section addresses issues regarding the social and behavioural context.
The social and behavioural context of sleep disturbance
The issue of chronic disease management is a major public health concern. The burden of chronic health conditions such as obesity, diabetes, and hypertension are disproportionately borne by racial/ethnic minorities and those of lower socio-economic position (Centers for Disease Control and Prevention, 2011). This may be an important reason why life expectancy is lower among many racial/ethnic minority groups compared to white groups (Levine et al., 2001). Understanding the nature of these disparities is an important step in developing strategies for ameliorating them. In the context of sleep and health, it is important to understand the degree to which the burden of poor sleep is experienced among racial/ ethnic minorities and those who are socio-economically disadvantaged. In addition, it is important to consider other behavioural and social factors that may explain some of this increased burden and point to potential intervention components.
Sleep and race/ethnicity
Several studies have shown that racial/ethnic minorities are more likely to be short sleepers. For example, Stamatakis and colleagues found that African-American and ‘other’ race/ethnicities (but not Hispanic) were consistently associated with short sleep duration (Stamatakis et al., 2007). Also, data from Hale and Do showed that black/African-Americans were also more likely to be long sleepers (Hale & Do, 2006).
A recent study by Whinnery and colleagues represents the most comprehensive analysis of sleep duration among racial/ethnic minorities in the US (Whinnery et al., 2014). This study used data from the 2007–2008 National Health and Nutrition Examination Survey (NHANES). In adjusted analyses, black/African-Americans, non-Mexican Hispanics/Latinos, and Asians/others were more likely to report very short sleep (< 5 h), black/African-Americans and Asians/others were more likely to report short sleep (5–6 h), Mexican-Americans were less likely to report long sleep (9 + h), those born in Mexico were less likely to report short sleep, and speaking Spanish only at home was associated with lower likelihood of reporting very short sleep. Other studies have found other patterns. For example, Hale and Do found that in addition to short sleep, black/African-Americans were more likely to report longer sleep as well (Hale & Do, 2007). The effect of the environment may also be a major explanatory factor. For example, Gamaldo and colleagues studied middle-aged urban adults and found no significant differences in the likelihood of short or long sleep between non-Hispanic white adults and black/African-Americans (Gamaldo et al., 2013a). Interestingly, within groups, different factors predicted shorter sleep, with stress and education playing a larger role in the non-Hispanic white group, and inflammation playing a larger role in the black/African-American group.
To assess patterns in sleep disturbance, Grandner and colleagues used the BRFSS 2006 sleep disturbance item (described above)(Grandner et al., 2010b). Among men, no significant differences were seen between non-Hispanic white groups and black/ African-Americans, Hispanics/Latinos, or those reporting more than one race/ethnicity, and a significant difference was seen among Asians/others, who reported fewer sleep disturbances. Among women, non-Hispanic white women reported significantly more sleep disturbance than black/African-Americans, Hispanics/Latinos, and Asians/others, and less sleep disturbance than those reporting more than one race/ethnicity. This finding was surprising – despite increased likelihood of short sleep duration and associated outcomes, racial/ethnic minorities tended to report fewer sleep disturbances.
A subsequent study by Grandner and colleagues examined the 2007–2008 NHANES data (Grandner et al., 2013d). In this case, rather than sleep disturbance, specific variables were assessed: sleep latency > 30 min, difficulty initiating sleep, difficulty maintaining sleep, early morning awakenings, non-restorative sleep, daytime sleepiness, choking/gasping during sleep (suggesting sleep apnoea), and snoring. In models that included age, sex, race/ethnicity, marital status, immigrant status, income, education, access to health insurance, and household food security, the following results were found. Black/African-Americans were 58% more likely to report sleep latency > 30 min, compared to non-Hispanic white adults, and no other differences were seen for this variable. Contrastingly, self-reported difficulty falling asleep was reported less frequently among black/ African-Americans (odds ratio (OR) = 0.57), Mexican-Americans (OR = 0.59), and other Hispanics/ Latinos (OR = 0.73). Similarly, difficulty maintaining sleep, early morning awakenings, non-restorative sleep, and daytime sleepiness were all reported less frequently among black/African-Americans and Mexican-Americans, and both non-restorative and sleep daytime sleepiness were also reported less frequently among other Hispanics/Latinos. The non-Mexican Hispanics/Latinos were more likely to report choking/gasping during sleep and snoring, however.
These findings mirror those of other studies, which inconsistently describe sleep disturbances among racial/ethnic minorities. For example, some studies have found overall sleep quality to be worse among minorities (Boss et al., 2011; Kripke et al., 2004; Moore et al., 2011; Patel et al., 2010b), whereas others have failed to find this association (Gellis et al., 2005; Grandner et al., 2010b; Hale & Rivero-Fuentes, 2011). Several reasons for this are possible. First, it is possible that differences in measurement introduce error across studies. Second, there may be fundamental differences among cohorts. Third, effects of geographical location and other environmental variables may moderate these relationships (Grandner et al., 2012b). Fourth, there may be differences in levels of bias in reporting. For example, questions asked in a non-judgemental way (e.g. sleep duration, sleep latency) may lead to different reporting patterns than questions asked in a way that asks for an explicit complaint (e.g. difficulty initiating or maintaining sleep). Another important issue is a potential interaction between race/ethnicity and poverty. Patel et al. (2010) and colleagues found that, relative to the non-poor non-Hispanic white group, poor individuals of all races/ethnicities (including the non-Hispanic white group) had worse sleep overall. The only non-poor group to differ from the non-poor white group was non-poor black/African-Americans, who had worse sleep overall, but to a lesser degree than their poor counterparts.
Sleep and socio-economic position
Socio-economic position is frequently assessed using variables such as education level, household income, and occupational status. Previous studies that have examined associations between sleep quality and socio-economic factors have tended to report increased sleep disturbance (represented as general overall sleep disturbance or specific symptoms such as difficulty falling asleep, staying asleep or waking too early) among individuals of lower socio-economic position (Friedman et al., 2005; Gellis et al., 2005; Geroldi et al., 1996; Grandner et al., 2010b, 2013c; Hall et al., 2009; Nomura et al., 2010; Patel et al., 2010a). In addition, several studies have shown that those of lower socio-economic position are more likely to be short sleepers (Stamatakis et al., 2007; Whinnery et al., 2014). Although few studies have measured this relationship using sleep assessed with polysomnography, these have also have generally found that lower socio-economic position is associated with poorer sleep quality (Friedman et al., 2005; Hall et al., 2009; Mezick et al., 2008; Tomfohr et al., 2010).
In the aforementioned study of general sleep disturbance measured using the BRFSS 2006 (Grandner et al., 2010b), increased sleep disturbance was more likely amongst those who did not graduate college, were of lower income (especially < US$25,000 among men), and were unemployed. In the study by Whinnery and colleagues (Whinnery et al., 2014), in fully adjusted models, very short sleep was more likely to be reported among those earning < $20,000 and $65,000–75,000 (relative to > $75,000), those who did not complete college, those with public insurance only (relative to no health insurance at all), and those with marginal or very low food security. Short sleep was more likely to be reported among those who attended high school but did not graduate from college, and those with very low food security. Long sleep was more frequently reported among those earning $20,000–25,000 per year and those with public insurance only. In the complementary study by Grandner and colleagues (Grandner et al., 2013d), sleep latency > 30 min was associated with less than a college education, no access to private health insurance, and marginal, low, or very low food security. Insomnia symptoms (difficulty falling asleep, difficulty maintaining sleep and early morning awakenings) were only associated with food security, and not other factors. Non-restorative sleep, sleepiness, snorting/gasping and snoring were all associated with lower education levels and lower food security. Interestingly, relationships to income (seen in unadjusted analyses) were rendered non-significant after adjustment. For income, it seems that the once the benefits of increased income are realized (e.g. access to employment, education, food and healthcare, little unique variance is explained by income alone.
Sleep and other social/environmental factors
In addition to (and perhaps interacting with) race/ ethnicity and socio-economic position, a whole host of contextual factors play a role in sleep disturbance. For example, recent studies have shown that geography explains sleep disturbance (Grandner et al., 2012b) and insufficient sleep (Grandner et al., 2013f) in the US. Preliminary analyses suggest that a whole host of factors may be relevant to these geographical differences, including differences in racial make-up of the population, temperature and weather patterns, regional health issues, regional healthcare access, and others (Grandner et al., 2012b). On a more granular level, there is a growing body of literature showing that neighbourhood factors play a role in sleep quality. Not only have studies shown that neighbourhoods that are perceived to be dirty, dangerous, and disconnected are also home to individuals more likely to report poor sleep quality (Hale & Do, 2006; Hale et al., 2010, 2013; Hill et al., 2009), but this increased poor sleep quality may partially mediate the detrimental effect that these neighbourhoods have on health (Hale et al., 2010).
In addition to geographic factors, there may be cultural beliefs and attitudes about sleep that may play a role. For example, Sell and colleagues found that Mexican-Americans who were not acculturated to American norms were less likely to be able to demonstrate basic knowledge about sleep disorders and what the symptoms of sleep disorders look like (Sell et al., 2009). In addition, a study in the Philadelphia area compared beliefs and attitudes about sleep matched for income level among older women matched for income (Grandner et al., 2013b). This study found that black/African-American women were less likely to report beliefs and attitudes consistent with healthy sleep habits. These data are consistent with other studies that show that black individuals who are at higher risk of obstructive sleep apnoea demonstrate more dysfunctional beliefs and attitudes about sleep (Pandey et al., 2011). Some cultural differences in sleep-related beliefs and attitudes may be reflected early. For example, Sadeh and colleagues (Sadeh et al., 2011) found that the definition of a child’s ‘sleep problem’ differed between countries relative to primary race/ethnicity. In primarily white countries, a ‘sleep problem’ was largely determined by length of sleep episode, nocturnal wakefulness, number of night wakings and total sleep time, whereas in primarily Asian countries, children’s ‘sleep problems’ were more likely to be determined by socio-demographic and/or socio-economic factors, such as the child’s age, parents’ age, and parental education level.
In summary, poor sleep is a health concern that may be disproportionately felt by minorities and/or individuals of lower socio-economic position. As Simon Williams writes, ‘When we sleep, where we sleep, and with whom we sleep are all important markers or indicators of social status, privilege, and prevailing power relations’ (Williams, 2005). The next section considers the upstream determinants of sleep disturbance and the downstream cardio-metabolic (and other) adverse effects of sleep disturbance to consider a theoretical framework for considering the role of sleep in public health and the development and implementation of public health interventions.
Conceptualizing sleep disturbance in the context of public health
Although most, if not all, sleep disorders have targeted interventions (e.g. PAP therapies for sleep apnoea (Avlonitou et al., 2012; Bertisch & Patel, 2012; Campbell et al., 2012; Ciccone et al., 2012; Filtness et al., 2012; Hegglin et al., 2012; Zhao et al., 2012), hypnotics or cognitive behavioural therapy for insomnia (CBTI) (Doghramji, 2010; Ebben & Narizhnaya, 2012; Gehrman & Gooneratne, 2010; Hall-Porter et al., 2010; Jarnefelt et al., 2012; Krystal, 2009; Morin & Willett, 2009; Pinto et al., 2010; Saddichha, 2010; Siebern & Manber, 2010; Siebern et al., 2012; Sullivan, 2010; Taylor et al., 2010), dopamine agonism for RLS and PLMD (Aurora et al., 2012; Benes et al., 2009; Earley et al., 2011; Earley & Silber, 2010; Garcia-Borreguero & Williams, 2010; Khalid et al., 2009; Natarajan, 2010; Quilici et al., 2008; Trenkwalder & Paulus, 2010; Wetter, 2010)), no intervention has been developed or evaluated for insufficient sleep or other aspects of general sleep disturbance. One potential reason is that the data needed to frame this form of sleep as important and relevant has only been recently aggregated in a way that makes the case compelling.
Sleep as a domain of health behaviour
One way to conceptualize insufficient sleep and other aspects of general sleep disturbance sleep disturbance is as a disorder. This perspective is one that has been adopted by the sleep medicine community, given the inclusion of insufficient sleep syndrome in the International Classification of Sleep Disorders (American Academy of Sleep Medicine et al., 2006). Another way to conceptualize insufficient sleep is as a domain of health behaviour. Insufficient sleep shares many characteristics with other behaviours, such as smoking, poor diet, and lack of physical activity, in that the reasons for insufficient sleep involve beliefs and attitudes (Carney & Edinger, 2006; Grandner et al., 2013b, 2014b; Smith et al., 2004; Yang et al., 2011), home and work demands (Basner & Dinges, 2009; Basner et al., 2007; Grandner et al., 2014b), and environmental constraints (Barclay et al., 2012; Buxton et al., 2012; Grandner et al., 2014b; McCall et al., 2012; Roenneberg et al., 2013; Verhaert et al., 2011; Yoder et al., 2012).
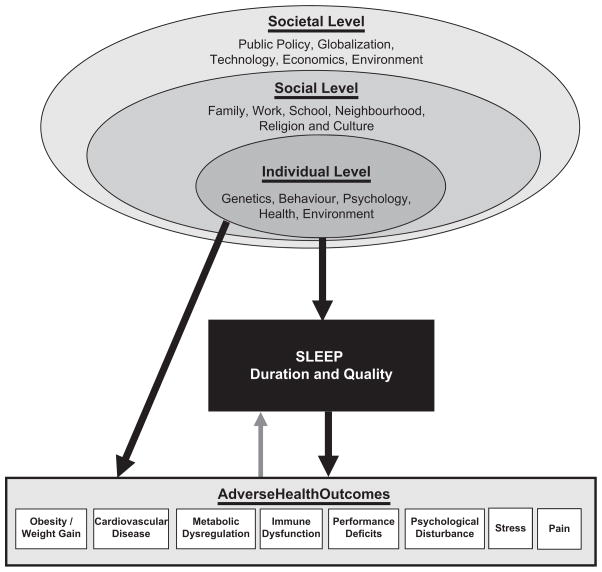
With this in mind, a social-ecological model of sleep and health was recently proposed by our group (Grandner et al., 2010c), based on the social-ecological theory of Bronfenbrenner (1977). This model is summarized in Figure 1 and suggests that sleep disturbances (and resultant adverse outcomes) depend on individual-level factors (e.g. behaviour, physiology), which are embedded within social factors (e.g. neighbourhood, work), which are themselves embedded within societal factors (e.g. 24/7 society).
Figure 1.
Socio-ecological model of sleep and health. Adapted from Grandner et al. (2010c).
This model brings together the upstream social/ behavioural determinants of sleep and the downstream adverse consequences of sleep disturbance. According to the model, the individual-level factors (which would include that individuals’ specific demographics, genetics, physiology, health, knowledge, beliefs, attitudes, and choices) directly impact on that individual’s sleep. However, the model goes a step further and recognizes that all of these individual factors are embedded within a social framework that includes people that exist separate from the individual but directly or indirectly impact the individual-level factors. For example, a noisy or crime-ridden neighbourhood, a culture that engenders certain beliefs/attitudes, a racial or ethnic group that is subject to certain social stresses, a work schedule that impinges on sleep time, family obligations that may begin early in the morning, or a social network that encourages unhealthy attitudes, all play a role in an individual’s sleep. The model even goes further to recognize that even these social-level factors exist in the context of larger, societal-level factors such as globalization, technology, 24/7 society, and others, which play a role in shaping social and eventually individual attitudes, beliefs, and practices regarding sleep.
This model suggests that all of these levels are relevant to understanding sleep. For example, recognizing the social-level factors aids in the understanding of the effects of neighbourhoods on sleep and sleep disorders (Brouillette et al., 2011; Hale & Do, 2006; Hale et al., 2010, 2013; Hill et al., 2009; Omlin et al., 2011; Platt et al., 2009), geographic patterns of sleep (Grandner et al., 2012b; McKnight-Eily et al., 2009), sleep and the physical environment (e.g. green space, weather, and traffic and other noise) (Astell-Burt et al., 2013; Buxton et al., 2012; Elmenhorst et al., 2012; Pandey et al., 2005; Pirrera et al., 2010), family and relationship factors (Ailshire & Burgard, 2012; Lallukka et al., 2013; Rhoades et al., 2012; Rogojanski et al., 2013; Troxel, 2010; Troxel et al., 2010b, 2010c; von Kaanel et al., 2012), and even the effects of a changing technological landscape (Brunborg et al., 2011; Crowder et al., 2012; Garmy et al., 2012; Lowden et al., 2011; Munezawa et al., 2011; Thomee et al., 2011, 2012) suggest that individual-level factors need to be viewed in the context of these social-level and societal-level factors. For example, the study by Gamaldo and colleagues mentioned above found no differences in sleep duration by race/ethnicity when the individuals were otherwise relatively homogeneous in terms of age and living environment (Gamaldo et al., 2013a).
This model also has implications for the development of interventions to address sleep and health. To change sleep-related behaviours, individual-level factors that affect sleep need to be taken into account, including knowledge, beliefs, attitudes, and practices. But social-level factors also need to be considered, such as work/school obligations, home/family obligations, and the social and environmental context. For example, community-based approaches may work better for some versus other groups. Thus, a behavioural intervention for insufficient sleep needs to address sleep as an aspect of health behaviour, cast in a larger context.
Developing a sleep health intervention
To develop an intervention it would be important to consider models of health behaviour change. These models are necessary because unhealthy behaviours (such as poor diet, sedentary lifestyle, smoking) are complex and resistant to change. Examples include the health belief model, the transtheoretical model, and behavioural economics. Briefly, the health belief model (Champion & Skinner, 2008; Rosenstock, 1966) states that individuals will alter their behaviour based on (1) perceived risk of maintaining existing behaviour, (2) perceived benefits of the new behaviour, (3) perceived barriers in engaging in the new behaviour, (4) perceived ability to carry out the behaviour, and (5) cues to action (reminders). The transtheoretical model (Prochaska & DiClemente, 1982; Prochaska et al., 2008, 2009), briefly, posits that behaviour change occurs on a dimension of readiness to change, which includes precontemplation (not yet thought about change), contemplation (thought but no decision to act), preparation (decision but no action), action, and maintenance. The model dictates that interventions based on action should only be implemented by those who are ready to engage in that action. Behavioural economics theory (Hursh, 1984), briefly, deals with the ways that complex health-promoting and health-compromising behaviours are/may be supported by complementary reinforcers (e.g. alcohol reinforces smoking behaviour) and weakened by alternative reinforcers (e.g. nicotine patch delivers nicotine without smoking). Since many health-compromising behaviours have benefits that may vary across individuals, financial incentives may act as a universal, simple, adaptable, alternative reinforcer (Halpern et al., 2011; John et al., 2011, 2012; Kim et al., 2012; Kimmel et al., 2012; Kullgren et al., 2013; Loewenstein et al., 2013; Shea et al., 2012; Volpp et al., 2008, 2011). As such, several studies have documented the benefits of financial incentives to change relatively intractable health-compromising behaviours (Cahill & Perera, 2011; Halpern et al., 2011; John et al., 2011; Kim et al., 2012; Kimmel et al., 2012; Kullgren et al., 2013; Volpp et al., 2008). It should also be noted that financial incentives may backfire in that they may serve to undermine an individual’s intrinsic motivation to improve their health (Hagger et al., 2014; Ma et al., 2014; Moller et al., 2012, 2013; Promberger & Marteau, 2013).
Does addressing sleep disturbance impact chronic disease?
There are no studies of interventions for general sleep disturbance, so it is difficult to say whether such an intervention would have a long-term impact on chronic disease. Despite this, if sleep shares characteristics with other health behaviours, it is reasonable to suspect that a sleep intervention could have lasting impact. After all, reduced cardiometabolic risk has been produced with interventions that successfully improve diet and exercise (Stephens et al., 2014) and reduce smoking (Rigotti & Clair, 2013). Regarding insomnia, although there are several studies showing associations with cardiometabolic disease, there are still no studies investigating whether insomnia treatment improves cardiometabolic outcomes. Regarding sleep apnoea, there are several studies that have shown improvements in cardiovascular outcomes (Gottlieb et al., 2013). For example, in studies by Martinez-Garcia and colleagues, treatment of sleep apnoea was associates with significantly reduced cardiovascular mortality (Campos-Rodriguez et al., 2012; Martinez-Garcia et al., 2009).
Future directions
Eventually, the goal is that there will be a more complete understanding of the role of sleep in health, and successful interventions will be ready for implementation. This is an important goal and was expressed by reports from the Institute of Medicine (Colten et al., 2006), the US Department of Health and Human Services (Office of Disease Prevention and Health Promotion, 2011) and the Joint Task Force of the American Academy of Sleep Medicine and Sleep Research Society (Zee et al., 2014). To achieve this goal, much work is needed.
First, the concept of ‘sleep disturbance’ (and the similar concept of ‘sleep deficiency’) needs to be unpacked. What is it about poor sleep that confers risk? What are the additive or interactive contributions of difficulty initiating sleep, difficulty maintaining sleep, decreased sleep ability, sleep fragmentation, insufficient sleep, excessive sleep, and so on? For example, epidemiological studies should move away from non-standard survey item measures of sleep and begin to adopt standardized, validated items or screening questionnaires. Also, retrospective, subjective measures of sleep need to be accompanied by prospective measures (e.g. sleep diary) and objective measures (e.g. actigraphy). This will allow for conceptual and methodological clarity regarding the role of sleep. Existing studies focused on specific sleep disorders help in this regard, but it should be noted that the domain of ‘sleep disturbance’ extends beyond the boundaries of traditional sleep disorders.
Second, additional research needs to clarify the causal relationships between sleep and cardiometabolic disease. Epidemiological studies should focus more on longitudinal designs. Since most cardiometabolic outcomes (e.g. obesity, metabolic syndrome, coronary heart disease) represent years of accumulated risk, and sleep assessments typically only retrospectively capture 1–4 weeks, it is difficult to argue in cross-sectional designs that the sleep problems preceded the cardiometabolic problems. For this reason, prospective, longitudinal designs are critical. Intervention studies may also help. If a sleep-based intervention can improve cardiometabolic health risk factors or even outcomes, this would lend further support to the notion that sleep changes are causally related to changes in cardiometabolic health.
Third, there needs to be an increased recognition of the role of sleep in health beyond the traditional construct of sleep disorders. Sleep likely represents a major domain of health behaviour. Studying sleep this way requires a different conceptual framework, likely involves different intervention strategies, and necessitates the examination of social and behavioural influences on health such as race/ethnicity and socio-economics. These perspectives will aid in gaining a better understanding of who is at increased risk of poor sleep (Grandner et al., 2013d; Whinnery et al., 2014), how this increased risk may be differentially associated with poor outcomes (Grandner et al., 2013a, 2014a; Sands-Lincoln et al., 2013a), and how to develop more effective interventions.
Summary and conclusions
‘Sleep disturbance’ is variously defined. It may represent short or long sleep duration (potentially reflecting insufficient or excessive sleep), or it may represent inadequate sleep quality, represented as a sleep disorder (or symptoms of a sleep disorder), such as insomnia, a circadian rhythm sleep disorder, or sleep-disordered breathing. Alternatively, it may be represented as a general perception of overall sleep quality. Taken together, the evidence suggests that sleep disturbances (especially short sleep duration, short sleep in the context of insomnia, and sleep disordered breathing) have been reliably associated with cardiometabolic disease. Less data is available for insomnia without short sleep, circadian-rhythm disorders, and general poor sleep quality, though there are a number of studies to suggest that these, too, may be associated with cardiometabolic disease. These relationships seem to generally remain consistent across domains, suggesting the importance of sleep in regulating cardiometabolic function. Several studies have evaluated potential mechanistic pathways, including metabolic, inflammatory, and neurocognitive possibilities. Overall, though, sleep represents an important issue for public health.
In examining the role of sleep in the context of public health, several upstream determinants of poor sleep have been explored. In particular, evidence that minority status and lower socio-economic position is associated with poor sleep represents the majority of this literature. Other studies, though, have addressed acculturation, geographic environment, beliefs and attitudes, and other factors. Putting all of this evidence together, a social-ecological model of sleep and health shows sleep at the intersection of these upstream determinants and downstream consequences. This conceptualization may be useful in framing the role of sleep in health and may also aid in the development of some of the first interventions for poor sleep at the population level.
Several important future directions are evident from the available data. First, the concept of ‘sleep disturbance’ needs to be more fully fleshed out. Put another way, more research is needed to understand the various types of situations in which someone does or does not report or experience ‘sleep disturbance’. This will help us better understand the phenotype of interest and better target mechanistic or interventional studies. Second, more research is needed to elucidate the causal pathways. More longitudinal research is needed especially in this regard. Third, the role of sleep alongside other more recognized components of lifestyle and cardiometabolic health (such as diet and exercise) needs to be better conceptualized. With this research agenda realized, we can begin to develop and implement successful interventions for healthy sleep at the population level and potentially help to ameliorate the cardiometabolic disease burden attributable to poor sleep.
Acknowledgments
I wish to thank Michael Perlis for insight and comments regarding the ideas presented in this review. The social-ecological model was originally developed with the assistance of Nirav Patel, Lauren Hale, and Melisa Moore, and was refined with the assistance of Orfeu Buxton and Girardin Jean-Louis.
This work was supported by the National Heart, Lung and Blood Institute (K23HL110216), the National Institute of Environmental Health Science (R21ES022931), and the Clinical and Translational Science award of the University of Pennsylvania (UL1RR024134).
Footnotes
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Abe T, Aoki T, Yata S, Okada M. Sleep duration is significantly associated with carotid artery atherosclerosis incidence in a Japanese population. Atherosclerosis. 2011;217:509–513. doi: 10.1016/j.atherosclerosis.2011.02.029. [DOI] [PubMed] [Google Scholar]
- Adenekan B, Pandey A, McKenzie S, Zizi F, Casimir GJ, Jean-Louis G. Sleep in America: Role of racial/ethnic differences. Sleep Medicine Reviews. 2013;17:255–262. doi: 10.1016/j.smrv.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailshire JA, Burgard SA. Family relationships and troubled sleep among US adults: Examining the influences of contact frequency and relationship quality. Journal of Health and Social Behavior. 2012;53:248–262. doi: 10.1177/0022146512446642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Jawder SE, Bahammam AS. Comorbid insomnia in sleep-related breathing disorders: An under-recognized association. Sleep and Breathing. 2012;16:295–304. doi: 10.1007/s11325-011-0513-1. [DOI] [PubMed] [Google Scholar]
- Alam A, Chengappa KN, Ghinassi F. Screening for obstructive sleep apnea among individuals with severe mental illness at a primary care clinic. General Hospital Psychiatry. 2012;34:660–664. doi: 10.1016/j.genhosppsych.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Altman NG, Izci-Balserak B, Schopfer E, Jackson N, Rattanaumpawan P, Gehrman PR, Grandner MA. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep Medicine. 2012;13:1261–1270. doi: 10.1016/j.sleep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman JW, Kotagal S, Olson CM, Scammell T, Schenck C, Spielman A. American Academy of Sleep Medicine. The International Classification of Sleep Disorders. 2. Westchester, IL: American Academy of Sleep Medicine; 2006. [Google Scholar]
- Astell-Burt T, Feng X, Kolt GS. Does access to neighbourhood green space promote a healthy duration of sleep? Novel findings from a cross-sectional study of 259 319 Australians. BMJ Open. 2013;3(8):e003094. doi: 10.1136/bmjopen-2013-003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora RN, Kristo DA, Bista SR, Rowley JA, Zak RS, Casey KR, Rosenberg RS. The treatment of restless legs syndrome and periodic limb movement disorder in adults – An update for 2012: Practice parameters with an evidence-based systematic review and meta-analyses: An American Academy of Sleep Medicine Clinical Practice Guideline. Sleep. 2012;35:1039–1062. doi: 10.5665/sleep.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avlonitou E, Kapsimalis F, Varouchakis G, Vardavas CI, Behrakis P. Adherence to CPAP therapy improves quality of life and reduces symptoms among obstructive sleep apnea syndrome patients. Sleep and Breathing. 2012;16:563–569. doi: 10.1007/s11325-011-0543-8. [DOI] [PubMed] [Google Scholar]
- Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on hypothalamo–pituitary–adrenal axis activity. International Journal of Endocrinology. 2010;2010:759234. doi: 10.1155/2010/759234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. Journal of Clinical Sleep Medicine. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- Bannai A, Tamakoshi A. The association between long working hours and health: A systematic review of epidemiological evidence. Scandanavian Journal of Work and Environmental Health. 2014;40:5–18. doi: 10.5271/sjweh.3388. [DOI] [PubMed] [Google Scholar]
- Barclay NL, Eley TC, Buysse DJ, Maughan B, Gregory AM. Nonshared environmental influences on sleep quality: A study of monozygotic twin differences. Behavioral Genetics. 2012;42:234–244. doi: 10.1007/s10519-011-9510-1. [DOI] [PubMed] [Google Scholar]
- Basner M, Dinges DF. Dubious bargain: Trading sleep for Leno and Letterman. Sleep. 2009;32:747–752. doi: 10.1093/sleep/32.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basner M, Fomberstein KM, Razavi FM, Banks S, William JH, Rosa RR, Dinges DF. American time use survey: Sleep time and its relationship to waking activities. Sleep. 2007;30:1085–1095. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Annals of Epidemiology. 2009;19:351–357. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Benes H, von Eye A, Kohnen R. Empirical evaluation of the accuracy of diagnostic criteria for restless legs syndrome. Sleep Medicine. 2009;10:524–530. doi: 10.1016/j.sleep.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Bertisch S, Patel SR. CPAP for obstructive sleep apnea and the metabolic syndrome. New England Journal of Medicine. 2012;366:963–964. doi: 10.1056/NEJMc1200497. author reply 965–966. [DOI] [PubMed] [Google Scholar]
- Besteiro Gonzalez JL, Suarez Fernandez TV, Arboleya Rodriguez L, Muniz J, Lemos Giraldez S, Alvarez Fernandez A. Sleep architecture in patients with fibromyalgia. Psicothema. 2011;23:368–373. [PubMed] [Google Scholar]
- Bianchi MT, Eiseman NA, Cash SS, Mietus J, Peng CK, Thomas RJ. Probabilistic sleep architecture models in patients with and without sleep apnea. Journal of Sleep Research. 2012;21:330–341. doi: 10.1111/j.1365-2869.2011.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlenga D, van der Heijden KB, Breuk M, van Someren EJ, Lie ME, Boonstra AM, Kooij JJ. Associations between sleep characteristics, seasonal depressive symptoms, lifestyle, and ADHD symptoms in adults. Journal of Attentional Disorders. 2013;17:261–275. doi: 10.1177/1087054711428965. [DOI] [PubMed] [Google Scholar]
- Boss EF, Smith DF, Ishman SL. Racial/ethnic and socioeconomic disparities in the diagnosis and treatment of sleep-disordered breathing in children. International Journal of Pediatric Otorhinolaryngology. 2011;75:299–307. doi: 10.1016/j.ijporl.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Boudreau P, Dumont GA, Boivin DB. Circadian adaptation to night shift work influences sleep, performance, mood and the autonomic modulation of the heart. PLoS One. 2013;8(7):e70813. doi: 10.1371/journal.pone.0070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U. Toward an experimental ecology of human development. American Psychologist. 1977;32:513–531. [Google Scholar]
- Brouillette RT, Horwood L, Constantin E, Brown K, Ross NA. Childhood sleep apnea and neighborhood disadvantage. Journal of Pediatrics. 2011;158:789–795. doi: 10.1016/j.jpeds.2010.10.036. [DOI] [PubMed] [Google Scholar]
- Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adi-pocytes after experimental sleep restriction: A randomized, crossover study. Annals of Internal Medicine. 2012;157:549–557. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunborg GS, Mentzoni RA, Molde H, Myrseth H, Skouveroe KJ, Bjorvatn B, Pallesen S. The relationship between media use in the bedroom, sleep habits and symptoms of insomnia. Journal of Sleep Research. 2011;20:569–575. doi: 10.1111/j.1365-2869.2011.00913.x. [DOI] [PubMed] [Google Scholar]
- Butt M, Dwivedi G, Khair O, Lip GY. Obstructive sleep apnea and cardiovascular disease. International Journal of Cardiology. 2010;139:7–16. doi: 10.1016/j.ijcard.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Ellenbogen JM, Wang W, Carballeira A, O’Connor S, Cooper D, Solet JM. Sleep disruption due to hospital noises: A prospective evaluation. Annals of Internal Medicine. 2012;157:170–179. doi: 10.7326/0003-4819-157-3-201208070-00472. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Social Science and Medicine. 2010;71:1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–2133. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byberg S, Hansen AL, Christensen DL, Vistisen D, Aadahl M, Linneberg A, Witte DR. Sleep duration and sleep quality are associated differently with alterations of glucose homeostasis. Diabetes Medicine. 2012;29:e354–360. doi: 10.1111/j.1464-5491.2012.03711.x. [DOI] [PubMed] [Google Scholar]
- Cahill K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database of Systematic Reviews. 2011;4:CD004307. doi: 10.1002/14651858.CD004307.pub4. [DOI] [PubMed] [Google Scholar]
- Campbell A, Neill A, Lory R. Ethnicity and socioeconomic status predict initial continuous positive airway pressure compliance in New Zealand adults with obstructive sleep apnoea. Internal Medicine Journal. 2012;42:e95–101. doi: 10.1111/j.1445-5994.2010.02360.x. [DOI] [PubMed] [Google Scholar]
- Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: A cohort study. Annals of Internal Medicine. 2012;156:115–122. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. European Heart Journal. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Miller MA. Are short bad sleep nights a hindrance to a healthy heart? Sleep. 2011;34:1457–1458. doi: 10.5665/sleep.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CE, Edinger JD. Identifying critical beliefs about sleep in primary insomnia. Sleep. 2006;29:444–453. [PubMed] [Google Scholar]
- Cengic B, Resic H, Spasovski G, Avdic E, Alajbegovic A. Quality of sleep in patients undergoing hemodialysis. International Urology and Nephrology. 2012;44:557–567. doi: 10.1007/s11255-010-9881-x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. CDC Health Disparities and Inequalities Report – United States, 2011. Atlanta, GA: Centers for Disease Control and Prevention; 2011. [Google Scholar]
- Chakravorty S, Grandner MA, Kranzler HR, Mavandadi S, Kling MA, Perlis ML, Oslin DW. Insomnia in alcohol dependence: Predictors of symptoms in a sample of veterans referred from primary care. American Journal on Addictions. 2013;22:266–270. doi: 10.1111/j.1521-0391.2012.12009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion VL, Skinner CS. The health belief model. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education: Theory, Research, and Practice. San Francisco: Jossey-Bass; 2008. pp. 45–65. [Google Scholar]
- Chang AM, Santhi N, St Hilaire M, Gronfier C, Bradstreet DS, Duffy JF, Czeisler CA. Human responses to bright light of different durations. Journal of Physiology. 2012a;590(13):3103–3112. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JK, Koo M, Kao VY, Chiang JK. Association of sleep duration and insulin resistance in Taiwanese vegetarians. BMC Public Health. 2012b;12:666. doi: 10.1186/1471-2458-12-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput JP, Despres JP, Bouchard C, Astrup A, Tremblay A. Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: Analyses of the Quebec Family Study. Sleep Medicine. 2009;10:919–924. doi: 10.1016/j.sleep.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec Family Study. Obesity (Silver Spring, MD) 2007;15:253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Du CL, Hwang JJ, Chen IS, Chen MF, Su TC. Working hours, sleep duration and the risk of acute coronary heart disease: A case-control study of middle-aged men in Taiwan. International Journal of Cardiology. 2014;171:419–422. doi: 10.1016/j.ijcard.2013.12.035. [DOI] [PubMed] [Google Scholar]
- Ciccone MM, Favale S, Scicchitano P, Mangini F, Mitacchione G, Gadaleta F, Carratu P. Reversibility of the endothelial dysfunction after CPAP therapy in OSAS patients. International Journal of Cardiology. 2012;158:383–386. doi: 10.1016/j.ijcard.2011.01.065. [DOI] [PubMed] [Google Scholar]
- Colten HR, Altevogt BM Institute of Medicine Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: Institute of Medicine, National Academies Press; 2006. [PubMed] [Google Scholar]
- Copinschi G. Metabolic and endocrine effects of sleep deprivation. Essential Psychopharmacology. 2005;6:341–347. [PubMed] [Google Scholar]
- Corbett RW, Middleton B, Arendt J. An hour of bright white light in the early morning improves performance and advances sleep and circadian phase during the Antarctic winter. Neuroscience Letters. 2012;525:146–151. doi: 10.1016/j.neulet.2012.06.046. [DOI] [PubMed] [Google Scholar]
- Crowder JS, Sisson SB, Ramey E, Arnold SH, Richardson S, DeGrace BW. How did the television get in the child’s bedroom? Analysis of family interviews. Preventive Medicine. 2012;55:623–628. doi: 10.1016/j.ypmed.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Cruz IA, Drummond M, Winck JC. Obstructive sleep apnea symptoms beyond sleepiness and snoring: Effects of nasal APAP therapy. Sleep and Breathing. 2012;16:361–366. doi: 10.1007/s11325-011-0502-4. [DOI] [PubMed] [Google Scholar]
- Darukhanavala A, Booth JN, III, Bromley L, Whitmore H, Imperial J, Penev PD. Changes in insulin secretion and action in adults with familial risk for type 2 diabetes who curtail their sleep. Diabetes Care. 2011;34:2259–2264. doi: 10.2337/dc11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwent D, Dawson D, Roach GD. A model of shiftworker sleep/wake behaviour. Accident Analysis and Prevention. 2012;45:S6–10. doi: 10.1016/j.aap.2011.09.017. [DOI] [PubMed] [Google Scholar]
- De Zambotti M, Covassin N, De Min Tona G, Sarlo M, Stegagno L. Sleep onset and cardiovascular activity in primary insomnia. Journal of Sleep Research. 2011;20:318–325. doi: 10.1111/j.1365-2869.2010.00871.x. [DOI] [PubMed] [Google Scholar]
- Doghramji K. The evaluation and management of insomnia. Clinical Chest Medicine. 2010;31:327–339. doi: 10.1016/j.ccm.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Dowling GA, Mastick J. Effects of light on the elderly. In: Pandi-Perumal SR, Monti JR, Monjan AA, editors. Principles and Practice of Geriatric Sleep Medicine. Cambridge: Cambridge; 2010. pp. 423–430. [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in Neurology. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Kuwabara H, Wong DF, Gamaldo C, Salas R, Brasic J, Allen RP. The dopamine transporter is decreased in the striatum of subjects with restless legs syndrome. Sleep. 2011;34:341–347. doi: 10.1093/sleep/34.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley CJ, Silber MH. Restless legs syndrome: Understanding its consequences and the need for better treatment. Sleep Medicine. 2010;11(9):807–815. doi: 10.1016/j.sleep.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Ebben MR, Narizhnaya M. Cognitive and behavioral treatment options for insomnia. Mount Sinai Journal of Medicine. 2012;79:512–523. doi: 10.1002/msj.21320. [DOI] [PubMed] [Google Scholar]
- Eguchi K, Hoshide S, Ishikawa S, Shimada K, Kario K. Short sleep duration is an independent predictor of stroke events in elderly hypertensive patients. Journal of the American Society of Hypertension. 2010;4:255–262. doi: 10.1016/j.jash.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Elmenhorst EM, Pennig S, Rolny V, Quehl J, Mueller U, Maass H, Basner M. Examining nocturnal railway noise and aircraft noise in the field: Sleep, psychomotor performance, and annoyance. Science of the Total Environment. 2012;424:48–56. doi: 10.1016/j.scitotenv.2012.02.024. [DOI] [PubMed] [Google Scholar]
- Fang J, Wheaton AG, Keenan NL, Greenlund KJ, Perry GS, Croft JB. Association of sleep duration and hypertension among US adults varies by age and sex. American Journal of Hypertension. 2012;25:335–341. doi: 10.1038/ajh.2011.201. [DOI] [PubMed] [Google Scholar]
- Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Medicine Reviews. 2012a;16(2):137–149. doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Faraut B, Touchette E, Gamble H, Royant-Parola S, Safar ME, Varsat B, Leger D. Short sleep duration and increased risk of hypertension: A primary care medicine investigation. Journal of Hypertension. 2012b;30:1354–1363. doi: 10.1097/HJH.0b013e32835465e5. [DOI] [PubMed] [Google Scholar]
- Fava C, Montagnana M, Favaloro EJ, Guidi GC, Lippi G. Obstructive sleep apnea syndrome and cardiovascular diseases. Seminars in Thrombosis and Hemostasis. 2011;37:280–297. doi: 10.1055/s-0031-1273092. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Kennaway DJ, Baker A, Lamond N, Dawson D. Sleep and circadian rhythms in mining operators: Limited evidence of adaptation to night shifts. Applied Ergonomics. 2012;43:695–701. doi: 10.1016/j.apergo.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN, Bixler EO, Singareddy R, Shaffer ML, Calhoun SL, Liao D. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep. 2012a;35:689–697. doi: 10.5665/sleep.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, Bixler EO. Insomnia with objective short sleep duration and incident hypertension: The Penn State Cohort. Hypertension. 2012b;60:929–935. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filtness AJ, Reyner LA, Horne JA. One night’s CPAP withdrawal in otherwise compliant OSA patients: Marked driving impairment but good awareness of increased sleepiness. Sleep and Breathing. 2012;16:865–871. doi: 10.1007/s11325-011-0588-8. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, Ryff CD. Social relationships, sleep quality, and interleukin-6 in aging women. Proceedings of the National Academy of Science of the United States of Amerca. 2005;102:18757–18762. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicchio L, Kalesan B. Sleep duration and mortality: A systematic review and meta-analysis. Journal of Sleep Research. 2009;18:148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- Gamaldo AA, McNeely JM, Shah MT, Evans MK, Zonderman AB. Racial differences in self-reports of short sleep duration in an urban-dwelling environment. Journal of Gerontology B: Psycholical Science and Social Science. 2013a doi: 10.1093/geronb/gbt117. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaldo CE, Spira AP, Hock RS, Salas RE, McArthur JC, David PM, Smith MT. Sleep, function and HIV: A multi-method assessment. AIDS and Behavior. 2013b;17(8):2808–2815. doi: 10.1007/s10461-012-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obesity Reviews. 2009;10(Suppl 2):37–45. doi: 10.1111/j.1467-789X.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Malaspina D. Short sleep duration as a risk factor for hypertension: Analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Malaspina D, Babiss LA, Opler MG, Posner K, Shen S, Ginsberg HN. Short sleep duration as a risk factor for hypercholesterolemia: Analyses of the National Longitudinal Study of Adolescent Health. Sleep. 2010a;33:956–961. doi: 10.1093/sleep/33.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Malaspina D, Posner K, Babiss LA, Heymsfield SB, Turner JB, Pickering TG. Insomnia and sleep duration as mediators of the relationship between depression and hypertension incidence. American Journal of Hypertension. 2010b;23:62–69. doi: 10.1038/ajh.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Borreguero D, Williams AM. Dopaminergic augmentation of restless legs syndrome. Sleep Medicine Reviews. 2010;14:339–346. doi: 10.1016/j.smrv.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Garland SN, Carlson LE, Antle MC, Samuels C, Campbell T. I-CAN SLEEP: Rationale and design of a non-inferiority RCT of Mindfulness-based Stress Reduction and Cognitive Behavioral Therapy for the treatment of Insomnia in CANcer survivors. Contemporary Clinical Trials. 2011;32(5):747–754. doi: 10.1016/j.cct.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: A randomized, partially blinded, noninferiority trial. Journal of Clinical Oncology. 2014;32:449–457. doi: 10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- Garmy P, Nyberg P, Jakobsson U. Sleep and television and computer habits of Swedish school-age children. Journal of School Nursing. 2012;28:469–476. doi: 10.1177/1059840512444133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrman PR, Gooneratne NS. Non-pharmacological treatment of insomnia in the elderly: Cognitive behavioral therapies. In: Pandi-Perumal SR, Monti JR, Monjan AA, editors. Principles and Practice of Geriatric Sleep Medicine. Cambridge: Cambridge University Press; 2010. pp. 384–393. [Google Scholar]
- Gellis LA, Lichstein KL, Scarinci IC, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Socioeconomic status and insomnia. Journal of Abnormal Psychology. 2005;114:111–118. doi: 10.1037/0021-843X.114.1.111. [DOI] [PubMed] [Google Scholar]
- Geroldi C, Frisoni GB, Rozzini R, De Leo D, Trabucchi M. Principal lifetime occupation and sleep quality in the elderly. Gerontology. 1996;42:163–169. doi: 10.1159/000213788. [DOI] [PubMed] [Google Scholar]
- Glidewell RN, Roby EK, Orr WC. Is insomnia an independent predictor of obstructive sleep apnea? Journal of the American Board of Family Medicine. 2012;25:104–110. doi: 10.3122/jabfm.2012.01.110123. [DOI] [PubMed] [Google Scholar]
- Golbidi S, Badran M, Ayas N, Laher I. Cardiovascular consequences of sleep apnea. Lung. 2012;190:113–132. doi: 10.1007/s00408-011-9340-1. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Craig SE, Lorenzi-Filho G, Heeley E, Redline S, McEvoy RD, Duran-Cantolla J. Sleep apnea cardiovascular clinical trials – Current status and steps forward: The International Collaboration of Sleep Apnea Cardiovascular Trialists. Sleep. 2013;36:975–980. doi: 10.5665/sleep.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Buxton OM, Jackson N, Sands-Lincoln M, Pandey A, Jean-Louis G. Extreme sleep durations and increased C-reactive protein: Effects of sex and ethnoracial group. Sleep. 2013a;36:769–779. doi: 10.5665/sleep.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Medicine. 2014a;15:42–50. doi: 10.1016/j.sleep.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Medicine Reviews. 2007;11:341–360. doi: 10.1016/j.smrv.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Jackson N, Gooneratne NS, Patel NP. The development of a questionnaire to assess sleep-related practices, beliefs, and attitudes. Behavioral Sleep Medicine. 2014b;12:123–142. doi: 10.1080/15402002.2013.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Jackson NJ, Pak VM, Gehrman PR. Sleep disturbance is associated with cardiovascular and metabolic disorders. Journal of Sleep Research. 2012a;21:427–433. doi: 10.1111/j.1365-2869.2011.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Jackson NJ, Pigeon WR, Gooneratne NS, Patel NP. State and regional prevalence of sleep disturbance and daytime fatigue. Journal of Clinical Sleep Medicine. 2012b;8:77–86. doi: 10.5664/jcsm.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Kripke DF. Self-reported sleep complaints with long and short sleep: A nationally representative sample. Psychosomatic Medicine. 2004;66:239–241. doi: 10.1097/01.psy.0000107881.53228.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Patel NP, Gehrman PR, Perlis ML, Pack AI. Problems associated with short sleep: Bridging the gap between laboratory and epidemiological studies. Sleep Medicine Reviews. 2010a;14:239–247. doi: 10.1016/j.smrv.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Patel NP, Gehrman PR, Xie D, Sha D, Weaver T, Gooneratne N. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep disturbance. Sleep Medicine. 2010b;11:470–479. doi: 10.1016/j.sleep.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Patel NP, Hale L, Moore M. Mortality associated with sleep duration: The evidence, the possible mechanisms, and the future. Sleep Medicine Reviews. 2010c;14:191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Patel NP, Jean-Louis G, Jackson N, Gehrman PR, Perlis ML, Gooneratne NS. Sleep-related behaviors and beliefs associated with race/ethnicity in women. Journal of the National Medical Association. 2013b;105:4–15. doi: 10.1016/s0027-9684(15)30080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Patel NP, Perlis ML, Gehrman PR, Xie D, Sha D, Gooneratne NS. Obesity, diabetes, and exercise associated with sleep-related complaints in the American population. Journal of Public Health. 2011;19:463–474. doi: 10.1007/s10389-011-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Petrov MER, Rattanaumpawan P, Jackson N, Platt A, Patel NP. Sleep symptoms, race/ethnicity, and socioeconomic position. Journal of Clinical Sleep Medicine. 2013c;9:897–905. doi: 10.5664/jcsm.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Petrov ME, Rattanaumpawan P, Jackson N, Platt A, Patel NP. Sleep symptoms, race/ethnicity, and socioeconomic position. Journal of Clinical Sleep Medicine. 2013d;9:897–905. 905A–905D. doi: 10.5664/jcsm.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nature and Science of Sleep. 2013e;5:93–107. doi: 10.2147/NSS.S31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Smith T, Jackson NJ, Jackson T, Burgard S, Branas C. Geographic distribution of insufficient sleep across the USA: A county-level hotspot analysis. Sleep. 2013f;36(Suppl):A335–A336. doi: 10.1016/j.sleh.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette FX, Morley JL, Weaver MD, Patterson PD, Hostler D. The effect of shift length on fatigue and cognitive performance in air medical providers. Prehospital Emergency Care. 2013;17:23–28. doi: 10.3109/10903127.2012.710719. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Keatley DA, Chan DC, Chatzisarantis NL, Dimmock JA, Jackson B, Ntoumanis N. The goose is (half) cooked: A consideration of the mechanisms and interpersonal context is needed to elucidate the effects of personal financial incentives on health behaviour. International Journal of Behavioral Medicine. 2014;21:197–201. doi: 10.1007/s12529-013-9317-y. [DOI] [PubMed] [Google Scholar]
- Hale L, Do DP. Sleep and the inner city: How race and neighborhood context relate to sleep duration. Paper presented at the Population Association of America Annual Meeting; Los Angeles, CA. 2006. pp. 3/30–4/1. [Google Scholar]
- Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–1103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L, Hill TD, Burdette AM. Does sleep quality mediate the association between neighborhood disorder and self-rated physical health? Preventive Medicine. 2010;51:275–278. doi: 10.1016/j.ypmed.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Hale L, Hill TD, Friedman E, Nieto FJ, Galvao LW, Engelman CD, Peppard PE. Perceived neighborhood quality, sleep quality, and health status: Evidence from the Survey of the Health of Wisconsin. Social Science and Medicine. 2013;79:16–22. doi: 10.1016/j.socscimed.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L, Rivero-Fuentes E. Negative acculturation in sleep duration among Mexican immigrants and Mexican Americans. Journal of Immigrant and Minority Health. 2011;13:402–407. doi: 10.1007/s10903-009-9284-1. [DOI] [PubMed] [Google Scholar]
- Hall-Porter JM, Curry DT, Walsh JK. Pharmacologic treatment of primary insomnia. Sleep Medicine Clinics. 2010;5(4):609–625. [Google Scholar]
- Hall MH, Matthews KA, Kravitz HM, Gold EB, Buysse DJ, Bromberger JT, Sowers M. Race and financial strain are independent correlates of sleep in midlife women: The SWAN sleep study. Sleep. 2009;32:73–82. [PMC free article] [PubMed] [Google Scholar]
- Halpern SD, Kohn R, Dornbrand-Lo A, Metkus T, Asch DA, Volpp KG. Lottery-based versus fixed incentives to increase clinicians’ response to surveys. Health Services Research. 2011;46:1663–1674. doi: 10.1111/j.1475-6773.2011.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazaki Y, Morikawa Y, Nakamura K, Sakurai M, Miura K, Ishizaki M, Nakagawa H. The effects of sleep duration on the incidence of cardiovascular events among middle-aged male workers in Japan. Scandanavian Journal of Work and Environmental Health. 2011;37:411–417. doi: 10.5271/sjweh.3168. [DOI] [PubMed] [Google Scholar]
- Hayes AL, Xu F, Babineau D, Patel SR. Sleep duration and circulating adipokine levels. Sleep. 2011;34:147–152. doi: 10.1093/sleep/34.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegglin A, Schoch OD, Korte W, Hahn K, Hurny C, Munzer T. Eight months of continuous positive airway pressure (CPAP) decrease tumor necrosis factor alpha (TNFA) in men with obstructive sleep apnea syndrome. Sleep and Breathing. 2012;16:405–412. doi: 10.1007/s11325-011-0512-2. [DOI] [PubMed] [Google Scholar]
- Hida A, Kitamura S, Mishima K. Pathophysiology and pathogenesis of circadian rhythm sleep disorders. Journal of Physiological Anthropology. 2012;31:7. doi: 10.1186/1880-6805-31-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TD, Burdette AM, Hale L. Neighborhood disorder, sleep quality, and psychological distress: Testing a model of structural amplification. Health and Place. 2009;15:1006–1013. doi: 10.1016/j.healthplace.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Hsieh SD, Muto T, Murase T, Tsuji H, Arase Y. Association of short sleep duration with obesity, diabetes, fatty liver and behavioral factors in Japanese men. Internal Medicine. 2011;50:2499–2502. doi: 10.2169/internalmedicine.50.5844. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics. Journal of the Experimental Analysis of Behavior. 1984;42(3):435–452. doi: 10.1901/jeab.1984.42-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel B, Buysse DJ, Krafty RT, Begley A, Miewald J, Hall M. Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: For some measures, one night is enough. Sleep. 2012;35:1285–1291. doi: 10.5665/sleep.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain SK, Kahlon G, Morehead L, Lieblong B, Stapleton T, Hoeldtke R, Levine SN. The effect of sleep apnea and insomnia on blood levels of leptin, insulin resistance, IP-10, and hydrogen sulfide in type 2 diabetic patients. Metabolic Syndrome and Related Disorders. 2012;10:331–336. doi: 10.1089/met.2012.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnefelt H, Lagerstedt R, Kajaste S, Sallinen M, Savolainen A, Hublin C. Cognitive behavior therapy for chronic insomnia in occupational health services. Journal of Occupational Rehabilitation. 2012;22:511–521. doi: 10.1007/s10926-012-9365-1. [DOI] [PubMed] [Google Scholar]
- Jaussent I, Bouyer J, Ancelin ML, Berr C, Foubert-Samier A, Ritchie K, Dauvilliers Y. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35:1201–1207. doi: 10.5665/sleep.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Association of short and long sleep durations with insulin sensitivity in adolescents. Journal of Pediatrics. 2011;158:617–623. doi: 10.1016/j.jpeds.2010.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ME, Gibson PG, Collins CE, Hilton JM, Latham-Smith F, Wood LG. Increased sleep latency and reduced sleep duration in children with asthma. Sleep and Breathing. 2013;17:281–287. doi: 10.1007/s11325-012-0687-1. [DOI] [PubMed] [Google Scholar]
- Jirong Y, Changquan H, Hongmei W, Bi-Rong D. Association of sleep quality and dementia among long-lived Chinese older adults. Age (Dordrecht, Netherlands) 2013;35:1423–1432. doi: 10.1007/s11357-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John LK, Loewenstein G, Troxel AB, Norton L, Fassbender JE, Volpp KG. Financial incentives for extended weight loss: A randomized, controlled trial. Journal of General Internal Medicine. 2011;26:621–626. doi: 10.1007/s11606-010-1628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John LK, Loewenstein G, Volpp KG. Empirical observations on longer-term use of incentives for weight loss. Preventive Medicine. 2012;55:S68–74. doi: 10.1016/j.ypmed.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Jungquist CR, O’Brien C, Matteson-Rusby S, Smith MT, Pigeon WR, Xia Y, Perlis ML. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Medicine. 2010;11:302–309. doi: 10.1016/j.sleep.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachi Y, Ohwaki K, Yano E. Association of sleep duration with untreated diabetes in Japanese men. Sleep Medicine. 2012;13:307–309. doi: 10.1016/j.sleep.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Kakizaki M, Kuriyama S, Nakaya N, Sone T, Nagai M, Sugawara Y, Tsuji I. Long sleep duration and cause-specific mortality according to physical function and self-rated health: The Ohsaki Cohort Study. Journal of Sleep Research. 2013;22:209–216. doi: 10.1111/j.1365-2869.2012.01053.x. [DOI] [PubMed] [Google Scholar]
- Kavcic P, Rojc B, Dolenc-Groselj L, Claustrat B, Fujs K, Poljak M. The impact of sleep deprivation and nighttime light exposure on clock gene expression in humans. Croatian Medical Journal. 2011;52:594–603. doi: 10.3325/cmj.2011.52.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent BD, Ryan S, McNicholas WT. Obstructive sleep apnea and inflammation: Relationship to cardiovascular co-morbidity. Respiratory Physiology and Neurobiology. 2011;178:475–481. doi: 10.1016/j.resp.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Clinton SM, Simpson DN, Bedrosian TA, Bernard R, Akil H, Watson SJ. Inborn differences in environmental reactivity predict divergent diurnal behavioral, endocrine, and gene expression rhythms. Psychoneuroendocrinology. 2012;37:256–269. doi: 10.1016/j.psyneuen.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid I, Rana L, Khalid TJ, Roehrs T. Refractory restless legs syndrome likely caused by olanzapine. Journal of Clinical Sleep Medicine. 2009;5:68–69. [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Grugle NL, Balkin TJ. Gambling when sleep deprived: Don’t bet on stimulants. Chronobiology International. 2012;29:43–54. doi: 10.3109/07420528.2011.635230. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Grugle NL, Killgore DB, Leavitt BP, Watlington GI, McNair S, Balkin TJ. Restoration of risk-propensity during sleep deprivation: Caffeine, dextroamphetamine, and modafinil. Aviation, Space, and Environmental Medicine. 2008a;79(9):867–874. doi: 10.3357/asem.2259.2008. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Grugle NL, Reichardt RM, Killgore DB, Balkin TJ. Executive functions and the ability to sustain vigilance during sleep loss. Aviation, Space, and Environmental Medicine. 2009a;80:81–87. doi: 10.3357/asem.2396.2009. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Kahn-Greene ET, Grugle NL, Killgore DB, Balkin TJ. Sustaining executive functions during sleep deprivation: A comparison of caffeine, dextroamphetamine, and modafinil. Sleep. 2009b;32:205–216. doi: 10.1093/sleep/32.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Kahn-Greene ET, Lipizzi EL, Newman RA, Kamimori GH, Balkin TJ. Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Medicine. 2008b;9:517–526. doi: 10.1016/j.sleep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Killgore DB, Day LM, Li C, Kamimori GH, Balkin TJ. The effects of 53 hours of sleep deprivation on moral judgment. Sleep. 2007;30:345–352. doi: 10.1093/sleep/30.3.345. [DOI] [PubMed] [Google Scholar]
- Killgore WDS. Effects of sleep deprivation on cognition. In: Kerkhof GA, Van Dongen HPA, editors. Human Sleep and Cognition, Part I: Basic Research. Amsterdam: Elsevier; 2010. pp. 105–129. [Google Scholar]
- Kim AE, Towers A, Renaud J, Zhu J, Shea JA, Galvin R, Volpp KG. Application of the RE-AIM framework to evaluate the impact of a worksite-based financial incentive intervention for smoking cessation. Journal of Occupational and Environmental Medicine. 2012;54:610–614. doi: 10.1097/JOM.0b013e31824b2171. [DOI] [PubMed] [Google Scholar]
- Kimmel SE, Troxel AB, Loewenstein G, Brensinger CM, Jaskowiak J, Doshi JA, Volpp K. Randomized trial of lottery-based incentives to improve warfarin adherence. American Heart Journal. 2012;164:268–274. doi: 10.1016/j.ahj.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. Journal of the American Medical Association. 2008;300:2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL. Sleep duration and cardiometabolic risk: A review of the epidemiologic evidence. Best Practices Ressearch in Clinical Endocrinology and Metabolism. 2010;24(5):731–743. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL. Does inadequate sleep play a role in vulnerability to obesity? American Journal of Human Biology. 2012;24:361–371. doi: 10.1002/ajhb.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL. Sociodemographic and cultural determinants of sleep deficiency: Implications for cardiometabolic disease risk. Social Science and Medicine. 2013;79:7–15. doi: 10.1016/j.socscimed.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Medicine Reviews. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Annals of the New York Academy of Science. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E, Rathouz PJ, Yan LL, Hulley SB, Liu K, Lauderdale DS. Association between sleep and blood pressure in midlife: The CARDIA sleep study. Archives of Internal Medicine. 2009;169:1055–1061. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T. Association between weight gain, obesity, and sleep duration: A large-scale 3-year cohort study. Sleep and Breathing. 2012;16:829–833. doi: 10.1007/s11325-011-0583-0. [DOI] [PubMed] [Google Scholar]
- Kohyama J. Sleep health and asynchronization. Brain and Development. 2011;33:252–259. doi: 10.1016/j.braindev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Jean-Louis G, Elliott JA, Klauber MR, Rex KM, Tuunainen A, Langer RD. Ethnicity, sleep, mood, and illumination in postmenopausal women. BMC Psychiatry. 2004;4:8. doi: 10.1186/1471-244X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Rex KM, Ancoli-Israel S, Nievergelt CM, Klimecki W, Kelsoe JR. Delayed sleep phase cases and controls. Journal of Circadian Rhythms. 2008;6:6. doi: 10.1186/1740-3391-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD. A compendium of placebo-controlled trials of the risks/benefits of pharmacological treatments for insomnia: The empirical basis for US clinical practice. Sleep Medicine Reviews. 2009;13:265–274. doi: 10.1016/j.smrv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Kullgren JT, Troxel AB, Loewenstein G, Asch DA, Norton LA, Wesby L, Volpp KG. Individual- versus group-based financial incentives for weight loss: A randomized, controlled trial. Annals of Internal Medicine. 2013;158:505–514. doi: 10.7326/0003-4819-158-7-201304020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: Final data for 2005. National Vital Statistics Reports. 2008;56:1–120. [PubMed] [Google Scholar]
- Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012;141:1601–1610. doi: 10.1378/chest.11-2214. [DOI] [PubMed] [Google Scholar]
- Lallukka T, Arber S, Laaksonen M, Lahelma E, Partonen T, Rahkonen O. Work–family conflicts and subsequent sleep medication among women and men: A longitudinal registry linkage study. Social Science and Medicine. 2013;79:66–75. doi: 10.1016/j.socscimed.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Lamont EW, James FO, Boivin DB, Cermakian N. From circadian clock gene expression to pathologies. Sleep Medicine. 2007;8:547–556. doi: 10.1016/j.sleep.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Landis CA. Sleep, pain, fibromyalgia, and chronic fatigue syndrome. In: Montagna P, Chokroverty S, editors. Sleep Disorders. Edinburgh: Elsevier; 2011. pp. 613–637. [DOI] [PubMed] [Google Scholar]
- Lattova Z, Keckeis M, Maurovich-Horvat E, Wetter TC, Wilde-Frenz J, Schuld A, Pollmacher T. The stress hormone system in various sleep disorders. Journal of Psychiatric Research. 2011;45:1223–1228. doi: 10.1016/j.jpsychires.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Laugsand LE, Strand LB, Platou C, Vatten LJ, Janszky I. Insomnia and the risk of incident heart failure: A population study. European Heart Journal. 2013 doi: 10.1093/eurheartj/eht019. [DOI] [PubMed] [Google Scholar]
- Laugsand LE, Vatten LJ, Bjorngaard JH, Hveem K, Janszky I. Insomnia and high-sensitivity C-reactive protein: The HUNT study, Norway. Psychosomatic Medicine. 2012;74:543–553. doi: 10.1097/PSY.0b013e31825904eb. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kang HW, Lee LH. The relationship between the Epworth Sleepiness Scale and polysomnographic parameters in obstructive sleep apnea patients. European Archives of Otorhinolaryngology. 2012;269:1143–1147. doi: 10.1007/s00405-011-1808-3. [DOI] [PubMed] [Google Scholar]
- Levine RS, Foster JE, Fullilove RE, Fullilove MT, Briggs NC, Hull PC, Hennekens CH. Black–white inequalities in mortality and life expectancy, 1933–1999: Implications for healthy people 2010. Public Health Reports. 2001;116:474–483. doi: 10.1093/phr/116.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PC, Chen CH, Pan SM, Pan CH, Chen CJ, Chen YM, Wu MT. Atypical work schedules are associated with poor sleep quality and mental health in Taiwan female nurses. International Archives of Occupational and Environmental Health. 2012;85:877–884. doi: 10.1007/s00420-011-0730-8. [DOI] [PubMed] [Google Scholar]
- Lindstrom V, Andersson K, Lintrup M, Holst G, Berglund J. Prevalence of sleep problems and pain among the elderly in Sweden. Journal of Nutrition, Health, and Aging. 2012;16:180–183. doi: 10.1007/s12603-011-0356-2. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang A, Li L. Sleep duration and overweight/obesity in children: Review and implications for pediatric nursing. Journal of Specialty Pediatric Nursing. 2012a;17:193–204. doi: 10.1111/j.1744-6155.2012.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Liu X, Arguelles LM, Patwari PP, Zee PC, Chervin RD, Wang X. A population-based twin study on sleep duration and body composition. Obesity (Silver Spring, MD) 2012b;20:192–199. doi: 10.1038/oby.2011.274. [DOI] [PubMed] [Google Scholar]
- Liu R, Zee PC, Chervin RD, Arguelles LM, Birne J, Zhang S, Wang X. Short sleep duration is associated with insulin resistance independent of adiposity in Chinese adult twins. Sleep Medicine. 2011;12:914–919. doi: 10.1016/j.sleep.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein G, Asch DA, Volpp KG. Behavioral economics holds potential to deliver better results for patients, insurers, and employers. Health Affairs (Millwood) 2013;32:1244–1250. doi: 10.1377/hlthaff.2012.1163. [DOI] [PubMed] [Google Scholar]
- Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: A systematic review and meta-analysis. Circulation Cardiovascular Quality Outcomes. 2012;5:720–728. doi: 10.1161/CIRCOUTCOMES.111.964783. [DOI] [PubMed] [Google Scholar]
- Lowden A, Akerstedt T, Ingre M, Wiholm C, Hillert L, Kuster N, Arnetz B. Sleep after mobile phone exposure in subjects with mobile phone-related symptoms. Bioelectromagnetics. 2011;32:4–14. doi: 10.1002/bem.20609. [DOI] [PubMed] [Google Scholar]
- Lucassen EA, Rother KI, Cizza G. Interacting epidemics? Sleep curtailment, insulin resistance, and obesity. Annals of the New York Academy of Science. 2012;1264:110–134. doi: 10.1111/j.1749-6632.2012.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie A. Obstructive sleep apnea in adults: Epidemiology, clinical presentation, and treatment options. Advances in Cardiology. 2011;46:1–42. doi: 10.1159/000327660. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jin J, Meng L, Shen Q. The dark side of monetary incentive: How does extrinsic reward crowd out intrinsic motivation. Neuroreport. 2014;25:194–198. doi: 10.1097/WNR.0000000000000113. [DOI] [PubMed] [Google Scholar]
- Machi MS, Staum M, Callaway CW, Moore C, Jeong K, Suyama J, Hostler D. The relationship between shift work, sleep, and cognition in career emergency physicians. Academic Emergency Medicine. 2012;19:85–91. doi: 10.1111/j.1553-2712.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- Magee CA, Iverson DC, Caputi P. Factors associated with short and long sleep. Preventive Medicine. 2009;49:461–467. doi: 10.1016/j.ypmed.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Mahan RP, Carvalhais AB, Queen SE. Sleep reduction in night-shift workers: Is it sleep deprivation or a sleep disturbance disorder? Perceptual and Motor Skills. 1990;70(3):723–730. doi: 10.2466/pms.1990.70.3.723. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP., Jr Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academy of Science of the United States of America. 2013;110:5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, Soriano Y, Roman-Sanchez P, Illa FB, Duran-Cantolla J. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: A 5-year follow-up study. American Journal of Respiratory and Critical Care Medicine. 2009;180:36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- Martinez-Gomez D, Eisenmann JC, Gomez-Martinez S, Hill EE, Zapatera B, Veiga OL, Marcos A. Sleep duration and emerging cardiometabolic risk markers in adolescents. The AFINOS study. Sleep Medicine. 2011;12:997–1002. doi: 10.1016/j.sleep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Martinez D, do Lenz MC. Circadian rhythm sleep disorders. Indian Journal of Medicine Research. 2010;131:141–149. [PubMed] [Google Scholar]
- McCall WV, Boggs N, Letton A. Changes in sleep and wake in response to different sleeping surfaces: A pilot study. Applied Ergonomics. 2012;43:386–391. doi: 10.1016/j.apergo.2011.06.012. [DOI] [PubMed] [Google Scholar]
- McKnight-Eily LR, Liu Y, Perry GS, Presley-Cantrell LR, Strine TW, Lu H, Croft JB. Perceived insufficient rest or sleep among adults – United States, 2008. Morbidity and Mortality Weekly Reports. 2009;58:1175–1179. [PubMed] [Google Scholar]
- Mendlewicz J. Disruption of the circadian timing systems: Molecular mechanisms in mood disorders. CNS Drugs. 2009;23(Suppl 2):15–26. doi: 10.2165/11318630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: A meta-analysis of prospective cohort studies. Hypertension Research. 2013;36(11):985–995. doi: 10.1038/hr.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall M, Strollo PJ, Jr, Buysse DJ, Kamarck TW, Reis SE. Influence of race and socioeconomic status on sleep: Pittsburgh Sleep-SCORE project. Psychosomatic Medicine. 2008;70(4):410–416. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NL, Tvaryanas AP, Shattuck LG. Accommodating adolescent sleep–wake patterns: The effects of shifting the timing of sleep on training effectiveness. Sleep. 2012;35:1123–1136. doi: 10.5665/sleep.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Journal of the American Medical Association. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Moller AC, Buscemi J, McFadden HG, Hedeker D, Spring B. Financial motivation undermines potential enjoyment in an intensive diet and activity intervention. Journal of Behavioral Medicine. 2013 doi: 10.1007/s10865-013-9542-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller AC, McFadden HG, Hedeker D, Spring B. Financial motivation undermines maintenance in an intensive diet and activity intervention. Journal of Obesity. 2012;2012:740519. doi: 10.1155/2012/740519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan K, Redline S. Role of obstructive sleep apnea in cardiovascular disease. Current Opinions in Cardiology. 2011;26:541–547. doi: 10.1097/HCO.0b013e32834b806a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Redline S. Correlates of adolescent sleep time and variability in sleep time: The role of individual and health related characteristics. Sleep Medicine. 2011;12:239–245. doi: 10.1016/j.sleep.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes W, Poyares D, Zalcman I, de Mello MT, Bittencourt LR, Santos-Silva R, Tufik S. Association between body mass index and sleep duration assessed by objective methods in a representative sample of the adult population. Sleep Medicine. 2013;14:312–318. doi: 10.1016/j.sleep.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Morin AK, Willett K. The role of eszopiclone in the treatment of insomnia. Advanced Therapy. 2009;26:500–518. doi: 10.1007/s12325-009-0026-5. [DOI] [PubMed] [Google Scholar]
- Morselli LL, Guyon A, Spiegel K. Sleep and metabolic function. Pflugers Archives. 2012;463:139–160. doi: 10.1007/s00424-011-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motivala SJ. Sleep and inflammation: Psychoneuroimmunology in the context of cardiovascular disease. Annals of Behavioral Medicine. 2011;42:141–152. doi: 10.1007/s12160-011-9280-2. [DOI] [PubMed] [Google Scholar]
- Munezawa T, Kaneita Y, Osaki Y, Kanda H, Minowa M, Suzuki K, Ohida T. The association between use of mobile phones after lights out and sleep disturbances among Japanese adolescents: A nationwide cross-sectional survey. Sleep. 2011;34:1013–1020. doi: 10.5665/SLEEP.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Tomata Y, Watanabe T, Kakizaki M, Tsuji I. Association between sleep duration, weight gain, and obesity for long period. Sleep Medicine. 2013;14(2):206–210. doi: 10.1016/j.sleep.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Nakazaki C, Noda A, Koike Y, Yamada S, Murohara T, Ozaki N. Association of insomnia and short sleep duration with atherosclerosis risk in the elderly. American Journal of Hypertension. 2012;25:1149–1155. doi: 10.1038/ajh.2012.107. [DOI] [PubMed] [Google Scholar]
- Natarajan R. Review of periodic limb movement and restless leg syndrome. Journal of Postgraduate Medicine. 2010;56:157–162. doi: 10.4103/0022-3859.65284. [DOI] [PubMed] [Google Scholar]
- Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. American Journal of Clinical Nutrition. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen LS, Danielsen KV, Sorensen TI. Short sleep duration as a possible cause of obesity: Critical analysis of the epidemiological evidence. Obesity Reviews. 2011;12:78–92. doi: 10.1111/j.1467-789X.2010.00724.x. [DOI] [PubMed] [Google Scholar]
- Nomura K, Yamaoka K, Nakao M, Yano E. Social determinants of self-reported sleep problems in South Korea and Taiwan. Journal of Psychosomatic Research. 2010;69:435–440. doi: 10.1016/j.jpsychores.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Office of Disease Prevention and Health Promotion. Healthy People 2020 Objective Topic Areas. Washington, DC: US Department of Health and Human Services; 2011. [Google Scholar]
- Okawa M. Delayed sleep phase syndrome and depression. Sleep Medicine. 2011;12:621–622. doi: 10.1016/j.sleep.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Okun ML, Reynolds CF, III, Buysse DJ, Monk TH, Mazumdar S, Begley A, Hall M. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosomatic Medicine. 2011;73:142–150. doi: 10.1097/PSY.0b013e3182020d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omlin S, Bauer GF, Brink M. Effects of noise from non-traffic-related ambient sources on sleep: Review of the literature of 1990–2010. Noise and Health. 2011;13(53):299–309. doi: 10.4103/1463-1741.82963. [DOI] [PubMed] [Google Scholar]
- Padilha HG, Crispim CA, Zimberg IZ, De-Souza DA, Waterhouse J, Tufik S, de-Mello MT. A link between sleep loss, glucose metabolism and adipokines. Brazilian Journal of Medical and Biological Research. 2011;44:992–999. doi: 10.1590/s0100-879x2011007500113. [DOI] [PubMed] [Google Scholar]
- Pak VM, Grandner MA, Pack AI. Circulating adhesion molecules in obstructive sleep apnea and cardiovascular disease. Sleep Medicine Reviews. 2014;18:25–34. doi: 10.1016/j.smrv.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A, Gekhman D, Gousse Y, Mckenzie S, White M, Zizi F, Jean-Louis G. Short sleep and dysfunctional beliefs and attitudes toward sleep among Black men. Sleep. 2011;34(Suppl):A261–262. [Google Scholar]
- Pandey J, Grandner M, Crittenden C, Smith MT, Perlis ML. Meteorologic factors and subjective sleep continuity: A preliminary evaluation. International Journal of Biometeorology. 2005;49:152–155. doi: 10.1007/s00484-004-0227-1. [DOI] [PubMed] [Google Scholar]
- Parhami I, Siani A, Rosenthal RJ, Lin S, Collard M, Fong TW. Sleep and gambling severity in a community sample of gamblers. Journal of Addiction Disorders. 2012;31:67–79. doi: 10.1080/10550887.2011.642754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NP, Grandner MA, Gehrman P, Xie D, Sha D, Weaver TE, Gooneratne N. Effects of sociodemographic and socioeconomic factors on sleep complaints depend on an individual’s race/ethnicity. Sleep. 2010a;33(Suppl):A307. [Google Scholar]
- Patel NP, Grandner MA, Xie D, Branas CC, Gooneratne N. ‘Sleep disparity’ in the population: Poor sleep quality is strongly associated with poverty and ethnicity. BMC Public Health. 2010b;10:475. doi: 10.1186/1471-2458-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR. Reduced sleep as an obesity risk factor. Obesity Reviews. 2009;10(Suppl 2):61–68. doi: 10.1111/j.1467-789X.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- Patel SR, Blackwell T, Ancoli-Israel S, Stone KL Osteoporotic Fractures in Men–MrOS Research Group. Sleep characteristics of self-reported long sleepers. Sleep. 2012;35:641–648. doi: 10.5665/sleep.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Hu FB. Short sleep duration and weight gain: A systematic review. Obesity (Silver Spring, MD) 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29:881–889. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Bootzin RR, Dikman ZV, Fleming GM, Drummond SP, Rose MW. Alpha sleep and information processing, perception of sleep, pain, and arousability in fibromyalgia. International Journal of Neuroscience. 1997;89:265–280. doi: 10.3109/00207459708988479. [DOI] [PubMed] [Google Scholar]
- Phillips B, Mannino DM. Do insomnia complaints cause hypertension or cardiovascular disease? Journal of Clinical Sleep Medicine. 2007;3:489–494. [PMC free article] [PubMed] [Google Scholar]
- Phillips CL, Butlin M, Wong KK, Avolio AP. Is obstructive sleep apnoea causally related to arterial stiffness? A critical review of the experimental evidence. Sleep Medicine Reviews. 2013;17:7–18. doi: 10.1016/j.smrv.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Pinto LR, Jr, Alves RC, Caixeta E, Fontenelle JA, Bacellar A, Poyares D, Tavares S. New guidelines for diagnosis and treatment of insomnia. Arquivos de Neuro-Psiquiatria. 2010;68:666–675. doi: 10.1590/s0004-282x2010000400038. [DOI] [PubMed] [Google Scholar]
- Pirrera S, De Valck E, Cluydts R. Nocturnal road traffic noise: A review on its assessment and consequences on sleep and health. Environment International. 2010;36:492–498. doi: 10.1016/j.envint.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Platt AB, Field SH, Asch DA, Chen Z, Patel NP, Gupta R, Kuna ST. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep. 2009;32:799–806. doi: 10.1093/sleep/32.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primhak R, Kingshott R. Sleep physiology and sleep-disordered breathing: The essentials. Archives of Disisease in Childhood. 2012;97:54–58. doi: 10.1136/adc.2010.186676. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Transtheoretical therapy: Toward a more integrative model of change. Psychotherapy: Theory, Research and Practice. 1982;19:276–288. [Google Scholar]
- Prochaska JO, Johnson S, Lee P. The transtheoretical model of behavior change. In: Shumaker SA, Ockene JK, Riekert KA, editors. Handbook of Health Behavior Change. 3. New York: Springer; 2009. pp. 59–83. [Google Scholar]
- Prochaska JO, Redding CA, Evers KE. The transtheoretical model and stages of change. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education: Theory, Research, and Practice. San Francisco: Jossey-Bass; 2008. pp. 97–121. [Google Scholar]
- Promberger M, Marteau TM. When do financial incentives reduce intrinsic motivation? Comparing behaviors studied in psychological and economic literatures. Health Psychology. 2013;32:950–957. doi: 10.1037/a0032727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilici S, Abrams KR, Nicolas A, Martin M, Petit C, Lleu PL, Finnern HW. Meta-analysis of the efficacy and tolerability of pramipexole versus ropinirole in the treatment of restless legs syndrome. Sleep Medicine. 2008;9:715–726. doi: 10.1016/j.sleep.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Zee PC. Circadian rhythm disorders. Seminars in Neurology. 2009;29:393–405. doi: 10.1055/s-0029-1237120. [DOI] [PubMed] [Google Scholar]
- Reynolds AC, Dorrian J, Liu PY, Van Dongen HP, Wittert GA, Harmer LJ, Banks S. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PLoS One. 2012;7(7):e41218. doi: 10.1371/journal.pone.0041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades KA, Leve LD, Harold GT, Mannering AM, Neiderhiser JM, Shaw DS, Reiss D. Marital hostility and child sleep problems: Direct and indirect associations via hostile parenting. Journal of Family Psychology. 2012;26:488–498. doi: 10.1037/a0029164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Clair C. Managing tobacco use: The neglected cardiovascular disease risk factor. European Heart Journal. 2013;34:3259–3267. doi: 10.1093/eurheartj/eht352. [DOI] [PubMed] [Google Scholar]
- Roach GD, Sargent C, Darwent D, Dawson D. Duty periods with early start times restrict the amount of sleep obtained by short-haul airline pilots. Accident Analysis and Prevention. 2012;45:S22–26. doi: 10.1016/j.aap.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Robillard R, Naismith SL, Rogers NL, Ip TK, Hermens DF, Scott EM, Hickie IB. Delayed sleep phase in young people with unipolar or bipolar affective disorders. Journal of Affective Disorders. 2013;145:260–263. doi: 10.1016/j.jad.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kantermann T, Juda M, Vetter C, Allebrandt KV. Light and the human circadian clock. Handbook of Experimental Pharmacology. 2013;217:311–331. doi: 10.1007/978-3-642-25950-0_13. [DOI] [PubMed] [Google Scholar]
- Rogojanski J, Carney CE, Monson CM. Interpersonal factors in insomnia: A model for integrating bed partners into cognitive behavioral therapy for insomnia. Sleep Medicine Reviews. 2013;17:55–64. doi: 10.1016/j.smrv.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Roscoe JA, Kaufman ME, Matteson-Rusby SE, Palesh OG, Ryan JL, Kohli S, Morrow GR. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12:S35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- Ruggiero JS, Redeker NS, Fiedler N, Avi-Itzhak T, Fischetti N. Sleep and psychomotor vigilance in female shiftworkers. Biological Research in Nursing. 2012;14:225–235. doi: 10.1177/1099800411408413. [DOI] [PubMed] [Google Scholar]
- Saberi P, Comfort M, Sheon N, Johnson MO. Qualitative study of the quality of sleep in marginalized individuals living with HIV. Patient Preference and Adherence. 2013;7:499–507. doi: 10.2147/PPA.S44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddichha S. Diagnosis and treatment of chronic insomnia. Annals of the Indian Academy of Neurology. 2010;13:94–102. doi: 10.4103/0972-2327.64628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Dahl RE, Shahar G, Rosenblat-Stein S. Sleep and the transition to adolescence: A longitudinal study. Sleep. 2009;32:1602–1609. doi: 10.1093/sleep/32.12.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Mindell J, Rivera L. ‘My child has a sleep problem’: A cross-cultural comparison of parental definitions. Sleep Medicine. 2011;12:478–482. doi: 10.1016/j.sleep.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Saini C, Suter DM, Liani A, Gos P, Schibler U. The mammalian circadian timing system: Synchronization of peripheral clocks. Cold Spring Harbor Symposium in Quantitative Biology. 2011;76:39–47. doi: 10.1101/sqb.2011.76.010918. [DOI] [PubMed] [Google Scholar]
- Sands-Lincoln M, Grandner M, Whinnery J, Keenan BT, Jackson N, Gurubhagavatula I. The association between obstructive sleep apnea and hypertension by race/ ethnicity in a nationally representative sample. Journal of Clinical Hypertension (Greenwich, CT) 2013a;15:593–599. doi: 10.1111/jch.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands-Lincoln M, Loucks EB, Lu B, Carskadon MA, Sharkey K, Stefanick ML, Eaton CB. Sleep duration, insomnia, and coronary heart disease among post-menopausal women in the Women’s Health Initiative. Journal of Women’s Health (Larchmont, NY) 2013b;22:477–486. doi: 10.1089/jwh.2012.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands MR, Lauderdale DS, Liu K, Knutson KL, Matthews KA, Eaton CB, Loucks EB. Short sleep duration is associated with carotid intima-media thickness among men in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Stroke. 2012;43:2858–2864. doi: 10.1161/STROKEAHA.112.660332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxvig IW, Pallesen S, Wilhelmsen-Langeland A, Molde H, Bjorvatn B. Prevalence and correlates of delayed sleep phase in high school students. Sleep Medicine. 2012;13:193–199. doi: 10.1016/j.sleep.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Sell RE, Bardwell W, Palinkas L, Ancoli-Israel S, Dimsdale J, Loredo JS. Ethnic differences in sleep-health knowledge. Sleep. 2009;32(Suppl):A392. [Google Scholar]
- Shankar A, Syamala S, Kalidindi S. Insufficient rest or sleep and its relation to cardiovascular disease, diabetes and obesity in a national, multiethnic sample. PLoS ONE. 2010;5(11):e14189. doi: 10.1371/journal.pone.0014189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KM, Carskadon MA, Figueiro MG, Zhu Y, Rea MS. Effects of an advanced sleep schedule and morning short wavelength light exposure on circadian phase in young adults with late sleep schedules. Sleep Medicine. 2011;12:685–692. doi: 10.1016/j.sleep.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Hansen CH, O’Connor M, Thekkumpurath P, Walker J, Kleiboer A, Sharpe M. Sleep problems in cancer patients: Prevalence and association with distress and pain. Psycho-Oncology. 2012;21:1003–1009. doi: 10.1002/pon.2004. [DOI] [PubMed] [Google Scholar]
- Shea JA, Weissman A, McKinney S, Silber JH, Volpp KG. Internal medicine trainees’ views of training adequacy and duty hours restrictions in 2009. Academic Medicine. 2012;87:889–894. doi: 10.1097/ACM.0b013e3182582583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebern AT, Manber R. Insomnia and its effective non-pharmacologic treatment. Medical Clinics of North America. 2010;94:581–591. doi: 10.1016/j.mcna.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Siebern AT, Suh S, Nowakowski S. Non-pharmacological treatment of insomnia. Neurotherapeutics. 2012;9:717–727. doi: 10.1007/s13311-012-0142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson NS, Banks S, Arroyo S, Dinges DF. Effects of sleep restriction on adiponectin levels in healthy men and women. Physiology and Behavior. 2010;101:693–698. doi: 10.1016/j.physbeh.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Lang C, Sullivan K, Warren J. Two new tools for assessing patients’ knowledge and beliefs about obstructive sleep apnea and continuous positive airway pressure therapy. Sleep Medicine. 2004;5:359–367. doi: 10.1016/j.sleep.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Solarz DE, Mullington JM, Meier-Ewert HK. Sleep, inflammation and cardiovascular disease. Frontiers in Bioscience (Elite Edition) 2012;4:2490–2501. doi: 10.2741/e560. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. Journal of Clinical Endocrinology and Metabolism. 2004a;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annals of Internal Medicine. 2004b;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder K, Fuchs L, Ladwig J, Kyle SD, Nissen C, Voderholzer U, Riemann D. Heart rate and heart rate variability in subjectively reported insomnia. Journal of Sleep Research. 2011;20:137–145. doi: 10.1111/j.1365-2869.2010.00863.x. [DOI] [PubMed] [Google Scholar]
- St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. American Journal of Clinical Nutrition. 2012;95:818–824. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. American Journal of Clinical Nutrition. 2011;94(2):410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: Population prevalence and growing disparities during 34 years of follow-up. Annals of Epidemiology. 2007;17:948–955. doi: 10.1016/j.annepidem.2007.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PK, Pu Y. Heart rate variability, sleep and sleep disorders. Sleep Medicine Reviews. 2012;16(1):47–66. doi: 10.1016/j.smrv.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Stephens S, Cobiac L, Veerman L. Improving diet and physical activity to reduce population prevalence of overweight and obesity: An overview of current evidence. Preventive Medicine. 2014 doi: 10.1016/j.ypmed.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Strand LB, Laugsand LE, Wisloff U, Nes BM, Vatten L, Janszky I. Insomnia symptoms and cardiorespiratory fitness in healthy individuals: The Nord-Trondelag Health Study (HUNT) Sleep. 2013;36:99–108. doi: 10.5665/sleep.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suka M, Yoshida K, Sugimori H. Persistent insomnia is a predictor of hypertension in Japanese male workers. Journal of Occupational Health. 2003;45:344–350. doi: 10.1539/joh.45.344. [DOI] [PubMed] [Google Scholar]
- Sullivan SS. Insomnia pharmacology. Medical Clinics North America. 2010;94(3):563–580. doi: 10.1016/j.mcna.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Sunwoo BY, Jackson N, Maislin G, Gurubhagavatula I, George CF, Pack AI. Reliability of a single objective measure in assessing sleepiness. Sleep. 2012;35:149–158. doi: 10.5665/sleep.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szosland D. Shift work and metabolic syndrome, diabetes mellitus and ischaemic heart disease. International Journal of Occupational Medicine and Environmental Health. 2010;23:287–291. doi: 10.2478/v10001-010-0032-5. [DOI] [PubMed] [Google Scholar]
- Taibi DM. Sleep disturbances in persons living with HIV. Journal of the Association of Nurses in AIDS Care. 2013;24:S72–85. doi: 10.1016/j.jana.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA, Achermann P. Dissipation of sleep pressure is stable across adolescence. Neuroscience. 2012;216:167–177. doi: 10.1016/j.neuroscience.2012.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveras EM, Rifas-Shiman SL, Rich-Edwards JW, Gunderson EP, Stuebe AM, Mantzoros CS. Association of maternal short sleep duration with adiposity and cardiometabolic status at 3 years postpartum. Obesity (Silver Spring, MD) 2011;19:171–178. doi: 10.1038/oby.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, Grieser EA, Tatum JI. Other nonpharmacological treatments of insomnia. In: Sateia MJ, Buysse DJ, editors. Insomnia: Diagnosis and Treatment. Colchester: Informa; 2010. pp. 290–298. [Google Scholar]
- Thomee S, Harenstam A, Hagberg M. Mobile phone use and stress, sleep disturbances, and symptoms of depression among young adults – A prospective cohort study. BMC Public Health. 2011;11:66. doi: 10.1186/1471-2458-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomee S, Harenstam A, Hagberg M. Computer use and stress, sleep disturbances, and symptoms of depression among young adults – A prospective cohort study. BMC Psychiatry. 2012;12:176. doi: 10.1186/1471-244X-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomfohr LM, Ancoli-Israel S, Dimsdale JE. Childhood socioeconomic status and race are associated with adult sleep. Behavioral Sleep Medicine. 2010;8:219–230. doi: 10.1080/15402002.2010.509236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenkwalder C, Paulus W. Restless legs syndrome: Pathophysiology, clinical presentation and management. Nature Reviews Neurology. 2010;6:337–346. doi: 10.1038/nrneurol.2010.55. [DOI] [PubMed] [Google Scholar]
- Troxel WM. It’s more than sex: Exploring the dyadic nature of sleep and implications for health. Psychosomatic Medicine. 2010;72:578–586. doi: 10.1097/PSY.0b013e3181de7ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Buysse DJ, Matthews KA, Kip KE, Strollo PJ, Hall M, Reis SE. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010a;33:1633–1640. doi: 10.1093/sleep/33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Buysse DJ, Matthews KA, Kravitz HM, Bromberger JT, Sowers M, Hall MH. Marital/cohabitation status and history in relation to sleep in midlife women. Sleep. 2010b;33:973–981. doi: 10.1093/sleep/33.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Buysse DJ, Monk TH, Begley A, Hall M. Does social support differentially affect sleep in older adults with versus without insomnia? Journal of Psychosomatic Research. 2010c;69:459–466. doi: 10.1016/j.jpsychores.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer C, Miller Wolman D, Johns MME Institute of Medicine Committee on Optimizing Graduate Medical Trainee (Resident) Hours and Work Schedules to Improve Patient Safety. Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- Van Cauter E, Knutson K. Sleep and the epidemic of obesity in children and adults. European Journal of Endocrinology. 2008;159:S59–66. doi: 10.1530/EJE-08-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Medicine. 2008;9:S23–28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaert V, Haex B, De Wilde T, Berckmans D, Verbraecken J, de Valck E, Vander Sloten J. Ergonomics in bed design: The effect of spinal alignment on sleep parameters. Ergonomics. 2011;54:169–178. doi: 10.1080/00140139.2010.538725. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Medicine Reviews. 2013;17:241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009a;32:491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, Bixler EO. Insomnia with short sleep duration and mortality: The Penn State cohort. Sleep. 2010;33:1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009b;32:1980–1985. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp KG, Asch DA, Galvin R, Loewenstein G. Redesigning employee health incentives – Lessons from behavioral economics. New England Journal of Medicine. 2011;365:388–390. doi: 10.1056/NEJMp1105966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: A randomized trial. Journal of the American Medical Association. 2008;300:2631–2637. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kaanel R, Mausbach BT, Ancoli-Israel S, Dimsdale JE, Mills PJ, Patterson TL, Grant I. Sleep in spousal Alzheimer caregivers: A longitudinal study with a focus on the effects of major patient transitions on sleep. Sleep. 2012;35:247–255. doi: 10.5665/sleep.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JS, DiBonaventura MD, Chandran AB, Cappelleri JC. The association of sleep difficulties with health-related quality of life among patients with fibromyalgia. BMC Musculoskeletal Disorders. 2012;13:199. doi: 10.1186/1471-2474-13-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia HK, Hayes AL, Przepyszny KA, Karumanchi P, Patel SR. Clinical presentation of shift workers to a sleep clinic. Sleep and Breathing. 2012;16:543–547. doi: 10.1007/s11325-011-0540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallhausser-Franke E, Schredl M, Delb W. Tinnitus and insomnia: Is hyperarousal the common denominator? Sleep Medicine Reviews. 2013;17:65–74. doi: 10.1016/j.smrv.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: A systematic review and meta-analysis. Hypertension Research. 2012;35:1012–1018. doi: 10.1038/hr.2012.91. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: A large-scale prospective study. Sleep. 2010;33(2):161–167. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep. 2010;33:1201–1209. doi: 10.1093/sleep/33.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter TC. Restless legs syndrome and periodic limb movement disorder. In: Overeem S, Reading P, editors. Sleep Disorders in Neurology: A Practical Approach. Hoboken NJ: Wiley-Blackwell; 2010. pp. 87–99. [Google Scholar]
- Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and Long Sleep Duration Associated with Race/Ethnicity, Sociodemographics, and Socioeconomic Position. Sleep. 2014;37:601–611. doi: 10.5665/sleep.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. Sleep and Society: Sociological Ventures into the (Un)known. London: Taylor & Francis; 2005. [Google Scholar]
- Woodward SC. Cognitive-behavioral therapy for insomnia in patients with cancer. Clinical Journal of Oncological Nursing. 2011;15:E42–52. doi: 10.1188/11.CJON.E42-E52. [DOI] [PubMed] [Google Scholar]
- Yang CM, Chou CP, Hsiao FC. The association of dysfunctional beliefs about sleep with vulnerability to stress-related sleep disturbance in young adults. Behavioral Sleep Medicine. 2011;9:86–91. doi: 10.1080/15402002.2011.557990. [DOI] [PubMed] [Google Scholar]
- Yoder JC, Staisiunas PG, Meltzer DO, Knutson KL, Arora VM. Noise and sleep among adult medical inpatients: Far from a quiet night. Archives of Internal Medicine. 2012;172:68–70. doi: 10.1001/archinternmed.2011.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Lu BS, Wang B, Wang H, Yang J, Li Z, Wang X. Short sleep duration and adiposity in Chinese adolescents. Sleep. 2007;30:1688–1697. doi: 10.1093/sleep/30.12.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee PC, Badr MS, Kushida C, Mullington JM, Pack AI, Parthasarathy S, Watson NF. Strategic opportunities in sleep and circadian research: Report of the joint task force of the Sleep Research Society and American Academy of Sleep Medicine. Sleep. 2014;37:219–227. doi: 10.5665/sleep.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Liu ZH, Luo Q, Zhao ZH, Zhang HL, Wang Y. Effects of continuous positive airway pressure on blood pressure and daytime sleepiness in obstructive sleep apnea patients with coronary heart diseases under optimal medications. Sleep and Breathing. 2012;16:341–347. doi: 10.1007/s11325-011-0498-9. [DOI] [PubMed] [Google Scholar]
- Zhou QP, Jung L, Richards KC. The management of sleep and circadian disturbance in patients with dementia. Current Neurology and Neuroscience Reports. 2012;12:193–204. doi: 10.1007/s11910-012-0249-8. [DOI] [PubMed] [Google Scholar]
- Zhou X, Ferguson SA, Matthews RW, Sargent C, Darwent D, Kennaway DJ, Roach GD. Sleep, wake and phase dependent changes in neurobehavioral function under forced desynchrony. Sleep. 2011;34:931–941. doi: 10.5665/SLEEP.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimberg IZ, Damaso A, Del Re M, Carneiro AM, de Sa Souza H, de Lira FS, de Mello MT. Short sleep duration and obesity: Mechanisms and future perspectives. Cell Biochemistry and Function. 2012;30:524–529. doi: 10.1002/cbf.2832. [DOI] [PubMed] [Google Scholar]
- Zuo H, Shi Z, Yuan B, Dai Y, Hu G, Wu G, Hussain A. Interaction between physical activity and sleep duration in relation to insulin resistance among non-diabetic Chinese adults. BMC Public Health. 2012;12:247. doi: 10.1186/1471-2458-12-247. [DOI] [PMC free article] [PubMed] [Google Scholar]