Abstract
Introduction
We sought to determine if early placental size, as measured by 3-dimensional ultrasonography, is associated with an increased risk of delivering a macrosomic or large-for-gestational age (LGA) infant.
Methods
We prospectively collected 3-dimensional ultrasound volume sets of singleton pregnancies at 11–14 weeks and 18–24 weeks. Birth weights were collected from the medical records. After delivery, the ultrasound volume set were used to measure the placental volume (PV) and placental quotient (PQ=PV/gestational age), as well as the mean placental and chorionic diameters (MPD and MCD, respectively). Placental measures were analyzed as predictors of macrosomia (birth weight ≥4000 grams) and LGA (birth weight ≥90th percentile).
Results
The 578 pregnancies with first trimester volumes included 44 (7.6%) macrosomic and 43 (7.4%) LGA infants. 373 subjects also had second trimester volumes available. A higher PV and PQ were both significantly associated with macrosomia and LGA in both the first and second trimesters. Second trimester MPD was significantly associated with both outcomes as well, while second trimester MCD was only associated with LGA. The above associations remained significant after adjusting for maternal demographic variables such as race, ethnicity, age and diabetes. Adjusted models yielded moderate prediction of macrosomia and LGA (AUC: 0.71–0.77).
Conclusions
Sonographic measurement of the early placenta can identify pregnancies at greater risk of macrosomia and LGA. Macrosomia and LGA are already determined in part by early placental growth and development.
Keywords: fetal growth, large for gestational age, macrosomia, placenta, ultrasound
Introduction
Macrosomia is associated with an increased risk of adverse short- and long-term neonatal outcomes including brachial plexus injury and other birth trauma which can result in long term disability.[1–7] In addition, macrosomia has been associated with longer term morbidities secondary to fetal programming, such as childhood obesity and adult cardiovascular and metabolic disorders.[8–12] Delivery of a macrosomic infant is associated with several maternal risks as well, including cesarean delivery and perineal trauma with potential for long term pelvic dysfunction. [1–3, 5, 13]
Many of the known risk factors for macrosomia, especially some of the potentially modifiable variables, such as maternal weight gain in pregnancy, development of gestational diabetes and post-term delivery, are not knowable until late in gestation. Therefore, there are currently no effective early prediction models to identify pregnancies at greatest risk for delivering a macrosomic infant.
Placental size at delivery has been shown to correlate with neonatal birth weight. [15, 16] In fact, a small placenta at delivery can indicate that placental insufficiency played a role in limiting the growth potential of that fetus.[14, 17, 18] This association has led investigators to explore whether prenatal ultrasonography can be used to detect a small placenta during pregnancy, thereby identifying pregnancies at risk for developing fetal growth restriction. Both 2- and 3-dimensional sonographic measurements of placental size have been associated with subsequent delivery of a small-for-gestational-age (SGA) infant. [19–26]
While these research efforts have focused almost exclusively on indentifying pregnancies at risk for fetal growth restriction, little attention has been placed on whether assessment of in-utero placental size can be helpful in predicting macrosomia.
We sought to determine if early placental size, as measured by 3-dimensional ultrasound, is associated with the delivery of a macrosomic infant.
Methods
For this study, we utilized a research database from a prospective cohort study conducted at the Hospital of the University of Pennsylvania for which 3-dimensional sonographic measures of early placental size were performed to identify pregnancies at increased risk of adverse outcomes.[27] In that study, conducted between May 2010 and March 2012, women with singleton pregnancies electing to undergo a nuchal translucency exam at 11–13 6/7 weeks’ gestation were recruited and informed consent obtained. All gestational dating was confirmed by first trimester ultrasound. In cases of unknown LMP or where first trimester sonographic gestational dating differed from LMP dating by >6 days, the ultrasound-based dates were used.
A transabdominal 3-dimensional sweep of the placenta was obtained during that exam (GE Voluson E8 machines, GE Healthcare, Milwaukee, WI) and stored offline for analysis. Subjects also consented to having another placental volume set obtained at the time of their anatomic survey (18–24 weeks’ gestation). Pregnancies were allowed to proceed per routine clinical management without study-mandated intervention. Any additional ultrasound examinations or other surveillance was conducted at the discretion of the clinician following routine clinical practice. Pregnancy outcomes were collected via individual chart review. Of note, standard practice in our institution does not include macrosomia or a large estimated fetal weight as an indication for a scheduled delivery.
Placental volume sets were analyzed offline using 4DVIEW software (GE Healthcare, Zipf, Austria) by investigators blinded to pregnancy outcomes using previously described techniques.[24] Briefly, placental volume (PV) was measured with the Virtual Organ Computer-Aided analysis (VOCAL) application using a 30°angle of rotation. The placental quotient (PQ) was then calculated to normalize the PV to gestational age (PQ=PV/GA in days). We chose to use gestational age rather than crown-rump length so that comparisons could be made between first and second trimester values. In addition, we also calculated a mean placental diameter (MPD) as the average of four traced distances measured along the maternal surface of the placenta at 45°intervals. Similarly, we obtained a mean chorionic diameter (MCD) by employing the same approach to the fetal surface of the placenta. (Figure 1)
Figure 1.
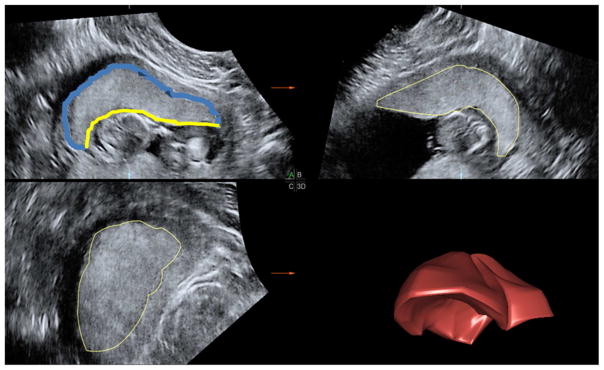
Display of a placental volume using 4DVIEW. Quadrants A, B, and C correspond to the 3 orthogonal planes. Successive tracings in plane A are rendered using VOCAL into a 3-dimensional placental volume depicted in the lower right quadrant (3D). The placenta diameter (blue) and chorionic diameter (yellow) were measured in both A and B planes before and after a 45° rotation around the y-axis.
To demonstrate the reliability of these measurement techniques, two investigators (NS, HQ) performed duplicate measurements for 25 second trimester placental volume sets. Bland-Altman [28] and concordance correlation coefficients were computed for PV, MPD and MCD. Correlations were high for volume (0.82) and MPD (0.75), and moderate for MCD (0.59). There were no systematic differences in measurements between the two investigators.
In this investigation, we queried the study database for all singleton gestations for which first trimester placental volume measurements and delivery outcome data were available. In the original study, subjects were excluded from the second trimester analysis if no second trimester volume set was saved, the entire placenta was not included in the volume sweep, or if there was significant shadowing by the fetus that obscured the placental margins. If there was a question as to whether a second trimester volume set was of sufficient quality, two investigators (N.S. and H.S.Q.) reviewed the volume together and reached a consensus as to whether to exclude that volume.
The primary outcome for this study was delivery of a macrosomic infant, defined as birth weight ≥4000 grams. As an earlier gestational age at delivery may limit the incidence of macrosomia, we also analyzed large-for-gestational-age (LGA), defined as birth weight ≥90th percentile for gestational age[29], as a secondary outcome.
Using the observed 7.6% (44/578) prevalence of macrosomia and the 578 subjects with first trimester placental data available in the database, we performed a post-hoc power calculation using the powerlog function in Stata (Stata version 12.1, College Station, TX) and found that the available sample size achieves an 80% power to detect an odds ration of 1.5 for a 1 Standard Deviation increase in placental volume, with an alpha of 0.05. The second trimester analyses, which had 373 subjects available, had 80% power to detect an odds ratio of 1.6.
Parametric and non-parametric tests were used as appropriate. Demographic variables such as maternal age, nulliparity, race, body-mass index (BMI) and presence of pre-gestational diabetes were analyzed as potential confounders. Backward elimination multivariable regression was used to determine the covariates that remained significant in each prediction model. Receiver operator characteristic curve (ROC) analysis yielded an area under the curve (AUC) as a metric of the predictive ability of univariable and multivariable models for those placental measures that demonstrated a trend towards a significant association with the outcome of interest (i.e. P<0.1). The predicted probability from the multivariable logistic models were used as the input variable into the ROCCOMP procedure in Stata to evaluate and test the ability of each multivariable model to discriminate between macrosomic vs nonmacrosomic infants.[30] This study was approved by the Institutional Review Board of the University of Pennsylvania (Protocol #811129).
Results
A first trimester placental volume set was obtained and analyzed for 578 subjects at a mean (SD) gestational age of 12.6 (0.5) weeks. Two additional first trimester volume sets were excluded due to a bi-lobed placental mass not allowing for clear measurements of the placenta. Forty-four (7.6%) subjects delivered a macrosomic infant. Interestingly, as the 90th percentile at 40 completed weeks or later ranges from 4060g to 4098g, there were 7 macrosomic infants that were not actually LGA. Demographic data can be seen in Table 1. The median maternal BMI at the time of first prenatal visit was significantly higher in pregnancies delivering a macrosomic infant (26.4 vs 24.6 kg/m2; P=0.01), while the difference in median maternal age was not statistically significant (33 vs 32 years; P=0.09). The median gestational age at delivery was greater in the macrosomic group (40.2 vs 39.2 weeks; P<0.001) as well.
Table 1.
Maternal Characteristics
| AGA (N=534) | Macrosomia (N=44) | P-value | |
|---|---|---|---|
| Age in years, median (IQR) | 32 (27–35) | 33 (29.5–35) | 0.09* |
| BMI, median (IQR) | 24.6 (22–30.8) | 26.4 (23.4–34.7) | 0.01* |
| Black race, N (%) | 219 (41) | 15 (34) | 0.37^ |
| Nulliparity, N (%) | 106 (19.9) | 2 (4.6) | 0.008§ |
| Pregestational diabetes, N (%) | 11 (2.1) | 3 (6.8) | 0.08§ |
| Gestational Diabetes, N (%) | 24 (4.6%) | 2 (4.9%) | 1.0 |
| Pregestational or gestational diabetes), N (%) | 35 (6.6%) | 5 (11.4%) | 0.2 |
Wilcoxon rank sum;
Chi-square;
Fisher’s exact
First trimester PV (P=0.006) and PQ (P=0.002) were both significantly greater in pregnancies that subsequently delivered macrosomic infants. (Table 2) In addition, there was a trend towards a larger MPD in the macrosomic group (P=0.06). ROC curve analysis demonstrated that both PV (AUC=0.625) and PQ (AUC=0.6396) had moderate predictive value in identifying pregnancies destined to deliver a macrosomic infant, although the AUC for PQ was significantly greater (P=0.048). (Table 2) Potential confounders were considered using a backwards elimination strategy which yielded a multivariable model including maternal BMI, nulliparity, and pregestational diabetes as the significant covariates. Although the AUCs for the adjusted model were not significantly higher than the AUC for a model using only clinical risk factors, PV, PQ and MPD each retained their statistically significant association with the outcome in adjusted analyses demonstrating that there remains a significant association between these early placental measures and macrosomia. (Table 2) No significant difference in AUC was seen when comparing the different multivariable models (P=0.4). The ROC curve for PQ can be seen in Figure 2.
Table 2.
First trimester placental measurements as predictors of macrosomia
| Placental Measures, median (IQR) | AGA (N=534) | Macrosomia (N=44) | P-value | Unadjusted AUC | Adjusted P-value § | Adjusted AUC§ |
|---|---|---|---|---|---|---|
| Placental volume (cc) | 65.8 (52.3–80.0) | 74.5 (65.2–85.7) | 0.006* | 0.625 | 0.009 | 0.724 |
| Placental Quotient (cc/days GA) | 0.74 (0.60–0.90) | 0.87 (0.75–0.98) | 0.002* | 0.640 | 0.004 | 0.731 |
| Mean placental diameter (cm) | 11.3 (11.1–11.4) | 11.7 (11.3–12.2) | 0.06^ | 0.592 | 0.036 | 0.709 |
| Mean chorionic diameter (cm) | 8.2 (8.1–8.3) | 8.4 (8.1–8.7) | 0.22^ | -- | -- | -- |
Wilcoxon rank sum;
T-test;
Model adjusted for maternal BMI, nulliparity and pregestational diabetes. A model with these clinical risk factors alone would have an AUC of 0.684.
Figure 2.
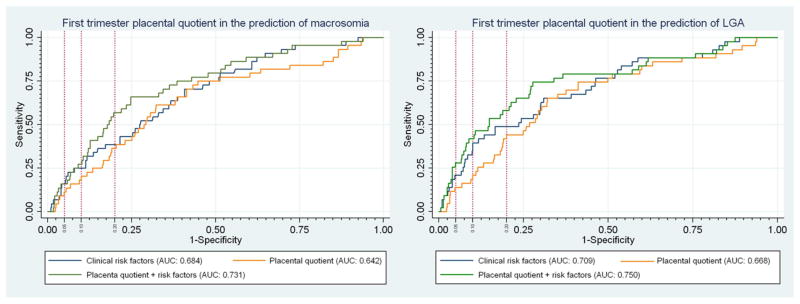
ROC curves for first trimester placental quotient (PQ) as a predictor of macrosomia and LGA. Vertical reference lines were placed to represent a false-positive rate of 5%, 10% and 20%. The corresponding sensitivity for the model can be seen by following the horizontal reference line to the y-axis.
Forty-three pregnancies (7.4%) delivered LGA fetuses, 37 of which were also macrosomic. First trimester PV (P=0.002) and PQ (P=0.003) were both significantly larger in pregnancies destined to deliver LGA infants. Once again, PQ (AUC=0.667) was significantly more predictive than PV (AUC=0.644; P<0.002) on univariate ROC analysis. (Table 3) In addition, a larger MPD was also significantly associated with the delivery of an LGA infant (P=0.008) and demonstrated a moderate ability (AUC-0.635) to predict LGA that was similar to that of PQ (P=0.25). (Table 3) Multivariable regression analysis demonstrated that maternal age, BMI, nulliparity and pregestational diabetes were all significant risk factors for macrosomia. Once again, the AUCs for the adjusted model were not significantly higher than the AUC for a model using only clinical risk factors; however, PV, PQ and MPD each retained their statistically significant association with the outcome. (Table 3) No significant difference in AUC was seen when comparing the different multivariable models (P=0.24). The ROC curve for PQ can be seen in Figure 2.
Table 3.
First trimester placental measurements as predictors of LGA
| Placental Measures, median (IQR) | AGA (N=534) | LGA (N=43) | P-value | Unadjusted AUC | Adjusted P-value§ | Adjusted AUC§ |
|---|---|---|---|---|---|---|
| Placental volume (cc) | 65.8 (52.2–79.9) | 77.7 (64.7–86.3) | 0.002* | 0.644 | 0.003 | 0.741 |
| Placental Quotient (cc/days GA) | 0.75 (0.60–0.90) | 0.88 (0.74–1.01) | 0.0003* | 0.667 | 0.001 | 0.750 |
| Mean placental diameter (cm) | 11.3 (11.1–11.4) | 11.9 (11.5–12.4) | 0.008^ | 0.635 | 0.005 | 0.745 |
| Mean chorionic diameter (cm) | 8.2 (8.1–8.3) | 8.4 (8.1–8.8) | 0.18^ | -- | -- | -- |
Wilcoxon rank sum;
T-test;
Model adjusted for maternal age, BMI, nulliparity and pregestational diabetes. A model with these clinical risk factors alone would have an AUC of 0.709.
Of the 578 subjects included in the first trimester analysis, 114 (19.7%) did not have second trimester volume sets stored at the time of the anatomic survey. An additional 63 (10.9%) were deemed inadequate for measurement and 28 (4.7%) were not attempted in the original second trimester study as the sample size for that study was attained, leaving 373 (64.5%) subjects with both first and second trimester placental volume data available for analysis. These ultrasounds were obtained at a mean gestational age of 20.4 (0.6) weeks. Second trimester PV (PV2), PQ (PQ2), and MPD (MPD2) were all significantly larger both in pregnancies that delivered macrosomic infants, as well as in those that delivered LGA infants, compared to pregnancies that resulted in normally grown infants. (Tables 4 and 5) In addition, there was a trend towards a larger second trimester MCD (MCD2) in pregnancies with macrosomia (P=0.08) and a significantly larger MCD2 with LGA deliveries (P=0.01). Unadjusted AUCs remained moderate for the prediction of macrosomia and LGA. (Tables 4 and 5) Comparison of the unadjusted AUCs revealed no significant differences among the various placental measures. A multivariable model including MPD2, pregestational diabetes and nulliparity yielded the highest adjusted AUC (0.772) for the prediction of macrosomia. (Figure 3) For LGA, a model including PQ2, maternal age, nulliparity and pregestational diabetes yielded the highest adjusted AUC (0.774) for predicting LGA. (Figure 3) While the placental measures retained their significant association with macrosomia and LGA after adjusting for the clinical risk factors, there was no statistically significant improvement in the AUC. Overall, the adjusted AUCs for the second trimester models were not significantly higher than the first trimester models (P>0.1).
Table 4.
Second trimester placental measurements as predictors of macrosomia
| Placental Measures, median (IQR) | AGA (N=343) | Macrosomia (N=30) | P-value | Unadjusted AUC | Adjusted P-value§ | Adjusted AUC§ |
|---|---|---|---|---|---|---|
| Placental volume (cc) | 237.9 (199.9–274.2) | 265.8 (231.9–321.9) | 0.005* | 0.655 | 0.001 | 0.738 |
| Placental Quotient (cc/days GA) | 1.65 (1.40–1.92) | 1.89 (1.67–2.17) | 0.002* | 0.667 | <0.001 | 0.746 |
| Mean placental diameter (cm) | 15.5 (15.3–15.7) | 16.7 (16.0–17.4) | 0.0007^ | 0.687 | 0.001 | 0.772 |
| Mean chorionic diameter (cm) | 13.5 (13.3–13.6) | 13.9 (13.4–14.4) | 0.08^ | 0.598 | 0.08 | 0.695 |
Wilcoxon rank sum;
T-test;
Model adjusted for nulliparity and pregestational diabetes.
-A clinical model would include nulliparity and maternal BMI and yield an AUC of 0.708.
Table 5.
Second trimester placental measurements as predictors of LGA
| Placental Measures, median (IQR) | AGA (N=343) | LGA (N=29) | P-value | Unadjusted AUC | Adjusted P-value | Adjusted AUC |
|---|---|---|---|---|---|---|
| Placental volume (cc) | 237.5 (199.3–274.2) | 265.4 (239.4–294.0) | 0.001* | 0.682 | <0.001§ | 0.767§ |
| Placental Quotient (cc/days GA) | 1.65 (1.40–1.90) | 1.89 (1.7–2.0) | 0.004* | 0.697 | <0.001§ | 0.774§ |
| Mean placental diameter (cm) | 15.5 (15.3–15.7) | 16.5 (15.8–17.2) | 0.008^ | 0.656 | 0.003¶ | 0.715¶ |
| Mean chorionic diameter (cm) | 13.5 (13.3–13.6) | 14.1 (13.7–14.6) | 0.01^ | 0.648 | 0.005¶ | 0.712¶ |
Wilcoxon rank sum;
T-test;
Model adjusted for maternal age, nulliparity and pregestational diabetes
Model adjusted for maternal age and pregestational diabetes
-A model of clinical risk factors alone would include age, pregestational diabetes and maternal BMI and yield an AUC of 0.707.
Figure 3.
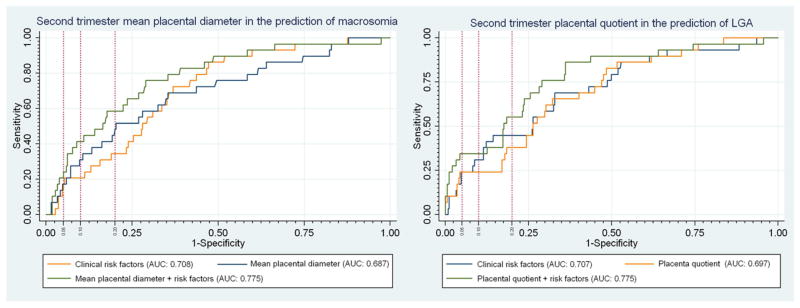
ROC curves for second trimester placental quotient as a predictor of macrosomia and second trimester mean placental diameter (MPD) as a predictor of LGA. Vertical reference lines were placed to represent a false-positive rate of 5%, 10% and 20%. The corresponding sensitivity for the model can be seen by following the horizontal reference line to the y-axis.
Finally, the per week rate of change for PV and PQ from first to second trimester yielded similar results to the second trimester values alone with no added predictive ability for either outcome of interest. (data not shown)
Discussion
Our study demonstrates that early placental size is a significant marker of fetal growth potential. While much of the literature investigating the potential significance of early placental size has concentrated on identifying patients at risk for fetal growth restriction, our results indicate that a large first trimester placenta is a risk factor for macrosomia and LGA.
Macrosomia and LGA have been associated with several adverse maternal, neonatal and adult-onset outcomes.[1–7, 9, 13, 31] Macrosomia is most commonly defined as a birth weight of either 4,000 or 4,500 grams. [32] We chose to use a birth weight of ≥4000 grams as this weight remains a significant risk factor for adverse outcomes compared with the general population and represents approximately 10% of all births in the United States.[32] In addition, while the risk for maternal and early neonatal morbidities is even greater at weights ≥4,500 grams, much of the data for longer term morbidities related to fetal programming of adult disease has used 4,000 grams to define large birth weights. [8, 9, 11, 12, 33]
Accurate prediction of macrosomia and LGA would allow for improved patient counseling regarding pregnancy risks. More importantly, there may be nutritional and behavioral modifications that can be implemented in order to reduce the risk of these outcomes. For example, gestational weight gain and carbohydrate intake are potentially modifiable risk factors for the development of LGA.[34–36] Furthermore, regular exercise in pregnancy has been linked to decreased odds of delivering an LGA infant.[37] Unfortunately, early randomized trials of interventions to limit maternal weight gain and improve perinatal outcomes have not been successful.[38] Thus it remains unclear whether behavioral interventions to modify maternal risk factors can ultimately improve outcomes.
Currently there are no early prediction models that reliably detect pregnancies destined to develop macrosomia or LGA. Poon et al.[39] published a retrospective cohort of greater than 30,000 pregnancies which showed that nuchal translucency measurement and first trimester PAPP-A and beta-HCG are associated with macrosomia. However, a multivariable model which included these three factors as well as maternal variables yielded a modest adjusted AUC of 0.727. Similarly, Plasencia et al.[40] added uterine artery Doppler pulsatility index to a similar prediction model which yielded an adjusted AUC of 0.722. Our adjusted model based on placental measures yielded similar AUCs and the clinical prediction remains suboptimal. However, While it may be that the addition of other known predictor variables or biomarkers can improve the ability to predict the development of macrosomia and LGA, neither first trimester PAPP-a multiple of the median (macrosomia: P=0.9; LGA: P=0.7) not uterine artery Doppler pulsatility index (macrosomia: P=0.2; LGA: P=0.2) were significantly associated with these outcomes in our cohort (data not shown).
Interestingly, second trimester placental measurements were not significantly more predictive of macrosomia or LGA compared to first trimester placental measurements. This may have been due, in part, to the smaller sample size in the second trimester analyses. If confirmed, this would further support the notion that it is the early, first trimester trophoblastic invasion and placental development where the more critical scaffolding for nutrient exchange is being formed. Hypothetically, a larger first trimester placenta may allow for hyper-efficient nutrient transfer to the fetus that may persist even if the maternal diet and weight gain are optimized. Consequently, it would appear that the development of macrosomia is already partly pre-destined from an early stage in pregnancy.
Our second trimester analysis was potentially limited by the smaller sample size available for analysis. However, given that most of the placental measures had statistically significant associations with our outcomes of interest, the effect of this limitation is less concerning. However, it may be that mean MPD and MCD are indeed significantly associated with our outcomes, but that we are underpowered to detect this association in our cohort. In fact, several investigators have already demonstrated an association between 2-dimensional placental diameters and SGA.[21, 23, 25, 26] Further investigation exploring the potential associations between diameter measurements and macrosomia is warranted as these measurements are simpler to perform and can be adapted to 2-dimensional ultrasound.
Another potential limitation in the second trimester cohort relates to the exclusion of 10% of volumes due to poor quality sweeps. While many of these were likely related to fetal shadowing, it remains possible that larger placentas were preferentially excluded due to a difficulty including the entire placental mass into the 3D sweep. However, as the rates of macrosomia and LGA were actually slightly lower (6.6%) among those subjects excluded from the second trimester analysis, it is unlikely that a significant bias limited the performance of the second trimester placental measurements in predicting macrosomia or LGA. Furthermore, as the user can see if there are portions of the placenta that are missing or too obscured to accurately trace, we are confident that those placental measurements included were of reasonable quality.
Overall, despite the significant associations between placental measurements and macrosomia and LGA, we are still left with a suboptimal tool for the early prediction of these outcomes. However, an important conclusion we can draw from our results is that the size of the early placenta is significantly associated with macrosomia and LGA. Therefore, many of the clinical and genetic influences placing certain women at increased risk for delivering a large infant may be acting through influence on the gross development of the early placenta as early as the first trimester. The association between first trimester placental size and fetal overgrowth underscores the fact that a significant portion of the etiologic factors that lead to macrosomia have already taken root early in pregnancy, even before modifiable risk factors, such as excessive weight gain and gestational diabetes, have become relevant, possibly explaining the failure of clinical interventions targeting these risk factors. Therefore, modifiable risk factors being targeted for interventions, such as weight gain and glycemic control should be addressed in the context of early placental development. In addition, as effective, early prediction of macrosomia would improve patient counseling and allow for clinical trials studying interventions to target those pregnancies at greatest risk of these outcomes, further research should focus on other potential predictors of these outcomes so that a clinically reliable prediction model can be generated.
Highlights.
Early placental size is significantly associated with fetal overgrowth
Macrosomia and LGA are already determined in part in the first trimester
Sonographic placental measures may have a role in early prediction of macrosomia
Acknowledgments
Funding for this work was provided by:
1R03HD069742-01A1 (NS)
The Penn Presbyterian George L. and Emily McMichael Harrison Fund for Research in Obstetrics and Gynecology (NS)
Abbreviations
- LGA
Large for gestational age
- MCD
mean chorionic diameter
- MPD
mean placental diameter
- PQ
placental quotient (defined as: placental volume/gestational age in days)
- PV
placental volume
- SGA
small for gestational age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boulet SL, Salihu HM, Alexander GR. Mode of delivery and birth outcomes of macrosomic infants. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2004 Sep;24(6):622–9. doi: 10.1080/01443610400007828. [DOI] [PubMed] [Google Scholar]
- 2.Modanlou HD, Dorchester WL, Thorosian A, Freeman RK. Macrosomia--maternal, fetal, and neonatal implications. Obstet Gynecol. 1980 Apr;55(4):420–4. [PubMed] [Google Scholar]
- 3.Spellacy WN, Miller S, Winegar A, Peterson PQ. Macrosomia--maternal characteristics and infant complications. Obstet Gynecol. 1985 Aug;66(2):158–61. [PubMed] [Google Scholar]
- 4.Walsh JM, Kandamany N, Ni Shuibhne N, Power H, Murphy JF, O’Herlihy C. Neonatal brachial plexus injury: comparison of incidence and antecedents between 2 decades. Am J Obstet Gynecol. 2011 Apr;204(4):324, e1–6. doi: 10.1016/j.ajog.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the united states: determinants, outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003 May;188(5):1372–8. doi: 10.1067/mob.2003.302. [DOI] [PubMed] [Google Scholar]
- 6.Gregory KD, Henry OA, Ramicone E, Chan LS, Platt LD. Maternal and infant complications in high and normal weight infants by method of delivery. Obstet Gynecol. 1998 Oct;92(4 Pt 1):507–13. doi: 10.1016/s0029-7844(98)00224-5. [DOI] [PubMed] [Google Scholar]
- 7.Lipscomb KR, Gregory K, Shaw K. The outcome of macrosomic infants weighing at least 4500 grams: Los Angeles County + University of Southern California experience. Obstet Gynecol. 1995 Apr;85(4):558–64. doi: 10.1016/0029-7844(95)00005-C. [DOI] [PubMed] [Google Scholar]
- 8.Harder T, Roepke K, Diller N, Stechling Y, Dudenhausen JW, Plagemann A. Birth weight, early weight gain, and subsequent risk of type 1 diabetes: systematic review and meta-analysis. Am J Epidemiol. 2009 Jun 15;169(12):1428–36. doi: 10.1093/aje/kwp065. [DOI] [PubMed] [Google Scholar]
- 9.Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reproductive toxicology. 2011 Sep;32(2):205–12. doi: 10.1016/j.reprotox.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Tian JY, Cheng Q, Song XM, Li G, Jiang GX, Gu YY, et al. Birth weight and risk of type 2 diabetes, abdominal obesity and hypertension among Chinese adults. European journal of endocrinology/European Federation of Endocrine Societies. 2006 Oct;155(4):601–7. doi: 10.1530/eje.1.02265. [DOI] [PubMed] [Google Scholar]
- 11.Wei JN, Sung FC, Li CY, Chang CH, Lin RS, Lin CC, et al. Low birth weight and high birth weight infants are both at an increased risk to have type 2 diabetes among schoolchildren in taiwan. Diabetes care. 2003 Feb;26(2):343–8. doi: 10.2337/diacare.26.2.343. [DOI] [PubMed] [Google Scholar]
- 12.Yu ZB, Han SP, Zhu GZ, Zhu C, Wang XJ, Cao XG, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011 Jul;12(7):525–42. doi: 10.1111/j.1467-789X.2011.00867.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferber A. Maternal complications of fetal macrosomia. Clin Obstet Gynecol. 2000 Jun;43(2):335–9. doi: 10.1097/00003081-200006000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Salafia CM, Charles AK, Maas EM. Placenta and fetal growth restriction. Clin Obstet Gynecol. 2006 Jun;49(2):236–56. doi: 10.1097/00003081-200606000-00007. Epub 2006/05/25. eng. [DOI] [PubMed] [Google Scholar]
- 15.Salafia CM, Maas E, Thorp JM, Eucker B, Pezzullo JC, Savitz DA. Measures of placental growth in relation to birth weight and gestational age. Am J Epidemiol. 2005 Nov 15;162(10):991–8. doi: 10.1093/aje/kwi305. Epub 2005/09/30. eng. [DOI] [PubMed] [Google Scholar]
- 16.Salafia CM, Zhang J, Charles AK, Bresnahan M, Shrout P, Sun W, et al. Placental characteristics and birthweight. Paediatr Perinat Epidemiol. 2008 May;22(3):229–39. doi: 10.1111/j.1365-3016.2008.00935.x. Epub 2008/04/23. eng. [DOI] [PubMed] [Google Scholar]
- 17.Biswas S, Ghosh SK. Gross morphological changes of placentas associated with intrauterine growth restriction of fetuses: a case control study. Early Hum Dev. 2008 Jun;84(6):357–62. doi: 10.1016/j.earlhumdev.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Salafia CM, Vintzileos AM, Silberman L, Bantham KF, Vogel CA. Placental pathology of idiopathic intrauterine growth retardation at term. American journal of perinatology. 1992 May;9(3):179–84. doi: 10.1055/s-2007-999316. [DOI] [PubMed] [Google Scholar]
- 19.Hafner E, Metzenbauer M, Hofinger D, Munkel M, Gassner R, Schuchter K, et al. Placental growth from the first to the second trimester of pregnancy in SGA-foetuses and pre-eclamptic pregnancies compared to normal foetuses. Placenta. 2003 Apr;24(4):336–42. doi: 10.1053/plac.2002.0918. Epub 2003/03/27. eng. [DOI] [PubMed] [Google Scholar]
- 20.Hafner E, Philipp T, Schuchter K, Dillinger-Paller B, Philipp K, Bauer P. Second-trimester measurements of placental volume by three-dimensional ultrasound to predict small-for-gestational-age infants. Ultrasound Obstet Gynecol. 1998 Aug;12(2):97–102. doi: 10.1046/j.1469-0705.1998.12020097.x. Epub 1998/09/23. eng. [DOI] [PubMed] [Google Scholar]
- 21.McGinty P, Farah N, Dwyer VO, Hogan J, Reilly A, Turner MJ, et al. Ultrasound assessment of placental function: the effectiveness of placental biometry in a low-risk population as a predictor of a small for gestational age neonate. Prenat Diagn. 2012 Jul;32(7):620–6. doi: 10.1002/pd.3870. [DOI] [PubMed] [Google Scholar]
- 22.Odibo AO, Goetzinger KR, Huster KM, Christiansen JK, Odibo L, Tuuli MG. Placental volume and vascular flow assessed by 3D power Doppler and adverse pregnancy outcomes. Placenta. 2011 Mar;32(3):230–4. doi: 10.1016/j.placenta.2011.01.010. Epub 2011/02/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proctor LK, Toal M, Keating S, Chitayat D, Okun N, Windrim RC, et al. Placental size and the prediction of severe early-onset intrauterine growth restriction in women with low pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 2009 Sep;34(3):274–82. doi: 10.1002/uog.7308. Epub 2009/08/13. eng. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz N, Coletta J, Pessel C, Feng R, Timor-Tritsch IE, Parry S, et al. Novel 3-dimensional placental measurements in early pregnancy as predictors of adverse pregnancy outcomes. J Ultrasound Med. 2010 Aug;29(8):1203–12. doi: 10.7863/jum.2010.29.8.1203. Epub 2010/07/28. eng. [DOI] [PubMed] [Google Scholar]
- 25.Toal M, Chan C, Fallah S, Alkazaleh F, Chaddha V, Windrim RC, et al. Usefulness of a placental profile in high-risk pregnancies. Am J Obstet Gynecol. 2007 Apr;196(4):363, e1–7. doi: 10.1016/j.ajog.2006.10.897. Epub 2007/04/04. eng. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz N, Wang E, Parry S. Two-dimensional sonographic placental measurements in the prediction of small-for-gestational-age infants. Ultrasound Obstet Gynecol. 2012 Dec;40(6):674–9. doi: 10.1002/uog.11136. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz N, Sammel MD, Leite R, Parry S. First-trimester placental ultrasound and maternal serum markers as predictors of small-for-gestational-age infants. Am J Obstet Gynecol. 2014 Mar 4; doi: 10.1016/j.ajog.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludbrook J. Confidence in Altman-Bland plots: a critical review of the method of differences. Clinical and experimental pharmacology & physiology. 2010 Feb;37(2):143–9. doi: 10.1111/j.1440-1681.2009.05288.x. [DOI] [PubMed] [Google Scholar]
- 29.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996 Feb;87(2):163–8. doi: 10.1016/0029-7844(95)00386-X. Epub 1996/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 30.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982 Apr;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. Epub 1982/04/01. eng. [DOI] [PubMed] [Google Scholar]
- 31.Hermann GM, Dallas LM, Haskell SE, Roghair RD. Neonatal macrosomia is an independent risk factor for adult metabolic syndrome. Neonatology. 2010;98(3):238–44. doi: 10.1159/000285629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ACOG Practice Bulletin No. 22: Fetal Macrosomia. 2000, reaffirmed in 2013.
- 33.Mu M, Wang SF, Sheng J, Zhao Y, Li HZ, Hu CL, et al. Birth weight and subsequent blood pressure: a meta-analysis. Archives of cardiovascular diseases. 2012 Feb;105(2):99–113. doi: 10.1016/j.acvd.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Johnson J, Clifton RG, Roberts JM, Myatt L, Hauth JC, Spong CY, et al. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine guidelines. Obstet Gynecol. 2013 May;121(5):969–75. doi: 10.1097/AOG.0b013e31828aea03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romon M, Nuttens MC, Vambergue A, Verier-Mine O, Biausque S, Lemaire C, et al. Higher carbohydrate intake is associated with decreased incidence of newborn macrosomia in women with gestational diabetes. Journal of the American Dietetic Association. 2001 Aug;101(8):897–902. doi: 10.1016/S0002-8223(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 36.Waters TP, Huston-Presley L, Catalano PM. Neonatal body composition according to the revised institute of medicine recommendations for maternal weight gain. J Clin Endocrinol Metab. 2012 Oct;97(10):3648–54. doi: 10.1210/jc.2012-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owe KM, Nystad W, Bo K. Association between regular exercise and excessive newborn birth weight. Obstet Gynecol. 2009 Oct;114(4):770–6. doi: 10.1097/AOG.0b013e3181b6c105. [DOI] [PubMed] [Google Scholar]
- 38.Walsh JM, McGowan CA, Mahony R, Foley ME, McAuliffe FM. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): randomised control trial. BMJ. 2012;345:e5605. doi: 10.1136/bmj.e5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poon LC, Karagiannis G, Stratieva V, Syngelaki A, Nicolaides KH. First-trimester prediction of macrosomia. Fetal diagnosis and therapy. 2011;29(2):139–47. doi: 10.1159/000318565. [DOI] [PubMed] [Google Scholar]
- 40.Plasencia W, Gonzalez Davila E, Tetilla V, Padron Perez E, Garcia Hernandez JA, Gonzalez Gonzalez NL. First-trimester screening for large-for-gestational-age infants. Ultrasound Obstet Gynecol. 2012 Apr;39(4):389–95. doi: 10.1002/uog.9060. [DOI] [PubMed] [Google Scholar]


