Abstract
Aims
Continuous infusion of prostacyclin analogues improves survival in advanced pulmonary arterial hypertension. In addition to its vasodilatory effects, prostacyclin has the potential to decrease inflammation, thrombosis, and smooth muscle proliferation. The aim of this retrospective study was to determine whether pathological data support the ability of prostanoids to prevent progression of vascular disease.
Methods and results
Twenty-two autopsied patients with World Health Organization category 1 pulmonary arterial hypertension (primarily idiopathic and connective tissue disease-associated) were divided into those who received long-term prostacyclin (n = 12, PG-long, mean treatment 3.9 years) and those who received 0–1 month of prostacyclin (n = 10, PG-short). Surprisingly, PG-long patients had larger plexiform lesions (P < 0.05), with no decrease in medial and intimal thicknesses as compared with PG-short patients. Plexiform lesion size and density increased with increasing treatment time. Also, PG-long patients had fewer platelet thrombi and more frequent acute diffuse alveolar haemorrhage. Quantification of macrophages and T cells revealed no differences in inflammatory infiltrates.
Conclusions
Although long-term prostacyclin therapy may have an antithrombotic effect in addition to its vasodilatory actions, it was not associated with the prevention of advanced vascular lesions. The mechanism by which prostacyclin analogues improve survival in pulmonary arterial hypertension remains uncertain.
Keywords: histopathology, plexiform lesions, prostaglandins, vascular remodelling
Introduction
The pulmonary arteries of patients with pulmonary arterial hypertension (PAH) demonstrate plexiform lesions, muscularization of arterioles, concentric intimal thickening and medial hypertrophy of arteries,1,2 and thrombosis in situ with platelet aggregates.3 Development of these lesions is thought to involve endothelial dysfunction, increased proliferation and impaired apoptosis of pulmonary artery smooth muscle cells (PASMCs), inflammatory cell infiltration, and increased deposition of extracellular matrix.4
Prostacyclin analogues are approved therapies for advanced PAH. In addition to vasodilatation, prostacyclin has anti-thrombotic and anti-inflammatory properties,5,6 and an anti-proliferative effect on PASMCs in vitro.7 These effects may contribute to the long-term benefits of prostacyclin, the only agent proven to increase survival in PAH.5 However, there has been little histopathological examination of its effects, which is relevant to the emerging recognition that prostacyclins may have beneficial effects on the right ventricle despite persistence of pulmonary vascular disease.8,9
Despite the use of the Heath–Edwards grading system to evaluate the potential reversibility of pulmonary hypertension in congenital heart disease, there are limited data regarding the regression of pulmonary vascular lesions and their timing. In children with congenital shunts, reductions in arterial wall thickness have been demonstrated several years after pulmonary artery banding.10,11 Two case studies have reported partial regression of idiopathic PAH following single lung transplantation, and one of them histologically demonstrated regression of vascular lesions.12,13 In rats, regression of arterial changes induced by hypoxia begins within 1 week of normoxia, but is not complete even after 1 month.14,15Although prostacyclin does not reverse arterial changes in rats,16 a variety of other therapies do. These include inhibitors of pyruvate dehydrogenase kinase, agents that enhance the nitric oxide pathway, and multikinase inhibitors.4 One human study in PAH found no effect of prostacyclin on plexiform lesions or arterial wall thickness.17
On the basis of a recent case report in which we noted an excellent 18-year response to eproprostenol in a patient despite severe arteriopathy, we postulated that prostacyclin might not protect against the progression of the underlying pulmonary vascular disease, and that prolonged survival might be attributable to beneficial effects on right ventricular function.18 We now report a retrospective analysis of autopsied patients with World Health Organization (WHO) category 1 PAH in which we compare the histological features of 21 additional patients who were treated with prostacyclin with those of patients who were not treated or were treated for less than 1 month (partial results were reported in an abstract19).
Materials and methods
PAH patients were identified from autopsy records (1982–2009), with additional data being obtained from clinical records. Cases with significant coexisting interstitial lung disease were excluded. All pulmonary slides (4–20 slides per case, average 11.4) were reviewed and scored on a blinded basis. Arterial intimal fibrosis was scored as follows: 0, none; 1, rare (<1 per slide); 2, occasional; and 3, frequent (>50% of vessels). Recanalization was scored as follows: 0, none; 1, rare (<1 per two slides); 2, occasional; and 3, frequent (>1 per slide). Plexiform lesion size was determined by multiplying the shortest and longest diameters (average of 24 lesions per case). Their association with prealveolar vessels was determined as previously described.20
Representative elastic stained slides (average 3.1 slides per case; 22 arteries per case) were used for arterial measurements on arteries of 100–400 μm cut in cross-section (circular or nearly circular). Measurements were performed across the shortest diameter.11
One representative slide was selected for immunohistochemistry and Prussian blue staining. Immunohistochemistry for CD3 (SP7; Neomarkers, Fremont, CA, USA) and CD68 (KP-1; Dako, Carpintera, CA, USA) was performed with the DakoCytomation Envision+ horseradish peroxidase system. Immunostaining for CD61 (clone Y2/51l) was performed following antigen retrieval in cell conditioning solution 2 for 30 min, with the Ventana BenchMark XT Instrument (Ventana Medical Systems, Tucson, AZ, USA), according to the manufacturer’s protocol, with the UltraView Universal DAB kit.
CD3 and CD68 staining was quantified with the Automated Cellular Imaging System (ACIS) (Clarient, San Juan Capistrano, CA, USA). Colour-specific thresholds were set on the ACIS to determine brown positivity. The average number of brown pixels per lymphocyte or macrophage was calculated on the basis of at least two areas with manually counted cells that were used to convert pixels to estimated cell number. The involved arteries and lesions were outlined, including the adventitia and excluding intravascular spaces. For adventitia continuous with bronchial adventitia, the adventitia was evenly divided.
STATISTICAL ANALYSIS
Because of small sample sizes, non-parametric analysis was performed with the Mann–Whitney test to compare groups, with box plots showing median and first and third quartiles. Spearman’s correlation was used to evaluate the relationship between treatment time and vascular morphology parameters. Testing for haemorrhage was performed with Fisher’s exact test. A P-value of <0.05 was considered to be statistically significant.
Results
Lungs from 22 autopsied patients were analysed (Tables 1 and S1). Complete clinical data were not always available, particularly for untreated patients who were transferred from other institutions in a critical condition. There were six patients with idiopathic PAH, six with scleroderma-associated-PAH, five with other types of connective tissue disease-associated PAH, and five additional cases, including human immunodeficiency virus-associated, cirrhosis-associated or anorexigen-associated PAH, and one patient with pulmonary capillary haemangiomatosis (PCH). In the majority of patients, the immediate cause of death was acute right heart failure or sepsis. Survival on prostacyclin ranged from 4 days to 18 years. As vascular remodelling is not expected to be a quick process (taking more than 1 month to occur, on the basis of rodent studies), we grouped patients who had been treated for less than 1 month with untreated patients (PG-short, n = 10), and compared them with patients treated for 1 month or more (PG-long, n = 12, mean treatment time 47 ± 63 months). Approximately half of the patients in each group had been treated with additional agents. All patients except three were female, and the average age (47 versus 42 years) was similar. The mean pulmonary artery pressure was not significantly different (PG-long 52.6 versus PG-short 60 mmHg), although pulmonary vascular resistance was higher in PG-short patients (21.1 versus 11.8 units, P = 0.016).
Table 1.
Clinical characteristics of autopsied patients
| Prostaglandin status |
Demographics | Other therapy | Hemodynamics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| PG* >1mo |
PG Time |
Etiology of PAH |
Age | Sex | Duration of PAH |
Cause of death |
Other PAH treatment |
War farin |
Other coagulation abnormalities/ Antithrombotic treatments |
PAP† | PVR‡ | |
| 1 | Yes | 18 years | Idiopathic | 53 | F | 23 years | Colon cancer |
none | Yes | Thrombo- cytopenia |
63 | 8 |
| 2 | Yes | 7 years | Anorexigen | 44 | F | 10 years | RV failure | Sildenafil x 3 years |
Yes | None | 54 | N/A§ |
| 3 | Yes | 6 years | Idiopathic | 48 | F | 7 years | RV failure | Sildenafil x 6 years |
Yes | None | 59 | 16 |
| 4 | Yes | 6 years | Idiopathic | 29 | F | 7 years | RV failure | Sildenafil x 1 year |
Yes | None | 52 | 11 |
| 5 | Yes | 5 years | Lupus | 48 | F | 5 years | RV failure | None | Yes | None | 54 | 16 |
| 6 | Yes | 1 year | Anorexigen | 48 | F | N/A | RV failure | None | No | Thrombo - cytopenia |
75 | 21 |
| 7 | Yes | 1 year | Cirrhosis | 52 | F | 8 years | sepsis | Sildenafil x3 years |
No | None | 43 | 3 |
| 8 | Yes | 10 months | Scleroderma | 55 | F | 3 years | RV failure | None | No | None | 49 | 11 |
| 9 | Yes | 7 months | Idiopathic | 51 | M | 3.5 years | RV failure | None | No | None | 53 | 13 |
| 10 | Yes | 6 months | Scleroderma | 56 | M | 3 years | RV failure | Sildenafil x 2 years |
No | None | 46 | N/A |
| 11 | Yes | 5 months | Scleroderma | 48 | F | 10 months | RV failure | Sildenafil x 6 months Bosentan x 9 months |
No | None | 40 | 9 |
| 12 | Yes | 3 months | PCH | 30 | F | 7 months | sepsis | None | Yes | None | 43 | 11 |
| 13 | No | 2 weeks | Scleroderma | 63 | F | 1 year | RV failure | Sildenafil x 5 months |
None | 52 | 19 | |
| 14 | No | 1 weeks | Sjogren’s syndrome | 45 | F | 6 months | RV failure | none | No | Hirudin, thrombo- cytopenia |
55 | 15 |
| 15 | No | 4 days | Scleroderma | 72 | F | 1 year | RV failure | Bosentan x 6 months |
No | None | 67 | 32 |
| 16 | No | 0 | Idiopathic | 27 | F | N/A | RV failure | none | No | Heparin | N/A | N/A |
| 17 | No | 0 | Idiopathic | 47 | F | 7 years | RV failure | None | Yes | None | N/A | N/A |
| 18 | No | 0 | Lupus | 54 | F | 1 year | RV failure | Bosentan x 3 months Sildenafil x 3 months |
Yes | None | N/A | N/A |
| 19 | No | 0 | Lupus, HIV, cirrhosis | 26 | M | N/A | RV failure | None | No | Aspirin, thrombo- cytopenia |
N/A | N/A |
| 20 | No | 0 | MCTD | 34 | F | N/A | sepsis | none | No | IVC filter | N/A | N/A |
| 21 | No | 0 | HIV | 3 | F | N/A | sepsis | None | No | Intermittent thrombo - cytopenia and coagulopathy |
N/A | N/A |
| 22 | No | 0 | Scleroderma | 53 | F | 1 year | RV failure | Sildenafil x 3 months |
Yes | Plavix | 66 | 18 |
PG = prostaglandin,
PAP = mean pulmonary artery pressure (mm Hg),
PVR = pulmonary vascular resistance (Wood units),
N/A = not available
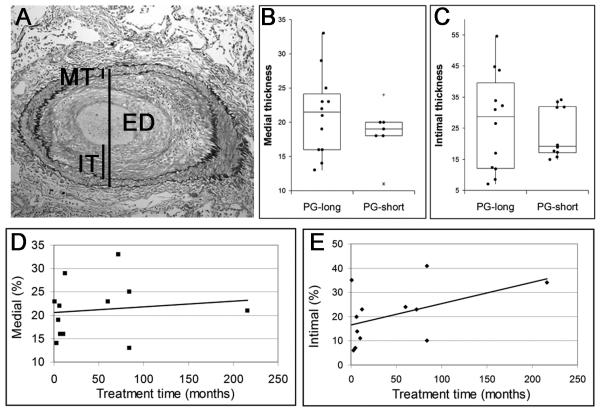
Although most patients had occasional or frequent intimal fibrosis, recanalization lesions were rare (Table 2). The degree of medial hypertrophy and intimal fibrosis was highly variable. Treated patients had a non-significant increase in medial hypertrophy (P = 0.13) (Figure 1). To further examine the relationship between prostacyclin treatment and arterial remodelling, we used Spearman’s correlation to evaluate the duration of therapy in the PG-long patients. As treatment time increased, PG-long patients showed no decrease in medial thickness and a non-significant trend towards more severe intimal disease (ρ10 = 0.38, P = 0.22) (Figure 1).
Table 2.
Histological findings in autopsied patients
| Plexi form |
Con centric intimal fibrosis |
Eccen -tric intimal fibrosis |
Recanal ization |
Hemorrhage | |
|---|---|---|---|---|---|
| 1 | Yes | 3 | 2 | 1 | Diffuse severe |
| 2 | Yes | 2 | 2 | 1 | Diffuse mild, Focally severe |
| 3 | Yes | 3 | 1 | 1 | Diffuse severe |
| 4 | Yes | 3 | 2 | 0 | Diffuse severe |
| 5 | Yes | 3 | 2 | 1 | Diffuse mild |
| 6 | Yes | 3 | 2 | 1 | Diffuse mild, Focally severe |
| 7 | Yes | 3 | 2 | 1 | Diffuse mild |
| 8 | No | 2 | 2 | 0 | Diffuse mild Focally severe |
| 9 | Yes | 1 | 2 | 1 | Diffuse mild, Focally severe |
| 10 | No | 3 | 2 | 1 | Focally severe |
| 11 | No | 1 | 2 | 0 | Diffuse mild |
| 12 | No | 2 | 3 | 0 | Focally severe |
| 13 | No | 3 | 2 | 0 | Focally mild |
| 14 | Yes | 1 | 2 | 2 | Diffuse severe |
| 15 | No | 3 | 2 | 0 | Focally severe |
| 16 | Yes | 3 | 2 | 0 | None |
| 17 | Yes | 0 | 0 | 0 | Diffuse mild |
| 18 | Yes | 3 | 2 | 2 | None |
| 19 | Yes | 2 | 2 | 0 | None |
| 20 | Yes | 2 | 2 | 1 | None |
| 21 | Yes | 2 | 1 | 3 | Diffuse mild |
| 22 | No | 3 | 1 | 1 | Diffuse mild Focally severe |
Figure 1.
Arterial remodeling. A, Elastic stained muscular artery of a prostacyclin-treated patient with intimal fibrosis showing measurements of medial thickness (MT), intimal thickness (IT), and external diameter (ED). B, Quantification of medial and intimal thicknesses in PG-short and PG-long patients showed no significant difference in medial or intimal thicknesses. With increasing prostacyclin treatment time, there was no decrease in medial thickness (C) and there was a non-significant trend towards increased intimal thickness (D).
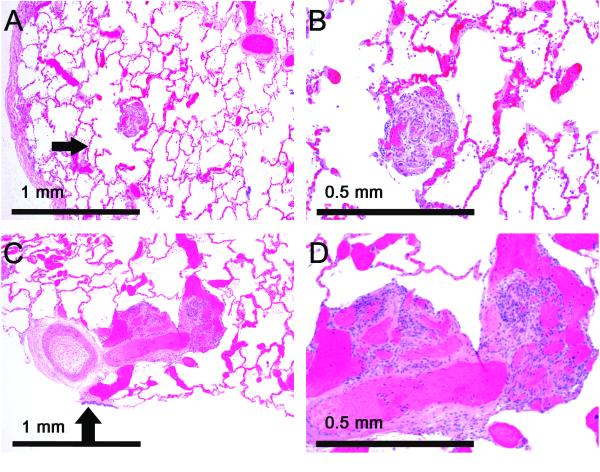
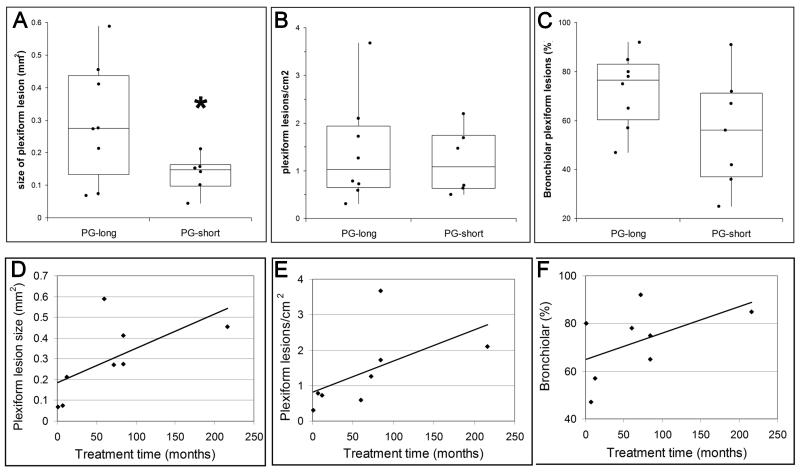
Plexiform lesions were not identified in any of the scleroderma patients, but were frequent in PAH patients with other connective tissue disorders, and were present in every case of idiopathic PAH. Classical small plexiform lesions in distal arteries (Figure 2) were seen in untreated patients. Treated patients frequently had much larger lesions with large dilatations associated with more proximal vessels (Figure 2). Quantification revealed that PG-long patients had significantly larger lesions (P = 0.040), although there was no increase in lesion density (P = 0.61) (Figure 3). Although it has been reported that plexiform lesions in idiopathic PAH are usually located in distal vessels rather than in prealveolar (bronchiolar or supernumerary) arteries,20 we found that almost all patients had more lesions associated with prealveolar arteries, with a non-significant increase in prealveolar lesions in PG-long patients (P = 0.12) (Figure 3). To further examine the relationship between prostacyclin treatment and plexiform lesions, we used Spearman’s correlation to evaluate the duration of therapy in the PG-long patients. Surprisingly, plexiform lesion size (ρ6 = 0.75, P = 0.031) and density (ρ6 = 0.86, P = 0.006) significantly increased with longer treatment time, whereas the increase in bronchiolar vessel location (ρ6 = 0.32, P = 0.43) was not significant (Figure 3).
Figure 2.
Patients treated with prostacyclin have large, proximal vascular lesions. A,B, Small, distal plexiform lesion in an untreated patient. The lesion is alongside an alveolar duct (arrow). C,D, Large, prealveolar plexiform lesion in a prostacyclin-treated patient. The lesion is at a small artery branching off a larger artery accompanying a bronchiole (arrow).
Figure 3.
Effect of prostacyclin treatment on plexiform lesions. A, Prostacyclin-treated patients had significantly (*P = 0.040) larger plexiform lesions. B, Plexiform lesion density in prostacyclin-treated patients was not significantly different from that in untreated patients. C, Plexiform lesions in prostacyclin-treated patients were not significantly more likely to be associated with more proximal vessels. D, Plexiform lesions were significantly larger with increasing treatment time (P = 0.031). E, Plexiform lesion density significantly increased with increasing treatment time (P = 0.006). F, Plexiform lesion association with bronchiolar arteries showed a non-significant increase with treatment time.
To exclude the possibility that patients who died of causes other than acute right heart failure might have less severe disease, we also repeated the analysis with inclusion only of patients dying of right ventricular failure. We found a similar increase in plexiform size in PG-long patients, although the difference was no longer significant (P = 0.158), probably because of a smaller sample size (data not shown). Similar results were obtained after exclusion of the patients with 4–14 days of prostacyclin exposure from the PG-short group (n = 7; P = 0.062, data not shown). There were no significant differences between patients with idiopathic disease and those with connective tissue disease (Figure S1).
In order to exclude the possibility that treatment times of less than 1 year might be insufficient to demonstrate regression, we repeated the analysis with the PG-long group restricted to patients treated for at least 1 year (n = 7), and found that the median intimal and medial thicknesses were greater than in the extended PG-long group (Figure S2), consistent with the gradual trend seen with increasing treatment time (Figure 1C,D).
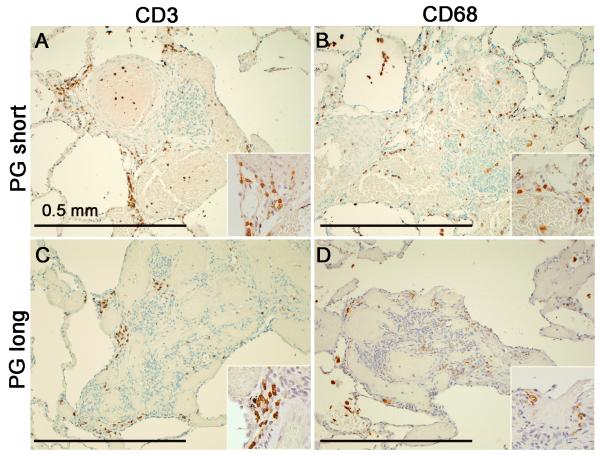
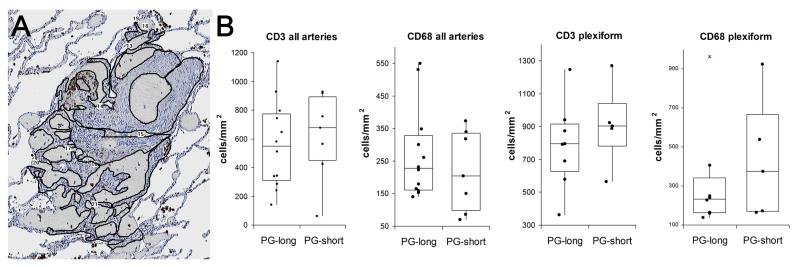
Similar to previous reports21 of inflammation in PAH, we found that most of the inflammatory cells were CD3-positive T cells and CD68-positive macrophages (Figure 4). Only rare CD20-positive B cells (data not shown) were present. Quantification with the ACIS (Figure 5) showed no significant differences in CD3 or CD68 staining between PG-short and PG-long patients in either plexiform lesions or in the affected arterial walls (Figure 5). Similar results were obtained when patients treated for less than 1 month were excluded from the PG-short group (data not shown). There were no significant differences between patients with idiopathic and connective tissue disease-associated PAH (Figure S1).
Figure 4.
Inflammation in pulmonary arterial hypertension, ×100. A,B, CD3-positive T cells (A) and CD68-positive macrophages (B) in an untreated PAH patient. C,D, CD3-positive T cells (C) and CD68-positive macrophages (D) in a prostacyclin-treated patient.
Figure 5.
Quantification of arterial inflammation. A, Outline of plexiform lesion in the Automated Cellular Imaging System (ACIS) to select areas for quantification. B, Quantification of inflammation showed no significant difference between PG-short and PG-long patients.
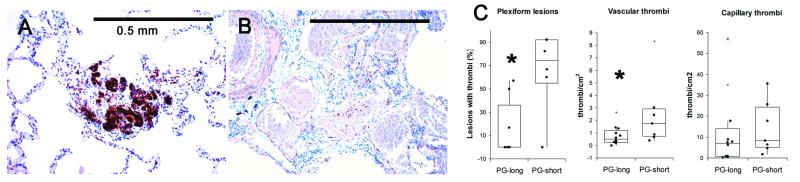
To evaluate platelet aggregation within the pulmonary vasculature, we performed immunohistochemistry for CD61 (Figure 6). PG-long patients had significantly fewer platelet thrombi within plexiform lesions (P = 0.021) and within arteries or veins (P = 0.013); however, there was no significant effect on aggregates within capillaries (P = 0.15) (Figure 6). The comparison remained significant when patients treated for less than 1 month were excluded from the PG-short group (data not shown).
Figure 6.
Effect of prostacyclin on platelet aggregates. A, CD61 immunohistochemistry highlights platelet aggregates within a plexiform lesion of an untreated patient. B, CD61 immunohistochemistry shows no platelet aggregates in a plexiform lesion from a prostacyclin-treated patient. C, Quantification of platelet aggregates shows significantly fewer aggregates in plexiform lesions and vessels, but no significant increase in capillary aggregates in PG-long patients as compared with PG-short patients.
Chronic haemorrhage was evaluated with a Prussian blue stain, which did not demonstrate increased haemosiderin-laden macrophages in PG-long patients (data not shown); however, all PG-long patients had at least some acute haemorrhage, and most (10/12) had diffuse haemorrhage (Table 2). In contrast, significantly fewer PG-short patients had diffuse haemorrhage (3/10, P = 0.027), and three had no haemorrhage at all. Severe diffuse pulmonary oedema was seen only in one prostacyclin-treated case.
Discussion
Prostacyclin is an effective therapy in PAH; however, its mechanisms of action have seldom been evaluated on a histopathological basis. We examined autopsy cases of PAH, and showed that prostacyclin use was not associated with a demonstrable reduction or prevention of arterial remodelling or arterial inflammatory cells. Prostacyclin was associated with increased acute alveolar haemorrhage and decreased platelet aggregates.
Given the good response to treatment, the impressive pulmonary vascular remodelling found at autopsy was unexpected. We found a correlation between treatment time and increasing plexiform lesion size in two dimensions, and in density. Unlike three-dimensional modelling of plexiform lesions,22 a two-dimensional estimate does not determine whether a particular lesion is genuinely small or merely appears small because of sectioning through the periphery. However, when averaged across many randomly sectioned lesions, it provides a non-biased size estimate. Prostacyclin treatment time closely correlated with duration of disease (Table 1), suggesting that lesion severity could reflect the underlying progression of the disease during the prolonged lifespan of these patients. Our findings are consistent with those of a previous study that found no changes in arterial remodelling in prostacyclin-treated patients, although the authors suggested that their cohort might represent a group that responded less favourably to prostacyclin.17 However, the overall survival of PAH patients, even when optimally treated, is only 58% at 3 years,23 and our group included several patients with prolonged survival, some of whom died of causes other than right heart failure.
A role for inflammation in the pathology of PAH is suggested by its association with connective tissue diseases and by the presence of macrophages and T lymphocytes within arterial walls.21,24 Although no large-scale clinical trial has evaluated immunosuppression as a treatment for PAH,25 some dramatic responses have been seen in patients with connective tissue disease-associated PAH.26 In vitro, prostacyclin decreases monocyte production of inflammatory cytokines.27 In paediatric patients, prostacyclin and an endothelin receptor antagonist decreased expression of HLA-DR by endothelial cells,28 probably by decreasing the levels of cytokines promoting endothelial activation and inflammatory cell recruitment.
Despite these potential anti-inflammatory effects, a previous study found generally increased pulmonary inflammation in prostacyclin-treated patients,17 and another group found increased T cells in the capillaries of prostacyclin-treated PAH patients as compared with healthy controls.29 We specifically quantified arterial wall inflammation, and found no effect in treated patients. However, prostacyclin might influence inflammation in other compartments of the lung, or could affect the relative proportions of T-cell subsets or their activities. The role of inflammation is likely to vary with the aetiology of PAH; therefore, in the absence of a more comprehensive understanding, it will be difficult to assess the effects of prostacyclin on this aspect of the disease.
Platelet aggregates can be seen in plexiform lesions, and multiple circulating markers of endothelial and platelet function are abnormal in PAH patients.30 The contribution of these aggregates to PAH pathology is unclear, although platelets have been increasingly recognized to have a role in vascular repair and inflammation in the lung.31 Prostacyclin inhibits platelet aggregation32 and can normalize platelet function values in PAH.33 It has not been established whether prostacyclin affects thrombosis within the pulmonary vasculature. We found that PG-long patients had fewer thrombi in plexiform lesions. Both PG-long and PG-short patients were anticoagulated (unless they had a contraindication), and thus any differential effect on platelet accumulation in the vasculature probably relates to prostacyclin. However, accumulation of platelet aggregates is relatively common in autopsies, and might reflect terminal events rather than a stable state in untreated patients.
It has been reported that prostacyclin treatment increases acute and chronic alveolar haemorrhage and pulmonary oedema.17 We found that prostacyclin-treated patients frequently had focal to diffuse severe haemorrhage, but they rarely had many haemosiderin-laden macrophages, suggesting that haemorrhage was a relatively acute preterminal phenomenon. In the case of PG-long patients, this might be attributable to prostacyclin or to increased warfarin use in this subset of patients, but it is unlikely to represent a characteristic of prostacyclin treatment in patients who are otherwise stable.
None of the parameters evaluated provide evidence for an effect of prostacyclin on the pulmonary vasculature beyond its immediate vasodilatory effect. Although it remains possible that prostacyclin has some effect on slowing the progression of the underlying disease, there is no way to document this short of serial open lung biopsy, which is not the standard of care in adult PAH. There were no previous biopsies available for any of our patients. Recently, it has been shown that right heart failure in PAH may be attributable to more than just elevated pulmonary arterial pressures.34 Animal models show decreased capillaries in the right ventricle, and this can be prevented by treatment with prostacyclin.9,34It is therefore possible that, in addition to acute vasodilatory effects, prostacyclin can prolong survival through direct effects on the myocardium. This should be addressed in future studies.
There are important limitations to this retrospective study that need to be highlighted. The number of cases analysed was relatively small, as is common in histopathological reports of PAH,11,17,20,21,28 and we included patients with varied aetiologies of WHO category 1 PAH (Table 1). This group is defined by disease directly affecting the pulmonary arteries, and, with the exception of PCH, also includes patients thought to fall in the ‘plexogenic’ pathological category of pulmonary hypertension. Thus, with few patients in each subcategory, we cannot determine to what extent this might contribute to variability in the response. Although recent reports suggest that scleroderma-associated PAH is histologically distinct from other types of PAH,35 and rarely has plexiform lesions,2,21,36 it continues to be classified as WHO category 1, and, in this study, scleroderma patients were equally divided between the two groups. Because of the limited sample size, we also could not evaluate the effects of other vasodilators for PAH.
Full clinical data were not always available. Where available, right heart catheterization data supported the diagnosis of PAH, but in this retrospective study the time between catheterization and death was highly variable, and may not reflect the severity of disease at death. Moreover, previous studies have shown that there is no strong association between histological findings and haemodynamics.37 However, as all patients had advanced pulmonary hypertension, which was confirmed at autopsy, it is reasonable to assume that a cardiac catheterization would have led to similarly marked elevations in pulmonary arterial pressure and resistance.
Our finding of increased acute pulmonary haemorrhage should be interpreted cautiously, given that these are autopsy samples, and coagulopathies are common in the immediate premortem period. It is also possible that the differences in platelet thrombi reflect differences in individuals’ end-stage courses rather than a treatment effect, and might not be seen during stable disease.
Conclusions
In summary, our observations argue against prostacyclin-induced prevention or reversal of vascular arteriopathy as the basis for its clinical benefits in PAH. Indeed, patients successfully treated with prostacyclin have more severe arterial remodelling at autopsy, possibly because of the longer survival, which allowed the disease to progress. Prostacyclin-treated patients had fewer platelet thrombi than untreated patients and more severe acute haemorrhage. Although intravenous prostacyclin remains the most effective treatment for PAH, it appears that it does not work by protecting against the development of advanced pulmonary vascular obstruction. It is more likely that its benefit relates to its effects on pulmonary vascular tone and right ventricular function. Indeed, prostacyclin has been used as an inotrope in the treatment of left heart failure.38,39New therapies need to be developed that are targeted at effectively halting the progression or inducing regression of the vascular disease in PAH.
Supplementary Material
Acknowledgements
S. L. Archer is supported by the NIH (RO1-HL071115) and (1RC1HL099462-01), the American Heart Association (AHA) and the Roche Foundation for Anemia Research, and has received grand rounds honoraria at various universities.
Abbreviations
- ACIS
automated cellular imaging system
- PAH
pulmonary arterial hypertension
- PASMC
pulmonary artery smooth muscle cell
- PCH
pulmonary capillary haemangiomatosis
- WHO
World Health Organization
Footnotes
Authorship J. E. Pogoriler contributed to conception and design, data acquisition and analysis, and writing the manuscript. S. Rich contributed to conception and design, data acquisition, and drafting the manuscript for important intellectual content. S. L. Archer contributed to conception and design, data analysis, and drafting the manuscript for important intellectual content. A.N. Husain contributed to conception and design, data acquisition and analysis, and drafting the manuscript for important intellectual content, and is responsible for the final approved version of the manuscript.
J. E. Pogoriler, S. Rich and A. N. Husain have no conflicts of interest.
References
- 1.Stewart S, Rassl D. Advances in the understanding and classification of pulmonary hypertension. Histopathology. 2009;54:104–116. doi: 10.1111/j.1365-2559.2008.03180.x. [DOI] [PubMed] [Google Scholar]
- 2.Pietra GG, Capron F, Stewart S, et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J. Am. Coll. Cardiol. 2004;43(12 Suppl. S):25S–32S. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation. 1984;70:580–587. doi: 10.1161/01.cir.70.4.580. [DOI] [PubMed] [Google Scholar]
- 4.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N. Engl. J. Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 6.Murata T, Ushikubi F, Matsuoka T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 7.Olschewski H, Olschewski A, Rose F, et al. Physiologic basis for the treatment of pulmonary hypertension. J. Lab. Clin. Med. 2001;138:287–297. doi: 10.1067/mlc.2001.119329. [DOI] [PubMed] [Google Scholar]
- 8.Piao L, Fang YH, Cadete VJ, et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J. Mol. Med. 2010;88:47–60. doi: 10.1007/s00109-009-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Albada ME, Berger RM, Niggebrugge M, van Veghel R, Cromme-Dijkhuis AH, Schoemaker RG. Prostacyclin therapy increases right ventricular capillarisation in a model for flow-associated pulmonary hypertension. Eur. J. Pharmacol. 2006;549:107–116. doi: 10.1016/j.ejphar.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Dammann JF, Jr, McEachen JA, Thiompson WM, Jr, Smith R, Muller WH., Jr The regression of pulmonary vascular disease after the creation of pulmonary stenosis. J. Thorac. Cardiovasc. Surg. 1961;42:722–734. [PubMed] [Google Scholar]
- 11.Wagenvoort CA, Wagenvoort N, Draulans-Noe Y. Reversibility of plexogenic pulmonary arteriopathy following banding of the pulmonary artery. J. Thorac. Cardiovasc. Surg. 1984;87:876–886. [PubMed] [Google Scholar]
- 12.Levy NT, Liapis H, Eisenberg PR, Botney MD, Trulock EP. Pathologic regression of primary pulmonary hypertension in left native lung following right single-lung transplantation. J. Heart Lung Transplant. 2001;20:381–384. doi: 10.1016/s1053-2498(00)00153-4. [DOI] [PubMed] [Google Scholar]
- 13.Deb S, Yun J, Burton N, Omron E, Thurber J, Nathan SD. Reversal of idiopathic pulmonary arterial hypertension and allograft pneumonectomy after single lung transplantation. Chest. 2006;130:214–217. doi: 10.1378/chest.130.1.214. [DOI] [PubMed] [Google Scholar]
- 14.Meyrick B, Reid L. Hypoxia-induced structural changes in the media and adventitia of the rat hilar pulmonary artery and their regression. Am. J. Pathol. 1980;100:151–178. [PMC free article] [PubMed] [Google Scholar]
- 15.Hislop A, Reid L. Changes in the pulmonary arteries of the rat during recovery from hypoxia-induced pulmonary hypertension. Br. J. Exp. Pathol. 1977;58:653–662. [PMC free article] [PubMed] [Google Scholar]
- 16.van Albada ME, van Veghel R, Cromme-Dijkhuis AH, Schoemaker RG, Berger RM. Treprostinil in advanced experimental pulmonary hypertension: beneficial outcome without reversed pulmonary vascular remodeling. J. Cardiovasc. Pharmacol. 2006;48:249–254. doi: 10.1097/01.fjc.0000248229.87510.9b. [DOI] [PubMed] [Google Scholar]
- 17.Achcar RO, Yung GL, Saffer H, Cool CD, Voelkel NF, Yi ES. Morphologic changes in explanted lungs after prostacyclin therapy for pulmonary hypertension. Eur. J. Med. Res. 2006;11:203–207. [PubMed] [Google Scholar]
- 18.Rich S, Pogoriler J, Husain AN, Toth PT, Gomberg-Maitland M, Archer SL. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest. 2010;138:1234–1239. doi: 10.1378/chest.09-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pogoriler JE, Rich S, Gomberg-Maitland M, Archer SL, Husain AN. Effect of prostacyclin treatment on lesions of pulmonary arterial hypertension. Mod. Pathol. 2010;23:12A. [Google Scholar]
- 20.Jamison BM, Michel RP. Different distribution of plexiform lesions in primary and secondary pulmonary hypertension. Hum. Pathol. 1995;26:987–993. doi: 10.1016/0046-8177(95)90088-8. [DOI] [PubMed] [Google Scholar]
- 21.Cool CD, Kennedy D, Voelkel NF, Tuder RM. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Hum. Pathol. 1997;28:434–442. doi: 10.1016/s0046-8177(97)90032-0. [DOI] [PubMed] [Google Scholar]
- 22.Cool CD, Stewart JS, Werahera P, et al. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am. J. Pathol. 1999;155:411–419. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 24.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am. J. Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 25.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur. Respir. J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez O, Sitbon O, Jais X, Simonneau G, Humbert M. Immunosuppressive therapy in connective tissue diseases-associated pulmonary arterial hypertension. Chest. 2006;130:182–189. doi: 10.1378/chest.130.1.182. [DOI] [PubMed] [Google Scholar]
- 27.Strassheim D, Riddle SR, Burke DL, Geraci MW, Stenmark KR. Prostacyclin inhibits IFN-gamma-stimulated cytokine expression by reduced recruitment of CBP/p300 to STAT1 in a SOCS-1-independent manner. J. Immunol. 2009;183:6981–6988. doi: 10.4049/jimmunol.0901045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall S, Brogan P, Haworth SG, Klein N. Contribution of inflammation to the pathology of idiopathic pulmonary arterial hypertension in children. Thorax. 2009;64:778–783. doi: 10.1136/thx.2008.106435. [DOI] [PubMed] [Google Scholar]
- 29.Austin ED, Rock MT, Mosse CA, et al. T lymphocyte subset abnormalities in the blood and lung in pulmonary arterial hypertension. Respir. Med. 2010;104:454–462. doi: 10.1016/j.rmed.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cella G, Bellotto F, Tona F, et al. Plasma markers of endothelial dysfunction in pulmonary hypertension. Chest. 2001;120:1226–1230. doi: 10.1378/chest.120.4.1226. [DOI] [PubMed] [Google Scholar]
- 31.Bozza FA, Shah AM, Weyrich AS, Zimmerman GA. Amicus or adversary: platelets in lung biology, acute injury, and inflammation. Am. J. Respir. Cell Mol. Biol. 2009;40:123–134. doi: 10.1165/rcmb.2008-0241TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dogne JM, de Leval X, Delarge J, David JL, Masereel B. New trends in thromboxane and prostacyclin modulators. Curr. Med. Chem. 2000;7:609–628. doi: 10.2174/0929867003374868. [DOI] [PubMed] [Google Scholar]
- 33.Friedman R, Mears JG, Barst RJ. Continuous infusion of prostacyclin normalizes plasma markers of endothelial cell injury and platelet aggregation in primary pulmonary hypertension. Circulation. 1997;96:2782–2784. doi: 10.1161/01.cir.96.9.2782. [DOI] [PubMed] [Google Scholar]
- 34.Bogaard HJ, Natarajan R, Henderson SC, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 35.Overbeek MJ, Vonk MC, Boonstra A, et al. Pulmonary arterial hypertension in limited cutaneous systemic sclerosis: a distinctive vasculopathy. Eur. Respir. J. 2009;34:371–379. doi: 10.1183/09031936.00106008. [DOI] [PubMed] [Google Scholar]
- 36.Yi ES, Kim H, Ahn H, et al. Distribution of obstructive intimal lesions and their cellular phenotypes in chronic pulmonary hypertension. A morphometric and immunohistochemical study. Am. J. Respir. Crit. Care Med. 2000;162(4 Pt 1):1577–1586. doi: 10.1164/ajrccm.162.4.9912131. [DOI] [PubMed] [Google Scholar]
- 37.Pietra GG, Edwards WD, Kay JM, et al. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation. 1989;80:1198–1206. doi: 10.1161/01.cir.80.5.1198. [DOI] [PubMed] [Google Scholar]
- 38.Mebazaa A, Martin LD, Robotham JL, Maeda K, Gabrielson EW, Wetzel RC. Right and left ventricular cultured endocardial endothelium produces prostacyclin and PGE2. J. Mol. Cell Cardiol. 1993;25:245–248. doi: 10.1006/jmcc.1993.1031. [DOI] [PubMed] [Google Scholar]
- 39.Montalescot G, Drobinski G, Meurin P, et al. Effects of prostacyclin on the pulmonary vascular tone and cardiac contractility of patients with pulmonary hypertension secondary to end-stage heart failure. Am. J. Cardiol. 1998;82:749–755. doi: 10.1016/s0002-9149(98)00439-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.