Abstract
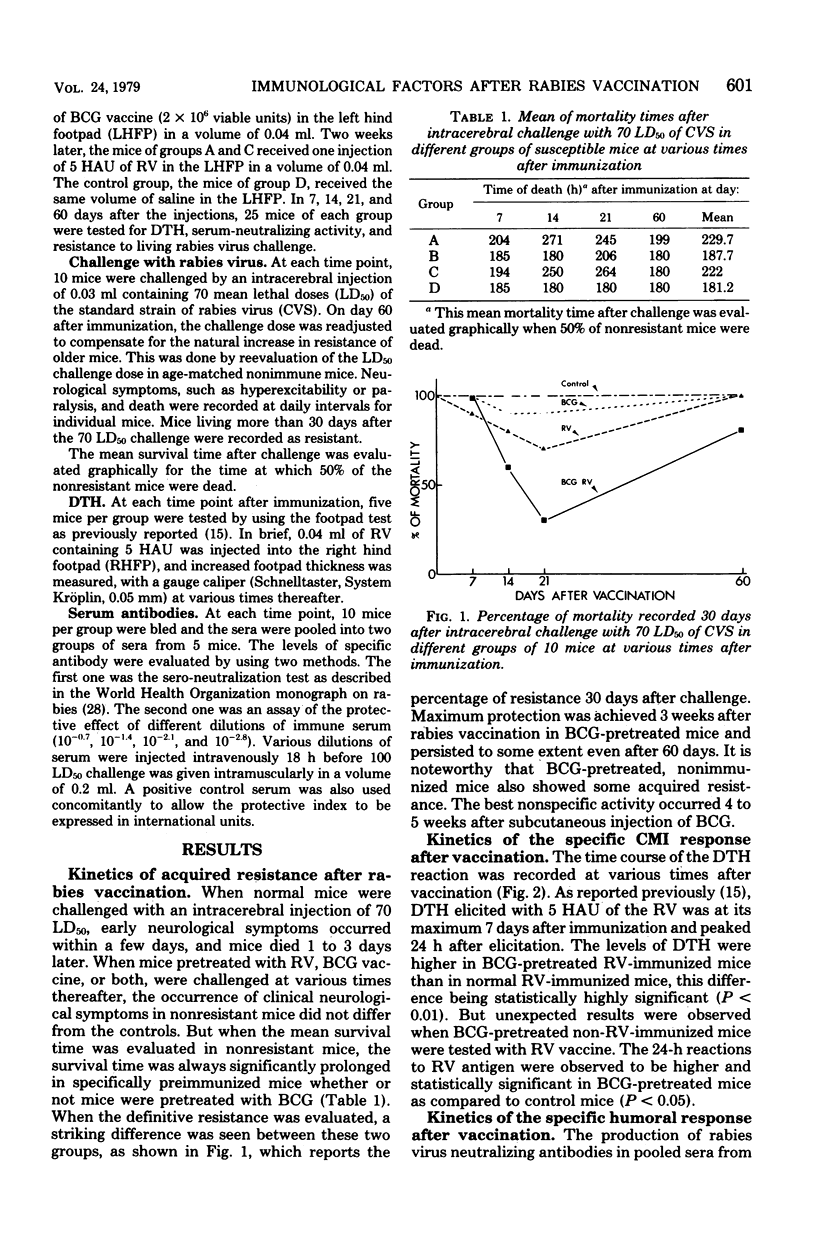
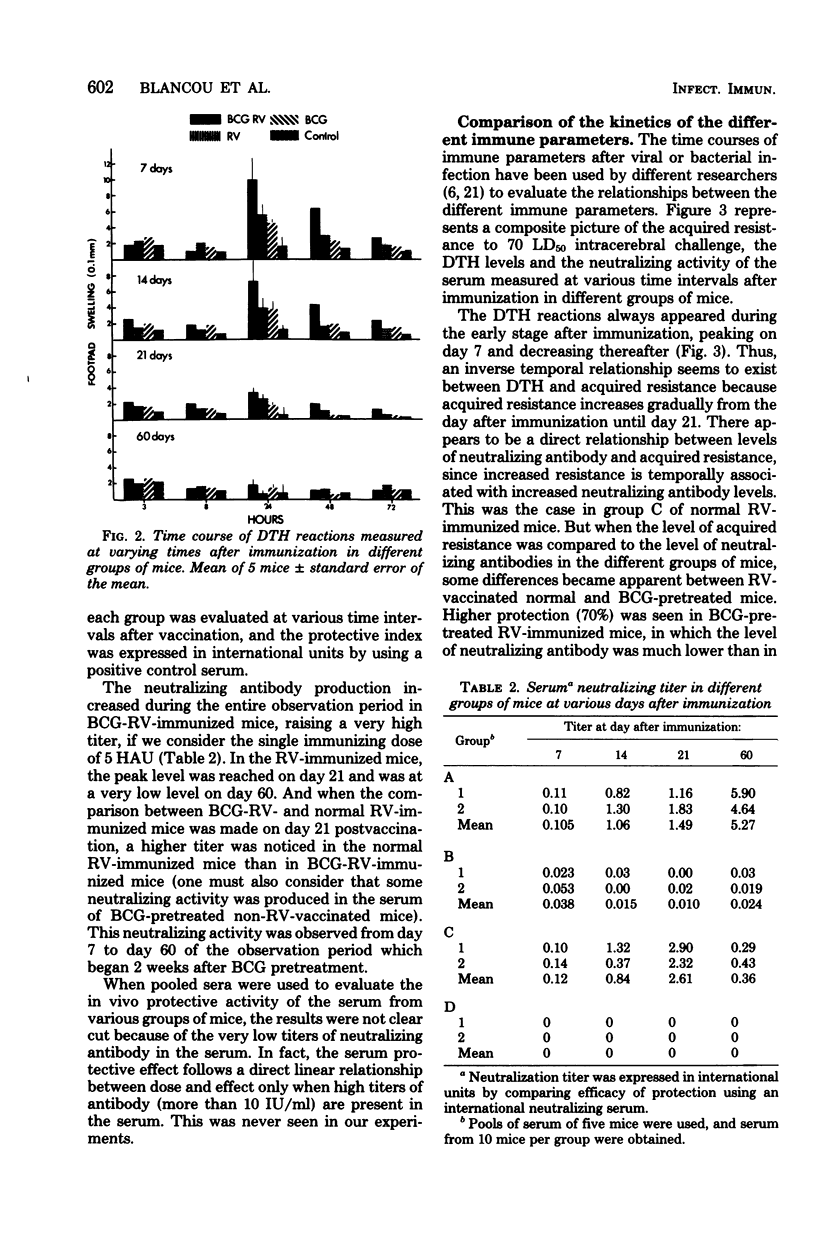
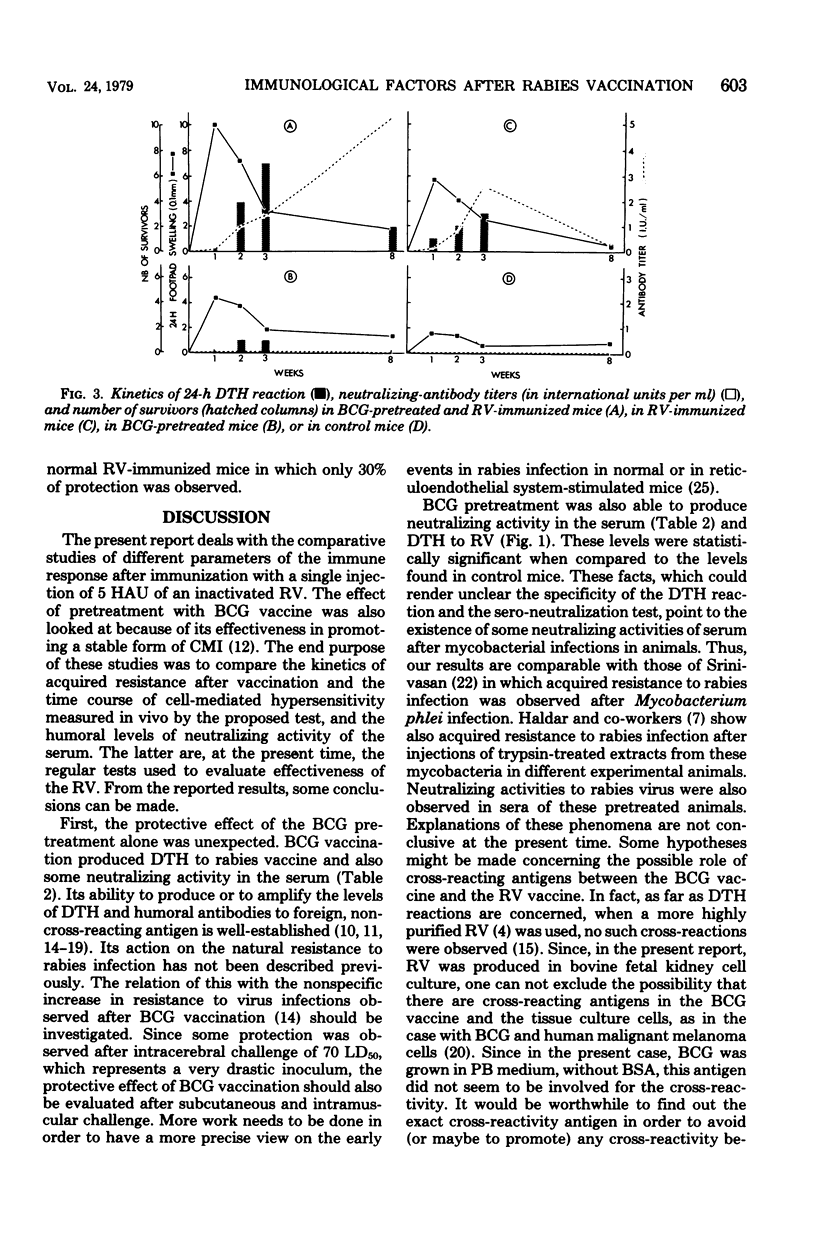
A significant protection to an intracerebral challenge of 70 mean lethal doses of a standard live rabies virus strain was obtained in BCG-pretreated mice or in normal mice which had been immunized with a single subcutaneous injection of a beta-propiolactone-inactivated rabies vaccine. Concomitantly, levels of delayed-type hypersensitivity (measured in vivo by the footpad test) and serum-neutralizing activity were evaluated at various times after immunization. All immune criteria were significantly augmented in the BCG-pretreated, rabies-immune mice as compared to normal, rabies-immune mice. However, peak levels of protection, delayed-type hypersensitivity, and serum-neutralizing activity did not occur at the same times. For instance, in the BCG-pretreated, rabies-immune mice, delayed-type hypersensitivity peaked on day 7, protection peaked on day 21, and serum-neutralizing activity peaked on day 60. In BCG-pretreated mice, which did not receive the rabies vaccine, positive delayed-type hypersensitivity, some protection, and serum neutralizing activity were observed 4 to 5 weeks after BCG pretreatment. The possible relationships between specific and nonspecific immunity provoked by rabies virus antigens, tissue culture cell-associated antigens (derived from the bovine fetal kidney cells in which the rabies virus was grown, and BCG are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Interactions of antibodies, complement components and various cell types in immunity against viruses and pyogenic bacteria. Transplant Rev. 1974;19(0):3–55. doi: 10.1111/j.1600-065x.1974.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Atanasiu P., Nozaki-Renard J., Savy V., Eyquem A. Evaluation de l'immunité cellulaire après vaccination rabique chez l'Homme. C R Acad Sci Hebd Seances Acad Sci D. 1977 Nov 7;285(12):1187–1190. [PubMed] [Google Scholar]
- Atanasiu P., Tsiang H., Perrin P., Favre S., Sisman J. Extraction d'un antigène soluble (glycoprotéine) par le Triton X100. A partir d'un vaccin antirabique de culture tissulaire de premier explant. Résultats d'immunisation et pouvoir protecteur. Ann Microbiol (Paris) 1974 Dec;125(4):540–557. [PubMed] [Google Scholar]
- Atanasiu P., Tsiang H., Reculard P., Aguilon F., Lavergne M., Adamovicz P. Zonal centrifuge purification of human rabies vaccine obtained on bovine fetal kidney cells. Biological results. Dev Biol Stand. 1978;40:35–44. [PubMed] [Google Scholar]
- Blanden R. V. T cell response to viral and bacterial infection. Transplant Rev. 1974;19(0):56–88. doi: 10.1111/j.1600-065x.1974.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Haldar S. K., Singh S. P., Mallick B. B., Kathuria B. K. Non-specific resistance against rabies virus in mice, rats & guineapigs. Indian J Exp Biol. 1977 May;15(5):370–372. [PubMed] [Google Scholar]
- Koprowski H., Mocarelli P., Wiktor T. J. Antibody response in vitro to an animal virus: production of rabies virus neutralizing antibodies by mouse cells in culture. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2433–2436. doi: 10.1073/pnas.69.9.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange P. H., Hurtrel B., Ravisse P. La réaction locale granulomateuse après injection sous-cutanée de BCG chez la souris. I--Description. Ann Immunol (Paris) 1978 Apr-Jun;129 100(4):529–546. [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B. A stable form of delayed-type hypersensitivity. J Exp Med. 1975 Jan 1;141(1):82–96. doi: 10.1084/jem.141.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Influence of dose and route of antigen injection on the immunological induction of T cells. J Exp Med. 1974 Mar 1;139(3):528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange P. H., Tsiang H., Hurtrel B., Ravisse P. Delayed-type hypersensitivity to rabies virus in mice: assay of active or passive sensitization by the footpad test. Infect Immun. 1978 Sep;21(3):931–939. doi: 10.1128/iai.21.3.931-939.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Auclair D. J., Lagrange P. H. Immunopotentiation with BCG. I. Immune response to different strains and preparations. J Natl Cancer Inst. 1973 Nov;51(5):1655–1667. doi: 10.1093/jnci/51.5.1655. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Ishibashi T. The modifying effect of BCG on the immunological induction of T cells. J Exp Med. 1974 Jun 1;139(6):1540–1552. doi: 10.1084/jem.139.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden P., Sharpton T. R., McClatchy J. K. Shared antigens between human malignant melanoma cells and Mycobacterium bovis (BCG). J Immunol. 1976 May;116(5):1407–1414. [PubMed] [Google Scholar]
- North R. J., Mackaness G. B., Elliott R. W. The histogenesis of immunologically committed lymphocytes. Cell Immunol. 1972 Apr;3(4):680–694. doi: 10.1016/0008-8749(72)90130-x. [DOI] [PubMed] [Google Scholar]
- Turner G. S., Ballard R. Interaction of mouse peritoneal macrophages with fixed rabies virus in vivo and in vitro. J Gen Virol. 1976 Feb;30(2):223–231. doi: 10.1099/0022-1317-30-2-223. [DOI] [PubMed] [Google Scholar]
- Turner G. S. Humoral and cellular immune responses of mice to rabies and smallpox vaccines. Nat New Biol. 1973 Jan 17;241(107):90–92. doi: 10.1038/newbio241090a0. [DOI] [PubMed] [Google Scholar]
- Turner G. S. Thymus dependence of rabies vaccine. J Gen Virol. 1976 Dec;33(3):535–538. doi: 10.1099/0022-1317-33-3-535. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J., Doherty P. C., Koprowski H. In vitro evidence of cell-mediated immunity after exposure of mice to both live and inactivated rabies virus. Proc Natl Acad Sci U S A. 1977 Jan;74(1):334–338. doi: 10.1073/pnas.74.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Kamo I., Koprowski H. In vitro stimulation of rabbit lymphocytes after immunization with live and inactivated rabies vaccines. J Immunol. 1974 Jun;112(6):2013–2019. [PubMed] [Google Scholar]



