Abstract
Objective
Fat accumulation around the heart and aorta may impact cardiovascular (CV) health. The purpose of this study was to conduct a systematic investigation to examine potential associations of these fat depots with risk factors for CV events, which has not been done before.
Methods
Pericardial fat, periaortic fat around the ascending aorta (AA), descending aorta (DA) and aortic arch, and abdominal subcutaneous and visceral fat were measured by MRI in older adults with (n=385, 69±8 years, 52% female) and without (n=50, 69±8 years, 58% female) risk factors for a CV event.
Results
Individuals with CV risk factors exhibited greater fat volumes across all fat depots compared to those without risk factors. In analysis of covariance accounting for age, gender, race/ethnicity, diabetes, hypertension, coronary artery disease, smoking, and BMI, individuals with risk factors possessed higher epicardial, pericardial, AA, DA, and abdominal visceral fat (p<0.05). When matched one-to-one on age, gender, race/ethnicity, and BMI, AA and DA fat were higher in those with versus without CV risk factors (p<0.01).
Conclusions
Older adults with a high risk for CV events have greater periaortic fat than low-risk adults, even after accounting for BMI. More studies are needed to determine whether greater periaortic fat predicts future CV events.
Keywords: pericardial fat, periaortic fat, aging, cardiovascular risk
Introduction
Obesity is associated with increased cardiovascular (CV) morbidity and mortality.1 However, not all overweight or obese individuals experience CV events,2 particularly among older adults.3 Age-related changes in body composition and fat distribution, mainly increased abdominal visceral fat and ectopic fat deposition, appear to play a major role in the pathogenesis of CV disease.4, 5 While research has largely focused on abdominal obesity, excess fat accumulation around the heart (i.e. pericardial fat) and proximal aorta (i.e. periaortic fat) may have more adverse effects on CV health given their anatomic location.6 These fat depots may impact adjacent tissues and organs through locally secreted biochemical factors that adversely affect neighboring cardiomyocytes and vascular endothelial and smooth muscle cells.6-9 Thus, direct assessment of fat around CV organs may enable better risk stratification for CV disease.
Data from the Framingham Heart Study and other studies have identified positive associations between total pericardial and/or periaortic fat volume and CV disease.10-15 Moreover, associations with pericardial fat may depend on the particular location of fat relative to the pericardium, and fat distribution around the aorta may also be important.13, 16 To our knowledge, there has been no systematic investigation of these fat depots in older adults and their relationship to CV risk. This issue remains important to address as both pericardial and periaortic fat are positively associated with age.13, 17 The purpose of this study was to perform a comprehensive assessment of epicardial fat (located within the pericardium), paracardial fat (located superficial to the pericardium), pericardial fat (epicardial and paracardial fat combined) and periaortic fat around the ascending aorta (AA), descending aorta (DA) and aortic arch in older adults. We also examined whether older adults with risk factors for a CV event have greater fat deposition than similarly aged adults without risk factors, accounting for overall adiposity.
Materials and Methods
Study Participants
This analysis included older men and women enrolled in two clinical studies at Wake Forest School of Medicine. All study participants provided written informed consent, and each study protocol was approved by the Wake Forest School of Medicine Institutional Review Board.
Individuals with CV Risk Factors
The Pulmonary Edema and Vascular Stiffness (PREDICT) study is a prospective study in middle-aged and older individuals designed to identify vascular abnormalities that predict the future development of congestive heart failure. Eligible participants were 55-85 years of age and were at high risk for their first episode of flash pulmonary edema based on the presence of at least one of the following risk factors:18 diabetes defined as a fasting glucose level ≥126 mg/dl;19 hypertension defined as a systolic blood pressure >140 mmHg, a diastolic blood pressure >85 mmHg, or use of anti-hypertensive medications;20 or coronary artery disease based on standards established by the American Heart Association and the American College of Cardiology.21 Major exclusion criteria included contraindication to MRI scanning (i.e. implanted electronic devices, intracranial metal, or claustrophobia); left main or 3-vessel coronary artery disease; moderate to severe aortic stenosis or other significant valvular disease; and myocardial infarction, acute coronary syndrome, or angina within the preceding year.
Individuals without CV Risk Factors
The HEALTHY cohort was recruited to provide a healthy, normal comparison group for studies of age-related health conditions. Community-dwelling older adults aged ≥60 years who were non-smokers; sedentary (<120 minutes of vigorous exercise per week);had no acute or chronic medical disorders, including hypertension or diabetes; and were not taking any medications except aspirin for primary prevention were recruited for this cohort.22
MRI Assessment of Fat Volume
MRI scans were performed on a 1.5T Siemens Avanto scanner (Siemens Medical Solution, Erlangen, Germany) with a phased-array surface coil applied to optimize signal to noise within the images. To determine the volume of pericardial and periaortic fat, axial images were acquired of the chest from the diaphragm to the aortic arch using a prospectively ECG-gated, T1-weighted, breath-held, black-blood, single-shot, turbo-spin echo sequence. Abdominal fat was determined in a single axial slice positioned at the level of the second lumbar vertebra, as described previously.23, 24 Images acquired for the purpose of determining thoracic and abdominal fat volumes were analyzed offline using sliceOmatic™ software (version 4.2 rev10, Tomovision, Montreal, CA). Regions of interest were drawn, and the cross-sectional fat area was quantified by summing the pixels within the segmented portions of the image and multiplying by the pixel surface area. Fat volumes were calculated by multiplying the cross-sectional areas by the slice thickness.
Analysis of Thoracic Fat Depots
The Threshold mode was used to separate fat from the thorax and the remaining portions of the heart using specific anatomic landmarks. In brief, the anterior border of the volume was defined by the chest wall and the posterior border by the descending aorta and spinal column. Epicardial fat (Figure 1, red color) and paracardial fat (Figure 1, blue color) were measured from the bottom of the left ventricle (LV) to the aortic root (at the level of the mitral valve leaflets) (Figure 1, slices 1-5). To define the pericardium (Figure 1, green color), 8-10 control points were manually placed and a smooth closed contour was automatically generated based on a Catmull-Rom spline function. Pericardial fat volumes(i.e. the sum of epicardial fat and paracardial fat) were calculated for two regions from 1) the LV to the aortic root (Figure 1, slices 1-5) and 2) the aortic root to the main pulmonary artery (Figure 1, orange color, slices 6-8). Pericardial fat was then summed across all 8 slices to obtain a total pericardial fat volume. Fat around the AA and DA was measured in a single slice at the level of the main pulmonary artery, prior to the bifurcation. The slice cutting through the middle of the pulmonary artery was identified (Figure 1, slice 9), and then AA fat (purple color) was defined by a circle with a diameter 10 mm greater than the aortic diameter, while DA fat (yellow color) was defined by the pulmonary vein anteriorly, the spinal column and spinal vessel posteriorly and postero-medially, and the lung fields laterally. Aortic arch fat, defined by the manubrium anteriorly, lung fields laterally, and spinal column posteriorly, was measured from above the pulmonary bifurcation to the top of the arch, prior to the branching vessels (Figure 1, slices 10-11, pink color). A coronal image highlighting the corresponding axial slices used to quantify pericardial and periaortic fat is shown in Figure 2.
Figure 1.
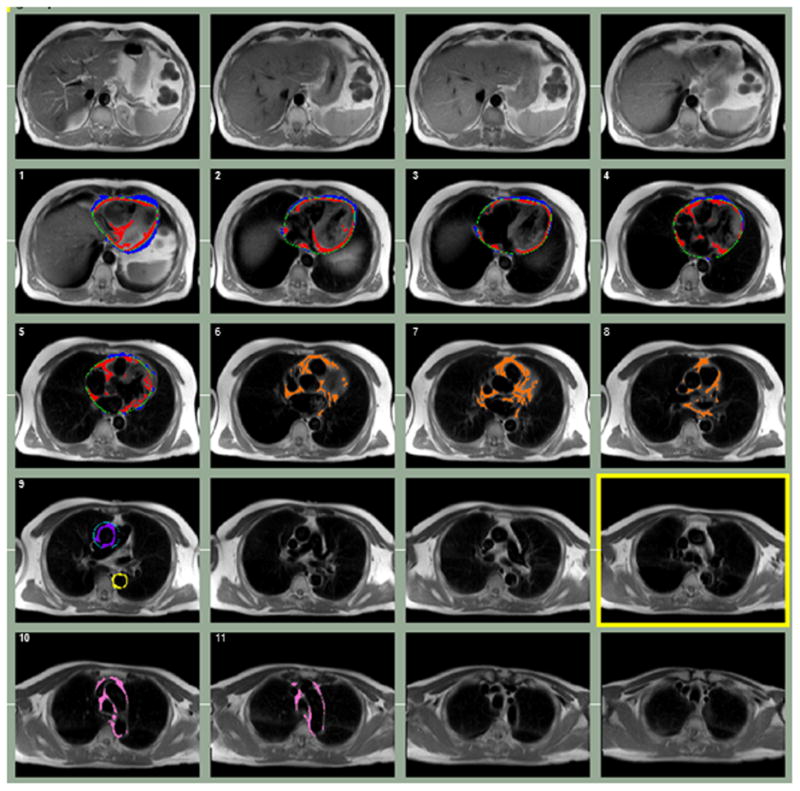
Segmentation of epicardial fat (red), paracardial fat (blue), pericardial fat above the aortic root (orange), AA fat (purple), DA fat (yellow), and aortic arch fat (pink) from a representative PREDICT participant. The pericardium (green) was manually drawn to distinguish epicardial and paracardial fat on slices from the bottom of the LV to the aortic root.
Figure 2.
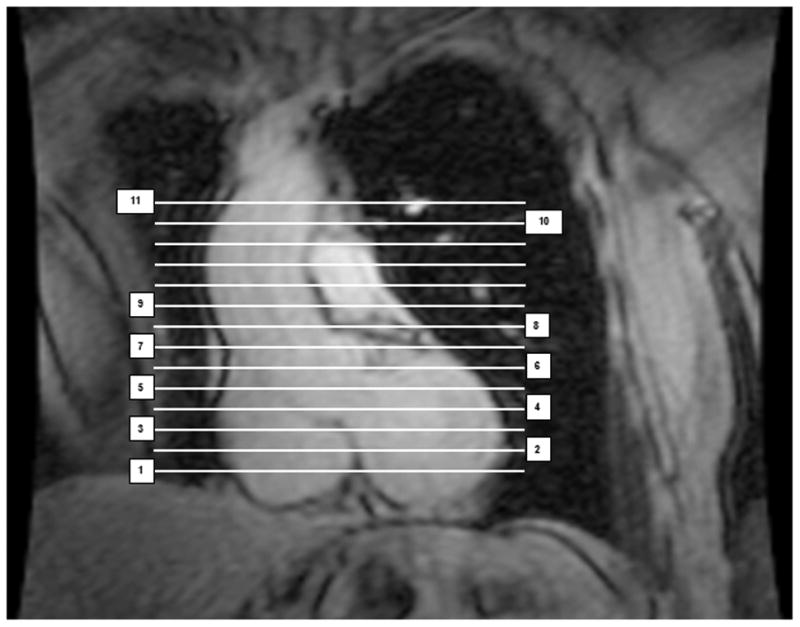
Coronal image corresponding to the axial planes used to segment pericardial and periaortic fat depots. Slices 1-5 were used to quantify epicardial fat, paracardial fat, and pericardial fat (below the aortic root). Slices 6-8 were used to quantify pericardial fat from the aortic root to the main pulmonary artery (i.e. above the aortic root). Slice 9 was used to quantify AA and DA fat at the level of the main pulmonary artery, prior to the bifurcation. Slices 10-11 were used to quantify aortic arch fat from above the pulmonary bifurcation to the top of the arch, prior to the branching vessels.
All scans were analyzed by a single reader. To determine the intra-observer variability of the fat volume measurements within each compartment, images from 12 randomly selected PREDICT participants were reanalyzed and intra-class correlation coefficients (ICC) were calculated. The intra-observer ICC were 0.87 for epicardial fat, 0.98 for paracardial fat, 0.89 for pericardial fat (above the aortic root), 0.95 for AA fat, 0.90 for DA fat, and 0.91 for aortic arch fat.
Analysis of Abdominal Fat Depots
Abdominal fat was segmented into subcutaneous and visceral compartments. Visceral fat was defined as fat within the mesentery and omentum and bounded by the muscular abdominal wall. Subcutaneous fat was defined as fat lying outside of the abdominal wall. Abdominal fat compartments were segmented using an automated algorithm (Morpho mode) followed by manual correction of misclassified pixels. The inter-observer ICC were 0.944-0.976 and 0.996 for visceral and subcutaneous fat, respectively.24
Measurement of Other Clinical Variables
Demographics (i.e. age, gender, race/ethnicity) and smoking history were obtained by questionnaire. Systolic and diastolic blood pressures were measured using a conventional mercury sphygmomanometer. Body weight was measured using a standard calibrated scale. Height was measured using a wall-mounted stadiometer. Blood samples were collected by venipuncture in the morning following an overnight fast. Laboratory variables, including lipids, glucose, and C-reactive protein, were measured using standardized procedures.
Statistical Analysis
All statistical analyses were performed using SAS 9.3 (Cary, NC). Clinical characteristics and fat volumes were compared between individuals with and without CV risk factors using Chi-square tests for categorical variables and Student's t-tests for continuous variables. Spearman correlation coefficients were used to examine relationships among pericardial, periaortic, and abdominal fat depots and BMI for each risk group. Analysis of covariance was used to examine whether the individual fat depots were independently associated with risk group after accounting for age, gender, race/ethnicity, diabetes, hypertension, coronary artery disease, smoking and BMI. An interaction term was initially included to determine whether the associations between risk group and the fat depots were modified by BMI. We found no significant group × BMI interactions; therefore, interaction terms were not included in the final models. In order to more carefully control for the effects of total adiposity, we also performed one-to-one matching to identify a subset of individuals with CV risk factors who were similar to individuals without CV risk factors based on gender (100%), race (100%), age (±4 years), and BMI (±5 kg/m2).Similar comparison analyses were performed for the matched individuals. For all tests, a p-value ≤0.05 was considered statistically significant.
Results
Clinical Characteristics of Individuals With and Without CV Risk Factors
Compared to individuals without CV risk factors, those with risk factors were more likely to be non-white (p=0.002), with higher body weight (p<0.0001), BMI (p<0.0001), and serum triglyceride (p=0.0005), glucose (p<0.0001), and C-reactive protein (p<0.0001) levels. These individuals also had lower serum levels of total (p=0.002) and HDL cholesterol (p<0.0001) (Table 1), and ∼60% were taking statins. As shown in Table 2, all of the fat volumes (except for abdominal subcutaneous fat) were higher in individuals with versus without risk factors (all p<0.01). In the analysis of individuals matched one-to-one on age, gender, race and BMI, differences in aortic arch fat were slightly attenuated (p=0.052), however, all other fat depots remained higher in those with risk factors for a CV event (p<0.05).
Table 1. Clinical characteristics of study participants according to the presence (PREDICT) or absence (HEALTHY) of CV risk factors.
| Characteristics | n | PREDICT Study (All Participants) |
n | PREDICT Study (Matched Subset#) |
n | HEALTHY Study |
|---|---|---|---|---|---|---|
| Age, yrs | 385 | 69.12 ± 8.21 | 50 | 68.82 ± 7.29 | 50 | 69.08 ± 7.51 |
| Female | 385 | 201 (52%) | 50 | 29 (58%) | 50 | 29 (58%) |
| White | 385 | 298 (77%)† | 50 | 48 (96%) | 50 | 48 (96%) |
| Diabetes | 385 | 146 (38%) | 50 | 16 (32%) | 50 | 0 (0) |
| Hypertension | 385 | 355 (92%) | 50 | 44 (88%) | 50 | 0 (0) |
| Coronary artery disease | 385 | 104 (27%) | 50 | 11 (22%) | 50 | 0 (0) |
| Smoker | 376 | 159 (41%) | 49 | 17 (34%) | 50 | 0 (0) |
| Height, cm | 380 | 1.69 ± 0.10 | 50 | 1.69 ± 0.10 | 50 | 1.69 ± 0.09 |
| Weight, kg | 380 | 85.86 ± 18.03‡ | 50 | 76.83 ± 13.59 | 50 | 74.82 ± 14.29 |
| BMI, kg/m2 | 380 | 30.21 ± 6.01‡ | 50 | 26.81 ± 4.00 | 50 | 26.15 ± 4.40 |
| Systolic BP, mmHg | 336 | 139.52 ± 17.21‡ | 43 | 137.88 ± 20.65‡ | 50 | 117.90 ± 13.35 |
| Diastolic BP, mmHg | 336 | 77.90 ± 12.67‡ | 43 | 73.26 ± 13.46* | 50 | 67.08 ±11.87 |
| Heart rate, bpm | 336 | 64.69 ± 11.03 | 43 | 66.12 ± 13.57 | 50 | 64.02 ± 10.41 |
| Total cholesterol, mg/dl | 362 | 173.54 ± 43.65† | 47 | 177.38 ± 46.35 | 32 | 192.53 ± 29.41 |
| HDL cholesterol, mg/dl | 362 | 44.93 ± 13.83‡ | 47 | 44.21 ± 12.04‡ | 32 | 60.31 ± 17.62 |
| LDL cholesterol, mg/dl | 360 | 103.54 ± 38.33 | 46 | 106.20 ± 41.91 | 32 | 113.81 ± 28.13 |
| Triglycerides, mg/dl | 362 | 126.66 ± 71.41† | 47 | 138.06 ± 77.64† | 32 | 91.84 ± 47.51 |
| Glucose, mg/dl | 362 | 120.17 ± 45.06‡ | 47 | 110.60 ± 17.31‡ | 50 | 91.38 ± 15.30 |
| C-reactive protein, mg/l | 351 | 4.54 ± 12.07‡ | 45 | 7.66 ± 31.01 | 43 | 0.20 ± 0.21 |
Table values are mean ± standard deviation or n (%);
Matched to the HEALTHY participants on age, gender, race, and BMI,
p<0.05,
p<0.01,
p<0.0001
Table 2. Unadjusted mean epicardial, paracardial, pericardial, periaortic, and abdominal fat volumes according to the presence (PREDICT) or absence (HEALTHY) of CV risk factors.
| Characteristics | n | PREDICT Study (All Participants) |
n | PREDICT Study (Matched Subset#) |
n | HEALTHY Study |
|---|---|---|---|---|---|---|
| Epicardial fat, cm3 | 384 | 80.5 ± 25.9‡ | 49 | 75.3 ± 24.0‡ | 50 | 54.9 ± 20.6 |
| Paracardial fat, cm3 | 384 | 87.6 ± 55.2‡ | 49 | 71.7 ± 46.7* | 50 | 53. 9 ± 36.7 |
| Pericardial fat – below aortic root, cm3 | 384 | 168.1 ± 73.7‡ | 49 | 147.0 ± 66.5† | 50 | 108.8 ± 52.9 |
| Pericardial fat – above aortic root, cm3 | 385 | 62.2 ± 29.7‡ | 50 | 57.4 ± 32.7* | 50 | 45.6 ± 23.3 |
| Pericardial fat – total, cm3 | 384 | 230.4 ± 91.2† | 49 | 204.8 ± 85.0† | 50 | 154.4 ± 69.2 |
| AA Fat, cm3 | 384 | 4.3 ± 1.4‡ | 50 | 3.9 ± 1.1‡ | 50 | 2.5 ± 0.7 |
| DA fat, cm3 | 383 | 2.9 ± 0.8‡ | 50 | 2.7 ± 0.7‡ | 50 | 1.7 ± 0.6 |
| Aortic arch fat, cm3 | 383 | 31.1 ± 17.9* | 50 | 26.5 ± 14.1 | 11 | 17.4 ± 12.6 |
| Abdominal subcutaneous fat, cm3 | 304 | 217.5 ± 120.7‡ | 37 | 167.2 ± 67.9 | 50 | 154.5 ± 84.9 |
| Abdominal visceral fat, cm3 | 304 | 197.3 ± 100.1‡ | 37 | 173.1 ± 77.5† | 50 | 121.4 ± 92.5 |
Table values are mean ± standard deviation.
Matched to the HEALTHY participants on age, gender, race, and BMI;
p<0.05,
p<0.01,
p<0.0001
Correlations Among Pericardial, Periaortic, and Abdominal Fat Depots and BMI
Correlations among all the adiposity measures can be found in Supplementary Tables 1 and 2 for individuals with and without CV risk factors, respectively. Epicardial, paracardial, and pericardial fat depots were correlated with the periaortic fat depots in those with risk factors(r=0.29-0.66, p<0.0001 for all), while only DA fat was associated with these fat depots in individuals without risk factors. Correlations between epicardial and paracardial fat(r=0.61-0.68, p<0.0001) and between AA and DA fat (r=0.38-0.39, p<0.01) were modest, but significant, in both groups. Pericardial fat (below the aortic root) and total pericardial fat were highly correlated with their components (r=0.74-0.97, p<0.0001 for all). In general, epicardial, paracardial, pericardial and periaortic fat depots were correlated with abdominal visceral fat in both groups (r=0.35-0.75, p≤0.01). In contrast, abdominal subcutaneous fat was only associated with paracardial fat and pericardial fat depots (p<0.05). BMI was associated with paracardial fat, all of the pericardial fat depots, and DA fat in individuals without CV risk factors. All fat depots except pericardial fat (above the aortic root) and AA fat were associated with BMI in those with CV risk factors.
Linear Regression Models for the Association of Study Group with Fat Depot
The mean differences in fat volumes between individuals with and without CV risk factors based on analysis of covariance are shown in Table 3. These results indicated that after accounting for age, gender, race/ethnicity, diabetes, hypertension, coronary artery disease, smoking, and BMI, individuals with CV risk factors had significantly greater epicardial fat (p=0.02), total pericardial fat (p=0.02), AA fat (p<0.0001), DA fat (p<0.0001), and abdominal visceral fat (p=0.006) compared to those without CV risk factors. In the matched analysis, AA and DA fat remained significantly different between groups, with the high-risk group having approximately 1.6 cm3 more AA fat (p=0.001) and 0.8 cm3 more DA fat (p=0.006) than the low-risk group. The differences in AA and DA fat between groups were unchanged after further adjustment for abdominal visceral fat (1.7±0.5 cm3, p=0.006 and 1.0±0.3 cm3, p=0.003, respectively).
Table 3. Adjusted differences in fat volumes (least squared means ± standard errors) between individuals with and without CV risk factors.
| All Study Participants (n=435)# | Matched Study Participants (n=100)## | |||
|---|---|---|---|---|
|
|
|
|||
| Fat Depot (cm3) | Mean ± SE | P-value | Mean ± SE | P-value |
| Epicardial fat | 13.7 ± 5.7 | 0.02 | 6.0 ± 11.2 | 0.59 |
| Paracardial fat | 12.4 ± 10.4 | 0.23 | 6.8 ± 18.9 | 0.72 |
| Pericardial fat – below aortic root | 26.1 ± 14.1 | 0.07 | 12.8 ± 2.8 | 0.65 |
| Pericardial fat – above aortic root | 13.4 ± 6.0 | 0.03 | 19.8 ± 13.8 | 0.15 |
| Pericardial fat – total | 39.3 ± 17.0 | 0.02 | 30.2 ± 34.5 | 0.38 |
| AA fat | 1.8 ± 0.3 | <0.0001 | 1.6 ± 0.5 | 0.001 |
| DA fat | 0.8 ± 0.2 | <0.0001 | 0.8 ± 0.3 | 0.006 |
| Arch fat | 7.4 ± 5.1 | 0.15 | 7.0 ± 7.2 | 0.33 |
| Abdominal subcutaneous fat | 12.7 ± 17.4 | 0.47 | 2.3 ± 27.8 | 0.94 |
| Abdominal visceral fat | 47.8 ± 17.3 | 0.006 | 36.7 ± 30.9 | 0.24 |
Based on analysis of covariance with study group (independent variable) modeled as high-risk=1, low-risk=0; models are adjusted for age, gender, race/ethnicity, diabetes, hypertension, coronary artery disease, smoking, and BMI.
Includes all PREDICT and HEALTHY participants;
Includes all HEALTHY participants and the 50 PREDICT participants who were matched based on age, gender, race, and BMI
Discussion
This study compared pericardial and periaortic fat depots in older adults with and without risk factors for a CV event. The main finding was that individuals with CV risk factors had significantly greater pericardial and periaortic fat volumes compared to those without risk factors, even after accounting for BMI. In addition, when directly matched one-to-one for age, gender, race/ethnicity and BMI, periaortic fat around the AA and DA remained different between risk groups. These findings suggest that excess accumulation of periaortic fat is strongly associated with CV risk in this population. Thus, measurement of periaortic fat deposition may provide additional information regarding CV risk stratification in older adults.
Previous data from the Framingham Heart Study demonstrate that higher volumes of DA fat are strongly associated with an adverse CV risk profile, as well as greater aortic diameter, calcification and stiffness.15, 17, 25, 26 Interestingly, among men and women with normal amounts of abdominal visceral fat, those with high DA fat(i.e. >90th percentile of healthy individuals) had a significantly greater prevalence of CV disease compared to those with normal DA fat, even after adjusting for the total volume of abdominal visceral fat.15 The authors suggested that increased DA fat in the absence of increased abdominal visceral fat may reflect a type of “metabolic obesity” that contributes to increased CV risk. Moreover, Britton et al. recently reported that higher DA fat is associated with a 31% higher risk of incident CV disease.27 The results of the present study are consistent with an adverse role of DA fat and extend the findings to AA fat, which has never been examined before. Although animal studies support the pathological relevance of aortic arch fat,8, 28 the small number of low-risk individuals with these data precluded our ability to fully test for an association. Nevertheless, our findings highlight the importance of increased periaortic fat and its potential utility as a clinical risk determinant of future CV events in older adults.
There are several potential mechanisms that could explain the higher periaortic fat volume observed in individuals with CV risk factors. Nearly all blood vessels are surrounded by a layer of fat that is thought to play a role in modulating vascular tone through the secretion of an adipocyte-derived relaxing factor.29-31 However, this anti-contractile effect becomes impaired in pathological settings, which may reflect adipose tissue dysfunction.32 Animal studies show that with both aging and diet-induced obesity periaortic fat is increased, accompanied by increased inflammation, oxidative stress, and vascular smooth muscle cell proliferation.28, 33, 34 Similarly, in humans, increased macrophage infiltration and altered adipokine expression have been found in periaortic fat surrounding atherosclerotic aortas.8, 35 Thus, periaortic fat exhibits a local paracrine effect that may ultimately contribute to obesity-related CV disease in older adults.
A number of studies have shown that epicardial, paracardial, and/or pericardial fat also have local effects that adversely influence LV structure, diastolic function, coronary atherosclerosis, and myocardial perfusion.12, 36, 37 However, we found no association between these fat depots and CV risk after matching the study groups on age, gender, race/ethnicity, and BMI. Similarly, abdominal visceral fat was not independently associated with risk group in the matched analysis, despite its known role in the pathophysiology of CV disease. These data suggest that pericardial and abdominal fat depots are not strongly related to CV risk in this population of older adults after closely accounting for overall adiposity.
In conclusion, in this study, we performed a comprehensive assessment of fat around the heart and aorta in two clinically-relevant groups of older men and women: one that included individuals with major risk factors for congestive heart failure and another that included healthy, age-matched individuals without these risk factors. We found positive correlations between pericardial, periaortic, and abdominal fat depots and BMI in both groups that varied in strength. While all of the fat volumes were significantly greater in the individuals with CV risk factors, after carefully matching the study groups to account for total adiposity, we found that only AA and DA fat were significantly higher in those with versus without risk factors for a CV event. The use of both analysis of covariance and matching allowed for better control of potentially confounding variables, demonstrating that group differences in the fat volumes were largely explained by differences in CV risk factors. However, since this was a cross-sectional study, we cannot determine whether excess periaortic fat contributes to the future development of hypertension, diabetes, and coronary artery disease, or accumulates as a consequence of pre-existing risk factors. In addition, the potential underlying mechanisms cannot be determined, as we were not able to examine local factors associated with increased fat deposition. Nevertheless, the present findings indicate that older individuals at high CV risk have greater fat deposition around the aorta, which likely reflects not only increased visceral adiposity, but also vascular abnormalities that predispose to CV events. The PREDICT study involves a well-characterized cohort with comprehensive assessments of visceral adiposity, CV structure and function, and CV events over 7 years of follow-up. Thus, future analyses will allow us to answer important questions related to the prognostic ability of CV fat depots in this high-risk population.
Supplementary Material
Acknowledgments
This research was supported by NIH grants K01-AG033652, R37-AG18915, R01-HL076438, and R01-HL093713 as well as the Wake Forest Claude D. Pepper Older Americans Independence Center (P30-AG21332) and General Clinical Research Center (M01-RR07122).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Supplementary information is available at the International Journal of Obesity's website.
References
- 1.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–976. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 2.Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. J Am Coll Cardiol. 2011;58:1343–1350. doi: 10.1016/j.jacc.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 3.Alam I, Ng TP, Larbi A. Does inflammation determine whether obesity is metabolically healthy or unhealthy? The aging perspective. Mediators Inflamm. 2012;2012:456456. doi: 10.1155/2012/456456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci. 2003;24:276–283. doi: 10.1016/S0165-6147(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 5.Zamboni M, Armellini F, Harris T, Turcato E, Micciolo R, Bergamo-Andreis IA, et al. Effects of age on body fat distribution and cardiovascular risk factors in women. Am J Clin Nutr. 1997;66:111–115. doi: 10.1093/ajcn/66.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Thalmann S, Meier CA. Local adipose tissue depots as cardiovascular risk factors. Cardiovasc Res. 2007;75:690–701. doi: 10.1016/j.cardiores.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 9.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 10.Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis MESA) Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009 doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding J, Kritchevsky SB, Harris TB, Burke GL, Detrano RC, Szklo M, et al. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16:1914–1919. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 14.Taguchi R, Takasu J, Itani Y, Yamamoto R, Yokoyama K, Watanabe S, et al. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001;157:203–209. doi: 10.1016/s0021-9150(00)00709-7. [DOI] [PubMed] [Google Scholar]
- 15.Britton KA, Pedley A, Massaro JM, Corsini EM, Murabito JM, Hoffmann U, et al. Prevalence, distribution, and risk factor correlates of high thoracic periaortic fat in the Framingham Heart Study. J Am Heart Assoc. 2012;1:e004200. doi: 10.1161/JAHA.112.004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlett CL, Massaro JM, Lehman SJ, Bamberg F, O'Donnell CJ, Fox CS, et al. Novel measurements of periaortic adipose tissue in comparison to anthropometric measures of obesity, and abdominal adipose tissue. Int J Obes (Lond) 2009;33:226–232. doi: 10.1038/ijo.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehman SJ, Massaro JM, Schlett CL, O'Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: The Framingham Heart Study. Atherosclerosis. 2010 doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC., Jr Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 19.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 21.Fraker TD, Jr, Fihn SD, Gibbons RJ, Abrams J, Chatterjee K, Daley J, et al. 2007 chronic angina focused update of the ACC/AHA 2002 Guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 Guidelines for the management of patients with chronic stable angina. Circulation. 2007;116:2762–2772. doi: 10.1161/CIRCULATIONAHA.107.187930. [DOI] [PubMed] [Google Scholar]
- 22.Stehle JR, Jr, Leng X, Kitzman DW, Nicklas BJ, Kritchevsky SB, High KP. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J Gerontol A Biol Sci Med Sci. 2012;67:1212–1218. doi: 10.1093/gerona/gls178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chughtai HL, Morgan TM, Hamilton CA, Charoenpanichkit C, Ding J, Brinkley TE, et al. Intraperitoneal fat is associated with thickening of the thoracic aorta in individuals at high risk for cardiovascular events. Obesity (Silver Spring) 2011;19:1784–1790. doi: 10.1038/oby.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chughtai HL, Morgan TM, Rocco M, Stacey B, Brinkley TE, Ding J, et al. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension. 2010;56:901–906. doi: 10.1161/HYPERTENSIONAHA.110.157370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Britton KA, Wang N, Palmisano J, Corsini E, Schlett CL, Hoffmann U, et al. Thoracic periaortic and visceral adipose tissue and their cross-sectional associations with measures of vascular function. Obesity (Silver Spring) 2013;21:1496–1503. doi: 10.1002/oby.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thanassoulis G, Massaro JM, Corsini E, Rogers I, Schlett CL, Meigs JB, et al. Periaortic adipose tissue and aortic dimensions in the Framingham Heart Study. J Am Heart Assoc. 2012;1:e000885. doi: 10.1161/JAHA.112.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62:921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol. 2004;286:H1107–H1113. doi: 10.1152/ajpheart.00656.2003. [DOI] [PubMed] [Google Scholar]
- 30.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 31.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 32.Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci. 2004;25:647–653. doi: 10.1016/j.tips.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Bailey-Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, et al. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68:780–792. doi: 10.1093/gerona/gls238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol. 2005;289:H1807–H1813. doi: 10.1152/ajpheart.01259.2004. [DOI] [PubMed] [Google Scholar]
- 35.Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb. 2010;17:115–130. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- 36.Bucci M, Joutsiniemi E, Saraste A, Kajander S, Ukkonen H, Saraste M, et al. Intrapericardial, but not extrapericardial, fat is an independent predictor of impaired hyperemic coronary perfusion in coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31:211–218. doi: 10.1161/ATVBAHA.110.213827. [DOI] [PubMed] [Google Scholar]
- 37.Malavazos AE, Ermetici F, Coman C, Corsi MM, Morricone L, Ambrosi B. Influence of epicardial adipose tissue and adipocytokine levels on cardiac abnormalities in visceral obesity. Int J Cardiol. 2007;121:132–134. doi: 10.1016/j.ijcard.2006.08.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


