Abstract
Rationale
The structural and functional integrity of the endothelium is crucial in maintaining vascular homeostasis and preventing atherosclerosis. Patients with systemic lupus erythematosus (SLE) have an increased risk of developing endothelial dysfunction and premature cardiovascular disease. Neutrophil extracellular trap (NET) formation is increased in SLE and has been proposed to contribute to endothelial damage but the mechanism remains unclear.
Objective
To determine the mechanism by which enhanced NET formation by low-density granulocytes (LDGs) in SLE contributes to endothelial damage and disrupts the endothelium.
Results
The putative role of NET-externalized matrix metalloproteinases (MMPs) in altering the functional integrity of the endothelium was examined. MMP-9 externalized by lupus LDGs during NET formation specifically impaired murine aortic endothelium-dependent vasorelaxation and induced endothelial cell apoptosis. Endothelial dysfunction correlated with the activation of endothelial MMP-2 by MMP-9 present in NETs, while inhibition of MMP-2 activation restored endothelium-dependent function and decreased NET-induced vascular cytotoxicity. Moreover, immunogenic complexes composed of MMP-9 and anti-MMP-9 were identified in SLE sera. These complexes, as well as anti-MMP-9 autoantibodies induced NETosis and enhanced MMP-9 activity.
Conclusion
These observations implicate activation of endothelial MMP-2 by MMP-9 contained in NETs as an important player in endothelial dysfunction, and MMP-9 as a novel self-antigen in SLE. These results further support that aberrant NET formation plays pathogenic roles in SLE.
Keywords: Endothelium, endothelial dysfunction, metalloproteinase, neutrophil extracellular traps, lupus
Introduction
Neutrophil extracellular traps (NETs) are networks of extracellular decondensed chromatin fibers decorated with histones, granule-derived enzymes and several cytoplasmic proteins.[1] Besides their physiologic antimicrobial functions, NETs may play pathogenic roles in several conditions including atherosclerosis,[2] rheumatoid arthritis (RA),[3] systemic vasculitides,[4] and systemic lupus erythematosus (SLE).[5 6]
Recently, a central role for neutrophils in lupus pathogenesis has been proposed. Human and mouse lupus neutrophils are primed to make NETs, and this is particularly the case for lowdensity granulocytes (LDGs),[5-8] a proinflammatory neutrophil subset isolated from human lupus PBMC fractions.[6 9] Further, sera from a subset of lupus patients shows impairment in the capacity to degrade NETs.[10] While the mechanisms associated to enhanced NETosis in SLE remain to be fully characterized, cytokines, immune complexes (ICs) and autoantibodies are putative inducers.[5-7 11] Importantly, LDG NETs are deleterious to the endothelium, with potential implications in the development of premature atherosclerosis in SLE.[6 9] Indeed, impaired endothelium-dependent vasorelaxation, a phenomenon that predicts atherogenesis, is highly prevalent in SLE.[12 13] However, the mechanisms by which NETs damage the endothelium remain to be fully characterized.
Due to their capacity to degrade the extracellular matrix, regulate tissue architecture, and induce proteolysis, matrix metalloproteinases (MMPs) are implicated in atherogenesis and vascular damage.[14 15] In SLE, elevated serum levels of MMPs, in particular MMP-9, have been reported.[16] As MMPs are present in neutrophil granules and may be externalized during NET formation,[1] we further examined how they may alter the functional integrity of the endothelium.
Materials and Methods
Results
MMP-9 is upregulated in lupus LDG NETs
The source of elevated MMP-9 in SLE sera remains unclear. Since MMP-9 is present in neutrophils granules, we investigated whether this molecule is externalized in NETs. Proteins from digested NETs obtained from control neutrophils (through LPS stimulation) or spontaneously formed in SLE normal density neutrophils and in SLE LDGs, were separated in a SDS-gradient gel. Western blot analysis showed that MMP-9 is present in NETs from control and lupus neutrophils (Figure 1A). Densitometry analysis demonstrated that MMP-9 is externalized at significantly higher levels in LDG NETs, when compared to healthy control or to normal density SLE neutrophils (Figures 1B). Zymography analysis of NETs demonstrated the presence of another neutrophil metalloproteinase, MMP-25 (Figure 1C), an endopeptidase specifically expressed in leukocytes.[17 18] Immunofluorescence microscopy confirmed the presence of MMP-9 and MMP-25 in NETs (Figure 1D). These results indicate that specific MMPs decorate the NETs and that this phenomenon is enhanced in lupus LDGs. As lupus LDGs distinctly upregulate MMPs in NETs and are primed for enhanced NETosis, subsequent experiments compared solely control neutrophils with lupus LDGs and did not examine normal density lupus neutrophils.
Figure 1. Active MMP-9 and MMP-25 decorate NETs and this is enhanced in lupus LDGs.
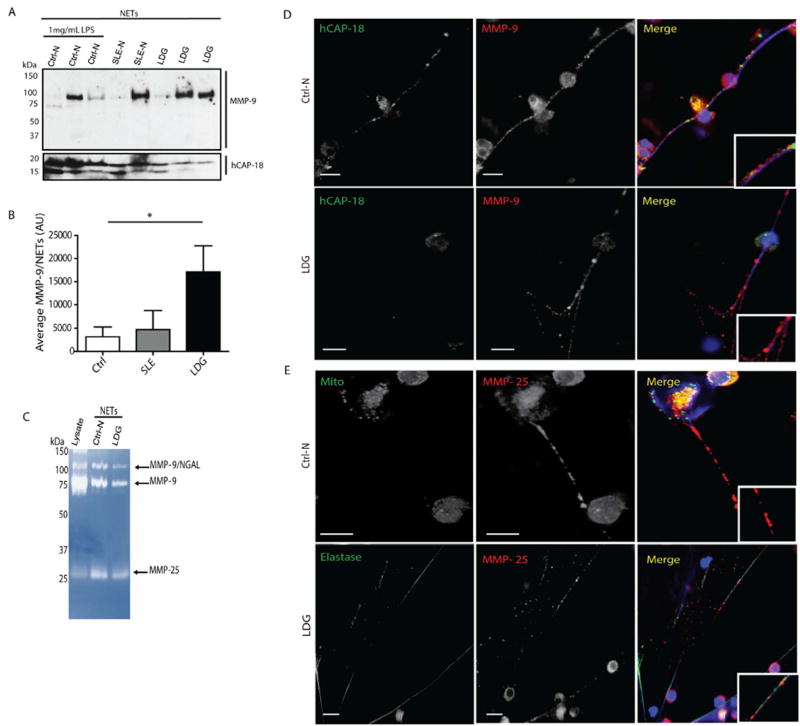
A. MMP-9 and human cationic antimicrobial protein (h-CAP18; LL-37 precursor) were quantified by immunoblot in purified NETs from LPS-stimulated controls (Ctrl-N) and spontaneously formed NETs by lupus neutrophils (SLE-N) and lupus LDGs (LDG) (n=2-3/group). B. Densitometry of MMP-9 in NETs is displayed mean arbitrary units (AUs) ± SEM; n=3 per group; p<0.05); C. Zymogram displaying MMP-9 and MMP-25 activities in purified NETs, when compared to neutrophil lysate. D. and E Representative images where red represents MMP-9 or MMP-25; green represents hCAP-18, mitochondria (Mito) or elastase, and blue is Hoechst. Scale bars, 10 μm.
MMPs contained in NETs damage the endothelium and activate MMP-2
To gain further insight into the role of LDGs NETs in endothelial cell (EC) damage, HUVECs were incubated with LPS-induced digested or intact NETs from control neutrophils, degranulated supernatant from LPS-induced neutrophils or with spontaneously-generated (unstimulated) NETs from lupus LDGs. Distinct morphological changes were evident after 3 h incubation with digested or intact NETs, when compared to untreated HUVECs or to cells exposed to supernatants from degranulated neutrophils (Figure 2A and Figure S1A). A significant proportion of HUVECs exposed to NETs lost their “cobblestone” appearance and acquired round-like shape. NETs modified contour, area and perimeter of ECs, indicating NETinduced endothelial cytotoxicity. Accumulation of NET-derived MMP-9 in HUVECs’ membrane was evident after incubation with control neutrophil and lupus LDG-NETs, although it was more prominent in the latter (Figure 2B). These observations further support that LDG-derived NETs externalize higher amounts of MMP-9 than control NETs. That MMP-9 was bound to NETs was further confirmed by assessing co-localization of this molecule with LL-37 (Figures 2C and Figure S2), an antimicrobial peptide previously reported to be externalized in NETs.[5 11] NET-derived MMP-9 was in direct contact with HUVECs’ membranes and clustered in HUVEC plasma membrane after 3 h of co-incubation. Vesicle-like structures containing MMP-9 and LL-37 present in distinct areas of the cell plasma membrane were visualized in the HUVECs (Figure 2B-C and Figure S2).
Figure 2. NETs activate endothelial MMP-2 and promote endothelial damage.
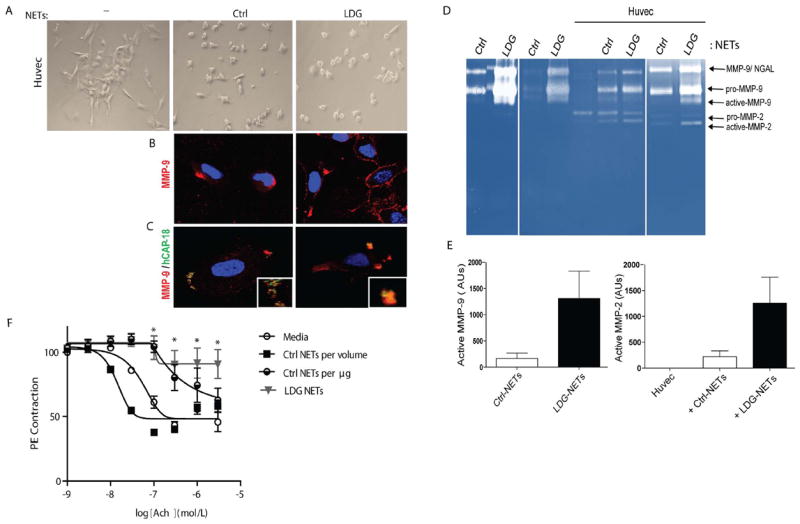
A. HUVECs were incubated for 3 h in the absence (-) or presence of control neutrophil (Ctrl) or lupus LDGs NETs. Images display morphological changes induced by NETs. B-C. Localization of NET proteins in HUVECs’ plasma membrane. D. Zymography analysis of MMP activity in control and lupus NETs before and after 3 h incubation with HUVECs; arrows indicate inactive and active MMP-2, MMP-9 and a complex between MMP-9 and neutrophil gelatinase associated lipocalin (NGAL). E. Elevated levels of active MMP-9 in LDGs-NET is associated with activation of endothelial MMP-2 following incubation with NETs. Results are presented as AUs and represent 3 experiments. F. NETs impair endothelium-dependent vasorelaxation in response to acetylcholine (Ach). Comparisons are made using NETs isolated from the same number of LPS-stimulated control neutrophils (Ctrl NETs per volume) and spontaneously-generated in lupus LDGs (LDG NETs), or equal concentrations of control NETs (Ctrl NETs per μg) and LDG NETs. Results represent mean ± SEM % relaxation of 3 independent experiments; *p< 0.05.
Latent MMP-2 is secreted by ECs and undergoes activation through interaction with others MMPs.[19] Analysis of supernatants from HUVECs alone or incubated with NETs, demonstrated activation of endothelial pro-MMP-2 when NETs were present, but not by exposure to supernatants from degranulated neutrophils (Figure 2D-E and Figure S1C). To further examine the role of NETs on endothelial function, thoracic aorta rings from C57BL/6 female mice were incubated with NETs purified from LPS-stimulated control neutrophils, from spontaneously-formed LDG NETs obtained from equal amounts of cells, or from heat-inactivated NETs, followed by assessment of endothelium-dependent vasorelaxation in response to acetylcholine (Figure 2F and Figure S1B). Significant impairment in endothelium-dependent vasorelaxation was observed in the presence of LDG-NETs, compared with control NETs (Figure 2F). To determine whether differences in vasorelaxation between lupus and controls NETs were related to different amounts generated/cell number, we adjusted the concentration of NETs generated by the control neutrophils neutrophils to NETs generated by lupus LDGs. Under these conditions, control NETs induced significant impairment in vasorelaxation, similar to LDG NETs (Figures 2F). These results indicate that lupus LDG-NETs more potently induce impaired vasorelaxation, likely due to their enhanced capacity to form these structures and to externalization of higher concentrations of molecules displaying negative effects on endothelial function.
MMP-9 promotes endothelial dysfunction
Histones are the most abundant proteins in NETs[20] and have been implicated in endothelial damage, in particular histone H2A.[21] To assess the putative role of MMP-9 in the activation of pro-MMP-2 and its possible implications in endothelial damage, when compared to other NET components, HUVECs and human aortic ECs (HAECs) were incubated with anti-H2A, anti-MMP-9, NETs, NETs depleted of MMP-9, NETs depleted of histone H2A, NETs plus neutralizing anti-MMP-9, or NETs plus MMP inhibitors. Upon depletion or neutralization of MMP-9 or broad inhibition of MMPs, ECs displayed improvements in morphology and cell-cell contact (Figure 3A and figure S3A) and decreases in apoptosis (Figure 3B and supplementary figure 3B). Furthermore, activation of endothelial MMP-2, following addition of NETs, was significantly decreased when MMP-9 was depleted or neutralized in the NETs, when compared to untouched NETs or NETs depleted of histone H2A (Figure 3C-D). These results suggest that MMP-9 externalized in the NETs plays a prominent role in activation of endothelial MMP-2 and contributes to the development of NET-induced vascular cytotoxicity. Indeed, aortic endothelium dependent vasorelaxation was significantly improved when when MMP-9 was neutralized or inhibited following exposure to LDG NETs (Figure 3D). These observations support the hypothesis that MMP-9 externalized in NETs plays a prominent role in inducing EC death and vascular dysfunction.
Figure 3. MMP-9 inhibition abrogates endothelial dysfunction induced by NETs.
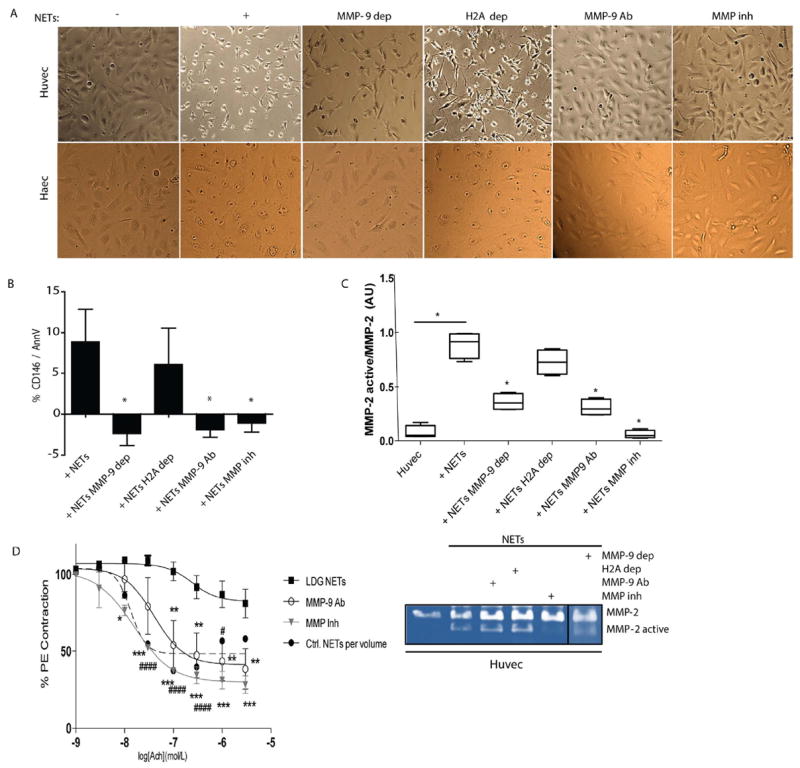
A. HUVECs and HAECs were incubated for 3 h in the absence or presence of NETs (+), MMP-9-depleted (MMP-9 dep)-NETs, histone H2A-depleted (H2A dep)-NETs, NETs plus neutralizing anti-MMP-9 (MMP-9 Ab) or NETs plus MMP inhibitor (MMP inh). Images display HUVEC morphological changes induced by NETs. B. Apoptotic ECs were quantified by FACS. Results are adjusted to background levels (no NETs) and represent mean ± SEM of six independent experiments. C. Zymography analysis performed on supernatants after 3 h incubation. MMP-2 (representative image, bottom) was quantified by densitometry. Results are presented active MMP-2/pro-MMP-2 ratio and expressed as median AUs ± SEM. D Improvement in aortic endothelium-dependent vasorelaxation after incubation with lupus LDG NETs with and without neutralizing anti-MMP-9 (MMP-9 Ab) or MMP inhibitor (MMP inh). Results represent mean ± SEM of two experiments. *p< 0.05, **p< 0.005, ***p<0.001, #### p< 0.0001 for Ctrl vs lupus LDG comparison; acetylcholine (Ach).
Active MMP-9 activates MMP-2 and triggers endothelial cytotoxicity
To corroborate that activation of endothelial MMP-2 by MMP-9 induces endothelial dysfunction, ECs were incubated with pro-MMP-9 or active-MMP-9 for 3 h in serum-free media. Pro-MMP-9 addition induced no changes in morphology or cell-cell contact (Figure 4A). In contrast, ECs incubated with active MMP-9 displayed enhanced apoptosis (Figure 4B) and activation of MMP-2 (Figure 4C). These results support the hypothesis that activation of endothelial MMP-2 by active MMP-9 triggers endothelial dysfunction.
Figure 4. Active MMP-9 induces endothelial damage through MMP-2 activation.
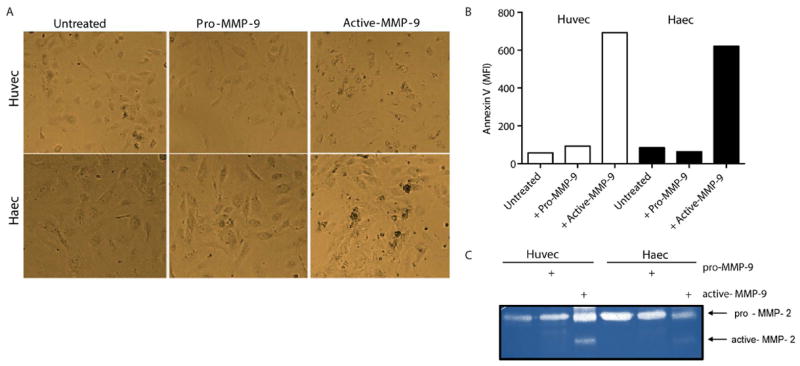
A. Representative images display EC morphological changes induced by MMP-9. B. EC death was quantified by FACS. C. MMP-2 activity was quantified on the supernatants after 3 h of incubation with MMP-9.
ICs containing MMP-9 and anti-MMP-9 Abs are present in SLE and stimulate NET formation
While detectable levels of MMP-9 were identified in all serum samples from healthy controls and SLE patients, significantly higher concentrations were detected in lupus samples (Figure 5A). As ICs play prominent pathogenic roles in SLE, we investigated whether MMP-9 is detected in these complexes in lupus patients. Total IgG was purified from sera obtained from SLE patients with high levels of anti-dsDNA (74-83 IU/mL). Zymography analysis showed that MMP-9 is present in purified IgG fractions isolated from SLE patients, but not from healthy donors (Figure 5B). ICs containing MMP-9, but not irrelevant ICs, significantly enhanced NET formation (Figure 5C and F). To characterize the signaling pathways involved in NET formation induced by lupus MMP-9-containing ICs, we used pharmacologic inhibition of reactive oxygen species (ROS) generation. Both diphenyleneiodonium (DPI, NOX inhibitor) and N-acetylcysteine (NAC, ROS scavenger) significantly decreased NET formation following stimulation with MMP-9-containing ICs (Figure 5C). These results indicate that NETosis induced by SLE-ICs containing MMP-9 requires intact ROS/NOX pathways.
Figure 5. Anti-MMP-9 Abs and ICs containing MMP-9 stimulate NETosis.
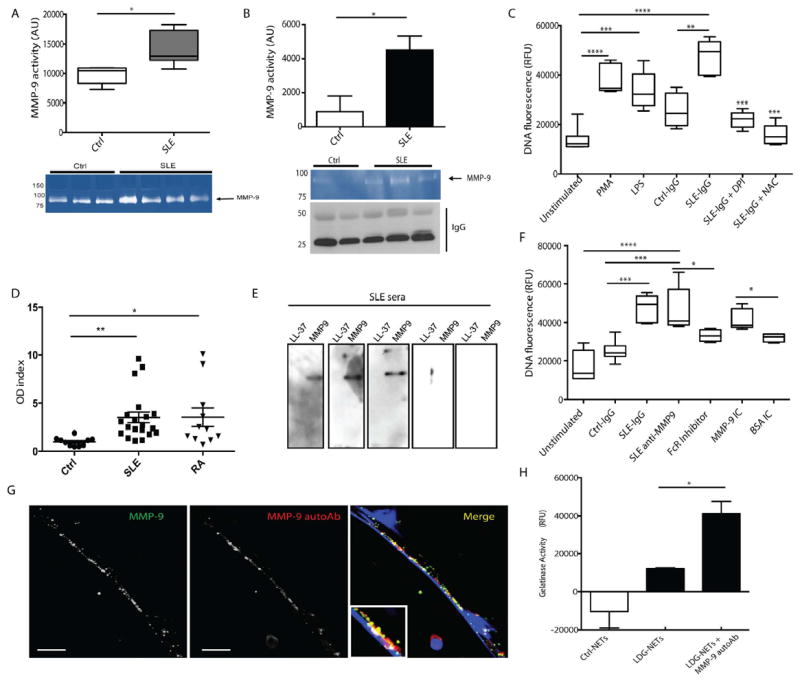
A. MMP-9 activity was quantified in control and SLE sera by zymography. Results represent median AUs ± SEM of three independent experiments. B. MMP-9 activity was quantified in IgG fractions purified from healthy control sera (n=2) or SLE sera with high anti-dsDNA titers (n= 3). Results are expressed as mean AU ± SEM. C. Purified SLE IgG enhances NETosis in control neutrophils through ROS and NOX-dependent processes. PMA and LPS are positive controls. Diphenyleneiodonium chloride (DPI); N-Acetylcysteine (NAC). Results are expressed as relative fluorescent units (RFUs) of DNA fluorescence using SYTOX Green and represent mean ± SEM of three independent experiments. D. Quantification of anti-MMP-9 Abs in SLE (n= 20), RA (n=12) or healthy control (n= 10) sera. Results are expressed as optical density (OD) index (OD in patient serum/ mean OD in control sera). E. Recombinant LL-37 and MMP-9 were resolved in a gradient gel. Membrane was incubated with SLE sera (n=5) and signal detected by chemiluminescence. F. Anti-MMP-9 isolated from SLE sera and MMP-9 ICs enhances NETosis in control neutrophils. This is blocked by FcγR inhibition. Results represent mean ± SEM of three independent experiments. NETs were detected as in 5C. G. Anti-MMP-9 Abs bind to MMP-9 externalized in NETs. H. MMP-9 activity was measured in NETs in presence or absence of anti-MMP-9 autoAb using FITC-coupled gelatin. Results represent mean RFUs± SEM of three independent experiments. Scale bars, 10 μm. *p< 0.05, **p< 0.005, ***p<0.001
Autoantibodies to MMP-9 are present in SLE and stimulate NETosis
Considering the presence of MMP-9 in ICs, we hypothesized that SLE patients develop autoantibodies against MMP-9. Elevated levels of anti-MMP-9 IgG were detected in SLE and RA (another condition characterized by increased NET formation and enhanced CVD risk) sera, compared to control sera (Figure 5D). To corroborate this finding, immunoblot was employed using both recombinant MMP-9 and LL-37. Although none of the SLE sera tested demonstrated anti-LL-37 Abs, three out of five showed positivity for anti-MMP-9 (Figure 5E). Further, enhanced NET formation in control neutrophils was observed after 1 hr incubation with lupus anti-MMP-9 Abs and with MMP-9 ICs but not with irrelevant ICs (Figure 5F), while anti-MMP-9 Abs bound to MMP-9 externalized in the NETs (Figure 5G). Anti-MMP-9 Abs enhanced MMP-9 activity in LDG-NETs (Figure 5H). These results suggest that autoantibodies against MMP-9 are generated and can induce NETosis in SLE, enhancing NET-MMP-9 activity.
Discussion
The cardiovascular risk described in SLE is not fully explained by the Framingham risk equation.[22 23] While accelerated EC apoptosis associated to vascular dysfunction has been reported in SLE,[12 24] the mechanisms leading to this phenomenon remain unclear. Recent evidence implicates accelerated NETosis in endothelial damage in lupus and other conditions. While histones contained in the NETs have been implicated in vessel dysfunction,[21] other molecules present in these structures may also contribute to endothelial damage.
Dysregulation of MMPs has been implicated in atherosclerosis development,[15] but their role in SLE vascular damage had not been systematically addressed. In this study, we found that MMP-9 was externalized at higher levels in LDG NETs and induced EC death and vascular dysfunction through the activation of endothelial MMP-2. Furthermore, MMP-9 autoantibodies and MMP-containing ICs are generated in SLE and contribute to NETosis. In turn, NETs externalize MMP-9 and this phenomenon may perpetuate a vicious cycle leading to endothelial dysfunction (Figure 6).
Figure 6. Proposed model of the role of NET-bound MMP-9 in endothelial dysfunction.
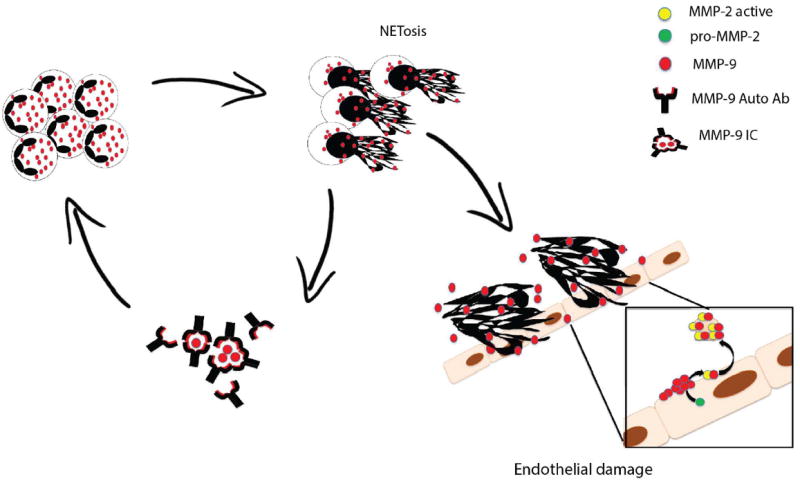
ICs containing MMP-9 and anti-MMP-9 enhance NETosis in LDGs. In turn, LDG-NETs are a source of active MMP-9 that activates pro-MMP-2 in ECs, triggering endothelial dysfunction. LDG-NETs also serve as a source of MMP-9 autoantigen and promote further propagation of a vicious cycle.
Previous work reported that, compared to controls, PBMCs from SLE patients synthesize and release more MMP-9;[25] since LDGs are purified from mononuclear cell fractions,[9 26] it is possible that they represent the main source of this molecule in peripheral blood. Anti-malarials inhibit MMP-9 secretion and restore MMP-9/TIMP-1 balance in SLE patients.[27] As such, the findings in this manuscript suggest an additional mechanism by which these drugs may be immunomodulatory and vasculoprotective in lupus.
This study further supports the concept that lupus autoantibodies contribute to increased NETosis in this disease. Anti-MMP-9 Abs were detected in lupus sera and directly bound to NETs. Further, both ICs containing MMP-9 and anti-MMP-9 Abs triggered NET release and enhance NET-MMP-9 activity; this provides a putative mechanism of enhanced NETosis, similar to what was described for autoAbs to other neutrophil proteins such as LL-37.[3 5] While not fully specific for SLE, as we also detected them in a subset of RA patients, anti-MMP-9 antibodies may represent a marker of vascular risk in autoimmunity that deserves further exploration.
Increased MMP activity in NETs could also contribute to kidney damage in SLE. Deposition of ICs containing MMP-9 disturbs glomerular basement membrane integrity,[28] ICs can induce MMP expression,[29 30] and MMP-9 upregulates gelatinolytic activity within the glomeruli[28] and correlates with chromatin-IgG complex deposition[28] in lupus-prone mice. Therefore, increased MMP activity could alter membrane composition/integrity and promote IC deposition. All these results point at a potentially crucial interaction between MMPs and endothelial health in various compartments in SLE.
MMP-2 activation by MMP-9 can damage endothelial integrity and function.[31] Recent work has demonstrated that MMP-25 can also activate MMP-2.[32] While it is known that neutrophils synthesize and store MMP-9 and MMP-25,[17 18 33] we now demonstrate that they are externalized during NETosis and activate endothelial pro-MMP-2. While we focused on MMP-9, we cannot exclude that MMP-25 also plays a role in endothelial MMP-2 activation, since a broad MMP inhibitor induced better vasorelaxation than anti-MMP-9. While previous evidence indicates that endothelial dysfunction is triggered by histones present in NETs,[21] no significant reduction in cytotoxicity after histone depletion was observed in our system. During NETosis, histones are citrullinated,[34] a phenomenon with potential immunoregulatory properties.[35] Previous studies evaluating EC damage by histones using unmodified histones; therefore, it is possible that posttranslational modifications during NETosis render histones less cytotoxic to the endothelium. This hypothesis should be tested in future studies.
When equal number of control neutrophils and lupus LDGs were compared, control NETs did not induce endothelial dysfunction, supporting the notion that the amount of MMP-9 released during NETosis could determine endothelial integrity. ECs exposed to NETs displayed MMP-9 clusters in the plasma membrane that led to generation of vesicle-like structures. This observation is in agreement with evidence that shed vesicles act as protease carriers[36-38] that may be involved in autocrine regulation. It is possible that vesicles containing MMP-9 could account for increased levels of this metalloproteinase in SLE.
MMP inhibitors have been explored as potential therapeutic approaches in cardiovascular disease and also show promise in animal models.[39] While broad-spectrum MMP inhibitors have given disappointing outcomes in some clinical studies, further research is needed to identify more selective MMP inhibitors and targets to better define therapeutic potential of these molecules. Defining molecular mechanism by which NETs modulate endothelial damage in SLE could provide new potential therapeutic targets in this disease and its associated vascular complications.
Supplementary Material
Acknowledgments
None
Funding information: This work was supported by the National Institutes of Health through the Intramural Research Program at NIAMS and by PHS grants HL088419 and T32AR007080.
Footnotes
Contributorship: Carmelo Carmona-Rivera designed and performed experiments and drafted the manuscript, Wenpu Zhao performed experiments and contributed to statistical analysis, Srilakshmi Yalavarthi performed experiments and contributed to statistical analysis, Mariana Kaplan conceived and designed study and drafted the manuscript.
Competing interests: None
References
- 1.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Knight JS, Luo W, O’Dell AA, et al. Peptidylarginine Deiminase Inhibition Reduces Vascular Damage and Modulates Innate Immune Responses in Murine Models of Atherosclerosis. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.114.303312. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessenbrock K, Krumbholz M, Schonermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15(6):623–5. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lande R, Ganguly D, Facchinetti V, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3(73):73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villanueva E, Yalavarthi S, Berthier CC, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187(1):538–52. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Romo GS, Caielli S, Vega B, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3(73):73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight JS, Zhao W, Luo W, et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013 doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denny MF, Yalavarthi S, Zhao W, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184(6):3284–97. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakkim A, Furnrohr BG, Amann K, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107(21):9813–8. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahlenberg JM, Carmona-Rivera C, Smith CK, et al. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol. 2013;190(3):1217–26. doi: 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajagopalan S, Somers EC, Brook RD, et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103(10):3677–83. doi: 10.1182/blood-2003-09-3198. [DOI] [PubMed] [Google Scholar]
- 13.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26(5):1235–41. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 14.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George SJ. Tissue inhibitors of metalloproteinases and metalloproteinases in atherosclerosis. Curr Opin Lipidol. 1998;9(5):413–23. doi: 10.1097/00041433-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Faber-Elmann A, Sthoeger Z, Tcherniack A, et al. Activity of matrix metalloproteinase-9 is elevated in sera of patients with systemic lupus erythematosus. Clin Exp Immunol. 2002;127(2):393–8. doi: 10.1046/j.1365-2249.2002.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pei D. Leukolysin/MMP25/MT6-MMP: a novel matrix metalloproteinase specifically expressed in the leukocyte lineage. Cell Res. 1999;9(4):291–303. doi: 10.1038/sj.cr.7290028. [DOI] [PubMed] [Google Scholar]
- 18.Kang T, Yi J, Guo A, et al. Subcellular distribution and cytokine- and chemokine-regulated secretion of leukolysin/MT6-MMP/MMP-25 in neutrophils. J Biol Chem. 2001;276(24):21960–8. doi: 10.1074/jbc.M007997200. [DOI] [PubMed] [Google Scholar]
- 19.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 20.Urban CF, Reichard U, Brinkmann V, et al. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8(4):668–76. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 21.Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7(2):e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42(2):338–46. doi: 10.1002/1529-0131(199902)42:2<338::AID-ANR17>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2331–7. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 24.Denny MF, Thacker S, Mehta H, et al. Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 2007;110(8):2907–15. doi: 10.1182/blood-2007-05-089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matache C, Stefanescu M, Dragomir C, et al. Matrix metalloproteinase-9 and its natural inhibitor TIMP-1 expressed or secreted by peripheral blood mononuclear cells from patients with systemic lupus erythematosus. J Autoimmun. 2003;20(4):323–31. doi: 10.1016/s0896-8411(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 26.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–23. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lesiak A, Narbutt J, Sysa-Jedrzejowska A, et al. Effect of chloroquine phosphate treatment on serum MMP-9 and TIMP-1 levels in patients with systemic lupus erythematosus. Lupus. 2010;19(6):683–8. doi: 10.1177/0961203309356455. [DOI] [PubMed] [Google Scholar]
- 28.Tveita A, Rekvig OP, Zykova SN. Glomerular matrix metalloproteinases and their regulators in the pathogenesis of lupus nephritis. Arthritis Res Ther. 2008;10(6):229. doi: 10.1186/ar2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blom AB, Radstake TR, Holthuysen AE, et al. Increased expression of Fcgamma receptors II and III on macrophages of rheumatoid arthritis patients results in higher production of tumor necrosis factor alpha and matrix metalloproteinase. Arthritis Rheum. 2003;48(4):1002–14. doi: 10.1002/art.10871. [DOI] [PubMed] [Google Scholar]
- 30.Gibbs DF, Warner RL, Weiss SJ, et al. Characterization of matrix metalloproteinases produced by rat alveolar macrophages. Am J Respir Cell Mol Biol. 1999;20(6):1136–44. doi: 10.1165/ajrcmb.20.6.3483. [DOI] [PubMed] [Google Scholar]
- 31.Fert-Bober J, Leon H, Sawicka J, et al. Inhibiting matrix metalloproteinase-2 reduces protein release into coronary effluent from isolated rat hearts during ischemia-reperfusion. Basic Res Cardiol. 2008;103(5):431–43. doi: 10.1007/s00395-008-0727-y. [DOI] [PubMed] [Google Scholar]
- 32.Velasco G, Cal S, Merlos-Suarez A, et al. Human MT6-matrix metalloproteinase: identification, progelatinase A activation, and expression in brain tumors. Cancer Res. 2000;60(4):877–82. [PubMed] [Google Scholar]
- 33.Kjeldsen L, Bjerrum OW, Askaa J, et al. Subcellular localization and release of human neutrophil gelatinase, confirming the existence of separate gelatinase-containing granules. Biochem J. 1992;287(Pt 2):603–10. doi: 10.1042/bj2870603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu CL, Tangsombatvisit S, Rosenberg JM, et al. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res Ther. 2012;14(1):R25. doi: 10.1186/ar3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilsgard O, A P, Malmsten M, Nordin SL, Linge HM, Eliasson M, Sorenson E, Erjefalt JS, Bylund J, Olin AI, Sorensen OE, Egesten A. Peptidylarginine deiminase present in the airways during tobacco smoking and inflammation can citrullinate the host defense peptide LL-37, resulting in altered activities. Am J Respir Cell Mol Biol. 2012;(46):240–48. doi: 10.1165/rcmb.2010-0500OC. [DOI] [PubMed] [Google Scholar]
- 36.Taraboletti G, D’Ascenzo S, Borsotti P, et al. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160(2):673–80. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ginestra A, Monea S, Seghezzi G, et al. Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT1080 fibrosarcoma cells. J Biol Chem. 1997;272(27):17216–22. doi: 10.1074/jbc.272.27.17216. [DOI] [PubMed] [Google Scholar]
- 38.Dolo V, D’Ascenzo S, Violini S, et al. Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin Exp Metastasis. 1999;17(2):131–40. doi: 10.1023/a:1006500406240. [DOI] [PubMed] [Google Scholar]
- 39.Whittaker M, Floyd CD, Brown P, et al. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem Rev. 1999;99(9):2735–76. doi: 10.1021/cr9804543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


