Abstract
Sleep supports the formation of a variety of declarative and non-declarative memories, and sleep deprivation often impairs these types of memories. In human subjects, natural sleep either during a nap or overnight leads to long-lasting improvements in visuomotor and fine motor tasks, but rodent models recapitulating these findings have been scarce. Here we present evidence that 5 hours of acute sleep deprivation impairs mouse skilled reach learning compared to a matched period of ad libitum sleep. In sleeping mice, the duration of total sleep time during the 5 hours of sleep opportunity or during the first bout of sleep did not correlate with ultimate gain in motor performance. In addition, we observed that reversal learning during the skilled reaching task was also affected by sleep deprivation. Consistent with this observation, 5 hours of sleep deprivation also impaired reversal learning in the water-based Y-maze. In conclusion, acute sleep deprivation negatively impacts subsequent motor and reversal learning and memory.
Keywords: sleep deprivation, skilled reach learning, reversal learning, Y-maze, motor learning
1. Introduction
Mounting evidence supports a role for sleep in both declarative and non-declarative forms of learning and memory (Diekelmann and Born, 2010; Havekes et al., 2012). In human subjects, one of the most robust and reproducible benefits of sleep has been observed with improved motor performance as assessed through a finger tap motor sequence test (MST) (Fischer et al., 2002; Walker et al., 2002). In addition, a greater benefit of sleep was appreciated with increasing complexity of the MST (Kuriyama et al., 2004). Disruption of sleep as a consequence of obstructive sleep apnea is associated with deficits in MST performance (Djonlagic et al., 2012) and in motor cortex plasticity induced by theta burst stimulation (Opie et al., 2013). Anatomically, performance of a MST after sleep preferentially activated the contralateral primary motor cortex, medial prefrontal lobe, hippocampus, and ipsilateral cerebellum (Walker et al., 2005).
A motor learning paradigm in rodents that engages similar circuits to the MST in humans is skilled reach learning, in which an animal learns to reach through a narrow window for a sugar reward pellet (Whishaw et al., 1986) with increases in accuracy over time. A previous study investigating how sleep architecture changes as a function of intensive skilled reach learning in rats showed an increase in slow wave activity in the cortex contralateral to the trained paw (Hanlon et al., 2009); however, change in performance as a function of subsequent sleep or sleep loss was not tested. In this study, we determined the effects acute sleep deprivation mice on subsequent skilled reaching performance.
Cortical circuits mediating reversal learning may also be affected by sleep deprivation. There is evidence both supporting and refuting a role for sleep deprivation on reversal learning, depending on the exact nature of the reversal task. Because the skilled reaching task we utilized herein incorporates a form of reversal learning, we determined the effects of acute sleep deprivation on reversal learning in this task. Choice reversal in a water-based Y-maze is a behavioral task that also allows for assessment of reversal learning by training animals to locate a rescue platform in one arm of the Y-maze and subsequently testing the frequency of reversing this behavior when the rescue platform is moved to the untrained arm. The results of our studies indicate that 5 hours of sleep deprivation after task acquisition impaired motor accuracy and reversal learning during skilled reaching as well as reversal learning in a water-based Y-maze.
2. Methods
2.1. Subjects
Adult C57BL/6 male mice (3–6 months of age) were kept on a 12 h/12 h light/dark schedule with lights on at 7:00 AM (zeitgeber time (ZT) 0) (8:00 AM during daylight savings). Mice were generally group housed (3–4 mice per cage) but were singly housed during skilled reach experiments. Food and water were generally available ad libitum, but food was limited during skilled reach experiments (see 2.3.3 below for details). All experiments were approved by the Institution of Animal Care and Use Committee of the New York University and were carried out in accordance with all National Institutes of Health guidelines.
2.2 Sleep Deprivation and Sleep Quantification
To achieve sleep deprivation (SD) in mice, we used the gentle handling technique involving graded interventions of manual cage tapping, bedding disturbance, and gentle animal stroking (Havekes et al., 2012). Electroencephalographic recordings have shown that these interventions effectively retain animals in a state of wakefulness for several hours (Meerlo et al., 2001) without substantial changes in serum cortisol (Vecsey et al., 2009; Hagewoud et al., 2010a). Acute SD always lasted 5 hours in duration. During skilled reach experiments, SD occurred between ZT 5 and ZT 10. SD during this circadian time was selected based on prior work showing changes in memory behavior, electrophysiology, and cellular signaling as a consequence of SD (Vecsey et al., 2009). Because of the extended duration of the Y-maze experiments, SD occurred between ZT 1 and ZT 6 during those experiments.
Quantification of sleep time in control mice was estimated in real time by a direct observer. Sleep was characterized by behavioral arrest, eye closure, and huddled body posture, or behavioral arrest for > 40 seconds if accompanied by huddled body posture without eye closure. Prior work assessing the inactivity of C57BL/6 mice for > 40 seconds showed 92% agreement with sleep/wake states evaluated by polysomnography (Pack et al., 2007).
2.3 Skilled Reaching Task
2.3.1 Skilled Reaching Chamber
We designed a skilled reaching chamber for mice as previously described (Farr and Whishaw, 2002; Diep et al., 2012). The chamber is a rectangular box constructed of clear acrylic measuring 23 cm long × 6.25 cm wide × 25 cm tall. A 1 cm wide window that runs up the front of the box was centered on the front (narrowest) wall. A shelf (6.25 cm long × 4 cm wide) was mounted at the base of this window outside of the box and was 1.2 cm above the floor. Two indentations spaced 1 cm away from the window and centered on its edges were placed in the shelf to hold sucrose pellets (this placement was intended to urge the mice to use their forepaw to obtain pellets and prevent reaching pellets simply with their tongue).
2.3.3 Habituation and Motivation
A 13-day protocol was used. Single-housed mice were placed on a diet on day 1 in order to achieve 85–90% of their baseline body weight (typically, grams of chow equal to 10% of their baseline weight). Water was available ad libitum throughout. Weights were monitored 6 times across the 13 days. During days 1 and 2, mice were habituated to the skilled reaching chamber for 10-minute periods twice per day. On days 1 and 2, mice were separately habituated to 20 mg sucrose pellets by placing 10 pellets/mouse in the home cages. On days 3 and 4, mice were maintained on the diet but did not undergo further habituation.
2.3.4 Initial Training
Beginning on day 5, mice spent two 15-minute sessions per day in the skilled reaching chamber, once between ZT 3 and ZT 5 and once between ZT 10 and ZT 12. During the initial sesssions in the skilled reach chamber, sucrose pellets were available on the floor, on the shelf within tongue-distance, and on the shelf within reaching distance. As the mice learned that pellets existed within reaching distance across trials, the pellets on the floor and on the shelf within tongue distance were removed, thus making pellets only accessible through reaching. In addition, as mice displayed paw-preference, pellets within reaching distance were placed in only one of the two indentations to reinforce use of the preferred paw. In some instances, mice would experience one rather than two sessions in a day, but all mice completed 11–14 total training sessions across days 5–11.
2.3.5 Skilled Reach Training and Testing with the Non-Preferred Paw
In order to better isolate the effect of sleep deprivation on the motor component of the task (independent of the novel taste and contextual learning), on days 12 and 13 mice were given 10 minutes to reach for sucrose pellets with the non-preferred paw by placing the pellet in the indentation opposite to the one the mouse had been trained on. On day 12, a training session with the non-preferred paw occurred between ZT 4 and ZT 5. Immediately after this, mice experienced conditions of either sleep deprivation via gentle handling or ad libitum sleep for 5 hours. For those mice that were allowed to sleep ad libitum, estimations of total sleep were made by direct observation. An initial immediate 10-minute testing session occurred between ZT 9 and ZT 10 (T5). On day 13, a delayed 10-minute testing session occurred between ZT 4 and ZT 5 (T24). Each 10-minute testing session was recorded with a digital video camcorder (Sony). Primary performance metrics recorded were gain in total success rate (i.e. number successfully consumed pellets per total number of reaches) and fraction of non-preferred paw reaches (i.e. number of reaches with the non-preferred paw per total number of reaches).
2.4 Water Y-Maze Reversal Task
A slightly modified version of the water Y-maze reversal task previously described was utilized (Hoeffer et al., 2008). Mice were habituated to the maze for 15 minutes on day 1 and then returned to their home cage. On day 2 the mice were trained to locate a submerged escape platform (in a pool of obscured water) in either arm of a Y-shaped maze (simple always right or always left arm pattern) for 20 trials. The mice were returned to their home cages after day two of training. On Day 3, the mice were tested to determine whether they achieved an escape success criterion of 4/5 correct. For mice that achieved this criterion, the escape arm was reversed on day 4. Mice had two initial opportunities to find the new escape location before undergoing a period of 5 hours of either ad libitum sleep or sleep deprivation via gentle handling. After this 5-hour period, mice underwent another 30 trials to find the new escape location. Mice were allowed a maximum of 60 seconds to make an arm choice. Mice were not directed to the correct arm if they made an error. If mice made an error in arm choice, they were trapped in the incorrect arm for 20 seconds before being rescued. The inter-trial interval during Days 2 through 4 was 10 minutes. Mice were assigned randomly to either left or right arms at the beginning of training and the researcher was blind to sleep condition during testing.
2.5 Data Analysis
Data were analyzed using SigmaPlot version 11.0. For normally distributed data (gain in fraction of non-preferred paw reaches), analysis consisted of using a two-way repeated measures ANOVA with sleep group (SD vs. ad libitum sleep) as the between-subjects independent variable and time (T5 vs. T24) as the within-subjects independent variable. Post-hoc analyses were completed with Bonferonni correction. Because gain in total success rate was continuous but not normally distributed, Wilcoxon signed rank tests were used to draw comparisons across time within any sleep condition and across sleep conditions at any time point. P-values were adjusted for multiple comparisons. Because Y-maze reversal data was binary (mice scored a 1 for successfully reaching the escape platform and a 0 for not reaching the platform) and not normally distributed, chi-square comparisons of proportions were completed for the sum of all testing blocks. Correlation data was calculated using a simple linear regression model. Results were considered significant at p < 0.05.
3. Results
3.1 Sleep Imparts Gains in Motor Success Across Time That Are Not Achieved After Acute Sleep Deprivation
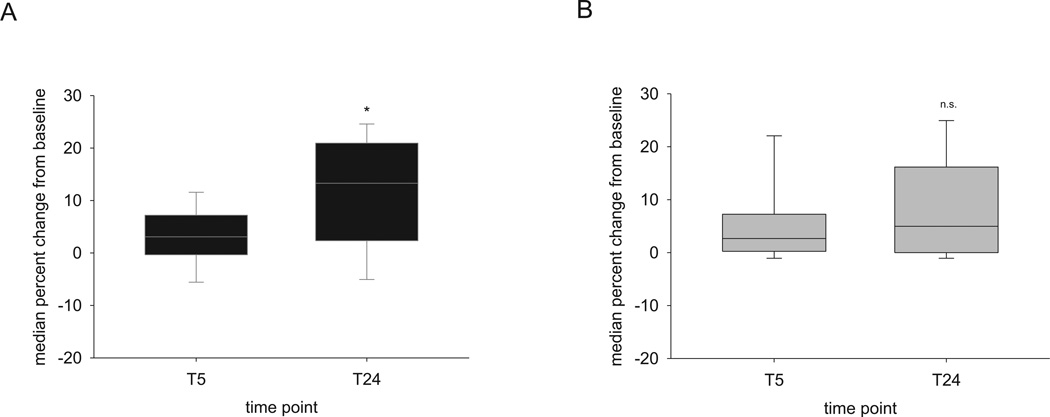
After one week of prior acclimation to the task using the preferred paw, mice were given an initial training period using the non-preferred paw for the first time in the skilled reaching task. There were no significant differences in performance with the preferred paw either in the last three trials during the acclimation period (p = 0.23, Mann-Whitney rank sum test) or in performance during the first opportunity to use the non-preferred paw (p = 0.30, Mann-Whitney rank sum test) between mice subsequently sleep-deprived or not. We then examined the impact of 5 hours of acute sleep deprivation on gain in skilled reaching success using the non-preferred paw immediately after this sleep condition (T5) and one day later (T24). Across mice, when a period of sleep ad libitum occurred after the initial training session with the non-preferred paw, there was a significant increase in the gain in total success rate at T24 compared to T5 (median gain at T24 = 13.3%, 1st quartile = 2.9%, 3rd quartile = 20.2% vs. median gain at T5 = 3.1%, 1st quartile = −0.3, 3rd quartile = 6.2%, Wilcoxon signed rank test, p(adj) = 0.016) that was not seen in mice that experienced 5 hours of acute sleep deprivation (median gain at T24 = 5%, 1st quartile = 0.7, 3rd quartile = 13.6% vs median gain at T5 = 2.7%, 1st quartile = 0.3, 3rd quartile = 6.7%, Wilcoxon signed rank test, p(adj) = 0.152) (Figure 1). Although mice that slept ad libitum showed greater gains in total success rate on average, there were not significantly different gains in total success rate across sleep groups at either T5 or T24.
Figure 1. Gains in motor success during skilled reaching across time do not occur following sleep deprivation.
(A) Box plots showing significant gains in skilled reach success are seen 24 hours following initial training when sleep is intact. (B) There is no significant gain in motor success when sleep is deprived for 5 hours after initial training. n = 14 mice with ad libitum sleep; n = 19 mice with sleep deprivation. *p(adj) = 0.016, Wilcoxon signed rank test. n.s. = p > 0.05.
3.2. Acute Sleep Deprivation Affects Ability of Mice to Adopt Use of Non-Preferred Paw
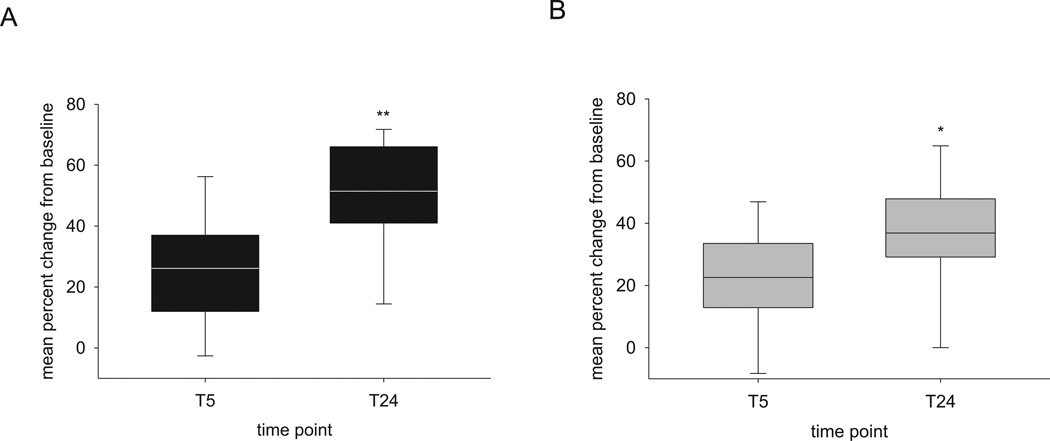
Although the skilled reaching task is designed primarily to test motor performance, the task as we have designed it incorporates a form of reversal learning: switching from using the previously trained preferred paw to the non-preferred paw. During the initial training session using the non-preferred paw, we observed that mice tended to perseverate by using the preferred paw and only slowly switching to using the non-preferred paw. A two way repeated measures ANOVA of gain in fraction of non-preferred paw attempts showed a significant interaction between sleep group and time (F(1,31) = 4.53, p = 0.041), suggesting that the size of the effect of time depends upon the sleep condition. Animals experiencing 5 hours of acute sleep deprivation showed smaller gains in fraction of non-preferred paw attempts, particularly at T24 (36.9% +/− 4.6% for SD vs. 51.5% +/− 5.1% for ad libitum sleep), but simple main effects of time were observed for both sleep conditions (F(1,31) = 57.91, p < 0.001) (Figure 2).
Figure 2. Degree of reversal learning during skilled reaching depends on sleep condition.
A two way repeated measures ANOVA of gain in fraction of non-preferred paw attempts showed a significant interaction between sleep group and time, suggesting that the size of the effect of time depends upon the sleep condition (F(1,31) = 4.53, **p = 0.041). A main effect of time was appreciated in both groups (F(1,31) = 57.91, *p < 0.001). (A) changes with ad libitum sleep (B) changes with acute sleep deprivation. n = 14 mice with ad libitum sleep; n = 19 mice with sleep deprivation.
3.3 Sleep Quantity During Ad Libitum Sleep Does Not Correlate with Changes in Skilled Reaching Performance or Use of Non-Preferred Paw
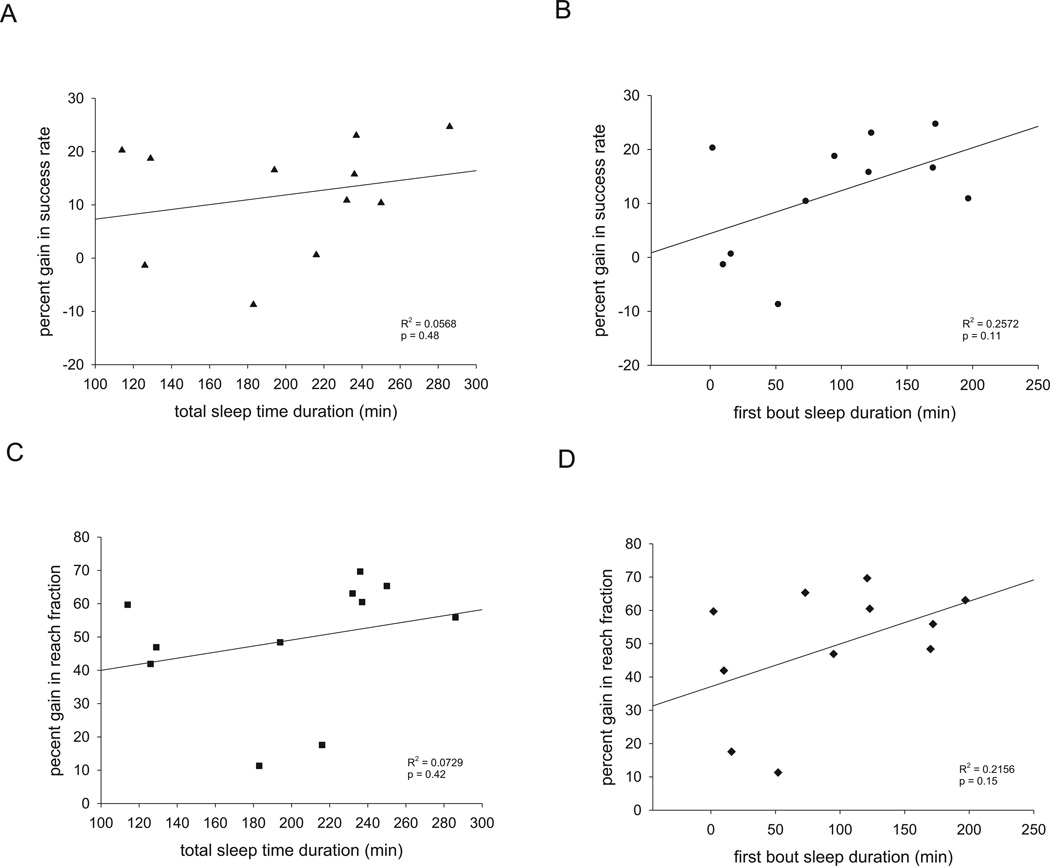
Because mice may sleep for variable amounts of time in any 5 hour period of ad libitum sleep, we examined whether associations existed between either total sleep time or the duration of first bout of sleep and gains in either skilled reaching success or fraction of non-preferred paw reaches in 11 of the 14 mice control mice in whom sleep data was recorded. Neither performance metric was correlated with either measures of total sleep time or the duration of first bout of sleep during the 5 hours of ad libitum sleep (Figure 3).
Figure 3. No correlation between sleep durations and ultimate performance gains.
No correlation existed between (A) total sleep time and gain in success rate at 24 hours, (B) duration of first sleep bout and gain in success rate at 24 hours, (C) total sleep time and gain in fraction of non-preferred paw reaches at 24 hours, or (D) duration of first sleep bout and gain in fraction of non-preferred paw reaches at 24 hours. p > 0.05 for all correlations by simple linear regression.
3.4 Acute Sleep Deprivation Impairs Reversal Learning in the Water Y-Maze
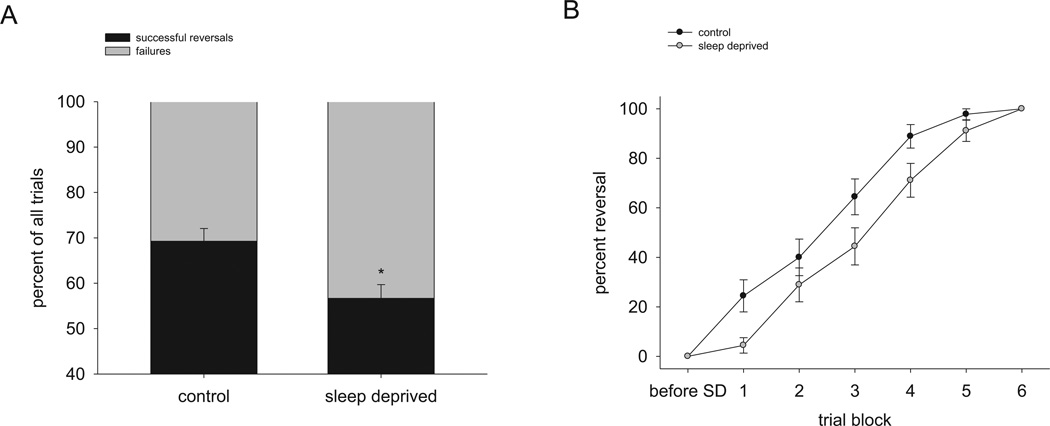
Based on the observation that acute sleep deprivation impaired paw reversal learning during the skilled reaching task, we proceeded to test the effect of acute sleep deprivation on an alternative form of reversal learning using a water-based Y-maze. Mice were trained to locate a rescue platform in one arm of the maze and tested 24 hours later to ascertain long-term memory. Then the mice were give two opportunities to locate the platform in the new opposite location. Following a period of subsequent sleep deprivation for 5 hours, mice perseverated on returning to the previously trained platform location significantly more than mice that slept ad libitum across all 30 trials (56.7% +/− 3.0% reversal success for SD vs. 69.3% +/− 2.8% reversal success for ad libitum sleep, chi-square = 8.65, df = 1, p = 0.003) (Figure 4). Thus, consistent with our finding that acute sleep deprivation impairs reversal learning during skilled reaching, acute sleep deprivation also impairs reversal learning in the water-based Y-maze.
Figure 4. Reversal learning in the water-based Y-maze is impaired following sleep deprivation.
(A) Mice experiencing 5 hours of acute sleep deprivation are impaired in reversal learning in the water Y-maze across all subsequent trials. *p = 0.003, chi-square test. (B) Changes in reversal learning by blocks of 5 trials. SD = sleep deprivation. n = 9 mice per condition.
4. Discussion
In this study, we investigated the effect of 5 hours acute sleep deprivation on skilled reach learning in mice, a form of motor learning that engages cortical plasticity. The vast majority of prior studies investigating relationships between sleep and motor learning have been done in human and non-human primate models. To our knowledge, this is the first study demonstrating a detrimental effect of sleep deprivation on motor learning in a rodent model.
The effect of sleep deprivation on motor learning was most prominent at the delayed test point (24 hours after the start of sleep deprivation) even though a more proximal test period occurred after sleep deprivation. There are two potential interpretations of this observation, which are not mutually exclusive. One interpretation is that the period of sleep deprivation disrupted some offline consolidation of the skills learned during the initial training period (T0), which did not become behaviorally manifested until one day later. Another interpretation is that, because subsequent testing sessions offer continued opportunities to acquire the motor skills, the period of sleep deprivation impaired the opportunity to further acquire these motor skills (i.e. impairs learning) at T5. The inability to optimally improve the motor skill at T5 in the sleep deprived animals then resulted in poorer performance when tested at T24. This latter interpretation is supported by the observation that the gain in reversal percentage in a land-based Y-maze without an additional training trial directly following the period of sleep deprivation was equivalent to mice that had slept normally, although absolute levels of reversal percentage in the sleep deprived mice was less than control mice (Hagewoud et al., 2010b). The observation that neither total sleep time nor duration of the first bout of sleep among animals during the 5 hours of ad libitum sleep correlated with the gain in success rate at T24 perhaps favors also the latter interpretation. However, we did not capture electrophysiological aspects of sleep, such as slow wave activity or sleep spindles, which are thought correlate more strongly with motor success (Huber et al., 2004; Fogel and Smith, 2006; Nishida and Walker, 2007). In addition, although classifying 40 seconds of inactivity as sleep shows 92% agreement with polysomnographically-confirmed sleep/wake states (Pack et al., 2007), this method tends to slightly overestimate total sleep time.
Current models divide motor learning into fast and slow components with “fast” and “slow” being relative terms that depend on the complexity of the motor task being learned (Dayan and Cohen, 2011). The fast component has been associated with decreased activity in the dorsolateral prefrontal cortex, primary motor cortex (M1), and presupplementary motor area and increased activity in the premotor cortex, supplementary motor area, parietal regions, striatum, and parts of the cerebellum as assessed via PET and fMRI (Dayan and Cohen, 2011). Molecular mechanisms underlying M1 cortical plasticity in skilled reaching depend in part on protein synthesis as well as the balance of formation and retraction of dendritic spines. Injection of the protein synthesis inhibitor anisomycin into rat primary motor cortex significantly attenuated skilled reach performance, with no such impairment when anisomycin was injected into parietal cortex or cerebellum (Luft et al., 2004). A smaller but significant effect on skilled reach performance was appreciated when anisomycin was injected into dorsal striatum (Wachter et al., 2010). Anisomycin similarly degraded cortical motor maps resulting from skilled reach training and reduced motor cortical synapse density (Kleim et al., 2003). De novo protein synthesis may have various roles in motor learning, but could be contributing to cortical synaptogenesis (Kleim et al., 2004) and/or dendritic spine formation (Xu et al., 2009) that is observed in the cortex following skilled reach training.
The role of de novo protein synthesis in motor learning and memory is relevant to sleep because the sleep state is generally thought to promote protein synthesis, and sleep disruption is believed to reduce protein synthesis. Radiolabeled amino acid uptake in both rat (Ramm and Smith, 1990) and non-human primate (Nakanishi et al., 1997) brain occurred in a state-dependent fashion, particularly in cortex, with new protein synthesis particularly associated with slow wave sleep. Conversely, analysis of mouse hippocampus after sleep deprivation showed a decrease in transcripts related to mRNA translation in general and decreases more specifically in both total and phosphorylated levels of mammalian target of rapamycin (mTOR), a key regulator of mRNA translation initiation (Vecsey et al., 2012). In addition, cortical spine retraction is regulated by sleep (Yang and Gan, 2012).
The skilled reach motor task as we designed it contains an intrinsic requirement for reversal learning: disinhibiting use of the trained preferred paw for use of the non-preferred paw. We observed deficits in reversal learning after 5 hours of acute sleep deprivation in the skilled reaching task as well as in a water-based Y-maze, a task designed more specifically to test reversal learning. These observations are consistent with prior studies showing deficits in reversal learning in a land Y-maze with food rewards following chronic sleep deprivation (Hagewoud et al., 2010b), although interestingly, in that study a greater deficit in reversal was seen when the chronic sleep deprivation occurred after training trials than after reversal trials. While some forms of spatial reversal learning in rats appear to be resistant to either REM sleep deprivation (Walsh et al., 2011) or 12 hours of total sleep deprivation prior to the opportunity for reversal (Leenaars et al., 2012a), our findings are predominantly consistent with effects of sleep deprivation on related tasks requiring prefrontal and orbitofrontal cortex such as task-switching in both rats (Leenaars et al., 2012b) and humans (Bratzke et al., 2009) and in extra-dimensional set shifting in rats (McCoy et al., 2007).
Overall our results support a negative effect of sleep deprivation on motor learning as well as reversal learning. How the consequences of sleep deprivation compare to either natural sleep or sleep fragmentation, as is observed in many clinical sleep disorders, remains an area of active investigation. Expanding the repertoire of learning and memory affected by sleep deprivation in mouse models is useful for further delineation of the role of sleep stages, their electrophysiological hallmarks, and molecular mechanisms in memory function.
Acute sleep deprivation impairs gains in skilled reach motor learning across time
Acute sleep deprivation impairs reversal learning in a mouse skilled reaching task
Acute sleep deprivation impairs reversal learning in a water-based Y-maze
Acknowledgments
We thank Drs. David Rapoport and Indu Ayappa for experimental insight and critical appraisal of the manuscript and Dr. Akifumi Kishi for statistical help. This work was supported by the philanthropy of the James Kuhn Friends of Sleep Medicine, the NYU CTSA grant UL1TR000038 from the National Center for the Advancement of Translational Science (NCATS) (A.W.V), the American Sleep Medicine Foundation Physician Scientist Training Award (A.W.V.), by NIEHS Training Grant T32ES007267-20 (Principal Investigator William N. Rom), and by NINDS grants NS34007 and NS047384 (E.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bratzke D, Rolke B, Steinborn MB, Ulrich R. The effect of 40 h constant wakefulness on task-switching efficiency. J Sleep Res. 2009;18(2):167–172. doi: 10.1111/j.1365-2869.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72(3):443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Diep AA, Hunsaker MR, Kwock R, Kim K, Willemsen R, Berman RF. Female CGG knock-in mice modeling the fragile X premutation are impaired on a skilled forelimb reaching task. Neurobiol Learn Mem. 2012;97(2):229–234. doi: 10.1016/j.nlm.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djonlagic I, Saboisky J, Carusona A, Stickgold R, Malhotra A. Increased sleep fragmentation leads to impaired off-line consolidation of motor memories in humans. PLoS One. 2012;7(3):e34106. doi: 10.1371/journal.pone.0034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr TD, Whishaw IQ. Quantitative and qualitative impairments in skilled reaching in the mouse (Mus musculus) after a focal motor cortex stroke. Stroke. 2002;33(7):1869–1875. doi: 10.1161/01.str.0000020714.48349.4e. [DOI] [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proc Natl Acad Sci U S A. 2002;99(18):11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Smith CT. Learning-dependent changes in sleep spindles and Stage 2 sleep. J Sleep Res. 2006;15(3):250–255. doi: 10.1111/j.1365-2869.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- Hagewoud R, Havekes R, Novati A, Keijser JN, Van der Zee EA, Meerlo P. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res. 2010a;19(2):280–288. doi: 10.1111/j.1365-2869.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- Hagewoud R, Havekes R, Tiba PA, Novati A, Hogenelst K, Weinreder P, Van der Zee EA, Meerlo P. Coping with sleep deprivation: shifts in regional brain activity and learning strategy. Sleep. 2010b;33(11):1465–1473. doi: 10.1093/sleep/33.11.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon EC, Faraguna U, Vyazovskiy VV, Tononi G, Cirelli C. Effects of skilled training on sleep slow wave activity and cortical gene expression in the rat. Sleep. 2009;32(6):719–729. doi: 10.1093/sleep/32.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes R, Vecsey CG, Abel T. The impact of sleep deprivation on neuronal and glial signaling pathways important for memory and synaptic plasticity. Cell Signal. 2012;24(6):1251–1260. doi: 10.1016/j.cellsig.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Tang W, Wong H, Santillan A, Patterson RJ, Martinez LA, Tejada-Simon MV, Paylor R, Hamilton SL, Klann E. Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron. 2008;60(5):832–845. doi: 10.1016/j.neuron.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430(6995):78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, Monfils MH, Sutherland RJ, Nader K. Functional organization of adult motor cortex is dependent upon continued protein synthesis. Neuron. 2003;40(1):167–176. doi: 10.1016/s0896-6273(03)00592-0. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004;24(3):628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama K, Stickgold R, Walker MP. Sleep-dependent learning and motor-skill complexity. Learn Mem. 2004;11(6):705–713. doi: 10.1101/lm.76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenaars CH, Joosten RN, Kramer M, Post G, Eggels L, Wuite M, Dematteis M, Feenstra MG, Van Someren EJ. Spatial reversal learning is robust to total sleep deprivation. Behav Brain Res. 2012a;230(1):40–47. doi: 10.1016/j.bbr.2012.01.047. [DOI] [PubMed] [Google Scholar]
- Leenaars CH, Joosten RN, Zwart A, Sandberg H, Ruimschotel E, Hanegraaf MA, Dematteis M, Feenstra MG, van Someren EJ. Switch-task performance in rats is disturbed by 12 h of sleep deprivation but not by 12 h of sleep fragmentation. Sleep. 2012b;35(2):211–221. doi: 10.5665/sleep.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM, Ringer T, Dichgans J, Schulz JB. Motor skill learning depends on protein synthesis in motor cortex after training. J Neurosci. 2004;24(29):6515–6520. doi: 10.1523/JNEUROSCI.1034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy JG, Tartar JL, Bebis AC, Ward CP, McKenna JT, Baxter MG, McGaughy J, McCarley RW, Strecker RE. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep. 2007;30(1):52–60. doi: 10.1093/sleep/30.1.52. [DOI] [PubMed] [Google Scholar]
- Meerlo P, de Bruin EA, Strijkstra AM, Daan S. A social conflict increases EEG slow-wave activity during subsequent sleep. Physiol Behav. 2001;73(3):331–335. doi: 10.1016/s0031-9384(01)00451-6. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Sun Y, Nakamura RK, Mori K, Ito M, Suda S, Namba H, Storch FI, Dang TP, Mendelson W, et al. Positive correlations between cerebral protein synthesis rates and deep sleep in Macaca mulatta. Eur J Neurosci. 1997;9(2):271–279. doi: 10.1111/j.1460-9568.1997.tb01397.x. [DOI] [PubMed] [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2(4):e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie GM, Catcheside PG, Usmani ZA, Ridding MC, Semmler JG. Motor cortex plasticity induced by theta burst stimulation is impaired in patients with obstructive sleep apnoea. Eur J Neurosci. 2013 doi: 10.1111/ejn.12203. [DOI] [PubMed] [Google Scholar]
- Pack AI, Galante RJ, Maislin G, Cater J, Metaxas D, Lu S, Zhang L, Von Smith R, Kay T, Lian J, et al. Novel method for high-throughput phenotyping of sleep in mice. Physiol Genomics. 2007;28(2):232–238. doi: 10.1152/physiolgenomics.00139.2006. [DOI] [PubMed] [Google Scholar]
- Ramm P, Smith CT. Rates of cerebral protein synthesis are linked to slow wave sleep in the rat. Physiol Behav. 1990;48(5):749–753. doi: 10.1016/0031-9384(90)90220-x. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li XY, Descalzi G, et al. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461(7267):1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Peixoto L, Choi JH, Wimmer M, Jaganath D, Hernandez PJ, Blackwell J, Meda K, Park AJ, Hannenhalli S, et al. Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus. Physiol Genomics. 2012;44(20):981–991. doi: 10.1152/physiolgenomics.00084.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter T, Rohrich S, Frank A, Molina-Luna K, Pekanovic A, Hertler B, Schubring-Giese M, Luft AR. Motor skill learning depends on protein synthesis in the dorsal striatum after training. Exp Brain Res. 2010;200(3–4):319–323. doi: 10.1007/s00221-009-2027-7. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35(1):205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G. Sleep-dependent motor memory plasticity in the human brain. Neuroscience. 2005;133(4):911–917. doi: 10.1016/j.neuroscience.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Walsh CM, Booth V, Poe GR. Spatial and reversal learning in the Morris water maze are largely resistant to six hours of REM sleep deprivation following training. Learn Mem. 2011;18(7):422–434. doi: 10.1101/lm.2099011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, O'Connor WT, Dunnett SB. The contributions of motor cortex, nigrostriatal dopamine and caudate-putamen to skilled forelimb use in the rat. Brain. 1986;109(Pt 5):805–843. doi: 10.1093/brain/109.5.805. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462(7275):915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Gan WB. Sleep contributes to dendritic spine formation and elimination in the developing mouse somatosensory cortex. Dev Neurobiol. 2012;72(11):1391–1398. doi: 10.1002/dneu.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]