Summary
Chagas disease, caused by the infection with Trypanosoma cruzi, is endemic in all Latin America. Due to the increase in population migration, Chagas disease has spread worldwide and is now considered a health issue not only in endemic countries. While most chronically infected individuals remain asymptomatic, approximately 30% of the patients develop a potentially deadly cardiomyopathy. The exact mechanisms that underlie the establishment and maintenance of the cardiac pathology are not clear. However, there is consistent evidence that immunoregulatory cytokines are critical for orchestrating the immune response and, thus, influence disease development or control. While the asymptomatic (indeterminate) form represents a state of balance between the host and the parasite, the establishment of the cardiac form represents the loss of this balance. Analysis of data obtained from several studies have led to the hypothesis that the indeterminate form is associated with an anti-inflammatory cytokine profile, represented by high expression of IL-10, while cardiac form is associated with a high production of IFN-gamma and TNF-alpha in relation to IL-10, leading to an inflammatory profile. Here, we discuss the immunoregulatory events that might influence disease outcome, as well as the mechanisms that influence the establishment of these complex immunoregulatory networks.
Keywords: Chagas disease, immunoregulation, cytokines, Trypanosoma cruzi, pathology, neuroendocrine
Chagas disease: a complex host-parasite interaction drives polar clinical outcomes
Chagas disease is a major health problem in Latin America, where it affects over 13 million people and millions are at risk of infection (1). Infection with Trypanosoma cruzi via contact with contaminated vectors is still the main route of transmission in endemic countries. However, Chagas disease transmission through blood transfusion and organ transplantation is a serious problem, especially in non-endemic areas (2). The main issue here is the fact that Chagas disease screening was not performed in blood banks in non-endemic countries. Since many donors are asymptomatic and sometimes do not even know that they have the disease, they would donate blood (or organs) and transmit the infection to the healthy recipients. This situation became so critical that several countries, such as the United States, now have a mandatory screening for Chagas disease in blood banks. Two major points arise here: (1) it is critical that this control is maintained, and (2) doctors from non-endemic countries need to become aware of the disease – how to deal with it in terms of diagnostics, clinical care and treatment.
Infection via the oral route has also become very important epidemiologically, as an alarming number of acute cases due to ingestion of contaminated sugar cane or açaí juices have been reported in the past ten years, especially in Brazil. These cases point to the re-emergence of Chagas disease in areas where its transmission was considered interrupted due to successful vector control programs (3). The variety of transmission routes and the spreading of the disease highlight an important issue: although Chagas disease is still a socioeconomic problem, its sustainable control requires joint efforts.
Individuals infected with T. cruzi undergo a short acute phase, characterized by high numbers of parasites in the bloodstream and tissues. Specific therapy is successful in about 70% of the individuals diagnosed shortly after infection (4, 5), but often associated with toxic side effects. If infection is not treated, roughly 5% of individuals die of acute myocarditis, but most individuals progress to the chronic phase, which is accompanied by sub-patent levels of parasitemia. Approximately 60–70% of the chronic patients develop the indeterminate form of Chagas disease and show no clinical symptoms associated with the infection, which is only identified following specific laboratory tests. Over time, however, chronically infected patients may develop the symptomatic forms of Chagas disease, affecting digestive and/or cardiac tissues. The cardiac clinical form is characterized by conductive and/or contractile disruptions in the heart accompanied by high morbidity and mortality (6). Chronic Chagas cardiomyopathy (CCC) poses an economic burden of over $1billion/year due to death or disability, with over 10,000 deaths/year. A critical issue is why some patients remain asymptomatic while others develop such devastating cardiac disease?
Several studies have approached the important issue of what are the events that drive the differential clinical evolution of Chagas disease but, as expected, the answer is multifactorial. Given the fact that the disease is the result of a complex interaction between a parasite and its host, it is natural (and necessary) to consider both parasite- and host-related factors in order to understand this issue. Parasite variability and its possible influence in the immune response and disease evolution are discussed in Box 1.
Box 1. Trypanosoma cruzi variability might influence the different clinical outcomes of Chagas disease.
T. cruzi reproduces predominantly by binary fission. However it is known that sexual events have occurred and, thus, shaped the genetic structure of the current T. cruzi population. These events seem to be rare enough to allow for the propagation of clonal genotypes over time (7). The classification of T. cruzi clonal populations has been highly controversial over the years and numerous authors have attempted to characterize the structure of the T. cruzi population. Analysis of the genetic variability of the T. cruzi population has allowed for its classification into different groups. This genetic variability also translates into biological differences, as demonstrated by the fact that the different groups display distinct host preferences, antigenic variability and tissue tropism, amongst other characteristics (8).
In a satellite meeting held in 1999 during the International Symposium to celebrate the 90th anniversary of the discovery of Chagas disease, T. cruzi was classified into two major groups, named T. cruzi I and T. cruzi II (Anonymous 1999) (8). Advances in the understanding of T. cruzi population diversity collected over the years were discussed in a second satellite meeting, which led to a new consensus in T. cruzi nomenclature published in 2009 by Zingales and colleagues. The new consensus classifies T. cruzi into six “discrete type units” (DTUs), from Tc I to VI (9). Each DTU groups together a set of stocks that are genetically more similar to each other than to any other stock, and are identifiable by common genetic, molecular, or immunological markers (10). Many experimental parameters were used to perform this classification (11) including; in vitro growth and infectivity, pathogenicity in mice, transmissibility through triatomine bugs, and in vitro and in vivo drug sensitivity, as summarized in table 1. However, the fact that there are still variations amongst individuals belonging to the same DTU indicates the importance of clearly determining the biological significance of the DTUs (7).
In Brazil, Tc I and Tc II groups correspond to parasite strains associated with sylvatic and domestic transmission cycles, respectively (Anonymous 1999). Despite the presence of Tc I/T cII populations in vectors and reservoirs in Brazil, the majority of chronic patients carry Tc II strains (19–21). In an outbreak of acute Chagas disease in the south of Brazil in the state of Santa Catarina, a mixed pattern of Tc I/Tc II strains was identified in patients during the acute phase, while only Tc II strains were found after disease chronification (21). This suggests that Tc I is either eliminated by the host’s immune system or is hidden somewhere in the host. While evidence supports the first hypothesis, the second one cannot be completely ruled out. TcI is prevalent in the northern region of Brazil, Central and North America (9, 22). Interestingly, parasites belonging to other DTUs may be found in patients from other Latin American countries. In the northern portion of South America and in Central America, chronic human infection is often associated with Tc I (23). However, Tc V was found to be the most common amongst patients in Bolívia, Argentina and Paraguay, whereas TcVI is the predominant type in the Gran Chaco region (24, 25).
Andrade et al., working with in vitro infection of cardiomyocytes from BALB/c and DBA-2 mice, demonstrated that co-infections with JG (TcII) and Col1.7G2 clone (Tc I) progressed with the selection of the JG strain (Tc II) over time. This selection was due to higher intracellular multiplication of the JG strain (26). Another study conducted using a mixture of Tc I and Tc II strains in murine and human macrophages also showed that Tc II strains have a higher proliferative ability, as well as lower doubling time inside macrophages, as compared to Tc I (27). Such characteristics partially explain the predominance of Tc II in humans. However, the fact that Tc I may be found in humans in other areas such as Bolivia, Chile and Venezuela (17), suggest that distinct genetic characteristics of the population strains can potentially influence the immune response and may also be critical for parasite control/persistence.
It is well established that different T. cruzi clones display distinct tissue tropism. This model is known as the “clonal histotropic model” of Chagas disease (28). Differential tissue tropism was seen in rats co-infected with JG and CL Brener strains (Tc II and Tc VI, respectively). At the end of the acute phase, only JG strain occurred in cardiac muscle, while CL-Brener was only detectable in skeletal muscle and other organs. During the acute phase, CL-Brener was no longer detected and its disappearance prevented CL-Brener-induced mortality (29). Moreover, the different tissue tropisms were correlated with different MHC gene expression in the murine host (30). Studies with Chagas patients also suggested that some MHC alleles could be associated with clinical forms of chronic Chagas disease (31, 32). Despite these findings, it is still difficult to establish a relationship between the genetic diversity of parasite and clinical manifestation of human Chagas disease. While Vago et al. 2000 clearly demonstrated that parasites with different genetic profiles can be found in different tissues (esophagus and heart) in the same patient, D’Ávila et al. were not able to establish a correlation between the genetic profiles of the T. cruzi isolates and different clinical forms of Chagas disease (33). Accordingly, del Puerto et al., analyzing blood samples from 306 chronic Bolivian patients did not find associations between Tc types and clinical manifestation of Chagas disease (34).
An important question is whether parasites belonging to distinct DTUs elicit different immune reactivity in the host, which could be associated with distinct clinical outcomes. Given that genetic variability can influence protein expression, it is possible that differential expression of antigenic molecules by parasites belonging to distinct DTUs influences host immune responses. Burgos and colleagues observed that trans-sialidase (TS) family genes have different distribution among T. cruzi DTUs (35). In murine models, TS proteins are recognized by CD8+ T cells (36) and are also capable of activating CD4+ T cells (37). These results could point to distinct patterns of T cell responses against the proteins that result from the polymorphisms of TS genes. However, a systematic study of the response to TS derived from parasites belonging to different DTUs has not yet been performed.
A study using the experimental model of T. cruzi infection demonstrated that infection with three different strains (all from Tc II) led to the development of cardiac inflammation, associated with high production of IFN-γ and TNF-α and low production of IL-10 during the acute phase of infection, regardless of the strain used in infection (38). It is known that the production of inflammatory cytokines is important for the control of the infection with T. cruzi. Amastigotes from a low virulent Tc I strain failed to trigger a patent infection in vivo due the high susceptibility to IFN-γ (39). Another study showed that strains from Tc I and Tc II present similar susceptibility to IFN-γ-activated macrophages in vitro with similar production of nitrite (27). Thus, the genetic diversity of T. cruzi and its relationship with the host’s immunity is an important aspect that should be clarified in order to provide more information that could aid in the understanding of how different strains influence the host’s immune response and, thus, pathology development and/or control. Moreover, considering the possibility of mixed infections, it is critical to understand the interaction between the different parasite strains. Lastly, clarifying whether the host can preferentially clear the infection with particular strains, or if they become undetectable because they “hide,” is an important point, with consequences for clinical management and treatment.
Host immune responses and the establishment of pathology: immunoregulatory mechanisms and their influence in clinical outcome
The activation of specific cell populations that will perform effector functions is critical for the control of the parasite during the acute phase of Chagas disease. Few studies are available in the literature covering a qualitative analysis of human immune response during acute infection with T. cruzi. However, it has been shown that antibody production, as well as activation of innate mechanisms, seem to be important for parasite control during the acute phase (40). It is noteworthy to mention that parasite control during the acute phase is highly efficient, given the parasitemia reaches subpatent levels as patients enter the chronic phase.
Studies in experimental models of T. cruzi infection have shown that a robust inflammatory response is triggered in the acute phase, with production of inflammatory cytokines, such as IFN-gamma and TNF-alpha, which activate cells to eliminate the parasite (41). This postulate is supported by the findings that IFN-gamma and TNF-alpha knockout animals are highly susceptible to T. cruzi infection, as are nitric oxide-deficient mice (42). Although scarce, studies during the acute phase of human Chagas disease have also shown the production of inflammatory cytokines. This production has been associated with parasite control. Acute myocarditis is also observed in animals (41) and patients (43) during the acute phase, possibly due to this intense inflammatory reaction elicited during the acute phase. However, this inflammation is transient in the great majority of patients, and a likely side effect resulting from the effective response to control the parasitemia that also depends on cell activation and effector functions.
The transition from the acute to the chronic phase is accompanied by a decrease in tissue and blood parasitemia, as well as the control of the inflammatory response observed in the acute phase. This suggests the presence of immunoregulatory mechanisms in the control of this inflammatory response. It is well established that anti-inflammatory cytokines play a key role in controlling inflammatory cytokine production. Amongst the most potent anti-inflammatory cytokines is IL-10, a cytokine produced by immune cells such as monocytes and T cells. Whereas data concerning IL-10 production during the acute phase of human Chagas disease is lacking, it is known that chronic patients produce IL-10 (44). In fact, IL-10 is detected in patients from the early stages of the chronic phase through the late phases (45, 46). Thus, it is possible that the control of the intense inflammatory reaction observed in the acute phase is due to IL-10 production. Interestingly, this control seems to be a well balanced since the production of IL-10 does not immunosuppress the cellular response enough to allow for increased levels of parasites, but it seems to be sufficient to control the establishment of inflammation during the chronic phase, at least in indeterminate patients. Even in patients who develop CCC, disease evolution may take many years, suggesting that some level of immunoregulatory control is present. Analyzing a collection of data published by us, as well as other groups, we hypothesize that while the indeterminate form of Chagas disease is associated with the predominance of an anti-inflammatory environment, the cardiac form is associated with the predominance of an inflammatory profile. Thus, by as of yet undiscovered mechanisms, the evolution to the cardiac clinical form is related to the loss of ability to control the inflammatory immune response, leading to tissue destruction. Host genetic susceptibility seems to play an important role in this process but other unknown mechanisms, such as epigenetic and post-translational events might also be important. Figure 1 summarizes the data that support our hypothesis, which are presented in more detail in the following sections.
Figure 1.

Cytokines play a key role in the orchestration of the immune response: the yin/yang of immunoregulation. This figure depicts the involvement of cytokines, produced by specific cell populations, in the development of Chagas disease. Upon infection, it is important to produce inflammatory cytokines such as IFN-gamma and TNF-alpha, which will activate macrophages to kill the intracellular parasites. However, these cytokines favor the establishment of an inflammatory environment that, if not controlled, may lead to tissue destruction and, thus, the establishment of the cardiac clinical form. On the other hand, if the initial inflammatory environment is controlled by the expression of anti-inflammatory cytokines such as IL-10, this may lead to a balanced response, and the maintenance of the indeterminate clinical form. Several studies have shown that TNF-alpha and IL-10 are mainly produced by monocytes/macrophages, although CD4+ and CD4−CD8−T cells also express these cytokines. IFN-gamma and IL-17 are mainly produced by CD4+ T cells, but CD8+ and CD4−CD8− T cells also express significant percentages of these cytokines. While patients with both clinical forms express inflammatory and anti-inflammatory cytokines, the predominance of an inflammatory environment is observed in cardiac patients, whereas an anti-inflammatory environment is predominantly observed in indeterminate patients.
Indeterminate clinical form: role of IL-10 and IL-17 in maintaining the host/parasite balance
In most patients that proceed to the chronic phase (untreated patients or treated and not cured), the disease is silent. Despite positive serology, exuberant antibody production and a vigorous T cell response to parasite and host antigens (47), such patients, named indeterminate, do not present clinical disease. These patients represent an “ideal” balance between the host and the parasite, given they carry the parasite for years and do not develop disease. Studies concerning immunoregulatory mechanisms in indeterminate patients have shown that, while these patients produce inflammatory cytokines such as TNF-alpha and IFN-gamma (likely important for keeping the parasite in check), they also produce anti-inflammatory cytokines, especially IL-10. In fact, a higher frequency of regulatory T cells (48, 49), monocytes and lymphocytes-producing IL-10 (44) can be found in indeterminate, as compared to cardiac patients. More importantly, the balance between inflammatory/anti-inflammatory cytokines is shifted towards the anti-inflammatory arm of the response in indeterminate patients (44). Recent studies have shown that IL-17 plays a protective role in human Chagas disease, as well as in experimental infection models of T. cruzi infection (50, 51). It has been shown that infection with T. cruzi in murine models lead to the production of IL-17 by CD4+ T cells, CD8+ T cells, NKT and γδT cells (51). In an attempt to study the role of IL-17 in protection, a series of experiments were performed using IL-17A knock out mice, as well as anti-IL-17 treatment. Results showed that while increased IL-17 expression was associated with susceptibility to T. cruzi infection in a murine model using bradykinin receptor 2−/− mice (52), other studies have shown that mice treated with anti-IL-17 and IL-17−/− mice exhibited earlier mortality as compared to controls, and that inhibition of IL-17 also resulted in greater heart inflammation (51, 53). Studies of IL-17 expression in Chagas patients showed that indeterminate patients display a higher frequency of IL-17+ T cells as compared to cardiac patients (50). Moreover, the use of captopril, an anti-hypertensive drug commonly used in Chagas patients that has cardioprotective properties, leads to an increase in IL-17 expression (54), suggesting an association between cardioprotection and IL-17 expression, as observed in experimental models. These studies do not address the direct role of IL-17, but they suggest that this cytokine is important in maintaining the immunoregulatory balance, allowing for parasite control during the chronic phase. Taken together, these data shape the argument that the modulatory environment observed in indeterminate patients allows for the maintenance of the asymptomatic state in these patients. Recent studies have shown that higher production of IL-10 or IL-17 are correlated with better cardiac function in Chagas patients, emphasizing a protective role for these cytokines (50, 55, 56). Thus, in order to avoid pathology establishment, it is critical to understand the mechanisms that control the production of anti-inflammatory cytokines, such as IL-10. A study by our group has shown an association between the occurrence of a functional gene polymorphism in the promoter region of the IL10 gene and the cardiac form of Chagas disease (55). This polymorphism was also related to lower IL-10 production (57). Thus, it is possible that genetic susceptibility to be a lower IL-10 producer may predispose individuals to develop a more intense inflammatory response upon infection with T. cruzi and, thus, cardiomyopathy. While this association poses an interesting possibility, it is also noteworthy to mention that not all cardiac patients display the IL-10 “low-producer” genotype. Thus, other mechanisms of IL-10 expression control, such as epigenetic and post-translational mechanisms, may play an important role in influencing IL-10 production.
Evolution to cardiac disease: lack of immunological control of TNF-alpha and IFN-gamma activities leading to heart inflammation, dysfunction and death
In contrast to the immunoregulatory events observed in indeterminate patients, cardiac individuals display a predominance of inflammatory cytokines in relation to IL-10. A series of studies have shown that, despite the fact that IL-10 production is also observed in cardiac patients, cardiac pathology is associated with an inflammatory response, in which TNF-alpha producing cells are predominant in the damaged tissue (58, 59). Also, T cell clones derived from lymphocytes isolated from heart tissue from cardiac patients display a predominant expression of IFN-gamma over other cytokines (60). Not only locally but also systemically, the production of inflammatory cytokines is evident in cardiac Chagas patients. Ex vivo analysis of cytokine expression showed that cells from cardiac patients display higher production of TNF-alpha as well as IFN-gamma (61). Interestingly, IFN-gamma production has been associated with parasitological cure (62), and a decrease in IFN-gamma expression was also observed in cardiac patients, in another study (63). The association between IFN-gamma and treatment emphasizes the need of an activating-response to potentiate parasitological cure. However, subsequent control of an initial inflammatory response seems critical to avoid pathology, as discussed above.
While monocytes seem to be the predominant source of TNF-alpha within mononuclear cells from cardiac Chagas patients (44), CD4+ T cells seem to be the major source of IFN-gamma in these patients (64). Interestingly, a small subset of lymphocytes, the CD4−CD8− T cells, are important producers of cytokines, despite their relatively low frequency in the peripheral blood of indeterminate and cardiac patients (56). These cells expand upon exposure to trypomastigote forms of T. cruzi in vitro and their cytokine profile expression is consistent with the immunoregulatory mechanisms taking place in indeterminate versus cardiac patients: while in the former they express predominantly IL-10, in the latter their cytokine expression is predominantly of TNF-alpha.
The production of TNF-alpha and IFN-gamma has also been correlated with clinical signs of cardiac pathology. Individuals with higher TNF-alpha production display worse cardiac function, as measured by left ventricular ejection fraction, and a worse prognosis of disease progression (65). Additionally, a study that classified cardiac patients according to the degree of cardiac damage, showed that the worse the degree of cardiomyopathy, the higher the production of the inflammatory cytokine IFN-gamma (64). Thus, it is clear that the immunoregulatory network upon human infection with T. cruzi is very different in indeterminate versus cardiac patients; such profiles also have a clear association with the lack or presence of inflammation-related pathology in indeterminate or cardiac patients, respectively, as summarized in Figure 1.
Although the misbalance between inflammatory and anti-inflammatory cytokine expression is clearly associated with the different clinical outcomes of Chagas disease, it is very difficult to establish whether it is a cause or a consequence of the disease development. Longitudinal studies would be necessary to clarify this point. One can hypothesize, based on the data currently available, that cytokine expression could change during disease progression and that these changes would modify the microenvironment, which would lead to pathology. Now: what leads to such changes? Even in individuals with a genetic predisposition to produce “pathogenic” levels of cytokines, what triggers the misbalanced production? Why is the time of disease evolution different amongst patients? Recent evidence has been suggesting that the interaction of the host immune system with the neuro-endocrine network might play a role in the immunoregulation of Chagas disease. Thus, other apparently unrelated events may interfere with the cytokine expression and could influence disease outcome. While still speculative, this intriguing aspect could shine new light on the understanding of Chagas disease pathology. Below we discuss some pioneering data addressing this point.
Interactions between the neuroendocrine and immune systems in T. cruzi infection: possible pathway of immunoregulation
The immune system is regulated by the central nervous system either directly, by way of the hypothalamic-pituitary-adrenal system (HPA axis) or by the autonomic nervous system (66). Interactions between immune and neuroendocrine systems are largely due to the anatomical connections and sharing of common mediators and cellular receptors. Communication between these various systems appears to be multidirectional with specific hormones, neuropeptides, cytokines and growth factors serving as mediators. Immune cells, in their resting state, or upon activation, express cell surface receptors for these hormones and peptides permitting responses to ligands. Similarly, cells within the neurvous and endocrine systems can express receptors to various immune-derived cytokines, chemokines and growth factors. During states of physical or psychological stress, these various systems release mediators to facilitate crosstalk with eachother, controlling cytokine production and general immune activation. Controlled interactions between these systems are believed to be critical for the maintenance of a homeostatic balance within the body and alterations in these systems in response to disease, stress, injury and/or metabolic alterations can lead to significant changes in immune responsiveness and susceptibility to infections and autoimmune disease states (67).
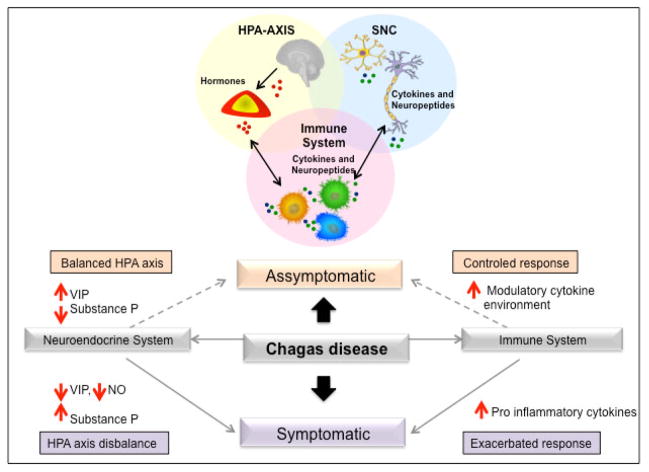
The hypothalamus–pituitary–adrenal (HPA) axis plays a key role in stress responses. Corticotropin-releasing factor (CRH), adrenocorticotropin (ACTH) and glucocorticoids, through activation of the stress response, modulation of pro-inflammatory cytokine secretion, and regulation of peripheral immune response, mediate the control of neuroendocrine–immune interactions (68). Thus, the HPA axis function can be considered as a target in experimental and human Chagas disease. Figure 2 shows a proposed model of the interaction between the HPA-axis, SNC and immune system, as well as the influence of neuropeptides, particularly VIP and substance P, in the development of the different clinical forms of Chagas disease. The data that supports this model is discussed in the following paragraphs. Corrêa-de-Santana and co-workers (69), investigating the HPA axis in mice acutely infected by T. cruzi, observed that parasites can be found in the adrenal gland, whereas a T. cruzi specific polymerase chain reaction (PCR) gene amplification product was found in both adrenal and pituitary glands of infected mice. Moreover, a decrease in CRH and an increase in corticosterone content were detected in the hypothalamus and serum of infected animals, respectively. In contrast, significant changes in the levels of ACTH were not found in sera of infected animals. These findings suggest an imbalance in the circuitry of the HPA axis during the course of infection. Interestingly, it is known that IL-6 is able to directly stimulate both glucocorticoid and ACTH release. In T. cruzi–infected mice, the apparent ACTH-independent increase of glucocorticoid secretion at the adrenal cortex is likely due to an enhancement of circulating IL-1 and/or IL-6 (69). Another study evaluated a possible up-regulation of the cellular immune response triggered by prolactin (PRL), one of several hormones involved in immunoregulation, in rats infected by T. cruzi (70). The data demonstrated that PRL induces the proliferation of T lymphocytes, coupled with an activation of macrophages and the production of nitric oxide (NO), leading to a reduction in the number of blood trypomastigotes during the peak of parasitemia. This work suggests that PRL might be an alternative hormone able to up-regulate the host’s immune system, consequently lowering the pathological effects of T. cruzi infection (70). Interestingly, chagasic patients showed no alterations in PRL levels (71).
Figure 2.
The crosstalk between the HPA-axis, nervous and immune systems. Neuroendocrine hormones, neuropeptides and cytokines produced by immune and neuroendocrine cells are recognized by common receptors expressed in both systems. The released neuroendocrine mediators act on immune cells influencing their function. In turn, molecules produced and released by immune cells, such as cytokines and neuropeptides, affect the response of neuroendocrine system. VIP and substance P influence the expression of immunoregulatory cytokines. While an increase in VIP and decrease in substance P lead to a modulatory cytokine environment, decrease in VIP and increase in substance P lead to an inflammatory cytokine environment, which are associated with the different clinical forms of Chagas disease.
In order to investigate the correlation between potential immunoneuroendocrine abnormalities and the diverse clinical manifestations in Chagas disease, Perez and co-workers (71) studied the features of parallel immunoneuroendocrine responses in patients with different degrees of chronic Chagas myocarditis (indeterminate, mild/moderate or severe cardiopathy). Corroborating with previous studies, they found a systemic inflammatory scenario in patients with severe myocarditis characterized by higher serum levels of TNF-α, IL-6, IL-17, CCL-2, IFN-γ and NO when compared to values found in healthy subjects. This was paralleled by a decreased concentration of dehydroepiandrosterone-sulfate (DHEA-s) and an unbalanced cortisol/DHEA-s ratio. Given that DHEA-s is an adrenal androgen implicated in immunomodulatory mechanisms, the data suggest a lack of satisfactory anti-inflammatory control that might contribute to pathology evolution (71). The determination of the role of such neuroendocrine changes in the pathophysiology of human Chagas disease is still open to investigation, considering the scarcity of clinical studies and the absence of strong evidence of hormonal involvement in chagasic cardiopathy.
Neuropeptides are mediators of the immune and neuroendocrine systems and their participation in Chagas disease development, especially in digestive tract, has been investigated in experimental infection by T. cruzi and in chronically infected patients. Digestive Chagas’ disease is frequently characterized by massive myenteric neuronal loss resulting in megacolon with severely and irreversibly disturbed motility. The distribution of substance P, a neuropeptide with pro-inflammatory activities and vasoactive intestinal peptide (VIP), a neuropeptide with modulatory activities, were investigated in myenteric plexus of mice infected with T. cruzi. There was less intense staining of VIP- and substance P-positive nerve fibers in the infected mice than in the controls. The decrease of substance P and VIP could be the result of denervation of the myenteric plexus observed in those mice, and could be related to the disturbances in intestinal motility observed in the chronic phase of Chagas disease (72). Nascimento and co-workers studied the expression of substance P and VIP in megaesophagus of chagasic individuals, which are known to activate or inhibit local immune cells, respectively (73). Morphometric analyses revealed increased substance P- and decreased VIP-reactive areas in esophageal sections from patients with megaesophagus. Furthermore, in the group of Chagas patients without megaesophagus, the loss of VIP positively correlated with the denervation process. The data suggest that an imbalance between VIP and substance P production results in the reestablishment and maintenance of the inflammatory process, leading to denervation and, consequently, promoting the development of megaesophagus (73). Since in the chronic phase of Chagas disease, the destruction of enteric nervous system (ENS) components leads to megacolon development, a study was performed to verify the regeneration rate of ENS components expressing several neuropeptides (cChat, Substance P, NPY, VIP and NOS) (74). The results demonstrated that the levels of cChat, Substance P, and NPY after regeneration were similar in Chagas patients and non-infected individuals. However, levels of VIP and NOS neuropeptides were increased in Chagas patients when compared with non-infected individuals. It is possible that the increase in the regeneration occurs due to an enhanced destruction of selective neuronal types, since previous studies pointed to selective destruction of VIP and NOS neurons in Chagas patients (75). Silveira and coworkers (76) demonstrated that neurons from myenteric and submucosal plexuses from the dilated portions of the colon from chagasic patients with megacolon presented high levels of Substance P and low levels of NK1 receptor when compared with non-dilated portions of the colon from the same patients, and with non-infected individuals. It is well known that Substance P participates in acute and chronic intestinal inflammation via binding to NK1 receptors inducing secretion of pro-inflammatory cytokines. On the other hand, the expression of pro-inflammatory cytokines inhibits the expression of NK1 receptor, which is a shared element used by both the enteric nervous system and immune system cells to restrict the magnitude of inflammation in the colon (76). Jabari and coworkers (77) have found a decrease in submucosal neuron number and a decrease in mucosal nerve fiber density in chagasic/megacolonic segments. Partial selective survival of special nerve elements was also observed. In the submucosa/mucosa, neurons and nerve fibers staining positive for calretinin (CALR) survive better than those that stain for somatostatin (SOM). VIP is widely co-localized with CALR in both submucosal neurons and mucosal nerve fibers. The same group (78), focusing on the balance of intramuscular excitatory (positive for choline acetyltransferase [ChAT]) and inhibitory (positive for neuronal nitric oxide synthase [NOS], as well as vasoactive intestinal peptide [VIP]) nerve fibers, observed that the intramuscular nerve fiber density was significantly reduced in megacolon segments from chagasic patients. Interestingly, in the myenteric plexus, neurons co-staining for NOS and VIP showed a significant increase. Thus, in chronic Chagas’ disease, VIP might have both neuro-protective functions. The peptide could protect CALR/VIP-neurons and NOS/VIP-neurons from cell death and thereby allow patients to survive for decades through its protective role on the intestinal mucosa, despite the presence of irreversibly disturbed colonic motility (77, 78).
Our group has studied the expression of VIP in Chagas patients with the cardiac form of the disease. In these studies, we have found that VIP expression is lower in patients with worse cardiac function (79). We believe that a decrease of neuropeptides with anti-inflammatory activities might contribute to the exacerbation of the immune response and, indirectly, participate in tissue lesions observed in Chagas disease. However, further research relating neuroendocrine-immune system interactions and its influence in Chagas disease outcome is necessary to elucidate the role of these systems in disease development.
Concluding remarks
There is compelling evidence that immunoregulatory mechanisms are associated with the different clinical forms of Chagas disease. However, the mechanisms that control the establishment of immunoregulatory networks are not completely understood. Genetic and epigenetic events, as well as control of cytokine secretion and signal transduction via cytokine receptors are important issues that remain to be investigated in the context of Chagas disease. However, these mentioned events only deal with the direct response to cytokines. As pointed out in this review, other issues may also influence cytokine responses, such as the neuro-immuno-endocrine interactions. Little is known about this area and further studies will certainly strengthen our knowledge. While Chagas disease is an old disease, the issues that surround its associated pathology are contemporary and, given the fact that the disease is far from being controlled – in fact, we would say the opposite – understanding the immunoregulatory dynamics will form the basis for proposing alternative immune-based therapies and/or adjuvants, as well as prophylactic methods.
Table 1.
Characteristics of the different T. cruzi populations, based on the current classification into discrete type units (DTUs)
| DTU | Pathogenicity in mice | Sensitivity to anti- trypanosome drugs | Transmissibility through triatomine vectors | Cycle | References |
|---|---|---|---|---|---|
| I | High pathogenicity | Low sensitivity | High transmissibility | Domestic and sylvatic | (12), (13), (14), (15), (16). |
| II | Low pathogenicity | High sensitivity | Low transmissibility | Domestic | (17, 18), (13), (14), (15), (16). |
| III | ND | ND | ND | Sylvatic | (17) |
| IV | ND | ND | ND | Sylvatic | (17) |
| V | Low pathogenicity | High sensitivity | Low transmissibility | Domestic | (17), (13), (14), (16). |
| VI | - | - | - | Domestic | (17, 18) |
ND= Not determined
Acknowledgments
We would like to acknowledge all authors whose contributions drove the rational discussed in this article, as well as so many others whose important work we did not cite, given the focus of this paper and space limitations. We also would like to acknowledge the funding agencies: WOD and KJG are CNPq fellows; LMDM is a CAPES fellow; all authors are part of the National Institute of Science and Technology – Tropical Diseases (INCT-DT) and of the Program of Excellence in Chagas disease Research (PRONEX-Chagas/FAPEMIG-CNPq) and thank CNPq, CAPES, FAPEMIG and NIH-NIAID (grant number AI06604403) for support for these studies. All authors wrote the review and generated some of the data cited here.
References
- 1.Moncayo A, Ortiz Yanine MI. An update on Chagas disease (human American trypanosomiasis) Ann Trop Med Parasitol. 2006;100:663–677. doi: 10.1179/136485906X112248. [DOI] [PubMed] [Google Scholar]
- 2.Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas’ Disease in the United States. Clin Microbiol Rev. 2011;24:655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coura JR, Vinas PA. Chagas disease: a new worldwide challenge. Nature. 2010;465:S6–7. doi: 10.1038/nature09221. [DOI] [PubMed] [Google Scholar]
- 4.Sosa-Estani S, Segura EL. Etiological treatment in patients infected by Trypanosoma cruzi: experiences in Argentina. Curr Opin Infect Dis. 2006;19:583–587. doi: 10.1097/01.qco.0000247592.21295.a5. [DOI] [PubMed] [Google Scholar]
- 5.Rassi A, Luquetti AO, Rassi A, Jr, et al. Specific treatment for Trypanosoma cruzi: lack of efficacy of allopurinol in the human chronic phase of Chagas disease. Am J Trop Med Hyg. 2007;76:58–61. [PubMed] [Google Scholar]
- 6.Rocha MO, Teixeira MM, Ribeiro AL. An update on the management of Chagas cardiomyopathy. Expert Rev Anti Infect Ther. 2007;5:727–743. doi: 10.1586/14787210.5.4.727. [DOI] [PubMed] [Google Scholar]
- 7.Brisse S, Henriksson J, Barnabe C, et al. Evidence for genetic exchange and hybridization in Trypanosoma cruzi based on nucleotide sequences and molecular karyotype. Infect Genet Evol. 2003;2:173–183. doi: 10.1016/s1567-1348(02)00097-7. [DOI] [PubMed] [Google Scholar]
- 8.Zingales B, Stolf BS, Souto RP, Fernandes O, Briones MR. Epidemiology, biochemistry and evolution of Trypanosoma cruzi lineages based on ribosomal RNA sequences. Mem Inst Oswaldo Cruz. 1999;94 (Suppl 1):159–164. doi: 10.1590/S0074-02761999000700020. [DOI] [PubMed] [Google Scholar]
- 9.Zingales B, Andrade SG, Briones MR, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 10.Tibayrenc M. Genetic epidemiology of parasitic protozoa and other infectious agents: the need for an integrated approach. Int J Parasitol. 1998;28:85–104. doi: 10.1016/s0020-7519(97)00180-x. [DOI] [PubMed] [Google Scholar]
- 11.Tibayrenc M. Genetic subdivisions within Trypanosoma cruzi (Discrete Typing Units) and their relevance for molecular epidemiology and experimental evolution. Kinetoplastid Biol Dis. 2003;2:12. doi: 10.1186/1475-9292-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brisse S, Dujardin JC, Tibayrenc M. Identification of six Trypanosoma cruzi lineages by sequence-characterised amplified region markers. Mol Biochem Parasitol. 2000;111:95–105. doi: 10.1016/s0166-6851(00)00302-9. [DOI] [PubMed] [Google Scholar]
- 13.Laurent JP, Barnabe C, Quesney V, Noel S, Tibayrenc M. Impact of clonal evolution on the biological diversity of Trypanosoma cruzi. Parasitology. 1997;114 ( Pt 3):213–218. doi: 10.1017/s0031182096008414. [DOI] [PubMed] [Google Scholar]
- 14.Revollo S, Oury B, Laurent JP, et al. Trypanosoma cruzi: impact of clonal evolution of the parasite on its biological and medical properties. Exp Parasitol. 1998;89:30–39. doi: 10.1006/expr.1998.4216. [DOI] [PubMed] [Google Scholar]
- 15.de Lana M, da Silveira Pinto A, Barnabe C, Quesney V, Noel S, Tibayrenc M. Trypanosoma cruzi: compared vectorial transmissibility of three major clonal genotypes by Triatoma infestans. Exp Parasitol. 1998;90:20–25. doi: 10.1006/expr.1998.4304. [DOI] [PubMed] [Google Scholar]
- 16.Pinto AS, de Lana M, Bastrenta B, et al. Compared vectorial transmissibility of pure and mixed clonal genotypes of Trypanosoma cruzi in Triatoma infestans. Parasitol Res. 1998;84:348–353. doi: 10.1007/s004360050409. [DOI] [PubMed] [Google Scholar]
- 17.Brisse S, Barnabe C, Tibayrenc M. Identification of six Trypanosoma cruzi phylogenetic lineages by random amplified polymorphic DNA and multilocus enzyme electrophoresis. Int J Parasitol. 2000;30:35–44. doi: 10.1016/s0020-7519(99)00168-x. [DOI] [PubMed] [Google Scholar]
- 18.Brisse S, Verhoef J, Tibayrenc M. Characterisation of large and small subunit rRNA and mini-exon genes further supports the distinction of six Trypanosoma cruzi lineages. Int J Parasitol. 2001;31:1218–1226. doi: 10.1016/s0020-7519(01)00238-7. [DOI] [PubMed] [Google Scholar]
- 19.Di Noia JM, Buscaglia CA, De Marchi CR, Almeida IC, Frasch AC. A Trypanosoma cruzi small surface molecule provides the first immunological evidence that Chagas’ disease is due to a single parasite lineage. J Exp Med. 2002;195:401–413. doi: 10.1084/jem.20011433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macedo AM, Machado CR, Oliveira RP, Pena SD. Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of chagas disease. Mem Inst Oswaldo Cruz. 2004;99:1–12. doi: 10.1590/s0074-02762004000100001. [DOI] [PubMed] [Google Scholar]
- 21.Steindel M, Kramer Pacheco L, Scholl D, et al. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brazil. Diagn Microbiol Infect Dis. 2008;60:25–32. doi: 10.1016/j.diagmicrobio.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Anez N, Crisante G, da Silva FM, et al. Predominance of lineage I among Trypanosoma cruzi isolates from Venezuelan patients with different clinical profiles of acute Chagas’ disease. Trop Med Int Health. 2004;9:1319–1326. doi: 10.1111/j.1365-3156.2004.01333.x. [DOI] [PubMed] [Google Scholar]
- 23.Miles MA, Cedillos RA, Povoa MM, de Souza AA, Prata A, Macedo V. Do radically dissimilar Trypanosoma cruzi strains (zymodemes) cause Venezuelan and Brazilian forms of Chagas’ disease? Lancet. 1981;1:1338–1340. doi: 10.1016/s0140-6736(81)92518-6. [DOI] [PubMed] [Google Scholar]
- 24.Breniere SF, Bosseno MF, Noireau F, et al. Integrate study of a Bolivian population infected by Trypanosoma cruzi, the agent of Chagas disease. Mem Inst Oswaldo Cruz. 2002;97:289–295. doi: 10.1590/s0074-02762002000300002. [DOI] [PubMed] [Google Scholar]
- 25.Zingales B, Miles MA, Campbell DA, et al. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Andrade LO, Galvao LM, de Meirelles MN, Chiari E, Pena SD, Macedo AM. Differential tissue tropism of Trypanosoma cruzi strains: an in vitro study. Mem Inst Oswaldo Cruz. 2010;105:834–837. doi: 10.1590/s0074-02762010000600018. [DOI] [PubMed] [Google Scholar]
- 27.Pena DA, Eger I, Nogueira L, et al. Selection of TcII Trypanosoma cruzi population following macrophage infection. J Infect Dis. 2011;204:478–486. doi: 10.1093/infdis/jir292. [DOI] [PubMed] [Google Scholar]
- 28.Macedo AM, Pena SD. Genetic Variability of Trypanosoma cruzi:Implications for the Pathogenesis of Chagas Disease. Parasitol Today. 1998;14:119–124. doi: 10.1016/s0169-4758(97)01179-4. [DOI] [PubMed] [Google Scholar]
- 29.Franco DJ, Vago AR, Chiari E, Meira FC, Galvao LM, Machado CR. Trypanosoma cruzi: mixture of two populations can modify virulence and tissue tropism in rat. Exp Parasitol. 2003;104:54–61. doi: 10.1016/s0014-4894(03)00119-x. [DOI] [PubMed] [Google Scholar]
- 30.Freitas JM, Andrade LO, Pires SF, et al. The MHC gene region of murine hosts influences the differential tissue tropism of infecting Trypanosoma cruzi strains. PLoS One. 2009;4:e5113. doi: 10.1371/journal.pone.0005113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruz-Robles D, Reyes PA, Monteon-Padilla VM, Ortiz-Muniz AR, Vargas-Alarcon G. MHC class I and class II genes in Mexican patients with Chagas disease. Hum Immunol. 2004;65:60–65. doi: 10.1016/j.humimm.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Menezes CA, Sullivan AK, Falta MT, et al. Highly conserved CDR3 region in circulating CD4(+)Vbeta5(+) T cells may be associated with cytotoxic activity in Chagas disease. Clin Exp Immunol. 2012;169:109–118. doi: 10.1111/j.1365-2249.2012.04608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Avila DA, Macedo AM, Valadares HM, et al. Probing population dynamics of Trypanosoma cruzi during progression of the chronic phase in chagasic patients. J Clin Microbiol. 2009;47:1718–1725. doi: 10.1128/JCM.01658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.del Puerto R, Nishizawa JE, Kikuchi M, et al. Lineage analysis of circulating Trypanosoma cruzi parasites and their association with clinical forms of Chagas disease in Bolivia. PLoS Negl Trop Dis. 2010;4:e687. doi: 10.1371/journal.pntd.0000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgos JM, Risso MG, Breniere SF, Barnabe C, Campetella O, Leguizamon MS. Differential distribution of genes encoding the virulence factor trans-sialidase along Trypanosoma cruzi Discrete typing units. PLoS One. 2013;8:e58967. doi: 10.1371/journal.pone.0058967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin DL, Weatherly DB, Laucella SA, et al. CD8+ T-Cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2006;2:e77. doi: 10.1371/journal.ppat.0020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todeschini AR, Nunes MP, Pires RS, et al. Costimulation of host T lymphocytes by a trypanosomal trans-sialidase: involvement of CD43 signaling. J Immunol. 2002;168:5192–5198. doi: 10.4049/jimmunol.168.10.5192. [DOI] [PubMed] [Google Scholar]
- 38.Guedes PM, Veloso VM, Caliari MV, et al. Trypanosoma cruzi high infectivity in vitro is related to cardiac lesions during long-term infection in Beagle dogs. Mem Inst Oswaldo Cruz. 2007;102:141–147. doi: 10.1590/s0074-02762007005000003. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues AA, Saosa JS, da Silva GK, et al. IFN-gamma plays a unique role in protection against low virulent Trypanosoma cruzi strain. PLoS Negl Trop Dis. 2012;6:e1598. doi: 10.1371/journal.pntd.0001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krettli AU. The utility of anti-trypomastigote lytic antibodies for determining cure of Trypanosoma cruzi infections in treated patients: an overview and perspectives. Mem Inst Oswaldo Cruz. 2009;104 (Suppl 1):142–151. doi: 10.1590/s0074-02762009000900020. [DOI] [PubMed] [Google Scholar]
- 41.Teixeira MM, Gazzinelli RT, Silva JS. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol. 2002;18:262–265. doi: 10.1016/s1471-4922(02)02283-3. [DOI] [PubMed] [Google Scholar]
- 42.Silva JS, Vespa GN, Cardoso MA, Aliberti JC, Cunha FQ. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect Immun. 1995;63:4862–4867. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marques J, Mendoza I, Noya B, Acquatella H, Palacios I, Marques-Mejias M. ECG manifestations of the biggest outbreak of Chagas disease due to oral Infection in Latin-America. Arq Bras Cardiol. 2013 doi: 10.5935/abc.20130144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souza PE, Rocha MO, Rocha-Vieira E, et al. Monocytes from patients with indeterminate and cardiac forms of Chagas’ disease display distinct phenotypic and functional characteristics associated with morbidity. Infect Immun. 2004;72:5283–5291. doi: 10.1128/IAI.72.9.5283-5291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sathler-Avelar R, Lemos EM, Reis DD, et al. Phenotypic features of peripheral blood leucocytes during early stages of human infection with Trypanosoma cruzi. Scand J Immunol. 2003;58:655–663. doi: 10.1111/j.1365-3083.2003.01340.x. [DOI] [PubMed] [Google Scholar]
- 46.Vitelli-Avelar DM, Sathler-Avelar R, Teixeira-Carvalho A, et al. Strategy to assess the overall cytokine profile of circulating leukocytes and its association with distinct clinical forms of human Chagas disease. Scand J Immunol. 2008;68:516–525. doi: 10.1111/j.1365-3083.2008.02167.x. [DOI] [PubMed] [Google Scholar]
- 47.Dutra WO, Colley DG, Pinto-Dias JC, et al. Self and nonself stimulatory molecules induce preferential expansion of CD5+ B cells or activated T cells of chagasic patients, respectively. Scand J Immunol. 2000;51:91–97. doi: 10.1046/j.1365-3083.2000.00648.x. [DOI] [PubMed] [Google Scholar]
- 48.Araujo FF, Gomes JA, Rocha MO, et al. Potential role of CD4+CD25HIGH regulatory T cells in morbidity in Chagas disease. Front Biosci. 2007;12:2797–2806. doi: 10.2741/2273. [DOI] [PubMed] [Google Scholar]
- 49.de Araujo FF, Correa-Oliveira R, Rocha MO, et al. Foxp3+CD25(high) CD4+ regulatory T cells from indeterminate patients with Chagas disease can suppress the effector cells and cytokines and reveal altered correlations with disease severity. Immunobiology. 2012;217:768–777. doi: 10.1016/j.imbio.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Magalhaes LM, Villani FN, do Nunes MC, Gollob KJ, Rocha MO, Dutra WO. High interleukin 17 expression is correlated with better cardiac function in human Chagas disease. J Infect Dis. 2013;207:661–665. doi: 10.1093/infdis/jis724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyazaki Y, Hamano S, Wang S, Shimanoe Y, Iwakura Y, Yoshida H. IL-17 is necessary for host protection against acute-phase Trypanosoma cruzi infection. J Immunol. 2010;185:1150–1157. doi: 10.4049/jimmunol.0900047. [DOI] [PubMed] [Google Scholar]
- 52.Monteiro AC, Schmitz V, Morrot A, et al. Bradykinin B2 Receptors of dendritic cells, acting as sensors of kinins proteolytically released by Trypanosoma cruzi, are critical for the development of protective type-1 responses. PLoS Pathog. 2007;3:e185. doi: 10.1371/journal.ppat.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.da Matta Guedes PM, Gutierrez FR, Maia FL, et al. IL-17 produced during Trypanosoma cruzi infection plays a central role in regulating parasite-induced myocarditis. PLoS Negl Trop Dis. 2010;4:e604. doi: 10.1371/journal.pntd.0000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coelho dos Santos JS, Menezes CA, Villani FN, et al. Captopril increases the intensity of monocyte infection by Trypanosoma cruzi and induces human T helper type 17 cells. Clin Exp Immunol. 2010;162:528–536. doi: 10.1111/j.1365-2249.2010.04270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costa GC, da Costa Rocha MO, Moreira PR, et al. Functional IL-10 gene polymorphism is associated with Chagas disease cardiomyopathy. J Infect Dis. 2009;199:451–454. doi: 10.1086/596061. [DOI] [PubMed] [Google Scholar]
- 56.Villani FN, Rocha MO, do Nunes MC, et al. Trypanosoma cruzi-induced activation of functionally distinct alphabeta and gammadelta CD4− CD8− T cells in individuals with polar forms of Chagas’ disease. Infect Immun. 2010;78:4421–4430. doi: 10.1128/IAI.00179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 58.Reis DD, Jones EM, Tostes S, Jr, et al. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-alpha+ cells and dominance of granzyme A+, CD8+ lymphocytes. Am J Trop Med Hyg. 1993;48:637–644. doi: 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]
- 59.Higuchi MD, Ries MM, Aiello VD, et al. Association of an increase in CD8+ T cells with the presence of Trypanosoma cruzi antigens in chronic, human, chagasic myocarditis. Am J Trop Med Hyg. 1997;56:485–489. doi: 10.4269/ajtmh.1997.56.485. [DOI] [PubMed] [Google Scholar]
- 60.Abel LC, Rizzo LV, Ianni B, et al. Chronic Chagas’ disease cardiomyopathy patients display an increased IFN-gamma response to Trypanosoma cruzi infection. J Autoimmun. 2001;17:99–107. doi: 10.1006/jaut.2001.0523. [DOI] [PubMed] [Google Scholar]
- 61.Dutra WO, Gollob KJ, Pinto-Dias JC, et al. Cytokine mRNA profile of peripheral blood mononuclear cells isolated from individuals with Trypanosoma cruzi chronic infection. Scand J Immunol. 1997;45:74–80. doi: 10.1046/j.1365-3083.1997.d01-362.x. [DOI] [PubMed] [Google Scholar]
- 62.Bahia-Oliveira LM, Gomes JA, Rocha MO, et al. IFN-gamma in human Chagas’ disease: protection or pathology? Braz J Med Biol Res. 1998;31:127–131. doi: 10.1590/s0100-879x1998000100017. [DOI] [PubMed] [Google Scholar]
- 63.Laucella SA, Postan M, Martin D, et al. Frequency of interferon- gamma -producing T cells specific for Trypanosoma cruzi inversely correlates with disease severity in chronic human Chagas disease. J Infect Dis. 2004;189:909–918. doi: 10.1086/381682. [DOI] [PubMed] [Google Scholar]
- 64.Gomes JA, Bahia-Oliveira LM, Rocha MO, Martins-Filho OA, Gazzinelli G, Correa-Oliveira R. Evidence that development of severe cardiomyopathy in human Chagas’ disease is due to a Th1-specific immune response. Infect Immun. 2003;71:1185–1193. doi: 10.1128/IAI.71.3.1185-1193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Talvani A, Rocha MO, Barcelos LS, Gomes YM, Ribeiro AL, Teixeira MM. Elevated concentrations of CCL2 and tumor necrosis factor-alpha in chagasic cardiomyopathy. Clin Infect Dis. 2004;38:943–950. doi: 10.1086/381892. [DOI] [PubMed] [Google Scholar]
- 66.Pozo D, Delgado M. The many faces of VIP in neuroimmunology: a cytokine rather a neuropeptide? FASEB J. 2004;18:1325–1334. doi: 10.1096/fj.03-1440hyp. [DOI] [PubMed] [Google Scholar]
- 67.Taub DD. Neuroendocrine interactions in the immune system. Cell Immunol. 2008;252:1–6. doi: 10.1016/j.cellimm.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Webster Marketon JI, Glaser R. Stress hormones and immune function. Cell Immunol. 2008;252:16–26. doi: 10.1016/j.cellimm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Correa-de-Santana E, Paez-Pereda M, Theodoropoulou M, et al. Hypothalamus-pituitary-adrenal axis during Trypanosoma cruzi acute infection in mice. J Neuroimmunol. 2006;173:12–22. doi: 10.1016/j.jneuroim.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 70.del Filipin MV, Brazao V, Santello FH, Caetano LC, Toldo MP, do Prado JC., Jr Prolactin: does it exert an up-modulation of the immune response in Trypanosoma cruzi-infected rats? Vet Parasitol. 2011;181:139–145. doi: 10.1016/j.vetpar.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 71.Perez AR, Silva-Barbosa SD, Berbert LR, et al. Immunoneuroendocrine alterations in patients with progressive forms of chronic Chagas disease. J Neuroimmunol. 2011;235:84–90. doi: 10.1016/j.jneuroim.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 72.Maifrino LB, Amaral SO, Watanabe I, Liberti EA, De Souza RR. Trypanosoma cruzi: preliminary investigation of NADH-positive and somatostatin-immunoreactive neurons in the myenteric plexus of the mouse colon during the infection. Exp Parasitol. 2005;111:224–229. doi: 10.1016/j.exppara.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Nascimento RD, Martins PR, de Souza Lisboa A, Adad SJ, Morais da Silveira AB, Reis D. An imbalance between substance P and vasoactive intestinal polypeptide might contribute to the immunopathology of megaesophagus after Trypanosoma cruzi infection. Hum Pathol. 2013;44:269–276. doi: 10.1016/j.humpath.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 74.Moreira MD, Brehmer A, de Oliveira EC, et al. Regenerative process evaluation of neuronal subclasses in chagasic patients with megacolon. Hum Immunol. 2013 Feb;74(2):181–8. doi: 10.1016/j.humimm.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 75.da Silveira AB, D’Avila Reis D, de Oliveira EC, et al. Neurochemical coding of the enteric nervous system in chagasic patients with megacolon. Dig Dis Sci. 2007;52:2877–2883. doi: 10.1007/s10620-006-9680-5. [DOI] [PubMed] [Google Scholar]
- 76.da Silveira AB, Freitas MA, de Oliveira EC, et al. Substance P and NK1 receptor expression in the enteric nervous system is related to the development of chagasic megacolon. Trans R Soc Trop Med Hyg. 2008 Nov;102(11):1154–6. doi: 10.1016/j.trstmh.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 77.Jabari S, da Silveira AB, de Oliveira EC, et al. Selective survival of calretinin- and vasoactive intestinal peptide containing nerve elements in human chagasic submucosa and mucosa. Cellular Tissue Research. 2012;349:473–481. doi: 10.1007/s00441-012-1406-8. [DOI] [PubMed] [Google Scholar]
- 78.Jabari S, da Silveira AB, de Oliveira EC, et al. Preponderance of inhibitory versus excitatory intramuscular nerve fibres in human chagasic megacolon. International Journal of Colorectal diseases. 2012;27:1181–1189. doi: 10.1007/s00384-012-1500-0. [DOI] [PubMed] [Google Scholar]
- 79.Correa MV, da Costa Rocha MO, de Sousa GR, et al. Low levels of vasoactive intestinal peptide are associated with Chagas disease cardiomyopathy. Hum Immunol. 2013 Oct;74(10):1375–81. doi: 10.1016/j.humimm.2013.06.028. [DOI] [PubMed] [Google Scholar]