Abstract
Scope
The long-term effect of exposure to relevant dietary levels of genistein (GEN) on estrogen receptor-positive (ER+) human breast cancer (MCF-7) progression after GEN withdrawal in athymic mice xenograft model was studied.
Materials and methods
Feeding studies were conducted to determine the estrogenic effect of diets on MCF-7 tumor growth: 1) implantation (19 weeks) and withdrawal (6 weeks) of 17β-estradiol (E2); 2) dietary GEN 500 and 750 ppm during treatment/withdrawal for 23/10 and 15/9 weeks, respectively; and, 3) dietary soy protein isolate (SPI) containing GEN 180 ppm for 31/9 weeks of treatment/withdrawal. MCF-7 tumors grew fast in the presence of E2 implantation and abruptly regressed completely after E2 withdrawal. At different rates, dietary GEN alone (500 and 750 ppm) and GEN (180 ppm)-containing SPI stimulated MCF-7 tumor growth. After removal of the stimulus diet, tumors induced by 750 ppm GEN, but not 500 ppm GEN or SPI, regressed completely. The protein expression of epidermal growth factor receptor 2 (HER2) was higher in the GEN- and SPI-induced non-regressing (GINR) tumors compared to MCF-7 and E2 controls.
Conclusion
Long-term consumption of low GEN doses (≤500 ppm) promotes MCF-7 tumor growth and results in GINR tumors with more aggressive and advanced growth phenotypes.
Keywords: athymic mice, breast cancer progression, genistein, MCF-7, soy protein isolate
1 INTRODUCTION
GEN is the major isoflavone in soybeans well-known for its estrogenic properties through binding to estrogen receptors (ER), albeit with different affinities [1]. Preclinical and in vitro studies indicate that GEN induces the transcriptional activation of several estrogen-responsive genes, preferentially through ERβ than ERα, at physiologically relevant doses typical for adults consuming soy foods [2,3]. Soy-containing foods and dietary supplements are the most significant dietary sources of isoflavones [1]. The rise in popularity of products containing isoflavones has come from epidemiological studies in which soy foods, soy protein or isoflavones were associated with health benefits related to menopause, cardiovascular disease and osteoporosis [4]. These health claims have been only partially supported, and have been challenged by new evidence [5].
Breast cancer (BC) is the second leading cause of cancer deaths in U.S. women. In 2013, 232,340 new cases and 39,620 deaths from BC were predicted [6]. Early epidemiological evidence demonstrated a disparity in BC risk between Eastern and Western countries, where 1 in 8 women in the U.S. will be diagnosed with BC during their lifetime as compared to 1 in 30 in Japan [7]. Soy phytoestrogens were singled out as a major contributing factor in the lower BC incidence in Asian countries, despite other prominent differences between these populations [8]. This association, however, has been difficult to explain as elevated levels of circulating estrogens and administration of hormone replacement therapy (HRT) have been associated with increased postmenopausal BC risk while ovariectomy or use of anti-estrogens such as tamoxifen (TAM) and aromatase inhibitors (AI) significantly reduce postmenopausal BC risk [9,10]. As estrogens induce proliferation of normal and malignant mammary cells, public health concerns regarding phytoestrogens and their potential role in BC progression warrants further investigation.
Our group has focused on understanding the role of phytoestrogens, from diets and dietary supplements, on the etiology of BC using ER+ human BC (MCF-7) cells xenografted into ovariectomized athymic mice [11]. This model, which requires estrogen to promote MCF-7 cell growth as xenografts, has been used extensively to study and develop anti-estrogens such as TAM, non-steroidal anti-estrogens (e.g. ICI 182,780) and the TAM analogue idoxifene [12,13]. Using this model, we demonstrated that GEN at physiological concentrations, in either purified glycoside or aglycone forms, or from soy-based sources, stimulated the growth of ER+ human BC and the expression of several ER target genes [11]; however, tumor growth rate was significantly lower than that from E2-stimulated tumors [14,15]. Additionally, we found the extent of soy processing modulated the metabolism of dietary GEN in vivo and affected tumor growth in direct proportion to the internal exposure to GEN aglycone [16,17]. Dietary GEN (15, 150, and 300 ppm)-containing soy protein isolate (SPI, ~90% protein), stimulated the growth of MCF-7 tumors dose dependently, while the less-processed soy flour diet containing equivalent levels of GEN did not [16,18]. These disparities were attributed to the presence of other bioactive components in soybeans that are altered by the degree of processing into soy foods and supplements. Because consumption of dietary supplements, including soy isoflavones, has increased in the US population, even among BC survivors (i.e., to alleviate symptoms of TAM therapy) [19], we studied the combinational effects of TAM or letrozole (a third generation AI) treatment along with GEN consumption. We found that dietary GEN can negate the inhibitory effects of TAM and letrozole on the growth of ER+ MCF-7 tumors in vivo [20,21]. Overall, these studies showed that GEN can act as an ER agonist resulting in the proliferation of ER+ human BC tumors in vivo and that its consumption could interfere with BC therapy.
An important observation from our previous work was that although dietary GEN at low doses (≤500 ppm) stimulated MCF-7 tumor growth, GEN withdrawal did not elicit the same pattern of tumor regression observed with E2 or GEN at higher doses (≥750 ppm) (unpublished data). The current study builds on our previous work by contrasting the effects of low vs. high doses of GEN on BC progression in vivo after long-term GEN consumption followed by its withdrawal.
2 MATERIALS AND METHODS
2.1 Materials
We used Minimal Essential Medium (MEM, without gentamicin, with glutamine) and phenol red free MEM (Mediatech, Inc., Herndon, VA), bovine calf serum (Hyclone, Logan, UT), penicillin/streptomycin and trypsin/EDTA (Invitrogen, Carlsbad, CA), GEN (Indofine Chemical, Inc., Hillsborough, NJ), PROFAM-873 soy protein isolate (local distributor for ADM products, ADM, Decatur, IL), E2 (Sigma, St. Louis, MO), reagents for RNA extraction and purification (Qiagen, Valencia, CA), reagents for cDNA synthesis and qRT-PCR (Bio-Rad, Hercules, CA), reagents for Western blot (Fisher Scientific, Inc., Rockford, IL), antibodies (Santa Cruz Biotech. Inc., Santa Cruz, CA), primers (Integrated DNA Technologies, Inc., Coralville, IA), and laboratory animal diet and dietary components (Dyets, Bethlehem, PA).
2.2 Cell Maintenance
ER+ MCF-7 cells were purchased from American Type Culture Collection, and maintained in MEM supplemented with 5% bovine calf serum, 1% penicillin (100 U/ml)/streptomycin (100 μg/mL), and 1 nM E2. MCF-7 cells were maintained at 37°C in a humidified atmosphere of 5% CO2 in air as a monolayer culture in plastic culture plates (100×20 mm). One week before the cell proliferation assay or the injection of MCF-7 cells into athymic mice, the media was switched to phenol red-free MEM containing 5% charcoal dextran stripped-bovine calf serum [14] and 1% penicillin/streptomycin.
2.3 Diet Formulation
Semi-purified diet (AIN-93G) (Dyets, Bethlehem, PA) served as the control diet. Treatment animals were fed AIN-93G diet plus GEN (500 and 750 ppm) as previously reported [17]. In another study arm, soy protein isolate (PROFAM-873, ADM, Decatur, IL), delivering a low dose of GEN (0.18 g/kg), replaced casein in the AIN-93G diet, similar to that reported earlier [16].
2.4 Animals
Female, ovariectomized athymic BALB/c mice (Charles River Laboratories, Wilmington, MA), were fed AIN-93G diet and acclimated for a week. Mice were maintained under standard 12-hour light/dark cycle) throughout the study. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Illinois.
2.5 Preparation of E2 Pellets and Silastic Implants
E2 supplementation was provided through either silastic implants (Study 1) or pellets (Studies 2 & 3) as described previously by Ju et al. [14,18]. Implant was placed subcutaneously in the interscapular region of each mouse before MCF-7 cell injections.
2.6 Animal Studies
2.6.1 Animal Model Considerations
Using the hormone dependent model of MCF-7 tumor growth, we have observed three distinct phases: initial growth and randomization, treatment, and withdrawal. During the first phase, all animals are implanted with MCF-7 cells on their four flanks and receive E2 implants to promote tumor growth. In the presence of E2 tumors grow fast, while upon its withdrawal, these tumors reduce growth rapidly. Once continuous growth is achieved (tumor cross-sectional area ~20-40 mm2), animals are randomized based on number of tumors, tumor area and body weight into control and treatment groups; and E2 implants are removed from all animals. Animals in the E2 (positive control) and MCF-7 (negative control) group continue on base diets; however, animals in the E2 group receive a new E2 implant. During the treatment phase, animals receive diets modified according to experiments. Tumor growth is monitored weekly by measuring cross-sectional area. Once tumors have achieved a pre-established cut-off area, animals are placed back on control diets; starting the withdrawal phase. Then, tumor growth is monitored until there is no evidence of further growth or until tumor growth reaches an area similar to the treatment phase.
2.6.2 Study 1. Effect of E2 treatment and withdrawal on the growth of E2-stimulated MCF-7 cells implanted into athymic mice
E2 pellets and MCF-7 cells (1×105 cells/40 μL Matrigel®, 4 sites/mouse, 10 mice/group) were implanted into all the mice (n=20). Tumor size was determined using the formula: length/2×width/2×π [14]. Two weeks after E2 pellet implantation, average tumor size reached 18.7 mm2. Mice were divided into two groups, MCF-7 control (no estrogen) and E2 (1:31=E2:cholesterol, 3 mg), and E2 pellets removed from all animals. Mice in the E2 group, however, received E2 (1:31) silastic implants. This dose produces 35-120 pM plasma E2 [15,22], similar to circulating levels observed in postmenopausal women. E2 treatment was monitored for 19 weeks, followed by a withdrawal period (6 weeks) and a re-implantation period (6 weeks) to a total of 31 weeks. Mice were on AIN-93G diet throughout the study.
2.6.3 Study 2. Effect of dietary GEN alone (500 and 750 ppm) during progression and withdrawal on the growth of MCF-7 tumors in athymic mice
Results and more details on MCF-7 tumor growth within the treatment phase with GEN (and in glycoside form) at 750 ppm were published earlier [14,18]. E2 pellets (2 mg E2, 18 mg cholesterol) and MCF-7 cells (1×105 cells/40μL Matrigel®, 4 sites per mouse) were implanted in all mice. When tumors reached an average tumor size of 40±12 mm2, E2 pellets were removed from all animals, and animals were randomly assigned into treatment groups: E2 (13 and 9 mice from 500 and 750 ppm studies, respectively), MCF-7 control (13 and 12 mice from 500 and 750 ppm studies, respectively), 500 ppm GEN (14 mice) and 750 ppm GEN (24 mice). Mice in the E2 and MCF-7 groups continued on AIN-93G diet. Mice in 500 and 750 ppm GEN groups were fed an AIN-93G containing GEN at specified amounts. In the 500 ppm GEN study, the average tumor size reached 95.1 mm2 at week 23. At this point, some animals were euthanized and the rest were placed on a GEN-free diet. Tumor area was monitored for ten weeks (weeks 24-33) during the withdrawal phase.
In the 750 ppm GEN study, the average tumor area (combining aglycone and glycoside observations) reached 114.0 mm2 at week 15. At this point, half of the animals were euthanized and the rest received a GEN free AIN-93G diet. Tumor area was monitored for nine weeks (11 mice total) during the withdrawal phase. At the end of the 9th week of the withdrawal phase, average tumor size was 16.2 mm2. Animals (5 mice) were then placed back on the GEN diet (750 ppm). Tumor area was monitored for seven additional weeks.
2.6.4 Cell Growth Assay In Vitro
To further characterize the non-regressing tumors from the 500 ppm GEN study, six tumors were randomly collected from six mice (404, 444, 472, 474, 486 and 488). Cells from tumors were maintained in media containing 5% charcoal-dextran stripped calf serum for two months. Cell viability of the GEN induced non-regressing (GINR) cells was measured for 4 days using a modified colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The order of cell viability at day 4 was 404=444=472=474=488>486>MCF-7 (Data not shown).
Cells from 472 GINR tumor were tested for their response to E2 and anti-estrogens (TAM, 4-hydroxy TAM, and ICI 182,780) using the MTT assay. Different doses of E2 (0.1, 1, and 10 nM), TAM (1, 5, and 10 μM), 4-hydroxy TAM (4OHTAM; 1, 5, and 10 μM), and ICI 182,780 (0.1, 0.5, and 1 μM) were tested. MCF-7 or GINR cells (1.5×104) were seeded in 1 mL of estrogen-free culture media in a 24-well polystyrene culture plate. After 24 h, cells in each well were washed with 1 mL PBS, and treated with anti-estrogens with or without E2 every 48 h. Finally, cells were treated with MTT reagent for 5 h and with 10% SDS (in 0.01 M HCl) for 12-18 h thereafter. OD was measured at 570 nm and normalized to the number of cells based on a standard curve.
2.6.5 Study 3: Effect of SPI treatment and withdrawal on the growth of MCF-7 tumors
Mice consumed the control AIN-93G diet for three weeks before pellet implantation. Once average tumor cross sectional area reached ~40 mm2, E2 implants were removed from all mice and mice were randomized into three groups: MCF-7 control (10 mice), E2 group (12 mice), and SPI group (18 mice). Animals in the E2 group received new E2 pellets. MCF-7 and E2 groups received AIN-93G diet, and the SPI group consumed AIN-93G with SPI (PROFAM: 180 ppm GEN, 72 ppm daidzein 18 ppm glycitein [23]), replacing casein as the protein source [17]. Tumors from mice in the E2 group were collected after 6 weeks from randomization. After 32 weeks, average tumor area reached 100 mm2. SPI diet was then replaced with AIN-93G control diet. During withdrawal phase, tumor size was monitored until the termination of the study, where animals were euthanized and tumors were collected for further analyses.
2.7 Western Blotting Analysis
Protein expression of HER2 and ERα were examined in GINR (GEN 500) and SPI tumors. Frozen tumors (4 tumors/control and 6 tumors/SPI) were pulverized with mortar and pestle in liquid N2 and lysed and homogenized in radioimmunoprecipitation assay buffer. Homogenates were centrifuged (10,000×g, 10 min, 4°C) and supernatant collected for analysis. Protein from tumors (10-15 μg) was loaded into a 7.5% SDS/PAGE gel, electrophoresed and transferred to nitrocellulose membrane. The membrane was blocked (1 h) in 5% milk with Tris buffered saline with Tween 20- TBST at room temperature, washed 3× with TBST, incubated overnight at 4°C in primary antibody (HER2 and ERα; washed 3× with TBST, incubated (1 h) at room temperature with secondary antibody (goat anti-rabbit-HRP, bovine anti-mouse-HRP, goat anti-rabbit-HRP respectively), and activated with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Inc., Rockford IL). The membrane was exposed to film and developed. Blots were stripped and re-probed with antibody that recognizes HER2, ERα, or β-actin and were analyzed using Image J (NIH: Bethesda, MD). Bands were normalized to β-actin.
2.8 Quantification of Plasma Isoflavones
Total GEN (aglycone plus conjugated forms) in plasma was determined in all animals at the end of the progression phase. Total plasma GEN was determined in mice from the SPI study during the diet withdrawal phase only. Total GEN was determined after complete enzymatic hydrolysis using a H. pomatia preparation containing glucuronidase, sulfatase, and glucosidase activities using a LC-ES/MS/MS method based on isotope dilution quantification of GEN [24].
2.9 Statistics
Data from tumor growth studies and gene expression assays were analyzed using one-way or repeated-measures ANOVA according to the characteristics of the data set using SAS (SAS, Inc.). If the overall treatment F-ratio was significant (P<0.05), the differences between treatment means were tested with Fisher’s least significant difference (LSD) test. All analyses were conducted using SAS.
3 RESULTS
3.1 Study 1: Effect of E2 on the growth of MCF-7 tumors
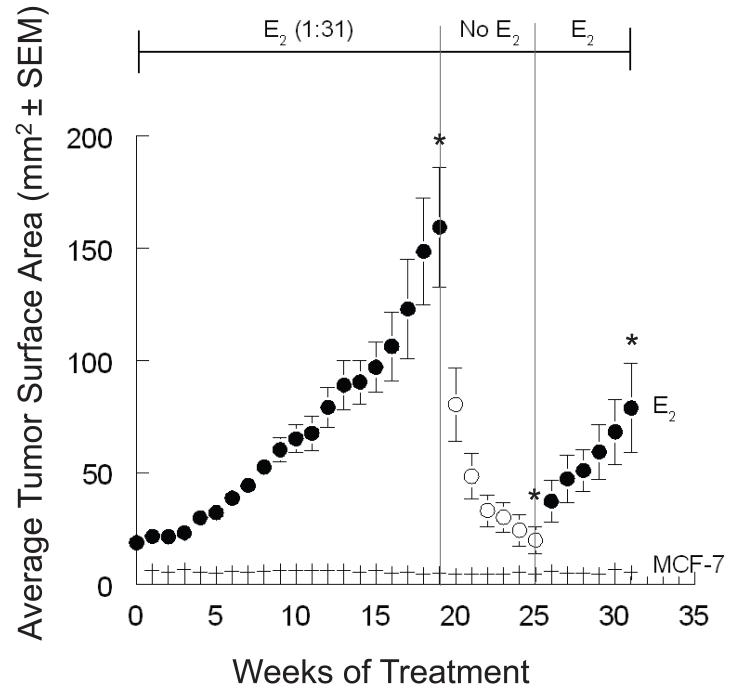
Nineteen weeks after E2 implantation, the average tumor size of this group reached 159.4 mm2. A large proportion of tumors (~88%) grew in the presence of the E2 implant; with only a small minority slowly growing or not at all. Six weeks after the E2 removal (at week 25), the average tumor size was 19.8 mm2. Sixty three percent of tumors regressed to the size of the MCF-7 control. E2 was reimplanted into the mice in this group. Six weeks after E2 reimplantation (week 31), the average tumor size of this group was 78.8 mm2, and the study was terminated (Figure 1).
Figure 1. Effect of E2 implantation and withdrawal on the growth of MCF-7 cells implanted into ovariectomized athymic mice.
Animals were randomly assigned to two treatment groups: MCF-7 (n = 10), and 1:31 E2 (n = 10). A large proportion of tumors in the MCF-7 control were very small for quantification. At week 19, the average tumor size of the E2 group reached 159.4 mm2, and their E2 implants removed. At week 25, the average tumor size of the E2 group was 19.8 mm2, and E2 (1:31) was reimplanted to mice in this group. At week 31, the average tumor size of this group was 78.8 mm2, and the study was terminated. Time points represent tumor surface area (±SEM). Superscripts (*) for the specified time endpoints on the E2 tumor growth curve represent significant differences compared to MCF-7 control (P<0.05).
3.2 Study 2: Effect of dietary GEN (750 and 500 ppm) treatment and withdrawal on the growth of MCF-7 tumors
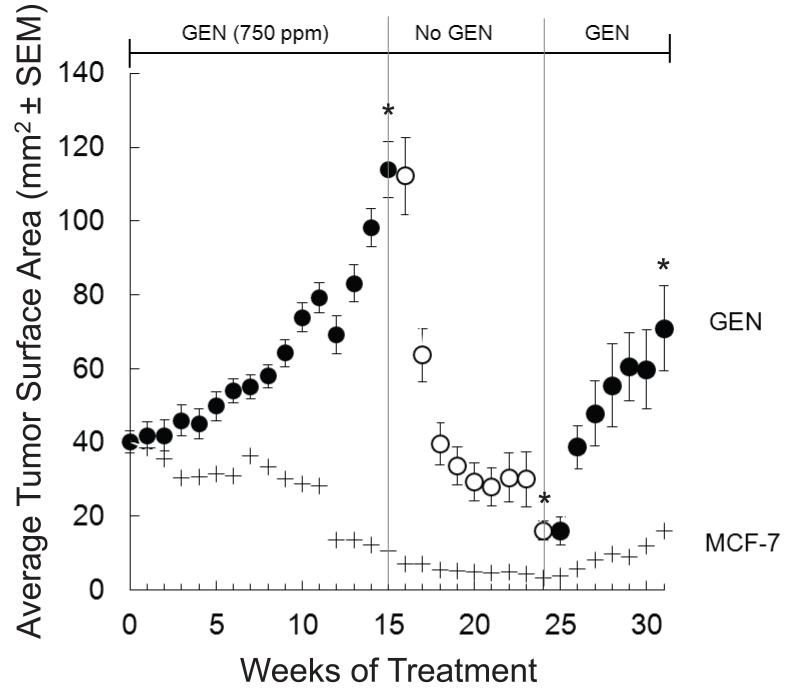
Average tumor size in the GEN 750 ppm group reached 114 mm2 by week 15 (Figure 2). Seventy four percent of implanted tumors grew. A group of mice (11 mice, 42 tumors) were euthanized for analysis and the remaining (11 mice, 42 tumors) were switched to a control diet. Rapid tumor regression was observed, with the average cross-sectional area of 16.2 mm2 (91% of tumors) still statistically larger than those from MCF-7 control mice (Figure 2). A subset of animals from this group (5 mice, 19 tumors) was placed back on the GEN 750 ppm diet and tumor growth was monitored for seven additional weeks. During this period, tumors grew rapidly reaching an average cross-sectional area of 70.9 mm2 by week 31.
Figure 2. Effect of dietary GEN (750 ppm) treatment, withdrawal and re-treatment on the growth of MCF-7 cells implanted into ovariectomized athymic mice.
E2 (2 mg) pellets and MCF-7 cells were subcutaneously implanted into ovariectomized athymic mice. At the randomization point (week 0, 40.0 mm2), E2 pellets were removed from the mice and these were divided into two groups: MCF-7 negative control (AIN-93G, n=12) and 750 GEN (n=24). Mice consumed diets until the average tumor size in the 750 GEN group reached 114.0 mm2 at week 15. At this point, half of the animals were euthanized and the rest were put on a GEN-free AIN-93G diet. Tumor regression was monitored for nine more weeks (11 mice total). At the end of the 9th week of the regression phase, average tumor size was 16.2 mm2. Some animals (n=5) were placed back on the GEN diet (750 ppm). Tumor progression was monitored for seven additional weeks. Tumor growth and body weight were measured weekly. Time points represent tumor surface area (±SEM). Superscripts (*) for the specified time endpoints on the GEN tumor growth curve represent significant differences compared to the MCF-7 control (P<0.05).
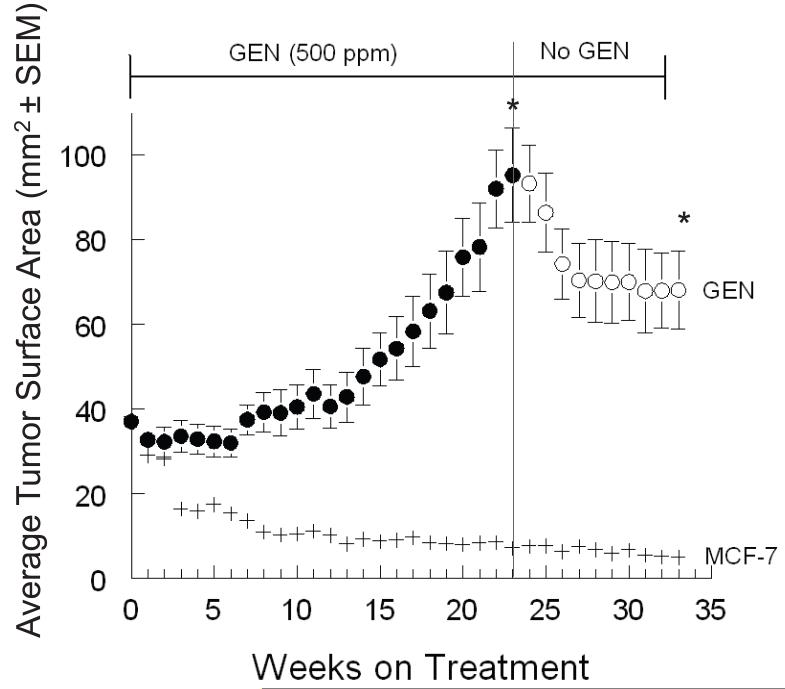
Average tumor size in the GEN 500 group reached 95.1 mm2 at week 23 (Figure 3). Tumor growth was slow in this group. Six mice (total 23 tumors) from GEN 500 and control groups were selected for the withdrawal phase and monitored for nine weeks. During the first three weeks (weeks 24 to 26, Figure 3) a reduction of average tumor size was observed, reaching 74.1 mm2 at week 26. Further size reduction was observed by week 33 (68.0 mm2), however not significant from week 26. At the end of the treatment phase, GEN values were 2.1 ±0.14 μM and 1.79 ±0.32 μM for GEN (total) in plasma of animals from the 750 and 500 ppm groups, respectively [25,26].
Figure 3. Effect of dietary GEN (500 ppm) treatment and withdrawal on the growth of MCF-7 cells implanted into ovariectomized athymic mice.
E2 (2 mg) pellets and MCF-7 cells were subcutaneously implanted into ovariectomized athymic mice. At randomization point (week 0), E2 pellets were removed from mice and these divided into two groups: MCF-7 control (AIN-93G only) and GEN 500 ppm (n=8 mice). Mice consumed treatment diets until the average tumor size of the 500 GEN group was 95.1 mm2 at week 23. At that time, mice in the GEN group were switched to the control diet until week 33, and non-regressing tumors were collected. Time points represent tumor surface area (±SEM). Superscripts (*) for the specified time endpoints on the GEN tumor growth curve represent significant differences compared to the MCF-7 control (P<0.05).
3.3 Study 3: Effect of dietary SPI treatment and withdrawal on the growth of MCF-7 tumors
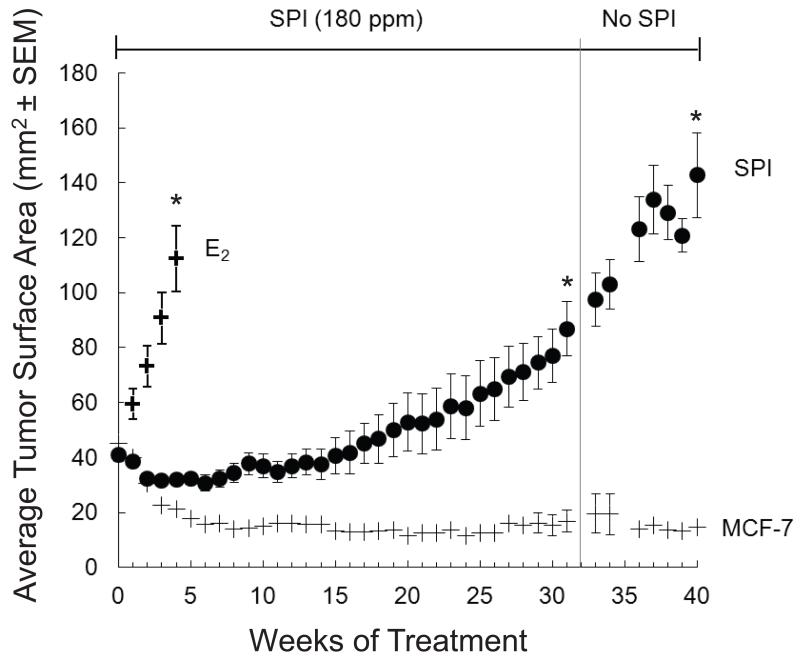
Upon E2 pellet removal and diet change, tumors slowly regressed from randomization (40 mm2), reaching an average cross-sectional area of 30 mm2 by week 12 of the treatment phase (Figure 4). Thereafter, tumors grew slowly. After 32 weeks, SPI diets were replaced with control diets for those animals with growing tumors at or above 100 mm2 (11 animals, 44 tumors). Fifty eight percent of tumors continued growing to a final tumor size of 142.7 mm2, and only twelve percent regressed completely (Figure 4). Diets were not replaced in seven of the original cohort of animals consuming SPI diet during the withdrawal period; these were only used to determine isoflavone content in plasma and tumor growth data were not included in analyses. Total GEN and daidzein levels in plasma of animals in the E2, MCF-7 and SPI (withdrawal) groups at the end of the study were essentially at the limits of detection (<0.05 μM). Plasma amounts for GEN, daidzein and equol(−) in the separate group of animals that consumed the SPI diet throughout the study were (mean±SEM): 0.462±0.402 μM, 0.275±0.201 μM and 0.044±0.012 μM, respectively.
Figure 4. Effect of dietary SPI (GEN 180 ppm) treatment and withdrawal on the growth of MCF-7 cells implanted into ovariectomized athymic mice.
E2 (2 mg) pellets and MCF-7 cells were subcutaneously implanted into ovariectomized athymic mice for 6 weeks. When the average tumor size reached 45 mm2 (week 0), E2 pellets were removed from the mice, and mice were divided into three groups: E2 control (12 mice), MCF-7 control (10 mice) and SPI group (18 mice). Control animals continued on the AIN-93G diet and the SPI group consumed AIN-93G with SPI containing 180 ppm GEN. The E2 positive control group was terminated at five weeks, while the SPI group continued on the diet until tumor area reached 100 mm2. At this point, SPI diet was replaced with the AIN-93G control diet. Change in tumor size was monitored until the termination of the study. Time points represent tumor surface area (±SEM). Superscripts (*) for the specified time endpoints on the SPI tumor growth curve represent significant differences compared to the MCF-7 control (P<0.05).
3.4 Molecular characterization of GEN and SPI treatment induced ER(+)/HER2(+) non-regressing tumors
To evaluate molecular changes in GEN- and SPI-induced non-regressing tumors (end of withdrawal phase), we performed Western blot analysis and compared to MCF-7 control (end of withdrawal phase) and E2-induced tumors (end of treatment phase).
GINR tumors
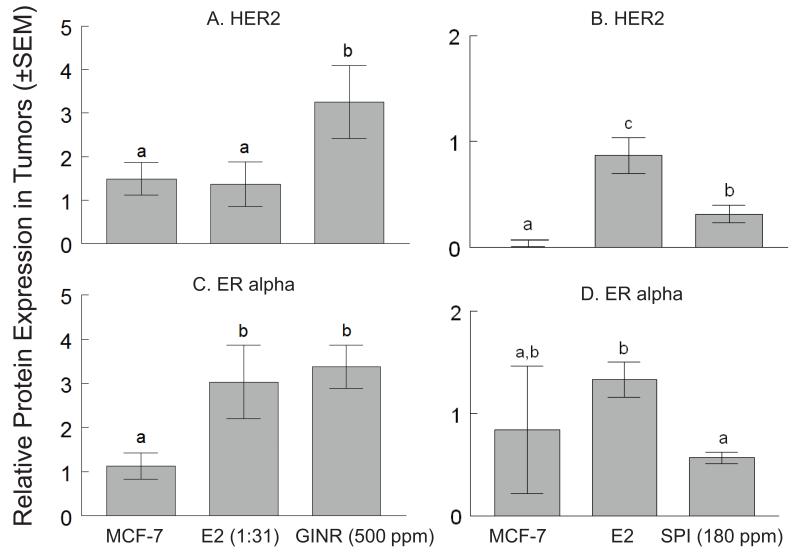
HER2 and ERα protein expression were compared among the MCF-7 control (n=3), E2-induced (n=5), and 500 ppm GINR tumors (n=5). HER2: Relative HER2 protein expression was 1.49±0.37, 1.37±0.51, and 3.25±0.84 for the MCF-7 control, E2 control, and GINR tumors, respectively. HER2 expression was increased (by 237%) in the GINR tumors compared to the E2 group (P<0.05) (Figure 5A). ERα: Relative ERα protein expression was 1.13±0.30, 3.03±0.83, and 3.37±0.49 for the MCF-7 control, E2 control, and GINR tumors, respectively. There was no difference in ERα expression between the E2 and GINR tumors (Figure 5C).
Figure 5. HER2 and ERα protein expression in GINR and SPI tumors.
Western blot analyses for HER2 (A,B) and ERα (C,D) were conducted on tumors collected at the end of withdrawal phase (i.e. MCF-7 negative control, GINR-500 ppm, SPI-180 ppm) or treatment phase (i.e. E2). Ratio of the target protein to the standard protein expression level (bars ±SEM) is displayed on the Y axis. β-actin was used for normalization of all protein data. Bars with different letters are significantly different (P<0.05).
SPI induced tumors
HER2 and ERα protein expressions were compared among the MCF-7 control tumors (n=6), E2-induced tumors (n=11), and SPI (GEN 180 ppm)-induced non-regressing MCF-7 tumors (n=6). HER2: Relative HER2 protein expression was 0.00±0.01, 0.87±0.17, and 0.32±0.08 for the MCF-7 control, E2 control, and SPI groups, respectively (Figure 5B). HER2 expression in SPI-induced non-regressing tumors was higher (320%, P<0.05) than that in MCF-7 tumors, but lower (63.8%, P<0.05) than that in E2-induced tumors. ERα: Relative ERα protein expression was 0.84±0.64, 1.33±0.17, and 0.57±0.06 for the MCF-7 control, E2, and SPI tumors, respectively (Figure 5D). ERα expression in SPI-induced non-regressing tumors was lower (57.4%, P<0.05) than that in E2-induced tumors, but not different than MCF-7 control tumors.
3.5 Characterization of cell growth from non-regressing tumors (GEN 500 ppm study)
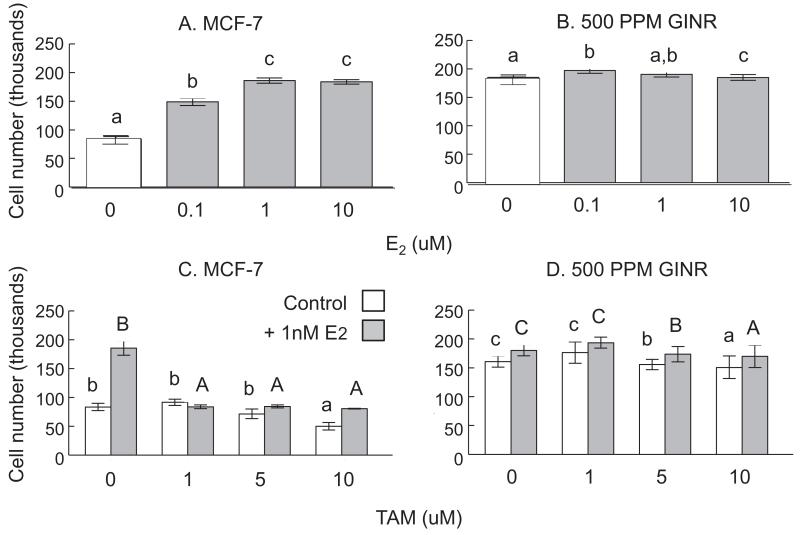
Cells harvested from the non-regressing tumors in this study were further characterized by treating cells in culture with E2, TAM, 4OHTAM and ICI 182,780 (data only shown for E2 and TAM). GINR cells were less responsive to E2 (Figure 6A). E2 (≥0.1 nM) stimulated the growth of MCF-7 cells in a dose-dependent manner (Figure 6A). However, E2 at the selected concentration range did not change the growth of GINR cells (Figure 6B).
Figure 6. Effect of E2 and TAM on MCF-7 and GINR (GEN 500 ppm) cell viability.
Cells (1.5×104 cells) were treated with E2 (A and B; 0, 0.1, 1, and 10 nM) or TAM (C and D; 0, 1, 5, and 10 μM) for 72 h. In TAM studies, grey bars represent addition of 1 nM E2. Cell viability was measured using MTT assay. Bars (±SEM) with different superscript letters are significantly different (P<0.05). In TAM experiments, lower or upper case superscript letters represent comparisons within treatments (MCF-7 with or without 1 nM E2).
E2 (1 nM) stimulated MCF-7 cell growth by 2.22-fold, and TAM (≥1 μM) completely blocked this E2 effect (Figure 6C). GINR cells were not stimulated by 1 nM E2 and were less responsive to TAM. TAM inhibited GINR cells by 4.46% and 40.91% at 5 μM and 10 μM, respectively (P<0.05) (Figure 6D).
4OHTAM (≥1 μM) completely blocked 1 nM E2-induced MCF-7 cells (data not shown). GINR cells were not stimulated by 1 nM E2 and were less responsive to 4OHTAM. 4OHTAM inhibited GINR cells by 12.85% at 5 μM, and by 42.79% at 10 μM, respectively (P<0.05).
ICI 182,780 (≥0.1 μM) completely blocked 1 nM E2-induced MCF-7 cells (data not shown). GINR cells were not stimulated by 1 nM E2 and were less responsive to ICI 182,780. ICI 182,780 inhibited GINR cells by 14.29% at 0.5 μM, and by 18.41% at 1 μM, respectively (P<0.05).
4 DISCUSSION
These results are the first reported to indicate that long-term dietary exposure to low doses of GEN (500 ppm) or GEN-containing SPI (180 ppm) stimulates MCF-7 tumor growth, and that after withdrawal of stimulus, these luminal A subtype tumor cells are reprogrammed towards a luminal B subtype, a more aggressive phenotype. Gene profiling has led to several predictive markers of important therapeutic targets in BC, such as ERα and HER2, which are strongly influenced by the proliferative status of the tumor [27]. There are different breast carcinomas subtypes: luminal (A & B), ER negative (basal- and normal-like) and HER2 enriched; with distinctive phenotypes and clinical outcomes [28]. As luminal A (ER+, HER2-) BC subtype is the most common, the ER has been the main target of molecular therapy, exemplified by the widespread use of anti-estrogens; a strategy responsible for major improvements in cure rates [29]. TAM is an anti-estrogen used for the hormonal management of BC for over 40 years [30]. After extended TAM exposure, however, ER+ tumors typically progress over time to become estrogen-independent or endocrine-resistant (luminal B and HER2 subtypes), which is a serious therapeutic problem [31]. As one of the earliest BC treatment drugs, much research has focused on mechanisms associated with TAM-resistance. Our results highlight certain similarities of the long-term effects of TAM and low dose GEN on MCF-7 tumor growth.
Evidence from both cell culture and xenograft animal models have shown that long-term estrogen-deprived or TAM-treated MCF-7 cells re-grow despite continued treatment, marking a conversion to estrogen-independence or endocrine-resistance [13]. Continuous exposure to low estrogen environments elicits changes in ER+ cells toward estrogen-independence adaptations [32]. The cellular mechanisms that produce endocrine-resistance in response to TAM appear to involve alterations in various signaling pathways through cross-talk with ERs including the MAPK pathway [12,33,34]. Overall, it is clear that progression to endocrine resistant BC is complex and involves modification in the expression of ERs and HER2.
Our results suggest that, similar to long-term exposure to TAM and estrogen deprivation, long-term exposure to dietary GEN and GEN-containing SPI at low, but physiologically relevant levels (≤ 500 ppm), induced GINR MCF-7 tumors. GEN and SPI diets during the treatment phase promoted tumor growth, establishing the estrogenic nature of GEN and SPI at all doses, as previously shown [18]. During the withdrawal phase, however, immediate tumor regression observed in animals withdrawn from E2 implants (Figure 1) was observed only in animals initially fed the GEN 750 ppm diet (Figure 2). Tumor regression was observed in animals immediately after GEN 500 ppm withdrawal, but regression terminated at a tumor size significantly larger than that of the MCF-7 negative control (Figure 3). Tumor regression during the withdrawal phase was not observed in the SPI group, where tumors continued growing (Figure 4). Purified isoflavones and those present in SPI are highly bioavailable in mice, even in very small amounts [23]. Although SPI contains other isoflavones (i.e. daidzein and glycitein) besides GEN, it is expected that tumor growth stimulation during the treatment phase was mainly due to GEN and not the other isoflavones because ER binding affinities are different (GEN>daidzein>glycitein) [35]. Also, daidzein and equol(+/−) did not promote tumor growth in the same model [36]. Using a similar model, Saarinen and colleagues reported that SPI (GEN at 320 ppm) promoted MCF-7 tumor growth during a 25-week treatment period, with a large proportion of tumors actively growing (>40%) and only a minor proportion completely regressed (<17%) [37]. Although our results confirm their observations, these authors did not examine tumor growth after withdrawal, limiting any potential conclusions on progression to estrogen-independence. It remains unknown whether GEN alone or its interaction with the whole SPI matrix sensitized MCF-7 tumors promoting their growth even after stimulus withdrawal.
To understand the molecular underpinnings of this stimulus-independent phenotype, we characterized the GINR tumors and the cells collected from GINR tumors. We observed increased protein expression of HER2 in GINR tumors from mice withdrawn from GEN 500 ppm and SPI diets (Figure 5). HER2 is an oncoprotein of the type 1 tyrosine kinase growth factor receptor family involved in cellular differentiation, adhesion, and motility. Higher expression of HER2 is observed in 20-25% of BC, which correlates with a poor disease prognosis [38]. Also, it is associated with endocrine resistance; with tumors of higher proliferative rates and of higher grade [39-41]. Moreover, long-term TAM treatment or E2-deprivation also induces overexpression of HER2 [12,39]. Thus, the increased expression of HER2 in GINR tumors provides insights for a plausible mechanism of the progression of endocrine resistant GINR tumors. Whereas no difference in ERα expression between SPI (180 ppm GEN) -induced non-regressing and MCF-7 control tumors (Figure 5) was observed, ERα expression in GINR (500 ppm GEN) tumors was higher than those in the MCF-7 control. Although it is unclear how ERα and HER2 crosstalk during the progression of GINR tumors, we can draw some similarities from studies with TAM-resistant cells. Usually, TAM-resistant breast tumors do not lose ERα [42]. Inhibition of HER2 and MAPK in TAM-resistant cells can restore the inhibitory effect of TAM on ERα transcriptional activity and cell growth [43,44], suggesting that ERα in TAM-resistant cells is present and functional. Fan et al. [45] reported enhanced translocation of ERα to the cytoplasm in TAM-resistant cells, which suggest a lesser number of membrane bound ERαs involved in ER-regulated pathways. It is possible that the SPI-induced non-regressing tumors have lost ERα, whereas the GINR tumors are yet to reach this no/low ERα stage. It appears that GEN-induced endocrine resistance involves non-monotonous dependence on GEN dose, exposure time and ER expression level.
Cells obtained from the GINR tumors were able to grow in E2-free media and addition of E2 (>1 nM) did not enhance further growth. Several anti-estrogens at different concentrations (1-10 μM) were effective in reducing E2-stimulated MCF-7 cell growth in vitro. This effect was absent in cells from GINR tumors even at the highest concentrations, where the aggregate reduction for all anti-estrogen agents at 10 μM was close to 1/3 of the almost complete growth inhibition observed in MCF-7 cells. These findings are different than those of Osborne et al. [46] who explored the long term effects of TAM administration using the athymic xenograft model and found that although cells were able to grow in vivo in the presence of TAM, TAM-resistant tumors remained endocrine-responsive. Altogether our evidence suggests that dietary GEN and SPI may alter MCF-7 cells from an ER+, luminal A subtype to an ER+/HER2+, luminal B or HER2 enriched subtype of breast tumors, which could potentially be progressing toward endocrine-resistance.
Our findings of enhanced tumor progression even after GEN withdrawal are of particular concern. Evidence from epidemiological studies in Asian populations, however, supports vegetable and soy consumption as a part of a healthy diet to reduce BC risk and disease recurrence [47,48]. To explain this discrepancy, it is critical to review the context in which the supportive effects of soy on BC risk have been observed. The U.S. population embraces a variety of lifestyles from the degree of physical activity to dietary preferences, different than their counterparts in Asia [49]. For example, in relation to soy foods and isoflavone consumption, dose, foods and time of exposure differ. Asians mostly consume whole soybean-foods (e.g. soy milk, tofu, tempeh, miso, natto) throughout their lives. Soy products consumed in Western markets are based on processed soy ingredients, such as soy protein concentrate, SPI, and texturized soy protein for use in beverage powders, infant formulas, liquid nutritional meals, power bars, and meat analogs. Average daily isoflavone consumption in China is 25-50 mg, with ~5% of the population consuming more than 100 mg/day. In the U.S. and Europe average consumption is less than 3 mg/day [50]. Although consumption of soy foods is low in the US, plasma levels of GEN among postmenopausal women range widely (1.9 - 3.0 μM), from those consuming no soy at all to those consuming isoflavone supplements [51]. Thus, based on our evidence, there are populations that will benefit from consuming soy products, but this benefit will depend on environmental factors such as food/isoflavone type, dose and life stage of exposure, physical activity, diet and culture, and intrinsic ones such as gene make up, age, gender and health status.
Consumption of whole soy products in the US is growing, mostly among populations that embrace certain lifestyles such as vegetarianism, high protein diets, or to reduce/prevent certain health conditions [52]. More specifically, cancer survivors alter their food choices from the belief that some foods may be better than others in preventing cancer [53]. Based on our observations and that of others, we contend that while the risk of consuming whole soy products is low, the potential risk of consuming high doses of purified isoflavones, especially in women with BC, is unknown. This is significant as there has been a dramatic increase in the number of women with, or at high risk for BC, who consume supplemental dietary botanical estrogens as part of nutrient supplement therapy (calcium and GEN), holistic or over-the-counter hormone remedies (e.g. black cohosh and GEN), or from a more processed soy matrix, which they perceive as “natural and safe” alternatives to HRT for the relief of post- or menopausal symptoms. Women taking purified isoflavone supplements might inadvertently be accelerating progression of BC from a benign, treatable subtype to a more aggressive, less treatable one.
In summary, low doses of GEN during long-term dietary treatment elicit changes in MCF-7 cells after stimulus withdrawal leading to a more aggressive and advanced tumor growth phenotype. In SPI-fed mice, tumor growth did not stop, even after diet withdrawal. Observed changes were accompanied with the modulation of BC biomarkers such as HER2, suggesting a potential molecular pathway that could explain the tumor stimulatory effects of GEN and GEN-containing SPI after prolonged treatment/withdrawal. In future studies, we will examine the molecular mechanisms associated with GINR, including ER-associated transcription, MAPK/ERK1/2 and PI3K/Akt pathways.
Acknowledgements
This work was supported by Grant Number P01AG024387 from the National Institute On Aging [W.G.H. and Y.H.J.]; and Grant Number P50AT006268 from the National Center for Complementary and Alternative Medicines (NCCAM), the Office of Dietary Supplements (ODS) and the National Cancer Institute (NCI) [W.G.H. and D.D.] Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS, NCI, or the National Institutes of Health.
Abbreviations
- BC
Breast cancer
- E2
17β-estradiol
- ER
estrogen receptor
- GEN
genistein
- GINR
genistein induced non-regressing
- HER2
epidermal growth factor receptor 2
- SPI
soy protein isolate
- TAM
tamoxifen
Footnotes
Conflict of Interest
The authors have declared no conflicts of interest.
REFERENCES
- [1].Barnes S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat. Res. Biol. 2010;8:89–98. doi: 10.1089/lrb.2009.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kuiper GG, Lemmen JG, Carlsson B, Corton JC, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- [3].Jefferson WN, Williams CJ. Circulating levels of genistein in the neonate, apart from dose and route, predict future adverse female reproductive outcomes. Reprod. Toxicol. 2011;31:272–279. doi: 10.1016/j.reprotox.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Branca F, Lorenzetti S. Health effects of phytoestrogens. Forum. Nutr. 2005;(57):100–111. doi: 10.1159/000083773. [DOI] [PubMed] [Google Scholar]
- [5].Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010;31:400–419. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- [7].Bouker KB, Hilakivi-Clarke L. Genistein: does it prevent or promote breast cancer? Environ. Health Perspect. 2000;108:701–8. doi: 10.1289/ehp.00108701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr. Cancer. 1994;21:113–31. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- [9].Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, Banerjee S, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J. Natl. Cancer Inst. 1995;87:190–7. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- [10].Mokbel K. Risk-reducing strategies for breast cancer--a review of recent literature. Int J Fertil. Womens Med. 2003;48:274–7. [PubMed] [Google Scholar]
- [11].Helferich WG, Andrade JE, Hoagland MS. Phytoestrogens and breast cancer: a complex story. Inflammopharmacology. 2008;16:219–26. doi: 10.1007/s10787-008-8020-0. [DOI] [PubMed] [Google Scholar]
- [12].Johnston SR, Head J, Pancholi S, Detre S, et al. Integration of signal transduction inhibitors with endocrine therapy: an approach to overcoming hormone resistance in breast cancer. Clin. Cancer Res. 2003;9:524S–32S. [PubMed] [Google Scholar]
- [13].Osborne CK, Coronado EB, Robinson JP. Human breast cancer in the athymic nude mouse: cytostatic effects of long-term antiestrogen therapy. Eur. J. Cancer Clin. Oncol. 1987;23:1189–96. doi: 10.1016/0277-5379(87)90154-4. [DOI] [PubMed] [Google Scholar]
- [14].Ju YH, Allred CD, Allred KF, Karko KL, et al. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J. Nutr. 2001;131:2957–2962. doi: 10.1093/jn/131.11.2957. [DOI] [PubMed] [Google Scholar]
- [15].Ju YH, Allred KF, Allred CD, Helferich WG. Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis. 2006;27:1292–9. doi: 10.1093/carcin/bgi370. [DOI] [PubMed] [Google Scholar]
- [16].Allred CD, Allred KF, Ju YH, Goeppinger TS, et al. Soy processing influences growth of estrogen-dependent breast cancer tumors. Carcinogenesis. 2004;25:1649–1657. doi: 10.1093/carcin/bgh178. [DOI] [PubMed] [Google Scholar]
- [17].Allred CD, Twaddle NC, Allred KF, Goeppinger TS, et al. Soy processing affects metabolism and disposition of dietary isoflavones in ovariectomized BALB/c mice. J. Agric. Food Chem. 2005;53:8542–8550. doi: 10.1021/jf051246w. [DOI] [PubMed] [Google Scholar]
- [18].Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61:5045–5050. [PubMed] [Google Scholar]
- [19].Van Patten CL, Olivotto IA, Chambers GK, Gelmon KA, et al. Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: a randomized, controlled clinical trial. J. Clin. Oncol. 2002;20:1449–55. doi: 10.1200/JCO.2002.20.6.1449. [DOI] [PubMed] [Google Scholar]
- [20].Ju YH, Doerge DR, Allred KF, Allred CD, Helferich WG. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. 2002;62:2474–7. [PubMed] [Google Scholar]
- [21].Ju YH, Doerge DR, Woodling KA, Hartman JA, et al. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis. 2008;29:2162–2168. doi: 10.1093/carcin/bgn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Du M, Yang X, Hartman JA, Cooke PS, et al. Low-dose dietary genistein negates the therapeutic effect of tamoxifen in athymic nude mice. Carcinogenesis. 2012;33:895–901. doi: 10.1093/carcin/bgs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Andrade JE, Twaddle NC, Helferich WG, Doerge DR. Absolute bioavailability of isoflavones from soy protein isolate-containing food in female BALB/c mice. J. Agric. Food Chem. 2010;58:4529–36. doi: 10.1021/jf9039843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Twaddle NC, Churchwell MI, Doerge DR. High-throughput quantification of soy isoflavones in human and rodent blood using liquid chromatography with electrospray mass spectrometry and tandem mass spectrometry detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;777:139–45. doi: 10.1016/s1570-0232(02)00275-1. [DOI] [PubMed] [Google Scholar]
- [25].Naaz A, Yellayi S, Zakroczymski MA, Bunick D, et al. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology. 2003;144:3315–3320. doi: 10.1210/en.2003-0076. [DOI] [PubMed] [Google Scholar]
- [26].Hsieh CY, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58:3833–3838. [PubMed] [Google Scholar]
- [27].Chuthapisith S, Eremin JM, Eremin O. Predicting response to neoadjuvant chemotherapy in breast cancer: molecular imaging, systemic biomarkers and the cancer metabolome (Review) Oncol. Rep. 2008;20:699–703. [PubMed] [Google Scholar]
- [28].Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J. Clin. Oncol. 1984;2:1102–9. doi: 10.1200/JCO.1984.2.10.1102. [DOI] [PubMed] [Google Scholar]
- [29].Harlan LC, Abrams J, Warren JL, Clegg L, et al. Adjuvant therapy for breast cancer: practice patterns of community physicians. J. Clin. Oncol. 2002;20:1809–1817. doi: 10.1200/JCO.2002.07.052. [DOI] [PubMed] [Google Scholar]
- [30].Mayo Medical Laboratories The pharmacogenetics of tamoxifen therapy. Mayo Reference Services Communiqué. 2007;32(1):7. [Google Scholar]
- [31].Fisher B, Dignam J, Bryant J, DeCillis A, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J. Natl. Cancer Inst. 1996;88:1529–42. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- [32].Herman ME, Katzenellenbogen BS. Alterations in transforming growth factor-alpha and -beta production and cell responsiveness during the progression of MCF-7 human breast cancer cells to estrogen-autonomous growth. Cancer Res. 1994;54:5867–5874. [PubMed] [Google Scholar]
- [33].Berstein LM, Zheng H, Yue W, Wang JP, et al. New approaches to the understanding of tamoxifen action and resistance. Endocr. Relat. Cancer. 2003;10:267–77. doi: 10.1677/erc.0.0100267. [DOI] [PubMed] [Google Scholar]
- [34].Shim WS, Conaway M, Masamura S, Yue W, et al. Estradiol hypersensitivity and mitogen-activated protein kinase expression in long-term estrogen deprived human breast cancer cells in vivo. Endocrinology. 2000;141:396–405. doi: 10.1210/endo.141.1.7270. [DOI] [PubMed] [Google Scholar]
- [35].Song TT, Hendrich S, Murphy PA. Estrogenic activity of glycitein, a soy isoflavone. J. Agric. Food Chem. 1999;47:1607–1610. doi: 10.1021/jf981054j. [DOI] [PubMed] [Google Scholar]
- [36].Ju YH, Fultz J, Allred KF, Doerge DR, Helferich WG. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis. 2006;27:856–863. doi: 10.1093/carcin/bgi320. [DOI] [PubMed] [Google Scholar]
- [37].Saarinen NM, Power K, Chen J, Thompson LU. Flaxseed attenuates the tumor growth stimulating effect of soy protein in ovariectomized athymic mice with MCF-7 human breast cancer xenografts. Int. J. Cancer. 2006;119:925–31. doi: 10.1002/ijc.21898. [DOI] [PubMed] [Google Scholar]
- [38].Harbeck N, Pegram MD, Ruschoff J, Mobus V. Targeted Therapy in Metastatic Breast Cancer: The HER2/neu Oncogene. Breast Care (Basel) 2010;5:3–7. doi: 10.1159/000285714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dowsett M. Overexpression of HER-2 as a resistance mechanism to hormonal therapy for breast cancer. Endocr. Relat. Cancer. 2001;8:191–5. doi: 10.1677/erc.0.0080191. [DOI] [PubMed] [Google Scholar]
- [40].Sihto H, Lundin J, Lehtimaki T, Sarlomo-Rikala M, et al. Molecular subtypes of breast cancers detected in mammography screening and outside of screening. Clin. Cancer Res. 2008;14:4103–10. doi: 10.1158/1078-0432.CCR-07-5003. [DOI] [PubMed] [Google Scholar]
- [41].Nicholson RI, Hutcheson IR, Harper ME, Knowlden JM, et al. Modulation of epidermal growth factor receptor in endocrine-resistant, oestrogen receptor-positive breast cancer. Endocr. Relat. Cancer. 2001;8:175–82. doi: 10.1677/erc.0.0080175. [DOI] [PubMed] [Google Scholar]
- [42].Encarnacion CA, Ciocca DR, McGuire WL, Clark GM, et al. Measurement of steroid hormone receptors in breast cancer patients on tamoxifen. Breast Cancer Res. Treat. 1993;26:237–246. doi: 10.1007/BF00665801. [DOI] [PubMed] [Google Scholar]
- [43].Bayliss J, Hilger A, Vishnu P, Diehl K, El-Ashry D. Reversal of the estrogen receptor negative phenotype in breast cancer and restoration of antiestrogen response. Clin. Cancer Res. 2007;13:7029–7036. doi: 10.1158/1078-0432.CCR-07-0587. [DOI] [PubMed] [Google Scholar]
- [44].Bobustuc GC, Smith JS, Maddipatla S, Jeudy S, et al. MGMT inhibition restores ERalpha functional sensitivity to antiestrogen therapy. Mol. Med. 2012;18:913–929. doi: 10.2119/molmed.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fan P, Wang J, Santen RJ, Yue W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res. 2007;67:1352–1360. doi: 10.1158/0008-5472.CAN-06-1020. [DOI] [PubMed] [Google Scholar]
- [46].Osborne CK, Coronado E, Allred DC, Wiebe V, DeGregorio M. Acquired tamoxifen resistance: correlation with reduced breast tumor levels of tamoxifen and isomerization of trans-4-hydroxytamoxifen. J. Natl. Cancer Inst. 1991;83:1477–1482. doi: 10.1093/jnci/83.20.1477. [DOI] [PubMed] [Google Scholar]
- [47].Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br. J. Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shu XO, Zheng Y, Cai H, Gu K, et al. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–43. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- [50].Messina M. A brief historical overview of the past two decades of soy and isoflavone research. J. Nutr. 2010;140:1350S–4S. doi: 10.3945/jn.109.118315. [DOI] [PubMed] [Google Scholar]
- [51].Setchell KD. Absorption and metabolism of soy isoflavones-from food to dietary supplements and adults to infants. J. Nutr. 2000;130:654S–5S. doi: 10.1093/jn/130.3.654S. [DOI] [PubMed] [Google Scholar]
- [52].United Soybean Board . Consumer Attitudes Report about Nutrition: Insights into nutrition, health and soyfoods. 2011. p. 18. [Google Scholar]
- [53].Maskarinec G, Murphy S, Shumay DM, Kakai H. Dietary changes among cancer survivors. Eur. J. Cancer. Care. (Engl) 2001;10:12–20. doi: 10.1046/j.1365-2354.2001.00245.x. [DOI] [PubMed] [Google Scholar]