Abstract
Studies examining the impact of stressors on diabetes self-care have been limited by focusing on a single stressor or have been largely qualitative. Therefore, we assessed the stressors experienced by a high-risk population with type 2 diabetes, and tested whether having more stressors was associated with less adherence to multiple self-care behaviors. Participants were recruited from a Federally Qualified Health Center and 192 completed a stressors checklist. Experiencing more stressors was associated with less adherence to diet recommendations and medications among participants who were trying to be adherent, but was not associated with adherence to other self-care behaviors. Because having more stressors was also associated with more depressive symptoms, we further adjusted for depressive symptoms; stressors remained associated with less adherence to medications, but not to diet recommendations. For adults engaged in adherence, experiencing numerous chronic stressors presents barriers to adherence that are distinct from associated depressive symptoms.
Keywords: diabetes, self-care, stressor, stress, medication adherence, diet, depression
Decades of research on the effects of exposure to acute and chronic stressors have shown consistent deleterious associations with health outcomes (Segerstrom & Miller, 2004). In diabetes, this literature has largely focused on single stressors, or on physiological responses to acute decontextualized stressors of questionable relevance to the populations studied (e.g., (Wiesli, Krayenbuhl, Kerwer, Seifert, & Schmid, 2007). Such a focus may have excluded the experiences of individuals with overlapping vulnerabilities, particularly racial/ethnic minorities and persons with low socioeconomic status (SES) whose stressors tend to be aggregated (e.g., inability to pay for medical care or afford transportation), chronic in nature (e.g., financial hardship, disability), and sociological in that they are embedded in both relationships (e.g., unemployment) and contexts (e.g., living in unsafe neighborhood), thereby being affected by and affecting family members, kin networks, neighborhoods and communities. The relationship between these types of cumulative stressors and the performance of diabetes self-care behaviors may be one of several mechanisms underlying disparities in diabetes outcomes.
Disparities in Stressors and Diabetes
In the general population, racial/ethnic minorities and persons with low SES are more likely than non-Hispanic Whites (NHW) and higher SES groups to experience multiple chronic stressors, including experiencing unfair treatment and/or racial discrimination (Mulia, Ye, Zemore, & Greenfield, 2008), alcohol-related problems (Mulia et al., 2008), financial hardship (Mulia et al., 2008), family-related stress (Mulia et al., 2008), being exposed to criminal activity (Neckerman et al., 2009), and living in unsafe neighborhoods (Neckerman et al., 2009). Among adults with type 2 diabetes mellitus (T2DM), racial/ethnic minorities are more likely than their NHW counterparts to report experiencing financial strain (Jeon, Essue, Jan, Wells, & Whitworth, 2009), and studies have identified several stressors disproportionately affecting racial/ethnic minorities with T2DM, including financial strain (Jiang, Beals, Whitesell, Roubideaux, & Manson, 2008; Russell et al., 2010), family dysfunction (Elstad, Tusiofo, Rosen, & McGarvey, 2008; Jiang et al., 2008; Mendenhall et al., 2012; Russell et al., 2010), daily hassles (Spencer et al., 2006), and discrimination (Jiang et al., 2008; Wagner et al., 2011).
Differential exposure to chronic stressors may be one of many factors underlying disparities (Lantz, House, Mero, & Williams, 2005) in the prevalence of diabetes and diabetes outcomes. Both chronic stressors (e.g., discrimination, addiction problems, or family dysfunction (Jiang et al., 2008) and perceived chronic stress (Novak et al., 2013) have been associated with an increased odds of being diagnosed with T2DM, and chronic life stress has been associated with suboptimal glycemic control (Morris, Moore, & Morris, 2011). Furthermore, blood glucose readings tend to be more erratic on days when problems (e.g., interpersonal problems, environmental stressors) are considered more stressful than on days when problems are less stressful (Goetsch, Wiebe, Veltum, & Van Dorsten, 1990).
However, to our knowledge no study has examined the relationship between the accumulation of chronic stressors and the performance of diabetes self-care behaviors, which may be a mechanism underlying disparities in diabetes outcomes. Some quantitative studies have reported that the presence of a single stressor is associated with deleterious effects on behavior. For example, after adjustment for demographics and health status, experiencing food insecurity was associated with medication underuse (Billimek & Sorkin, 2012a), experiencing financial strain was associated with nonadherence to medications due to costs (Ngo-Metzger, Sorkin, Billimek, Greenfield, & Kaplan, 2012), and living in an unsafe neighborhood was associated with delays in filling prescriptions and obtaining needed medical care (Billimek & Sorkin, 2012b). These findings are consistent with a handful of qualitative studies reporting that, across racially, culturally and linguistically diverse groups, stressors such as financial hardship (Jeon et al., 2009; Russell et al., 2010), repeated racial stressors (Wagner et al., 2011), and multi-caregiving responsibilities (Samuel-Hodge et al., 2000) compromise the performance of diabetes self-care activities.
Being socially disadvantaged is not only associated with exposure to more stressors, it is also associated with experiencing more depressive symptoms. Living in poverty, the frequency of unfair treatment, and consciousness of racial/ethnic stigma all covary with having depressive symptoms (Mulia et al., 2008). This is particularly relevant for socially disadvantaged persons with diabetes, as having a diabetes diagnosis increases one’s risk of having elevated depressive symptoms (Osborn et al., 2011). Moreover, continuous depressive symptom severity scores have been shown to be a better predictor of nonadherence to diet, exercise, and medications than categorically defined major depression (Gonzalez et al., 2007). Therefore, any examination of the effect of exposure to stressors on diabetes self-care in low SES samples should carefully control for the influence of depressive symptoms.
Purpose
As reviewed above, the literature to date suggests specific stressors are prevalent among certain populations with T2DM, and experiencing a single stressor is associated with less medication adherence, or compromised self-care defined more broadly. However, less is known about the number of stressors certain patient populations experience and what stressors are more or less likely to be reported. It also remains unclear whether experiencing more stressors is associated with less adherence to multiple self-care behaviors and, if so, if those relationships are better accounted for by depressive symptoms. In an effort to fill these gaps in the literature, we conducted a cross-sectional study with a diverse sample of low SES adults with T2DM and assessed the type and number of experienced stressors, and the relationships between an accumulation of these stressors and self-care behaviors before and after accounting for any effects of depressive symptoms.
Methods
Sample and Recruitment
From June 2010 to November 2012, we enrolled 314 English- and Spanish-speaking adults (age ≥18 years) with a diagnosis of T2DM receiving outpatient care at a Federally Qualified Health Center in Nashville, TN to participate in a cross-sectional study examining predictors of diabetes medication adherence. A trained English-speaking or bilingual research assistant (RA) worked with clinic personnel to recruit patients who arrived for a clinic appointment and satisfied eligibility criteria, approaching potential participants in the clinic waiting room and advertising the study on flyers located in the clinic. Eligible patients were English- or Spanish-speaking adults prescribed medications for T2DM, and were excluded if their medications were administered by a caregiver. Potential participants were also excluded if they did not have a social security number needed for compensation, exhibited unintelligible speech, or a lack of orientation to person, time, or place, or a severe hearing impairment. Of the 588 adult patients with a T2DM diagnosis who arrived for a clinic appointment during the recruitment period, RAs approached 507 (86.2%); 58 were not interested in participating and 135 did not meet eligibility criteria: 74 did not speak English/Spanish, 52 were not prescribed diabetes medications, 4 had no social security number, 3 exhibited an intellectual disorder or unintelligible speech, and 2 had medications administered by a caregiver. The remaining 314 were enrolled and participated. In June 2011, we added the Tool for Assessing Patients’ Stressors (TAPS) described below to the original battery of instruments, resulting in a subset of 192 participants with these data. The Vanderbilt University Institutional Review Board approved all study procedures prior to enrollment.
Data and Procedure
RAs took interested and eligible persons to a private room to complete informed consent and self-report instruments before and/or after their clinic appointment. RAs read all self-report items and response options aloud to ensure participants’ responses were not confounded by their ability to read. RAs also presented a printed response scale for each instrument, allowing participants to provide a verbal response and/or point to their response. Participation lasted approximately one hour and participants received $20 compensation for their time.
Demographic and diabetes characteristics
We collected self-reported age, gender, race, ethnicity, income, education, insurance status, and diabetes duration (i.e., years since diabetes diagnosis). RAs reviewed participants’ medical records to collect body mass index (BMI) and to obtain the number and type of prescribed diabetes medications. Clinic nurses administered the Bayer A1CNow point-of-care HbA1C (%) test, which is a valid and reliable measure of glycemic control (Kennedy & Herman, 2005).
Stressors
We used the English and Spanish versions of the 20-item Tool for Assessing Patients’ Stressors (TAPS) to assess participants’ stressors (Rothberg, DuVal, Luciano, Frederici, & Welch, 2011; Welch et al., 2011). Each item on the TAPS (Table 1) assesses a stressor commonly reported by racially and ethnically diverse populations with low SES, and was created by reviewing the salient literature coupled with qualitative input from FQHC healthcare providers and diabetes educators, and behavioral researchers familiar with the target population (Rothberg et al., 2011). For each item, respondents are asked, “In the past year (12 months), have any of the following family issues been stressful for you?” with 1=yes and 0=no as response options. In the current study, items 1–17 were administered to all participants; items 18–20 account for stressors most relevant to Hispanic groups, and were administered to Hispanic participants only. The TAPS score is calculated by summing the “yes” responses across items for a possible range of 0–20, representing the number of stressors experienced by the respondent in the last year.
Table 1.
Tool for Assessing Patients’ Stressors (TAPS) item frequencies.
| In the past year (12 months), have any of the following family issues been stressful for you? | % Yes |
|---|---|
| Sickness or disability in my family or myself, or death in the family | 57.3 |
| Not enough money for food, rent or mortgage, or clothes for my family or myself | 56.3 |
| Problems with depression or anxiety in my family or myself | 48.7 |
| Difficulty paying for medications, doctor’s visits, or medical equipment for my family or myself | 48.2 |
| Taking care of my family’s different needs and problems | 45.3 |
| Family members working in unsafe, low paying, or stressful jobs, or being unemployed | 34.2 |
| Having conflict or arguments among family members | 29.3 |
| Lack of affordable local transport for my family or myself (car, bus, taxi) | 28.3 |
| Problems reading or understanding written information (newspapers, bills, official forms, letters) | 24.6 |
| Legal problems for my family or myself (fines, arrests, court appearances, immigration problems, detention or prison) | 15.8 |
| Our neighborhood looks run down and neglected | 15.2 |
| Living in an unsafe neighborhood (crime, violence, conflict) | 14.2 |
| Problems at school for our children or teens (poor conduct, grades, or attendance) | 14.2 |
| Overcrowding or lack of privacy in the house | 13.1 |
| Family members or myself experiencing discrimination or racism at work or in public | 11.5 |
| Problems with alcohol or drug abuse in my family or myself | 9.4 |
| Problems with violence or physical abuse in my family or myself | 7.3 |
| Hispanic respondents only | N=19 |
| Difficulty affording the cost of travel back home to visit friends and family? | 50.0 |
| Difficulties adjusting to American culture and language? | 38.9 |
| Difficulty affording to send money or gifts back home to friends and family? | 33.3 |
Depressive symptoms
We assessed the presence and severity of depressive symptoms with the Personal Health Questionnaire-9 (PHQ-9; Kroenke, Spitzer, & Williams, 2001). The PHQ-9 items ask respondents to report how often, over the last two weeks, they have experienced a depressive symptom such as “little interest or pleasure in doing things,” or “trouble falling asleep or sleeping too much” on a scale from 0=not at all to 3=nearly every day. Responses are summed to create a score ranging from 0–27, with higher scores indicating more depressive symptoms.
Self-care
Two self-report instruments assessed diabetes self-care behavior: the Summary of Diabetes Self-Care Activities (SDSCA) and the Adherence to Refills and Medications Scale for Diabetes (ARMS-D). The SDSCA is comprised of subscales measuring the frequency of performing different self-care behaviors in the last 7 days. We used the SDSCA to assess participants’ adherence to a general diet (followed a healthful diet), a specific diet (ate fruits and vegetables/low fat diet/low carbohydrate diet), exercise, and blood sugar testing (Toobert, Hampson, & Glasgow, 2000). Each SDSCA subscale ranges from 0–7, with higher scores indicating greater adherence. Because the SDSCA medications subscale has identified few instances of nonadherence to medications and has low variability (Toobert et al., 2000), we relied on the 11-item ARMS-D to assess medication adherence, which has been shown to be more sensitive to nonadherence and more predictive of glycemic control than the SDSCA medications subscale (Mayberry, Gonzalez, Wallston, Kripalani, & Osborn, 2013). ARMS-D response options range from 1=none of the time to 4=all of the time and are summed to create a score ranging from 11–44, with higher scores indicating more problems with medication adherence. For our analyses, we reverse-scored the ARMS-D, so higher scores indicate greater adherence and are in the same direction as the SDSCA subscale scores.
Data Analyses
All statistical analyses were conducted with Stata version 12. Descriptive statistics were used to summarize participants’ demographic and diabetes characteristics, stressors, depressive symptoms, and self-care behaviors. We used non-parametric tests (i.e., Spearman’s rho correlation coefficients, Mann-Whitney U tests, and Kruskal-Wallis one-way analysis of variance tests) to examine the relationships between demographic characteristics (i.e., age, gender, race, ethnicity, and SES – income, education, and insurance status) and the number of stressors.
We used unadjusted and adjusted ordinary least squares (OLS) regression models, and adjusted logistic quantile regression (LQR) models to estimate the relationships between having more stressors and each self-care behavior. Adjusted models included specific demographic and diabetes characteristics (i.e., age, gender, race/ethnicity, education, insurance status, and insulin status). When having more stressors was significantly associated with a behavior in OLS regression models (i.e., regression at the sample mean), we conducted an adjusted LQR model to examine the relationship between having more stressors and a behavior for participants scoring low, moderate, or high on the sample’s distribution of scores for that behavior (Bottai, Cai, & McKeown, 2010). Finally, we introduced depressive symptoms into the adjusted models to determine if having more stressors remained significantly associated with a given behavior after accounting for psychological distress.
Results
Participants were 192 adults with a T2DM diagnosis and an average age of 51.6 ± 10.9 years; 70% were female; 56% were African American/Black; 10% reported Hispanic ethnicity and 11 interviews were conducted in Spanish. Nearly half (47%) were uninsured; 31% had less than a high school degree; and 44% had incomes less than $10K. The sample’s average HbA1C was 7.9% ± 2.0% (range 4.4–13.0), 62% had suboptimal glycemic control (HbA1C ≥7.0%), and almost half (47%) were on insulin (Table 2).
Table 2.
Participant characteristics.
| N = 192 | Mean ± SD or n (%) |
|---|---|
| Age, years | 51.6 ± 10.9 |
| Gender | |
| Male | 57 (29.7) |
| Female | 135 (70.3) |
| Race/Ethnicity | |
| Non-Hispanic White | 62 (32.3) |
| Non-White | 130 (67.7) |
| African American/Black | 107 (55.7) |
| Hispanic | 19 (9.9) |
| Other | 4 (2.1) |
| Education, years | 12.0 ± 3.0 |
| Income | |
| <$10,000 | 78 (43.6) |
| $10,000 – $14,999 | 49 (27.4) |
| $15,000 – $19,999 | 27 (15.0) |
| ≥$20,000 | 25 (14.0) |
| Insurance status | |
| Uninsured | 90 (46.9) |
| Public Insurance | 87 (45.3) |
| Private Insurance | 15 (7.8) |
| Diabetes duration, years | 7.7 ± 7.2 |
| Body mass index | 36.1 ± 8.7 |
| Insulin status, taking insulin | 90 (46.9) |
| Glycemic control (HbA1C, %) | 7.9 ± 2.0 |
| <7.0% | 73 (39.0) |
| ≥7.0% | 119 (62.0) |
| Stressors (TAPS) | 4.8 ± 3.9 |
| Depressive Symptoms (PHQ-9) | 8.4 ± 6.6 |
| Adherence to Self-Care Activities (SDSCA) | |
| General diet | 4.1 ± 2.3 |
| Specific diet | 4.0 ± 1.6 |
| Exercise | 2.6 ± 2.2 |
| Blood sugar testing | 4.8 ± 2.8 |
| Medication Adherence (ARMS-D) | 39.44 ± 4.9 |
ARMS-D = Adherence to Refills and Medications Scale for Diabetes, reverse scores; PHQ-9 = Personal Health Questionnaire-9; SD = standard deviation; SDSCA = Summary of Diabetes Self-Care Activities; TAPS = Tool for Assessing Patients’ Stressors
According to the TAPS score, the sample reported 4.8 ± 3.9 (sample range 0–19) of 20 queried stressors in the last year. There was wide variability in the number of stressors experienced by participants: 13% reported no stressors, 47% reported 1 to 5 stressors, 30% reported 6 to 10 stressors, and 10% reported more than 10 stressors. As shown in Table 1, the five most common stressors were: “sickness or disability in my family or myself, or death in the family” (57.3%); “not enough money for food, rent or mortgage, or clothes for my family or myself” (56.3%); “problems with depression or anxiety in my family or myself” (48.7%); “difficulty paying for medications, doctor's visits, or medical equipment for my family or myself” (48.2%); and “taking care of my family’s different needs and problems” (45.3%). Furthermore, 50% of Hispanic respondents reported “difficulty affording to send money or gifts back home to friends and family.”
The sample’s average PHQ-9 score was 8.0 ± 6.6 (sample range 0–27). One-third (33%) reported moderate to severe depressive symptoms (PHQ-9 score 10–27) and one-third (32%) reported mild depressive symptoms (PHQ-9 score 5–9). Having more stressors was significantly and strongly associated with more depressive symptoms (rho=0.54, p<.001).
Of the various self-care behaviors, participants reported the highest adherence to blood sugar testing (4.8 ± 2.8 days last week), followed by adherence to general diet (4.1 ± 2.3 days last week) and specific diet (4.0 ± 1.6 days last week), and the lowest adherence to exercise (2.6 ± 2.2 days last week). Participants’ average medication adherence score on the reverse-scored ARMS-D was 39.4 ± 4.9 (sample range 16–44 of a possible 11–44).
Older age was associated with fewer stressors (rho=−0.15, p=.04). However, there were no relationships between gender, race/ethnicity (NHW vs. non-White), education (years), income (<$10K vs. $10K-$14,999 vs. $15K-$19,999 vs. ≥20K), or insurance status (uninsured vs. public vs. private) and the number of stressors reported.
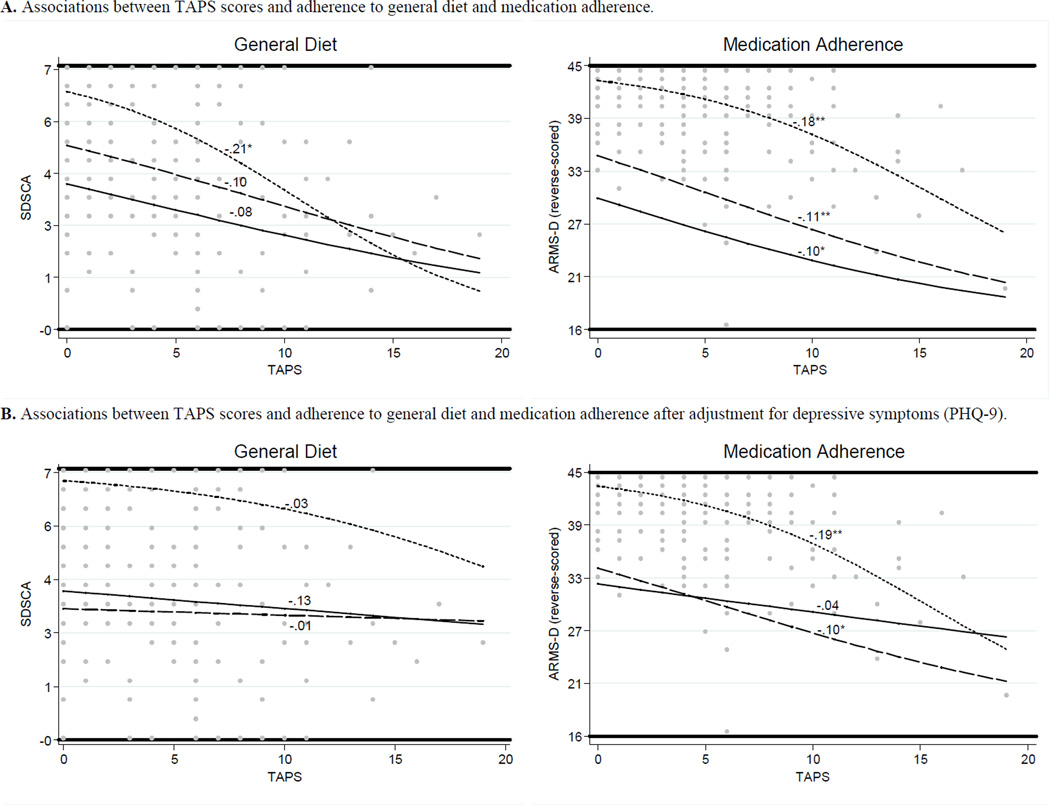
In OLS regression models, having more stressors was associated with less adherence to general diet (β = −0.23, p<.001 unadjusted; β = −0.21, p=.002 adjusted) and less medication adherence (β = −0.38, p<.001 unadjusted; β = −0.36, p<.001 adjusted), but having more stressors was not associated with adherence to specific diet, exercise, or blood sugar testing. Table 3 shows all OLS and LQR regression coefficients for both adherence to general diet and medications. Figure 1 shows prediction lines for the relationships between having more stressors and adherence to general diet and medications for participants scoring at the lower (25th), median (50th), and upper (75th) quartiles of the outcome distribution, after adjustment for demographic and diabetes characteristics (Figure 1A) and with additional adjustment for depressive symptoms (Figure 1B).
Table 3.
Regression models of the unadjusted and adjusted associations between stressors and adherence to general diet and medications, and of the adjusted association between depressive symptoms and adherence to general diet and medications.
| General Diet | Medications | |||||||
|---|---|---|---|---|---|---|---|---|
| OLS | LQR | OLS | LQR | |||||
| β | 25th | 50th | 75th | β | 25th | 50th | 75th | |
| TAPS, unadjusted | −0.23*** | — | — | — | −0.38*** | — | — | — |
| TAPS, adjusteda | −0.21** | −0.08 | −0.10 | −0.21* | −0.36*** | −0.10** | −0.11*** | −0.18*** |
| TAPS, adjusteda + PHQ-9 | −0.08 | −0.03 | −0.01 | −0.13 | −0.23* | −0.04 | −0.10** | −0.19** |
| PHQ-9, adjusteda + TAPS | −0.23* | −0.08 | −0.07* | −0.10* | −0.21* | −0.07* | −0.01 | 0.02 |
Adjusted for age, gender, race/ethnicity, education insurance status, and insulin status.
PHQ-9 = Personal Health Questionnaire-9; TAPS = Tool Assessing Patients’ Stressors.
p < 0.05,
p < 0.01,
p < 0.001.
Figure 1.
Scatter-plot of the TAPS scores (i.e., number of stressors) (dots) with prediction lines and coefficients for general diet and medication adherence scores at lower (solid line), median (dashed line), and upper (dotted line) quartiles of the outcome distributions. All models adjusted for demographic and diabetes characteristics (i.e., age, gender, race/ethnicity, education, insurance status, and insulin status) and used bootstrapped (100) standard errors. * p < 0.05, ** p < 0.01, *** p < 0.001.
Adherence to General Diet
After adjusting for demographic and diabetes characteristics, having more stressors was only associated with less adherence to general diet for participants who were the most adherent (i.e., those scoring at the upper (75th) quartile of the sample’s distribution of general diet scores; Table 3 and Figure 1A). After also adjusting for depressive symptoms, having more stressors was no longer associated with adherence to general diet for participants scoring at any quartile of the sample’s distribution of general diet scores (Table 3 and Figure 1B). Instead, depressive symptoms were significantly associated with less adherence to general diet for participants scoring at the median and upper quartiles of the sample’s distribution of general diet scores (Table 3; not shown in Figure 1).
Medication Adherence
After adjusting for demographic and diabetes characteristics, having more stressors was associated with less medication adherence for participants scoring at all quartiles of the sample’s distribution of medication adherence scores (Table 3 and Figure 1A). After also adjusting for depressive symptoms, having more stressors remained associated with less medication adherence for participants scoring at the median and upper quartiles of the sample’s distribution of medication adherence scores (Table 3 and Figure 1B). For participants scoring at the lower quartile, depressive symptoms were a significant predictor of less medication adherence whereas having more stressors was not (Table 3; not shown in Figure 1).
Discussion
In a sample of racially and ethnically diverse low SES adults with T2DM, we found that experiencing multiple chronic stressors was associated with less adherence to certain self-care behaviors, specifically less adherence to general diet and diabetes medications; and the relationship between having more stressors and less adherence to general diet varied by level of adherence. Having more stressors was also associated with more depressive symptoms, and whether stressors or depressive symptoms were associated with self-care depended on the behavior in question and participants’ level of adherence to that behavior. In short, for participants who were highly adherent, stressors exerted a stronger effect whereas depressive symptoms exerted a stronger effect for participants who were less adherent. To our knowledge, this is the first study to measure the amount of chronic stressors experienced in a high risk patient population with T2DM, and assess the quantitative relationship between having more stressors and adherence to multiple self-care behaviors. We found that 40% of this sample reported experiencing six or more stressors in the past year and 10% reported experiencing more than ten. Given the serious nature of the stressors queried, this indicates that a significant degree of life stress that may be interfering with patients’ ability to adhere to self-care recommendations.
The first main finding was that stressors interfered with adherence to general diet and medication adherence. However, for general diet, the relationship between having more stressors and less adherence was only evident among participants reporting high levels of adherence (i.e., 5.5 ± 0.7 days adherence). For these participants, an increase in the number of stressors from 5 to 10 was associated with 2 fewer days of adherence to general diet. Whereas, for medication adherence, the relationship between having more stressors and less adherence was apparent regardless of participants’ level of adherence, although there was a stronger association between stressors and adherence for participants reporting higher rates of adherence (i.e., when they were trying to adhere). There was a sharper decline in medication adherence when the number of stressors increased from 5 to 10 for participants reporting moderate and high medication adherence (i.e., a 4–5-point decrease in ARMS-D scores) compared to participants reporting low medication adherence (i.e., a 2-point decrease in ARMS-D scores).
We speculate that the different effect of having more stressors on adherence to general diet versus medication adherence is attributed to the absolute levels of adherence we observed for each of these behaviors. Our data suggest a minimum level of performance of a self-care behavior must be in place in order for stressors to interfere with that behavior. Consistent with previous studies (Tol et al., 2012; Toobert et al., 2000), our sample was more adherent to medications than to general diet. Put another way, the scores in the lowest quartile for medication adherence indicated higher adherence than did the scores in the lowest quartile of general diet.
The second main finding was that depressive symptoms had a different effect on the relationship between stressors and adherence to general diet than it did on the relationship between stressors and medication adherence. In general, depressive symptoms were more predictive of adherence to general diet, whereas having more stressors was more predictive of adherence to diabetes medications. Diagnostic criteria for depression include changes in appetite, eating, and weight, and the PHQ-9 assesses “poor appetite or overeating” as a depressive symptom. Thus, depression may exert a stronger effect on dietary adherence than stressors do, per se. As stated previously, our overall findings suggest that higher levels of adherence are disrupted by stressors whereas lower levels of adherence are not. Stressors remained independently associated with less medication adherence, even after adjusting for depressive symptoms among participants who were relatively adherent to their medications. Depressive symptoms interfered with medication adherence only among those who were the least adherent to their medications. Less medication adherence may be one manifestation of the reduced motivation, fatigue, and poor concentration that are symptomatic of depression (Gonzalez et al., 2007). When motivation to adhere is low, stressors may have no adherence to disrupt, whereas when motivation to adhere is high, stressors may be formidable barriers to medication adherence. In summary, our results suggest experiencing numerous chronic stressors presents barriers to adherence that are distinct from associated depressive symptoms for adults engaged in adherence.
We did not find a relationship between having more stressors and adherence to specific diet, exercise, or blood sugar testing. Although the sample means for specific diet and general diet were similar, the low variability in adherence to specific diet may explain why having more stressors had no association with adherence to that behavior. Because we found stressors were more strongly related to behaviors that patients were more adherent to, we speculate the lack of a relationship between stressors and adherence to exercise is due to participants reporting the least amount of adherence to this behavior. As mentioned previously, the effect of stressors was strongest for participants reporting higher rates of adherence to both general diet and medication adherence, suggesting an accumulation of chronic stressors may only disrupt efforts at self-care when patients are indeed trying to perform a behavior. A relationship between stressors and frequency of blood sugar testing may be detected in future studies using more sensitive measures than the SDSCA. However, we are not aware of any studies finding a relationship between stress and adherence to exercise, specific diet, or blood sugar testing.
The number of stressors reported did not vary by race or SES. This may be due to the unique characteristics of patients who receive care at FQHCs. FQHCs disproportionately serve low-income, uninsured or publicly insured patients (Fiandt, Doeschot, Lanning, & Latzke, 2010) and are located in areas identified as “high-need” due to elevated poverty rates and few practicing physicians. The populations served by FQHCs often experience overlapping vulnerabilities related to (a) being socially disadvantaged (e.g., unemployment, less education, and less incomes), (b) having complex clinical needs (e.g., comorbid chronic illnesses, disabilities, or co-occurring mental health and substance problems), and (c) being racial/ethnic minorities and/or immigrants (Fiandt et al., 2010). We expect that, in other clinic settings with a wider range of SES, the number of stressors reported on the TAPS may be strongly associated with SES. However, this measure was designed to address the specific experiences of FQHC patient populations. In this context, scores on the TAPS do not represent a proxy for SES, but rather may identify specific areas of need uniquely prevalent in FQHC patient populations. Reflective of the overlapping vulnerabilities of patients served by FQHCs, our participants had relatively high rates of stressors in their lives.
There are study limitations to acknowledge. First, our results speak most clearly to the population under study (i.e., racially/ethnically diverse, indigent patients), and should be replicated with comparable samples as well as with racially/ethnically homogenous groups from all socioeconomic strata with stressor checklists (such as the TAPS) relevant to each population, as our results may not generalize to certain patient populations. Second, the uniqueness of the TAPS instrument and items limits our knowledge of its psychometric properties. We were unable to identify a measure comparable to the TAPS to be able to establish convergent validity. The checklist nature of the TAPS and diversity of the items precludes interpretation of internal-consistency reliability coefficients. Third, our cross-sectional data did not allow assessment of the instrument’s test-retest reliability. However, like most checklist instruments that are carefully constructed based on extensive qualitative input, the TAPS is face valid. It was also designed with input from healthcare providers and diabetes educators at FQHCs, and the number of stressors most commonly reported by our sample were similar to the most common stressors among other samples who have completed the TAPS (Rothberg et al., 2011; Welch et al., 2011), boosting our confidence in the instruments’ utility. Finally, the TAPS assesses stressors experienced in the past year, whereas the SDSCA assesses self-care adherence in the past week, and the ARMS-D assesses diabetes medication adherence in general. While differences in the assessed timeframe between measures may offer an alternative explanation for the findings, we have found that experiencing a single, chronic stressor (e.g., racism) at any point during the lifespan has harmful effects on diabetes self-care (Wagner et al., 2011).
Furthermore, our cross-sectional data cannot speak to whether reporting more stressors at one time is associated with continued or future compromised self-care performance, nor can we draw causal conclusions about the relationships between having more stressors and compromised adherence. While we did not examine the mechanisms through which stressors decrease self-care, we hypothesized that they may deplete psychological resources necessary to perform self-care activities (Matthews & Gallo, 2011). As stressors accumulate, they may consume available psychological resources (Gailliot et al., 2007), leaving fewer resources to perform self-care. Stress management interventions can increase psychological resources (e.g., coping skills) and have been beneficial for patients with diabetes (Soo & Lam, 2009). Future prospective research should investigate the longitudinal effects of such stressors on the performance of self-care behaviors overtime and whether allocating resources to address these stressors improves patients’ adherence. Future research should also assess diabetes distress and whether it predicts self-care independently of the presence and accumulation of chronic stressors.
Despite these limitations, this is the first study to our knowledge to associate the number of chronic stressors experienced by low SES persons with compromised diabetes self-care behaviors. We found that most participants reported numerous chronic stressors, and that these stressors were associated with more depressive symptoms and less adherence to general diet and medications. Although some of the stressors assessed on the TAPS are not modifiable (e.g., death of a loved one), and others are beyond the scope of the efforts of a medical setting (e.g., discrimination), some of the stressors can, in fact, be addressed by healthcare providers (e.g., substance abuse). The TAPS or other population-specific stressor checklist can be used in regular clinical care to identify areas that might require additional screening (e.g., depression, anxiety, substance abuse, or literacy limitations). This may be particularly true for “problems with depression or anxiety,” which was among the most frequently reported stressors on the TAPS. Approaches to managing depression that include nurses, such as collaborative care (Huang, Wei, Wu, Chen, & Guo, 2013) and case management (Morgan et al., 2013), have been shown to improve depressive symptoms which underscores the utility of regular screening for depressive symptoms by nurses. Because of the complexities of successful depression management in person with diabetes, nurses are encouraged to establish collaborations with mental health professionals and provide team based-care whenever possible. The use of stressor checklists in routine clinical care may help nurses and other providers understand the needs of the populations they are serving, justify additional services to support these patients, and ultimately allow for treating the whole patient and not just a specific health problem.
Acknowledgements
This research study was funded with support from the Vanderbilt Clinical Translational Scientist Award (UL1TR000445) from the National Center for Advancing Translational Sciences. Study authors were supported by a Career Development Award (K01DK087894) and a National Research Service Award (F32DK097880) from the National Institute of Diabetes and Digestive and Kidney Diseases, and from a Baystate Medical Center Incubator Fund Research Grant. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent official views of these funding entities. The authors would like to thank Cecilia C. Quintero, Sahbina Ebba, Karen Calderon, Leo Cortes, Anne Crook, Carmen Mekhail, the Vine Hill Community Clinic personnel, and the participants for their contributions to this research.
Footnotes
Contribution Statement: C.Y.O. designed the parent study, oversaw data collection, wrote the introduction, methods, and discussion, and edited the manuscript. L.S.M. managed data, conducted analyses, wrote the methods and results, and edited the manuscript. J.A.W. contributed substantially to the introduction and discussion, and reviewed and edited the manuscript. G.W.W. wrote the abstract, contributed to the discussion, and reviewed the manuscript.
Duality of Interest: The authors have no conflicts of interest.
References
- Billimek J, Sorkin DH. Food insecurity, processes of care, and self-reported medication underuse in patients with type 2 diabetes: Results from the California Health Interview Survey. Health Services Research. 2012a;47(6):2159–2168. doi: 10.1111/j.1475-6773.2012.01463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billimek J, Sorkin DH. Self-reported neighborhood safety and nonadherence to treatment regimens among patients with type 2 diabetes. Journal of General Internal Medicine. 2012b;27(3):292–296. doi: 10.1007/s11606-011-1882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottai M, Cai B, McKeown RE. Logistic quantile regression for bounded outcomes. Statistics in Medicine. 2010;29:309–317. doi: 10.1002/sim.3781. [DOI] [PubMed] [Google Scholar]
- Elstad E, Tusiofo C, Rosen RK, McGarvey ST. Living with Ma'i Suka: Individual, familial, cultural, and environmental stress among patients with type 2 diabetes mellitus and their caregivers in American Samoa. Preventing Chronic Disease. 2008;5(3):A79. [PMC free article] [PubMed] [Google Scholar]
- Fiandt K, Doeschot C, Lanning J, Latzke L. Characteristics of risk in patients of nurse practitioner safety net practices. Journal of the American Academy of Nurse Practitioners. 2010;22(9):474–479. doi: 10.1111/j.1745-7599.2010.00536.x. [DOI] [PubMed] [Google Scholar]
- Gailliot MT, Baumeister RF, DeWall CN, Maner JK, Plant EA, Tice DM, Schmeichel BJ. Self-control relies on glucose as a limited energy source: Willpower is more than a metaphor. Journal of Personality and Social Psychology. 2007;92(2):325–336. doi: 10.1037/0022-3514.92.2.325. [DOI] [PubMed] [Google Scholar]
- Goetsch VL, Wiebe DJ, Veltum LG, Van Dorsten B. Stress and blood glucose in type II diabetes mellitus. Behaviour Research and Therapy. 1990;28(6):531–537. doi: 10.1016/0005-7967(90)90140-e. [DOI] [PubMed] [Google Scholar]
- Gonzalez JS, Safren SA, Cagliero E, Wexler DJ, Delahanty L, Wittenberg E, Grant RW. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30(9):2222–2227. doi: 10.2337/dc07-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wei X, Wu T, Chen R, Guo A. Collaborative care for patients with depression and diabetes mellitus: A systematic review and meta-analysis. BMC Psychiatry. 2013;13:260. doi: 10.1186/1471-244X-13-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YH, Essue B, Jan S, Wells R, Whitworth JA. Economic hardship associated with managing chronic illness: A qualitative inquiry. BMC Health Services Research. 2009;9:182. doi: 10.1186/1472-6963-9-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Beals J, Whitesell NR, Roubideaux Y, Manson SM. Stress burden and diabetes in two American Indian reservation communities. Diabetes Care. 2008;31(3):427–429. doi: 10.2337/dc07-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy L, Herman WH. Glycated hemoglobin assessment in clinical practice: Comparison of the A1CNow point-of-care device with central laboratory testing (GOAL A1C Study) Diabetes Technology & Therapeutics. 2005;7(6):907–912. doi: 10.1089/dia.2005.7.907. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz PM, House JS, Mero RP, Williams DR. Stress, life events, and socioeconomic disparities in health: Results from the Americans' Changing Lives Study. Journal of Health and Social Behavior. 2005;46(3):274–288. doi: 10.1177/002214650504600305. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annual Review of Psychology. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberry LS, Gonzalez JS, Wallston KA, Kripalani S, Osborn CY. The ARMS-D out performs the SDSCA, but both are reliable, valid, and predict glycemic control. Diabetes Research and Clinical Practice. 2013;102(2):96–104. doi: 10.1016/j.diabres.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall E, Shivashankar R, Tandon N, Ali MK, Narayan KM, Prabhakaran D. Stress and diabetes in socioeconomic context: a qualitative study of urban Indians. Social Science & Medicine. 2012;75(12):2522–2529. doi: 10.1016/j.socscimed.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, Coates MJ, Dunbar JA, Reddy P, Schlicht K, Fuller J. The TrueBlue model of collaborative care using practice nurses as case managers for depression alongside diabetes or heart disease: a randomised trial. BMJ Open. 2013;3(1) doi: 10.1136/bmjopen-2012-002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris T, Moore M, Morris FS. Stress and chronic illness: The case of diabetes. Journal of Adult Development. 2011;18(2):70–80. [Google Scholar]
- Mulia N, Ye Y, Zemore SE, Greenfield TK. Social disadvantage, stress, and alcohol use among black, Hispanic, and white Americans: Findings from the 2005 U.S. National Alcohol Survey. Journal of Studies on Alcohol and Drugs. 2008;69(6):824–833. doi: 10.15288/jsad.2008.69.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckerman KM, Lovasi GS, Davies S, Purciel M, Quinn J, Feder E, Rundle A. Disparities in urban neighborhood conditions: Evidence from GIS measures and field observation in New York City. Journal of Public Health Policy. 2009;30(Suppl. 1):S264–S285. doi: 10.1057/jphp.2008.47. [DOI] [PubMed] [Google Scholar]
- Ngo-Metzger Q, Sorkin DH, Billimek J, Greenfield S, Kaplan SH. The effects of financial pressures on adherence and glucose control among racial/ethnically diverse patients with diabetes. Journal of General Internal Medicine. 2012;27(4):432–437. doi: 10.1007/s11606-011-1910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak M, Björck L, Giang K, Heden-Ståhl C, Wilhelmsen L, Rosengren A. Perceived stress and incidence of Type 2 diabetes: A 35-year follow-up study of middle-aged Swedish men. Diabetic Medicine. 2013;30(1):e8–e16. doi: 10.1111/dme.12037. [DOI] [PubMed] [Google Scholar]
- Osborn CY, Patel KA, Liu J, Trott HW, Buchowski MS, Hargreaves MK, Schlundt DG. Diabetes and co-morbid depression among racially diverse, low-income adults. Annals of Behavioral Medicine. 2011;41(3):300–309. doi: 10.1007/s12160-010-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg M, DuVal T, Luciano G, Frederici J, Welch GW. A scale for measuring social stress among diabetic patients. Paper presented at the 34th Society of General Internal Medicine Scientific Meeting; Phoenix, AZ. 2011. May, [Google Scholar]
- Russell BE, Gurrola E, Ndumele CD, Landon BE, O'Malley JA, Keegan T, Hicks LS. Perspectives of non-Hispanic Black and Latino patients in Boston's urban community health centers on their experiences with diabetes and hypertension. Journal of General Internal Medicine. 2010;25(6):504–509. doi: 10.1007/s11606-010-1278-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel-Hodge CD, Headen SW, Skelly AH, Ingram AF, Keyserling TC, Jackson EJ, Elasy TA. Influences on day-to-day self-management of type 2 diabetes among African-American women: Spirituality, the multi-caregiver role, and other social context factors. Diabetes Care. 2000;23(7):928–933. doi: 10.2337/diacare.23.7.928. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo H, Lam S. Stress management training in diabetes mellitus. Journal of Health Psychology. 2009;14(7):933–943. doi: 10.1177/1359105309341146. [DOI] [PubMed] [Google Scholar]
- Spencer MS, Kieffer EC, Sinco BR, Palmisano G, Guzman JR, James SA, Heisler M. Diabetes-specific emotional distress among African Americans and Hispanics with type 2 diabetes. Journal of Health Care for the Poor and Underserved. 2006;17(2 Suppl):88–105. doi: 10.1353/hpu.2006.0095. [DOI] [PubMed] [Google Scholar]
- Tol A, Shojaeezadeh D, Eslami A, Alhani F, Mohajeritehrani M, Baghbanian A, Sharifirad G. Evaluation of self-care practices and relative components among type 2 diabetic patients. Journal of Education and Health Promotion. 2012;1:19. doi: 10.4103/2277-9531.99219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: Results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Osborn CY, Mendenhall EA, Budris LM, Belay S, Tennen HA. Beliefs about racism and health among African American women with diabetes: A qualitative study. Journal of the National Medical Association. 2011;103(3):224–232. doi: 10.1016/s0027-9684(15)30298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch GW, Osborn CY, Rotherber M, Zagarins SE, White RO, Friderici J. Level of social distress among Mexican and Puerto Rican American diabetes patients and relationship to glycemic control. Paper presented at the 71st Annual Scientific Session of the American Diabetes Association; San Diego, CA. 2011. Jun, [Google Scholar]
- Wiesli P, Krayenbuhl PA, Kerwer O, Seifert B, Schmid C. Maintenance of glucose control in patients with type 1 diabetes during acute mental stress by riding high-speed rollercoasters. Diabetes Care. 2007;30(6):1599–1601. doi: 10.2337/dc06-2102. [DOI] [PubMed] [Google Scholar]