Abstract
Endocannabinoids (eCBs) are endogenous lipid mediators involved in a variety of physiological, pharmacological, and pathological processes. While activation of the eCB system primarily induces inhibitory effects on both GABAergic and glutamatergic synaptic transmission and plasticity through acting on presynaptically-expressed CB1 receptors in the brain, accumulated information suggests that eCB signaling is also capable of facilitating or potentiating excitatory synaptic transmission in the hippocampus. Recent studies show that a long-lasting potentiation of excitatory synaptic transmission at Schaffer collateral (SC)-CA1 synapses is induced by spatiotemporally primed inputs, accompanying with a long-term depression of inhibitory synaptic transmission (I-LTD) in hippocampal CA1 pyramidal neurons. This input-timing-dependent long-lasting synaptic potentiation at SC-CA1 synapses is mediated by 2-arachidonoylglycerol (2-AG) signaling triggered by activation of postsynaptic NMDA receptors, group I metabotropic glutamate receptors (mGluRs), and a concurrent rise in intracellular Ca2+. Emerging evidence now also indicates that 2-AG is an important signaling mediator keeping brain homeostasis by exerting its anti-inflammatory and neuroprotective effects in response to harmful insults through CB1/2 receptor-dependent and/or independent mechanisms. Activation of the nuclear receptor protein peroxisome proliferator-activated receptor-γ (PPARγ) apparently is one of the important mechanisms in resolving neuroinflammation and protecting neurons produced by 2-AG signaling. Thus, the information summarized in this review suggests that the role of eCB signaling in maintaining integrity of brain function is greater than what we thought previously.
Keywords: Monoacylglycerol lipase, Alzheimer’s disease, chronic traumatic encephalopathy, input-timing-dependent plasticity, long-term depression of inhibitory synaptic transmission, PPARγ
Marijuana (cannabis) is a natural product derived from the cannabis plant and has been used for thousands of years as a medical treatment for a variety of medical conditions (Adams and Martin 1996). The most effective and well-known ingredient of cannabis is Δ9-tetrahydrocannabinol (Δ9-THC, Gaoni and Mechoulam 1964), which binds to cannabinoid receptors (CB1R and CB2R) to induce a series of physiological and psychological effects, including changes in heart rate, blood pressure, appetite, reaction time, and impairments in some forms of memory, suppression of motor skills, and induction of anxiety or addiction. Both CB1R and CB2R, which were cloned and identified in 1990 (Matsuda and others 1990) and 1993 (Munro and others 1993), respectively, are G protein-coupled receptors (GPCRs) with seven trans-membrane spanning domains. CB1R is abundantly expressed in the central nervous system (CNS) (Herkenham and others 1990, 1991; Howlett and others 1990; Matsuda and others 1990), while CB2R is primarily expressed in the immune system (Munro and others 1993; Galiegue and others 1995). CB2R expressed in CNS is primarily in astroglial cells (Cabral and others 2008; Morgan and others 2009). The discovery of CB1 and CB2 resulted in identification of two endogenous cannabinoids (endocannabinoids, eCBs) arachidonoyl-ethanolamide (Anandamide or AEA) and 2-arachidonoylglycerol (2-AG, Devane and others 1992; Mechoulam and others 1995; Sugiura and others 1995). Several eCB-like ligands have been reported (Hanus and Mechoulam 2010), but more compelling studies are needed to reassure their eCB identity.
Although 2-AG and AEA are derived from basic structure phospholipids in the plasma membrane, they have distinct synthesis pathways. AEA is primarily synthesized from N-arachidonoylphosphatidylethanolamine (NAPE) by phospholipase D (PLD), while 2-AG is largely produced from diacylglycerol (DAG) by diacylglycerol lipase (DAGL, Sugiura and others 2002; Wang and Ueda 2009). They also have different inactivation routes. Fatty acid amide hydrolase (FAAH) and monoacylglycerol (MAGL) are the primary enzymes to terminate the activity of AEA and 2-AG respectively (Cravatt and others 1996; Dinh and others 2002, 2004). While FAAH is located in postsynaptic site, MAGL is located in the presynaptic site (Gulyas and others 2004). Therefore, the re-uptake and inactivation of AEA and 2-AG occur at postsynaptic and presynaptic sites, respectively (Figure 1). In particular, it has been demonstrated that 85% of 2-AG in the brain is metabolized by MAGL (Blankman and others 2007; Long and others 2009a, b; Nomura and others 2011), suggesting a key role of MAGL in terminating 2-AG signaling. The primary metabolite of AEA or 2-AG by FAAH or MAGL is arachidonic acid (AA), a precursor of prostaglandins (PGs) and leukotrienes (LTA-E4) by the enzymes cyclooxygenase -1 and -2 (COX-1/2) and arachidonate 5-lipoxygenase (LOX, Kano and others 2009). Apparently, the eCB system, which consists of naturally biosynthesized eCBs, their corresponding CB receptors, the enzymes synthesizing and degrading eCBs, and transporters, is a complex and dynamic system (Figure 1 and 6). In this review, we primarily discuss the role of 2-AG signaling in synaptic plasticity and neuroprotection.
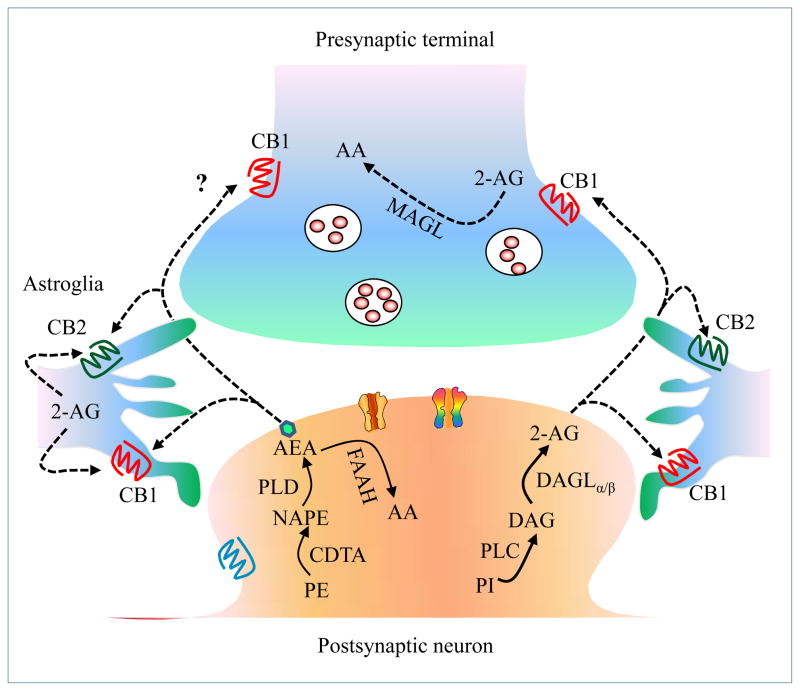
Figure 1. The endocannabinoid system.
A schematic diagram illustrates the endocannabinoid (eCB) system. CB1 receptors are expressed in presynaptic terminals and astroglial cells. CB2 receptors are primarily expressed in astroglial cells. 2-AG is synthesized in postsynaptic neurons from phosphatidylinositol (PI) lipid precursors through enzymatic activities of phospholipase C (PLC) and the DAGLα and DAGLβ enzymes and inactivated in presynaptic terminals by monoacylglycerol lipase (MAGL). AEA is mainly synthesized from phosphatidylethanolamine (PE) and N-acyl phosphatidylethanolamine (NAPE) by the enzymes calcium-dependent transacylase (CDTA) and phospholipase D (PLD) and inactivated by the enzyme fatty acid amide hydrolase (FAAH).
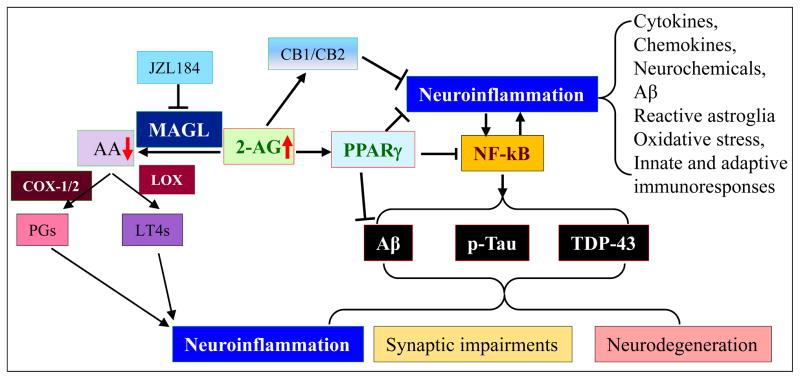
Figure 6. eCB-mediated anti-inflammation and neuroprotection.
eCB-mediated anti-inflammation and neuroprotection. Neuroinflammation is a key player in neurodegenerative diseases, including traumatic brain injury (TBI), Parkinson’s disease (PD), Alzheimer’s disease (AD), multiple sclerosis (MS), and amyotrophic Lateral Sclerosis (ALS). Inhibition of 2-AG metabolism by inactivating MAGL strengthens 2-AG signaling, which is anti-inflammatory and neuroprotective, and reduces proinflammatory and neurotoxic prostaglandins (PGs) synthesized by cyclooxygase-1 and -2 (COX-1/2) and leukotrienes (LT4s) synthesized through 5-lipoxygenase (LOX). The neuroprotective effects of 2-AG are likely mediated CB1/2 dependent and independent pathways. PPARγ, which suppresses NF-κB, is an important signaling mediator in 2-AG-induced CB1/2-independet neuroprotection, resulting in reductions of the factors causing neurodegenerative diseases. These factors include Aβ, tau phosphorylation, and TDP-43 protein.
eCBs in Synaptic Plasticity
Synaptic plasticity is a widespread phenomenon occurred both at excitatory and inhibitory synapses by changes in synaptic strength and efficacy in response to various activities and events. This activity-dependent potentiation or depression in synaptic efficacy is thought to be one of the important neurochemical foundations for learning, memory, and behavior adaptation. Since synaptic plasticity was first reported in 1973 (Bliss and Gardner-Medwin 1973; Bliss and Lomo 1973), this phenomenon has been extensively studied. Increased/decreased presynaptic release of neurotransmitters or/and altered postsynaptic capacities were the primary mechanisms contributing to the most of known forms of synaptic plasticity. The changes in the density of active receptors (including newly formed receptors and activating the silence receptors) expressed on a synapse can also modify synaptic efficacy (Gerrow and Triller 2010). The eCB system is involved in a variety of short-term and long-term synaptic plasticity both at excitatory and inhibitory synapses throughout the brain regions (Alger 2002; Wilson and Nicoll 2002; Chevaleyre and others 2006; Kano and others 2009; Xu and others 2010, 2012). Unlike the conventional neurotransmitters, which are released from presynaptic terminals to activate the target receptors located on the postsynaptic site, eCBs generated in the postsynaptic neurons as a retrograde messenger act on presynaptic CB1R, resulting in suppression of the neurotransmitter release (Freund and others 2003). It is generally accepted now that 2-AG likely is a retrograde messenger in mediating GABAergic and glutamatergic synaptic transmission in the brain (Kim and Alger 2004; Chevaleyre and others 2006; Kano and others 2009).
eCB-Mediated Short-Term Depression of Synaptic Transmission
Postsynaptically Ca2+-dependent depolarization-induced suppression of inhibition (DSI) is a form of short-term synaptic plasticity at GABAergic synapses, which was first demonstrated in rat CA1 pyramidal cells by Pitler and Alger (1992; 1994). It appeared that DSI relied on retrograde signaling (Alger and Pitler 1995). This led to the identification of eCBs as the retrograde messengers responsible for this short-term synaptic plasticity (Wilson and Nicoll 2001; Diana and Marty 2004). Thereafter, depolarization-induced suppression of excitation (DSE) at glutamatergic synapses was also found to be mediated by eCBs in the cerebellum (Kreitzer and Regehr 2001) and hippocampus (Ohno-Shosaku and others 2002). In addition, eCBs also likely mediate short-term reduction in the probability of neurotransmitter release in the hippocampus, amygdale, cerebellum, cerebral cortex, basal ganglia, brain stem, and hypothalamus. The suppression of short-term synaptic transmission mediated by eCBs is termed as eCB-STD.
A critical step of releasing eCBs in initiating eCB-STD is a rise in intracellular Ca2+ in the postsynaptic site via voltage-gated calcium channels (VGCC), group I metabotropic receptors (mGluRs), and muscarinic acetylcholine receptors, which mobilize stored Ca2+ via phospholipase C (PLC). Elevation of intracellular Ca2+ concentration by different means induces eCB-STD, while decrease of Ca2+ blocks eCB-STD (Llano and others 1991; Pitler and Alger 1992; Kreitzer and Regehr 2001; Wilson and Nicoll 2001; Kim and others 2002), suggesting that the increase of postsynaptic Ca2+ is necessary for the induction of eCB-STD. The nature of eCB formation (2-AG or AEA or both) is depending on the specific stimulus protocols and the locations of the synapses. Activation of the PLC pathway induces the AEA production, implying the involvement of AEA in synaptic driven DSI (Liu and others 2006). While eCB-STD mediated by AEA is still controversial (Kim and Alger 2004; Heinbockel and others 2005; Pan and others 2009), more evidence supports that 2-AG is the main retrograde messenger in eCB-STD (Kim and Alger 2004; Makara and others 2005; Pan and others 2009; Straiker and others 2009; Gao and others 2010).
eCB-Mediated Long-Term Depression of Synaptic Transmission
eCB-mediated long-term depression of synaptic transmission (eCB-LTD) is the form of long-term reduction of the neurotransmitter release at the same or nearby synapses by activation of presynaptic CB1R. These long-term changes of synaptic strength or weakness are important for reshaping various forms of memory and adaptive learning. The first eCB-LTD at excitatory synapses was reported in 2002 in the dorsal striatum and nucleus accumbens (Gerdeman and others 2002; Robbe and others 2002). Almost the same time, eCB-LTD at inhibitory synapses was reported in the amygdala (Marsicano and others 2002). Later, eCB-LTD was observed in a variety of brain regions, including in the hippocampus (Chevaleyre and Castillo 2003; Yasuda and others 2008), cerebellum (Safo and Regehr 2005), basolateral amygdale (Azad and others 2004), dorsal striatum (Kreitzer and Malenka 2005; Ronesi and Lovinger 2005), nucleus accumbens (Mato and others 2008), and neocortex (Sjostrom and others 2003).
Induction of eCB-LTD also requires an increase in intracellular Ca2+ and activation of postsynaptic mGluRs in most brain regions (Ito 2001; Robbe and others 2002; Chevaleyre and Castillo 2003; Bender and others 2006; Kreitzer and Malenka 2007; Lafourcade and others 2007). Similar to induction of eCB-STD, 2-AG is a retrograde signaling molecule in eCB-LTD (Chevaleyre and Castillo 2003, 2004; Jung and others 2005). However, the maintenance phase of eCB-LTD appears to be independent on CB1R after several minutes of continued activation at the initial induction step (Robbe and others 2002). The exact mechanism of sustaining the long-term depression of a neurotransmitter release after activation of CB1R within the short-time scale (minutes) is still unknown, but the distribution of CB1R largely determines the strength of eCB-mediated short- and long-term synaptic plasticity. Anatomically, hippocampal CA1 pyramidal neurons receive dual glutamatergic synaptic inputs from entorhinal perforant path (PP) and Schaffer-collateral (SC) path. The PP directly sends information from layer III neurons in the entorhinal cortex to the distal dendritic regions of CA1 pyramidal neuron, while the SC path sends information indirectly from layer II neurons in the entorhinal cortex to the proximal dendritic regions of CA1 pyramidal neuron via the trisynaptic circuit (Anderson and others 1971; Steward and Scoville 1976; Claiborne and others 1986; Amaral and Witter 1989; Ishizuka and others 1990). Recent study revealed that eCB-mediated LTD and DSE were weaker at the PP than that at SC. This is consistent with the different densities of CB1R on these two glutamatergic inputs. The differentially expressed synaptic plasticity in the same neuron suggests a potential impact on synaptic scaling, integration and plasticity of hippocampal CA1 pyramidal neurons (Xu and others 2010). The wide distributions of eCB-LTD, together with the prevalence of CB1 receptors throughout the brain, suggest an important modification role of eCB-LTD in synaptic function and behavior adaptation.
eCB-Mediated Long-Term Potentiation of Excitatory Synaptic Transmission
Given the fact that eCBs mediate several forms of short-term and long-term depressions by reducing presynaptic release of GABA, it was hypothesized that eCBs may be capable of indirectly mediating LTP of glutamatergic synaptic transmission via the disinhibition of postsynaptic neurons through the suppression of GABAergic synaptic release. This hypothesis was first proved by the study performed in the hippocampus (Carlson and others 2002), where fEPSPs and whole-cell EPSCs in single pyramidal cell were simultaneously recorded in the hippocampal CA1 region. A combined depolarization and weak stimulus together induces LTP of EPSCs but not fEPSPs. This highly localized LTP within the diffusion distance of eCBs raises the possibility that this form of LTP was likely mediated via eCBs. This speculation was confirmed by the observation in which EPSC-LTP was inhibited in the hippocampal slices pretreated with CB1 receptor antagonist AM251 (Carlson and others 2002). This indicates that eCB-STD at inhibitory synapses would facilitate LTP induction at glutamatergic synapses. Similarly, eCB-LTD at the inhibitory synapse (I-LTD) can also facilitate LTP induction at nearby glutamatergic synapse in hippocampal slices (Chevaleyre and Castillo 2004). This form of LTP is not expressed under the presence of GABAA antagonist, suggesting the requirement of GABAergic synaptic function in facilitating LTP induction. The facilitation of LTP induction mediated via eCBs was supported by the fact that CB1 inhibition or knockdown abolishes the priming protocol induced facilitation of LTP. This synaptic facilitation at glutamatergic synapses was confirmed by other studies where a primed low-frequency stimulation (LFS) with a weak theta-burst stimulation (TBS) protocol induces LTP at excitatory synapses (Zhu and Lovinger 2007). Although both the eCB-DSI/STD and eCB-I-LTD can trigger induction or facilitation of LTP at glutamatergic synapses, more research is needed to address the mechanisms underlying eCB-mediated LTP of excitatory synaptic transmission.
eCB-Mediated ITDP
The hippocampus is a primary brain structure with a major role in the cognitive learning and consolidation of memory and spatial navigation. The structure complexity of the hippocampus forms the base for a variety of forms of functional synaptic plasticity adapting to different activities or stimuli. Available evidence suggests that synaptic transmission at the PP and SC path is differentially modulated by neurotransmitters (Hasselmo and Schnell 1994; Otmakhova and Lisman 1999, 2000; Otmakhova and others 2005). Recent studies also show that there are differences in LFS- and DHPG-induced LTD between PP and SC synapses (Xu and others 2010). The differences are eliminated by pharmacological or genetic inhibition of CB1R, indicating that eCB signaling contributes to the differences in LTD between these two synapses (Xu and others 2010). In addition, there also exhibit differences in DSE between PP and SC synapses. The results from dendritic recordings and photolysis of caged Ca2+ confirm that DSE can be induced at SC but not PP synapses (Xu and others 2010). Currently, no information is available as to whether there is a difference in the release machinery of eCBs between the two synapses. However, lower density of CB1 expression in stratum lacunosum-moleculare (SLM) when compared with that in stratum radiatum (SR) may underlie differential modulation of short-term and long-term depression of excitatory synaptic transmission between PP and SC pathways (Xu and others 2010).
The inputs from the PP path have been reported to modulate induction or expression of LTP induced by activity or stimulus via the SC path (Colbert and Levy 1993; Remondes and Schuman 2002, 2004; Brun and others 2008; Izumi and Zorumski 2008). This suggest that these highly wired synaptic pathways in the brain may provide important learning rule for memory consolidation, storage, and retrieval (Eichenbaum 2000; Remondes and Schuman 2002, 2004; Nolan and others 2004). The role of the dual sensory inputs in hippocampal information processing and storage was revealed by a new form of synaptic plasticity, called input-timing-dependent heterosynaptic plasticity (ITDP), which is induced by a pairing stimulus paradigm at these synaptic inputs (Dudman and others 2007). This form of synaptic plasticity reflects the interaction between cortico-hippocampal activities. Specifically, both distal and proximal EPSPs or EPSCs were recorded in CA1 pyramidal neurons by focally stimulating PP and SC paths. An LTP-like potentiation at SC synapses, but not at PP synapses, was induced by pairing of stimuli at PP with 20ms preceded to stimuli at SC path. There are some unique features of IDTP when compared to classical forms of plasticity as previously reported at SC synapses (Dan and Poo, 2004). Induction of ITDP at SC does not require firing of somatic action potential of postsynaptic CA1 neurons, but requires a preceding stimulation at PP with an appropriate timing interval at 20ms, which matches the expected propagation delay of the signal from the entorhinal cortex through the trisynaptic circuit (Dudman and others 2007). Similar to other forms of synaptic plasticity, induction of ITDP also requires a rise in intracellular Ca2+ at proximal synapses through NMDA receptors and/or mGluRs. Apparently, an increase in Ca2+ is facilitated by distal inputs (Dudman and others 2007). ITDP likely serves as a predictive learning tool to shape the information flow within the hippocampal macro-circuit.
A rise in intracellular Ca2+ and activation of mGluRs are important features of ITDP (Dudman and others 2007). These features are identical to that of eCB modulation of synaptic transmission and plasticity at SC synapses (Alger 2002; Chevaleyre and Castillo 2004; Chevaleyre and others 2006; Hashimotodani and others 2007). It was noticed that the ratio of paired-pulse facilitation (PPR) is reduced during ITDP (Figure 2-A4; Xu and others 2012), suggests that ITDP likely is a form of postsynaptically induced and presynaptically expressed plasticity and may be mediated via a retrograde messenger (Xu and others 2012). Indeed, recent work provides compelling evidence that ITDP is mediated via eCB signaling by the fact that ITDP was inhibited by pharmacological or genetic inhibition of CB1 (Figure 2-A and B; Xu and others 2012). Importantly, inhibition of DAGL, the enzyme that biosynthesizes 2-AG, blocks ITDP, while inhibition of MAGL, the enzyme that metabolizes 2-AG, facilitates the potentiation, suggesting that 2-AG is a signaling mediator in induction of ITDP (Figure 2-C; Xu and others 2012). The mediation of ITDP by eCB signaling has been confirmed by the recent work where inhibition of CB1 greatly attenuates ITDP (Basu and others 2013). ITDP induction, which is Ca2+-dependent and requires activation of NMDA receptors and mGluRs, has been confirmed by the study (Xu and others 2012). However, there is a discrepancy between the observations made by Dudman and others (2007) and Xu and others (2012). In the study by Dudman and others (2007), ITDP was independent on GABAergic inhibition. However, Xu and others (2012) found that ITDP was not induced by a PP-SC pairing stimulus protocol when GABAergic synaptic transmission was inhibited by the antagonists. A recent study by Basu and others (2013) reports that ITDP is significantly reduced when GABAergic synaptic transmission is inhibited, supporting the finding by Xu and others (2012). This information suggests that GABAergic synapses may play a critical role in ITDP induction. In fact, the authors observed that there are at least two components that contribute to ITDP, including LTP at excitatory synapses (E-LTP) and LTD at inhibitory synapses (I-LTD, Basu and others 2013). It seems that E-LTP is independent of eCBs, while I-LTD likely relies on eCB signaling (Basu and others 2013). Through elegantly designed experiments, they found that I-LTD occurs during ITDP at synapses between CCK interneurons and pyramidal neurons (Basu and others 2013). This is consistent with the previous findings that eCBs reduce the release of presynaptic GABA from CCK interneuron (Castillo and others 2012) As stated in Basu and others (2013), this in-phase cortico-hippocampal activity provides a powerful heterosynaptic learning rule for long-term gating of information flow through the hippocampal excitatory macrocircuit by the silencing of the CCK inhibitory microcircuit. The participation of 2-AG signaling in ITDP suggests a new role of the eCB system in hippocampal long-term synaptic plasticity (Figure 2 and Figure 3).
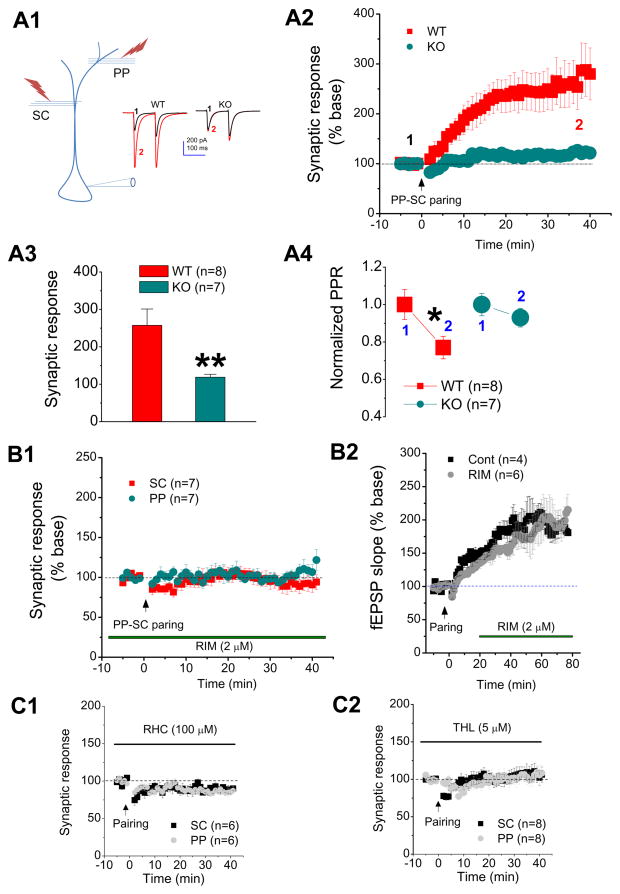
Figure 2. Input-timing-dependent plasticity is mediated via 2-AG signaling.
Input-timing-dependent plasticity is mediated via 2-AG signaling (adopted from Xu et al., J Physiol. 590: 2305–2315, 2012 with modification). A1, Patch clamp recording setup with pairing of distal and proximal synaptic stimuli in a CA1 pyramidal neuron. One stimulus electrode was placed at the site of the distal perforant path (PP) in the lacunosum-moleculare (SLM), and second stimulus electrode was placed at the site of Schaffer collateral (SC) path in the stratum radiatum (SR). Somatic recordings under the whole-cell voltage clamp mode were made in CA1 pyramidal neurons. Representative traces of EPSCs recorded at SC synapses of CA1 pyramidal neurons from a CB1 knockout (KO) or a wild-type controls (WT) mouse before and after pairing of PP-SC stimuli. A2, time courses of changes in synaptic response at the SC synapses in WT and KO mice. A3, mean values of EPSCs averaged from 36 to 40 min following PP-SC pairing. ** P < 0.01 compared with WT at the SC. A4, mean value of normalized paired pulse ratio (PPR) at the SC before and after PP-SC pairing recorded from WT and KO mice. * P < 0.05 compared with the baseline. B1, time courses of synaptic response recorded at PP and SC in rat CA1 pyramidal neurons in the presence of Rimonabant (RIM, 2 μm), a selective CB1 receptor antagonist. RIM was pre-treated and continuously perfused during recordings. B2, time courses of fEPSPS recorded at the SC in rat CA1 pyramidal neurons. RIM (2 μm) was applied 10 min following PP-SC pairing stimulation. There are no differences in the potentiation between RIM and the control. C1, time courses of EPSCs recorded before and after pairing at the PP and SC in the presence of RHC-80267 (RHC, 100 μm), an inhibitor for diacylglycerol kinase (DAGL) that synthesizes 2-AG, in rat hippocampal CA1 pyramidal neurons. C2, time courses of synaptic response recorded before and after PP-SC pairing at the PP and SC in the presence of tetrahydrolipstatin (THL, 5 μm), another DAGL inhibitor, in rat CA1 pyramidal neurons.
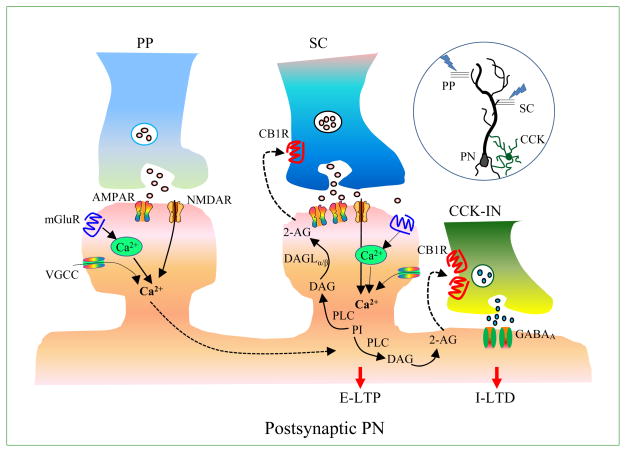
Figure 3. eCB-ITDP.
A schematic diagram illustrates eCB-mediated input-timing-dependent plasticity (eCB-ITDP). Hippocampal CA1 pyramidal neurons receive dual entorhinal glutamatergic afferent inputs via the proximal Schaffer-collateral (SC) path and the distal perforate path (PP). eCB-ITDP in pyramidal neurons (PN) is induced by a paring stimulus protocol, consisting of pairs of proximal and distal stimuli (1 Hz for 90 sec) in which the distal stimulus preceded the proximal stimulus by 20 msec. 2-AG is a retrograde messenger in ITDP, resulting in E-LTP at excitatory SC synapses and I-LTD at inhibitory cholecystokinin interneuron (CCK-IN) and PN synapses.
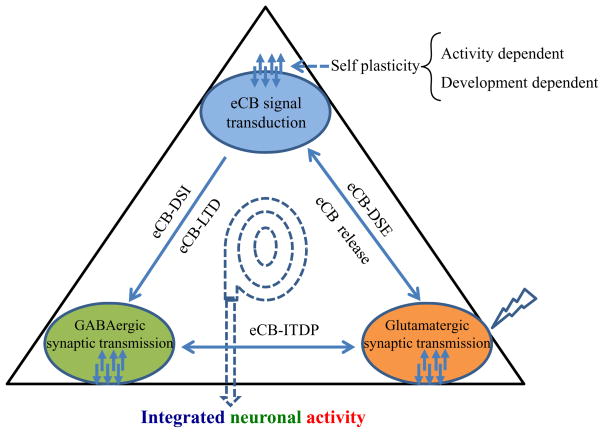
eCB Signaling in Integration of Inhibitory and Excitatory Synaptic Transmission
The emerging evidence strongly suggests that the eCB system serves as an important signaling mediator in integrating neuronal transmission during meta-synaptic plasticity in CNS. Based on the current available information and knowledge, a theoretical triangle framework is proposed to represent the integration of neural plasticity (the neuronal mechanism for learning and memory) that is regulated or modulated by eCB signaling (Figure 4). The temporal and spatial features of eCB signaling suggest an important control for the overall output to the final neuronal activity and behavior performance. Among integration strategies, the most important eCB-mediated synaptic plasticity is short- and long-term depression of inhibitory GABAergic synaptic transmission in a variety of brain regions, which have been extensively studied and reviewed (Alger 2009; Castillo and others 2012; Ohno-Shosaku and others 2012). Another important eCB-mediated synaptic plasticity is a short-term depression of excitatory glutamate release (i.e., eCB-DSE) and a long-term potentiation of excitatory synaptic transmission (i.e., eCB-ITDP). The discovery of ITDP (Dudman and others 2007) and 2-AG signaling in ITDP (Xu and others 2012; Basu and others 2013) provide new insight into the regulation of information processing in the hippocampus by eCB signaling. However, there is still limited information or knowledge available for the reverse regulation of eCB signaling from GABAergic and glutamatergic transmission during the eCB-mediated dynamic integration process. Interesting but not surprise, eCBs are capable of not only inducing synaptic plasticity at excitatory synapses via other neurotransmitters (i.e. GABA), they may also be able to undergo a long-term self plasticity by persistent synaptic activity (Zhu and Lovinger 2007). The inherent self plasticity of eCB signaling has important physiological implication in this proposed neuronal integration network due to its spatiotemporally functional feature. With the advance in experimental techniques, including rapid integration of computer simulation, real time electrophysiological recording, optogenetics, and high resolution imaging, we will better to understand the functional roles of the complex eCB system in integrating neural network and behavior adaptation as well as in clinical applications.
Figure 4. eCB signaling in dynamically integrating GABAergic and glutamatergic transmission.
eCB signaling in integration of inhibitory and excitatory synaptic transmission. Several forms of short-term and long-term homosynaptic synaptic plasticity can be induced at excitatory and inhibitory synapses separately. eCBs functioning as retrograde messengers are capable of integrating excitatory and/or inhibitory synaptic transmission to mediate new forms of heterosynaptic plasticity (i.e. eCB-ITDP). The self plasticity of eCB signal transduction may be important in the fine-tuning adjustment in integrating multiple synaptic plasticity.
2-AG Signaling in Neuroprotection
Growing evidence indicates that eCBs display profound anti-inflammatory and neuroprotective properties in response to harmful insults. There is a strong link between neuroinflammation and neurodegenerative processes. This suggest that anti-inflammatory properties of eCBs are important for the eCB-mediated neuroprotection (Sarne and Mechoulam 2005; van der Stelt and Di Marzo 2005; Eljaschewitsch and others 2006; Centonze and others 2007; Zhang and Chen 2008; Stella 2009; Arevalo-Martin and others 2010; Bisogno and Di Marzo 2010; Scotter and others 2010; Chen and others 2011; Du and others 2011; Shohami and others 2011). Available information indicates that the eCB system responds differently to different pathogenic events. Previous studies show that the levels of 2-AG are significantly increased in the brain following closed head injury and administration of 2-AG attenuates brain trauma-induced neuropathology (Panikashvili and others 2001, 2005 and others 2006). In contrast, administration of kainic acid rapidly raises the levels of AEA, but not 2-AG (Marsicano and others 2003). Interestingly, the release of 2-AG, but not AEA, is elevated in response to brain Aβ42 infusion (van der Stelt and others 2006). This suggests that AEA and 2-AG, the two important endogenous signaling molecules, are ‘on demand’ responsible for maintaining homeostasis of brain function. While the actions of eCBs in anti-inflammatory and neuroprotective effects are primarily mediated through CB1 and/or CB2 receptors, recent studies provide evidence of CB1- and CB2-independent effects in resolving neuroinflammation and reducing neurodegeneration by inhibition of 2-AG metabolism (Nomura and others 2011; Chen and others 2012; Piro and others 2012). This suggests that multiple signaling pathways are involved in the eCB-mediated neuroprotective effects. In this section, we primarily discuss the role of 2-AG signaling in inflammation associated neurodegenerative diseases.
Traumatic Brain Injury
Traumatic brain injury (TBI) is a sudden head injury that causes damage to the brain. Latest studies reveal that the risk of development of chronic traumatic encephalopathy (CTE), a long-term progressive neurodegenerative disease, is significantly increased in athlete and military personnel who have exposed to single or repetitive mild head injuries (Omalu and others 2005; Blennow and others 2012; Dekosky and others 2013; Jordan 2013; Lakis and others 2013; McKee and others 2013; Smith and others 2013; Yi and others 2013). This means that TBI may lead to a neurodegenerative disease and dementia.
The acute brain damage after TBI results from primary injury, which is the result of the external mechanical force leading to contusion, laceration, and diffuse injuries, and from secondary injury immediately followed by primary injury, which is associated with a complex cascade of molecular, cellular, and immune responses, resulting in neuroinflammation, excitotoxicity, oxidative stress, disruption of calcium homeostasis, mitochondrial dysfunction, neuronal injury, and death (Dardiotis and others 2012; Smith and others 2013; Zetterberg and others 2013). The inflammatory response associated with other processes likely plays a key role in leading to TBI-induced neuropathology, including Aβ formation, tau phosphorylation, TDP-43 protein aggregation, and white matter degeneration, which eventually cause synaptic and cognitive deficits, and dementia (Blennow and others 2012; Dekosky and others 2013). Neuroinflammation is one of the important hallmarks in TBI. It has been documented that inflammatory markers such as cytokines IL-1β, IL-6, and TNFα, and chemokines released from reactivated astroglial cells and infiltrated leukocytes are robustly elevated in the brain and cerebrospinal fluid after TBI (Dardiotis and others 2012; Woodcock and Morganti-Kossmann 2013; Zetterberg and others 2013). Although the primary injury immediately following TBI is not preventable, the secondary injury provides a window for interventions to prevent further brain damage after the primary injury. Appropriate and timely intervention during this critical window following the primary injury after TBI may significantly reduce secondary brain damage and eventually prevent occurrence of CTE. Thus, resolving neuroinflammation immediately following TBI may be the key to prevent further brain damage and neuropathological changes. Because of the anti-inflammatory property, the eCB system as an endogenous neuroprotective system may offer such a barrier to prevent excessive inflammatory response after TBI. In fact, it has been demonstrated that the levels of endogenous 2-AG in the brain are significantly elevated in a mouse model of closed head injury (Panikashvili and others 2001). Direct administration of 2-AG reduces edema formation, infarct volume, inflammatory IL-1β, IL-6, TNFα, and blood-brain-barrier permeability. The neuroprotective effects of 2-AG is partially blocked by rimonabant (RIM), a CB1R antagonist (Panikashvili and others 2001, 2005 and others 2006). This information suggests that the neuroprotective effects of 2-AG are not solely mediated via CB1R (Shohami and others 2011). In agreement with these previous studies, a recent study shows that attenuated neuronal degeneration in the dentate gyrus of TBI mice by inhibition of α/β hydrolase domain 6 (ABHD6), an enzyme hydrolyzes 2-AG, is partially blocked by AM280, CB1R antagonist or AM630, a CB2R antagonist (Tchantchou and Zhang 2013). However, the reduced lesion volume by ABHD6 inhibition in TBI mouse brain is only blocked by AM280, but not by AM630. This suggests there may be multiple signal transduction pathways involved in 2-AG-produced neuroprotection in TBI. Although the molecular mechanisms responsible for the actions produced by 2-AG in TBI warrant further investigation, the message that 2-AG is neuroprotective after TBI is clear. 2-AG is a very unstable fatty acid, easily and rapidly enzymatically metabolized upon its release. This suggests that strengthening 2-AG signaling by facilitating synthesis of 2-AG or inhibiting metabolism of 2-AG would be a good strategy for preventing neuroinflammatory and neurodegenerative cascades after TBI.
Parkinson’s Disease
Parkinson’s disease (PD) is a neurodegenerative disease characterized by the degeneration of dopaminergic neurons in the substantia nigra. Available information from epidemiological, genetic, and pharmacological studies indicates that neuroinflammation contributes to the neuropathogenesis of PD. Inflammatory responses are currently recognized as prominent features of PD manifested by increased production of inflammatory cytokines and chemokines, reactivation of astroglial cells, infiltration of immune cells, and oxidative stress (Tufekci and others 2012). The involvement of the eCBs system in PD was initially revealed by the studies where researchers observed that the levels of 2-AG, AEA or both are significantly elevated in the basal ganglia of PD animal models and in the cerebrospinal fluid of PD patients (Di Marzo and others 2000; Pisani and others 2005; van der Stelt and others 2005; Pisani and others 2010). Increased CB1 receptor expression has also been found at postmortem in the striatum of PD patients and MPTP-lesioned nonhuman primates (Lastres-Becker and others 2001). This indicates eCB signaling is involved in the pathophysiology of PD. The changes in endogenous eCB levels are likely associated with the inflammatory response in neuropathology of PD. A recent study shows that increase endogenous 2-AG by pharmacological or genetic inactivation of MAGL suppress inflammatory cytokines and microglial activation in response to a proinflammatory stimulus (Nomura and others 2011). Importantly, similar manipulations prevent degeneration of dopaminergic neurons and loss of dopamine in an MPTP model of Parkinson’s disease, suggesting that inhibition of 2-AG metabolism may have potential for a PD therapy. These anti-inflammatory and neuroprotective effects appear independent on CB1 or CB2 because these effects are not blocked by CB1 and/or CB2 antagonists or in CB1 or CB2 knockout animals (Nomura and others 2011). Since AA, a precursor of PGs, is the metabolite of 2-AG by MAGL, it is likely that these beneficial effects exerted by inhibition of 2-AG metabolism may be through lowering neuroinflammatory eicosanoid production (Nomura and others 2011; Mulvihill and Nomura 2013). However, it is still not clear what the functional role of the elevated 2-AG levels by inhibition of its metabolism plays in reducing neuroinflammation and protecting neurons in response to harmful insults or pathogenic events. This suggests that the molecular mechanisms underlying the beneficial effects of eCBs in inflammation and neurological disorders are still not completely understood.
Alzheimer’s Disease
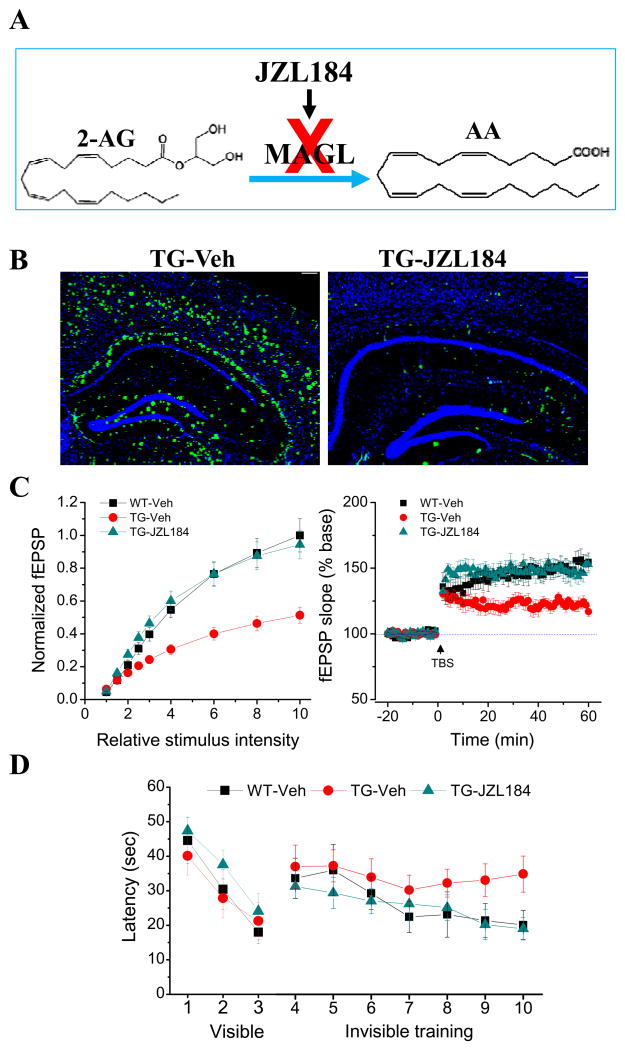
Alzheimer’s disease (AD) is also a neurodegenerative disorder characterized by accumulation and deposition of β-amyloid (Aβ) plaques and neurofibrillary tangles, neuroinflammation, synaptic dysfunction, progressive deterioration of cognitive function and loss of memory in association with widespread neuronal death. Currently there are few agents approved by the Food and Drug Administration for treatment of AD, but they only show modest effects in modifying the clinical symptoms for relatively short periods, and none has shown a clear effect on disease progression or prevention. Thus, there is a great public health need to discover or identify novel therapeutic targets for prevention and treatment of AD. Because neuroinflammation is important feature in AD, it is likely that the eCB system plays an important role in neurobiology of AD. It has been shown that expression of CB1R on neurons is reduced in the brains of AD humans, while expression of CB2 is dramatically up-regulated, particularly in the microglial cells surrounding Aβ plaques in AD humans (Westlake and others 1994; Benito and others 2003; Ramirez and others 2005; Solas and others 2013). Studies also observe that 2-AG signaling in terms of its synthesis and metabolism is altered both in AD humans and animals (Mulder and others 2011; Piro and others 2012). This indicates that 2-AG signaling is an important endogenous mediator in response to neuropathological changes in AD. Indeed, the release of 2-AG, but not AEA in the brain is increased when Aβ42 is administered into the brain (van der Stelt and others 2006). Also 2-AG or MAGL inhibitors reduce Aβ-induced neuroinflammation and neurodegeneration in culture (Chen and others 2011). Therefore, enhancing brain 2-AG signaling by inhibiting its metabolism may provide a therapeutic intervention for preventing or delaying development of AD. The research toward this end has achieved interesting and promising results by targeting MAGL, the key enzyme that degrades 85% of 2-AG in the brain (Blankman and others 2007; Long and others 2009a, b; Nomura and others 2011). Pharmacological or genetic inhibition of MAGL significantly reduces Aβ plaques, inflammatory cytokines and reactive astroglial, neurodegeneration and improves hippocampal synaptic plasticity and spatial learning in AD animals (Figure 5, Chen and others 2012; Piro and others 2012). As mentioned above, MAGL inhibition also suppresses inflammatory cytokines in LPS-treated animals and neurodegeneration in MPTP model of PD, suggesting that MAGL is an important therapeutic target for neurodegenerative diseases (Chen and others 2012).
Figure 5. Inhibition of MAGL reduces Aβ and improves synaptic and cognitive function in a mouse model of Alzheimer’s disease.
Inhibition of MAGL reduces Aβ and improves synaptic and cognitive function in a mouse model of Alzheimer’s disease (adopted from Chen et al., Cell Rep., 2: 1329–1339, 2012 with modification). A. 2-AG is primarily metabolized by monoacylglycerol lipase (MAG) in the brain. JZL184 is a selective and potent inhibitor for MAGL. Inhibition of MAGL by JZL184 increases 2-AG and reduces metabolites of 2-AG. B. Amyloid-β (Aβ) plaques, a neuropathological hallmark of Alzheimer’s disease (AD), is reduced by JZL184 in a mouse of model of AD. C. Inhibition of MAGL improves hippocampal basal synaptic transmission (input-output function) and long-term synaptic plasticity (LTP) in AD animals. D. MAGL inhibition prevents deteriorations in spatial learning in AD animals using the water maze test.
Similar to the beneficial effects produced by MAGL inactivation in proinflammatory LPS-treated animals and PD animals (Nomura and others 2011), reducing Aβ and neuroinflammation and improving synaptic cognitive function in AD animals apparently are independent of CB1 or CB2 (Chen and others 2012; Piro and others 2012). Inactivation of MAGL greatly elevates 2-AG levels and decreases AA and PGs and leukotrienes (Figure. 6). PGs produced by the enzymes COX-1 and COX-2 and leukotrienes produced by the enzyme LOX have been long known as important inflammatory mediators in neurodegenerative diseases such as AD (Salmon and Higgs 1987; Hein and O’Banion 2009). Recent evidence shows that a large proportion of PGs derives from metabolites of 2-AG by MAGL (Nomura and others 2011). This means that inhibition of 2-AG metabolism is crucial in regulating endocannabinoid and prostaglandin signaling that tunes neuroinflammatory and neurodegenerative processes in response various assaults in the brain, suggesting that MAGL inactivation would be a better strategy to strengthen 2-AG signaling, which is anti- inflammatory and neuroprotective, while reduce the levels of AA and its derivatives PGs and leukotrienes, which are proinflammatory and neurotoxic (Figure 6).
PPARγ in 2-AG-Produced Anti-Inflammation and Neuroprotection
Since administration 2-AG per se suppresses neuroinflammation and protects neurons from harmful insults (Panikashvili and others 2001, 2005 and others 2006; Zhang and Chen 2008; Chen and others 2011), we would have the reason to raise the question of other mechanisms mediating anti-inflammatory and neuroprotective effects produced by MAGL inhibition, which increases endogenous 2-AG levels. Studies have shown that 2-AG may be an endogenous agonist for peroxisome proliferator-activated receptor γ (PPARγ, Rockwell and others 2006; O’Sullivan 2007; Du and others 2011). Activation of PPARγ is capable of reducing neuropathology in AD and TBI (Kummer and Heneka 2008; Yi and others 2008; Sauerbeck and others 2011). This suggests that PPARγ is an important signaling mediator in 2-AG-induced resolution of neuroinflammation and protection of neurons against harmful insults. PPARγ is a family member of the nuclear receptors PPARs including PPARα, and PPARβ/δ. Although PPARγ was originally shown to regulate lipid metabolism and adipocyte differentiation, accumulated information indicates that PPARγ possesses anti-inflammatory and neuroprotective properties by regulating transcription of genes involving inflammation (Ricote and others 1998; Daynes and Jones 2002; Luna-Medina and others 2005; Drew and others 2006; Bensinger and Tontonoz 2008; Bright and others 2008; Jiang and others 2008; Necela and others 2008; Racke and Drew 2008). PPARγ regulates gene transcription by binding to conserved DNA sequences termed peroxisome proliferator response elements (PPRE) as heterodimers with retinoic X receptor (Bensinger and Tontonoz 2008). Growing evidence suggests that PPARγ is likely a target for eCBs (Lenman and Fowler 2007; O’Sullivan and Kendall 2010; Pertwee and others 2010; Pistis and Melis 2010; Du and others 2011). It has been demonstrated that 2-AG-induced suppression of interleukin-2 in Jurkat T cells is mediated by activation of PPARγ through a CB1/2 receptor-independent mechanism (Rockwell and others 2006), suggesting that 2-AG may be able to directly activate nuclear PPARγ by crossing both the plasma and nuclear membranes (O’Sullivan 2007). This suggests that 2-AG-initiated signaling events mediated through PPARγ prevent or inhibit inflammatory gene transcription, resulting in resolution of inflammation and neuroprotection. Presumably, anti-inflammatory and neuroprotective effects produced by PPARγ are primarily through limiting NF-κB transcription (Bensinger and Tontonoz 2008; Bright and others 2008; Du and others 2011). PPARγ agonist treatments have been shown to exhibit beneficial effects both in patients with AD and animal models of AD (Jiang and others 2008; Kummer and Heneka 2008; Sato and others 2011). MAGL inhibition, which boosts brain 2-AG and reduces AA and its metabolites, is also able to suppress neuroinflammation and counteract neuropathology of neurodegenerative diseases through CB1- and CB2-independent mechanisms. It is possible that the beneficial effects produced by MAGL inhibition are mediated via 2-AG signaling, which activates PPARγ (Figure 6).
Perspective
The complexity of the eCB system suggests a broad spectrum of biological functions mediated by eCB signaling. As discussed in this review, activation of the eCB system induces not only inhibitory effects on both GABAergic and glutamatergic synaptic transmissions, but also produces potentiation of synaptic transmission at glutamatergic synapses with concurrent depression at inhibitory synapses. Although the dual sensory inputs that the CA1 pyramidal neurons receive have been suggested to be essential for information processing, consolidation, storage and retrieval in the hippocampus (Eichenbaum 2000; Remondes and Schuman 2002, 2004), the precise function of ITDP at SC-CA1 synapses is still not clear (Basu and others 2013). In addition, inhibition of 2-AG metabolism by inactivating MAGL produces profound anti-inflammatory and neuroprotective effects (Nomura and others 2011; Chen and others 2012; Piro and others 2012), the molecular mechanisms are not completely elucidated yet. Recent studies show that Δ9-THC, the major psychoactive component in cannabis, reduces Aβ and neurodegeneration in AD animals (Chen and others 2013). While these effects appear to be mediated through increasing Aβ clearance by upregulation of neprilysin (NEP), an endopeptidase that degrades Aβ, MAGL inhibition-reduced Aβ and neurodegeneration in AD animals are likely associated with reducing Aβ formation by suppression of β-site amyloid precursor protein cleaving enzyme 1 (BACE1), a key enzyme that synthesizes Aβ (Chen and others 2012). Interestingly, the same type of CB1 receptors mediates opposite effects in suppression or induction of COX-2 upon binding to different agonists (Chen and others 2013). 2-AG suppresses COX-2 via a CB1-coupled Gαi subunit in response to proinflammatory stimuli (Zhang and Chen 2008). In contrast, Δ9-THC increases COX-2 through CB1-coupled Gβγ subunits (Chen and others 2013). This suggests that the role of eCB signaling in maintaining integrity of brain function is greater than what we thought previously.
Acknowledgments
We would like to thank Jian Zhang for her excellent technical assistance. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health grants R01NS054886, R01NS076815, and R21AG039669 (to C.C.).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 1996;91:1585–1614. [PubMed] [Google Scholar]
- Alger BE, Pitler TA. Retrograde signaling at GABAA-receptor synapses in the mammalian CNS. Trends Neurosci. 1995;18:333–340. doi: 10.1016/0166-2236(95)93923-l. [DOI] [PubMed] [Google Scholar]
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Alger BE. Endocannabinoid signaling in neural plasticity. Curr Top Behav Neurosci. 2009;1:141–172. doi: 10.1007/978-3-540-88955-7_6. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Andersen P, Bliss TV, Skrede KK. Lamellar organization of hippocampal pathways. Exp Brain Res. 1971;13:222–238. doi: 10.1007/BF00234087. [DOI] [PubMed] [Google Scholar]
- Arevalo-Martin A, Garcia-Ovejero D, Molina-Holgado E. The endocannabinoid 2-arachidonoylglycerol reduces lesion expansion and white matter damage after spinal cord injury. Neurobiol Dis. 2010;38:304–312. doi: 10.1016/j.nbd.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Srinivas KV, Cheung SK, Taniguchi H, Huang ZJ, Siegelbaum SA. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron. 2013;79:1208–1221. doi: 10.1016/j.neuron.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensinger ST, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Di Marzo V. Cannabinoid receptors and endocannabinoids: role in neuroinflammatory and neurodegenerative disorders. CNS Neurol Disord Drug Targets. 2010;9:564–573. doi: 10.2174/187152710793361568. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76:886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright JJ, Kanakasabai S, Chearwae W, Chakraborty S. PPAR regulation of inflammatory signaling in CNS diseases. PPAR Res. 2008;2008:1–12. doi: 10.1155/2008/658520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun VH, Leutgeb S, Wu HQ, Schwarcz R, Witter MP, Moser EI, Moser MB. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008;57:290–302. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Raborn ES, Griffin L, Dennis J, Marciano-Cabral F. CB2 receptors in the brain: role in central immune function. Br J Pharmacol. 2008;153:240–251. doi: 10.1038/sj.bjp.0707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci. 2002;5:723–724. doi: 10.1038/nn879. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Finazzi-Agrò A, Bernardi G, Maccarrone M. The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends Pharmacol Sci. 2007;28:180–187. doi: 10.1016/j.tips.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Chen R, Zhang J, Wu Y, Wang D, Feng G, Tang YP, Teng Z, Chen C. Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Rep. 2012;2:1329–1339. doi: 10.1016/j.celrep.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zhang J, Fan N, Teng Z, Wu Y, Yang H, Tang Y, Sun H, Song Y, Chen C. Δ9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell. 2013;155:1554–1565. doi: 10.1016/j.cell.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang J, Chen C. Endocannabinoid 2-arachidonoylglycerol protects neurons against β-amyloid insults. Neurosci. 2011;178:159–168. doi: 10.1016/j.neuroscience.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Levy WB. Long-term potentiation of perforant path synapses in hippocampal CA1 in vitro. Brain Res. 1993;606:87–91. doi: 10.1016/0006-8993(93)91573-b. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Dardiotis E, Karanikas V, Paterakis K. Traumatic brain injury and inflammation: Emerging role of innate and adaptive immunity. In: Agrawal Amit., editor. Brain Injury - Pathogenesis, Monitoring, Recovery and Management. 2012. pp. 23–38. [Google Scholar]
- Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- Dekosky ST, Blennow K, Ikonomovic MD, Gandy S. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat Rev Neurol. 2013;9:192–200. doi: 10.1038/nrneurol.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Diana MA, Marty A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE) Br J Pharmacol. 2004;142:9–19. doi: 10.1038/sj.bjp.0705726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Hill MP, Bisogno T, Crossman AR, Brotchie JM. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J. 2000;14:1432–1438. doi: 10.1096/fj.14.10.1432. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Kathuria S, Piomelli D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol Pharmacol. 2004;66:1260–1264. doi: 10.1124/mol.104.002071. [DOI] [PubMed] [Google Scholar]
- Drew PD, Xu J, Storer PD, Chavis JA, Racke MK. Peroxisome proliferator-activated receptor agonist regulation of glial activation: relevance to CNS inflammatory disorders. Neurochem Int. 2006;49:183–189. doi: 10.1016/j.neuint.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Du H, Chen X, Zhang J, Chen C. Inhibition of COX-2 expression by endocannabinoid 2-arachidonoylglycerol is mediated via PPAR-γ. Br J Pharmacol. 2011;163:1533–1549. doi: 10.1111/j.1476-5381.2011.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudman JT, Tsay D, Siegelbaum SA. A role for synaptic inputs at distal dendrites: instructive signals for hippocampal long-term plasticity. Neuron. 2007;56:866–879. doi: 10.1016/j.neuron.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, Ullrich O. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le FG, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang MY, Strassle BW, Lu P, Mark L, Piesla MJ, Deng K, Kouranova EV, Ring RH, Whiteside GT, Bates B, Walsh FS, Williams G, Pangalos MN, Samad TA, Doherty P. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–47. [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gerrow K, Triller A. Synaptic stability and plasticity in a floating world. Curr Opin Neurobiol. 2010;20:631–639. doi: 10.1016/j.conb.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Hanus LO, Mechoulam R. Novel natural and synthetic ligands of the endocannabinoid system. Curr Med Chem. 2010;17:1341–1359. doi: 10.2174/092986710790980096. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E. Laminar selectivity of the cholinergic suppression of synaptic transmission in rat hippocampal region CA1: computational modeling and brain slice physiology. J Neurosci. 1994;14:3898–3914. doi: 10.1523/JNEUROSCI.14-06-03898.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein AM, O’Banion MK. Neuroinflammation and memory: the role of prostaglandins. Mol Neurobiol. 2009;40:15–32. doi: 10.1007/s12035-009-8066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinbockel T, Brager DH, Reich CG, Zhao J, Muralidharan S, Alger BE, Kao JP. Endocannabinoid signaling dynamics probed with optical tools. J Neurosci. 2005;25:9449–9459. doi: 10.1523/JNEUROSCI.2078-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, De Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, De Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Bidaut-Russell M, Devane WA, Melvin LS, Johnson MR, Herkenham M. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13:420–423. doi: 10.1016/0166-2236(90)90124-s. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. Direct cortical inputs erase long-term potentiation at Schaffer collateral synapses. J Neurosci. 2008;28:9557–9563. doi: 10.1523/JNEUROSCI.3346-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Heneka M, Landreth GE. The role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in Alzheimer’s disease: therapeutic implications. CNS Drugs. 2008;22:1–14. doi: 10.2165/00023210-200822010-00001. [DOI] [PubMed] [Google Scholar]
- Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol. 2013;9:222–230. doi: 10.1038/nrneurol.2013.33. [DOI] [PubMed] [Google Scholar]
- Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Kummer MP, Heneka MT. PPARs in Alzheimer’s disease. PPAR Res. 2008;2008:1–8. doi: 10.1155/2008/403896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS One. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakis N, Corona RJ, Toshkezi G, Chin LS. Chronic traumatic encephalopathy - neuropathology in athletes and war veterans. Neurol Res. 2013;35:290–299. doi: 10.1179/1743132813Y.0000000177. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Cebeira M, de Ceballos ML, Zeng BY, Jenner P, Ramos JA, Fernández-Ruiz JJ. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson’s syndrome and of MPTP-treated marmosets. Eur J Neurosci. 2001;14:1827–1832. doi: 10.1046/j.0953-816x.2001.01812.x. [DOI] [PubMed] [Google Scholar]
- Lenman A, Fowler CJ. Interaction of ligands for the peroxisome proliferator-activated receptor gamma with the endocannabinoid system. Br J Pharmacol. 2007;151:1343–1351. doi: 10.1038/sj.bjp.0707352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Long JZ, Normura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009a;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009b;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Medina R, Cortes-Canteli M, Alonso M, Santos A, Martínez A, Perez-Castillo A. Regulation of inflammatory response in neural cells in vitro by thiadiazolidinones derivatives through peroxisome proliferatoractivated receptor γ activation. J Biol Chem. 2005;280:21453–21462. doi: 10.1074/jbc.M414390200. [DOI] [PubMed] [Google Scholar]
- Makara JK, Mor M, Fegley D, Szabo SI, Kathuria S, Astarita G, Duranti A, Tontini A, Tarzia G, Rivara S, Freund TF, Piomelli D. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci. 2005;8:1139–1141. doi: 10.1038/nn1521. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di MV, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di MV, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Mato S, Lafourcade M, Robbe D, Bakiri Y, Manzoni OJ. Role of the cyclic-AMP/PKA cascade and of P/Q-type Ca++ channels in endocannabinoid-mediated long-term depression in the nucleus accumbens. Neuropharmacology. 2008;54:87–94. doi: 10.1016/j.neuropharm.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, Lee HS, Hall G, Wojtowicz SM, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Morgan NH, Stanford IM, Woodhall GL. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology. 2009;57:356–368. doi: 10.1016/j.neuropharm.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Mulder J, Zilberter M, Pasquaré SJ, Alpár A, Schulte G, Ferreira SG, Köfalvi A, Martín-Moreno AM, Keimpema E, Tanila H, Watanabe M, Mackie K, Hortobágyi T, de Ceballos ML, Harkany T. Molecular reorganization of endocannabinoid signalling in Alzheimer’s disease. Brain. 2011;134:1041–1060. doi: 10.1093/brain/awr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill MM, Nomura DK. Therapeutic potential of monoacylglycerol lipase inhibitors. Life Sci. 2013;92:492–497. doi: 10.1016/j.lfs.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Necela BM, Su W, Thomson EA. Toll-like receptor 4 mediates cross-talk between peroxisom proliferator-activated receptor γ and nuclear factor-κB in macrophages. Immunol. 2008;125:344–358. doi: 10.1111/j.1365-2567.2008.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tanimura A, Hashimotodani Y, Kano M. Endocannabinoids and retrograde modulation of synaptic transmission. Neuroscientist. 2012;18:119–132. doi: 10.1177/1073858410397377. [DOI] [PubMed] [Google Scholar]
- Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57:128–134. doi: 10.1227/01.neu.0000163407.92769.ed. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152:576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SE, Kendall DA. Cannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory disease. Immunobiol. 2010;215:611–616. doi: 10.1016/j.imbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Lewey J, Asrican B, Lisman JE. Inhibition of perforant path input to the CA1 region by serotonin and noradrenaline. J Neurophysiol. 2005;94:1413–1422. doi: 10.1152/jn.00217.2005. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. Dopamine selectively inhibits the direct cortical pathway to the CA1 hippocampal region. J Neurosci. 1999;19:1437–1445. doi: 10.1523/JNEUROSCI.19-04-01437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. Dopamine, serotonin, and noradrenaline strongly inhibit the direct perforant path-CA1 synaptic input, but have little effect on the Schaffer collateral input. Ann N Y Acad Sci. 2000;911:462–464. doi: 10.1111/j.1749-6632.2000.tb06746.x. [DOI] [PubMed] [Google Scholar]
- Pan B, Wang W, Long JZ, Sun D, Hillard CJ, Cravatt BF, Liu QS. Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) Enhances retrograde endocannabinoid signaling. J Pharmacol Exp Ther. 2009;331:591–597. doi: 10.1124/jpet.109.158162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Mechoulam R, Beni SM, Alexandrovich A, Shohami E. CB1 cannabinoid receptors are involved in neuroprotection via NF-kappa B inhibition. J Cereb Blood Flow Metab. 2005;25:477–484. doi: 10.1038/sj.jcbfm.9600047. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Shein NA, Mechoulam R, Trembovler V, Kohen R, Alexandrovich A, Shohami E. The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol Dis. 2006;22:257–264. doi: 10.1016/j.nbd.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piro JR, Benjamin DI, Duerr JM, Pi Y, Gonzales C, Wood KM, Schwartz JW, Nomura DK, Samad TA. A Dysregulated Endocannabinoid-Eicosanoid Network Supports Pathogenesis in a Mouse Model of Alzheimer’s Disease. Cell Rep. 2012;1:617–623. doi: 10.1016/j.celrep.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Fezza F, Galati S, Battista N, Napolitano S, Finazzi-Agrò A, Bernardi G, Brusa L, Pierantozzi M, Stanzione P, Maccarrone M. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann Neurol. 2005;57:777–779. doi: 10.1002/ana.20462. [DOI] [PubMed] [Google Scholar]
- Pisani V, Moschella V, Bari M, Fezza F, Galati S, Bernardi G, Stanzione P, Pisani A, Maccarrone M. Dynamic changes of anandamide in the cerebrospinal fluid of Parkinson’s disease patients. Mov Disord. 2010;25:920–924. doi: 10.1002/mds.23014. [DOI] [PubMed] [Google Scholar]
- Pistis M, Melis M. From surface to nuclear receptors: the endocannabinoid family extends its assets. Curr Med Chem. 2010;17:1450–1467. doi: 10.2174/092986710790980014. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Depolarization-induced suppression of GABAergic inhibition in rat hippocampal pyramidal cells: G protein involvement in a presynaptic mechanism. Neuron. 1994;13:1447–1455. doi: 10.1016/0896-6273(94)90430-8. [DOI] [PubMed] [Google Scholar]
- Racke MK, Drew PD. PPARs in Neuroinflammation. PPAR Res. 2008;2008:638356. doi: 10.1155/2008/638356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature. 2002;416:736–740. doi: 10.1038/416736a. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70:101–111. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- Ronesi J, Lovinger DM. Induction of striatal long-term synaptic depression by moderate frequency activation of cortical afferents in rat. J Physiol. 2005;562:245–256. doi: 10.1113/jphysiol.2004.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Salmon JA, Higgs GA. Prostaglandins and leukotrienes as inflammatory mediators. Br Med Bull. 1987;43:285–296. doi: 10.1093/oxfordjournals.bmb.a072183. [DOI] [PubMed] [Google Scholar]
- Sarne Y, Mechoulam R. Cannabinoids: between neuroprotection and neurotoxicity. Curr Drug Targets CNS Neurol Disord. 2005;4:677–684. doi: 10.2174/156800705774933005. [DOI] [PubMed] [Google Scholar]
- Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. 2011;32:1626–1633. doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp Neurol. 2011;227:128–135. doi: 10.1016/j.expneurol.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotter EL, Abood ME, Glass M. The endocannabinoid system as a target for the treatment of neurodegenerative disease. Br J Pharmacol. 2010;160:480–498. doi: 10.1111/j.1476-5381.2010.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohami E, Cohen-Yeshurun A, Magid L, Algali M, Mechoulam R. Endocannabinoids and traumatic brain injury. Br J Pharmacol. 2011;163:1402–1410. doi: 10.1111/j.1476-5381.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solas M, Francis PT, Franco R, Ramirez MJ. CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiol Aging. 2013;34:805–808. doi: 10.1016/j.neurobiolaging.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Stella N. Endocannabinoid signaling in microglial cells. Neuropharmacol. 2009;56(Suppl 1):244–253. doi: 10.1016/j.neuropharm.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Straiker A, Hu SS, Long JZ, Arnold A, Wager-Miller J, Cravatt BF, Mackie K. Monoacylglycerol lipase limits the duration of endocannabinoid-mediated depolarization-induced suppression of excitation in autaptic hippocampal neurons. Mol Pharmacol. 2009;76:1220–1227. doi: 10.1124/mol.109.059030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Tchantchou F, Zhang Y. Selective Inhibition of Alpha/Beta-Hydrolase Domain 6 Attenuates Neurodegeneration, Alleviates Blood Brain Barrier Breakdown, and Improves Functional Recovery in a Mouse Model of Traumatic Brain Injury. J Neurotrauma. 2013;30:1–15. doi: 10.1089/neu.2012.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufekci KU, Meuwissen R, Genc S, Genc K. Inflammation in Parkinson’s disease. Adv Protein Chem Struct Biol. 2012;88:69–132. doi: 10.1016/B978-0-12-398314-5.00004-0. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Fox SH, Hill M, Crossman AR, Petrosino S, Di Marzo V, Brotchie JM. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson’s disease. FASEB J. 2005;19:1140–1142. doi: 10.1096/fj.04-3010fje. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. Cannabinoid receptors and their role in neuroprotection. Neuro Molecular Med. 2005;7:37–50. doi: 10.1385/NMM:7:1-2:037. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Mazzola C, Esposito G, Matias I, Petrosino S, De Filippis D, Micale V, Steardo L, Drago F, Iuvone T, Di Marzo V. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci. 2006;63:1410–1424. doi: 10.1007/s00018-006-6037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ueda N. Biology of endocannabinoid synthesis system. Prostaglandins Other Lipid Mediat. 2009;89:112–119. doi: 10.1016/j.prostaglandins.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer’s brains. Neurosci. 1994;63:637–652. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Frontiers Neurol. 2013;4:1–18. doi: 10.3389/fneur.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JY, Chen R, Zhang J, Chen C. Endocannabinoids differentially modulate synaptic plasticity in rat hippocampal CA1 pyramidal neurons. PLoS One. 2010;5:e10306. doi: 10.1371/journal.pone.0010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JY, Zhang J, Chen C. Long-lasting potentiation of hippocampal synaptic transmission by direct cortical input is mediated via endocannabinoids. J Physiol. 2012;590:2305–2315. doi: 10.1113/jphysiol.2011.223511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Huang Y, Tsumoto T. Regulation of excitability and plasticity by endocannabinoids and PKA in developing hippocampus. Proc Natl Acad Sci U S A. 2008;105:3106–3111. doi: 10.1073/pnas.0708349105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J, Padalino DJ, Chin LS, Montenegro P, Cantu RC. Chronic traumatic encephalopathy. Curr Sports Med Rep. 2013;12:28–32. doi: 10.1249/JSR.0b013e31827ec9e3. [DOI] [PubMed] [Google Scholar]
- Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res. 2008;1244:164–172. doi: 10.1016/j.brainres.2008.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol. 2013;9:201–210. doi: 10.1038/nrneurol.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen C. Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J Biol Chem. 2008;283:22601–20611. doi: 10.1074/jbc.M800524200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Persistent synaptic activity produces long-lasting enhancement of endocannabinoid modulation and alters long-term synaptic plasticity. J Neurophysiol. 2007;97:4386–4389. doi: 10.1152/jn.01228.2006. [DOI] [PubMed] [Google Scholar]