Abstract
Increasing evidence suggests critical functions of thrombospondins (TSPs) in a variety of physiological and pathological processes. With the growing understanding of the importance of these matricellular proteins, the need to understand the mechanisms of regulation of their expression and potential approaches to modulate their levels is also increasing. The regulation of TSPs expression is multi-leveled, cell- and tissue-specific, and very precise. However, the knowledge of mechanisms modulating the levels of TSPs is fragmented and incomplete. This review discusses the known mechanisms of regulation of TSPs levels and the gaps in our knowledge that prevent us from developing strategies to modulate the expression of these physiologically important proteins.
Keywords: Thrombospondin, regulation of expression, transcription, translation, miRNA
Introduction
Thrombospondins (TSPs) are secreted extracellular proteins that belong to a group of matricellular proteins - proteins that are present in the extracellular matrix (ECM), but do not play a primary structural role as other ECM proteins do. Matricellular proteins, including TSPs, have diverse functions that integrate ECM and cells: they interact with a number of cell surface receptors and with a variety of ECM proteins (e.g., structural proteins such as collagens, proteases, growth factors, etc.). The revolutionary classification of matricellular proteins proposed by late Paul Bornstein (1, 2) has promoted a better appreciation of ECM and its role in important physiological and pathological processes, as well as understanding that cells do not function in isolation from their environment and ECM.
The human TSP protein family consists of five members (TSP-1, TSP-2, TSP-3, TSP-4, and TSP-5, or cartilage oligomeric matrix protein, COMP), which are divided in two subgroups based on their domain structure (3-5) (TSP-1 and TSP-2 belong to subgroup A, while TSP-3, TSP-4, and TSP-5 compose subgroup B). In lower organisms, TSPs are represented by a single protein, as in sponge (6), or by multiple family members, e.g., five proteins in sea anemone (7), that have a structure similar to human TSPs of subgroup B. The second subgroup A appears later in the evolutionary development, coinciding with the development of the vascular system (6), and develops further as a reflection of the development of cardiovascular and immune systems where this group plays an important role.
Important physiological functions of TSPs have been discovered in many organs and systems [reviewed in (8-11)]. Regulation of angiogenesis and cancer progression (12), regulation of inflammation (8, 13-16), modulation of immune response (17, 18), formation of myotendinous junctions (19), maintenance of the myocardium integrity and function (20-27), regulation of fibrosis (28), and synaptogenesis (29) are just a few examples of their roles in physiology and pathology. To participate in the critical physiological processes, TSPs have to be present in the right location at the right time. This review summarizes the published information about the regulation of the expression of TSPs.
1. Similarities and distinctions between TSP family members
All TSPs share a high degree of homology at the protein level, especially in their “signature domain” - the C-terminal half of the human protein that includes 3-4 EGF-like repeats [1 – 5 in other species (4)], the Ca2+-binding domains with up to 26 metal binding sites per protein subunit, and the globular C-terminal domain [reviewed in (4)]. Table 1 summarizes the extent of homology between five TSPs. However, apart from the protein coding regions of the TSP genes (THBS), there is no homology in their DNA sequence, and the regulatory parts of TSP genes, either the untranslated regions of mRNA (UTR) or the gene promoters, are distinctly different at the first glance (30). Search for common DNA sequence motifs using SCOPE motif finder (http://genie.dartmouth.edu/scope)(31, 32) and comparison of the positions of common DNA sequence motifs in the promoters of five TSPs reveals some similarities between TSP promoters and suggests that in some conditions two or more TSPs may be regulated by the same stimuli simultaneously (Fig.2). However, there are no experimental data to support the predicted similarity of the DNA motifs.
Table 1.
Homology of human TSP proteins [calculated using algorithm described in (216)].
| TSP-1 | TSP-2 | TSP-3 | TSP-4 | |
|---|---|---|---|---|
| TSP-1 | ||||
| TSP-2 | 62.6 | |||
| TSP-3 | 52.2 | 52.9 | ||
| TSP-4 | 53.9 | 52.6 | 61 | |
| TSP-5 | 53.6 | 54.4 | 65.3 | 69.9 |
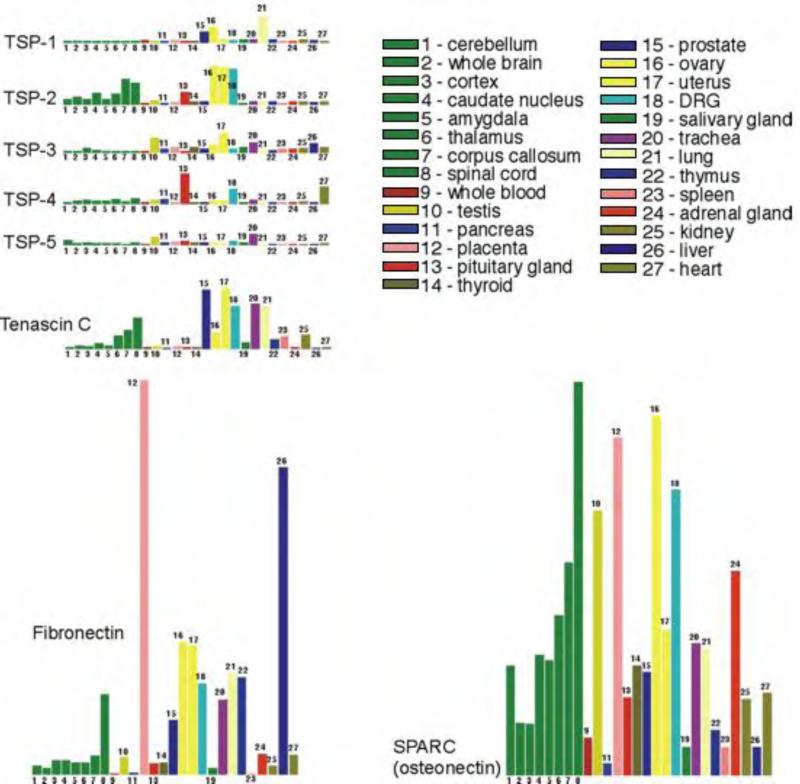
Figure 2. Expression of TSPs in human tissues and organs, comparison to tenascin C, SPARC and fibronectin expression.
The figure was prepared using the data from the database http://expression.gnf.org (33). Organs from left to right: cerebellum, whole brain, cortex, caudate nucleus, amygdala, thalamus, corpus collosum, spinal cord, whole blood, testis, pancreas, placenta, pituitary gland, thyroid, prostate, ovary, uterus, DRG, salivary gland, trachea, lung, thymus, spleen, adrenal gland, kidney, liver, heart.
The five proteins are located on different chromosomes (human THBS1 on chromosome 15 and mouse Thbs1 on chromosome 2, human THBS2 on chromosome 6 and mouse Thbs2 on chromosome 17, human THBS3 on chromosome 1 and mouse Thbs3 on chromosome 3, human THBS4 on chromosome 5 and mouse Thbs4 on chromosome 13, human THBS5 on chromosome 19 and mouse Thbs5 on chromosome 8).
In most tissues, TSPs are expressed at a low level compared to other non-structural ECM proteins, e.g., SPARC, tenascin C and fibronectin (Fig.3)(33). Similar to TSPs, tenascin C and SPARC are representatives of the matricellular protein family. SPARC and fibronectin regulate collagen deposition and ECM assembly and support the interaction between cells and ECM, similar to TSPs. These proteins have similar functions in disease regulation: e.g., they support interaction of cancer cells with stromal cells.
Figure 3.
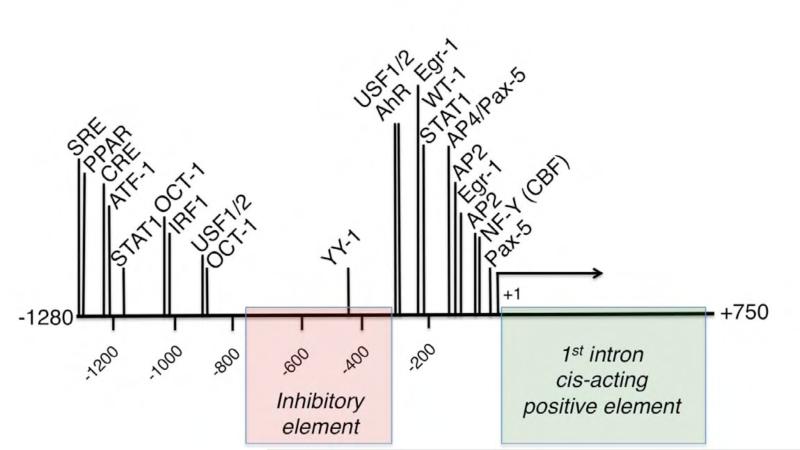
Promoter of TSP-1: regulatory regions and experimentally confirmed binding sites for transcription factors.
Emphasizing the potent effect of TSPs presence in the tissue and the need to tightly regulate their expression, there are rapid mechanisms upregulating TSPs at the transcriptional level (34-36) and mechanisms rapidly degrading mRNA or blocking its translation into a protein (37-40). The proteins appear to be unstable after they are secreted, stressing the importance of timely elimination of TSPs from the ECM and cell environment (41-43). In adult organisms, upregulation of TSPs is associated with specific stages of wound healing [e.g., (36, 44-48)] and tissue remodeling [e.g., (13, 24, 25, 49-52)], in which it can be either protective and beneficial or detrimental.
Distinct localization of TSPs in tissues suggests differential functions, despite the high homology between proteins and a number of shared cell surface receptors and binding partners. Even when two or more TSPs are found in the same tissue, they are localized to the different cell types or structures within the tissue. For example, both TSP-3 and TSP-5 are present in the atherosclerotic lesion of ApoE−/− mice, but they are clearly produced by different cell types in the lesion (13). Similarly, in the wall of smaller blood vessels TSP-3, TSP-4, and TSP-5 not only are produced by different cell types, but also are deposited in ECM in distinct patterns and in distinct localization within the vessel (53). In tendon, both TSP-3 and TSP-4 are abundant, but arranged in fibers of different orientation, stressing the distinctions in their functions (53). When TSPs are expressed in the same structures at the same time [e.g., TSP-4 and TSP-5 in tendon (53)], it is still unclear why both are required and what differential properties of tissue they support.
Differences in the expression profiles of individual TSPs in cancers and other cells and tissues [e.g., (48, 54, 55)] suggest that TSPs have differential functions in pathological processes, despite shared homologous domains and ligands. Several gene expression studies demonstrated opposite profiles for TSP-1 and TSP-4 in brain (56-58) and breast (59-61) cancers. Examination of the datasets from these studies reveals that TSP-4 upregulation is accompanied by downregulation of TSP-1 in the tumor samples (www.oncomine.org), clearly indicating distinct, probably even opposite, functions for these two proteins. Unexpectedly, TSP-2, belonging to the subgroup A and sharing high homology with TSP-1, exhibits a profile similar to the TSP-4 profile – it is upregulated in both brain and breast cancers, with the exception of one data set (57). In some datasets TSP-2 was in top 1% of upregulated genes, together with TSP-4. These comparisons of the three TSPs profiles from the same datasets clearly demonstrate that the three TSPs play important distinct roles in cancer growth, and the regulation of their expression is an efficient mechanism to support these still poorly understood functions (with the exception of TSP-1, whose downreguation is known to support angiogenesis in tumors). TSP-5 was upregulated similar to TSP-4 in multiple datasets from breast cancer studies (www.oncomine.org), while TSP-3 data were inconsistent, and both down- and up-regulation were observed (62, 63).
The expression studies in human samples and in animal tissues clearly suggest distinct functions and roles in physiological and pathological processes for five TSPs. However, the molecular mechanisms responsible for the differences in TSPs functions still have to be addressed and confirmed by examining specific functions of all five TSPs and by studying their interactions with cells and ligands. Precise regulation of TSPs expression is especially important in a view of TSPs homology and a number of shared ligands.
2. Mechanisms of regulation of TSPs expression
Most of the information about the regulation of TSPs expression comes from descriptive studies that either used array type experiments to identify highly upregulated or downregulated genes in tissues of interest or specifically looked at the TSPs expression (in most cases expression of TSP-1), based on known functions of TSPs. However, a few reports address specific molecular mechanisms of regulation of TSPs expression in cells and tissues.
2.1. Transcriptional regulation
2.1.1. TSP-1
2.1.1A Positive regulation
The first analysis of TSP-1 promoter and identification of the serum response element was reported by Framson and Bornstein in 1993 (64). The identified serum response element is bi-partite and includes a distal element at the position −1280 and a proximal element with NF-Y binding site at the position −65 (Fig.3). Interestingly, the proximal element is different in the mouse TSP-1 promoter: it harbors Egr-1 binding site in the position of human NF-Y site and does not participate in serum response. The Egr-1 binding site maintained constitutive activity of the promoter acting in concert with the adjacent GC-rich region binding SP1 (65). The importance of the first intron for the efficient transcription of the gene was noted (66): the deletion of this region results in 4-fold decrease in the reporter production, suggesting a cis-acting positive element in the first intron.
AP-1 binding to TSP-1 gene promoter mediates the activation of the promoter in response to protein kinase C (PKC) activation in human hepatoma cells (67). However, a similar study of the effects of PKC activation in porcine aortic endothelial cells resulted in opposite conclusions: a specific region of the promoter was responsible for the downregulation of the promoter activity (68). The cell-specificity is a very common theme in the studies of TSPs expression. The cell-and tissue-specific differences in regulation reflect the multi-functional nature of these proteins and the importance of a very strictly localized and timely production and elimination of TSPs.
The cell-specificity of TSP-1 gene promoter regulation was reported in several studies. The activity of a promoter is determined not only by its sequence, but also by availability of target binding sites in a specific cell type, presence of transcription factors capable of binding the promoter, and, finally, cell-specific signals to activate the transcription factors or protein co-activators. The regulatory complex assembled on TSP-1 gene promoter is often cell-specific, and the promoter regions involved in regulation are also different depending on the cell type. For example, in response to high glucose, TSP-1 mRNA is upregulated in major cell types of the vascular wall – endothelial cells, smooth muscle cells and fibroblasts (35). However, the time of upregulation is distinctly different – in endothelial cells upregulation can be detected earlier than in smooth muscle cells. The analysis of the promoter revealed that in two cell types the promoter activity is regulated by different transcriptional protein complex. In endothelial cells, a proximal regulatory region of the promoter binding Aryl Hydrocarbon Receptor is sufficient to drive the transcription (34, 69), while in smooth muscle cells the same promoter region is interacting with a distal part of the promoter through the formation of a single protein complex between transcription factors binding to the proximal and to the distal regions (69, 70). The regulation is determined by a cell-type-specific profile of the expression and the activation of transcription factors.
Transcriptional upregulation of TSP-1 in response to high glucose is probably the most dissected pathway among all the pathways regulating TSP-1 expression. TSP-1 has been implicated in development of vascular complications of diabetes, and its mRNA was dramatically upregulated in hyperglycemia in various tissues and organs (28, 35, 37, 38, 69, 71-78). The initial observation of upregulation of TSP-1 in response to high glucose in mesangial cells (73) was later confirmed in multiple cell types and tissues. The precise transcriptional mechanisms appear to vary depending on the cell type, with several transcriptional factors and regulatory regions being constant independently of the cell type – e.g., upstream stimulatory factors USF (74) that are common mediators of glucose effects. Another transcription factor activated by high glucose and essential for TSP-1 gene promoter activation is Aryl Hydrocarbon Receptor, or AhR (34, 69). The activation of the AhR pathway may provide a mechanistic connection between glucose and hypoxia regulatory pathways (AhR is a ligand for HIF1β or Arnt, whose other well studied ligand is HIF1α, a transcription factor that mediates many effects of hypoxia in complexes with Arnt). AhR is a member of the Clock family of proteins and can form complexes with several other proteins from this family that regulate circadian rhythms. The activation of AhR by glucose may provide insights into the well-known connection between food intake and maintenance of the circadian rhythms. TSP-1, as a major and highly upregulated target of AhR, may play a role in this regulation.
Increasingly appreciated significance of TSP-1 function in metabolic disorders (79, 80) prompted an investigation of transcriptional regulation of TSP-1 gene by leptin, a hormone implicated in development of obesity and diabetes. Leptin induced TSP-1 at the transcriptional level through the by JAK2/ERK/JNK-dependent mechanism (78).
The promoter of TSP-1 gene has an active Egr-1 binding site. The promoter activity is upregulated by Egr-1-inducing stimuli (81). TSP-1 mRNA can be very rapidly (within 10-15 min) upregulated in response to injury, both in vivo and in vitro (35), and, although not investigated, this upregulation most probably requires Egr-1, one of the first-response transcription factors. Egr-1 is responsible for transcriptional upregulation of TSP-1 by thrombin, together with MYC, in a thrombin receptor PAR-1, G(i/o), G(q), EBOX/EGRF-signaling cascade (82).
TSP-1 is a potent anti-angiogenic protein, and most of the reports on its expression and transcriptional regulation are related to regulation of angiogenesis in various tissues and pathological conditions. It is difficult to judge whether the focus on its anti-angiogenic function is due to the fact that this is truly the main function of TSP-1, whether this is due to the current popularity of the angiogenesis topics, or whether this is a result of a lack of sufficient understanding and knowledge about other functions of TSP-1. TSP-1 inhibits angiogenesis even in the presence of pro-angiogenic factors (83-85).
CCAAT box binding CCAAT-binding factor (CBF) mediates the effect of Histone Deacetylase (HDAC) inhibitors in regulation of angiogenesis and tumor growth: acetylated CBF specifically binds to TSP-1 promoter, and its activity is increased by the binding of acetylated H3 (86).
Recently, the role of TSP-1 in inflammation has been actively studied and confirmed in several models (87). Transcriptional regulation of TSP-1 in chronic inflammation was addressed in inflammatory joint disease (87): the orphan receptor 4A2 (NR4A2) was responsible for the downregulation of the promoter activity, and the anti-tumor necrosis factor treatment, which decreases NR4A2, increased TSP-1 expression and suggested that TSP-1 plays a role in resolution of inflammation.
Many studies reported the stimuli regulating transcription without addressing specific transcriptional mechanisms and transcription factors involved.
For example, TGFβ1 and TGFβ2 stimulated TSP-1 and TSP-2 production in bovine adrenocortical cells, and the effect was blocked by the transcription inhibitors (88).
2.1.1B Negative regulation
TSP-1 gene promoter appears to have an inhibitory element between −300 and −750 bp positions, as was reported in two independent studies (34, 89). Shorter promoter deletion constructs are significantly more active, as are the longer constructs, both constitutively and in response to stimuli.
The promoter was found to be sensitive to treatment with nickel (90): nickel downregulated the activity of the promoter and decreased the expression of endogenous TSP-1 in cultured hamster embryo cells. Activating Transcription Factor-1 (ATF-1) was identified as a component of the protein complex that bound to a negative regulatory site in the mouse TSP-1 gene promoter (91). ATF-1 binding to the region between positions −1210 and −1123 of the promoter harboring a cAMP-responsive element (CRE) induced the repression of the promoter in response to hepatocyte growth factor (HGF) and played a key role in tumor progression triggered by HGF (92).
Further analyses of THBS1 promoter identified many active binding sites for transcription factors that tightly regulate TSP-1 expression at the transcriptional level. YY-1 binds to a site at the position −440 of the promoter, which results in an interaction that can be weakened by the increased c-Jun levels, suggesting that the interaction of YY- and c-Jun decreases the promoter activity (89).
The well-documented suppression of TSP-1 production in tumors (upregulating angiogenesis to promote tumor growth) includes transcriptional downregulation: e.g., the product of Wilms' tumor suppressor gene, WT1, binds to the −210 region of the TSP-1 gene promoter and represses THBS1 transcription (93), while tumor suppressor protein p53 activates TSP-1 promoter (94). Another mechanism used by cancers to prevent TSP-1 promoter activation is hypermethylation of the promoter that has been detected in a variety of cancers [e.g., (95-98)]. TSP-1 promoter is also a target for a transcriptional factor Id1 that is known to regulate tumor angiogenesis (99).
Importantly, transcriptional repression is a common mechanism regulating the expression of TSP-1 – it results in a rapid effect, because both the protein and mRNA of TSP-1 are unstable (69, 70, 78, 100, 101).
MicroRNA regulation is usually associated with the modulation of mRNA translation or stability. However, miR-182 was reported to regulate the transcription of TSP-1 gene in colon cancer by modulating the function of transcription factors Egr-1 and Sp-1 function and their binding to THBS1 promoter (102).
Both TSP-1 and TSP-2 were downregulated in Akt−/− mice, and this downregulation was responsible for the increased angiogenesis in these mice. The TSP-2 promoter inactivation by dominant negative Akt suggested that the regulation is transcriptional, but the exact mechanism has not been addressed (103). Although the studies identifying the signals that lead to the transcriptional upregulation are informative in defining the stimuli regulating the expression of TSPs, without addressing the molecular mechanism and delineating the promoter elements involved in the regulation it is impossible to know whether the observed effects are direct or mediated by an autocrine mediator and whether the mechanism of the regulation in these examples is truly transcriptional.
2.1.2. TSP-2
The promoters of human and mouse TSP-2 genes were analyzed and compared in (30). Interestingly, tissue-specific differences in transcription sites were found, resulting in transcripts of different lengths depending on the tissue. This was the only report considering the features of THBS2 promoter. Putative transcription factor binding sites were analyzed, and comparison to THBS1 promoter did not reveal any similarities between two genes in the promoter regions.
2.1.3. Other TSPs
Dr. Bornstein's group reported on the promoter of THBS3 as well. Interaction between THBS3 gene and adjacent metaxin gene was revealed (104), and the active SP1-binding elements were identified in the common regulatory region of the two genes (105). Analysis of the transcription initiation sites revealed alternative transcripts (106). Since the last publication in 1999, there have been no additional reports on regulation of THBS3 transcription.
There is very little information about the transcriptional regulation of TSP-4 and TSP-5 genes, despite their reported importance in several physiological and pathological processes (13, 24-26, 29, 52, 107, 108). The promoter of TSP-5 (COMP) has been analyzed, and a region required for the activity in chondrocytes was identified within 375 bp of the translational start site, as well as enhancer elements between −1.0 kb and −1.7 kb (109).
2.2. Alternative splicing
With a large number of exons in TSPs genes (>20 in most TSPs), the possibility of alternative splicing and regulation of expression and functions by this mechanism is plausible. However, alternative splicing and its potential significance in regulation of TSPs has not been seriously addressed to date. Several publications reported alternative forms of TSP-2 and TSP-3 (106, 110). A production of a Lumbar-disc-herniation-associated form of TSP-2 is due to a polymorphism and the alternative splicing of TSP-2 that results in skipping exon 11 (111). Lack of exon 11 causes decreased interaction of TSP-2 with metalloproteinases. Alternative splicing of TSP-4 gene associated with change in cell motility was reported in cancer cells (112).
Multiple TSPs forms of different sizes are routinely observed (e.g., (35, 113)), but the origin of these forms has not been thoroughly explored, and the possibility of the alternative splicing has not been excluded.
2.3. Regulation of mRNA stability
As has been mentioned above, both TSP-1 mRNA and protein have a short half-life. The short half-life of TSP-1 mRNA is due, at least in part, to AU-rich elements in the 3’ untranslated regions (3'UTR) of the TSP-1 mRNA (100, 114). Unusually large regulatory 3’UTR of TSP-1 and TSP-2 mRNA suggest extensive post-transcriptional regulation at the level of mRNA (Table 2). The rest of TSPs have an average sized UTR; however, this does not exclude regulation of mRNA stability or translation. UTR of all TSPs have putative target binding sites for miRNAs. There is no information on regulation of TSP-3, TSP-4, and TSP-5 expression at the level of mRNA, but several reports described the regulation of TSP-1 and TSP-2 through the modulation of mRNA stability or translation.
Table 2.
Size of untranslated regions of mRNA of TSPs.
| 5’UTR | 3’UTR | |
|---|---|---|
| TSP-1 | 179 | 2130 |
| TSP-2 | 320 | 2061 |
| TSP-3 | 21 | 234 |
| TSP-4 | 191 | 156 |
| TSP-5 | 36 | 161 |
TSP-1 mRNA was destabilized by activation of myc oncoprotein (39). Further analysis of this mechanism revealed that destabilization is a result of increased expression of miR-17-92 family that bind TSP-1 mRNA (115). The decrease in the levels of the members of this family of miRNA, miR-18/19, was associated with increased TSP-1 level in cardiomyocytes of aging hearts, identifying the aging process (116). p53-responsive miR-194 decreased the levels of mature TSP-1 mRNA despite the increased levels of the primary transcript, prevented the production of TSP-1 protein, and promoted angiogenesis in colon cancers (117), presumably, due to destabilization of TSP-1 mRNA.
Increased TSP-1 mRNA stability was detected in response to heat shock (118), and a region of TSP-1 3’UTR responsible for the increased stability was identified as 968 – 1258 from the stop codon. TGFβ also increased TSP-1 expression in cultured osteosarcoma cells by stabilizing mRNA via p38 MAPK signaling (119).
There are no mechanistic reports describing the effects on TSP-2 mRNA stability. Although there are examples of post-trancriptional regulation of TSP-2 [e.g., in (120) describing the effect of c-myb on TSP-2 expression via a post-transcriptional mechanism], the details of this regulation remain unknown.
2.4. Regulation of mRNA translation
Due to the complexity of translational mechanisms and lack of detailed knowledge of these mechanisms, as well as to the requirement of specific skills and experience for the experimental manipulations with RNA, investigation of translational regulation of TSPs has not been the most popular of research topics. The large size of TSPs mRNA and, especially, the long regulatory UTR of TSP-1 and TSP-2 may have discouraged this type of study. However, studies of translational regulation of TSPs may prove very rewarding: there are multiple independent observations that increased levels of mRNA do not guarantee increased production of the corresponding protein and that the increased production of a protein may not always reflect the increased levels of mRNA [e.g., (37, 38, 43)]. As more examples of such discordant levels of protein and mRNA accumulate, we will be forced to address the translational regulation of TSPs.
TSP-1 mRNA is associated with rich in adenines and uracils ARE binding protein HuR. HuR facilitates translation, and it has been co-precipitated with TSP-1 mRNA from the cultured MCF7 breast cancer cells (121). Another example of translational regulation of TSP-1 production is translational silencing of TSP-1 mRNA in response to high glucose (37, 38) that is tissue-specific and regulated by a cell-specific induction of miR-467 and its binding to TSP-1 3’UTR.
2.5. Post-translational regulation – secretion
TSPs are secreted extracellular matrix proteins. Although some of them, e.g., TSP-1, TSP-4 and TSP-5, perform important functions inside the secretory pathways (25, 122-124), most of the widely known functions rely on the presence of TSPs outside the cell.
The regulation of secretion of pre-made protein is a very fast and a very efficient way to regulate the protein availability, and this type of regulation is used by the cells to control the extracellular TSPs levels. TSP-1 can be retained inside the cells, and the secretion depends on the Ca2+ regulation: TSP-1 retention inside the cells can be induced by a calcium chelator and is regulated in renal cell carcinoma cells by TRPC4 calcium channel expression (125). TSP-1 is a major component of platelets alpha granules: pre-synthetised protein is released upon platelets activation (126, 127). Macrophages also regulate TSP-1 secretion: the secretion is induced by LPS, and the threshold for the level of secretion is regulated by the environmental signals, including the mediators of the adaptive immune system, Th1 and Th2 cytokines (128). TSP-1 secretion depends on cell density in culture: lower density results in higher secretion of TSP-1 by fibroblasts, endothelial and smooth muscle cells without change in protein synthesis (129).
Both TSP-4 and TSP-5 appear to have additional intracellular functions in the secretory pathways: TSP-5 can associate with its co-secreted extracellular ligands and ER chaperones (122-124), and TSP-4 bind a transcriptional factor Aft6α promoting its translocation to the nucleus (25). The latter function is shared with TSP-1 and is thought to be a common feature of TSPs in protecting and augmenting ER function (25).
3. Descriptive studies of TSPs expression
More than 2,400 publications address the expression of TSPs in different tissues and various conditions. The findings clearly indicate the importance of the altered expression in physiology and pathology and stress the need to develop understanding of the mechanisms that control the expression. Most of the information about the expression of TSPs comes from descriptive studies, in which the mechanisms of regulation have not been addressed. This information is valuable in identifying physiological and pathological processes dependent on TSPs and in providing basis for future studies of precise mechanisms. Among all the descriptive studies, the most valuable are the ones where protein levels have been assessed. Whenever only the mRNA levels were evaluated, there is always a possibility that they do not translate into protein production or that the produced protein is not secreted, as seems to be a frequent occurrence with TSPs.
Although an overview of conditions resulting in altered expression of TSPs is not the goal of this article, several major themes in regulation of TSPs are very prominent and must be mentioned: growth and remodeling of tissues, altered angiogenesis and cancer, ischemia and reperfusion, activation of TGFβ, inflammation and immune response, aging, and embryonic development (Table 3). Any of these pathological or physiological situations consistently result in or are caused by the change in the levels of one or several TSPs. As usual, TSP-1 expression has been better studied than the expression of the other four TSPs, although many recent reports repeatedly find the associations of TSP-4 expression and pathological changes.
Table 3.
Summary of examples of pathological and physiological conditions associated with altered expression of TSPs (references to published reports). Green – upregulated expression, red – downregulated expression, blue – both the upregulated and the downregulated expression has been reported.
Altered expression of TSPs during the growth and remodeling of tissues is associated either with the need for angiogenesis, matrix remodeling, or the direct stimulation of cell growth. TSP-4 is upregulated in the mouse heart in response to pressure overload and in human hypertrophied hearts. It has a protective function regulating cardyomyocyte function and production of extracellular matrix (24-26, 52, 130-132). TSP-4 is expressed in atherosclerotic lesions and promotes atherosclerotic process by attracting and retaining macrophages in the lesion (13). Thbs4 is responsive to the ischemic injury in the brain cortex and plays an important role in post-injury astrogenesis (133). THBS4 expression increases with age in the brain gray matter and may be associated with Alzheimers disease and altered inflammatory response (134). Increased TSP-1 expression is also often found in tissue growth and remodeling, e.g., in embryo fibroblasts in response to c-Jun stimulation (135). Increased levels of TSP-1 and TSP-2 have been reported in remodeling large arteries in atherosclerotic lesions and after injury (13, 35, 51, 80).
Inflammation and immunity is an emerging field in TSP studies (16, 136-145). Although functions of TSPs in immune response and inflammation are not completely understood, these proteins are clearly important for the adequate immune response and the resolution of the inflammation.
Multiple studies documented changed expression of TSP-1 and TSP-2 associated with altered angiogenesis and cancer growth. Decreased TSP-1 and/or TSP-2 expression correlates with increased vascularity in non-small cell lung cancers (146), colon cancer (147, 148), invasive cervical cancer (149, 150), glioma cells (151), in cytomegalovirus (CMV) infection (152), and many other situations where angiogenesis is increased. Interestingly, the expression of group B thrombospodins (TSP-3, TSP-4 and TSP-5) increases in cancers. Increased levels of TSP-3 were associated with poor prognosis in osteosarcoma patients (153). TSP-4 expression was increased in invasive breast cancer and was suggested to facilitate the invasion of tumor cells (154). It was differentially expressed in lobular versus ductal breast tumors (155). THBS4 was found to be a powerful marker of diffuse-type gastric adenocarcinomas: its expression in fibroblasts was stimulated by tumor cells (156). Based on the existing data, THBS4 expression is a selective marker for specific cancers, although the regulation and the significance of the increased levels have not been explored.
Many stimuli able to reduce TSP-1 levels have been identified: e.g., all-trans-retinoid acid in smooth muscle cells (157); Hepatocyte growth factor/scatter factor that mediates angiogenesis through positive VEGF and negative TSP-1 regulation (158); shear stress (159), and hypoxia (160). Increase in TSP-1 levels is often detected in association with decreased angiogenesis and ischemia, e.g., in chronic leg ischemia (161). Increased levels of TSP-1 mRNA were detected upon stimulation with dexamethasone (162); progesterone (163); high glucose (35, 73), thrombin and angiotensin II (164). TSP-2 expression is down regulated by Cyp1B1, and this regulation is important for a proper capillary morphogenesis in a model of retinopathy of prematurity (a neovascular response during oxygen-induced ischemic retinopathy)(165). The activity of endothelial nitric oxide synthase (eNOS) negatively correlated with TSP-2 levels: deficiency in eNOS resulted in increased levels of TSP-2 and decreased angiogenesis in a mouse model, and NO repressed the promoter of TSP-2 (166). However, the precise transcriptional mechanisms were not dissected in these studies.
The extent of angiogenesis after ischemia and reperfusion (IR) correlates with the successful repair process in tissues and with the patient survival. Elevation of TSP-1 levels was detected after IR of brain, myocardium, lung, and kidney (139, 167-170). While the expression of TSP-2 was elevated at the peak of angiogenesis (2 weeks after reperfusion), the expression of TSP-1 was dramatically elevated early after the event and did not seem to represent a response to the angiogenic process. Although it is unclear what the role of TSP-1 is in tissues just hours after IR, it appears to have a protective effect in heart: e.g., myocardial infarction (MI) patients with a higher levels of TSP-1 in the platelet-poor plasma had a better outcome and fewer adverse cardiac events within 14 months after MI (171). In animals models of MI, TSP-1 mRNA was induced only 1 hour of ischemia and was elevated 3-7 days after reperfusion. TSP-1 was localized to the infarction border zone and reduced inflammation and granulation tissue formation protecting non-infarcted myocardium from fibrosis (168, 171). Interestingly, in kidneys increased expression of TSP-1 early after IR (only 3 – 12 hours, returning to the base line by 48 hours) mediated the injury, and Thbs1−/− mice showed significant protection from the damage after IR injury and from the renal failure (172). Clearly, the function and the effect of the altered expression of TSPs depend on the injury mechanism and the specific cellular events characteristic for a tissue.
TSP-1 is a major activator of TGFβ1, and there is a reciprocal feedback mechanism maintaining and amplifying the circuit: TGFβ was identified as TSP-1 expression regulator increasing the production of TSP-1 in multiple studies (162, 173). As a major activator of TGFβ1 (174), TSP-1 is often expressed in tissues producing TGFβ1, e.g., in cancers with high TGFβ1 levels (175) and in embryonic development (176). TSP-1 gene mutation was identified in a family with the familial pulmonary arterial hypertension (PAH)(177). The ability of the mutant Asp362Asn TSP-1 to activate TGFβ1 was reduced to ½ of the activity of the wild-type TSP-1. TSP-1 contributes to the development of PAH in more than one way: the levels of TSP-1 are increased in PAH patients and in animal models of PAH, and the interaction of TSP-1 with its receptor CD47 disrupts the constitutive interaction between CD47 and Caveolin-1, causing increased eNOS-dependent superoxide production and oxidative stress (178).
Levels of TSPs increase in multiple tissues with age (21, 116, 179-182). The significance of this increase in TSPs production is still unclear, although in some tissues it appears to be beneficial (21, 26, 179, 180). In others, TSPs are associated with aging-related pathological changes (27, 116, 182, 183).
While the significance of the altered expression of TSP-1 and TSP-2 is well understood in most situations, and specific consequences are expected (e.g., altered angiogenesis, matrix remodeling, TGFβ activation), we don't have a clear understanding of the significance of altered expression of group B TSPs in the majority of cases. Studies of the signals and molecular pathways regulating their expression would greatly increase our understanding of TSPs associations with specific pathological conditions and would suggest the functions performed by TSPs in physiological and pathological processes.
4. Expression of TSPs in developing embyo
TSPs are highly expressed in tissues of developing embryos of various species. In some cases, the significance of high expression has been examined and understood. For example, in drosophila, single TSP of the fly is expressed in tendon during the formation of myotendinous junctions (19, 184). Its expression is induced by the tendon-specific transcription factor Stripe. TSP accumulates in the myotendinous junction, and flies deficient in TSP fail to form functional somatic musculature.
Pluripotent human embryonic stem cells expressed and secreted TSP-1, and it was the highest expressed protein detected in expression profiling of these cells (185) and potentiating cell division in cardiomyocytes. This observation suggested that TSP-1 might participate in cardiogenesis and cardiac repair by stem cells. Similarly to the adult tissues, TSP expression is tightly regulated in embryos and is associated with specific stages of embryonic development. Increased TSP-1 expression at mid gestation was responsible for embryonic lethality with pronounced heart defects and vascular abnormalities (186). The expression of TSP-1 in mouse embryo brain was also time dependent with a peak at days 11 and 12 and down-regulation at the later stages (187). The expression patterns of TSP-1 suggested a role in early CNS development (188). Differential functions in developing embryo are supported by the distinct profile of expression of TSP-1, TSP-2 and TSP-3: TSP-1 was observed transiently in the neural tube, head mesenchyme, and cardiac cushions and constitutively in the resident megakaryocytes of the liver and in circulating megakaryocytes. In contrast, high expression of TSP-2 was detected in the connective tissue: e.g., in pericardium, pleura, perichondrium, periosteum, meninges, ligaments, reticular dermis, and cartilage and bone precursors (187, 189). TSP-2 was also expressed in blood vessels and skeletal myoblasts. mRNA of TSP-3 was restricted to brain, cartilage and lung. The overlapping expression of TSP-1 and TSP-2 was found only in the kidney and the gut (187). The dynamic pattern of TSPs expression in Xenopus and avian embryos also support the distinct non-overlapping functions for TSPs (190, 191). TSP-2 may be important in palate development: in the earlier stages of palatogenesis, it was found throughout the extracellular matrix of shelf mesenchyme (176), but later it was restricted to the EM and bone of the maxilla. TSP-1 was also abundantly expressed in developing head mesenchyme, including the palate. Developmental stage – dependent co-expression of TSP-1 and TSP-2 was proposed to regulate TGFβ1 activity. TSP-4 expression pattern suggests its role in regulation of neurite outgrowth and differentiation of cartilage, tendons, and bone (192, 193). In mouse embryo, TSP-5 is expressed in mesenchyme at the day E10, and by E19 it is clearly expressed in osteogenic and chondrogenic tissues (194-196). Its expression in both the embryonic and the adult tissues indicates its importance in skeletal and cartilage development (197-200).
Most of the studies that documented the expression profiles of TSPs in embryonic development are descriptive [in addition to the studies mentioned above (201, 202) and others]. Most molecular mechanisms of the regulation of the expression in the developing embryos have never been addressed, and the significance of the tightly timed distinct expression of each TSP is unclear in most cases. However, similar to the investigation into expression profiles and regulation of expression in adults, these studies suggest specific distinct functions for five TSPs in development.
5. Therapeutic regulation of expression
Traditionally, the regulation of expression was not the first choice in seeking to target specific proteins for therapeutic purpose. Difficulties of delivery of the molecular regulators into the nucleus and lack of specificity in the action of the expression activators and inhibitors prevented the practical use of expression regulation in most cases. In the case of TSPs, the regulation of expression is not only poorly understood, but is also multi-staged and complex, in part due to large regulatory regions of mRNA (TSP-1 and TSP-2) and numerous introns (all TSPs).
In an attempt to control the foreign body response and to prevent the granuloma formation in response to implantation and to increase angiogenesis, plasmids for the expression of a sense and an antisense cDNA for TSP-2 were delivered in a collagen solution, which was applied to biomaterials implanted subcutaleously (203). TSP-2 sense cDNA reversed the foreign body response in Thbs2−/− mice, and TSP-2 anti-sense cDNA reversed the granuloma formation in wild-type mice, demonstrating that manipulations with the expression of TSPs may be a valuable approach to regulate angiogenesis and matrix deposition. This approach was successfully used to alter the wound healing response in wild-type and Thbs2−/− mice using an in vivo murine excisional wound healing model with the improved polymer facilitating the delivery and release of the plasmid and increasing the efficiency of cell transfection in the healing wound (204).
The discovery and the rapid progression of our knowledge of miRNA rekindled the interest in therapeutic regulation of expression. Synthetic miRNA and their sequence-specific antisense antagonists can be relatively easily designed, produced and modified to stabilize the oligonucleotide and to facilitate its better delivery into cells (205-215). The synthetic oligonucleotides are stable, and they are retained in tissues for weeks. Despite a large number of predicted targets for each miRNA that can be found in public databases, the predictions based solely on the sequence match not necessarily become experimentally proven targets. The regulatory mechanisms mediated by miRNA now appear to be more complex than simple no-nonsense binding of miRNA to the target and degradation of the sequence-matched mRNA. Thus, if a mechanism operating through miRNA is dissected, targeting of this mechanism may be specific and efficient.
We have demonstrated that the antagonist of miR-467 efficiently prevents hyperglycemia-induced cancer angiogenesis and tumor growth in mouse models by targeting miR-467 interaction with 3’UTR of TSP-1, relieving the translational silencing of TSP-1, and increasing TSP-1 production (38). As the functions of TSPs in different pathologies are better understood and the miRNA regulation of TSPs is uncovered, targeting protein synthesis by miRNA may prove to be an efficient way to prevent or restore the production of a protein. In the case of miR-467, the pathway is signal- and tissue-specific (37, 38). For TSPs that are normally tightly regulated and expressed in large amounts only in specific and limited conditions, regulation with miRNA may prove to be an efficient and harmless therapeutic approach. The fact that all of the five knockout mice did not demonstrate an overt phenotype without challenge suggests that general non-specific downregulation of a single or several TSPs may be safe, at least for a short period of time.
6. Conclusion
Regulation of TSPs expression is not a well-studied area, with the exception of TSP-1 that attracted more attention, mostly due to the profound changes in its expression during tumor development and its potent effect on angiogenesis. The existing information suggests that the expression of TSPs is regulated at multiple molecular levels from transcriptional regulation and splicing to regulation of protein secretion. Furthermore, we learned that the regulation of expression is often not only cell- and tissue-specific, but also multi-leveled: simultaneous engagement of several levels of regulation results in discordant levels of mRNA and protein. Although investigation of different levels of regulation may appear tedious and complex, the detailed knowledge of complementary molecular mechanisms may eventually prove useful in developing effective, fast and specific therapeutic intervention.
Highlights.
Thrombospondins (TSPs) are highly expressed in embryonic development, tissue injury and remodeling, inflammation and immune response, and aging.
Expression patterns suggest distinct specific functions for each TSP.
Expression of TSPs is tightly regulated at several molecular levels: transcription, RNA processing and translation, protein stability and secretion.
Experimental regulation of TSPs expression modulates physiological and pathological processes dependent on TSPs.
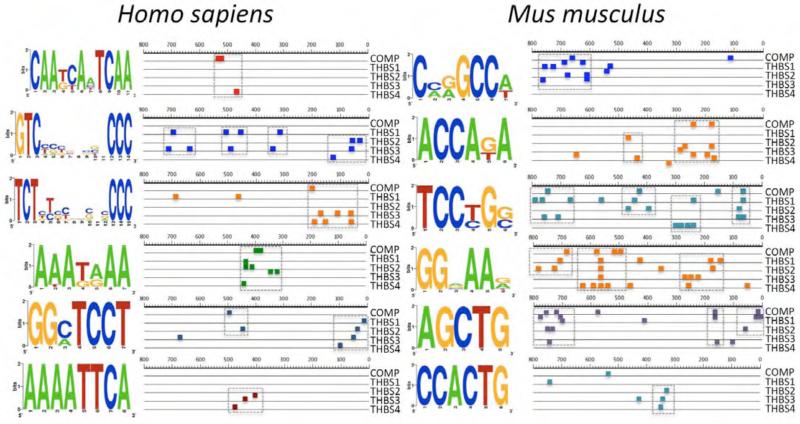
Figure 1. Common DNA sequence motifs in the promoters of TSPs.
The analysis of the promoters of TSP-1, TSP-2, TSP-3, TSP-4 and COMP was performed using SCOPE motif finder (http://genie.dartmouth.edu/scope) (31, 32). Motifs positioned similarly in the promoters of two or more TSPs are shown in the figure (6 out of 20 most significant matches).
Acknowledgments
The work was supported by R01 DK067532 and R01 HL117216.
The author would like to thank Nadia Hoppe and Maria Stenina for help with editing the text.
Abbreviations
- AhR
Aryl (aromatic) hydrocarbon receptor
- ARE
Adenylate-uridylate-rich element
- ATF-1
Activating Transcription Factor-1
- CBF
CCAAT-binding factor
- COMP
Cartilage oligomeric matrix protein
- Cyp1B 1
Cytochrome P450 1B1
- EBOX
Enhancer box
- ECM
Extracellular matrix
- Egr-1
Early growth response protein 1
- EGRF
Early growth response factor 1
- eNOS
Endothelial nitric oxide synthase
- ER
Endoplasmic reticulum
- H3
Histone 3
- HDAC
Histone Deacetylase
- HGF
Hepatocyte growth factor
- HuR
Human antigen R
- IR
Ischemia / reperfusion
- MAPK
Mitogen-activated protein kinases
- MI
Myocardial infarction
- MYC
Myelocytomatosis oncogene
- NO
Nitric oxide
- NR4A2
Nuclear receptor subfamily 4, group A, member 2
- PAH
Pulmonary arterial hypertension
- PAR-1
Protease-activated receptor-1
- SPAR C
Secreted protein acidic and rich in cysteine
- TGFβ
Transforming growth factor beta
- TRPC 4
Short transient receptor potential channel 4
- TSP
Thrombospondin
- UTR
Untranslated region
- WT1
Wilms' tumor suppressor
- YY-1
Yin Yang 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991;266:14831–14834. [PubMed] [Google Scholar]
- 2.Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams JC. Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol. 2001;17:25–51. doi: 10.1146/annurev.cellbio.17.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Bentley AA, Adams JC. The evolution of thrombospondins and their ligand-binding activities. Mol Biol Evol. 2010;27:2187–2197. doi: 10.1093/molbev/msq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tucker RP, Hess JF, Gong Q, Garvey K, Shibata B, Adams JC. A thrombospondin in the anthozoan Nematostella vectensis is associated with the nervous system and upregulated during regeneration. Biol Open. 2013;2:217–226. doi: 10.1242/bio.20123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stenina-Adognravi O. Thrombospondins: old players, new games. Curr Opin Lipidol. 2013;24:401–409. doi: 10.1097/MOL.0b013e3283642912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer JE, Iatridis JC, Chan D, Qureshi SA, Gottesman O, Hecht AC. Genetic polymorphisms associated with intervertebral disc degeneration. Spine J. 2013;13:299–317. doi: 10.1016/j.spinee.2013.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–688. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henkin J, Volpert OV. Therapies using anti-angiogenic peptide mimetics of thrombospondin-1. Expert Opin Ther Targets. 2011;15:1369–1386. doi: 10.1517/14728222.2011.640319. [DOI] [PubMed] [Google Scholar]
- 12.Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frolova EG, Pluskota E, Krukovets I, Burke T, Drumm C, Smith JD, Blech L, Febbraio M, Bornstein P, Plow EF, Stenina OI. Thrombospondin-4 regulates vascular inflammation and atherogenesis. Circ Res. 2010;107:1313–1325. doi: 10.1161/CIRCRESAHA.110.232371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanhoutte D, van Almen GC, Van Aelst LN, Van Cleemput J, Droogne W, Jin Y, Van de Werf F, Carmeliet P, Vanhaecke J, Papageorgiou AP, Heymans S. Matricellular proteins and matrix metalloproteinases mark the inflammatory and fibrotic response in human cardiac allograft rejection. Eur Heart J. 2013;34:1930–1941. doi: 10.1093/eurheartj/ehs375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mustonen E, Ruskoaho H, Rysa J. Thrombospondin-4, tumour necrosis factor-like weak inducer of apoptosis (TWEAK) and its receptor Fn14: novel extracellular matrix modulating factors in cardiac remodelling. Ann Med. 2012;44:793–804. doi: 10.3109/07853890.2011.614635. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: multiple paths to inflammation. Mediators Inflamm. 2011;2011:296069. doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller TW, Kaur S, Ivins-O'Keefe K, Roberts DD. Thrombospondin-1 is a CD47-dependent endogenous inhibitor of hydrogen sulfide signaling in T cell activation. Matrix Biol. 2013;32:316–24. doi: 10.1016/j.matbio.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin-Manso G, Navarathna DH, Galli S, Soto-Pantoja DR, Kuznetsova SA, Tsokos M, Roberts DD. Endogenous thrombospondin-1 regulates leukocyte recruitment and activation and accelerates death from systemic candidiasis. PLoS One. 2012;7:e48775. doi: 10.1371/journal.pone.0048775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian A, Wayburn B, Bunch T, Volk T. Thrombospondin-mediated adhesion is essential for the formation of the myotendinous junction in Drosophila. Development. 2007;134:1269–1278. doi: 10.1242/dev.000406. [DOI] [PubMed] [Google Scholar]
- 20.Chatila K, Ren G, Xia Y, Huebener P, Bujak M, Frangogiannis NG. The role of the thrombospondins in healing myocardial infarcts. Cardiovasc Hematol Agents Med Chem. 2007;5:21–27. doi: 10.2174/187152507779315813. [DOI] [PubMed] [Google Scholar]
- 21.Swinnen M, Vanhoutte D, Van Almen GC, Hamdani N, Schellings MW, D'Hooge J, Van der Velden J, Weaver MS, Sage EH, Bornstein P, Verheyen FK, VandenDriessche T, Chuah MK, Westermann D, Paulus WJ, Van de Werf F, Schroen B, Carmeliet P, Pinto YM, Heymans S. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation. 2009;120:1585–1597. doi: 10.1161/CIRCULATIONAHA.109.863266. [DOI] [PubMed] [Google Scholar]
- 22.van Almen GC, Swinnen M, Carai P, Verhesen W, Cleutjens JP, D'Hooge J, Verheyen FK, Pinto YM, Schroen B, Carmeliet P, Heymans S. Absence of thrombospondin-2 increases cardiomyocyte damage and matrix disruption in doxorubicin-induced cardiomyopathy. J Mol Cell Cardiol. 2011;51:318–328. doi: 10.1016/j.yjmcc.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, Porter JG, Evelo CT, Duisters R, van Leeuwen RE, Janssen BJ, Debets JJ, Smits JF, Daemen MJ, Crijns HJ, Bornstein P, Pinto YM. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res. 2004;95:515–522. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- 24.Cingolani OH, Kirk JA, Seo K, Koitabashi N, Lee DI, Ramirez-Correa G, Bedja D, Barth AS, Moens AL, Kass DA. Thrombospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circ Res. 2011;109:1410–1414. doi: 10.1161/CIRCRESAHA.111.256743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch JM, Maillet M, Vanhoutte D, Schloemer A, Sargent MA, Blair NS, Lynch KA, Okada T, Aronow BJ, Osinska H, Prywes R, Lorenz JN, Mori K, Lawler J, Robbins J, Molkentin JD. A thrombospondin-dependent pathway for a protective ER stress response. Cell. 2012;149:1257–1268. doi: 10.1016/j.cell.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frolova EG, Sopko N, Blech L, Popović ZB, Li J, Vasanji A, Drumm C, Krukovets I, Jain MK, Penn MS, Plow EF, Stenina OI. Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload. FASEB J. 2012;26:2363–73. doi: 10.1096/fj.11-190728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Risher WC, Eroglu C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol. 2012;31:170–177. doi: 10.1016/j.matbio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adolph KW, Liska DJ, Bornstein P. Analysis of the promoter and transcription start sites of the human thrombospondin 2 gene (THBS2). Gene. 1997;193:5–11. doi: 10.1016/s0378-1119(97)00070-x. [DOI] [PubMed] [Google Scholar]
- 31.Carlson JM, Chakravarty A, DeZiel CE, Gross RH. SCOPE: a web server for practical de novo motif discovery. Nucleic Acids Res. 2007;35:W259–264. doi: 10.1093/nar/gkm310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakravarty A, Carlson JM, Khetani RS, Gross RH. A novel ensemble learning method for de novo computational identification of DNA binding sites. BMC Bioinformatics. 2007;8:249. doi: 10.1186/1471-2105-8-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A. 2002;99:4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dabir P, Marinic TE, Krukovets I, Stenina OI. Aryl hydrocarbon receptor is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells. Circ Res. 2008;102:1558–1565. doi: 10.1161/CIRCRESAHA.108.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenina OI, Krukovets I, Wang K, Zhou Z, Forudi F, Penn MS, Topol EJ, Plow EF. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation. 2003;107:3209–3215. doi: 10.1161/01.CIR.0000074223.56882.97. [DOI] [PubMed] [Google Scholar]
- 36.Raugi GJ, Olerud JE, Gown AM. Thrombospondin in early human wound tissue. J Invest Dermatol. 1987;89:551–554. doi: 10.1111/1523-1747.ep12461198. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharyya S, Marinic TE, Krukovets I, Hoppe G, Stenina OI. Cell type-specific post-transcriptional regulation of production of the potent antiangiogenic and proatherogenic protein thrombospondin-1 by high glucose. J Biol Chem. 2008;283:5699–5707. doi: 10.1074/jbc.M706435200. [DOI] [PubMed] [Google Scholar]
- 38.Sanghamitra Bhattacharyya KS, Krukovets Irene, Nestor Carla, Li Jianbo, Stenina-Adognravi Olga. Novel Tissue-Specific mechanism of Regulation of Angiogenesis and Cancer Growth in Response to Hyperglycemia. Journal of the American Heart Association. 2012;1:e005967. doi: 10.1161/JAHA.112.005967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janz A, Sevignani C, Kenyon K, Ngo CV, Thomas-Tikhonenko A. Activation of the myc oncoprotein leads to increased turnover of thrombospondin-1 mRNA. Nucleic Acids Res. 2000;28:2268–2275. doi: 10.1093/nar/28.11.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy-Ullrich JE, Mosher DF. Interactions of thrombospondin with cells in culture: rapid degradation of both soluble and matrix thrombospondin. Semin Thromb Hemost. 1987;13:343–351. doi: 10.1055/s-2007-1003510. [DOI] [PubMed] [Google Scholar]
- 42.Murphy-Ullrich JE, Mosher DF. Interactions of thrombospondin with endothelial cells: receptor-mediated binding and degradation. J Cell Biol. 1987;105:1603–1611. doi: 10.1083/jcb.105.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manni A, Rager T, Kimball SR, Jefferson LS, Washington S, Hu X, Verderame MF. Effects of alpha-difluoromethylornithine on thrombospondin-1 production by human breast cancer cells. Int J Oncol. 2007;31:1187–1191. [PubMed] [Google Scholar]
- 44.Reed MJ, Puolakkainen P, Lane TF, Dickerson D, Bornstein P, Sage EH. Differential expression of SPARC and thrombospondin 1 in wound repair: immunolocalization and in situ hybridization. J Histochem Cytochem. 1993;41:1467–1477. doi: 10.1177/41.10.8245406. [DOI] [PubMed] [Google Scholar]
- 45.DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol. 1996;148:1851–1860. [PMC free article] [PubMed] [Google Scholar]
- 46.Streit M, Velasco P, Riccardi L, Spencer L, Brown LF, Janes L, Lange-Asschenfeldt B, Yano K, Hawighorst T, Iruela-Arispe L, Detmar M. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. Embo J. 2000;19:3272–3282. doi: 10.1093/emboj/19.13.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kyriakides TR, Tam JW, Bornstein P. Accelerated wound healing in mice with a disruption of the thrombospondin 2 gene. J Invest Dermatol. 1999;113:782–787. doi: 10.1046/j.1523-1747.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 48.Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161:831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stenina OI, Desai SY, Krukovets I, Kight K, Janigro D, Topol EJ, Plow EF. Thrombospondin-4 and its variants: expression and differential effects on endothelial cells. Circulation. 2003;108:1514–1519. doi: 10.1161/01.CIR.0000089085.76320.4E. [DOI] [PubMed] [Google Scholar]
- 50.Rysa J, Leskinen H, Ilves M, Ruskoaho H. Distinct upregulation of extracellular matrix genes in transition from hypertrophy to hypertensive heart failure. Hypertension. 2005;45:927–933. doi: 10.1161/01.HYP.0000161873.27088.4c. [DOI] [PubMed] [Google Scholar]
- 51.Pohjolainen V, Mustonen E, Taskinen P, Napankangas J, Leskinen H, Ohukainen P, Peltonen T, Aro J, Juvonen T, Satta J, Ruskoaho H, Rysa J. Increased thrombospondin-2 in human fibrosclerotic and stenotic aortic valves. Atherosclerosis. 2012;220:66–71. doi: 10.1016/j.atherosclerosis.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Mustonen E, Aro J, Puhakka J, Ilves M, Soini Y, Leskinen H, Ruskoaho H, Rysa J. Thrombospondin-4 expression is rapidly upregulated by cardiac overload. Biochem Biophys Res Commun. 2008;373:186–191. doi: 10.1016/j.bbrc.2008.05.164. [DOI] [PubMed] [Google Scholar]
- 53.Frolova EG, et al. Control of Organization and Function of Muscle and Tendon by Thrombospondin-4. Matrix Biology in press; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carron JA, Hiscott P, Hagan S, Sheridan CM, Magee R, Gallagher JA. Cultured human retinal pigment epithelial cells differentially express thrombospondin-1, -2, -3, and -4. Int J Biochem Cell Biol. 2000;32:1137–1142. doi: 10.1016/s1357-2725(00)00065-0. [DOI] [PubMed] [Google Scholar]
- 55.Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36:1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Liang Y, Diehn M, Watson N, Bollen AW, Aldape KD, Nicholas MK, Lamborn KR, Berger MS, Botstein D, Brown PO, Israel MA. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci U S A. 2005;102:5814–5819. doi: 10.1073/pnas.0402870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bredel M, Bredel C, Juric D, Harsh GR, Vogel H, Recht LD, Sikic BI. Functional network analysis reveals extended gliomagenesis pathway maps and three novel MYC-interacting genes in human gliomas. Cancer Res. 2005;65:8679–8689. doi: 10.1158/0008-5472.CAN-05-1204. [DOI] [PubMed] [Google Scholar]
- 58.Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey R, Rosenblum M, Mikkelsen T, Fine HA. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11:R7. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lonning PE, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turashvili G, Bouchal J, Baumforth K, Wei W, Dziechciarkova M, Ehrmann J, Klein J, Fridman E, Skarda J, Srovnal J, Hajduch M, Murray P, Kolar Z. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer. 2007;7:55. doi: 10.1186/1471-2407-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu K, Ganesan K, Tan LK, Laban M, Wu J, Zhao XD, Li H, Leung CH, Zhu Y, Wei CL, Hooi SC, Miller L, Tan P. A precisely regulated gene expression cassette potently modulates metastasis and survival in multiple solid cancers. PLoS Genet. 2008;4:e1000129. doi: 10.1371/journal.pgen.1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 64.Framson P, Bornstein P. A serum response element and a binding site for NF-Y mediate the serum response of the human thrombospondin 1 gene. J Biol Chem. 1993;268:4989–4996. [PubMed] [Google Scholar]
- 65.Shingu T, Bornstein P. Overlapping Egr-1 and Sp1 sites function in the regulation of transcription of the mouse thrombospondin 1 gene. J Biol Chem. 1994;269:32551–32557. [PubMed] [Google Scholar]
- 66.Laherty CD, Gierman TM, Dixit VM. Characterization of the promoter region of the human thrombospondin gene. DNA sequences within the first intron increase transcription. J Biol Chem. 1989;264:11222–11227. [PubMed] [Google Scholar]
- 67.Kim SA, Um SJ, Kang JH, Hong KJ. Expression of thrombospondin-1 in human hepatocarcinoma cell lines and its regulation by transcription factor Jun/AP-1. Mol Cell Biochem. 2001;216:21–29. doi: 10.1023/a:1011022822077. [DOI] [PubMed] [Google Scholar]
- 68.Kim SA, Hong KJ. Responsive site on the thrombospondin-1 promotor to down-regulation by phorbol 12-myristate 13-acetate in porcine aortic endothelial cells. Exp Mol Med. 2000;32:135–140. doi: 10.1038/emm.2000.23. [DOI] [PubMed] [Google Scholar]
- 69.Raman P, Harry C, Weber M, Krukovets I, Stenina OI. A novel transcriptional mechanism of cell type-specific regulation of vascular gene expression by glucose. Arterioscler Thromb Vasc Biol. 2011;31:634–642. doi: 10.1161/ATVBAHA.110.219675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raman P, Krukovets I, Marinic TE, Bornstein P, Stenina OI. Glycosylation mediates up-regulation of a potent antiangiogenic and proatherogenic protein, thrombospondin-1, by glucose in vascular smooth muscle cells. J Biol Chem. 2007;282:5704–5714. doi: 10.1074/jbc.M610965200. [DOI] [PubMed] [Google Scholar]
- 71.Wang JY, Zhang XM, Zhang HY. [The study of thrombospondin-I (TSP1) expression in the early stages of diabetic retinopathy induced by streptozotocin]. Fen Zi Xi Bao Sheng Wu Xue Bao. 2006;39:431–437. [PubMed] [Google Scholar]
- 72.Wang S, Wu X, Lincoln TM, Murphy-Ullrich JE. Expression of constitutively active cGMP-dependent protein kinase prevents glucose stimulation of thrombospondin 1 expression and TGF-beta activity. Diabetes. 2003;52:2144–2150. doi: 10.2337/diabetes.52.8.2144. [DOI] [PubMed] [Google Scholar]
- 73.Poczatek MH, Hugo C, Darley-Usmar V, Murphy-Ullrich JE. Glucose stimulation of transforming growth factor-beta bioactivity in mesangial cells is mediated by thrombospondin-1. Am J Pathol. 2000;157:1353–1363. doi: 10.1016/s0002-9440(10)64649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang S, Skorczewski J, Feng X, Mei L, Murphy-Ullrich JE. Glucose up-regulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J Biol Chem. 2004;279:34311–34322. doi: 10.1074/jbc.M401629200. [DOI] [PubMed] [Google Scholar]
- 75.Zhou Y, Poczatek MH, Berecek KH, Murphy-Ullrich JE. Thrombospondin 1 mediates angiotensin II induction of TGF-beta activation by cardiac and renal cells under both high and low glucose conditions. Biochem Biophys Res Commun. 2006;339:633–641. doi: 10.1016/j.bbrc.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 76.Belmadani S, Bernal J, Wei CC, Pallero MA, Dell'italia L, Murphy-Ullrich JE, Berecek KH. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol. 2007;171:777–789. doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, Hugo C. Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int. 2004;65:459–468. doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 78.Chavez RJ, Haney RM, Cuadra RH, Ganguly R, Adapala RK, Thodeti CK, Raman P. Upregulation of thrombospondin-1 expression by leptin in vascular smooth muscle cells via JAK2- and MAPK-dependent pathways. Am J Physiol Cell Physiol. 2012;303:C179–191. doi: 10.1152/ajpcell.00008.2012. [DOI] [PubMed] [Google Scholar]
- 79.Li Y, Tong X, Rumala C, Clemons K, Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS One. 2011;6:e26656. doi: 10.1371/journal.pone.0026656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moura R, Tjwa M, Vandervoort P, Van Kerckhoven S, Holvoet P, Hoylaerts MF. Thrombospondin-1 Deficiency Accelerates Atherosclerotic Plaque Maturation in ApoE−/− Mice. Circ Res. 2008;103:1181–1189. doi: 10.1161/CIRCRESAHA.108.185645. [DOI] [PubMed] [Google Scholar]
- 81.Moon Y, Bottone FG, Jr., McEntee MF, Eling TE. Suppression of tumor cell invasion by cyclooxygenase inhibitors is mediated by thrombospondin-1 via the early growth response gene Egr-1. Mol Cancer Ther. 2005;4:1551–1558. doi: 10.1158/1535-7163.MCT-05-0213. [DOI] [PubMed] [Google Scholar]
- 82.McLaughlin JN, Mazzoni MR, Cleator JH, Earls L, Perdigoto AL, Brooks JD, Muldowney JA, 3rd, Vaughan DE, Hamm HE. Thrombin modulates the expression of a set of genes including thrombospondin-1 in human microvascular endothelial cells. J Biol Chem. 2005;280:22172–22180. doi: 10.1074/jbc.M500721200. [DOI] [PubMed] [Google Scholar]
- 83.Almog N, Henke V, Flores L, Hlatky L, Kung AL, Wright RD, Berger R, Hutchinson L, Naumov GN, Bender E, Akslen LA, Achilles EG, Folkman J. Prolonged dormancy of human liposarcoma is associated with impaired tumor angiogenesis. FASEB J. 2006;20:947–949. doi: 10.1096/fj.05-3946fje. [DOI] [PubMed] [Google Scholar]
- 84.Zaslavsky A, Chen C, Grillo J, Baek KH, Holmgren L, Yoon SS, Folkman J, Ryeom S. Regional control of tumor growth. Mol Cancer Res. 2010;8:1198–1206. doi: 10.1158/1541-7786.MCR-10-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roudier E, Milkiewicz M, Birot O, Slopack D, Montelius A, Gustafsson T, Paik JH, DePinho RA, Casale GP, Pipinos II, Haas TL. Endothelial FoxO1 is an intrinsic regulator of thrombospondin 1 expression that restrains angiogenesis in ischemic muscle. Angiogenesis. 2013;16:759–772. doi: 10.1007/s10456-013-9353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang JH, Kim MJ, Chang SY, Sim SS, Kim MS, Jo YH. CCAAT box is required for the induction of human thrombospondin-1 gene by trichostatin A. J Cell Biochem. 2008;104:1192–1203. doi: 10.1002/jcb.21697. [DOI] [PubMed] [Google Scholar]
- 87.McMorrow JP, Crean D, Gogarty M, Smyth A, Connolly M, Cummins E, Veale D, Fearon U, Tak PP, Fitzgerald O, Murphy EP. Tumor necrosis factor inhibition modulates thrombospondin-1 expression in human inflammatory joint disease through altered NR4A2 activity. Am J Pathol. 2013;183:1243–1257. doi: 10.1016/j.ajpath.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 88.Negoescu A, Lafeuillade B, Pellerin S, Chambaz EM, Feige JJ. Transforming growth factors beta stimulate both thrombospondin-1 and CISP/thrombospondin-2 synthesis by bovine adrenocortical cells. Exp Cell Res. 1995;217:404–409. doi: 10.1006/excr.1995.1103. [DOI] [PubMed] [Google Scholar]
- 89.Kang JH, Chang SY, Yeom DH, Kim SA, Um SJ, Hong KJ. Weakening of the repressive YY-1 site on the thrombospondin-1 promoter via c-Jun/YY-1 interaction. Exp Mol Med. 2004;36:300–310. doi: 10.1038/emm.2004.41. [DOI] [PubMed] [Google Scholar]
- 90.Salnikow K, Cosentino S, Klein C, Costa M. Loss of thrombospondin transcriptional activity in nickel-transformed cells. Mol Cell Biol. 1994;14:851–858. doi: 10.1128/mcb.14.1.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salnikow K, Wang S, Costa M. Induction of activating transcription factor 1 by nickel and its role as a negative regulator of thrombospondin I gene expression. Cancer Res. 1997;57:5060–5066. [PubMed] [Google Scholar]
- 92.Ghoneim C, Soula-Rothhut M, Blanchevoye C, Martiny L, Antonicelli F, Rothhut B. Activating transcription factor-1-mediated hepatocyte growth factor-induced down-regulation of thrombospondin-1 expression leads to thyroid cancer cell invasion. J Biol Chem. 2007;282:15490–15497. doi: 10.1074/jbc.M610586200. [DOI] [PubMed] [Google Scholar]
- 93.Dejong V, Degeorges A, Filleur S, Ait-Si-Ali S, Mettouchi A, Bornstein P, Binetruy B, Cabon F. The Wilms’ tumor gene product represses the transcription of thrombospondin 1 in response to overexpression of c-Jun. Oncogene. 1999;18:3143–3151. doi: 10.1038/sj.onc.1202654. [DOI] [PubMed] [Google Scholar]
- 94.Su F, Pascal LE, Xiao W, Wang Z. Tumor suppressor U19/EAF2 regulates thrombospondin-1 expression via p53. Oncogene. 2010;29:421–431. doi: 10.1038/onc.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanai Y, Ushijima S, Kondo Y, Nakanishi Y, Hirohashi S. DNA methyltransferase expression and DNA methylation of CPG islands and peri-centromeric satellite regions in human colorectal and stomach cancers. Int J Cancer. 2001;91:205–212. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1040>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 96.Yang QW, Liu S, Tian Y, Salwen HR, Chlenski A, Weinstein J, Cohn SL. Methylation-associated silencing of the thrombospondin-1 gene in human neuroblastoma. Cancer Res. 2003;63:6299–6310. [PubMed] [Google Scholar]
- 97.Liu Y, Pang JC, Dong S, Mao B, Poon WS, Ng HK. Aberrant CpG island hypermethylation profile is associated with atypical and anaplastic meningiomas. Hum Pathol. 2005;36:416–425. doi: 10.1016/j.humpath.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 98.Lindner DJ, Wu Y, Haney R, Jacobs BS, Fruehauf JP, Tuthill R, Borden EC. Thrombospondin-1 expression in melanoma is blocked by methylation and targeted reversal by 5-Aza-deoxycytidine suppresses angiogenesis. Matrix Biol. 2013;32:123–132. doi: 10.1016/j.matbio.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Volpert OV, Pili R, Sikder HA, Nelius T, Zaichuk T, Morris C, Shiflett CB, Devlin MK, Conant K, Alani RM. Id1 regulates angiogenesis through transcriptional repression of thrombospondin-1. Cancer Cell. 2002;2:473–483. doi: 10.1016/s1535-6108(02)00209-x. [DOI] [PubMed] [Google Scholar]
- 100.McGray AJ, Gingerich T, Petrik JJ, LaMarre J. Rapid insulin-like growth factor-1-induced changes in granulosa cell thrombospondin-1 expression in vitro. J Reprod Dev. 2011;57:76–83. doi: 10.1262/jrd.10-045h. [DOI] [PubMed] [Google Scholar]
- 101.El Btaouri H, Morjani H, Greffe Y, Charpentier E, Martiny L. Role of JNK/ATF-2 pathway in inhibition of thrombospondin-1 (TSP-1) expression and apoptosis mediated by doxorubicin and camptothecin in FTC-133 cells. Biochim Biophys Acta. 2011;1813:695–703. doi: 10.1016/j.bbamcr.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 102.Amodeo V, Bazan V, Fanale D, Insalaco L, Caruso S, Cicero G, Bronte G, Rolfo C, Santini D, Russo A. Effects of anti-miR-182 on TSP-1 expression in human colon cancer cells: there is a sense in antisense? Expert Opin Ther Targets. 2013;17:1249–1261. doi: 10.1517/14728222.2013.832206. [DOI] [PubMed] [Google Scholar]
- 103.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Collins M, Rojnuckarin P, Zhu YH, Bornstein P. A far upstream, cell type-specific enhancer of the mouse thrombospondin 3 gene is located within intron 6 of the adjacent metaxin gene. J Biol Chem. 1998;273:21816–21824. doi: 10.1074/jbc.273.34.21816. [DOI] [PubMed] [Google Scholar]
- 105.Collins M, Bornstein P. SP1-binding elements, within the common metaxinthrombospondin 3 intergenic region, participate in the regulation of the metaxin gene. Nucleic Acids Res. 1996;24:3661–3669. doi: 10.1093/nar/24.19.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Adolph KW, Bornstein P. The human thrombospondin 3 gene: analysis of transcription initiation and an alternatively spliced transcript. Mol Cell Biol Res Commun. 1999;2:47–52. doi: 10.1006/mcbr.1999.0148. [DOI] [PubMed] [Google Scholar]
- 107.Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hecht JT, Nelson LD, Crowder E, Wang Y, Elder FF, Harrison WR, Francomano CA, Prange CK, Lennon GG, Deere M, et al. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat Genet. 1995;10:325–329. doi: 10.1038/ng0795-325. [DOI] [PubMed] [Google Scholar]
- 109.Posey KL, Davies S, Bales ES, Haynes R, Sandell LJ, Hecht JT. In vivo human Cartilage oligomeric matrix protein (COMP) promoter activity. Matrix Biol. 2005;24:539–549. doi: 10.1016/j.matbio.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 110.Adolph KW. Detection of exon skipping and retained introns in transcription of the human thrombospondin 2 gene. Biochem Biophys Res Commun. 1999;259:527–532. doi: 10.1006/bbrc.1999.0812. [DOI] [PubMed] [Google Scholar]
- 111.Hirose Y, Chiba K, Karasugi T, Nakajima M, Kawaguchi Y, Mikami Y, Furuichi T, Mio F, Miyake A, Miyamoto T, Ozaki K, Takahashi A, Mizuta H, Kubo T, Kimura T, Tanaka T, Toyama Y, Ikegawa S. A functional polymorphism in THBS2 that affects alternative splicing and MMP binding is associated with lumbar-disc herniation. Am J Hum Genet. 2008;82:1122–1129. doi: 10.1016/j.ajhg.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee JH, Horak CE, Khanna C, Meng Z, Yu LR, Veenstra TD, Steeg PS. Alterations in Gemin5 expression contribute to alternative mRNA splicing patterns and tumor cell motility. Cancer Res. 2008;68:639–644. doi: 10.1158/0008-5472.CAN-07-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stenina OI, Ustinov V, Krukovets I, Marinic T, Topol EJ, Plow EF. Polymorphisms A387P in thrombospondin-4 and N700S in thrombospondin-1 perturb calcium binding sites. Faseb J. 2005;19:1893–1895. doi: 10.1096/fj.05-3712fje. [DOI] [PubMed] [Google Scholar]
- 114.McGray AJ, Gingerich T, Petrik JJ, Lamarre J. Regulation of thrombospondin-1 expression through AU-rich elements in the 3′UTR of the mRNA. Cell Mol Biol Lett. 2011;16:55–68. doi: 10.2478/s11658-010-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dews M, Fox JL, Hultine S, Sundaram P, Wang W, Liu YY, Furth E, Enders GH, El-Deiry W, Schelter JM, Cleary MA, Thomas-Tikhonenko A. The myc-miR-17~92 axis blunts TGF{beta} signaling and production of multiple TGF{beta}-dependent antiangiogenic factors. Cancer Res. 2010;70:8233–8246. doi: 10.1158/0008-5472.CAN-10-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van Almen GC, Verhesen W, van Leeuwen RE, van de Vrie M, Eurlings C, Schellings MW, Swinnen M, Cleutjens JP, van Zandvoort MA, Heymans S, Schroen B. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell. 2011;10:769–779. doi: 10.1111/j.1474-9726.2011.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sundaram P, Hultine S, Smith LM, Dews M, Fox JL, Biyashev D, Schelter JM, Huang Q, Cleary MA, Volpert OV, Thomas-Tikhonenko A. p53-responsive miR-194 inhibits thrombospondin-1 and promotes angiogenesis in colon cancers. Cancer Res. 2011;71:7490–7501. doi: 10.1158/0008-5472.CAN-11-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kang JH, Kim SA, Hong KJ. Induction of TSP1 gene expression by heat shock is mediated via an increase in mRNA stability. FEBS Lett. 2006;580:510–516. doi: 10.1016/j.febslet.2005.12.065. [DOI] [PubMed] [Google Scholar]
- 119.Okamoto M, Ono M, Uchiumi T, Ueno H, Kohno K, Sugimachi K, Kuwano M. Up-regulation of thrombospondin-1 gene by epidermal growth factor and transforming growth factor beta in human cancer cells--transcriptional activation and messenger RNA stabilization. Biochim Biophys Acta. 2002;1574:24–34. doi: 10.1016/s0167-4781(01)00345-1. [DOI] [PubMed] [Google Scholar]
- 120.Bein K, Ware JA, Simons M. Myb-dependent regulation of thrombospondin 2 expression. Role of mRNA stability. J Biol Chem. 1998;273:21423–21429. doi: 10.1074/jbc.273.33.21423. [DOI] [PubMed] [Google Scholar]
- 121.Caux Y, Husson R, Maquin M. [In vitro demonstration of polymerization retraction of composites by capillary penetration phenomenon]. Chir Dent Fr. 1991;61:51–55. [PubMed] [Google Scholar]
- 122.Hecht JT, Hayes E, Snuggs M, Decker G, Montufar-Solis D, Doege K, Mwalle F, Poole R, Stevens J, Duke PJ. Calreticulin, PDI, Grp94 and BiP chaperone proteins are associated with retained COMP in pseudoachondroplasia chondrocytes. Matrix Biol. 2001;20:251–262. doi: 10.1016/s0945-053x(01)00136-6. [DOI] [PubMed] [Google Scholar]
- 123.Hecht JT, Montufar-Solis D, Decker G, Lawler J, Daniels K, Duke PJ. Retention of cartilage oligomeric matrix protein (COMP) and cell death in redifferentiated pseudoachondroplasia chondrocytes. Matrix Biol. 1998;17:625–633. doi: 10.1016/s0945-053x(98)90113-5. [DOI] [PubMed] [Google Scholar]
- 124.Hecht JT, Sage EH. Retention of the matricellular protein SPARC in the endoplasmic reticulum of chondrocytes from patients with pseudoachondroplasia. J Histochem Cytochem. 2006;54:269–274. doi: 10.1369/jhc.5C6834.2005. [DOI] [PubMed] [Google Scholar]
- 125.Veliceasa D, Ivanovic M, Hoepfner FT, Thumbikat P, Volpert OV, Smith ND. Transient potential receptor channel 4 controls thrombospondin-1 secretion and angiogenesis in renal cell carcinoma. FEBS J. 2007;274:6365–6377. doi: 10.1111/j.1742-4658.2007.06159.x. [DOI] [PubMed] [Google Scholar]
- 126.Lawler JW, Slayter HS. The release of heparin binding peptides from platelet thrombospondin by proteolytic action of thrombin, plasmin and trypsin. Thromb Res. 1981;22:267–279. doi: 10.1016/0049-3848(81)90119-5. [DOI] [PubMed] [Google Scholar]
- 127.Dawes J, Clemetson KJ, Gogstad GO, McGregor J, Clezardin P, Prowse CV, Pepper DS. A radioimmunoassay for thrombospondin, used in a comparative study of thrombospondin, beta-thromboglobulin and platelet factor 4 in healthy volunteers. Thromb Res. 1983;29:569–581. doi: 10.1016/0049-3848(83)90212-8. [DOI] [PubMed] [Google Scholar]
- 128.Fordham JB, Hua J, Morwood SR, Schewitz-Bowers LP, Copland DA, Dick AD, Nicholson LB. Environmental conditioning in the control of macrophage thrombospondin-1 production. Sci Rep. 2012;2:512. doi: 10.1038/srep00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mumby SM, Abbott-Brown D, Raugi GJ, Bornstein P. Regulation of thrombospondin secretion by cells in culture. J Cell Physiol. 1984;120:280–288. doi: 10.1002/jcp.1041200304. [DOI] [PubMed] [Google Scholar]
- 130.Gabrielsen A, Lawler PR, Yongzhong W, Steinbruchel D, Blagoja D, Paulsson-Berne G, Kastrup J, Hansson GK. Gene expression signals involved in ischemic injury, extracellular matrix composition and fibrosis defined by global mRNA profiling of the human left ventricular myocardium. J Mol Cell Cardiol. 2007;42:870–883. doi: 10.1016/j.yjmcc.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 131.Melenovsky V, Benes J, Skaroupkova P, Sedmera D, Strnad H, Kolar M, Vlcek C, Petrak J, Benes J, Jr., Papousek F, Oliyarnyk O, Kazdova L, Cervenka L. Metabolic characterization of volume overload heart failure due to aorto-caval fistula in rats. Mol Cell Biochem. 2011;354:83–96. doi: 10.1007/s11010-011-0808-3. [DOI] [PubMed] [Google Scholar]
- 132.Mustonen E, Leskinen H, Aro J, Luodonpaa M, Vuolteenaho O, Ruskoaho H, Rysa J. Metoprolol treatment lowers thrombospondin-4 expression in rats with myocardial infarction and left ventricular hypertrophy. Basic Clin Pharmacol Toxicol. 2010;107:709–717. doi: 10.1111/j.1742-7843.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- 133.Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, Sheng H, Warner DS, Liu C, Eroglu C, Kuo CT. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497:369–373. doi: 10.1038/nature12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cagliani R, Guerini FR, Rubio-Acero R, Baglio F, Forni D, Agliardi C, Griffanti L, Fumagalli M, Pozzoli U, Riva S, Calabrese E, Sikora M, Casals F, Comi GP, Bresolin N, Caceres M, Clerici M, Sironi M. Long-standing balancing selection in the THBS4 gene: influence on sex-specific brain expression and gray matter volumes in Alzheimer disease. Hum Mutat. 2013;34:743–753. doi: 10.1002/humu.22301. [DOI] [PubMed] [Google Scholar]
- 135.Mettouchi A, Cabon F, Montreau N, Vernier P, Mercier G, Blangy D, Tricoire H, Vigier P, Binetruy B. SPARC and thrombospondin genes are repressed by the c-jun oncogene in rat embryo fibroblasts. EMBO J. 1994;13:5668–5678. doi: 10.1002/j.1460-2075.1994.tb06905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yabkowitz R, Dixit VM, Guo N, Roberts DD, Shimizu Y. Activated T-cell adhesion to thrombospondin is mediated by the alpha 4 beta 1 (VLA-4) and alpha 5 beta 1 (VLA-5) integrins. J Immunol. 1993;151:149–158. [PubMed] [Google Scholar]
- 137.Zhao XM, Hu Y, Miller GG, Mitchell RN, Libby P. Association of thrombospondin-1 and cardiac allograft vasculopathy in human cardiac allografts. Circulation. 2001;103:525–531. doi: 10.1161/01.cir.103.4.525. [DOI] [PubMed] [Google Scholar]
- 138.Vallejo AN, Mugge LO, Klimiuk PA, Weyand CM, Goronzy JJ. Central role of thrombospondin-1 in the activation and clonal expansion of inflammatory T cells. J Immunol. 2000;164:2947–2954. doi: 10.4049/jimmunol.164.6.2947. [DOI] [PubMed] [Google Scholar]
- 139.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 140.Riessen R, Kearney M, Lawler J, Isner JM. Immunolocalization of thrombospondin-1 in human atherosclerotic and restenotic arteries. Am Heart J. 1998;135:357–364. doi: 10.1016/s0002-8703(98)70105-x. [DOI] [PubMed] [Google Scholar]
- 141.Lange-Asschenfeldt B, Weninger W, Velasco P, Kyriakides TR, von Andrian UH, Bornstein P, Detmar M. Increased and prolonged inflammation and angiogenesis in delayed-type hypersensitivity reactions elicited in the skin of thrombospondin-2--deficient mice. Blood. 2002;99:538–545. doi: 10.1182/blood.v99.2.538. [DOI] [PubMed] [Google Scholar]
- 142.Pluskota E, Stenina OI, Krukovets I, Szpak D, Topol EJ, Plow EF. Mechanism and effect of thrombospondin-4 polymorphisms on neutrophil function. Blood. 2005;106:3970–3978. doi: 10.1182/blood-2005-03-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Manna PP, Frazier WA. The mechanism of CD47-dependent killing of T cells: heterotrimeric Gi-dependent inhibition of protein kinase A. J Immunol. 2003;170:3544–3553. doi: 10.4049/jimmunol.170.7.3544. [DOI] [PubMed] [Google Scholar]
- 144.Papageorgiou AP, Swinnen M, Vanhoutte D, VandenDriessche T, Chuah M, Lindner D, Verhesen W, de Vries B, D'Hooge J, Lutgens E, Westermann D, Carmeliet P, Heymans S. Thrombospondin-2 prevents cardiac injury and dysfunction in viral myocarditis through the activation of regulatory T-cells. Cardiovasc Res. 2012;94:115–124. doi: 10.1093/cvr/cvs077. [DOI] [PubMed] [Google Scholar]
- 145.Grimbert P, Bouguermouh S, Baba N, Nakajima T, Allakhverdi Z, Braun D, Saito H, Rubio M, Delespesse G, Sarfati M. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25- T cells in response to inflammation. J Immunol. 2006;177:3534–3541. doi: 10.4049/jimmunol.177.6.3534. [DOI] [PubMed] [Google Scholar]
- 146.Oshika Y, Masuda K, Tokunaga T, Hatanaka H, Kamiya T, Abe Y, Ozeki Y, Kijima H, Yamazaki H, Tamaoki N, Ueyama Y, Nakamura M. Thrombospondin 2 gene expression is correlated with decreased vascularity in non-small cell lung cancer. Clin Cancer Res. 1998;4:1785–1788. [PubMed] [Google Scholar]
- 147.Tokunaga T, Nakamura M, Oshika Y, Abe Y, Ozeki Y, Fukushima Y, Hatanaka H, Sadahiro S, Kijima H, Tsuchida T, Yamazaki H, Tamaoki N, Ueyama Y. Thrombospondin 2 expression is correlated with inhibition of angiogenesis and metastasis of colon cancer. Br J Cancer. 1999;79:354–359. doi: 10.1038/sj.bjc.6690056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kawakami T, Tokunaga T, Hatanaka H, Tsuchida T, Tomii Y, Osada H, Onoda N, Morino F, Nagata J, Kijima H, Yamazaki H, Abe Y, Osamura Y, Ueyama Y, Nakamura M. Interleukin 10 expression is correlated with thrombospondin expression and decreased vascular involvement in colon cancer. Int J Oncol. 2001;18:487–491. doi: 10.3892/ijo.18.3.487. [DOI] [PubMed] [Google Scholar]
- 149.Kodama J, Hashimoto I, Seki N, Hongo A, Yoshinouchi M, Okuda H, Kudo T. Thrombospondin-1 and -2 messenger RNA expression in invasive cervical cancer: correlation with angiogenesis and prognosis. Clin Cancer Res. 2001;7:2826–2831. [PubMed] [Google Scholar]
- 150.Wu MP, Tzeng CC, Wu LW, Huang KF, Chou CY. Thrombospondin-1 acts as a fence to inhibit angiogenesis that occurs during cervical carcinogenesis. Cancer J. 2004;10:27–32. doi: 10.1097/00130404-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 151.Harada H, Nakagawa K, Saito M, Kohno S, Nagato S, Furukawa K, Kumon Y, Hamada K, Ohnishi T. Introduction of wild-type p53 enhances thrombospondin-1 expression in human glioma cells. Cancer Lett. 2003;191:109–119. doi: 10.1016/s0304-3835(02)00592-x. [DOI] [PubMed] [Google Scholar]
- 152.Cinatl J, Jr., Kotchetkov R, Scholz M, Cinatl J, Vogel JU, Driever PH, Doerr HW. Human cytomegalovirus infection decreases expression of thrombospondin-1 independent of the tumor suppressor protein p53. Am J Pathol. 1999;155:285–292. doi: 10.1016/S0002-9440(10)65122-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dalla-Torre CA, Yoshimoto M, Lee CH, Joshua AM, de Toledo SR, Petrilli AS, Andrade JA, Chilton-MacNeill S, Zielenska M, Squire JA. Effects of THBS3, SPARC and SPP1 expression on biological behavior and survival in patients with osteosarcoma. BMC Cancer. 2006;6:237. doi: 10.1186/1471-2407-6-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Amy EM, Song S, Kutasovic JR, Reid LE, Valle JM, Vargas AC, Smart CE, Simpson PT. Thrombospondin-4 expression is activated during the stromal response to invasive breast cancer. Virchows Arch. 2013;463:535–545. doi: 10.1007/s00428-013-1468-3. [DOI] [PubMed] [Google Scholar]
- 155.Korkola JE, DeVries S, Fridlyand J, Hwang ES, Estep AL, Chen YY, Chew KL, Dairkee SH, Jensen RM, Waldman FM. Differentiation of lobular versus ductal breast carcinomas by expression microarray analysis. Cancer Res. 2003;63:7167–7175. [PubMed] [Google Scholar]
- 156.Forster S, Gretschel S, Jons T, Yashiro M, Kemmner W. THBS4, a novel stromal molecule of diffuse-type gastric adenocarcinomas, identified by transcriptome-wide expression profiling. Mod Pathol. 2011;24:1390–1403. doi: 10.1038/modpathol.2011.99. [DOI] [PubMed] [Google Scholar]
- 157.Axel DI, Frigge A, Dittmann J, Runge H, Spyridopoulos I, Riessen R, Viebahn R, Karsch KR. All-trans retinoic acid regulates proliferation, migration, differentiation, and extracellular matrix turnover of human arterial smooth muscle cells. Cardiovasc Res. 2001;49:851–862. doi: 10.1016/s0008-6363(00)00312-6. [DOI] [PubMed] [Google Scholar]
- 158.Zhang YW, Su Y, Volpert OV, Vande Woude GF. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci U S A. 2003;100:12718–12723. doi: 10.1073/pnas.2135113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bongrazio M, Da Silva-Azevedo L, Bergmann EC, Baum O, Hinz B, Pries AR, Zakrzewicz A. Shear stress modulates the expression of thrombospondin-1 and CD36 in endothelial cells in vitro and during shear stress-induced angiogenesis in vivo. Int J Immunopathol Pharmacol. 2006;19:35–48. [PubMed] [Google Scholar]
- 160.Bienes-Martinez R, Ordonez A, Feijoo-Cuaresma M, Corral-Escariz M, Mateo G, Stenina O, Jimenez B, Calzada MJ. Autocrine stimulation of clear-cell renal carcinoma cell migration in hypoxia via HIF-independent suppression of thrombospondin-1. Sci Rep. 2012;2:788. doi: 10.1038/srep00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Favier J, Germain S, Emmerich J, Corvol P, Gasc JM. Critical overexpression of thrombospondin 1 in chronic leg ischaemia. J Pathol. 2005;207:358–366. doi: 10.1002/path.1833. [DOI] [PubMed] [Google Scholar]
- 162.Flugel-Koch C, Ohlmann A, Fuchshofer R, Welge-Lussen U, Tamm ER. Thrombospondin-1 in the trabecular meshwork: localization in normal and glaucomatous eyes, and induction by TGF-beta1 and dexamethasone in vitro. Exp Eye Res. 2004;79:649–663. doi: 10.1016/j.exer.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 163.Mirkin S, Archer DF. Effects of mifepristone on vascular endothelial growth factor and thrombospondin-1 mRNA in Ishikawa cells: implication for the endometrial effects of mifepristone. Contraception. 2004;70:327–333. doi: 10.1016/j.contraception.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 164.Scott-Burden T, Resink TJ, Hahn AW, Buhler FR. Induction of thrombospondin expression in vascular smooth muscle cells by angiotensin II. J Cardiovasc Pharmacol 16 Suppl. 1990;7:S17–20. [PubMed] [Google Scholar]
- 165.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood. 2009;113:744–754. doi: 10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.MacLauchlan S, Yu J, Parrish M, Asoulin TA, Schleicher M, Krady MM, Zeng J, Huang PL, Sessa WC, Kyriakides TR. Endothelial nitric oxide synthase controls the expression of the angiogenesis inhibitor thrombospondin 2. Proc Natl Acad Sci U S A. 2011;108:E1137–1145. doi: 10.1073/pnas.1104357108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lin TN, Kim GM, Chen JJ, Cheung WM, He YY, Hsu CY. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke. 2003;34:177–186. doi: 10.1161/01.str.0000047100.84604.ba. [DOI] [PubMed] [Google Scholar]
- 168.Sezaki S, Hirohata S, Iwabu A, Nakamura K, Toeda K, Miyoshi T, Yamawaki H, Demircan K, Kusachi S, Shiratori Y, Ninomiya Y. Thrombospondin-1 is induced in rat myocardial infarction and its induction is accelerated by ischemia/reperfusion. Exp Biol Med (Maywood) 2005;230:621–630. doi: 10.1177/153537020523000904. [DOI] [PubMed] [Google Scholar]
- 169.Lario S, Bescos M, Campos B, Mur C, Luque P, Alvarez R, Campistol JM. Thrombospondin-1 mRNA expression in experimental kidney transplantation with heart-beating and non-heart-beating donors. J Nephrol. 2007;20:588–595. [PubMed] [Google Scholar]
- 170.Sage E, Mercier O, Van den Eyden F, de Perrot M, Barlier-Mur AM, Dartevelle P, Eddahibi S, Herve P, Fadel E. Endothelial cell apoptosis in chronically obstructed and reperfused pulmonary artery. Respir Res. 2008;9:19. doi: 10.1186/1465-9921-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kaiser R, Grotemeyer K, Kalsch T, Graber S, Wilkens H, Elmas E. Decreased TSP-1 following percutaneous coronary intervention is associated with major adverse cardiac events in ST-elevation myocardial infarction. Clin Hemorheol Microcirc. 2013;54:59–73. doi: 10.3233/CH-2012-1565. [DOI] [PubMed] [Google Scholar]
- 172.Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, Barone S, Bevans S, Lentsch AB, Rabb H, Soleimani M. Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest. 2005;115:3451–3459. doi: 10.1172/JCI25461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bein K, Odell-Fiddler ET, Drinane M. Role of TGF-beta1 and JNK signaling in capillary tube patterning. Am J Physiol Cell Physiol. 2004;287:C1012–1022. doi: 10.1152/ajpcell.00101.2004. [DOI] [PubMed] [Google Scholar]
- 174.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 175.Kawataki T, Naganuma H, Sasaki A, Yoshikawa H, Tasaka K, Nukui H. Correlation of thrombospondin-1 and transforming growth factor-beta expression with malignancy of glioma. Neuropathology. 2000;20:161–169. doi: 10.1046/j.1440-1789.2000.00327.x. [DOI] [PubMed] [Google Scholar]
- 176.Melnick M, Chen H, Zhou Y, Jaskoll T. Thrombospondin-2 gene expression and protein localization during embryonic mouse palate development. Arch Oral Biol. 2000;45:19–25. doi: 10.1016/s0003-9969(99)00113-2. [DOI] [PubMed] [Google Scholar]
- 177.Maloney JP, Stearman RS, Bull TM, Calabrese DW, Tripp-Addison ML, Wick MJ, Broeckel U, Robbins IM, Wheeler LA, Cogan JD, Loyd JE. Loss-of-function thrombospondin-1 mutations in familial pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;302:L541–554. doi: 10.1152/ajplung.00282.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bauer PM, Bauer EM, Rogers NM, Yao M, Feijoo-Cuaresma M, Pilewski JM, Champion HC, Zuckerbraun BS, Calzada MJ, Isenberg JS. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res. 2012;93:682–693. doi: 10.1093/cvr/cvr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rogers NM, Roberts DD, Isenberg JS. Age-Associated Induction of Cell Membrane CD47 Limits Basal and Temperature-Induced Changes in Cutaneous Blood Flow. Ann Surg. 2012;258:184–91. doi: 10.1097/SLA.0b013e31827e52e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Frazier EP, Isenberg JS, Shiva S, Zhao L, Schlesinger P, Dimitry J, Abu-Asab MS, Tsokos M, Roberts DD, Frazier WA. Age-dependent regulation of skeletal muscle mitochondria by the thrombospondin-1 receptor CD47. Matrix Biol. 2011;30:154–161. doi: 10.1016/j.matbio.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Starr ME, Hu Y, Stromberg AJ, Carmical JR, Wood TG, Evers BM, Saito H. Gene expression profile of mouse white adipose tissue during inflammatory stress: age-dependent upregulation of major procoagulant factors. Aging Cell. 2013;12:194–206. doi: 10.1111/acel.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cai H, Yuan Z, Fei Q, Zhao J. Investigation of thrombospondin-1 and transforming growth factor-beta expression in the heart of aging mice. Exp Ther Med. 2012;3:433–436. doi: 10.3892/etm.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Agah A, Kyriakides TR, Letrondo N, Bjorkblom B, Bornstein P. Thrombospondin 2 levels are increased in aged mice: consequences for cutaneous wound healing and angiogenesis. Matrix Biol. 2004;22:539–547. doi: 10.1016/j.matbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 184.Gilsohn E, Volk T. Slowdown promotes muscle integrity by modulating integrin-mediated adhesion at the myotendinous junction. Development. 2010;137:785–794. doi: 10.1242/dev.043703. [DOI] [PubMed] [Google Scholar]
- 185.LaFramboise WA, Petrosko P, Krill-Burger JM, Morris DR, McCoy AR, Scalise D, Malehorn DE, Guthrie RD, Becich MJ, Dhir R. Proteins secreted by embryonic stem cells activate cardiomyocytes through ligand binding pathways. J Proteomics. 2010;73:992–1003. doi: 10.1016/j.jprot.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fouladkou F, Lu C, Jiang C, Zhou L, She Y, Walls JR, Kawabe H, Brose N, Henkelman RM, Huang A, Bruneau BG, Rotin D. The ubiquitin ligase Nedd4-1 is required for heart development and is a suppressor of thrombospondin-1. J Biol Chem. 2010;285:6770–6780. doi: 10.1074/jbc.M109.082347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Iruela-Arispe ML, Liska DJ, Sage EH, Bornstein P. Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn. 1993;197:40–56. doi: 10.1002/aja.1001970105. [DOI] [PubMed] [Google Scholar]
- 188.Adams JC, Tucker RP. The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Dev Dyn. 2000;218:280–299. doi: 10.1002/(SICI)1097-0177(200006)218:2<280::AID-DVDY4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 189.Tooney PA, Sakai T, Sakai K, Aeschlimann D, Mosher DF. Restricted localization of thrombospondin-2 protein during mouse embryogenesis: a comparison to thrombospondin-1. Matrix Biol. 1998;17:131–143. doi: 10.1016/s0945-053x(98)90026-9. [DOI] [PubMed] [Google Scholar]
- 190.Urry LA, Whittaker CA, Duquette M, Lawler J, DeSimone DW. Thrombospondins in early Xenopus embryos: dynamic patterns of expression suggest diverse roles in nervous system, notochord, and muscle development. Dev Dyn. 1998;211:390–407. doi: 10.1002/(SICI)1097-0177(199804)211:4<390::AID-AJA10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 191.Tucker RP, Hagios C, Chiquet-Ehrismann R, Lawler J. In situ localization of thrombospondin-1 and thrombospondin-3 transcripts in the avian embryo. Dev Dyn. 1997;208:326–337. doi: 10.1002/(SICI)1097-0177(199703)208:3<326::AID-AJA4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 192.Tucker RP, Adams JC, Lawler J. Thrombospondin-4 is expressed by early osteogenic tissues in the chick embryo. Dev Dyn. 1995;203:477–490. doi: 10.1002/aja.1002030410. [DOI] [PubMed] [Google Scholar]
- 193.Arber S, Caroni P. Thrombospondin-4, an extracellular matrix protein expressed in the developing and adult nervous system promotes neurite outgrowth. J Cell Biol. 1995;131:1083–1094. doi: 10.1083/jcb.131.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fang C, Carlson CS, Leslie MP, Tulli H, Stolerman E, Perris R, Ni L, Di Cesare PE. Molecular cloning, sequencing, and tissue and developmental expression of mouse cartilage oligomeric matrix protein (COMP). J Orthop Res. 2000;18:593–603. doi: 10.1002/jor.1100180412. [DOI] [PubMed] [Google Scholar]
- 195.Di Cesare PE, Fang C, Leslie MP, Tulli H, Perris R, Carlson CS. Expression of cartilage oligomeric matrix protein (COMP) by embryonic and adult osteoblasts. J Orthop Res. 2000;18:713–720. doi: 10.1002/jor.1100180506. [DOI] [PubMed] [Google Scholar]
- 196.Kipnes J, Carlberg AL, Loredo GA, Lawler J, Tuan RS, Hall DJ. Effect of cartilage oligomeric matrix protein on mesenchymal chondrogenesis in vitro. Osteoarthritis Cartilage. 2003;11:442–454. doi: 10.1016/s1063-4584(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 197.Chen AL, Fang C, Liu C, Leslie MP, Chang E, Di Cesare PE. Expression of bone morphogenetic proteins, receptors, and tissue inhibitors in human fetal, adult, and osteoarthritic articular cartilage. J Orthop Res. 2004;22:1188–1192. doi: 10.1016/j.orthres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 198.Lin Y, Luo E, Chen X, Liu L, Qiao J, Yan Z, Li Z, Tang W, Zheng X, Tian W. Molecular and cellular characterization during chondrogenic differentiation of adipose tissue-derived stromal cells in vitro and cartilage formation in vivo. J Cell Mol Med. 2005;9:929–939. doi: 10.1111/j.1582-4934.2005.tb00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kong L, Tian Q, Guo F, Mucignat MT, Perris R, Sercu S, Merregaert J, Di Cesare PE, Liu CJ. Interaction between cartilage oligomeric matrix protein and extracellular matrix protein 1 mediates endochondral bone growth. Matrix Biol. 2010;29:276–286. doi: 10.1016/j.matbio.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Roman-Blas J, Dion AS, Seghatoleslami MR, Giunta K, Oca P, Jimenez SA, Williams CJ. MED and PSACH COMP mutations affect chondrogenesis in chicken limb bud micromass cultures. J Cell Physiol. 2010;224:817–826. doi: 10.1002/jcp.22185. [DOI] [PubMed] [Google Scholar]
- 201.Laherty CD, O'Rourke K, Wolf FW, Katz R, Seldin MF, Dixit VM. Characterization of mouse thrombospondin 2 sequence and expression during cell growth and development. J Biol Chem. 1992;267:3274–3281. [PubMed] [Google Scholar]
- 202.Corless CL, Mendoza A, Collins T, Lawler J. Colocalization of thrombospondin and syndecan during murine development. Dev Dyn. 1992;193:346–358. doi: 10.1002/aja.1001930408. [DOI] [PubMed] [Google Scholar]
- 203.Kyriakides TR, Hartzel T, Huynh G, Bornstein P. Regulation of angiogenesis and matrix remodeling by localized, matrix-mediated antisense gene delivery. Mol Ther. 2001;3:842–849. doi: 10.1006/mthe.2001.0336. [DOI] [PubMed] [Google Scholar]
- 204.Kyriakides TR, Cheung CY, Murthy N, Bornstein P, Stayton PS, Hoffman AS. pH-sensitive polymers that enhance intracellular drug delivery in vivo. J Control Release. 2002;78:295–303. doi: 10.1016/s0168-3659(01)00504-1. [DOI] [PubMed] [Google Scholar]
- 205.Wang S, Olson EN. AngiomiRs--key regulators of angiogenesis. Curr Opin Genet Dev. 2009;19:205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Horwich MD, Zamore PD. Design and delivery of antisense oligonucleotides to block microRNA function in cultured Drosophila and human cells. Nat Protoc. 2008;3:1537–1549. doi: 10.1038/nprot.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Davis S, Lollo B, Freier S, Esau C. Improved targeting of miRNA with antisense oligonucleotides. Nucleic Acids Res. 2006;34:2294–2304. doi: 10.1093/nar/gkl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Scherr M, Venturini L, Battmer K, Schaller-Schoenitz M, Schaefer D, Dallmann I, Ganser A, Eder M. Lentivirus-mediated antagomir expression for specific inhibition of miRNA function. Nucleic Acids Res. 2007;35:e149. doi: 10.1093/nar/gkm971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wu L, Belasco JG. Let me count the ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol Cell. 2008;29:1–7. doi: 10.1016/j.molcel.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 210.Stenvang J, Kauppinen S. MicroRNAs as targets for antisense-based therapeutics. Expert Opin Biol Ther. 2008;8:59–81. doi: 10.1517/14712598.8.1.59. [DOI] [PubMed] [Google Scholar]
- 211.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 212.Krutzfeldt J, Kuwajima S, Braich R, Rajeev KG, Pena J, Tuschl T, Manoharan M, Stoffel M. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 2007;35:2885–2892. doi: 10.1093/nar/gkm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Stenvang J, Lindow M, Kauppinen S. Targeting of microRNAs for therapeutics. Biochem Soc Trans. 2008;36:1197–1200. doi: 10.1042/BST0361197. [DOI] [PubMed] [Google Scholar]
- 214.van Rooij E, Marshall WS, Olson EN. Toward microRNA-based therapeutics for heart disease: the sense in antisense. Circ Res. 2008;103:919–928. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stenvang J, Silahtaroglu AN, Lindow M, Elmen J, Kauppinen S. The utility of LNA in microRNA-based cancer diagnostics and therapeutics. Semin Cancer Biol. 2008;18:89–102. doi: 10.1016/j.semcancer.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 216.Huang X. a. M., W. A time-efficient, linear-space local similarity algorithm. In Advances in Applied Mathematics. 1991;12:337–357. [Google Scholar]