Abstract
Alpha-actinins (ACTNs) were originally identified as cytoskeletal proteins which cross-link filamentous actin to establish cytoskeletal architect that protects cells from mechanical stress and controls cell movement. Notably, unlike other ACTNs, alpha-actinin 4 (ACTN4) displays unique characteristics in signaling transduction, nuclear translocation, and gene expression regulation. Initial reports indicated that ACTN4 is part of the breast cancer cell motile apparatus and is highly expressed in the nucleus. These results imply that ACTN4 plays a role in breast cancer tumorigenesis. While several observations in breast cancer and other cancers support this hypothesis, little direct evidence links the tumorigenic phenotype with ACTN4-mediated pathological mechanisms. Recently, several studies have demonstrated that in addition to its role in coordinating cytoskeleton, ACTN4 interacts with signaling mediators, chromatin remodeling factors, and transcription factors including nuclear receptors. Thus, ACTN4 functions as a versatile promoter for breast cancer tumorigenesis and appears to be an ideal drug target for future therapeutic development.
1. INTRODUCTION
Alpha-actinins (ACTNs) are ubiquitously expressed proteins known to be cross-linked with filamentous actin (F-actin) to maintain cytoskeletal integrity and to control cell movement (Sjöblom, Salmazo, & Djinović-Carugo, 2008). ACTNs localize to cell–cell and cell–matrix contact sites, cellular protrusions, and stress fiber-dense regions and regulate diverse signaling pathways by linking membrane receptors with the cytoskeleton (Edlund, Lotano, & Otey, 2001; Otey & Carpen, 2004; Pavalko, Otey, Simon, & Burridge, 1991). Four ACTN family members, numbered 1–4, are present in humans (Beggs et al., 1992; Honda et al., 1998) and highly conserved in other mammals (Arimura et al., 1988; Fyrberg, Kelly, Ball, Fyrberg, & Reedy, 1990). ACTNs can be categorized as “muscle” or “non-muscle” due to their tissue-specific function and expression patterns (Oikonomou, Zachou, & Dalekos, 2011). ACTN2 and ACTN3 are muscle specific and are major components of contractile machinery connecting with actin filaments at Z lines in striated muscles or at dense bodies in smooth muscle cells (Mills et al., 2001). In contrast, ACTN1 and alpha-actinin 4 (ACTN4) are ubiquitously expressed. ACTN1 and ACTN4 share 80% and about 90% similarity in nucleotide and amino acid sequence, respectively (Honda et al., 1998). Despite the high level of similarity in their protein sequences, ACTN1 and ACTN4 are found in different subcellular compartments and are function distinct (Honda et al., 1998; Quick & Skalli, 2010). ACTN1 localizes with stress fibers and is present in adherent junctions, suggesting a major function in cytoskeleton regulation. By contrast, ACTN4 is more widely distributed in the cell compared to ACTN1 (Honda et al., 1998). In addition to stress fibers, ACTN4 is found in membrane ruffles and inhibition of PI3 kinase (PI3K) promotes ACTN4 nuclear translocation in breast cancer and several cancer cell lines (Araki, Hatae, Yamada, & Hirohashi, 2000; Honda et al., 1998). These observations strongly implicate ACTN4 as a mediator of signal transduction and a regulator of gene expression. Indeed, histological analyses of cancer tissues show a strong correlation between ACTN4 expression and tumorigenesis in several types of cancers, though the detailed mechanism is still elusive (Honda et al., 2005; Welsch et al., 2009; Yamamoto et al., 2007). It was demonstrated that ACTN4 functions as a transcriptional coactivator of nuclear receptors and interacts with other DNA binding transcription factors (Fig. 13.2; Babakov et al., 2008; Hayashida et al., 2005; Jasavala et al., 2007; Khurana, Chakraborty, Cheng, Su, & Kao, 2011; Khurana et al., 2012). Additionally, elevated expression of ACTN4 in cancer cells has been suggested as a biomarker for malignant cell invasion and drug resistance (Fellenberg, Dechant, Ewerbeck, & Mau, 2003; He et al., 2011; Kikuchi et al., 2008; Zhou et al., 2012). In this chapter, we focus on the function of ACTN4 in intracellular signal transduction, especially related to breast cancer tumorigenesis.
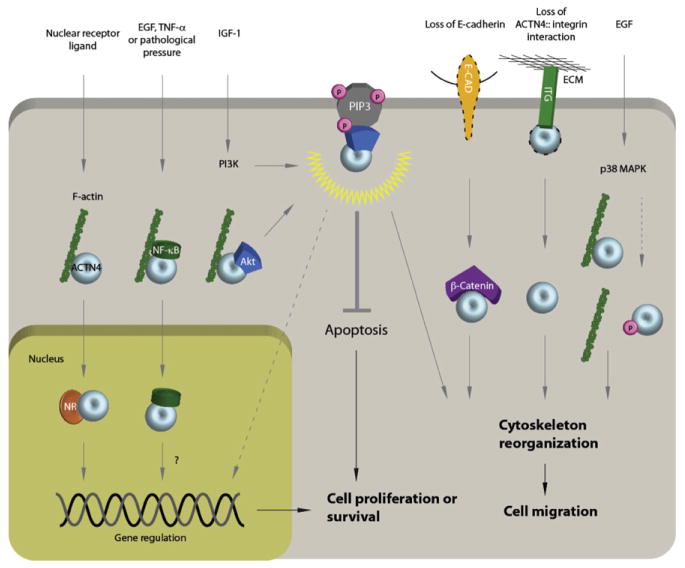
Figure 13.2.
A summary of key ACTN4-associated oncogenic pathways. In the nucleus, ACTN4 functions as a transcriptional coactivator to potentiate expression of nuclear receptor target genes, some of which promote cancer cell proliferation and survival. ACTN4 also interacts with NF-κB and chromatin remodeling factors, but the functional significance of these associations is currently not clear. In the cytosol, activated PI3K induces an association between ACTN4, Akt, and PIP3. Once anchored to PIP3, Akt is activated, thereby inhibiting apoptosis and promoting cell proliferation and migration. As a cytoskeletal protein, the interactions of ACTN4 and its cytoskeletal binding partners are subject to regulation by several signals. For example, loss of E-cadherin in colorectal cancer results in an increase in an ACTN4:β-catenin interaction which subsequently facilitates cytoskeletal reorganization and cancer cell migration. Similarly, disruption of the ACTN4:integrin or ACTN4:F-actin interactions results in acceleration of cytoskeletal reorganization and subsequent increases in cell migration.
2. OVERVIEW OF ACTNs
2.1. The evolutionarily conserved domain organization
All four ACTN family members, ACTN1–4, share a similar structural organization and are known to cross-link F-actin to maintain cell morphology and control cell movement (Otey & Carpen, 2004). The first member of ACTN, ACTN1, was identified 40 years ago because of its abundance in the striated muscle contractile apparatus (Maruyama & Ebashi, 1965). In humans, ACTN2 is preferentially expressed in cardiac and oxidative muscle fiber with some expression in brain, while ACTN3 is mainly detected in type II (fast) muscle fibers (Mills et al., 2001). In contrast, ACTN1 and ACTN4 are ubiquitously expressed with distinct tissue expression patterns, subcellular localizations, and biological functions from the muscle ACTNs (Edlund et al., 2001; Honda et al., 1998; Otey & Carpen, 2004). Although the major biological functions of muscle and nonmuscle ACTNs are different, their overall functional domain organization and F-actin attachment characteristics are conserved (Sheterline, Clayton, & Sparrow, 1995). The full-length protein structure has not been solved, but a schematic depiction of ACTNs can be created based on known structures of the individual domains (Fig. 13.1A; Atkinson et al., 2001; Djinović-Carugo, Young, Gautel, & Saraste, 1999; Franzot, Sjöblom, Gautel, & Djinović Carugo, 2005; Sjöblom et al., 2008; Tang, Taylor, & Taylor, 2001). Each ACTN is composed of three major structural domains, an N-terminal calponin-homology (CH) repeat, a central region consisting of four spectrin repeats (SR), followed by a C-terminal calmodulin (CaM)-like domain consisting of two EF-hand motifs (Sjöblom et al., 2008). The functional F-actin-associated ACTN unit is composed of two antiparallel 90° twisted single peptides (Ylänne, Scheffzek, Young, & Saraste, 2001). Actin-binding domain (ABD) at both ends can bind F-actin. The middle SR domain links the N-terminal ABD domain and C-terminal CaM-like domain to build a complete rod-shaped molecule by two mediate flexible neck structures (Atkinson et al., 2001; Djinovic-Carugo, Gautel, Ylänne, & Young, 2002; Djinović-Carugo et al., 1999; Franzot et al., 2005; Sjöblom et al., 2008). The SR domain typically consists of four SR motifs, named after their homology to the actin-binding spectrin family (Djinovic-Carugo et al., 2002). These provide structural elasticity and mechanical strength for ACTNs (Kusunoki, MacDonald, & Mondragón, 2004; Otey & Carpen, 2004). In addition to its physical function in cytoskeletal architecture, the SR repeats also serve as a protein–protein interaction platform and provide ACTN member-specific functions in signal transduction (Oikonomou et al., 2011; Trulsson et al., 2011; Ylänne et al., 2001). Notably, the conserved negatively charged surface of the ACTN rod has the potential to interact with membrane phospholipids and the cytoplasmic domains of various transmembrane receptors (Fig. 13.1A; Fraley, Pereira, Tran, Singleton, & Greenwood, 2005; Fukami, Sawada, Endo, & Takenawa, 1996; Otey, Pavalko, & Burridge, 1990; Ylänne et al., 2001).
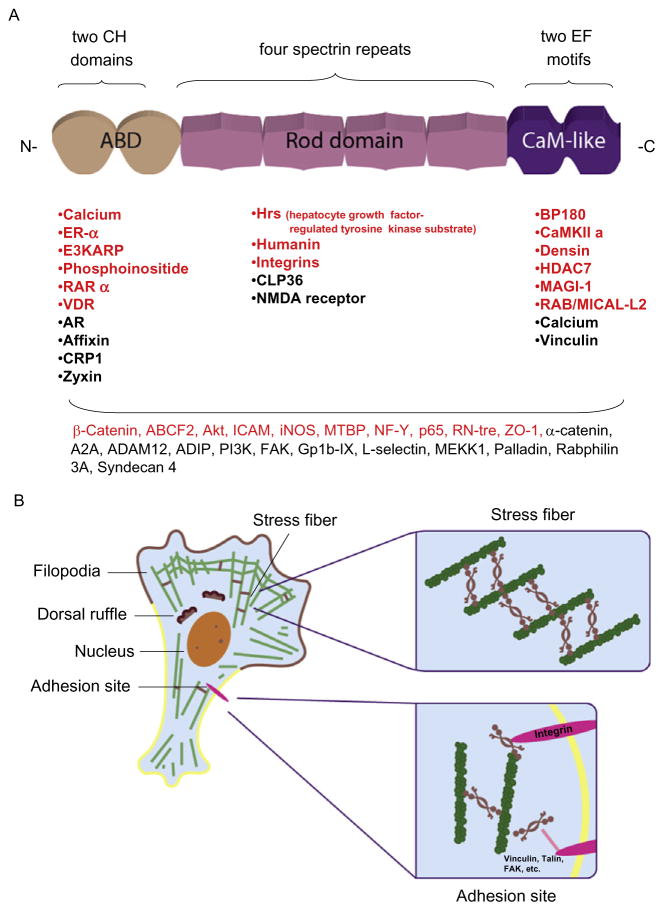
Figure 13.1.
(A) A schematic representation of the structural domains of ACTN. The N-terminal CH repeat constitutes a functional actin-binding domain (ABD), which can also bind to phosphoinositide and calcium. Following the ABD, four SRs form a rod-shaped structure that connects to the EF motif-integrated CaM-like domain. A list of ACTN binding partners is also shown under the corresponding domains. Among these binding partners, the proteins annotated in red are validated ACTN4 binding partners. Shown at the bottom are ACTN binding partners of which the interaction domains were not mapped. (B) A cartoon depicting a moving cell demonstrating the detailed localization of ACTN4 in stress fiber and adhesion sites. F-actin is shown in green and ACTN4 is shown as a brown line or a twisted cherry-like shape. Inside the cells, ACTN4 cross-links with F-actin to form stress fibers or locates in other motile apparatus components or in the nucleus. Close to the focal adhesion sites, ACTN4 directly links integrins with F-actin or other adhesion molecules, such as vinculin or talin.
2.2. Tissue expression and subcellular distribution
In muscle cells, ACTN2 and ACTN3 are predominantly localized in sarcomeric Z-discs (Masaki, Endo, & Ebashi, 1967). They bind F-actin filaments and interact with sarcomeric proteins to cross-link adjacent sarcomeres and maintain the overall cytoskeletal organization during muscle contraction (Squire, 1997). In contrast, nonmuscle ACTN1 and ACTN4 are widely expressed. ACTN4 is expressed at a lower level than ACTN1 in most nonmuscle cells with the exception of kidney cells (Oikonomou et al., 2011). Interestingly, ACTN4 expression is higher in motile cells than in static cells (Araki et al., 2000; Honda et al., 1998). In general, both ACTN1 and ACTN4 localize in stress fibers and focal adhesions. In stress fiber, nonmuscle ACTNs cross-link with F-actin to organize filamentous frame networks which provide a mechanical platform in maintaining cell shape (Fig. 13.1B; Edlund et al., 2001; Pavalko & Burridge, 1991). In focal adhesion sites, the rod-shaped ACTNs cooperate with membrane-associated cytoskeletal proteins including vinculin, talin, tensin, and zyxin to link actin filaments with membrane-bound integrin receptors (Otey & Carpen, 2004; Pavalko et al., 1991, 1995). Although ACTN1 and ACTN4 share approximately 80% and 86% similarity in DNA and protein sequence, respectively, they exhibit different characteristics and functions (Honda et al., 1998). For example, ACTN4 harbors fewer calcium (Ca2+)-binding motifs than ACTN1 and hence the interaction between ACTN1 and F-actin is more sensitive to Ca2+ (Imamura et al., 1994; Nikolopoulos et al., 2000). Additionally, although both ACTN1 and ACTN4 localize in stress fibers and focal contacts, ACTN1 is concentrated in adhesion junctions, whereas ACTN4 predominantly accumulates at dorsal circular ruffles and also can be found in the nucleus (Fig. 13.1B; Araki et al., 2000; Honda et al., 1998; Knudsen, Soler, Johnson, & Wheelock, 1995). This suggests that these two nonmuscle ACTNs may function in distinct signaling and cellular processes.
2.3. ACTN4 regulation and function
The subcellular distribution and binding partners of ACTN1 and ACTN4 strongly suggest that they link signaling and gene regulation to cell movement, the cell cycle, and cell proliferation (Honda et al., 1998; Khurana et al., 2011; Kumeta, Yoshimura, Harata, & Takeyasu, 2010; Otey & Carpen, 2004). The activity of nonmuscle ACTNs is controlled by four major mechanisms, processing by calpain protease, phosphorylation by kinases, binding to phosphatidylinositol intermediates, and Ca2+ binding (Otey & Carpen, 2004).
Calpain, a Ca2+-sensitive protease, predominantly localizes in focal adhesions where it cleaves various focal adhesion proteins, including ACTNs (Carragher, Fincham, Riley, & Frame, 2001; Carragher, Levkau, Ross, & Raines, 1999; Sprague, Fraley, Jang, Lal, & Greenwood, 2008). The calpain-mediated ACTN proteolysis has been implicated in focal contact protein disassembly, which triggers sequential rear adhesion detachment for cell migration (Dourdin et al., 2001; Laukaitis, Webb, Donais, & Horwitz, 2001). Several kinases, including tyrosine receptor kinases and cytoplasmic serine/threonine kinases, interact with ACTNs and phosphorylate it at different sites for various functions (Sjöblom et al., 2008). MEKK1 colocalizes with and binds to ACTNs in stress fibers and focal adhesions to facilitate calpain-mediated ACTNs proteolysis (Christerson, Vanderbilt, & Cobb, 1999; Cuevas et al., 2003). The regulatory subunit of PI3K, p85, also directly binds to ACTNs (Shibasaki, Fukami, Fukui, & Takenawa, 1994). Instead of direct targeting, activated PI3K disrupts ACTN:actin and ACTN:integrin interactions and promotes cytoskeletal remodeling (Fraley et al., 2005; Greenwood, Theibert, Prestwich, & Murphy-Ullrich, 2000). Furthermore, the PI3K downstream kinase, Akt, also interacts with ACTN4 (Ding et al., 2006; Vandermoere et al., 2007). This interaction is required for Akt activation and Akt-mediated cell survival through recruiting Akt to membrane ruffles (Fig. 13.2; Ding et al., 2006). In addition to serine/threonine kinases, integrin-activated focal adhesion kinase (FAK) phosphorylates Y12 on the ABD of ACTN, thereby reducing its affinity with F-actin (Izaguirre et al., 2001). Moreover, phosphorylation of ACTN4 at Y4 and Y31 by an epithelial growth factor (EGF) downstream kinase also reduces ACTN4:F-actin interaction (Fig. 13.2; Shao, Wu, & Wells, 2010). These observations indicate that extracellular signals are capable of regulating cytoskeletal architect by modulating ACTN4:F-actin interaction.
Two intracellular secondary messengers, phosphatidylinositol and Ca2+, bind to ACTN4 via the ABD and EF-hand motifs, respectively (Fukami et al., 1996; Nikolopoulos et al., 2000). Binding of phosphatidylinositol 3,4,5-trisphosphate (PIP3) or phosphatidylinositol 4,5-diphosphate (PIP2) also regulates the susceptibility of ACTNs to calpain-1 and -2-mediated proteolysis (Sprague et al., 2008). However, binding of Ca2+ to ACTN4 blocks its actin-binding activity. Differential Ca2+ sensitivity between ACTN1 and ACTN4 may play a role in tuning ACTN-mediated cytoskeleton organization (Nikolopoulos et al., 2000). Increasing PIP3 production by PDGF-activated PI3K blocks the ACTN4:integrin interaction, thereby disassembling focal adhesion structures (Greenwood et al., 2000). A further study suggested that the abundant PIP2 functions as a regulator to control the dynamics of ACTN:F-actin interaction. However, PI3K transiently produces PIP3 and regulates local ACTN:integrin binding, hence facilitating reorganization of focal adhesions (Fraley et al., 2005; Greenwood et al., 2000).
In addition to its well-established cytoplasmic function, ACTN4 is also found in the nucleus in some types of cells (Honda et al., 1998). Notably, inhibition of PI3K or depolymerization of actin filaments promotes ACTN4 nuclear accumulation, indicating that cytoplasmic signaling controls ACTN4 nuclear translocation and function. In tumor necrosis factor alpha (TNF-α) and EGF-treated epithelial carcinoma, the nuclear translocation of ACTN4 occurs with NF-κB, although the biological significance of this interaction remains elusive (Fig. 13.2; Babakov et al., 2008). Recently, Kumeta et al. (2010) found that the central SR domain of ACTN4 facilitates its nuclear translocation through the nuclear pore complex, whereas the chromosome region maintenance-1 promotes ACTN4 nuclear export. The distribution of cytoplasmic and nuclear ACTN4 is altered during cell cycle progression (Kumeta et al., 2010). In the nucleus, ACTN4 interacts with the INO80 complex and the rRNA transcriptional machinery to regulate a subset cell cycle-related genes (Kumeta et al., 2010). This suggests that ACTN4 also plays a role in cell cycle progression. In addition, ACTN4 is capable of interacting and cooperating with nuclear receptors, including vitamin D receptor, retinoic acid receptor alpha, estrogen receptor alpha (ER-α), and androgen receptor (AR) (Fig. 13.1A; Jasavala et al., 2007; Khurana et al., 2011, 2012).
3. THE FUNCTION OF ACTN4 IN BREAST CANCER
Breast cancer is a complex disease because it can be genetically heterogeneous and contains a variety of cell types. For a number of years, breast cancer researchers have tried to categorize these diverse tumors into different classes and identify distinct biomarkers in each subtype for clinical prognosis and therapeutic targets. Based on their responses to hormones and receptor status, breast cancer can be broadly classified into four major subtypes: (1) luminal A and (2) B breast cancer subtypes that are estrogen receptor (ER)-positive but distinct from different genetic mutation backgrounds; (3) human epidermal growth factor receptor 2 (HER2)-positive breast cancer; (4) basal-like breast cancer that is ER-, HER2-, and progesterone receptor (PR)-triple negative (Perou et al., 2000). Based on this molecular portrait of breast cancer and subsequent studies (Brenton, Carey, Ahmed, & Caldas, 2005; Kao et al., 2009; Perou et al., 2000), ER and HER2 have been considered the two major therapeutic targets for the treatment of breast cancer. The former is a hormone-responsive nuclear receptor that regulates specific genes involved in mammary cell proliferation and survival. Upon estrogen binding to ER in the cytoplasm, it translocates to the nucleus where it binds DNA sequence called estrogen response elements. This ligand-bound ER then recruits coregulators to turn on or off the expression of genes that promote mammary cell differentiation and proliferation. HER2 is a membrane-bound tyrosine kinase receptor possessing mitogenic activity that promotes mammary cell proliferation. Notably, ACTN4 has been shown to potentiate ER transcription activity (Khurana et al., 2011), while the HER2 activator, EGF, is known to modulate ACTN4 function (Shao, Wu, et al., 2010).
ACTN4 was first discovered based on its association with cell motility and breast cancer invasion (Honda et al., 1998). The same report showed that ACTN4 is ubiquitously expressed and was found in virtually every carcinoma they examined (Honda et al., 1998), suggesting that ACTN4 may play an important role in tumorigenesis. In contrast to ACTN1 localization, ACTN4 not only colocalizes in stress fibers but also is dispersed in the cytoplasm and the nucleus in fibroblasts (Fig. 13.1B; Honda et al., 1998). In some cancer cells that lack stress fibers, ACTN4 is distributed in the cytoplasm and accumulates at the edge of cell extended filopodia (Honda et al., 1998). Furthermore, β-catenin, a key oncogenic protein in colorectal cancer tumorigenesis, can bind ACTN4 and their colocalization in cytoplasm is elevated in the infiltrative colorectal cancer (Hayashida et al., 2005). The location, the expression pattern, and the ability of ACTN4 to enhance cell movement imply a function in cancer cell migration and metastasis. Importantly, ACTN4 was found exclusively in the nucleus of several breast and other cancer cell lines (Honda et al., 1998). Nuclear ACTN4 is regulated by PI3K and its abundance in the nucleus correlates with the malignant grade of breast cancer. Indeed, Khurana et al. demonstrated that ACTN4 is a transcriptional coactivator of ER-α, a critical nuclear hormone receptor in breast cancer tumorigenesis (Khurana et al., 2011). Since ACTN4 has a dual function in regulating gene expression and cytoskeletal organization, in this section, we will discuss the detailed mechanisms by which ACTN4 is regulated in breast cancer cells and how it promotes breast cancer proliferation and metastasis, respectively (Fig. 13.2).
3.1. ACTN4 promotes breast cancer proliferation
3.1.1 ACTN4 is a transcriptional coactivator of ER-α
The early observation that ACTN4 was also found in the nucleus of breast cancer cells suggested a role of ACTN4 in the nucleus. The first evidence that ACTN4 plays a role in transcriptional regulation was suggested by the findings that ACTN4 interacts with class II histone deacetylases (HDACs) and myocyte enhancer factor 2s (MEF2s) and functions as a coactivator for MEF2s (Chakraborty et al., 2006). Through sequence analyses, Khurana et al. further demonstrated that ACTN4 harbors a functional nuclear receptor interacting motif (or NR box), LXXLL, within its CH1 domain (Khurana et al., 2011). The ability of ACTN4 to activate ER-α reporter activity and ER-α target gene expression depends on the NR box. In ER-α positive MCF-7 breast cancer cells, estradiol (E2) promotes recruitment of ACTN4 to the promoter of pS2, an ER-α target gene (Khurana et al., 2011).
The fact that ACTN4 regulates ER-α-mediated transcriptional activation suggested that ACTN4 may also play a role in E2-mediated regulation of cell proliferation. Indeed, knockdown of ACTN4 in MCF-7 breast cancer cells significantly decreased E2-mediated induction of ER-α target genes, such as pS2 and PR, and abolished estrogen-mediated cancer cell proliferation (Khurana et al., 2011). Therefore, at least in some breast cancer subtypes, ACTN4 functions as a transcriptional coactivator to potentiate ER-α-mediated gene expression and cell proliferation. Together, these findings established a critical role of ACTN4 in transcriptional regulation and cell proliferation by ER-α.
3.1.2 Histone deacetylase 7 (HDAC7) and ACTN4 in breast cancer proliferation
Through a yeast-two hybrid screen, Chakraborty et al. (2006) isolated ACTN4 as a novel interacting protein bound to transcriptional corepressor HDAC7. HDAC7 belongs to class IIa HDACs known to mediate repression activity of MEF2s, a family of master transcription factors implicated in heart development, blood vessel development, neuron differentiation, and muscle differentiation (Dressel et al., 2001). Further domain-mapping studies indicated that there is a reciprocal protein–protein interaction between, MEF2, HDAC7, and ACTN4. Chakraborty et al. (2006) showed that the N-terminal MADS domain of MEF2s is capable of interacting with HDAC7 or ACTN4, while amino acids 72–172 of HDAC7 interacts with MEF2 or ACTN4. Similarly, the C-terminal CaM-like domain of ACTN4 is required to bind HDAC7 and MEF2. Indeed, a point mutant, L112A in HDAC7 abolished its ability to bind MEF2 and ACTN4 (Chakraborty et al., 2006). Functionally, ectopic expression of wild-type ACTN4, but not the mutant defective in MEF2A or HDAC7 binding, disrupts the association between HDAC7 and MEF2 and abolishes HDAC7-mediated transcription repression on MEF2 target genes (Chakraborty et al., 2006). These data suggest that ACTN4 and MEF2 binding sites on HDAC7 largely overlap. ACTN4 in the nucleus may compete with HDAC7 for MEF2 binding, thereby further relieving the repressive effect of HDAC7 on MEF2-stimulated genes (Chakraborty et al., 2006). MEF2-bound ACTN4 functions as a coactivator to potentiate MEF2-mediated transcription activation. Taken together, these data support a model in which ACTN4 antagonizes HDAC7-mediated MEF2 repression activity by competing with HDAC7 for MEF2 binding. This Ying-Yang effect is reminiscent of corepressor and coactivator action in signal-dependent transcriptional regulation. Indeed, the interplay between HDAC7 and ACTN4 in MEF2 target gene regulation is recapitulated in E2-mediated transcriptional regulation and breast cancer cell proliferation. In MCF-7 breast cancer cells, HDAC7 and ACTN4 associate with ER-α target gene promoters in a largely mutual exclusive pattern such that HDAC7 and ACTN4 do not associate with ER-α target genes simultaneously (Khurana et al., 2011). In contrast to the outcome following ACTN4 knockdown, knockdown of HDAC7 enhances MCF-7 cell growth, releases repression, and further promotes E2 activity for MCF-7 cell proliferation (Khurana et al., 2011). These data provide a model that accounts for elevated expression of ACTN4 observed in some breast cancers. It facilitates the switch from HDAC7-bound ER-α to ACTN4-bound ER-α and thereby contributes to increased cell proliferation.
3.1.3 NF-κB and ACTN4 in breast cancer cell proliferation
The Rel family of transcription factors includes the heterodimeric NF-κB that mediates the activity of multiple signaling pathways regulating cell proliferation, survival, and drug resistance (Ahmed, Cao, & Li, 2006; Piva, Belardo, & Santoro, 2006). Under nonstimulated conditions, the inhibitor of kappa B (I-κB) sequesters NF-κB in the cytoplasm. Signal-induced activation of the I-κB-kinase (IKK) complex promotes ubiquitination-dependent degradation of I-κB, releasing cytoplasmic NF-κB to translocate to the nucleus and bind specific DNA sequences that activate target gene expression (Karin & Ben-Neriah, 2000). In breast cancer, aberrant NF-κB activation has been observed predominantly in ER-α-negative breast cancer cell lines compared to ER-α-positive breast cancer cell lines (Nakshatri, Bhat-Nakshatri, Martin, Goulet, & Sledge, 1997; Pratt et al., 2003). Other studies suggest that NF-κB activation promotes ER-α-negative breast cancer survival, while inhibition of NF-κB in these cells inhibits cancer cell proliferation and induces apoptosis (Biswas et al., 2004; Liu et al., 2003). Although the role of NF-κB in ER-α-positive breast cancer is controversial, ablation of NF-κB activity in ER-α-positive or ER-α-negative breast cancer could be a plausible therapeutic approach for endocrine treatment-resistant breast cancer in the future.
In the cytoplasm, F-actin colocalizes with NF-κB, suggesting a potential mechanismfor theregulation of NF-κB nuclear translocation following cytoskeletal alternations (Are, Galkin, Pospelova, & Pinaev, 2000; Rosette & Karin, 1995). ACTN4 colocalizes with p65/RelA subunit of NF-κB in stress fibers and membrane lamellae in unstimulated epithelial carcinoma cells (Babakov et al., 2008). Upon TNF-α and EGF stimulation, a majority of ACTN4 and p65/RelA cotranslocates into the nucleus (Fig. 13.2; Babakov et al., 2008). Additionally, disassembly of p65/RelA- and ACTN4-associated actin filaments by cytochalasin D also results in nuclear translocation of p65/RelA and ACTN4 (Babakov et al., 2008). While it was proposed that cytoplasmic ACTN4 regulates p65/RelA activity and its nuclear translocation, experimental evidence is still lacking to support this hypothesis.
Since intratumoral and intravascular stress promote cancer cell survival, angiogenesis, and metastasis, Downey et al. proposed that ACTN4, as a stress-sensitive cytoskeletal component, senses the extracellular stress to facilitate malignant cancer cell progression. Indeed, depletion of ACTN4 significantly reduces experimental pressure-induced cell proliferation (Downey, Craig, & Basson, 2011). Furthermore, the experimental pressure enhances ACTN4 and NF-κB association, implying that this interaction has the potential to respond to pathological pressure and promote cancer cell proliferation (Downey et al., 2011). Interestingly, depletion of ACTN4 by siRNA did not block pressure-induced NF-κB activation. In summary, these observations suggest that ACTN4 interacts with NF-κB in response to extracellular stimuli, but the functional significance of this interaction in tumorigenesis warrants further investigation.
3.1.4 ACTN4 and AKT-1 in breast cancer cell proliferation
Akt is a serine/threonine kinase originally identified through its similarity to retroviral oncogene v-Akt (Staal, Hartley, & Rowe, 1977). Overexpression of Akt is often found in breast cancer but not in normal mammary epithelial cells (Stål et al., 2003). A major pathway to activate Akt is through PI3K–Akt axis. Upon extracellular stimuli, activated membrane-bound receptor tyrosine kinases induce PI3K activation and subsequent PIP3 production. The latter is a second lipid messenger that promotes Akt recruitment to cell membranes for activation (Castaneda, Cortes-Funes, Gomez, & Ciruelos, 2010). As described earlier, binding of PIP3 to ACTNs increases their susceptibility to calpain-1 and -2-mediated proteolysis (Sprague et al., 2008). Aberrant activation of Akt is a well-known mechanism for initiating tumorigenesis and subsequent cancer cell survival, and hence targeting Akt is a potential therapy for cancer treatment (Luo, Manning, & Cantley, 2003).
Through a retrovirus-based protein-fragment complementation assay, ACTN4 was shown to interact with Akt1 in mammalian cells (Ding et al., 2006). Under starvation conditions, the ACTN4–Akt complex is dispersed throughout the cytoplasm. Upon serum stimulation in HeLa cells, the complex surrounds nucleus with some preference for membrane ruffles (Ding et al., 2006). The serum-dependent ACTN4–Akt complex membrane translocation requires the presence of the PI3K downstream signal mediator, PIP3 (Fig. 13.2). Interestingly, knockdown of ACTN4 largely reduces Akt membrane translocation and phosphorylation and further enhances the abundance of the cyclin-dependent kinase inhibitor, p27Kip1, which is negatively regulated by Akt (Ding et al., 2006). These observations suggest a sequential signaling relay between PI3K and Akt via ACTN4. The overall effect of ACTN4 depletion results in reduced cell proliferation, at least partially because of a loss of ACTN4-mediated Akt activation. Using a proteomic approach, ACTN4 was also shown to coimmunoprecipitate with Akt by anti-Akt antibodies in MCF-7 breast cancer cells, suggesting that ACTN4–Akt complex-mediated cell growth and survival in HeLa cells can be recapitulated in an ER-α-positive breast cancer cell line (Vandermoere et al., 2007).
3.2. ACTN4 enhances breast cancer metastasis
As an actin-binding protein, it is not surprising that ACTN4 regulates cell motility and cancer metastasis. Histological investigations of ACTN4 expression levels in staged malignant cancers demonstrate a strong correlation between cytoplasmic ACTN4 expression and tumor grade (Honda et al., 1998, 2005; Kikuchi et al., 2008; Koizumi et al., 2010; Yamamoto et al., 2007). In invasive cancers, ACTN4 largely accumulates at the leading edge of invasive front. Knockdown of ACTN4 in several invasive cancers significantly attenuates their migration and metastatic ability (Barbolina et al., 2008; Koizumi et al., 2010; Yamada et al., 2010). Conversely, constitutive expression of ACTN4 in colorectal cancer enhances cell motility and promotes metastasis in a mouse model (Honda et al., 2005). These data support the hypothesis that high ACTN4 expression and its distinct pattern of cytoplasmic accumulation contribute toward a gain in metastatic potential.
In most malignant carcinoma cell lines, including epithelial A431 and invasive breast carcinoma R27, ACTN4 is diffusively distributed in the cytoplasm and cannot be detected in stress fibers since most malignant carcinomas lose substantial cytoskeletal fiber structure (Honda et al., 1998). Accumulation of ACTN4 in focal clusters was observed when cells exhibited an extended morphology poised for movement (Honda et al., 1998). Comparing ER-α-positive MCF-7 and R27, a tamoxifen-resistant MCF-7 subcell type, ACTN4 exhibited a different subcellular distribution pattern in which ACTN4 was detected in the nucleus in MCF-7 and in the cytoplasm in R27 (Honda et al., 1998). However, we observed ACTN4 in both nucleus and cytoplasm in MCF-7 cells (Khurana et al., 2011). Further clinical investigation indicates that ACTN4 predominantly localizes in the nucleus of low-infiltrative breast cancer and shows an exclusively cytoplasmic distribution in invasive mammary lobular carcinoma (Honda et al., 1998). Based on these observations, it was proposed that ACTN4 potentially contributes to cancer metastatic progression and a poor survival rate in patients with breast cancer of the nonnuclear ACTN4 subtype (Honda et al., 1998).
A clear picture of how cytoplasmic ACTN4 controls breast cancer migration and the nature of the upstream stimuli that trigger ACTN4-mediated breast cancer metastasis is still not well established. Metastasis is a complicated process in which distal tumor dissemination occurs after cancer cells in the primary tumor experience epithelial–mesenchymal transition (EMT) to disrupt intercellular junctions, reorganize the actin cytoskeleton, and increase cell motility for initial cancer cell spreading (Sarrió et al., 2008). Several stromal and endocrine growth factors, including insulin-like growth factor I (IGF-1), fibroblast growth factor, EGF, and angiogenin (ANG), regulate ACTN or ACTN4 binding to its cytoskeletal working partners and hence result in cell morphological changes and cytoskeletal reorganization, both of which may facilitate the metastatic process (Guvakova, Adams, & Boettiger, 2002; Shao, Wu, et al., 2010; Vandermoere et al., 2007; Wei, Gao, Du, Su, & Xu, 2011). In most breast cancers, the separation of invasive carcinoma from nearby adherent epithelium is an early step for metastases. Short-term IGF-1 stimulation in MCF-7 breast cancer cells causes cell morphological changes, loss of cell–cell contacts, and subsequent induction of cell separation (Guvakova et al., 2002). In the early transition state, IGF-1 treatment activates IGF-1R downstream PI3K, which disassembles stress fibers and facilitates ACTN and actin reorganization in a PIP3-dependent manner (Fig. 13.2; Guvakova et al., 2002). Both ACTNs and actin accumulate in the newly formed microspike structures at the border of separating cell–cell adherent regions. The scenario of IGF-1-induced ACTN reorganization in breast cancer cell separation resembles the accumulation of cytoplasmic ACTN4 in the protrusion edge of other metastatic cancer cells (Guvakova et al., 2002; Honda et al., 1998, 2005). Upon disruption of cell–cell adhesion, the subsequent cellular cytoskeletal reassembly promotes forward filopodia formation and rear detachment, both of which enhance cell migration. In normal macrophages and highly motile cells, ACTN4 accumulates in circular dorsal ruffles and extended filopodia to trigger cell forward movement (Fig. 13.1B; Araki et al., 2000; Shao, Wang, Pollak, & Wells, 2010). The same is true in mobile cancer cells. Downregulation of ACTN4 in fibroblasts and carcinomas blocks cell filopodia formation and contractility (Agarwal et al., 2013; Shao, Wang, et al., 2010), suggesting that ACTN4 not only increases cell forward spread but also assists cell detachment. Indeed, ACTN4 interacts with integrins, the major adherence receptors, and disruption of ACTN4:integrin interactions facilitates the motile cell detachment step (Kobayashi, Kamiie, Yasuno, Ogihara, & Shirota, 2011; Trulsson et al., 2011). In addition to its involvement in the detachment step in motile cells, the disassembly of ACTN4–integrin complexes may also play an important role in dissociation of cell–cell adhesion and cell–extracellular matrix junction in EMT. Integrins are key components that link extracellular matrix to intercellular contacts (Kobayashi et al., 2011; Pavalko et al., 1991). Along this line, in invasive colorectal cancer, downregulation of E-cadherin, a starting feature in the loss of intercellular contacts enhances β-catenin and ACTN4 interaction that results in their relocation in the protrusion region for cell movement (Fig. 13.2; Hayashida et al., 2005). The EGF induces ACTN4 phosphorylation and reduces its cross-link with actin to facilitate ACTN4-mediated cytoskeleton reorganization (Fig. 13.2; Shao, Wu, et al., 2010). Recently, ANG was reported to interact with ACTN4 and regulate ACTN4-associated cytoskeleton reorganization, focal adhesion, and cell migration (Wei et al., 2011). Taken together, ACTN4 can be viewed as a motility promoter, causing reorganization in the motile apparatus following growth factor exposure, resulting in enhanced cell migration and metastasis.
3.3. ACTN4 potentiates drug resistance for breast cancer therapy
Recent studies suggest that ACTN4 plays a role in drug resistance in cancer therapy. Using a proteomic approach to analyze samples prepared from 39 HER2-positive and triple-negative chemo-resistant breast cancer (TNCBC) patients, He and his colleague found elevated ACTN4 in TNCBC. They proposed that ACTN4 is one of the top 30 proteins associated with neoadjuvant chemotherapy resistance in docetaxel and carboplatin-treated TNCBC (He et al., 2011). Misregulation of ER-α-associated coregulators has been shown to enhance ER-α-positive breast cancer resistance to adjuvant endocrine therapy (Smith, Nawaz, & O’Malley, 1997; Stanya, Liu, Means, & Kao, 2008; Su et al., 2008). In another study, using high-performance liquid chromatography Mass spectrometry, Zhou et al. found that ACTN4 had higher expression levels in tamoxifen-resistant MCF-7 than the parental cells, suggesting that ACTN4 may promote cell mobility or enhance transcription activity to enhance tumor survival against tamoxifen therapy (Zhou et al., 2012).
Induction of several NF-κB target genes enhances cancer survival against radio-, chemo-, and endocrine therapy (Ahmed et al., 2006; Zhou, 2005). For example, activated NF-κB induces the expression of MnSOD, an anti-oxidant enzyme, to promote radiation-induced adaptive responses in radio-resistant MCF-7 cells (Guo et al., 2003). The HER2–PI3K–Akt axis has been shown to potentiate multiple drug resistance in breast cancer cells (Knuefermann et al., 2003), in part, by activating the antiapoptotic Bcl-2 family (Datta et al., 1997; Stål et al., 2003). Notably, overexpression of oncogenic HER2 enhances NF-κB activity via PI3K–Akt by a non-canonical, IKK-independent pathway in mammary gland tumors (Pianetti, Arsura, Romieu-Mourez, Coffey, & Sonenshein, 2001). Inhibition of either PI3K–Akt or NF-κB activity significantly enhances sensitivity of breast cancer cells to small molecule drugs or radiotherapy (Guo et al., 2003; Liang et al., 2003). As mentioned earlier, ACTN4 potentiates Akt function and promotes Akt-mediated cell survival by recruiting Akt to membrane ruffles (Ding et al., 2006; Vandermoere et al., 2007). Moreover, ACTN4 may regulate NF-κB-mediated stress responses (Babakov et al., 2008; Downey et al., 2011). In summary, these observations strongly support the notion that ACTN4 is a potential candidate for drug targeting for the treatment of adjuvant therapy-resistant breast cancer.
4. ACTN4 AND OTHER CANCERS
In addition to breast cancer, ACTN4 is also found aberrantly expressed in many other types of cancers (Table 13.1). Recent studies further suggest its role in the initiation of tumors and subsequent malignancy. The contribution of ACTN4 in other cancers is similar to that described in breast cancer. It enhances cancer cell proliferation, survival, migration, and metastasis. In this section, we discuss ACTN4 in several other cancers. Table 13.1 lists the major findings on oncogenic activity of ACTN4 in ovarian, prostate, colorectal, and pancreas cancers.
Table 13.1.
A summary of known ACTN4 function in various cancers
| Cancer type | Associated oncogenic factor | Observations and proposed function | References |
|---|---|---|---|
| Breast | ER Akt | Transcriptional coactivator; promote cell proliferation, survival, and metastasis | Honda et al. (1998), Khurana et al. (2011), and Vandermoere et al. (2007) |
| Bladder | N.D. | High ACTN4 mRNA and protein levels are found in bladder cancer; knockdown of ACTN4 attenuates bladder cancer invasive ability | Koizumi et al. (2010) |
| Colorectal cancer | β-Catenin | Increased ACTN4 found in 70% of colon cancer; overexpression of ACTN4 elevates colon cancer cell migration and metastasis; loss of epithelial marker, β-cadherin, in colon cancer increases β-catenin–ACTN4 association which may contribute to metastasis | Downey et al. (2011), Hayashida et al. (2005), and Honda et al. (2005) |
| Epithelial carcinoma | NF-κB | NF-κB and ACTN4 interact in the cytoplasm of epithelial cancer; translocate into nucleus upon EGF and TNF-D stimuli | Bolshakova et al. (2007) |
| Esophageal and oral cancer | N.D. | High expression of ACTN4 is found in advanced oral and neck cancers; knockdown of ACTN4 decreases oral carcinoma invasive ability; ACTN4 serves as a metastasis biomarker | Fu et al. (2007), Hatakeyama et al. (2006), and Yamada et al. (2010) |
| Hepatocellular carcinoma | CD81-PI4KIIβ | CD81-PI4KIIβ axis lowers hepatocellular carcinoma cell motility via sequestering ACTN4 in CD81 enriched vesicle | Mazzocca et al. (2008) |
| Glioblastoma | N.D. | Knockdown of ACTN4 alters cytoskeleton organization and reduces glioma motility | Henry et al. (2011) and Sen et al. (2009) |
| Neuroblastoma | N.D. | *Overexpression of ACTN4 in BE(2)-C human neuroblastoma cell line suppresses colony formation, tumor suppressor | *Nikolopoulos et al. (2000) |
| Lung carcinoma | N.D. | *Overexpression of ACTN4 (K122N) in lung carcinoma loses ACTN4 tumor suppression ability | *Menez et al. (2004) |
| Small cell lung cancer | N.D. | Expression of a spliced isoform of ACTN4 is found in patient with small cell lung cancer; may be considered as a diagnostic biomarker | Honda et al. (2004) |
| Ovary | N.D. | High expression of ACTN4 is found in advanced ovary cancer; knockdown of ACTN4 in ovary cancer reduces cell migration ability | Barbolina et al. (2008) and Yamamoto et al. (2007, 2009) |
| Pancreas | N.D. | High expression of ACTN4 correlates with advanced pancreatic cancer; knockdown of ACTN4 in pancreatic cancer reduces cancer invasive growth ability | Kikuchi et al. (2008) and Pan et al. (2009) |
In this table, the reported ACTN4-associated cancers are updated. Asterisks mark the studies in which ACTN4 is proposed to be a tumor suppressor. ACTN4 binding partners, which have been shown to facilitate ACTN4-mediated tumorigenesis, are listed.
4.1. Colorectal cancer
By histochemical analyses, it was found that 70% of tested colorectal cancers showed higher ACTN4 expression compared to normal intestinal epithelium (Honda et al., 2005). Most ACTN4-associated colorectal cancers harbor an aggressive propensity due to their lack of the epithelial marker E-cadherin and differentiated glandular structure (Honda et al., 2005). To further determine the role of ACTN4 in colorectal cancer malignancy, a stable colorectal cancer cell line expressing ACTN4 was established and showed higher extended morphology and migration activity (Honda et al., 2005). Using an animal model and clinical specimens, it was concluded that elevated expression of ACTN4 facilitates colon cancer EMT and promotes metastasis via lymph node colonization (Honda et al., 2005). In a later report, it was shown that the association of ACTN4 and β-catenin is regulated by E-cadherin (Hayashida et al., 2005). In E-cadherin-positive non-infiltrative colorectal cancer, the colocalization of ACTN4 and β-catenin was mainly observed in the nucleus. However, it was localized to membrane ruffles when E-cadherin was knocked down (Hayashida et al., 2005). In addition, elevated ACTN4 increased β-catenin colocalization at the leading edge of protrusion in DLD-1 colorectal cancer (Hayashida et al., 2005). In clinical colorectal cancer tissues, the subcellular distribution of β-catenin and ACTN4 was similar to those observed in aggressive cancer cell lines and showed overlap around membrane ruffles in the leading invasive border (Hayashida et al., 2005). In summary, ACTN4 plays an important role in the promotion of colorectal cancer cell migration through E-cadherin-and β-catenin-associated pathways. It may control cell proliferation by affecting β-catenin’s subcellular localization and function.
4.2. Ovarian cancer
Ovarian epithelial carcinoma is a major type of ovary cancer that shows a poor prognosis and outcome for patient therapy (Shih & Kurman, 2004). Increased expression of cytoplasmic ACTN4 has been observed in high-grade ovarian cancer in tissue microarray analyses (Yamamoto et al., 2007). Similar to those described in breast cancer, predominant nuclear ACTN4 is found in low-grade ovarian tumor (Yamamoto et al., 2007). These data imply that the role of ACTN4 in cytoskeleton assembly is highly correlated with ovarian cancer metastasis. Knockdown of ACTN4 in epithelial ovarian carcinoma resulted in significantly decreased cell migration (Barbolina et al., 2008). It was hypothesized that high expression of ACTN4 in ovarian cancer may result from the duplicated amplicon including ACTN4 in chromosome 19 of those advanced cancer cells (Yamamoto et al., 2009). In summary, current data support a role of ACTN4 in ovarian cancer metastasis, but the direct link and detailed mechanisms require further investigation.
4.3. Pancreatic cancer
Pancreatic cancer causes a higher mortality compared to other types of cancer but shows a better survival rate when surgery or other interventions are executed at an early stage. The etiology of this indocile tumor is still mysterious. Therefore, it is important to establish biomarkers to serve as early prognostic indicators. Histological and proteomic studies have indicated that ACTN4 is highly expressed in malignant pancreatic tissue and the surrounding stroma (Kikuchi et al., 2008; Pan et al., 2009). The increased expression of ACTN4 in pancreatic tumors is, in part, caused by the amplification of ACTN4 gene on chromosome 19 (Kikuchi et al., 2008). Injection of BxPC3 pancreatic cancer cells after ACTN4 knockdown in nude mice showed lower invasive growth compared to the control group (Kikuchi et al., 2008), indicating a role of ACTN4 in promoting pancreatic cancer invasiveness and proliferation. In total, these findings provide a strong rationale to understand ACTN4’s function in pancreatic tumorigenesis as a potential prognostic marker and for drug development.
4.4. Prostate cancer
Two groups have reported opposite activity of ACTN4 in the regulation of prostate cancer cells. In a study by Hara et al. (2007), ACTN4 expression was found to be lower in most prostate cancers than in normal prostate epithelial cells. Using a colony formation assay, they observed that over-expression of ACTN4 in androgen-independent prostate cancers, such as 22RV1 and PC-3, decreased colony formation, indicating that ACTN4 possesses tumor suppressor activity in these cells. These authors proposed that ACTN4 tumor suppression activity is related to its ability to regulate endocytosis. Surprisingly, Jasavala et al. (2007) found that ACTN4 showed high expression in malignant prostate tissue and exhibited distinct cytoplasmic and nuclear localization patterns during tumor progression. They further demonstrated that ACTN4 interacts with AR, a major nuclear receptor critical for prostate development and tumorigenesis, and functions as a coregulator to control AR transactivation activity (Jasavala et al., 2007). Therefore, it is likely that ACTN4 contributes to prostate tumorigenesis due to its coactivator function in AR-dependent transcriptional regulation. Whether the conflicting conclusions on ACTN4 in prostate cancer are due to the examinations in different prostate cancer types requires further experiments.
5. CONCLUSION AND FUTURE DIRECTION
Breast cancer is the most common cancer among women and ranked as the second leading cause of death in women among all cancers, except lung cancer (http://www.cdc.gov/cancer/breast/). While intensive efforts have been devoted to developing therapeutic agents targeting known breast cancer oncoproteins, such as ER-α and HER2, it is well established that some patients will develop resistance to these drugs (Osborne & Schiff, 2011; Pohlmann, Mayer, & Mernaugh, 2009). Therefore, identifying new drug targets and understanding the mechanisms underlying drug resistance are critical to combat drug-resistant breast cancer. ACTN4 seems to be one such drug target.
Since ACTN4 has the ability to bind ER-α, oncogenic transcription factors and chromatin remodeling factors (Fig. 13.1A; Babakov et al., 2008; Chakraborty et al., 2006; Downey et al., 2011; Jasavala et al., 2007; Khurana et al., 2011), it may function as a transcription coactivator to promote cell proliferation via induction of antiapoptotic, proproliferation, and prosurvival genes (Fig. 13.2). Transcriptional coactivators in breast cancer are known to play a role in adjuvant endocrine therapy resistance (Smith et al., 1997; Stanya et al., 2008; Su et al., 2008). Therefore, it might be beneficial in cancer therapy if one can identify ACTN4 binding partners related to breast cancer and exploit these interactions. There is substantial evidence that knockdown of ACTN4 decreases cancer cell migration and lowers cancer metastasis (Table 13.1; Barbolina et al., 2008; Koizumi et al., 2010; Yamada et al., 2010). However, few reports identify upstream signals in breast cancer that are important for stimulating ACTN4 activity to increase cell migration. To this end, the PI3K–Akt axis has been shown to induce ACTN4-mediated cytoskeletal reorganization which subsequently facilitates cell migration (Fig. 13.2; Honda et al., 1998; Sprague et al., 2008). Additionally, FAK or EGF downstream kinases may regulate the ACTN4:F-actin interaction to increase cell migration (Izaguirre et al., 2001; Shao, Wu, et al., 2010). Although these two pathways are known to promote ACTN4-mediated cell migration, the upstream signaling in breast cancer cells that drives this process still needs validation.
While several studies have proposed that ACTN4 participates in cancer resistance to adjuvant therapy (He et al., 2011; Zhou et al., 2012), direct evidence of its role in cancer cell survival is still missing. Further investigation into the function of ACTN4 in cell survival may help the development of therapeutic agents that alleviate drug resistance.
In summary, ACTN4 has dual functions that may contribute to cancer cell proliferation and migration due to its localization in both the nucleus and cytoplasm. Histologic data suggest that accumulation of ACTN4 in the leading edge of cancer cell protrusion coincides with cancer malignant transition (Hayashida et al., 2005; Honda et al., 1998, 2005). Along this line, we hypothesize a role of nuclear ACTN4 as an initiator of early benign tumor formation and that shuttling to the cytoplasm facilitates late malignant stages such as metastasis. As such, understanding the mechanisms underlying nucleocytoplasmic shuttling of ACTN4 is of particular importance.
Finally, although the majority of studies indicate that ACTN4 is a proto-oncogene (Table 13.1), several reports have suggested that ACTN4 suppresses tumor formation. This is particularly true in prostate cancer and neuroblastoma (Hara et al., 2007; Nikolopoulos et al., 2000). This issue requires an in-depth investigation in order to clarify these conflicting conclusions.
Acknowledgments
We thank Dr. Samols for discussion and Sandy Gu for her assistance in graphic art. This work was supported by National Institutes of Health, RO1 DK078965, and HL093269 to H. -Y. K.
References
- Agarwal N, Adhikari AS, Iyer SV, Hekmatdoost K, Welch DR, Iwakuma T. MTBP suppresses cell migration and filopodia formation by inhibiting ACTN4. Oncogene. 2013;32:462–470. doi: 10.1038/onc.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed KM, Cao N, Li JJ. HER-2 and NF-κB as the targets for therapy-resistant breast cancer. Anticancer Research. 2006;26:4235–4243. [PMC free article] [PubMed] [Google Scholar]
- Araki N, Hatae T, Yamada T, Hirohashi S. Actinin-4 is preferentially involved in circular ruffling and macropinocytosis in mouse macrophages: Analysis by fluorescence ratio imaging. Journal of Cell Science. 2000;113:3329–3340. doi: 10.1242/jcs.113.18.3329. [DOI] [PubMed] [Google Scholar]
- Are AF, Galkin VE, Pospelova TV, Pinaev GP. The p65/RelA subunit of NF-kappaB interacts with actin-containing structures. Experimental Cell Research. 2000;256:533–544. doi: 10.1006/excr.2000.4830. [DOI] [PubMed] [Google Scholar]
- Arimura C, Suzuki T, Yanagisawa M, Imamura M, Hamada Y, Masaki T. Primary structure of chicken skeletal muscle and fibroblast alpha-actinins deduced from cDNA sequences. European Journal of Biochemistry. 1988;177:649–655. doi: 10.1111/j.1432-1033.1988.tb14419.x. [DOI] [PubMed] [Google Scholar]
- Atkinson RA, Joseph C, Kelly G, Muskett FW, Frenkiel TA, Nietlispach D, et al. Ca2+-independent binding of an EF-hand domain to a novel motif in the alpha-actinin-titin complex. Nature Structural Biology. 2001;8:853–857. doi: 10.1038/nsb1001-853. [DOI] [PubMed] [Google Scholar]
- Babakov VN, Petukhova OA, Turoverova LV, Kropacheva IV, Tentler DG, Bolshakova AV, et al. RelA/NF-κB transcription factor associates with α-actinin-4. Experimental Cell Research. 2008;314:1030–1038. doi: 10.1016/j.yexcr.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Barbolina MV, Adley BP, Kelly DL, Fought AJ, Scholtens DM, Shea LD, et al. Motility-related actinin alpha-4 is associated with advanced and metastatic ovarian carcinoma. Laboratory Investigation. 2008;88:602–614. doi: 10.1038/labinvest.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs AH, Byers TJ, Knoll JH, Boyce FM, Bruns GA, Kunkel LM. Cloning and characterization of two human skeletal muscle alpha-actinin genes located on chromosomes 1 and 11. Journal of Biological Chemistry. 1992;267:9281–9288. [PubMed] [Google Scholar]
- Biswas DK, Shi Q, Baily S, Strickland I, Ghosh S, Pardee AB, et al. NF-κB activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10137–10142. doi: 10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakova A, Petukhova O, Turoverova L, Tentler D, Babakov V, Magnusson KE, et al. Extra-cellular matrix proteins induce re-distribution of alpha-actinin-1 and alpha-actinin-4 in A431 cells. Cell Biology International. 2007;31:360–365. doi: 10.1016/j.cellbi.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: Ready for clinical application? Journal of Cell Oncology. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- Carragher NO, Fincham VJ, Riley D, Frame MC. Cleavage of focal adhesion kinase by different proteases during src-regulated transformation and apoptosis. Distinct roles for calpain and caspases. Journal of Biological Chemistry. 2001;276:4270–4275. doi: 10.1074/jbc.M008972200. [DOI] [PubMed] [Google Scholar]
- Carragher NO, Levkau B, Ross R, Raines EW. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125FAK, Paxillin, and Talin. The Journal of Cell Biology. 1999;147:619–630. doi: 10.1083/jcb.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda C, Cortes-Funes H, Gomez H, Ciruelos E. The phosphatidyl inositol 3-kinase/AKT signaling pathway in breast cancer. Cancer and Metastasis Reviews. 2010;29:751–759. doi: 10.1007/s10555-010-9261-0. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Reineke EL, Lam M, Li X, Liu Y, Gao C, et al. α-Actinin 4 potentiates myocyte enhancer factor-2 transcription activity by antagonizing histone deacetylase 7. Journal of Biological Chemistry. 2006;281:35070–35080. doi: 10.1074/jbc.M602474200. [DOI] [PubMed] [Google Scholar]
- Christerson LB, Vanderbilt CA, Cobb MH. MEKK1 interacts with α-actinin and localizes to stress fibers and focal adhesions. Cell Motility and the Cytoskeleton. 1999;43:186–198. doi: 10.1002/(SICI)1097-0169(1999)43:3<186::AID-CM2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Cuevas BD, Abell AN, Witowsky JA, Yujiri T, Johnson NL, Kesavan K, et al. MEKK1 regulates calpain-dependent proteolysis of focal adhesion proteins for rear-end detachment of migrating fibroblasts. EMBO Journal. 2003;22:3346–3355. doi: 10.1093/emboj/cdg322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Ding Z, Liang J, Lu Y, Yu Q, Songyang Z, Lin SY, et al. A retrovirus-based protein complementation assay screen reveals functional AKT1-binding partners. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15014–15019. doi: 10.1073/pnas.0606917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djinovic-Carugo K, Gautel M, Ylänne J, Young P. The spectrin repeat: A structural platform for cytoskeletal protein assemblies. FEBS Letters. 2002;513:119–123. doi: 10.1016/s0014-5793(01)03304-x. [DOI] [PubMed] [Google Scholar]
- Djinović-Carugo K, Young P, Gautel M, Saraste M. Structure of the alpha-actinin rod: Molecular basis for cross-linking of actin filaments. Cell. 1999;98:537–546. doi: 10.1016/s0092-8674(00)81981-9. [DOI] [PubMed] [Google Scholar]
- Dourdin N, Bhatt AK, Dutt P, Greer PA, Arthur JSC, Elce JS, et al. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. Journal of Biological Chemistry. 2001;276:48382–48388. doi: 10.1074/jbc.M108893200. [DOI] [PubMed] [Google Scholar]
- Downey C, Craig DH, Basson MD. Isoform-specific modulation of pressure-stimulated cancer cell proliferation and adhesion by α-actinin. American Journal of Surgery. 2011;202:520–523. doi: 10.1016/j.amjsurg.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel U, Bailey PJ, Wang SCM, Downes M, Evans RM, Muscat GEO. A dynamic role for HDAC7 in MEF2-mediated muscle differentiation. Journal of Biological Chemistry. 2001;276:17007–17013. doi: 10.1074/jbc.M101508200. [DOI] [PubMed] [Google Scholar]
- Edlund M, Lotano MA, Otey CA. Dynamics of α-actinin in focal adhesions and stress fibers visualized with α-actinin-green fluorescent protein. Cell Motility and the Cytoskeleton. 2001;48:190–200. doi: 10.1002/1097-0169(200103)48:3<190::AID-CM1008>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Fellenberg J, Dechant MJ, Ewerbeck V, Mau H. Identification of drug-regulated genes in osteosarcoma cells. International Journal of Cancer. 2003;105:636–643. doi: 10.1002/ijc.11135. [DOI] [PubMed] [Google Scholar]
- Fraley TS, Pereira CB, Tran TC, Singleton C, Greenwood JA. Phosphoinositide binding regulates α-actinin dynamics. Mechanism for modulating cytoskeletal remodeling. Journal of Biological Chemistry. 2005;280:15479–15482. doi: 10.1074/jbc.M500631200. [DOI] [PubMed] [Google Scholar]
- Franzot G, Sjöblom B, Gautel M, Djinović Carugo K. The crystal structure of the actin binding domain from alpha-actinin in its closed conformation: Structural insight into phospholipid regulation of alpha-actinin. Journal of Molecular Biology. 2005;348:151–165. doi: 10.1016/j.jmb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Fu L, Qin YR, Xie D, Chow HY, Ngai SM, Kwong DLW, et al. Identification of alpha-actinin 4 and 67 kDa laminin receptor as stage-specific markers in esophageal cancer via proteomic approaches. Cancer. 2007;110:2672–2681. doi: 10.1002/cncr.23110. [DOI] [PubMed] [Google Scholar]
- Fukami K, Sawada N, Endo T, Takenawa T. Identification of a phosphatidylinositol 4,5-bisphosphate-binding site in chicken skeletal muscle α-actinin. Journal of Biological Chemistry. 1996;271:2646–2650. doi: 10.1074/jbc.271.5.2646. [DOI] [PubMed] [Google Scholar]
- Fyrberg E, Kelly M, Ball E, Fyrberg C, Reedy MC. Molecular genetics of Drosophila alpha-actinin: Mutant alleles disrupt Z disc integrity and muscle insertions. The Journal of Cell Biology. 1990;110:1999–2011. doi: 10.1083/jcb.110.6.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood JA, Theibert AB, Prestwich GD, Murphy-Ullrich JE. Restructuring of focal adhesion plaques by Pi 3-kinase regulation by PtdIns (3,4,5-P) 3 binding to α-actinin. The Journal of Cell Biology. 2000;150:627–642. doi: 10.1083/jcb.150.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, et al. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Molecular and Cellular Biology. 2003;23:2362–2378. doi: 10.1128/MCB.23.7.2362-2378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvakova MA, Adams JC, Boettiger D. Functional role of α-actinin, PI 3-kinase and MEK1/2 in insulin-like growth factor I receptor kinase regulated motility of human breast carcinoma cells. Journal of Cell Science. 2002;115:4149–4165. doi: 10.1242/jcs.00104. [DOI] [PubMed] [Google Scholar]
- Hara T, Honda K, Shitashige M, Ono M, Matsuyama H, Naito K, et al. Mass spectrometry analysis of the native protein complex containing actinin-4 in prostate cancer cells. Molecular & Cellular Proteomics. 2007;6:479–491. doi: 10.1074/mcp.M600129-MCP200. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Kondo T, Fujii K, Nakanishi Y, Kato H, Fukuda S, et al. Protein clusters associated with carcinogenesis, histological differentiation and nodal metastasis in esophageal cancer. Proteomics. 2006;6:6300–6316. doi: 10.1002/pmic.200600488. [DOI] [PubMed] [Google Scholar]
- Hayashida Y, Honda K, Idogawa M, Ino Y, Ono M, Tsuchida A, et al. E-Cadherin regulates the association between β-Catenin and Actinin-4. Cancer Research. 2005;65:8836–8845. doi: 10.1158/0008-5472.CAN-05-0718. [DOI] [PubMed] [Google Scholar]
- He J, Whelan SA, Lu M, Shen D, Chung DU, Saxton RE, et al. Proteomic-based biosignatures in breast cancer classification and prediction of therapeutic response. International Journal of Proteomics. 2011;2011:1–16. doi: 10.1155/2011/896476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry WI, Dubois J, Quick QA. The microtubule inhibiting agent epothilone B antagonizes glioma cell motility associated with reorganization of the actin-binding protein α-actinin 4. Oncology Reports. 2011;25:887–893. doi: 10.3892/or.2011.1145. [DOI] [PubMed] [Google Scholar]
- Honda K, Yamada T, Endo R, Ino Y, Gotoh M, Tsuda H, et al. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. The Journal of Cell Biology. 1998;140:1383–1393. doi: 10.1083/jcb.140.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Yamada T, Seike M, Hayashida Y, Idogawa M, Kondo T, et al. Alternative splice variant of actinin-4 in small cell lung cancer. Oncogene. 2004;23:5257–5262. doi: 10.1038/sj.onc.1207652. [DOI] [PubMed] [Google Scholar]
- Honda K, Yamada T, Hayashida Y, Idogawa M, Sato S, Hasegawa F, et al. Actinin-4 increases cell motility and promotes lymph node metastasis of colorectal cancer. Gastroenterology. 2005;128:51–62. doi: 10.1053/j.gastro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Imamura M, Sakurai T, Ogawa Y, Ishikawa T, Goto K, Masaki T. Molecular cloning of low-Ca(2+)-sensitive-type non-muscle alpha-actinin. European Journal of Biochemistry. 1994;223:395–401. doi: 10.1111/j.1432-1033.1994.tb19006.x. [DOI] [PubMed] [Google Scholar]
- Izaguirre G, Aguirre L, Hu YP, Lee HY, Schlaepfer DD, Aneskievich BJ, et al. The cytoskeletal/non-muscle isoform of α-actinin is phosphorylated on its actin-binding domain by the focal adhesion kinase. Journal of Biological Chemistry. 2001;276:28676–28685. doi: 10.1074/jbc.M101678200. [DOI] [PubMed] [Google Scholar]
- Jasavala R, Martinez H, Thumar J, Andaya A, Gingras AC, Eng JK, et al. Identification of putative androgen receptor interaction protein modules cytoskeleton and endosomes modulate androgen receptor signaling in prostate cancer cells. Molecular & Cellular Proteomics. 2007;6:252–271. doi: 10.1074/mcp.M600169-MCP200. [DOI] [PubMed] [Google Scholar]
- Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annual Review of Immunology. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Khurana S, Chakraborty S, Cheng X, Su YT, Kao HY. The actin-binding protein, actinin alpha 4 (ACTN4), is a nuclear receptor coactivator that promotes proliferation of MCF-7 breast cancer cells. Journal of Biological Chemistry. 2011;286:1850–1859. doi: 10.1074/jbc.M110.162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S, Chakraborty S, Lam M, Liu Y, Su YT, Zhao X, et al. Familial focal segmental glomerulosclerosis (FSGS)-linked α-actinin 4 (ACTN4) protein mutants lose ability to activate transcription by nuclear hormone receptors. Journal of Biological Chemistry. 2012;287:12027–12035. doi: 10.1074/jbc.M112.345421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S, Honda K, Tsuda H, Hiraoka N, Imoto I, Kosuge T, et al. Expression and gene amplification of actinin-4 in invasive ductal carcinoma of the pancreas. Clinical Cancer Research. 2008;14:5348–5356. doi: 10.1158/1078-0432.CCR-08-0075. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. The Journal of Cell Biology. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuefermann C, Lu Y, Liu B, Jin W, Liang K, Wu L, et al. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22:3205–3212. doi: 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]
- Kobayashi R, Kamiie J, Yasuno K, Ogihara K, Shirota K. Expression of nephrin, podocin, α-actinin-4 and α3-integrin in canine renal glomeruli. Journal of Comparative Pathology. 2011;145:220–225. doi: 10.1016/j.jcpa.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Koizumi T, Nakatsuji H, Fukawa T, Avirmed S, Fukumori T, Takahashi M, et al. The role of actinin-4 in bladder cancer invasion. Urology. 2010;75:357–364. doi: 10.1016/j.urology.2009.09.037. [DOI] [PubMed] [Google Scholar]
- Kumeta M, Yoshimura SH, Harata M, Takeyasu K. Molecular mechanisms underlying nucleocytoplasmic shuttling of actinin-4. Journal of Cell Science. 2010;123:1020–1030. doi: 10.1242/jcs.059568. [DOI] [PubMed] [Google Scholar]
- Kusunoki H, MacDonald RI, Mondragón A. Structural insights into the stability and flexibility of unusual erythroid spectrin repeats. Structure. 2004;12:645–656. doi: 10.1016/j.str.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of α5 integrin, paxillin, and α-actinin during formation and disassembly of adhesions in migrating cells. The Journal of Cell Biology. 2001;153:1427–1440. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Jin W, Knuefermann C, Schmidt M, Mills GB, Ang KK, et al. Targeting the phosphatidylinositol 3-kinase/Akt pathway for enhancing breast cancer cells to radiotherapy1. Molecular Cancer Therapeutics. 2003;2:353–360. [PubMed] [Google Scholar]
- Liu H, Lee ES, Gajdos C, Pearce ST, Chen B, Osipo C, et al. Apoptotic action of 17β-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. Journal of the National Cancer Institute. 2003;95:1586–1597. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]
- Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Ebashi S. α-Actinin, a new structural protein from striated muscle II. Action on actin. Journal of Biochemistry. 1965;58:13–19. doi: 10.1093/oxfordjournals.jbchem.a128158. [DOI] [PubMed] [Google Scholar]
- Masaki T, Endo M, Ebashi S. Localization of 6S component of a alpha-actinin at Z-band. Journal of Biochemistry. 1967;62:630–632. doi: 10.1093/oxfordjournals.jbchem.a128717. [DOI] [PubMed] [Google Scholar]
- Mazzocca A, Liotta F, Carloni V. Tetraspanin CD81-regulated cell motility plays a critical role in intrahepatic metastasis of hepatocellular carcinoma. Gastroenterology. 2008;135:244–256. e1. doi: 10.1053/j.gastro.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Menez J, Chansac BLM, Dorothée G, Vergnon I, Jalil A, Carlier MF, et al. Mutant alpha-actinin-4 promotes tumorigenicity and regulates cell motility of a human lung carcinoma. Oncogene. 2004;23:2630–2639. doi: 10.1038/sj.onc.1207347. [DOI] [PubMed] [Google Scholar]
- Mills M, Yang N, Weinberger R, Woude DLV, Beggs AH, Easteal S, et al. Differential expression of the actin-binding proteins, α-actinin-2 and −3, in different species: Implications for the evolution of functional redundancy. Human Molecular Genetics. 2001;10:1335–1346. doi: 10.1093/hmg/10.13.1335. [DOI] [PubMed] [Google Scholar]
- Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Sledge GW. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Molecular and Cellular Biology. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, Spengler BA, Kisselbach K, Evans AE, Biedler JL, Ross RA. The human non-muscle alpha-actinin protein encoded by the ACTN4 gene suppresses tumorigenicity of human neuroblastoma cells. Oncogene. 2000;19:380–386. doi: 10.1038/sj.onc.1203310. [DOI] [PubMed] [Google Scholar]
- Oikonomou KG, Zachou K, Dalekos GN. Alpha-actinin: A multidisciplinary protein with important role in B-cell driven autoimmunity. Autoimmunity Reviews. 2011;10:389–396. doi: 10.1016/j.autrev.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annual Review of Medicine. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey CA, Carpen O. α-Actinin revisited: A fresh look at an old player. Cell Motility and the Cytoskeleton. 2004;58:104–111. doi: 10.1002/cm.20007. [DOI] [PubMed] [Google Scholar]
- Otey CA, Pavalko FM, Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. The Journal of Cell Biology. 1990;111:721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Chen R, Reimel BA, Crispin DA, Mirzaei H, Cooke K, et al. Quantitative proteomics investigation of pancreatic intraepithelial neoplasia. Electrophoresis. 2009;30:1132–1144. doi: 10.1002/elps.200800752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Burridge K. Disruption of the actin cytoskeleton after microinjection of proteolytic fragments of alpha-actinin. The Journal of Cell Biology. 1991;114:481–491. doi: 10.1083/jcb.114.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Otey CA, Simon KO, Burridge K. Alpha-actinin: A direct link between actin and integrins. Biochemical Society Transactions. 1991;19:1065–1069. doi: 10.1042/bst0191065. [DOI] [PubMed] [Google Scholar]
- Pavalko FM, Walker DM, Graham L, Goheen M, Doerschuk CM, Kansas GS. The cytoplasmic domain of L-selectin interacts with cytoskeletal proteins via alpha-actinin: Receptor positioning in microvilli does not require interaction with alpha-actinin. The Journal of Cell Biology. 1995;129:1155–1164. doi: 10.1083/jcb.129.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Pianetti S, Arsura M, Romieu-Mourez R, Coffey RJ, Sonenshein GE. Her-2/neu overexpression induces NF-kappaB via a PI3-kinase/Akt pathway involving calpain-mediated degradation of IkappaB-alpha that can be inhibited by the tumor suppressor PTEN. Oncogene. 2001;20:1287–1299. doi: 10.1038/sj.onc.1204257. [DOI] [PubMed] [Google Scholar]
- Piva R, Belardo G, Santoro MG. NF-kappaB: A stress-regulated switch for cell survival. Antioxidants & Redox Signaling. 2006;8:478–486. doi: 10.1089/ars.2006.8.478. [DOI] [PubMed] [Google Scholar]
- Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clinical Cancer Research. 2009;15:7479–7491. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt MAC, Bishop TE, White D, Yasvinski G, Ménard M, Niu MY, et al. Estrogen withdrawal-induced NF-κB activity and Bcl-3 expression in breast cancer cells: Roles in growth and hormone independence. Molecular and Cellular Biology. 2003;23:6887–6900. doi: 10.1128/MCB.23.19.6887-6900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick Q, Skalli O. α-Actinin 1 and α-actinin 4: Contrasting roles in the survival, motility, and RhoA signaling of astrocytoma cells. Experimental Cell Research. 2010;316:1137–1147. doi: 10.1016/j.yexcr.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Rosette C, Karin M. Cytoskeletal control of gene expression: Depolymerization of microtubules activates NF-kappa B. The Journal of Cell Biology. 1995;128:1111–1119. doi: 10.1083/jcb.128.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrió D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Research. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- Sen S, Dong M, Kumar S. Isoform-Specific Contributions of α-Actinin to Glioma Cell Mechanobiology. PLoS One. 2009;4:e8427. doi: 10.1371/journal.pone.0008427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Wang JH, Pollak MR, Wells A. α-Actinin-4 is essential for maintaining the spreading, motility and contractility of fibroblasts. PLoS One. 2010;5:e13921. doi: 10.1371/journal.pone.0013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Wu C, Wells A. Phosphorylation of α-actinin 4 upon epidermal growth factor exposure regulates its interaction with actin. Journal of Biological Chemistry. 2010;285:2591–2600. doi: 10.1074/jbc.M109.035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheterline P, Clayton J, Sparrow J. Actin. Protein Profile. 1995;2:1–103. [PubMed] [Google Scholar]
- Shibasaki F, Fukami K, Fukui Y, Takenawa T. Phosphatidylinositol 3-kinase binds to alpha-actinin through the p85 subunit. Biochemical Journal. 1994;302:551–557. doi: 10.1042/bj3020551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih IM, Kurman RJ. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. American Journal of Pathology. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöblom B, Salmazo A, Djinović-Carugo K. Alpha-actinin structure and regulation. Cellular and Molecular Life Sciences. 2008;65:2688–2701. doi: 10.1007/s00018-008-8080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Nawaz Z, O’Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Molecular Endocrinology. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- Sprague CR, Fraley TS, Jang HS, Lal S, Greenwood JA. Phosphoinositide binding to the substrate regulates susceptibility to proteolysis by calpain. Journal of Biological Chemistry. 2008;283:9217–9223. doi: 10.1074/jbc.M707436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire JM. Architecture and function in the muscle sarcomere. Current Opinion in Structural Biology. 1997;7:247–257. doi: 10.1016/s0959-440x(97)80033-4. [DOI] [PubMed] [Google Scholar]
- Staal SP, Hartley JW, Rowe WP. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:3065–3067. doi: 10.1073/pnas.74.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stål O, Pérez-Tenorio G, Akerberg L, Olsson B, Nordenskjöld B, Skoog L, et al. Akt kinases in breast cancer and the results of adjuvant therapy. Breast Cancer Research. 2003;5:R37–R44. doi: 10.1186/bcr569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanya KJ, Liu Y, Means AR, Kao HY. Cdk2 and Pin1 negatively regulate the transcriptional corepressor SMRT. The Journal of Cell Biology. 2008;183:49–61. doi: 10.1083/jcb.200806172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q, Hu S, Gao H, Ma R, Yang Q, Pan Z, et al. Role of AIB1 for tamoxifen resistance in estrogen receptor-positive breast cancer cells. Oncology. 2008;75:159–168. doi: 10.1159/000159267. [DOI] [PubMed] [Google Scholar]
- Tang J, Taylor DW, Taylor KA. The three-dimensional structure of alpha-actinin obtained by cryoelectron microscopy suggests a model for Ca(2+)-dependent actin binding. Journal of Molecular Biology. 2001;310:845–858. doi: 10.1006/jmbi.2001.4789. [DOI] [PubMed] [Google Scholar]
- Trulsson M, Yu H, Gisselsson L, Chao Y, Urbano A, Aits S, et al. HAMLET binding to α-actinin facilitates tumor cell detachment. PLoS One. 2011;6:e17179. doi: 10.1371/journal.pone.0017179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermoere F, Yazidi-Belkoura IE, Demont Y, Slomianny C, Antol J, Lemoine J, et al. Proteomics exploration reveals that actin is a signaling target of the kinase Akt. Molecular & Cellular Proteomics. 2007;6:114–124. doi: 10.1074/mcp.M600335-MCP200. [DOI] [PubMed] [Google Scholar]
- Wei S, Gao X, Du J, Su J, Xu Z. Angiogenin enhances cell migration by regulating stress fiber assembly and focal adhesion dynamics. PLoS One. 2011;6:e28797. doi: 10.1371/journal.pone.0028797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch T, Keleg S, Bergmann F, Bauer S, Hinz U, Schmidt J. Actinin-4 expression in primary and metastasized pancreatic ductal adenocarcinoma. Pancreas. 2009;38:968–976. doi: 10.1097/MPA.0b013e3181b28d6f. [DOI] [PubMed] [Google Scholar]
- Yamada S, Yanamoto S, Yoshida H, Yoshitomi I, Kawasaki G, Mizuno A, et al. RNAi-mediated down-regulation of α-actinin-4 decreases invasion potential in oral squamous cell carcinoma. International Journal of Oral and Maxillofacial Surgery. 2010;39:61–67. doi: 10.1016/j.ijom.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Tsuda H, Honda K, Kita T, Takano M, Tamai S, et al. Actinin-4 expression in ovarian cancer: A novel prognostic indicator independent of clinical stage and histological type. Modern Pathology. 2007;20:1278–1285. doi: 10.1038/modpathol.3800966. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Tsuda H, Honda K, Onozato K, Takano M, Tamai S, et al. Actinin-4 gene amplification in ovarian cancer: A candidate oncogene associated with poor patient prognosis and tumor chemoresistance. Modern Pathology. 2009;22:499–507. doi: 10.1038/modpathol.2008.234. [DOI] [PubMed] [Google Scholar]
- Ylänne J, Scheffzek K, Young P, Saraste M. Crystal structure of the alpha-actinin rod reveals an extensive torsional twist. Structure. 2001;9:597–604. doi: 10.1016/s0969-2126(01)00619-0. [DOI] [PubMed] [Google Scholar]
- Zhou Y. The NFκB pathway and endocrine-resistant breast cancer. Endocrine-Related Cancer. 2005;12:S37–S46. doi: 10.1677/erc.1.00977. [DOI] [PubMed] [Google Scholar]
- Zhou C, Zhong Q, Rhodes LV, Townley I, Bratton MR, Zhang Q, et al. Proteomic analysis of acquired tamoxifen resistance in MCF-7 cells reveals expression signatures associated with enhanced migration. Breast Cancer Research. 2012;14:R45. doi: 10.1186/bcr3144. [DOI] [PMC free article] [PubMed] [Google Scholar]