Abstract
This study examines communication about limitations of genomic results interpretation for colon cancer risk during education and counseling of minority participants. As part of a larger study conducted from 2010 to 2012, participants recruited from a large primary care clinic were offered testing for a research panel of 3 genomic markers (single nucleotide polymorphisms or SNPs) for colorectal cancer risk. Genetic counselors conducted pre- and post-test sessions which included discussion of limitations of result interpretation due to the lack of racial/ethnic diversity in research populations from which risk data are derived. Sessions were audio-recorded, transcribed and thematically analyzed. Many participants did not respond directly to this limitation. Among the participants that responded directly to this race-related limitation, many responses were negative. However, a few participants connected the limited minority information about SNPs with the importance of their current research participation. Genetic counselor discussions of this limitation were bio-medically focused with limited explanations for the lacking data. The communication process themes identified included: low immediacy (infrequent use of language directly involving a participant), verbal dominance (greater speaking ratio of the counselor to the patient) and wide variation in the degree of interactivity (or the amount of turn-taking during the discussion). Placed within the larger literature on patient-provider communication, these present results provide insight into the dynamics surrounding race-related educational content for genomic testing and other emerging technologies. Clinicians may be better able to engage patients in the use of new genomic technology by increasing their awareness of specific communication processes and patterns during education or counseling sessions.
Keywords: United States, communication, genomic testing, race, limitations, cancer
Introduction
The field of genomics is identifying an increasing number of low-penetrance genetic variants that are associated with common, complex disease. Low-penetrance variants have to act in concert with other genetic and environmental factors to cause disease, as each low-penetrance variant confers only minimal to moderate risk. The clinical or public health applications of such variants are unclear, including whether knowledge of such genetic variants related to disease risk will lead to meaningful improvements in public health. The commercialization of genomic tests involving low-penetrance variants has outpaced our understanding of the communication, behavioral and social aspects of testing (Khoury et al., 2007; McBride et al., 2008).
Given genetic counselors’ relevant experience communicating about common disease genetics and testing, a one-on-one session with a genetic counselor is one model for the clinical application of genomic information (Waxler et al., 2012). Genetic counseling sessions vary in content and length based on patient needs, health condition, the counselor’s style and administrative aspects of the clinic. Traditionally, in the field of cancer genetic counseling for rare high-penetrance genetic mutations, education and counseling involves in-depth discussion of personal and family history risk factors, risk and risk perceptions, appropriateness and clinical utility of available testing, risk management options, privacy/confidentiality, and psychosocial and behavioral responses to the information (Clarke & Thirlaway, 2011; O'Daniel, 2010). Translational researchers are now investigating less resource intensive models of delivering genomic information to better match the low-penetrant and low-risk nature of the information and the potential public health applications (Waxler et al., 2012). Genomic risk communication research paves the way for future communication and educational efforts related to appropriately conveying the implications and limitations of whole genome sequencing and genomic medicine to diverse populations.
One specific concern about the interpretation of risk estimates for various health conditions involves the homogeneous samples from which the risk estimates were derived. To date, many of the large-scale studies used to estimate disease risk based on genomic information (called genome-wide association studies or GWAS) include samples of people of predominately European ancestral descent (Houlston et al., 2008). Although the ancestral diversity of participants included in GWAS studies is increasing (Fesinmeyer et al., 2013; Ku, Loy, Pawitan, & Chia, 2010), many published risk estimates cannot be universally applied given population-based differences in the frequency of genetic markers or alleles (Rotimi & Jorde, 2010). While the clinical utility of much genomic information is quite limited or unknown, the use of homogenous epidemiological study populations adds further uncertainty to the interpretation of genomic information.
Additional complications to the interpretation of genomic information for individuals from diverse ancestral, geographic and social backgrounds include the fluid definitions of race and ethnicity (Cho, 2006; Hunt & Megyesi, 2008; Shields et al., 2005). Although many genetic studies continue to use participants’ self-reported race and ethnicity, both the categories and participants’ socially defined self-reports likely may not adequately capture individuals’ true genetic admixture (Giri et al., 2009; Sucheston et al., 2012). The current categories of race and ethnicity are methodologically imprecise for use in genetic research based on genetic heterogeneity and unmeasured environmental, social and ecological factors (Cho, 2006; Hunt & Megyesi, 2008; Shields et al., 2005). Race will likely have decreasing relevance in decisions that are based primarily on physiologic processes and for which genomic information may be a better predictor than the social construct of race. For example, the recent discoveries in beta-blocker response (Liggett et al., 2008; Wells, Delaney, & Roden, 2012) and prostate cancer (Gudmundsson et al., 2012) exemplify the relevance of physiological mechanisms in a subset of health-outcome differences seen among races. Yet, as long as cultural differences and social inequities persist, race, and the accompanying imprecision of how it is defined, will likely remain a relevant topic in medicine (Bonham, 2010; Matthews-Juarez & Juarez, 2011).
On-going and recent engagement and methodological efforts are underway to address the issue related to the underrepresentation of individuals from various genetic and geographic backgrounds in genetic research (The International HapMap Consortium, 2005; Monda et al., 2013; Pasaniuc et al., 2011; Rotimi & Jorde, 2010; Shriner, Adeyemo, Ramos, Chen, & Rotimi, 2011). Yet, few studies have investigated how best to meaningfully communicate the limitations related to the interpretation of genomic data based on concepts of race. Improving our understanding of how to communicate these limitations have implications for future genomic education and, more broadly, communication strategies about other emerging technologies that may have slow translation into clinical care or larger public health efforts.
The purpose of the present analysis was to examine the communication about limitations to results interpretation involving the construct of race in the context of pre- and post-test sessions for colon cancer genomic risk assessment among minority participants. Among all cancers, colorectal cancer is the third most frequently diagnosed and the second leading cause of cancer death in men and women in the United States (American Cancer Society, 2013). Colorectal cancer incidence and mortality rates are highest among individuals of African American descent in the U.S., with incidence rates 12% higher and mortality rates 10% higher compared to non-Hispanic whites (American Cancer Society, 2013). We elected to examine risk for colorectal cancer given that it occurs in men and women, has effective screening/prevention options, modifiable lifestyle risk factors (e.g., physical activity, diet), the noted racially disparate outcomes, and a growing evidence base on the number of identified single nucleotide polymorphisms (SNPs) related to colorectal cancer risk (Dimou, Syrigos, & Saif, 2009; National Cancer Institute, 2011). This analysis was nested within a larger study in which participants of diverse self-reported racial backgrounds met individually with a certified genetic counselor to discuss information about colon cancer risk based on genomic, family history and personal risk factors.
The present study takes advantage of an opportunity to examine candid examples of the communication content related to genomic risk education. While not the primary aim of the larger study, for the present study, we sought to explore what is said, how it is communicated, and the meaning underlying that communication. Genetic counselors were not informed that we would be focusing on this specific limitation discussion, nor did we prompt participants to consider the limitations related to race any differently than the other noted limitations to result interpretation of single nucleotide polymorphisms (SNPs; a type of low-penetrance genetic variant that are common in the population). Qualitative methods are well suited to studies involving communication between a health care provider and a patient because qualitative approaches can illuminate nuanced social interactions and allow an in-depth examination of how beliefs are constructed during the exchange of information. Further, qualitative methods can provide documentation of facilitators and barriers to effective communication about genomic information (Timmermans, 2013). Our work was guided by two social science theories related to health care communication. Specifically, we explored the present analyses within the concepts of oral literacy, or focusing on patient-centered and meaningful communication that can be interpreted by the patient (Roter, Erby, Larson, & Ellington, 2007), and reciprocal engagement, or the focus on both the content and process of how patient education and counseling is delivered (Veach, Bartels, & Leroy, 2007). This study is one of the first to investigate the content and process of genomic risk assessment and testing services and adds novel insight on one aspect of how to best deliver genomic information to diverse populations.
Materials and Methods
Participants and Setting
We conducted a prospective mixed-methods study from 2010 to 2012 at the Lombardi Comprehensive Cancer Center at Georgetown University and the Division of General Internal Medicine at Georgetown University Hospital. Persons eligible for this study were male and female primary care patients aged 40 and older. Exclusion criteria were (a) inability to read or understand English or (b) cognitive impairment that precluded informed consent. Procedures were approved by the Institutional Review Board at Georgetown University/MedStar Health.
We used participants’ self-identified race in the present study for three reasons. First, self-identified race is a fluid contract, with evidence that self-perceptions of one’s race may change over time (Cho, 2006). Second, self-identified race has implications for the strength of associations between emerging genomic risk markers and health outcomes (Sharma et al., 2011). Finally, the categories we used reflect how race is described in similar translational genomics research (Grant et al., 2013; Reid et al., 2012) and are based on the categories provided by the Office of Management and Budget (OMB) as required for reporting purposes in federally-sponsored research. Specifically, we used the categories of Black or African American, White, Asian, American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, Other or More than One Race; the latter two had the availability of a write-in category.
Recruitment
We recruited participants through a mailed study invitation letter or by approaching potential participants in-person in the Division of General Internal Medicine primary care clinic at MedStar Georgetown University Hospital.
Procedures
After providing written informed consent, participants completed in-person or telephone-based pre- and post-test sessions with genetic counselors. These 20–30 minute sessions focused on biomedical risk information and genetic testing decision-making with less emphasis on the psychosocial aspects of cancer risk or genomic testing. The biomedical information discussed included colon cancer risk based on personal and family history, SNP testing for colon cancer risk and the pros and cons of SNP-testing to promote informed decision-making about such testing. Study-specific educational materials were used to structure and standardize the sessions (Graves et al., 2013). Participants were offered free genomic testing for a research panel of 3 SNPs related to colorectal cancer risk. Details of the development of the 3-SNP panel, risk algorithms, and educational materials are described elsewhere (Graves et al., 2013; Nusbaum et al., 2013).
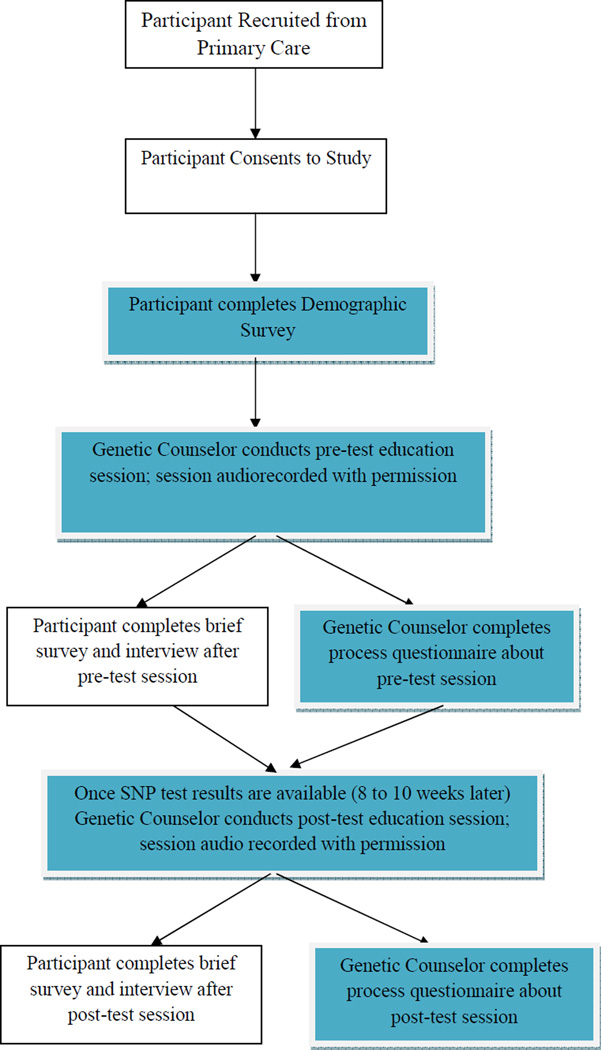
Following the first pre-test session (visit 1 or V2), participants who opted for SNP testing completed a second session (referred to as visit 2 or V2) with the genetic counselor approximately 8–10 weeks later (see Figure 1). During this post-test session, participants were provided with a personalized lifetime colon cancer risk estimate based on their SNP results although the limitations of SNP result interpretation were heavily emphasized. Participants were also informed of their colon cancer risk based on their personal and family history; SNP risk results and family history risk information were not combined. Participants were provided with a personalized results booklet and a technical report of their SNP results. These materials were mailed to participants who completed the pre- and post-test sessions by telephone. Relevant to the present paper, both the pre- and post-test booklets contained statements to help participants keep in mind the limitations to interpreting SNP-based genomic test results. These limitations were bulleted in the materials as follows: “When thinking about your SNP results, please keep in mind: 1) There is a lot we do not yet know about what SNP results mean. Your SNP-risk is just an estimate. 2) These results are based only on 3 specific SNPs. 3) We do not yet know how other colon cancer risk factors interact with these 3 SNPs. 4) We do not know how these SNPs specifically impact risk for people of minority race and ethnic groups.” This last limitation was not consistently discussed in sessions with non-Hispanic whites.
Figure 1.
Study Flow
Note: Blue boxes represent the data that was analyzed for the present study.
Both the pre- and post-test sessions were followed by in-person or telephone semi-structured qualitative interviews and quantitative surveys conducted by a trained research assistant to elicit participants’ experiences with and opinions about the session. All pre- and post-test sessions and interviews were audio-recorded and transcribed. Participants received gift cards for completion of the study interviews and surveys (total of up to $55 for their participation across study assessments). Genetic counselors also completed post-session process questionnaires to capture the counselors’ impressions of each session (e.g., genetic counselors’ perceptions of the participants’ understanding of the information).
Analysis
We used NVIVO 10 (NVIVO Software, QSR International), a qualitative analysis program to analyze transcripts of the pre- and post-test sessions for minority participants. We initially reviewed the in-depth interviews for relevant content, but found that this and closely related topics were not discussed and so the interview data was not included in the present analysis. Rather, we focused the analyses on the transcripts from each pre-test and post-test session and the process questionnaires completed by the genetic counselors. Analyses were informed by communication theories of oral literacy (Roter et al., 2007), and reciprocal engagement (Veach et al., 2007) and our empirical and clinical experiences related to cancer risk counseling and health care communication (Graves, Peshkin, Luta, Tuong, & Schwartz, 2011; Graves et al., 2011; Leventhal et al., 2013; Nusbaum et al., 2013).
Qualitative analyses
The present qualitative analyses focused only on the subset of sessions with minority participants. The interdependent nature of communication necessitated analysis of both genetic counselor and participant speech. Two coders (MB and LV), representing different disciplines, conducted all analyses. Prior to codebook development, the coders listened to a subset of the sessions and read all session transcripts thoroughly to gain an in-depth understanding of the participants’ experiences. Starting from the patterns observed in these early reads and attempting to remain close to the data, the patterns were organized into broad themes and iteratively refined by the coders as analysis progressed and as new transcripts from sessions with minority participants became available. Each coder coded all transcripts independently and double coding of the transcripts was supplemented with review of audio recordings or session notes, as needed for comprehensive interpretation. The iterative and collaborative refinement and application of the codebook supplants a measure of percent agreement for validity (Giacomini & Cook, 2000a; Giacomini & Cook, 2000b). Review of audio recordings supported and expanded upon the coders’ interpretation of the transcripts. For example, communication processes identified through the review of audio recordings that were not evident in the transcripts alone included pace changes (e.g., a participant may slow down her speech when formulating a question about this limitation or alternatively, reply with a quick forceful response) and changes in tone (e.g., decreasing speech volume when formulating a question).
As noted above, analyses were further guided by consideration of existing social science theories describing oral literacy (Roter et al., 2007) and reciprocal engagement (Veach et al., 2007). Both of these health care communication models focus on the exchange of genetic information; however, their underlying focus on communication dynamics make them applicable to other patient and provider communication. The theoretical model of “Oral literacy” described by Roter and colleagues emphasizes clinicians’ ability to provide more patient-centered and meaningful communication by limiting the use of unfamiliar technical terms and monitoring structural characteristics of dialogue, including density, verbal dominance, and interactivity. Additionally, the model of reciprocal engagement highlights the multifaceted nature of patient education and counseling with the centrality of both genetic information and relationship. Further, this model focuses on the extent to which patient and counselor influence each other, the communication, and relationship. For example, messages and modeling from the genetic counselor about what type of content is relevant (e.g., biomedical vs. socioemotional) continuously shapes what type of information is volunteered by the patient, which continuously shapes the relationship, the counselor’s perceptions of the patient, and the subsequent communication. Thus, it is essential to examine each party’s communication content and process within the context of the other party’s communication behavior.
Consideration of these literatures allowed for theoretical triangulation of the data, meaning the use of multiple theoretical perspectives to help interrogate and interpret the data (Miles & Huberman, 1994). The oral literacy and reciprocal engagement models reveal unique but complementary aspects of health care communication; thus by discussing their key concepts in the context of this data we were able to further identify relevant themes and patterns (Patton, 1990). We also held regular meetings with a third researcher (KG) to promote investigator triangulation and the validity of our analyses (Miles & Huberman, 1994). The researchers involved in discussing the findings come from different perspectives (genetic counseling, clinical health psychology and counseling psychology). Finally, we engaged in data triangulation by evaluating the qualitative content of the discussions held with participants and the quantitative process data of the communication dynamics from study sessions and ratings on genetic counselors’ post-session process questionnaires (Miles & Huberman, 1994). The iterative process of coding the transcripts continued until theoretical saturation (Belgrave, Zablotsky, & Guadagno, 2002; Giacomini & Cook, 2000b; Giacomini & Cook, 2000a).
Quantitative Analyses
Finally, we used quantitative data to from the demographic surveys to characterize the sociodemographics of participants (Table 1). We also calculated descriptive statistics to identify the communication dynamics between the participants and genetic counselors from the transcripts using the formulas from the oral literacy literature (Table 2). Specifically, verbal dominance was calculated by the total number of words on the transcript from the genetic counselor divided by total number of words by the participant. Total changes of floor was the number of times the speaker switched during the session, interactivity was calculated by dividing the total number of changes of floor by two divided by the session length in minutes, and density was calculated as words per turn (Roter et al., 2007). Statistics describing communication dynamics required a full, high-quality audio recording, which was available for the present sessions that were analyzed in NVIVO. Last, we used the ratings from the genetic counselors’ process questionnaires to evaluate counselors’ perceptions of whether participants understood the session content. We used t-tests to determine if counselors’ ratings differed by participant racial minority status.
Table 1.
Self-reported Demographics of Participants
| Characteristics | Racial Minority Participants (n=20) |
Non-Minority Participants (n=27) |
|---|---|---|
| n (%) | n(%) | |
| Age ≥50 | 16 (80%) | 21 (77.8%) |
| Female | 12 (60%) | 15 (55.6%) |
| Race | ||
| Non-Hispanic White | 0 (0%) | 27 (100%) |
| African American | 14 (70%) | 0 (0%) |
| Asian | 2 (10%) | 0 (0%) |
| Multi-racial | 4 (20%) | 0 (0%) |
| Education | ||
| < College | 7 (35%) | 1 (3.7%) |
| ≥ College | 13 (70%) | 26 (96.3%) |
Table 2.
Descriptive Statistics of Communication Dynamics (n=30)
| Variable | Mean (SD) | Minimum | Maximum |
|---|---|---|---|
| Total Words | 2744 (1325) | 695 | 6354 |
| Genetic Counselor | 2172 (951) | 658 | 3892 |
| Pt | 571 (616) | 37 | 2916 |
| Verbal Dominance | 6.37 (4.14) | 1.13 | 17.78 |
| Total Changes of Floor | 130.50 (92.91) | 17.00 | 399.00 |
| Interactivity | 3.17(1.76) | 0.69 | 7.30 |
| Density | |||
| Words/Genetic | 46.62 (20.75) | 12.17 | 90.32 |
| Counselor turn | |||
| Words/Patient turn | 8.32 (4.06) | 2.77 | 19.56 |
| Session length (mins) | 20.26 (6.81) | 5.30 | 32.16 |
Results
Participant characteristics
A total of 47 participants took part in the parent study, 20 primary care patients who self-identified as racial minorities (8 males, 12 females; 14 African American, 2 Asian, 2 multiracial) and 27 primary care patients who self-identified as White. Forty-five of the 47 participants opted for SNP testing (19/20 of the racial minority participants). Participant demographics are shown in Table 1. Overall, participants had a mean age of 58.3 years (SD = 10.4 years). Among the racial minority participants (n=20), four (20%) participants reported a family history of colorectal cancer and four (20%) participants reported a personal history of cancer (2 prostate, 1 bladder and 1 breast cancer). Two of 20 pre-test sessions with minority participants were completed by telephone and 9 of 19 post-test sessions with minority participants were completed by telephone (one participant chose not to test). Transcripts did not appear to meaningfully differ by personal or family history of cancer or in-person vs. telephone counseling. Four non-Hispanic white female genetic counselors conducted the pre- and post-test sessions.
The qualitative analyses in the present study focused on the 20 racial minority participants and quantitative analyses for the post-session process questionnaires included the entire sample of 47 participants (see Figure 1). We present the results in three sections: 1) data from genetic counselor and minority participant dialogue, 2) process-related aspects of the communication dynamics between the genetic counselors and minority participants, and 3) the post-session process questionnaires completed by genetic counselors after each pre-test and post-test session for all participants.
Counselor discussion
Overview of the presentation of limitations
The genetic counselors’ presentation of this race-related limitation often mirrored the presentation in the standardized educational materials (see supplementary Figure 2). These materials stated that “We do not yet know how these SNPs impact risk for people of minority race and ethnic groups. Because most studies related to SNPs and cancer have been done in Caucasians we often don’t know what the SNPs mean for people of other ethnicities.” The discussion around this limitation typically appeared in the transcripts of both the pre- and post-test sessions and were short in most cases (e.g., 2–3 sentences by the GC; 1–2 sentences or single word responses by the participant). However, the discussion of this limitation in the pre-test sessions were longer and more in-depth (Mean word count = 132 words, range 0–517 words) than the discussion of the limitation in the post-test sessions (Mean word count = 81 words, range 0 – 374). The quotes included in the results represent approximately 75% of the exchanges between genetic counselors and participants on this topic. The genetic counselors received no special training on this education point in particular, nor were they instructed explicitly how to deliver this information. The study principal investigator provided general feedback following the first few sessions for each genetic counselor; however, the discussion of limitations to interpreting SNP test results based on race or ethnicity was not part of the general feedback. There was some variability in how the study genetic counselors explained this limitation, with some genetic counselors providing more inclusive explanations of why this data is limited.
Explanations were limited
When the discussion of this limitation involved potential causes for the lacking data, genetic counselor explanations tended to focus on lower rates of research participation among minorities, specifically African Americans. This was the most frequent, and typically only, explanation given for the lacking data. Two examples are presented below:
2031 V1: Genetic counselor –Most of the studies have been done… in Caucasian populations…because there’s just a much lower rate of participation among African Americans in research studies.
2022 V1: Genetic counselor – So we don’t know how [the SNPs] would affect risk for other populations.
African American participant – Why is that?
Genetic counselor – African Americans are less likely to participate in research.
Other reasons, such as the failure of researchers to better engage and invite racial/ethnic minorities to participate in genetics and genomic research (Hartz et al., 2011) or the actual lower number of racial/ethnic minorities compared to number of people in the racial majority were not consistently discussed. Two examples of providing explanations other than lower rates of research participation are presented below:
2031 V1 (to an African American participant): Genetic counselor – Probably some combination including African Americans not being reached, in terms of being invited to participate in these studies.
2018 V2 (to an Asian participant): Genetic counselor - Given that most of the research over the last however long, 50 years or so, has been done in the United States and in Europe. We have universities in China and Japan, I mean there are. They just haven’t done as much. And more because of that there’s probably an overrepresentation in the published literature of data, information about people who are Caucasian.
Biomedical focus
Given the intended biomedical focus of these sessions in general, or other reasons, very few socio-emotional statements were made during the discussion of this specific limitation. One such example followed the subtle expression of disappointment:
2016 V1 (to an African American participant) Genetic counselor: I mean I wish there was data on risk versions in different groups but we don’t have that yet…
Participant responses
Minimal response or no direct comment on race-specific limitation
Approximately half of the transcripts included no greater than a minimal response (e.g., “okay”) to the presentation of the limitation related to the SNP data being from primarily non-Hispanic white populations. In some instances the limitation was presented within a list of caveats to testing and none of the limitations were explained further one-by-one. Other caveats include not being able to accurate estimate how much SNPs impact the risk for colon cancer or how to combine the SNP risk with other risk factors.
In other instances, this particular limitation was highlighted and the genetic counselor attempted to elicit input from the participant about how the race-specific limitation impacted their decision about genetic testing. An example of this pattern is presented below and further discussed in the next section.
2001 v1: Genetic counselor- But it’s important for us to tell you that a lot of these studies have been done in only Caucasians so the data in other ethnicities is really lacking.
African American participant- Ok.
Genetic counselor- And so we have to think about that when you’re thinking about whether or not you want to do this testing that most of this data is based on Caucasian population, so it may be different in African American populations.
African American participant- Ok.
2004 v2: Genetic counselor- So, this data that we’ve given you is based on Caucasians so it could be different in other people with different racial and ethnic backgrounds. Any questions about that?
African American participant: Nope.
Negative response to race-related limitation of SNP testing
Among the transcripts that included dialogue about this race-related limitation, several participants voiced their frustration and disappointment upon hearing the limitations to how SNP results can be interpreted in minorities. This frustration was never vehement, instead being somewhat muted although nonetheless palpable by transcript and audio-recording.
2018 V2: Genetic counselor - … there’s probably an overrepresentation in the published literature of data, information about people who are Caucasian.
Multi-racial participant: That’s too bad…
In some cases, this frustration was seen through the participant’s efforts to assess how systemic this deficit is in research by inquiring about the racial composition of the present study. In these and other examples, the frustration or disappointment documented in the transcript could also be heard through tone and pace changes in the audio-recording. Two examples are presented below:
2031 V1: African American participant - … Since we brought race into this, what is the percentage of African American and Latinos in the study that you’re currently doing?
2016 V2: African American participant - No, but then your study… Well, I don’t know. That’s fine, that’s fine. I guess what I’m saying is, is your study more integrated than just a one-race study?
In response to this question the genetic counselor explained the diverse recruitment of the present study but the cause of this question was not revisited.
Participant-generated positive reframe of limitation
Occasionally, participants interpreted this limitation as a call to action and it helped them construct meaning out of their current participation in research.
2001 V:1 Genetic counselor- We don’t know exactly what it means, we can tell you it probably increases your risk, but there haven’t been many studies, really any studies done in [minority] ethnicities, done other than [in] Caucasians.
African American participant: Well, we’re helping the research. It helps the education.
Process description
We identified several process themes in the context of the discussion on the limitations of interpreting genomic risk for racial and ethnic minorities. Process themes capture aspects of what transpires during a session beyond the content and including interactive effects between the genetic counselor’s and participant’s communication (Biesecker & Peters, 2001). Themes included: varying degrees of immediacy, structural characteristics of the dialogue, and genetic counselor techniques used to guide or regulate the discussion. Immediacy is the use of language that brings one in to direct involvement with the issue at hand and is related to the degree to which education is personalized or grounded in the participant’s experience. Structural dialogue characteristics include the relative amount of speech from each speaker as well as the frequency of turn taking. Both these themes have been previously described in other contexts and are associated with patient outcomes (Roter et al., 2007). Genetic counselor techniques to guide or regulate the discussion include dense presentation of material, initiating subject changes and positive reframing of the limitation.
Varying degrees of immediacy
This limitation was consistently introduced in terms of the limitations of existing genetic association studies (low immediacy). Two examples are presented below which demonstrate the abstract nature of low immediacy language:
2027 V2: Genetic counselor - We base our data on these papers here but they looked primarily at Caucasian populations so our estimates are even less certain than in minority populations because these numbers that we use here are heavily biased towards Caucasian populations.
2004 V1: Genetic counselor - Most of the data that we have on these SNPs is from Caucasians so that’s certainly a limitation. Do they mean the same thing in other populations? Is the distribution the same in other populations? We don’t exactly know.
The genetic counselors occasionally applied this limitation directly to the patient’s concrete, lived experience (high immediacy):
2016 V1: Genetic counselor - If I’m a patient I’m going to think to myself, “if we have no idea if this is really applicable to me, do I really want that information?”
In contrast, the participants were much more likely to directly apply or inquire about the meaning of the limitation (high immediacy). Two examples are presented below and they demonstrate the concrete language of high immediacy communication and its tendency to be grounded in what is directly seen or experienced:
2016 V1: African American participant - Well here’s a question – being that you don’t know the impact risk for people of minority, race and ethnic groups, how does that define my participation in this study?
2040 V1: African American participant – So I would be, I am the first African American to take this test?
Techniques used to regulate sessions
In order to work through the information relatively quickly, or for other reasons, the genetic counselors’ use of techniques to guide the pace and content session were notable. These generally fell in to two categories: initiating subject changes/dense presentation of limitations and positive reframing of the limitation.
Regarding subject changes or dense presentation of limitations, in some sessions, the race-related limitation was mentioned within a dense presentation of material or the discussion was moved to another point after minimal participant interjection. Two examples are presented below:
2007 V2: Genetic counselor - So we’re not really sure if these SNPs apply to you in the same way as they would in a Caucasian. The only way we can find out is by doing more studies and including more people of different ethnicities. That’s noted down here. Also, we’re looking at only 3 SNPs. There have been several other SNPs identified that are related to colon cancer. So we’re looking at a little piece of the big picture.
2018 V2: Genetic counselor - So it’s less clear how people of Asian ancestry may be affected by [the SNPs]. So we’re also going to give you this, which is your official test result. And it basically says the same thing as this in a lot more words and a lot more detail.
Additionally, in a few cases, the genetic counselor steered the session by providing the limitation with a positive spin, potentially reducing the participant’s full consideration of this point as a limitation.
2011 V1: Genetic Counselor – this… becomes a real limitation to interpreting your result because we don’t know if these risk versions mean the exact same thing in groups other than Caucasians. But on the other hand, it’s nice to have you in our study so we can learn more, right?
African American participant: Right.
Genetic counselor: Ok so for this study we’re offering testing for three SNPs. We’ll call them A, B, and C…
Structural characteristics of the communication
Verbal dominance and interactivity
Additionally, structural characteristics of the communication process were notable and are presented in table 2. In particular, there was wide variation among the sessions in the amount of verbal dominance and interactivity. Verbal dominance is characterized by a high ratio of genetic counselor to participant talk and this ranged from 1.13–17.78 Genetic counselor words per participant word (mean = 6.37, SD = 4.14). Interactivity reflects conversational turn-taking or the frequency with which speakers exchange the floor (Roter, Erby, Larson, & Ellington, 2009) and this ranged from 0.69–7.30 changes of speaker per minute (mean = 3.17, SD = 1.76). Relatedly and also notable is the density of the speaker turns: genetic counselors spoke an average 46.65 (SD = 20.75) words per turn while participants spoke an average of 8.32 (SD=4.06) words per turn.
Generally, sessions with high verbal dominance also had lower interactivity (two-tailed Pearson correlation = −0.45, p = 0.01). Further, sessions tended to maintain the patterns of verbal dominance and interactivity set in the beginning throughout the session. When this included high verbal dominance and lower interactivity, the session was unlikely to include dialogue about this specific race-related limitation, even with efforts to engage the participant on the topic.
Process Questionnaires
In addition to the process themes identified from the interactions between the genetic counselors and the patients during the pre-test and post-test sessions, we also evaluated quantitative survey data from the process questionnaires the genetic counselors completed after each session. These questionnaires (see Table 3) capture the genetic counselor’s impressions of each session, including whether the genetic counselor thought the patient understood the information, if questions were answered and if the patient might act on the information provided. For the present study, we evaluated whether genetic counselor responses differed by participants’ self-identified race. No statistically significant differences were found, although a trend was noted in the pre-test process questionnaires that genetic counselors felt slightly less able to adequately assess whether understanding of the information discussed with racial minority patients than with non-minority patients (t (df= 43) = 2.0, p = .05). No differences were found for genetic counselors’ impressions of their ability to assess understanding or answer participants’ questions for the post-test sessions (data not shown). Likewise, we did not find any differences in process questionnaire data when we compared the sessions held in-person vs. the sessions held by telephone.
Table 3.
Process Questionnaire Ratings by Genetic Counselors of Pre-Test Sessions (N=30)
| Counseling Element Rated by Genetic Counselor | Minority Participants M (SD) |
Non-Minority Participants M (SD) |
t-value |
|---|---|---|---|
| I was able to adequately cover all topics in the Educational materials. | 4.3 (0.7) | 4.6 (0.5) | t=−1.8, p=0.08 |
| I was able to adequately assess the participant’s understanding of the information we discussed. | 4.0 (0.8) | 4.4 (0.7) | t=−2.0, p =0.05 |
| I was able to assess the participant’s emotional response, well-being, and/or coping abilities. | 4.2 (0.8) | 4.3 (0.7) | t=−0.6, p=0.57 |
| I felt as though I had good rapport with the participant. | 4.1 (0.5) | 4.3 (0.8) | t=−0.9, p=0.34 |
| I was able to assess the participant’s intentions about obtaining SNP testing. | 4.7 (0.5) | 4.8 (0.3) | t= 1.05 p=0.30 |
| I was able to assess the participant’s intentions about communicating with relatives and other individuals about his/her SNP results. | 3.6 (1.3) | 2.4 (1.3) | t=0.62, p =0.54 |
| In general, I was able to identify and discuss key issues important to the participant in his/her decision-making. | 4.3 (0.5) | 4.3 (0.6) | t=0.41, p=0.70 |
| I was able to assist the participant with or facilitate decision-making about SNP testing. | 3.3 (0.6) | 3.6 (0.8) | t=−1.14, p=0.26 |
| The visual aids helped me to structure the education session. | 4.5 (0.5) | 4.5 (0.7) | t=−0.09, p=0.90 |
| The visual aids helped the participant understand important concepts covered in the session. | 4.2 (0.6) | 4.1 (0.8) | t=0.24, p=0.80 |
| There were specific concepts that were difficult to convey during the counseling session. | 2.7 (1.13) | 2.2 (0.8) | t= 1.58, p=0.12 |
| The length of the session seemed adequate. | 4.0 (0.7) | 4.3 (0.5) | t=−1.43, p=0.16 |
| The participant asked relevant questions. | 4.1 (1.0) | 4.5 (0.6) | t=−1.63, p=0.12 |
| The participant seemed to understand the information we discussed. | 4.0 (0.8) | 4.4 (0.6) | t=−1.85, p=0.07 |
| The participant seemed interested in the information presented. | 4.4 (0.6) | 4.5 (0.7) | t=−0.18, p=0.86 |
| The education session seemed like it was valuable to the participant. | 3.7 (0.5) | 3.9 (0.8) | t=−0.84, p=0.40 |
| The results of SNP testing would likely influence the exercise behaviors or diet of this participant. | 2.9 (0.6) | 2.8 (0.8) | t=0.51, p=0.60 |
| The results of SNP testing would likely influence the colon cancer screening intent of this participant. | 2.4 (0.8) | 2.6 (1.0) | t=−0.53, p=0.60 |
| The results of SNP testing would likely influence the degree of cancer worry of this participant. | 2.3 (1.1) | 2.2 (0.8) | t=0.32, p=0.70 |
| The results of SNP testing would likely influence the overall quality of life of this participant. | 1.9 (0.5) | 1.9 (0.5) | t=0.35, p=0.70 |
Discussion
The present study is among the first to explore how health professionals communicate limitations in the interpretation of low-penetrance genetic risk information to minorities. Our findings extend the growing literature on race and health care communication by focusing on the emerging technology of genomic testing. Past research in health care communication has identified potential impediments to communication between health care providers and patients, including lack of provider engagement and misreading of patients’ non-verbal communication (Levine & Ambady, 2013). Recent research that explored physicians’ discussion of clinical trials for cancer treatment with African American and non-Hispanic white patients identified that physicians spent less time discussing potential trial risks and more time discussing the voluntary nature of participation with African Americans compared to non-Hispanic whites (Eggly, Barton, Winckles, Penner, & Albrecht, 2013). The conversations analyzed for the present study were part of tailored one-on-one discussions occurring before and after SNP testing. Our findings indicate that many participants do not comment at all and a few express disappointment about this limitation. These results indicate that in the context of genomic testing, racial minority participants do not express strong concern about the limitations to result interpretation based on the current lack of diversity in genomic research. Importantly, almost all participants still opted for the free SNP testing for SNPs related to colon cancer risk. This high degree of interest in genetic testing contrasts with prior work in which African American women at increased risk for carrying a BRCA1/2 mutation had relatively modest rates of genetic counseling and testing update (Halbert et al., 2012).
Understanding the factors that contribute to differential rates of genomic and genetic testing uptake among minorities is an active area of study (Corbie-Smith et al., 2008; Hall et al., 2012; Sheppard, Mays, LaVeist, & Tercyak, 2013). Recent research suggests that participation of racial and ethnic minorities in genetic services may reflect informed decisions not to have testing (Halbert et al., 2012), challenges with the provision of referral to such services (Graves et al., 2011) or cultural influences that may decrease the salience of such information (Glenn, Chawla, & Bastani, 2012) is unclear. In one recent study, African Americans had lower rates of testing for a genomic test that described an increased risk for colorectal cancer based on a genetic and environmental interaction (Hall et al., 2012). The high rate of genomic testing uptake among minorities in the present study may have been due to the availability of free testing, although free testing has also been available in prior work with contrasting findings (Halbert et al., 2012; Hall et al., 2012). Future work can further explore the individual, social and contextual factors that may influence uptake of genomic testing.
Discussion of the limitation related to the interpretation of risk among minorities should be placed in the larger context of the overall limited clinical utility of SNP-based genomic risk information. The three SNPs account for only a fraction of the total risk for colon cancer, even among Caucasians, and the true interaction between the three SNPs and behavioral and family history risk factors is unknown. Therefore, the absolute decrease in clinical utility among minorities is quite small relative to the current limited utility among all people and thus perhaps a distinction without a difference. We have explored participants’ understanding of the “gist” of the genomic risk information in our prior work (Graves et al., 2013; Leventhal et al., 2013). Briefly, participants appear to understand that at present, genomic risk information may be one small piece of their overall risk for colorectal cancer given the uncertainties that surround the information. Given the sub-set of negative reactions to this limitation of very small absolute impact of clinical utility, this study documents opportunities to better engage and communicate more effectively about the additional limitation to the interpretation of the information for individuals of diverse ancestry within the context of low-clinical utility genomic testing. Simply put, highlighting this particular limitation within the overall context of low clinical utility may not necessarily contribute to more informed decisions about genomic testing. The present results could be interpreted as ‘null’ findings within the context of a qualitative study – we did not find strong responses to the presentation of the race-related limitations for interpreting genomic risk information. Gaining insights from studies that focus on the communication of SNP- or genome-based genomic risk through use of specific post-visit interview questions can inform future efforts to communicate risk to individuals from diverse backgrounds.
The remainder of the discussion describes interpretation of genetic counselor and participant dialogue in turn, and then proceeds to the communication dynamics themselves, ending with implications for the future translations of low-penetrance genomic information, especially for racial minority patients.
Discussion of limitation by genetic counselors
Within the context that some participants expressed frustration or disappointment about this limitation to SNP result interpretation, examining the counselors’ presentation of the limitation and handling of the response is relevant to guide future genomic education efforts. When further discussion of the limitation unfolded, it was primarily an explanation for why more SNP data exists for non-Hispanic whites. The explanations focused on lower rates of research participation among minorities. Interestingly, a 2006 meta-analysis of over 70,000 participants across multiple study types found no significant difference in rates of research participation among minorities and non-Hispanic whites, meaning that when offered participation, people from different racial groups participated at the same rates. This evidence contradicts the impression and individual studies that indicate minorities decline research at a greater rate than non-Hispanic whites and encourages a more nuanced or comprehensive explanation for lacking data among minorities (Wendler et al., 2006). As more evidence accumulates for the processes by which research participation is explained to individuals of different backgrounds, we may uncover specific strategies to help improve what and how much information is shared (Eggly et al., 2013).
In contrast, other explanations were inconsistently mentioned, including minority racial status (fewer individuals compared to the racial majority) or potentially limited efforts on behalf of investigators to engage and recruit minority communities into research. The most notable example of providing alternative explanations for lacking data was with one of the two Asian participants and in this case was given with much greater emphasis than with any African American participant. While the implications of homogeneous study populations are likely taught to all genetic counseling students and some version of communicating this limitation is modeled in their training, in-depth consideration of how to communicate about this limitation and respond to patient concerns is not routine. As evidence slowly accumulates from genetic research with individuals of diverse ancestry (Monda et al., 2013), it is possible the need for health professionals to highlight this specific limitation may diminish. Nonetheless, for the foreseeable future, meaningful communication around this topic is necessary.
Future research on unknown/low-clinical utility genomic risk information could consider communication approaches that emphasize that the limited utility of genomic risk estimate interpretation for individuals with different ancestral backgrounds likely falls within the overall limited utility of the genomic information under study. Importantly, while we promote informed decision making for all patients, the enumerating of all potential and real limits to clinical utility of low-penetrance genomic tests may be excessive; patients may not need each limitation delineated to grasp that interpretation of genomic risk information for common complex disease is not yet definitive. Future work can explore how patients interpret and respond to different explanations for understanding the limits to appropriately applying genomic information. Further, future research can determine if people are interested in learning how genetic markers of ancestry relate to the interpretation of results from genomic or clinical tests (Giri et al., 2009) or if ancestry-specific genomic risk markers resonate as more personally meaningful than risk markers derived without consideration of genetic ancestry.
Participant responses
Many participants had minimal response or no response to this specific limitation. We have several possible explanations for this lack of response. Some participants may have fully accepted the very limited clinical utility of these tests and understood that this specific limitation likely did not decrease the absolute low value of the test. For some participants, perhaps altruistic motivations for research participation subsumed any particular drawbacks to testing, as they were “helping the research” by participating (Michie, Henderson, Garrett, & Corbie-Smith, 2011). Additionally, some of these participants may have had an internal reaction to this limitation; however, the verbal dominance of the genetic counselor and low level of interactivity may have precluded them from sharing their thoughts about this specific limitation.
A few participants voiced frustration or disappointment about this race-specific limitation. For some, learning of the unknown utility among minorities appeared to have diminished the general sense of optimism about the knowledge to be gained through new technology. Perhaps for some participants, learning about this limitation may have raised personal or historical experiences with inequity. Linking this limitation with inquiries about the present study seemed to be an attempt to evaluate their immediate context for similar inequity. None of the participants expressed passionate anger or frustration, but for those who did voice discontent the expression was nonetheless palpable. Social norms around expression of anger in a medical setting may have shaped the somewhat muted responses. While a few participants made a quick positive reframe out of their present participation given this news, the majority of participants who expressed frustration or disappointment did not reframe the limitation positively. The topic was typically concluded with a wish for more minority research or the genetic counselor transitioning to the next discussion point.
Process description
As with any communication process, the process patterns within the present study involve the dynamic interchange between two participants. Subsequently, as an example, it is unlikely that a genetic counselor will be verbally dominant with a patient who volunteers a large amount of medical narrative and asks many questions, although in some instances a provider may insist on controlling the discussion. In health care settings, the clinician typically has more power, sets the tone for communication and implicitly or explicitly lets the patient know what type of interaction is desired (Roter & Hall, 2006). Greater verbal dominance of genetic counselors is associated with more anxiety after counseling for highly penetrant genetic mutations like BRCA1/2 (Dijkstra, Albada, Klockner, Ausems, & van Dulmen, 2013). Patient-physician communication in oncology treatment settings reveals greater verbal dominance by physicians, focused on biomedically-relevant topics and with communication patterns that differed by patients’ race (Siminoff, Graham, & Gordon, 2006). In non-acute encounters in the emergency department, physicians are also more verbally dominant than patients, yet the focus appears to be more on facilitation and activation than on biomedical topics alone (McCarthy et al., 2013). In recent research exploring structural patterns of communication in other health care contexts, physicians’ verbal dominance did not differ by race in discussion of osteoarthritis treatment (Hausmann et al., 2011).
Our examination of the communication process in the present study is framed by the theories describing oral literacy demand and reciprocal engagement. Our analysis reveals several findings. Notable were differences between the genetic counselors and participants in use of language conveying immediacy, or language that directly involves the issue at hand. Genetic counselors typically introduced the race-specific limitation by describing the homogenous study populations comprising the colon cancer SNP literature and uncertainty that implies for minorities. In contrast, participants were much more likely to apply the limitation directly to their personal current context, be it framed as research participation or deciding about SNP testing. Information presented with low immediacy by genetic counselors has been reported before (Roter et al., 2009) and this pattern has strong associations with subsequent patient knowledge scores, with lower immediacy correlating with lower subsequent knowledge (Roter et al., 2009). Moreover, this comprehension pattern also qualitatively appeared to occur in this data, although comprehension is explored in detail by Nusbaum and colleagues (Nusbaum et al., 2013). In the present study, genetic counselors directed the communication as noted by their use of few socio-emotional statements and techniques to regulate the information and pace. Moreover, while there was wide variation, the transcripts from multiple sessions demonstrated high genetic counselors verbal dominance and low levels of interactivity. These communication patterns may be due to the parameters of the study or other subtle influences related to communication between health care providers and patients.
First, the study was structured so that the sessions were conducted as brief health education interventions. While these study sessions depart from standard cancer genetic counseling sessions in length and family history detail, the communication patterns seen here are in line with other analyses of cancer genetic counseling communication (Ellington et al., 2005; Ellington et al., 2006; Ellington et al., 2007; Meiser, Irle, Lobb, & Barlow-Stewart, 2008; Roter & Hall, 2006). For example, such communication has been characterized as being dominated by genetic counselor talk, biomedical in focus, and typically implementing a teaching approach (as seen in this study). Evidence from these prior studies also suggests that higher levels of facilitation, empathic responses, lower verbal dominance and more interactivity are associated with more positive patient outcomes (e.g., knowledge, lower distress, satisfaction) (Meiser et al., 2008; Roter et al., 2007). Thus, future providers of genomic services could better engage patients, even when using a brief health education delivery model, by decreasing their verbal dominance and increasing the session interactivity and immediacy.
Second, the observed patterns of communication in the present study may reflect the subtle influence of broader social and economic communication patterns. While explicit biases are rare, social patterns related to race and socioeconomics from the dominant culture are evident in health care communication across almost all services and illnesses (Cooper et al., 2003; Gordon, Street, Jr., Sharf, & Souchek, 2006; Johnson, Roter, Powe, & Cooper, 2004; Smedley, Stith, & Nelson, 2003; Street, Jr., O'Malley, Cooper, & Haidet, 2008; Thornton, Powe, Roter, & Cooper, 2011). Fortunately, early evidence for clinicians’ ability to recognize and change these patterns is encouraging (Burgess, van Ryn, Dovidio, & Saha, 2007; Teal, Gill, Green, & Crandall, 2012; Wallaert, Ward, & Mann, 2010).
Limitations
Interpretation of these findings should consider the study’s limitations. First, only the transcripts of participants who self-reported African American, Asian or other minority racial categories were reviewed. We opted to focus on the transcripts of these participants because race-related caveats were not consistently discussed with non-Hispanic white participants. We chose to focus on discussion of and reactions to this specific content among racial and ethnic minority participants. We are thus unable to draw conclusions about differences in responses to the race-related caveats to result interpretation between non-Hispanic white vs. African American, Hispanic and Asian participants. Relatedly, we included all non-whites in the present analysis given the small sample size. Although other investigators justify alternative racial/ethnic groupings given the divergent experiences among racial/ethnic minority groups with healthcare (Bissell, Traulsen, & Haugbolle, 2003); we were interested in the experiences of all self- identified non-white participants, regardless of race or ethnicity. Our study was also conducted in the context of free testing for a panel of three research SNPs related to colon cancer. The availability of free testing may have reduced concerns participants had about the identified limitations to the interpretation of genomic risk estimates. Further, our small sample size limits our ability to detect differences in the structural components of communication between minority and non-minority participants. Last, insofar as qualitative research uses recruitment strategies that best serve qualitative questions, this study population may not be representative of other groups of people who may participate in future translational genomic health-care services.
Implications
Despite these caveats, this study is among the first to examine communication about race-related limitations to SNP interpretation. Although the focus of the study was in the area of genomics, our results have implications not only for genetics professionals but also for other health care providers. Specific to genetic counselors and other genomic service providers, our results suggest that these clinicians should carefully consider communication processes of verbal dominance, immediacy and interactivity when communicating with a patient. Beyond genetics professionals, results highlight the potential benefit of attending to both how and how much information is conveyed in discussions with all patients, but particularly minority patients (Eggly et al., 2013; Hagiwara et al., 2013). With continued focus on patient-centered communication and care as potential drivers of improved health outcomes (Epstein & Street, 2007), examining how clinicians describe limitations to results from emerging technologies can contribute to the identification of potential education or intervention targets. Results from the present study can help raise awareness of the communication issues related to the delivery of genomic- and other emerging medical services to minorities to better enable clinicians to meaningfully translate new technologies to the patients they serve.
Highlights.
Genomic risk results for racial minorities have limited interpretability.
When this limitation was explained, many minority participants did not respond.
Of participants who did respond, negative reactions were notable.
Specific communication processes during genetic counseling may enhance discussion.
Acknowledgements
Thank you to Clinton Finch, M.A. for his assistance with setting up the study databases, Matthew Archambault for transcribing interview audio files, and Susan Marx for her assistance with manuscript preparation. We also appreciate the time and contributions of all of our study participants.
Grant Support: This project was supported by funding through NCI K07CA131172-S2 (KG), NIH/NCI grant P30-CA051008 (which partially supports the Genomics and Epigenomics Shared Resource at the Lombardi Comprehensive Cancer Center) and the Fisher Center for Familial Cancer Research. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Cancer Society. What are the key statistics about colorectal cancer. 2013 Retrieved September 10, 2013 from http://www.cancer.org/cancer/colonandrectumcancer/detailedguide/colorectal-cancer-key-statistics.
- Belgrave LL, Zablotsky D, Guadagno MA. How do we talk to each other? Writing qualitative research for quantitative readers. Qualitative Health Research. 2002;12(10):1427–1439. doi: 10.1177/1049732302238753. [DOI] [PubMed] [Google Scholar]
- Biesecker BB, Peters KF. Process studies in genetic counseling: peering into the black box. American Journal of Medical Genetics. 2001;106(3):191–198. doi: 10.1002/ajmg.10004. [DOI] [PubMed] [Google Scholar]
- Bissell P, Traulsen JM, Haugbolle LS. (6) Researching "race", ethnicity and health: a critical review. International Journal of Pharmacy Practice. 2003;11:183–197. [Google Scholar]
- Bonham VL. The role of genetics in understanding racial and ethnic health disparities. JAAPA. 2010;23(1):49–50. doi: 10.1097/01720610-201001000-00012. [DOI] [PubMed] [Google Scholar]
- Burgess D, van Ryn M, Dovidio J, Saha S. Reducing racial bias among health care providers: lessons from social-cognitive psychology. Journal of General Internal Medicine. 2007;22(6):882–887. doi: 10.1007/s11606-007-0160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MK. Racial and ethnic categories in biomedical research: there is no baby in the bathwater. Journal of Law, Medicine & Ethics. 2006;34(3):497–9. doi: 10.1111/j.1748-720x.2006.00061.x. 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, Thirlaway K. 'Genomic counseling'? Genetic counseling in the genomic era. Genome Medicine. 2011;3(1):7. doi: 10.1186/gm221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Annals of Internal Medicine. 2003;139(11):907–915. doi: 10.7326/0003-4819-139-11-200312020-00009. [DOI] [PubMed] [Google Scholar]
- Corbie-Smith G, Blumenthal C, Henderson G, Garrett J, Bussey-Jones J, Moloney M, et al. Studying genetic research participants: lessons from the "Learning About Research in North Carolina" study. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(8):2019–2024. doi: 10.1158/1055-9965.EPI-07-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra H, Albada A, Klockner CC, Ausems MG, van Dulmen S. Nonverbal communication and conversational contribution in breast cancer genetic counseling: are counselors' nonverbal communication and conversational contribution associated with counselees' satisfaction, needs fulfillment and state anxiety in breast cancer genetic counseling? Patient Education and Counseling. 2013;93(2):216–223. doi: 10.1016/j.pec.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Dimou A, Syrigos KN, Saif MW. Disparities in colorectal cancer in African-Americans vs Whites: before and after diagnosis. World Journal of Gastroenterology. 2009;15(30):3734–3743. doi: 10.3748/wjg.15.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggly S, Barton E, Winckles A, Penner LA, Albrecht TL. A disparity of words: racial differences in oncologist-patient communication about clinical trials. Health Expectations. 2013 Aug 2; doi: 10.1111/hex.12108. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington L, Baty BJ, McDonald J, Venne V, Musters A, Roter D, et al. Exploring genetic counseling communication patterns: the role of teaching and counseling approaches. Journal of Genetic Counseling. 2006;15(3):179–189. doi: 10.1007/s10897-005-9011-6. [DOI] [PubMed] [Google Scholar]
- Ellington L, Maxwel A, Baty BJ, Roter D, Dudley WN, Kinney AY. Genetic counseling communication with an African American BRCA1 kindred. Social Science & Medicine. 2007;64(3):724–734. doi: 10.1016/j.socscimed.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington L, Roter D, Dudley WN, Baty BJ, Upchurch R, Larson S, et al. Communication analysis of BRCA1 genetic counseling. Journal of Genetic Counseling. 2005;14(5):377–386. doi: 10.1007/s10897-005-3660-3. [DOI] [PubMed] [Google Scholar]
- Epstein RM, Street RL., Jr . Patient-centered communication in cancer care: Promoting healing and reducing suffering. Bethesda, MD: National Cancer Institute, NIH publication no; 2007. pp. 07–6225. [Google Scholar]
- Fesinmeyer MD, North KE, Ritchie MD, Lim U, Franceschini N, Wilkens LR, et al. Genetic risk factors for BMI and obesity in an ethnically diverse population: results from the population architecture using genomics and epidemiology (PAGE) study. Obesity (Silver Spring, Maryland) 2013;21(4):835–846. doi: 10.1002/oby.20268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini MK, Cook DJ. Users' guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 2000a;284(3):357–362. doi: 10.1001/jama.284.3.357. [DOI] [PubMed] [Google Scholar]
- Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care B. What are the results and how do they help me care for my patients? Evidence-Based Medicine Working Group. JAMA. 2000b;284(4):478–482. doi: 10.1001/jama.284.4.478. [DOI] [PubMed] [Google Scholar]
- Giri VN, Egleston B, Ruth K, Uzzo RG, Chen DY, Buyyounouski M, et al. Race, genetic West African ancestry, and prostate cancer prediction by prostate-specific antigen in prospectively screened high-risk men. Cancer Prevention Research (Philadelphia) 2009;2(3):244–250. doi: 10.1158/1940-6207.CAPR-08-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn BA, Chawla N, Bastani R. Barriers to genetic testing for breast cancer risk among ethnic minority women: an exploratory study. Ethnicity & Disease. 2012;22(3):267–273. [PubMed] [Google Scholar]
- Gordon HS, Street RL, Jr, Sharf BF, Souchek J. Racial differences in doctors' information-giving and patients' participation. Cancer. 2006;107(6):1313–1320. doi: 10.1002/cncr.22122. [DOI] [PubMed] [Google Scholar]
- Grant RW, O'Brien KE, Waxler JL, Vassy JL, Delahanty LM, Bissett LG, et al. Personalized genetic risk counseling to motivate diabetes prevention: a randomized trial. Diabetes Care. 2013;36(1):13–19. doi: 10.2337/dc12-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves KD, Christopher J, Harrison TM, Peshkin BN, Isaacs C, Sheppard VB. Providers' perceptions and practices regarding BRCA1/2 genetic counseling and testing in African American women. Journal of Genetic Counseling. 2011;20(6):674–689. doi: 10.1007/s10897-011-9396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves KD, Leventhal KG, Nusbaum R, Salehizadeh Y, Hooker GW, Peshkin BN, et al. Behavioral and psychosocial responses to genomic testing for colorectal cancer risk. Genomics. 2013 doi: 10.1016/j.ygeno.2013.04.002. [Epub ahead of print, April 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves KD, Peshkin BN, Luta G, Tuong W, Schwartz MD. Interest in genetic testing for modest changes in breast cancer risk: implications for SNP testing. Public Health Genomics. 2011;14(3):178–189. doi: 10.1159/000324703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson J, Sulem P, Gudbjartsson DF, Masson G, Agnarsson BA, Benediktsdottir KR, et al. A study based on whole-genome sequencing yields a rare variant at 8q24 associated with prostate cancer. Nature Genetics. 2012;44(12):1326–1329. doi: 10.1038/ng.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N, Penner LA, Gonzalez R, Eggly S, Dovidio JF, Gaertner SL, et al. Racial attitudes, physician-patient talk time ratio, and adherence in racially discordant medical interactions. Social Science & Medicine. 2013;87:123–131. doi: 10.1016/j.socscimed.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CH, Kessler L, Collier A, Weathers B, Stopfer J, Domchek S, et al. Low rates of African American participation in genetic counseling and testing for BRCA1/2 mutations: racial disparities or just a difference? Journal Genetic Counseling. 2012;21(5):676–683. doi: 10.1007/s10897-012-9485-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MJ, Manne SL, Myers RE, Keenan EM, Balshem AM, Weinberg DS. Predictors of patient uptake of colorectal cancer gene environment risk assessment. Genome Medicine. 2012;4(11):92. doi: 10.1186/gm393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz SM, Johnson EO, Saccone NL, Hatsukami D, Breslau N, Bierut LJ. Inclusion of African Americans in genetic studies: what is the barrier? American Journal of Epidemiology. 2011;174(3):336–344. doi: 10.1093/aje/kwr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann LR, Hanusa BH, Kresevic DM, Zickmund S, Ling BS, Gordon HS, et al. Orthopedic communication about osteoarthritis treatment: Does patient race matter? Arthritis Care & Research (Hoboken) 2011;63(5):635–642. doi: 10.1002/acr.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlston RS, Webb E, Broderick P, Pittman AM, Di Bernardo MC, Lubbe S, et al. Meta-analysis of genome-wide association data identifies four new susceptibility loci for colorectal cancer. Nature Genetics. 2008;40(12):1426–1435. doi: 10.1038/ng.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt LM, Megyesi MS. The ambiguous meanings of the racial/ethnic categories routinely used in human genetics research. Social Science & Medicine. 2008;66(2):349–361. doi: 10.1016/j.socscimed.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. American Journal of Public Health. 2004;94(12):2084–2090. doi: 10.2105/ajph.94.12.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genetics in Medicine. 2007;9(10):665–674. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- Ku CS, Loy EY, Pawitan Y, Chia KS. The pursuit of genome-wide association studies: where are we now? Journal of Human Genetics. 2010;55(4):195–206. doi: 10.1038/jhg.2010.19. [DOI] [PubMed] [Google Scholar]
- Leventhal KG, Tuong W, Peshkin BN, Salehizadeh Y, Fishman MB, Eggly S, et al. "Is it really worth it to get tested?": primary care patients' impressions of predictive SNP testing for colon cancer. Journal of Genetic Counseling. 2013;22(1):138–151. doi: 10.1007/s10897-012-9530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine CS, Ambady N. The role of non-verbal behaviour in racial disparities in health care: implications and solutions. Medical Education. 2013;47(9):867–876. doi: 10.1111/medu.12216. [DOI] [PubMed] [Google Scholar]
- Liggett SB, Cresci S, Kelly RJ, Syed FM, Matkovich SJ, Hahn HS, et al. A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nature Medicine. 2008;14(5):510–517. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews-Juarez P, Juarez PD. Cultural competency, human genomics, and the elimination of health disparities. Social Work in Public Health. 2011;26(4):349–365. doi: 10.1080/19371918.2011.579043. [DOI] [PubMed] [Google Scholar]
- McBride CM, Alford SH, Reid RJ, Larson EB, Baxevanis AD, Brody LC. Putting science over supposition in the arena of personalized genomics. Nature Genetics. 2008;40(8):939–942. doi: 10.1038/ng0808-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, Buckley BA, Engel KG, Forth VE, Adams JG, Cameron KA. Understanding patient-provider conversations: what are we talking about? Academic Emergency Medicine. 2013;20(5):441–448. doi: 10.1111/acem.12138. [DOI] [PubMed] [Google Scholar]
- Meiser B, Irle J, Lobb E, Barlow-Stewart K. Assessment of the content and process of genetic counseling: a critical review of empirical studies. Journal of Genetic Counseling. 2008;17(5):434–451. doi: 10.1007/s10897-008-9173-0. [DOI] [PubMed] [Google Scholar]
- Michie M, Henderson G, Garrett J, Corbie-Smith G. "If I could in a small way help": motivations for and beliefs about sample donation for genetic research. Journal of Empirical Research on Human Research Ethics. 2011;6(2):57–70. doi: 10.1525/jer.2011.6.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles MB, Huberman AM. Qualitative data analysis: An expanded sourcebook. 2nd ed. Thousand Oaks, CA: Sage; 1994. [Google Scholar]
- Monda KL, Chen GK, Taylor KC, Palmer C, Edwards TL, Lange LA, et al. A meta-analysis identifies new loci associated with body mass index in individuals of African ancestry. Nature Genetics. 2013 doi: 10.1038/ng.2608. [Epub ahead of print, April 14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. variantGPS: SNP500Cancer. 2011 Retrieved October 7, 2013 from http://variantgps.nci.nih.gov/cgfseq/pages/snp500.do.
- Nusbaum R, Leventhal KG, Hooker GW, Peshkin BN, Butrick M, Salehizadeh Y, et al. Translational genomic research: protocol development and initial outcomes following SNP testing for colon cancer risk. Translational Behavioral Medicine. 2013;3(1):17–29. doi: 10.1007/s13142-012-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Daniel JM. The prospect of genome-guided preventive medicine: a need and opportunity for genetic counselors. Journal of Genetic Counseling. 2010;19(4):315–327. doi: 10.1007/s10897-010-9302-4. [DOI] [PubMed] [Google Scholar]
- Pasaniuc B, Zaitlen N, Lettre G, Chen GK, Tandon A, Kao WH, et al. Enhanced statistical tests for GWAS in admixed populations: assessment using African Americans from CARe and a Breast Cancer Consortium. PLoS Genetics. 2011;7(4):e1001371. doi: 10.1371/journal.pgen.1001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton MQ. Qualitative evaluation and research methods. 2nd ed. Newbury Park, CA: Sage; 1990. [Google Scholar]
- Reid RJ, McBride CM, Alford SH, Price C, Baxevanis AD, Brody LC, et al. Association between health-service use and multiplex genetic testing. Genetics in Medicine. 2012;14(10):852–859. doi: 10.1038/gim.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roter DL, Erby L, Larson S, Ellington L. Oral literacy demand of prenatal genetic counseling dialogue: Predictors of learning. Patient Education and Counseling. 2009;75(3):392–397. doi: 10.1016/j.pec.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Roter DL, Erby LH, Larson S, Ellington L. Assessing oral literacy demand in genetic counseling dialogue: preliminary test of a conceptual framework. Social Science & Medicine. 2007;65(7):1442–1457. doi: 10.1016/j.socscimed.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roter DL, Hall JA. Doctors talking with patients/Patients talking with doctors: Improving communication in medical visits. 2nd ed. Westport, CT: Praeger Publishers; 2006. [Google Scholar]
- Rotimi CN, Jorde LB. Ancestry and disease in the age of genomic medicine. The New England Journal of Medicine. 2010;363(16):1551–1558. doi: 10.1056/NEJMra0911564. [DOI] [PubMed] [Google Scholar]
- Sharma S, Murphy A, Howrylak J, Himes B, Cho MH, Chu JH, et al. The impact of self-identified race on epidemiologic studies of gene expression. Genetic Epidemiology. 2011;35(2):93–101. doi: 10.1002/gepi.20560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard VB, Mays D, LaVeist T, Tercyak KP. Medical mistrust influences black women's level of engagement in BRCA 1/2 genetic counseling and testing. Journal of the National Medical Association. 2013;105(1):17–22. doi: 10.1016/s0027-9684(15)30081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields AE, Fortun M, Hammonds EM, King PA, Lerman C, Rapp R, et al. The use of race variables in genetic studies of complex traits and the goal of reducing health disparities: a transdisciplinary perspective. The American Psychologist. 2005;60(1):77–103. doi: 10.1037/0003-066X.60.1.77. [DOI] [PubMed] [Google Scholar]
- Shriner D, Adeyemo A, Ramos E, Chen G, Rotimi CN. Mapping of disease-associated variants in admixed populations. Genome Biology. 2011;12(5):223. doi: 10.1186/gb-2011-12-5-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminoff LA, Graham GC, Gordon NH. Cancer communication patterns and the influence of patient characteristics: disparities in information-giving and affective behaviors. Patient Education and Counseling. 2006;62(3):355–360. doi: 10.1016/j.pec.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Smedley BD, Stith AY, Nelson AR. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- Street RL, Jr, O'Malley KJ, Cooper LA, Haidet P. Understanding concordance in patient-physician relationships: personal and ethnic dimensions of shared identity. Annals of Family Medicine. 2008;6(3):198–205. doi: 10.1370/afm.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucheston LE, Bensen JT, Xu Z, Singh PK, Preus L, Mohler JL, et al. Genetic ancestry, self-reported race and ethnicity in African Americans and European Americans in the PCaP cohort. PLoS One. 2012;7(3):e30950. doi: 10.1371/journal.pone.0030950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teal CR, Gill AC, Green AR, Crandall S. Helping medical learners recognise and manage unconscious bias toward certain patient groups. Medical Education. 2012;46(1):80–88. doi: 10.1111/j.1365-2923.2011.04101.x. [DOI] [PubMed] [Google Scholar]
- The International HapMap Consortium. International HapMap Project. 2005 Retrieved April 16, 2013 from http://hapmap.ncbi.nlm.nih.gov/ethicalconcerns.html.en.
- Thornton RL, Powe NR, Roter D, Cooper LA. Patient-physician social concordance, medical visit communication and patients' perceptions of health care quality. Patient Education and Counseling. 2011;85(3):e201–e208. doi: 10.1016/j.pec.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans S. Seven warrants for qualitative health sociology. Social Science & Medicine. 2013;77:1–8. doi: 10.1016/j.socscimed.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Veach PM, Bartels DM, Leroy BS. Coming full circle: a reciprocal-engagement model of genetic counseling practice. Journal of Genetic Counseling. 2007;16(6):713–728. doi: 10.1007/s10897-007-9113-4. [DOI] [PubMed] [Google Scholar]
- Wallaert M, Ward A, Mann T. Explicit control of implicit responses: simple directives can alter IAT performance. Social Psychology (Gottingen, Germany) 2010;41(3):152–157. doi: 10.1027/1864-9335/a000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxler JL, O'Brien KE, Delahanty LM, Meigs JB, Florez JC, Park ER, et al. Genetic counseling as a tool for type 2 diabetes prevention: a genetic counseling framework for common polygenetic disorders. Journal of Genetic Counseling. 2012;21(5):684–691. doi: 10.1007/s10897-012-9486-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells QS, Delaney JT, Roden DM. Genetic determinants of response to cardiovascular drugs. Current Opinion in Cardiology. 2012;27(3):253–261. doi: 10.1097/HCO.0b013e32835220e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler D, Kington R, Madans J, Van WG, Christ-Schmidt H, Pratt LA, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Medicine. 2006;3(2):e19. doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]