Abstract
Purpose:
To compare the performance of CADx analysis of pre-contrast HiSS MRI to that of clinical DCE-MRI in the diagnostic classification of breast lesions.
Materials and Methods:
Thirty-four malignant and seven benign lesions were scanned using 2D HiSS and clinical 4D DCE-MRI protocols. Lesions were automatically segmented. Morphological features were calculated for HiSS whereas both morphological and kinetic features were calculated for DCE-MRI. After stepwise feature selection, Bayesian artificial neural networks merged selected features, and ROC analysis evaluated the performance with leave-one-lesion-out validation.
Results:
AUC values of 0.92 ± 0.06 and 0.90 ± 0.05 were obtained using CADx on HiSS and DCE-MRI, respectively, in the task of classifying benign and malignant lesions. While we failed to show that the higher HiSS performance was significantly better than DCE-MRI, non-inferiority testing confirmed that HiSS was not worse than DCE-MRI.
Conclusion:
CADx of HiSS (without contrast) performed similarly to CADx on clinical DCE-MRI; thus, computerized analysis of HiSS may provide sufficient information for diagnostic classification. The results are clinically important for patients in whom contrast agent is contra-indicated. Even in the limited acquisition mode of 2D single slice HiSS, by using quantitative image analysis to extract characteristics from the HiSS images, similar performance levels were obtained as compared to those from current clinical 4D DCE-MRI. As HiSS acquisitions become possible in 3D, CADx methods can also be applied. Since HiSS and DCE-MRI are based on different contrast mechanisms, the use of the two protocols in combination may increase diagnostic accuracy.
Keywords: High spectral and spatial resolution (HiSS) MRI, computer-aided diagnosis (CADx), breast cancer, dynamic contrast enhanced MRI (DCE-MRI), contrast-agent induced nephrotoxicity
INTRODUCTION
Dynamic contrast-enhanced MRI (DCE-MRI) of the breast is being used increasingly for a variety of clinical purposes including screening of high-risk patients, post-treatment evaluation, and assessment of extent of disease (1-5). However, the specificity of DCE-MRI is limited, and improvements in specificity are desirable, particularly for early cancers (6, 7). DCE-MRI has a number of limitations. The requirement for high temporal resolution limits spatial resolution and signal-to-noise ratio. Contrast agents can cause increased blurring at the lesion margins, which is known as “blooming” (8, 9). The quality of 'subtraction' images may be degraded due to motion. These factors can compromise a radiologist’s ability to accurately assess lesion morphology with respect to internal architecture and lesion margins on DCE-MRI.
Non-contrast enhanced high spectral and spatial resolution (HiSS) MRI is being developed to quantitatively assess the distinct components of the water signal. The spectral information can be used to minimize blurring, and a more uniform fat suppression can be obtained by separating the water signal from the fat and background signals and discarding nonwater signals (10, 11). Furthermore, by imaging individual components of the water resonance, the image contrast in HiSS images is inherently different from the image contrast in conventional DCE-MRI. Previous work has correlated the structure of the water proton spectra with regions of high vascularity (12) and malignancy on biopsy (13). In previous studies analyzing breast lesions, radiologists found internal lesion definition, fat suppression, lesion conspicuity, and margin definition improved on pre-contrast HiSS images as compared to standard clinical DCE-MRI images (14-17). In a reader study, in which readers used BIRADs rating to assess the morphological characteristics, the diagnostic utility of HiSS images was compared to that of DCE-MRI images, and radiologists interpreting non-contrast enhanced 2D HiSS images performed similarly to their interpretation of 2D DCE-MRI (18).
Thus, with decreased blurring effects and improved fat suppression, non-contrast enhanced HiSS may potentially provide more accurate morphology assessment of breast lesions than DCE-MRI. In addition to improved morphologic detail, sensitivity to deoxygenated blood via the 'BOLD' effect (19, 20) provides functional information. This may be of high clinical importance to patients who cannot tolerate contrast administration due to risk of nephrogenic systemic fibrosis (21-23) or allergic reactions (24). HiSS MRI may also be employed as an adjunct to DCE-MRI for the optimization of lesion characterization. There is a well-known trade-off between spatial and temporal resolution in DCE-MRI (25). This limitation can be moderated by combining DCE-MRI with HiSS.
While the work reported here is based on single-slice HiSS, protocols are being developed to allow for full bilateral HiSS scans (17). Such pre-contrast bilateral HiSS could potentially be used to provide morphological assessment to identify suspect areas in the breast. Then, DCE-MRI with high temporal resolution might be utilized to focus on these suspicious areas with high temporal and spatial resolution, to provide improved kinetic characterization of lesions, especially ductal carcinoma in situ lesions (26).
Previous studies employed radiologists to evaluate non-contrast enhanced HiSS MRI (14-18). Computer-aided diagnosis (CADx) methods for automated lesion segmentation, feature extraction, and classification have been developed for breast MRI (27-35), and observer studies have demonstrated the potential usefulness of breast MRI CADx in the clinical arena (36, 37). The goal of automated computerized analysis of medical images is to obtain quantitative and reproducible indices for lesion characterization (38, 39).
The purpose of this pilot study is to perform a preliminary evaluation and compare the performance of automated computerized analysis of pre-contrast HiSS MRI to that of DCE-MRI in the classification of benign and malignant breast lesions, using our in-house CADx scheme. Our pilot study is the first use of quantitative CADx analysis methods to characterize morphology and evaluate the diagnostic utility of computer-extracted lesion features in pre-contrast HiSS MR images.
It is important to note that (a) our study focuses on diagnosis and not detection, and (b) the HiSS images were obtainable only in 2D while the full 4D DCE-MRI data were available for the comparative analysis. Note that in this study, we were not attempting to find better performance with HiSS as compared to DCE-MRI, but rather demonstrate similar performances, and thus demonstrate the potential for the use of HiSS, which does not involve contrast, in breast cancer imaging.
MATERIALS AND METHODS
Patient Population
This HIPAA-compliant study was conducted under a protocol approved by our institutional review board after informed consent was obtained from study participants. Forty women with suspicious breast lesions were recruited from our institution’s cancer risk clinic and breast imaging service. The biopsy-determined diagnoses of the malignant lesions yielded 22/34 invasive ductal carcinoma, 9/34 IDC with ductal carcinoma in situ, and 3/34 invasive lobular carcinoma. The biopsy-determined diagnoses of the benign lesions included 1/7 atypical ductal hyperplasia, 1/7 fibrocystic change (FCC), 1/7 sclerosing adenosis, 1/7 hyalinzed stroma with focal FCC, and 3/7 fibroadenomas. The average lesion size was 22mm ± 15.0mm with median size of 20mm, minimum size of 6mm, and maximum size of 81mm.
Image Acquisition
The patients were scanned in the prone position using a standard double breast coil on a 1.5T whole-body MRI system (GE Medical Systems, Milwaukee, WI). HiSS images were acquired using an EPSI pulse sequence, which was composed of a slice-selective excitation pulse (3-4 mm slice thickness), a phase-encoding gradient with 192 phase-encoding steps, and a train of 64-128 gradient echoes with 384 sampled points per gradient echo. The HiSS images had an in-plane resolution of 0.63-0.95 mm and 64-128 bins of spectral resolution. The gradient-echo train was sampled at a bandwidth of 62.5 kHz, so that the free induction decay (FID) was sampled for 192-384 msec, and the spacing between echoes was 3.0 msec. This resulted in a spectral resolution of 2.6-5.2 Hz, or 0.041-0.082 ppm, and a spectral bandwidth of 333 Hz. The acquisition time per slice under these conditions was 64-128 sec. Prior mammographic examination and/or the pre-contrast T2-weighted MRI examinations sequence were used to position the HiSS slice to capture the lesion in question. Shimming was performed on the slice immediately before HiSS imaging. One or two sagittal pre-contrast HiSS slices were imaged in each patient.
Following the pre-contrast HiSS MRI acquisition protocol, the DCE-MRI images were obtained using a T1-weighted 3D spoiled gradient recalled (SPGR) sequence (TR/TE = 7.7/4.2 ms, 30° flip angle). Fat suppression was not employed. After the acquisition of the pre-contrast series, Omniscan (gadolinium diethylenetriamine penta-acetic acid or Gd-DTPA BMA) contrast agent was delivered intravenously with a fixed dose of 20cc followed by a 20-mL saline flush. Three to five postcontrast series were obtained with a time interval of 68 seconds, covering approximately 5-6 min post-contrast. Each series contained 60 slices with a matrix of 256 × 256 pixels. The in-plane resolution was 1.4 × 1.4 mm2 and the slice thickness was in the range of 2 mm to 4 mm depending on breast size.
HiSS MRI Post-processing
The HiSS MRI data was processed by applying a 3D Fourier transform to the raw data to obtain a high-resolution proton spectrum in each voxel of the imaged slice. An in-house algorithm was used on the resulting spectra to detect the frequencies of the water and fat peaks (11). The high-resolution magnitude spectrum in each voxel was fitted using a Lorentzian function approximation for the fat, water, and any Nyquist spectral ghost peaks. The Lorentzian fits for fat peaks and ghost peaks as well as any constant background signal were subtracted to reveal the water signal. Because the water signal was frequently inhomogeneously broadened, the water peak height images were not obtained from the fit parameters but from the maximum value of the isolated water signal in each voxel. All processing software was developed in Interactive Data Language (IDL, ITT Visual Information Solutions, Boulder, CO).
Automated Computerized Lesion Segmentation
A region-of-interest (ROI) was placed around the lesion in the MR image; this was the only manual step in the image analysis process. Fuzzy c-means clustering method (FCM) was used to automatically segment the lesion in two dimensions for HiSS MR images and in three dimensions for DCE-MR images (30). For the HiSS MR images, voxel values within the ROI were used, while for the DCE-MR images, relative enhancement (the ratio of voxel value to the corresponding pre-contrast value) was used in the two-category FCM algorithm (32). The final output of the FCM was a membership map that classified each voxel as lesion or non-lesion. Connected-component labeling and hole-filling were the final steps in giving the segmentation outline of the lesion.
Automated Computer-Extracted Features
Mathematical lesion descriptors were grouped into five types of features: (i) textural; (ii) spiculation; (iii) geometric; (iv) kinetics; and (v) enhancement-variance kinetics. HiSS MRI features consisted of (i)-(iii) while DCE-MRI features comprised all types (i)-(v). It is important to note that the analysis of the HiSS images was in 2D, whereas the analysis of the DCE-MRI images was in 3D, with the kinetic analysis being in 4D.
Texture features were calculated from the gray-level co-occurrence matrix (GLCM) using the entire segmented lesion (40). In the 2D HiSS MR image, a pixel has 8 neighboring pixel-pairs in 4 independent directions while a voxel in the 3D DCE-MR image is surrounded by 26 neighboring voxels in 13 independent directions. Since the voxels were anisotropic in DCE-MR images, a linear interpolation was used before the construction of directional GLCMs to yield isotropic voxels. A non-directional GLCM was obtained by summing all the directional GLCMs. Fourteen texture features were extracted from the non-directional GLCM to quantify different textural characteristics of the lesion, including homogeneity, gray-level dependence, brightness, and randomness. The specific features were contrast, correlation, difference entropy, difference variance, energy, entropy, homogeneity, maximum correlation coefficient (MaxCC), sum average, sum entropy, sum variance, variance, and two information measures of correlation (IMC1, IMC2) (38).
Spiculation features were determined from an analysis of both radial edge gradients (radial gradient) and of maximum gradient (FWHM or full width half maximum) using the entire segmented lesion (41, 42). These measures were extracted not only along the margin (border) and within the margin of the mass (grown) but also in enlarged neighborhoods of the ROI minus the lesion (margin) as well as the entire ROI. Margin sharpness is the mean gradient magnitude along the margin of the lesion. Of these spiculation features, FWHM grown and margin sharpness were generalized to 3D MRI. Finally, three additional geometric features including lesion size, circularity, and irregularity were calculated; circularity quantifies the conformity of a lesion to a circle and irregularity measures the roughness of the lesion contour surface (43). Overall, twenty-six quantifiable HiSS MRI features were generated for each lesion.
For the DCE-MR images, we assessed lesion texture using the 3-D GLCM method (33) as well as calculated similar geometric features for sphericity, irregularity, and size or lesion volume (35) using the entire segmented lesion. For spiculation features, radial gradient index (RGI) is based on the radial gradient of the voxels within the lesion and its local environment, and corresponds to the FWHM grown feature for HiSS. In addition to margin sharpness, variance of margin sharpness, which is the variance of the image gradient at the lesion margin, was also calculated (35).
Kinetic features quantify the enhancement kinetics of the breast lesions on DCE-MR images and aim to describe the physiologic process of the uptake and washout of the contrast agent during the imaging time. First, the characteristic kinetic curve (CKC) of a lesion was automatically determined by FCM clustering, as follows (34). The signal-time curves obtained from each voxel in a segmented 3D lesion were clustered into a number of prototypal curves, and the category curve with the highest initial enhancement was selected as the characteristic kinetic curve representative of the specific lesion (34). Seven kinetic features were then extracted from the CKC including maximum contrast enhancement, time to peak (TTP), uptake rate, washout rate, curve shape index, enhancement at the first postcontrast time frame, and signal enhancement ratio.
Enhancement-variance kinetics features characterize the time course of the spatial variance of the enhancement over the entire segmented lesion. Similar to the kinetic features, the maximum enhancement-variance, time to peak, the increasing rate of enhancement-variance, and the decreasing rate of enhancement-variance were determined (35). Overall, thirty-one DCE-MRI features were generated for each lesion.
The overall and conditional Pearson correlation coefficients and p-values were calculated between the corresponding HiSS MRI and DCE-MRI features (44). The Holm t-test was used for multiple comparisons with the overall α-level set at 0.05 (45).
Feature Selection and Classification
Stepwise feature selection was performed using linear discriminant analysis (LDA) with a Wilks lambda cost function (46) in a leave-one-lesion-out (LOLO) method. Feature selection was utilized to select the subset of features that perform effectively in classifying the lesions as benign or malignant. The number of cases should be 3-10 times the number of computer-extracted features to allow for unbiased feature selection (47); however the number of features exceeded the number of cases for both HiSS and DCE-MRI. Thus, feature selection was performed in two steps: first using LOLO within each feature type for each protocol – texture, spiculation, and geometric for HiSS and texture, spiculation, geometric, kinetics, and enhancement-variance kinetics for DCE-MRI. The selected features were then grouped together (four features for HiSS and seven features for DCE-MRI), and LDA feature selection was performed a second time using LOLO for each protocol to give the final set of selected features (48, 49).
A two-class Bayesian artificial neural network (BANN) classifier merged the selected features to yield a computer-estimated probability of malignancy (50). The LOLO validation method was used in the performance evaluation. Receiver operating characteristic (ROC) analysis was used to evaluate the classification performance with the ROCKIT software package (51-53). The index of performance was the area under the maximum likelihood-fitted binormal ROC curve (AUC), and the difference in AUC performance was assessed using two-sided 95% confidence intervals (CI) (44). Non-inferiority was tested using one-sided 95% CI (54, 55) and since this is a preliminary study, the lower bound for non-inferiority was set at −0.1.
RESULTS
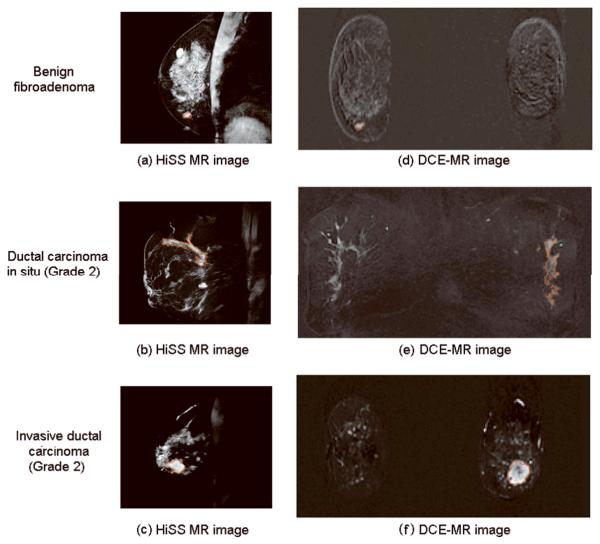
Figure 1 depicts the HiSS MRI and DCE-MRI images for three patients in the study to demonstrate the segmentation results. The lesions on the HiSS images have similar visual conspicuity to that of the contrast-enhanced lesions on the DCE-MRI images.
Figure 1.
Pre-contrast HiSS MR image (a - c) and DCE-MR images at first minute postinjection in coronal projection (d - f) for three patients with breast lesions (diagnosis, top to bottom: benign fibroadenoma, ductal carcinoma in situ (Grade 2), and invasive ductal carcinoma (Grade 2)). Computerized segmentations of lesions are shown in red.
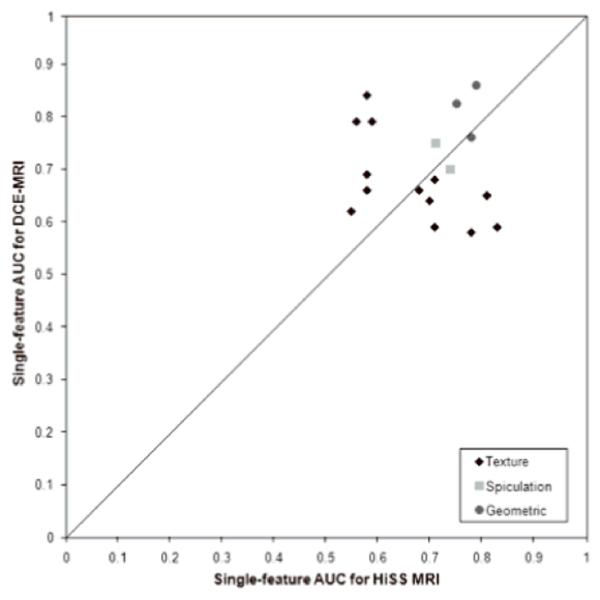
With respect to single feature AUC values (Table 1, Figure 2), HiSS MRI yielded higher AUC values for several GLCM-based texture features (contrast, correlation, difference in entropy, difference in variance, homogeneity, IMC1, IMC2, maximum correlation coefficient) while DCE-MRI had higher AUC values for the other texture features (energy, entropy, sum average, sum entropy, sum variance, variance). After application of the Holm t-test, the sum average feature and size showed statistically significant conditional correlation between HiSS MRI and DCE-MRI for the malignant lesions. The performance of the HiSS MRI spiculation features ranged from an AUC value of 0.53 (radial gradient margin) to 0.74 (FWHM border). HiSS MRI had a lower AUC value for margin sharpness but a slightly higher AUC value for the FWHM grown features compared to DCE-MRI.
Table 1.
Single-feature AUC (with 95% C.I. in parentheses) and the Pearson correlation coefficient (with p-value in parentheses) between corresponding pre-contrast HiSS and DCE-MRI features for overall and conditional correlations. Significant p-values that satisfy the Holm-Bonferroni multiple comparison correction are in bold. SE = standard error.
| Computer- Extracted Lesion Features |
AUC [95% C.I.] |
Pearson Correlation Coefficient (p-value) | ||||
|---|---|---|---|---|---|---|
| HiSS | DCE-MRI | Overall | Malignant | Benign | ||
| (i) Textural | Contrast | 0.68 [0.46, 0.90] |
0.66 [0.46, 0.86] |
−0.25 (0.12) | −0.18 (0.30) | −0.69 (0.09) |
| Correlation | 0.81 [0.65, 0.97] |
0.65 [0.39, 0.91] |
0.02 (0.88) |
0.07 (0.71) | −0.48 (0.28) | |
| Difference in entropy | 0.71 [0.49, 0.93] |
0.68 [0.48, 0.88] |
−0.37 (0.02) | −0.31 (0.08) | −0.62 (0.01) | |
| Difference in variance |
0.70 [0.48, 0.92] |
0.64 [0.42, 0.86] |
−0.19 (0.02) | −0.13 (0.46) | −0.51 (0.25) | |
| Energy | 0.58 [0.36, 0.80] |
0.69 [0.51, 0.87] |
0.1 (0.53) | 0.22 (0.22) | −0.47 (0.29) | |
| Entropy | 0.55 [0.33, 0.77] |
0.62 [0.40, 0.84] |
0.17 (0.28) | 0.28 (0.11) | −0.27 (0.55) | |
| Homogeneity | 0.71 [0.47, 0.95] |
0.59 [0.35, 0.83] |
−0.14 (0.39) | 0.02 (0.89) | −0.60 (0.15) | |
| Information measure of correlation 1 (IMC1) |
0.83 [0.69, 0.97] |
0.59 [0.35, 0.83] |
−0.13 9(0.42) | −0.01 (0.93) | −0.58 (0.18) | |
| Information measure of correlation 2 (IMC2) |
0.78 [0.62, 0.94] |
0.58 [0.34, 0.82] |
−0.12 (0.47) | −0.02 (0.91) | −0.60 (0.15) | |
| Maximum correlation coefficient |
0.81 [0.67, 0.95] |
0.65 [0.41, 0.82] |
−0.07 (0.68) | 0.01 (0.94) | −0.68 (0.09) | |
| Sum average | 0.58 [0.34, 0.82] |
0.66 [0.38, 0.94] |
−0.43 (0.01) | −0.43 (0.001) | −0.08 (0.86) | |
| Sum entropy | 0.58 [0.34, 0.82] |
0.84 [0.70, 0.98] |
−0.09 (0.56) | −0.13 (0.45) | −0.16 (0.73) | |
| Sum variance | 0.56 [0.28, 0.84] |
0.82 [0.62, 1.00] |
−0.34 (0.03) | −0.40 (0.02) | −0.41 (0.35) | |
| Variance | 0.59 [0.33, 0.85] |
0.79 [0.59, 0.99] |
−0.36 (0.02) | −0.45 (0.008) | −0.36 (0.43) | |
| (ii) Spiculation | Margin sharpness | 0.71 [0.47, 0.95] |
0.75 [0.49, 1.00] |
0.27 (0.09) | 0.49 (0.003) | −0.17 (0.71) |
| Variance in margin sharpness |
0.55 [0.29, 0.81] |
|||||
| FWHM grown (HiSS) Radial gradient index (DCE-MRI) |
0.74 [0.48, 1.00] |
0.70 [0.46, 0.94] |
0.06 (0.70) | 0.06 (0.72) | −0.11 (0.81) | |
| Radial gradient grown |
0.53 [0.35, 0.71] |
|||||
| FWHM border | 0.74 [0.50, 0.98] |
|||||
| Radial gradient border |
0.53 [0.27, 0.79] |
|||||
| FWHM ROI | 0.63 [0.39, 0.87] |
|||||
| Radial gradient ROI | 0.64 [0.44, 0.84] |
|||||
| FWHM margin | 0.53 [0.27, 0.79] |
|||||
| Radial gradient margin |
0.64 [0.44, 0.84] |
|||||
| (iii) Geometric | Size | 0.78 [0.66, 0.90] |
0.76 [0.54, 0.98] |
0.45 (0.007) | 0.48 (0.002) | 0.54 (0.21) |
| Circularity (HiSS) Sphericity (DCE- MRI) |
0.75 [0.51, 0.99] |
0.82 [0.62, 1.00] |
0.14 (0.39) | 0.24 (0.17) | −0.09 (0.86) | |
| Irregularity | 0.79 [0.57, 1.00] |
0.86 [0.68, 1.00] |
0.24 (0.13) | 0.05 (0.79) | 0.38 (0.40) | |
| (iv) Kinetics | Maximum enhancement |
0.63 [0.39, 0.87] |
||||
| Time to peak (TTP) | 0.91 [0.77, 1.00] |
|||||
| Uptake rate | 0.87 [0.75, 0.99] |
|||||
| Washout rate | 0.90 [0.72, 1.00] |
|||||
| Curve shape index | 0.86 [0.68, 1.00] |
|||||
| Enhancement at 1st
postcontrast timepoint |
0.65 [0.45, 0.85] |
|||||
| Signal enhancement ratio |
0.75 [0.51, 0.99] |
|||||
| (v) Enhancement-variance kinetics | Enhancement- variance maximum enhancement |
0.73 [0.51, 0.95] |
||||
| Enhancement- variance TTP |
0.88 [0.76, 1.00] |
|||||
| Enhancement- variance increasing rate |
0.89 [0.77, 1.00] |
|||||
| Enhancement- variance decreasing rate |
0.87 [0.69, 1.00] |
|||||
Figure 2.
Comparison of single-feature AUCs of pre-contrast HiSS features and DCE-MRI features for diagnostic classification.
For the geometric features, DCE-MRI yielded higher AUC values for circularity/sphericity and irregularity compared to HiSS MRI, and the overall correlation between the size features from the two MR imaging techniques was moderately high at 0.48. Several kinetic features (time to peak, uptake rate, washout rate, curve shape index) gave high AUC values from 0.86 to 0.91. The enhancement-variance kinetics features also performed well, with AUC values ranging from 0.73 to 0.89.
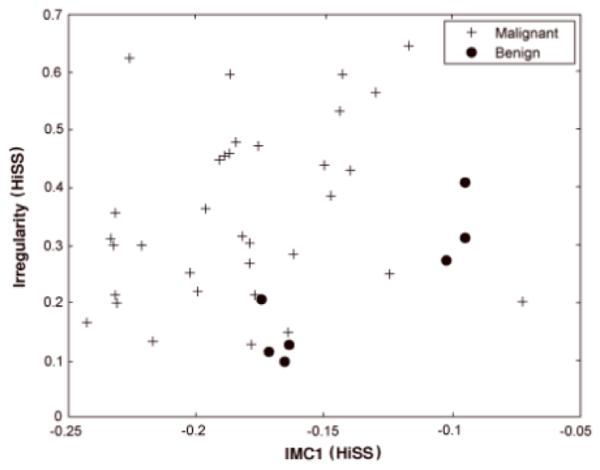
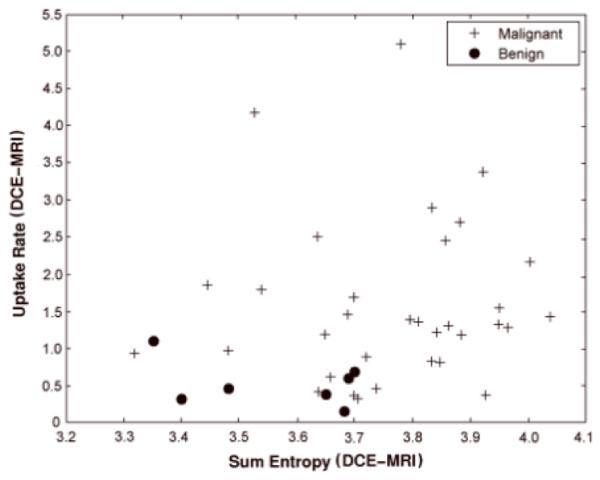
For the pre-contrast HiSS MRI classification task, the features IMC1 and irregularity were selected from the two-step feature selection process. IMC1 describes non-linear gray-level dependence within the lesion and is a measure of lesion heterogeneity. Malignant lesions tend to have higher values for irregularity and lower values for IMC1 as seen in Figure 3. For the DCE-MRI classification task, sum entropy and uptake rate were selected. Benign lesions tend to have lower uptake rate and sum entropy values than malignant lesions as illustrated in Figure 4. The Pearson correlation coefficient between HiSS MRI IMC1 and DCE-MRI sum entropy was 0.15 with a p-value of 0.40 and 95% CI of [-0.20, 0.46].
Figure 3.
Relationship between pre-contrast HiSS irregularity and GLCM-based information measure of correlation 1 (IMC1) for malignant and benign lesions.
Figure 4.
Relationship between DCE-MRI sum entropy and uptake rate for malignant and benign lesions.
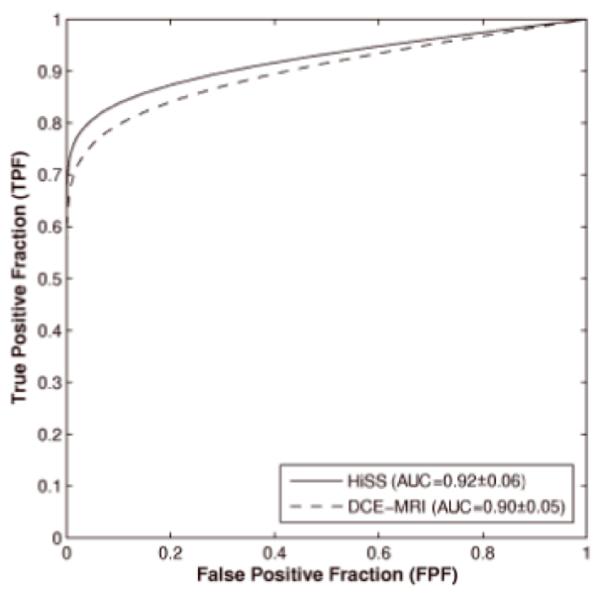
We achieved AUC values of 0.92 ± 0.06 and 0.90 ± 0.05 for the 2D HiSS MRI CADx and the 4D DCE-MRI CADx, respectively. The two-sided 95% CI for difference in AUC was [−0.10, 0.14]. The one-sided 95% CI was −0.08 which was larger than the lower bound for inferiority testing of −0.1. The selected features and performance in terms of AUC and standard error are shown in Table 2, and the corresponding ROC curves from LOLO analyses are plotted in Figure 5. The AUC for using all selected HiSS and DCE-MRI features in a combined CADx was 0.94 ± 0.04.
Table 2.
Selected features and classification performance of pre-contrast high spectral and spatial resolution (HiSS) and dynamic contrast-enhanced MRI (DCE-MRI) in terms of AUC, SE (standard error), and 95% confidence intervals (CI) for difference in AUC. IMC1 = information measure of correlation 1.
| Modality | Features Selected |
AUC ± SE | 95% CI for difference in AUC |
|---|---|---|---|
| Considering only pre-contrast HiSS features |
IMC1 Irregularity |
0.92 ± 0.06 | [−0.10, 0.14] |
| Considering only DCE-MRI features |
Sum Entropy Uptake Rate |
0.90 ± 0.05 |
Figure 5.
ROC curves for the classification performance for pre-contrast HiSS and DCE-MRI.
DISCUSSION
The diagnostic classification performance of pre-contrast 2D HiSS MRI CADx was similar to that of CADx applied on conventional clinical 4D DCE-MRI. Although we failed to show that the HiSS MRI classification [AUC = 0.92 ± 0.06] performance was significantly better than DCE-MRI [AUC = 0.90 ± 0.05], non-inferiority testing confirmed that pre-contrast HiSS MRI was not worse than DCE-MRI. In an earlier study (18) in which experienced radiologists used BIRADS rating to assess the morphological characteristics from pre-contrast 2D HiSS and 2D DCE-MRI images as part of the reader’s interpretation of benign and malignant lesions, the non-inferiority of HiSS MRI was also demonstrated. The current study improves on it by using automatic image analysis of both the 2D HiSS and the clinical 3D DCE-MRI, which eliminates potential subjectivity and inter- and intra-observer variability, and fully utilizes the morphological information in the HiSS and the temporal information in the DCE-MRI.
It is important to note that the statistical power of the current study was limited by the small size of the database. Yet, the results demonstrate that quantitative analysis of HiSS MRI may potentially provide adequate information to distinguish benign and malignant lesions without use of contrast agent. To our knowledge, this was the first use of CADx analysis methods to characterize morphology and evaluate the diagnostic utility of computer-extracted features in HiSS MR images. These promising preliminary results suggest that further testing of HiSS MRI with a larger group of patients is justified.
Radiologists have found that HiSS MR images show better margin delineation and internal lesion definition (14, 16). Thus, it is understandable that in the CADx analysis of HiSS images, irregularity and IMC1 were selected by the feature selection algorithm since the former is a measure of spiculation of the lesion, while the latter is a measure of internal lesion heterogeneity. As 3D HiSS imaging becomes available, we will be able to calculate such features in 3D as we have done already in 3D DCE-MRI and 3D T2w-MRI (56)
Of the 19 common features between HiSS MRI and DCE-MRI, sum average and size were significantly correlated. Sum average is a measure of the overall image brightness (i.e., average grey-level) in the tumor (33). The strong correlation may imply that the lesions appear as bright in water peak height images as the contrast-enhanced lesions on DCE-MRI as evident in Figure 1. Previous studies reported that HiSS MR images visually show better lesion conspicuity as compared to those on fat-saturated T1-weighted post-contrast images (14-17).
The differences in other 17 features may be attributed to the blurring effects of the contrast agent in DCE-MRI (8, 9) and fat suppression techniques (spectral separation in HiSS MRI vs. whole signal subtraction in DCE-MRI). The protocols also evaluated different physical properties of the lesion; HiSS MRI provides spectral information from the water protons in lesions while DCE-MRI measures the uptake and washout of contrast in the lesion to evaluate the vascularity of the lesion. There are also differences in in-plane resolution (0.63-0.95mm for HiSS MRI vs. 1.4mm for DCE-MRI), lesion size/volume evaluation (single slice for HiSS MRI vs entire lesion volume for DCE-MRI) and dimensional assessment (2D for HiSS MRI vs. 4D for DCE-MRI).
Thus, our quantitative evaluation of HiSS MRI features appear to be in agreement with the previous qualitative assessments of HiSS MRI (14-18), thereby supporting further tests of the deployment of HiSS imaging in the clinical area. Our results are clinically important for the patient population in whom administration of contrast agent is contra-indicated due to poor kidney function (21-23) or allergic reactions (24). These patients would benefit from a non-contrast HiSS scan with high diagnostic accuracy. In addition, the current results suggest applications of HiSS in the general patient population. While the current analysis focuses on HiSS imaging of one or two slices, more recent results demonstrate the viability of whole breast and bilateral HiSS imaging (17, 56). Bilateral pre-contrast HiSS scans could identify suspicious legions, and guide prescription of subsequent DCE-MRI, to sample these lesions with optimal spatial and temporal resolution. Improved sampling of contrast media kinetics would increase sensitivity to angiogenesis and therefore improve diagnostic accuracy. In addition, since HiSS and DCE-MRI are based on different contrast mechanisms, these two imaging methods may provide complementary information such that use of the two in combination may be beneficial. With our current study, classification with both the selected HiSS and DCE-MRI features yielded a slight increase in AUC of 0.94 ± 0.04 from the single protocol AUCs (HiSS with AUC of 0.92 ± 0.06 and DCE-MRI with AUC of 0.90 ± 0.05). With the large error bars, it is not possible to demonstrate significant improvement over DCE-MRI AUC, but the increase in AUC indicates the need for further studies. We are currently working on implementation of bilateral 3D HiSS imaging; however, due to the extremely large amounts of data generated by the scans, the challenge is in streamlining the acquisition, and data transfer and processing, in order to make 3D HiSS MRI feasible for clinical use.
A limitation to this pilot study is the small size of the database, thus constraining the statistical power of our study. The high prevalence of malignant lesions is expected in our lesion population since HiSS MRI would be employed for the diagnostic work-up of suspicious and malignant lesions. Another limitation is that the positioning in acquiring the HiSS MR image was dependent on mammography/MRI guidance, which introduces a possible confounding bias in the accuracy of HiSS MR imaging of breast lesions. However, with the development of faster data acquisition and processing techniques including parallel imaging and 2D multislice HiSS imaging of the whole breast is already achieved, negating the need for additional imaging for guidance (17, 57). The next step in our research would be to confirm the results of this preliminary study with CADx analysis of multi-slice HiSS MRI; we expect that computer-extracted features on 3D HiSS will yield better performance than those from 2D HiSS (33).
In conclusion, we demonstrated that HiSS MRI CADx can potentially provide sufficient information to characterize breast lesions without administration of contrast agent. Our results showed that automated quantitative image analysis of pre-contrast 2D HiSS MRI performs similarly to the CADx analysis of the current clinical 4D DCE-MRI in distinguishing benign and malignant breast lesions; however it is important to note the limitations in comparing single slice HiSS MRI to 3D DCE-MRI; even in the limited acquisition mode of 2D single slice HiSS, by using quantitative image analysis to extract characteristics from the HiSS images, similar performance levels were obtained as compared to those from current clinical 4D DCE-MRI. As HiSS acquisitions become possible in 3D, CADx methods can also be applied. Since HiSS and DCE-MRI are based on different contrast mechanisms, future research exploring 3D HiSS MRI as well as a combination of HiSS MRI and high temporal resolution perfusion MRI may ultimately improve lesion characterization.
Acknowledgments
The authors are grateful to Weijie Chen, PhD, for his help with the DCE-MRI database and breast CADx workstation.
MLG is a stockholder in R2 Technology/Hologic and receives royalties from Hologic, GE Medical Systems, MEDIAN Technologies, Riverain Medical, Mitsubishi and Toshiba. It is the University of Chicago Conflict of Interest Policy that investigators disclose publicly actual or potential significant financial interest that would reasonably appear to be directly and significantly affected by the research activities. Any opinions, findings and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the Department of Defense.
Grant Support
This work was supported in part by NIH R33-113800 and P50-CA125183, an NIHMedical Scientist Training Program (MSTP) grant, DOE grant DE-FG02-08ER6478, NIH S10 RR021039 and P30 CA14599, DOD grant W81XWH-10-1-0216, NIBIB RO1-EB003108, NCI RO1-CA78803, the NIH/NCI P50-CA125183, and the Segal Foundation.
References
- 1.Malich A, Fischer DR, Wurdinger S, et al. Potential MRI Interpretation Model: Differentiation of Benign from Malignant Breast Masses. AJR. 2005;185:964–970. doi: 10.2214/AJR.04.1073. [DOI] [PubMed] [Google Scholar]
- 2.Newstead GM. Role of MR in breast imaging. RSNA Categorical Course in Breast Imaging. 1999:287–293. [Google Scholar]
- 3.Warren RML, Bobrow LG, Earl HM, et al. Can breast MRI help in the management of women with breast cancer treated by neoadjuvant chemotherapy? British Journal of Cancer. 2004;90:1349–1360. doi: 10.1038/sj.bjc.6601710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartella L, Liberman L, Morris EA, Dershaw DD. Nonpalpable mammographically occult invasive breast cancers detected by MRI. AJR. 2006;186:865–870. doi: 10.2214/AJR.04.1777. [DOI] [PubMed] [Google Scholar]
- 5.Kuhl CK, Schild HH. Dynamic image interpretation of MRI of the breast. J Magn Reson Imaging. 2000;12:965–974. doi: 10.1002/1522-2586(200012)12:6<965::aid-jmri23>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Turnbull LW. Dynamic contrast-enhanced MRI in the diagnosis and management of breast cancer. NMR Biomed. 2009;22:28–39. doi: 10.1002/nbm.1273. [DOI] [PubMed] [Google Scholar]
- 7.Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246:116–124. doi: 10.1148/radiol.2461061298. [DOI] [PubMed] [Google Scholar]
- 8.Kovar DA, Al-Hallaq HA, Zamora MA, River JN, Karczmar GS. Fast spectroscopic imaging of water and fat resonances to improve the quality of MR images. Acad Radiol. 1998;5:269–275. doi: 10.1016/s1076-6332(98)80226-2. [DOI] [PubMed] [Google Scholar]
- 9.Penn A, Thrompson S, Brem R, et al. Morphological blooming in breast MRI as a characterization of margin for discriminating benign from malignant lesions. Acad Radiol. 2006;13:1344–1354. doi: 10.1016/j.acra.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medved M, Newstead GM, Fan X, et al. Fourier components of inhomogeneously broadened water resonances in breast: a new source of MRI contrast. Magn Reson Med. 2004;52:193–196. doi: 10.1002/mrm.20115. [DOI] [PubMed] [Google Scholar]
- 11. Authors identified in this reference. Citation hidden FOR REVIEW PURPOSES ONLY.
- 12.Foxley S, Fan X, Mustafi D, et al. Sensitivity to tumor microvasculature without contrast agents in high spectral and spatial resolution MR images. Magn Reson Med. 2009;61:291–298. doi: 10.1002/mrm.21801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medved M, Newstead GM, Fan X, et al. Fourier component imaging of water resonance in the human breast provides markers for malignancy. Phys Med Biol. 2009;54:5767–5779. doi: 10.1088/0031-9155/54/19/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du W, Du YP, Bick U, et al. Breast MR imaging with high spectral and spatial resolutions: preliminary experience. Radiology. 2002;224:577–585. doi: 10.1148/radiol.2242011022. [DOI] [PubMed] [Google Scholar]
- 15.Medved M, Newstead GM, Abe H, et al. High spectral and spatial resolution MRI of breast lesions: Preliminary clinical experience. AJR. 2006;186:30–37. doi: 10.2214/AJR.04.1704. [DOI] [PubMed] [Google Scholar]
- 16.Fan X, Abe H, Medved M, et al. Fat suppression with spectrally selective inversion vs. high spectral and spatial resolution MRI of breast lesions: Qualitative and quantitative comparisons. J Magn Reson Img. 2006;24:1311–1315. doi: 10.1002/jmri.20732. [DOI] [PubMed] [Google Scholar]
- 17. Authors identified in this reference. Citation hidden FOR REVIEW PURPOSES ONLY.
- 18.Medved M, Fan X, Abe H, et al. Non-contrast enhanced MRI for evaluation of breast lesions: comparison of non-contrast enhanced high spectral and spatial (HiSS) resolution images vs. contrast enhanced fat-suppressed images. Acad Radiol. 2011;18:1467–1474. doi: 10.1016/j.acra.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karczmar GS, Kuperman VY, River JN, Lewis MZ, Lipton MJ. Magnetic resonance measurement of response to hyperoxia differentiates tumors from normal tissue and may be sensitive to oxygen consumption. Invest Radiol. 1994;29(Suppl. 2):S161–163. doi: 10.1097/00004424-199406001-00053. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependence on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal R, Brunelli SM, Williams K, Mitchell MD, Feldman HI, Umscheid CA. Gadolinium-based contrast agents and nephrogenic systemic fibrosis:a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24:856–863. doi: 10.1093/ndt/gfn593. [DOI] [PubMed] [Google Scholar]
- 22.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: Suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359–2362. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- 23.Broome DR. Nephrogenic systemtic fibrosis associated with gadolinium based contrast agents: A summary of the medical literature reporting. Eur J Radiol. 2008;66:230–234. doi: 10.1016/j.ejrad.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Murphy K, Szopinski K, Cohan R, Mermillod B, Ellis J. Occurrence of adverse reactions to gadolinium-based contrast material and management of patients at increased risk: A survey of the American society of neuroradiology fellowship directors. Acad Radiol. 1999;6:656–664. doi: 10.1016/S1076-6332(99)80114-7. [DOI] [PubMed] [Google Scholar]
- 25.Kuhl CK, Schild HH, Morakkabati N. Dynamic bilateral contrast-enhanced MR imaging: Trade-off between Spatial and Temporal Resolution. Radiology. 2005;236:789–800. doi: 10.1148/radiol.2363040811. [DOI] [PubMed] [Google Scholar]
- 26.Jansen SA, Fan X, Medved M, et al. Characterizing early contrast uptake of ductal carcinoma in situ with High temporal résolution dynamic contrast-enhanced MRI of the breast : a pilot study. Phys Med Biol. 2010;55:N473–485. doi: 10.1088/0031-9155/55/19/N02. [DOI] [PubMed] [Google Scholar]
- 27.Ertaş G, Gülçür HO, Tunaci M. Improved Lesion Detection in MR Mammography: Three-Dimensional Segmentation, Moving Voxel Sampling, and Normalized Maximum Intensity–Time Ratio Entropy. Academic Radiology. 2007;14:151–161. doi: 10.1016/j.acra.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Penn AI, Kumar N, Thompson SF, Schnall MD. Preliminary performance analysis of breast MRI CAD system. Proc. SPIE. 2001;4322:1944. [Google Scholar]
- 29.Meyer-Baese A, Wismueller A, Lange O, Leinsinger G. Computer-aided diagnosis in breast MRI based on unsupervised clustering techniques. Proc. SPIE. 2004;5421:29. [Google Scholar]
- 30.Szabo BK, Wiberg MK, Bone B, Aspelin P. Application of artificial neural networks to the analysis of dynamic MR imaging features of the breast. Eur Rad. 2004;14:1217–1225. doi: 10.1007/s00330-004-2280-x. [DOI] [PubMed] [Google Scholar]
- 31.Bhooshan N, Giger ML, Jansen SA, Li H, Lan L, Newstead GM. Cancerous breast lesions on dynamic contrast-enhanced MR images: computerized characterization for image-based prognostic markers. Radiology. 2010;254:680–690. doi: 10.1148/radiol.09090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W, Giger ML, Bick U. A fuzzy c-means (FCM) based approach for computerized segmentation of breast lesions in dynamic contrast-enhanced MR images. Acad Radiol. 2006;13:63–72. doi: 10.1016/j.acra.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 33. Authors identified in this reference. Citation hidden FOR REVIEW PURPOSES ONLY.
- 34.Chen W, Giger ML, Bick U, Newstead GM. Automatic identification and classification of characteristic kinetic curves of breast lesions on DCE-MRI. Medical Physics. 2006;33:2878–2887. doi: 10.1118/1.2210568. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Giger ML, Lan L, Bick U. Computerized interpretation of breast MRI: Investigation of enhancement-variance dynamics. Medical Physics. 2004;31:1076–1082. doi: 10.1118/1.1695652. [DOI] [PubMed] [Google Scholar]
- 36.Shimauchi A, Giger ML, Bhooshan N, et al. Reader Study for the Evaluation of Radiologists’ Interpretation of Breast MRI using a CAD Breast MRI workstation. Radiology. 2011;258:696–704. doi: 10.1148/radiol.10100409. [DOI] [PubMed] [Google Scholar]
- 37.Meinel LA, Stolpen AH, Berbaum KS, Fajardo LL, Reinhardt JM. Breast MRI lesion classification: Improved performance of human readers with a backpropagation neural network computer-aided diagnosis (CAD) system. J Mag Res Img. 2007;25:89–95. doi: 10.1002/jmri.20794. [DOI] [PubMed] [Google Scholar]
- 38.Giger ML, Chan H-P, Boone J. Anniversary paper: History and status of CAD and quantitative image analysis: The role of Medical Physics and AAPM. Med Phys. 2008;35:5799–5820. doi: 10.1118/1.3013555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Y, Nishikawa RM, Schmidt RA, et al. Improving breast cancer diagnosis with computer-aided diagnosis. Acad Radiol. 1999;6:22. doi: 10.1016/s1076-6332(99)80058-0. [DOI] [PubMed] [Google Scholar]
- 40.Gibbs P, Turnbull LW. Textural analysis of contrast-enhanced MR images of the breast. Magn Reson Med. 2003;50:92–98. doi: 10.1002/mrm.10496. [DOI] [PubMed] [Google Scholar]
- 41.Huo Z, Giger ML, Vyborny CJ, Wolverton DE, Schmidt RA, Doi K. Automated Computerized Classification of Malignant and Benign Masses on Digitized Mammograms. Acad Radiol. 1998;5:155–168. doi: 10.1016/s1076-6332(98)80278-x. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Giger ML, Olopade O, Margolis A, Lan A, Chinander M. Computerized texture analysis of mammographic parenchymal patterns of digitized mammograms. Acad Rad. 2005;12:963–973. doi: 10.1016/j.acra.2005.03.069. [DOI] [PubMed] [Google Scholar]
- 43.Gilhuijs KGA, Giger ML, Bick U. Automated analysis of breast lesions in three dimensions using dynamic magnetic resonance imaging. Med Phys. 1998;25:1647–1654. doi: 10.1118/1.598345. [DOI] [PubMed] [Google Scholar]
- 44.Glantz S. Primer of Biostatistics. 5th McGraw-Hill; New York, NY: 2002. pp. 262–267. [Google Scholar]
- 45.Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Statist. 1979;6:65–70. [Google Scholar]
- 46.Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. 3rd Prentice-Hall; Englewood Cliffs, NJ: 1992. pp. 629–665. [Google Scholar]
- 47.Finette S, Bleier A, Swindell W. Breast tissue classification using diagnostic ultrasound and pattern recognition techniques: I. Methods of pattern recognition. Ultrasonic Imaging. 1983;5:55–70. doi: 10.1177/016173468300500106. [DOI] [PubMed] [Google Scholar]
- 48.Kupinski MA, Giger ML. Feature selection with limited datasets. Med Phys. 1999;26:2176–2182. doi: 10.1118/1.598821. [DOI] [PubMed] [Google Scholar]
- 49.Chan HP, Sahiner B, Wagner RF, Petrick N. Classifier design for computer-aided diagnosis: Effects of finite sample size on the mean performance of classical and neural network classifiers. Med Phys. 1999;26:2654–2668. doi: 10.1118/1.598805. [DOI] [PubMed] [Google Scholar]
- 50.MacKay DJS. Bayesian Methods for Adaptive Models. California Institute of Technology; Pasadena, California: 1992. Ph.D. thesis. [Google Scholar]
- 51.Metz CE. Some practical issues of experimental design and data analysis in radiological ROC studies. Invest. Radiol. 1989;24:234–245. doi: 10.1097/00004424-198903000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Metz CE, Herman BA, Roe CA. Statistical comparison of two ROC-curve estimates obtained from partially-paired datasets. Med Decision Making. 1998;18:110–121. doi: 10.1177/0272989X9801800118. [DOI] [PubMed] [Google Scholar]
- 53. Authors identified in this reference. Citation hidden FOR REVIEW PURPOSES ONLY.
- 54.Pocock SJ. The pros and cons of noninferiority trials. Fundam Clin Pharmacol. 2003;17:483–490. doi: 10.1046/j.1472-8206.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- 55.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJW. Reporting of noninferiority and equivalence randomized trials: An extension of the CONSORT statement. JAMA. 2006;295:1152–1160. doi: 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]
- 56.Bhooshan N, Giger ML, Lan L, Li H, Marquez A, Shimauchi A, Newstead GM. Combined use of T2-weighted MRI and T1-weighted dynamic contrast–enhanced MRI in the automated analysis of breast lesions. MRM. 2011;66:555–564. doi: 10.1002/mrm.22800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Authors identified in this reference. Citation hidden FOR REVIEW PURPOSES ONLY.