Abstract
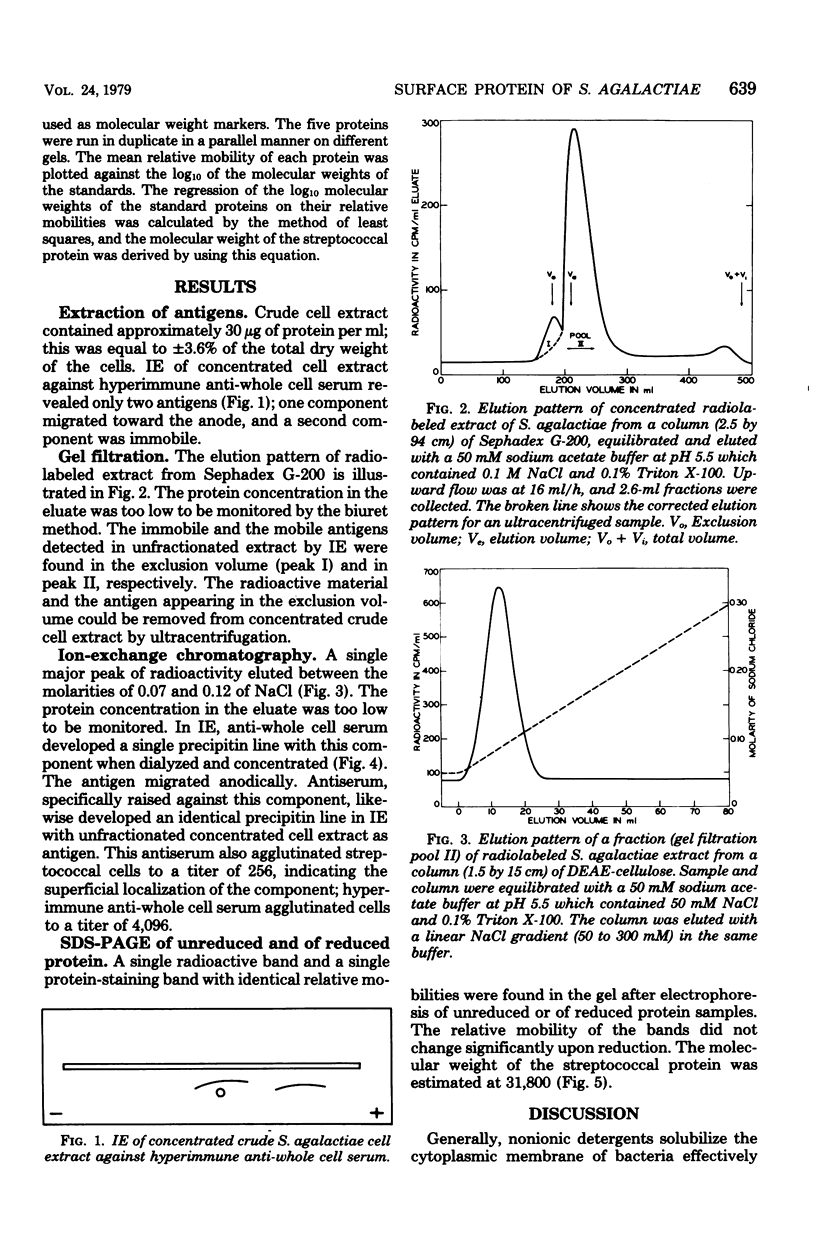
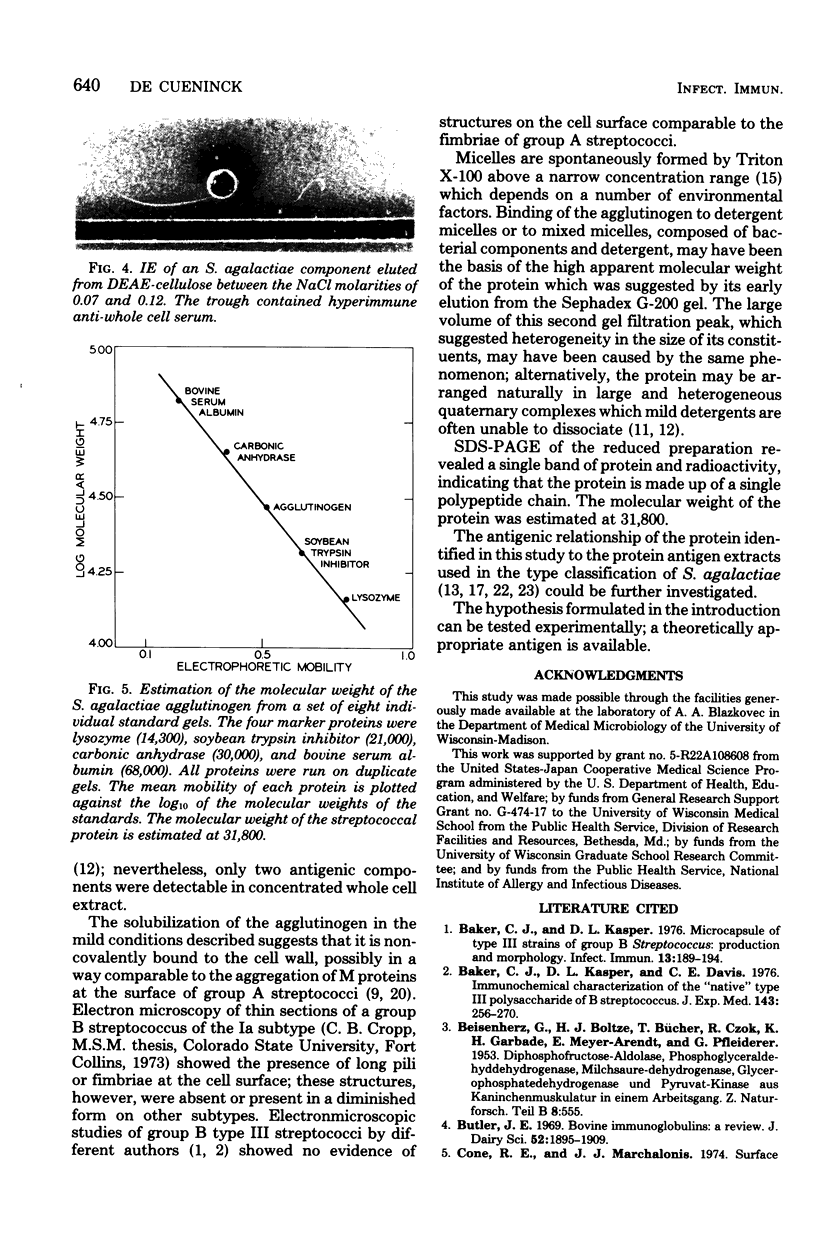
A Streptococcus agalactiae isolate of bovine origin was cultured in broth; log-phase cells were washed and radioiodinated and subsequently extracted at low pH in the presence of a nonionic detergent. A protein antigen was purified from concentrated extract by ultracentrifugation, gel filtration, and ion-exchange chromatography. The molecular weight of the protein was estimated at 31,800. The agglutinogenic character of the protein indicated its localization at the cell surface.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. J., Kasper D. L., Davis C. E. Immunochemical characterization of the "native" type III polysaccharide of group B Streptococcus. J Exp Med. 1976 Feb 1;143(2):258–270. doi: 10.1084/jem.143.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Microcapsule of type III strains of group B Streptococcus: production and morphology. Infect Immun. 1976 Jan;13(1):189–194. doi: 10.1128/iai.13.1.189-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. E., Marchalonis J. J. Surface proteins of thymus-derived lymphocytes and bone-marrow-derived lymphocytes. Selective isolation of immunoglobulin and the theta-antigen by non-ionic detergents. Biochem J. 1974 Jun;140(3):345–354. doi: 10.1042/bj1400345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Siviglia G., Zabriskie J. B. Streptococcal M protein extracted by nonionic detergent. I. Properties of the antiphagocytic and type-specific molecules. J Exp Med. 1976 Jul 1;144(1):32–53. doi: 10.1084/jem.144.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein W. N. Quantitative densitometry of 1-50 g protein in acrylamide gel slabs with Coomassie blue. Anal Biochem. 1972 Apr;46(2):388–401. doi: 10.1016/0003-2697(72)90312-0. [DOI] [PubMed] [Google Scholar]
- Frost A. J. Selective adhesion of microorganisms to the ductular epithelium of the bovine mammary gland. Infect Immun. 1975 Nov;12(5):1154–1156. doi: 10.1128/iai.12.5.1154-1156.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. The binding of detergents to lipophilic and hydrophilic proteins. J Biol Chem. 1972 Jun 10;247(11):3656–3661. [PubMed] [Google Scholar]
- LANCEFIELD R. C., PERLMANN G. E. Preparation and properties of a protein (R antigen) occurring in streptococci of group A, type 28 and in certain streptococci of other serological groups. J Exp Med. 1952 Jul;96(1):83–97. doi: 10.1084/jem.96.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. J., McDonald J. S. Streptococci isolated from bovine intramammary infections. Am J Vet Res. 1976 Apr;37(4):377–381. [PubMed] [Google Scholar]
- PATTISON I. H., MATTHEWS P. R., HOWELL D. G. The type classification of group-B streptococci, with special reference to bovine strains apparently lacking in type polysaccharide. J Pathol Bacteriol. 1955 Jan-Apr;69(1-2):51–60. [PubMed] [Google Scholar]
- Schalm O. W., Lasmanis J., Carroll E. J. Experimental Streptococcus agalactiae mastitis in cattle: attempts to superimpose the organism in lactating glands harboring unrelated bacterial infections and in glands with experimentally induced sterile inflammation. Am J Vet Res. 1967 May;28(124):685–695. [PubMed] [Google Scholar]
- Schalm O. W., Lasmanis J., Carroll E. J. Significance of leukocytic infiltration into the milk in experimental Streptococcus agalactiae mastitis in cattle. Am J Vet Res. 1966 Nov;27(121):1537–1546. [PubMed] [Google Scholar]
- Swanson J., Hsu K. C., Gotschlich E. C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969 Nov 1;130(5):1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilkinson H. W. Comparison of streptococcal R antigens. Appl Microbiol. 1972 Oct;24(4):669–670. doi: 10.1128/am.24.4.669-670.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson H. W., Eagon R. G. Type-specific antigens of group B type Ic streptococci. Infect Immun. 1971 Nov;4(5):596–604. doi: 10.1128/iai.4.5.596-604.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]




