Abstract
C-type lectins (CTLs) are a superfamily of calcium-dependent carbohydrate binding proteins containing at least one carbohydrate-recognition domain (CRD) and they are present in almost all metazoans. Insect CTLs may function as pattern-recognition receptors and play important roles in innate immunity. In this study, we selected five AsCTLs from the mosquito Armigeres subalbatus, a natural vector of filarial nematodes, and performed both in vitro and in vivo studies to elucidate their functions in innate immunity. AsCTLMA15, AsCTLGA5 and AsCTL15 were mainly expressed in hemocytes, AsCTL16 was expressed in fat body, while AsCTLMA11 was expressed in both hemocytes and fat body, and only AsCTLMA11 and AsCTL16 were expressed at high levels in adult females. In vitro binding assays showed that all five recombinant AsCTLs could bind to different microbial cell wall components, including lipopolysaccharide (LPS), lipid A, peptidoglycan (PG), lipoteichoic acid (LTA), zymosan and laminarin (beta-1,3-glucan). Recombinant AsCTLs also bound to several Gram-negative and Gram-positive bacteria, and could agglutinate bacterial cells. Injection of double-stranded RNAs (dsRNAs) could significantly reduce expression of the five AsCTL mRNAs, and the survival of mosquitoes treated with dsRNA to AsCTLGA5 was significantly decreased after Escherichia coli infection, but did not change significantly after Micrococcus luteus infection compared to the control groups, suggesting that Ar. subalbatus AsCTLGA5 may participate in innate immunity against E. coli.
Keywords: C-type lectin, Armigeres subalbatus, innate immunity, RNAi, survival
1. Introduction
Mosquitoes are disease vectors that can transmit malaria, lymphatic filariasis, dengue and yellow fevers, Japanese encephalitis, and some other diseases, which cause death and incapacity to millions of people every year (McGraw and O’Neill, 2013; Ramasamy and Surendran, 2012; Roberts, 2002). Mosquito-borne diseases have devastating effects because of enormous health and economic burdens on a large percentage of the population in the world, especially in the tropical and subtropical regions (Aliota et al., 2007; Christensen et al., 2005). Thus, it is necessary to develop new and efficient strategies for disease treatment and vector control. Mosquitoes lack the adaptive immune system and totally depend on the innate immune system to fight against pathogens (Hillyer, 2010; Hoffmann, 2003; Iwanaga and Lee, 2005; Osta, et al., 2004b). Therefore, identification of mosquito immune-related genes and gene products may help better understand mosquito defense mechanisms against invading pathogens (Aliota et al., 2007; Christensen et al., 2005; Waterhouse et al., 2007; Yassine and Osta, 2010). In order to trigger immune responses against different pathogens, mosquitoes must apply various pathogen recognition proteins/receptors, and studies on pathogen recognition in mosquitoes will provide valuable information for developing new methods in mosquito-borne disease control and for understanding the evolution of the innate immune system (Cirimotich et al, 2010; Hillyer, 2010; Osta et al., 2004b; Yassine and Osta, 2010).
The mosquito Armigeres subalbatus is a natural vector of filarial nematode parasites that cause lymphatic filariasis (Mayhew et al., 2007). Ar. subalbatus is an ideal model to study the innate immune system against filarial nematodes, since it is susceptible to the filarial nematode Brugia pahangi but refractory to B. malayi microfilariae with a strong melanotic encapsulation response (Aliota et al., 2007; Mayhew et al., 2007). Ar. subalbatus is one of a few mosquito species using melanotic encapsulation to protect against metazoan pathogens (Christensen et al., 2005). It is a vector that transmits Japanese encephalitis virus and also a laboratory vector for study of Plasmodium gallinaceum that causes avian malaria (Chen et al., 2000; Kanojia and Geevarghese, 2005; Mayhew et al., 2007).
Recognition of non-self pathogens is accomplished by germ-line encoded pattern recognition receptors (PRRs) (Hoffmann, 2003). PRRs can recognize pathogen-associated molecular patterns (PAMPs), the conserved molecular patterns that present on the pathogen surface but not on the host cells (Medzhitov and Janeway, 2002; Pal and Wu, 2009). Recognition of PAMPs by different PRRs can initiate various cellular and humoral immune responses, such as phagocytosis, nodulation, encapsulation and melanization, prophenoloxidase activation, and synthesis of antimicrobial peptides (AMPs) (Kanost et al., 2004; Osta et al., 2004b; Pal and Wu, 2009). C-type lectins (CTLs) are one major family of PRRs in innate immunity and one of the largest animal lectin families with binding ability to glycoproteins and glycolipids on the surface of pathogens (Cirimotich et al., 2010; Hardison and Brown, 2012; Kerrigan and Brown, 2011; Kingeter and Lin, 2012; Takeuchi and Akira, 2010; van den Berg et al., 2012). Typical vertebrate CTLs are calcium-dependent carbohydrate binding proteins, and most members contain one carbohydrate-recognition domain (CRD) for ligand binding (Kanost et al., 2004; Zelensky and Gready, 2005). Based on the conserved amino acid motifs for ligand binding and calcium coordination, classical vertebrate CTLs can be further divided into two groups: mannose-type and galactose-type (van Vliet et al., 2008a, 2008b). Mannose-type CTLs contain aGlu-Pro-Asn (EPN) motif in the CRD with predicted binding specificity for mannose, glucose and fucose, while a Gln-Pro-Asp (QPD) motif is present in the CRD of galactose-type CTLs for recognition of galactose and N-acetyl-D-galactosamine (GalNac)(van Vliet et al., 2008b). CTLs that do not contain the conserved EPN or QPD motif belong to the other-type CTLs.
There are more than 30 genes encoding C-type lectin domains (CTLDs) in the Drosophila melanogaster genome (Dodd and Drickamer, 2001). A galactose-specific CTL(called DL1) purified from D. melanogaster pupae can bind to Escherichia coli and Erwinia chrysanthemi, and agglutinate E. coli cells (Tanji et al., 2006), while two other Drosophila CTLs can enhance encapsulation and melanization (Ao et al., 2007). Drosophila lectin-24 A participates in defense against parasitic wasp infection (Keebaugh and Schlenke, 2012). The Anopheles gambiae genome contains 22 genes encoding proteins with CTLDs (Christophides et al., 2002). Two An. gambiae CTLs (AgCTL4 and AgCTLMA2) have been shown to protect Plasmodium parasites from melanization, and they can also form heterodimers to enhance clearance of Gram-negative bacteria (Osta et al., 2004a; Schnitger et al., 2009). A C-type lectin, mosGCTL-1 (AAEL000563 or AaCTLMA15) from the mosquito Aedes aegypti, is induced by West Nile virus (WNV) and can facilitate virus infection (Cheng et al., 2010). Several CTLs (called immulectins) containing dual CRDs from the tobacco hornworm, Manduca sexta, can stimulate phagocytosis, hemocyte encapsulation and melanization, prophenoloxidase activation, and protect larvae from bacterial infection (Ling and Yu, 2006; Yu and Kanost, 2000, 2003, 2004; Yu et al., 1999, 2005, 2006).
In Ar. subalbatus, a lectin containing a fibrinogen-like domain (as lectin or AL-1) has been cloned and characterized, and its mRNA expression is up-regulated by Gram-negative E. coli and Gram-positive Micrococcus luteus (Wang et al., 2004). AL-1 specifically recognizes N-acetyl-D-glucosamine (GlcNac) and can bind to both E. coli and M. luteus, and it may function as a pattern recognition receptor in innate immune response of Ar. subalbatus (Wang et al., 2004). There are 17 ESTs encoding proteins with CTLDs in Ar. subalbatus; however, functions of Ar. subalbatus CTLs (AsCTLs) in innate immunity have not been reported so far.
In this study, we selected five AsCTLs that are predicted to be secreted proteins and represent the three types of CTLs (two mannose-types, one galactose-type and two other-types), cloned the full-length cDNA sequences, expressed and purified recombinant lectins, and studied their potential functions in innate immune responses by in vitro and in vivo assays. Our results showed that the transcript levels of the five AsCTLs were up-regulated by E. coli and/or M. luteus, and recombinant AsCTLs could bind to different microbial cell wall components, such as lipopolysaccharide (LPS), lipid A, peptidoglycan (PG), lipoteichoic acid (LTA), laminarin and zymosan, and to several Gram-negative and Gram-positive bacteria. RNA interference (RNAi) experiments showed that expression of the five AsCTLs transcripts in female mosquitoes could be significantly down-regulated by injection of double-stranded RNAs (dsRNAs), and knockdown expression of AsCTLGA5 could significantly decrease survival of Ar. subalbatus after E. coli infection, but did not change the survival of mosquitoes significantly after M. luteus infection, suggesting that AsCTLGA5 may participate in immune defense against E. coli in Ar. subalbatus.
2. Material and methods
2.1 Mosquito rearing, microorganisms, microbial components and saccharides
Ar. subalbatus used in this study were maintained at the University of Missouri- Columbia following the methods described previously (Beerntsen et al., 1989; Wang et al., 2012).
Gram-positive Staphylococcus aureus, Bacillus subtilis, B. cereus, and M. luteus, Gram-negative E. coli DH5α, Serratia marcescens, Pseudomonas aeruginosa, and yeast Saccharomyces cerevisiae were originally from ATCC or Sigma and maintained in the laboratory. Smooth LPS from E. coli 055:B5 and 026:B6, S. marcescens, P. aeruginosa, Salmonella enterica, rough mutants of LPS from E. coli EH100 (Ra-LPS), E. coli F583 (Rd-LPS), E. coli J5 (Rc-LPS), and S. enterica serotype minnesota Re 595 (Re-LPS), mono-phosphoryl lipid A from E. coli F583 (Rd mutant), and di-phosphoryl lipid A from E. coli F583 (Rd mutant), laminarin (β-1,3-glucan), curdlan, mannan, zymosan (from S. cerevisiae), D-galactose, L-galactose, N-acetyl-D-galactosamine (GalNac), D-glucose, L-fucose, L-rhamnose, talose, xylose, lactose, sucrose, maltose, melibiose, chitotriose were all from Sigma-Aldrich (MO, USA). TLR grade LPS-K12 and PG-K12 from E. coli K12, PG-SA and LTA-SA from S. aureus, and PG-BS and LTA-BS from B. subtilis were from Invivogen (CA, USA).
2.2 Sequence alignment and data analysis
The five AsCTLEST sequences were obtained from the Ar. subalbatus database (https://asap.ahabs.wisc.edu/asap/full_text.php). The protein sequences were deduced from the cDNA sequences using the ExPASy program (http://web.expasy.org/translate/). Multiple sequence alignment was performed with ClustalW (http://www.ch.embnet.org/software/ClustalW.html). Figures were made using GraphPad Prism software (GraphPad, San Diego, CA) with one representative data set from at least three biological samples. Significant difference was determined by the unpaired t-test or one way ANOVA followed by a Tukey’s multiple comparison test (GraphPad, San Diego, CA).
2.3 Tissue distribution and developmental expression profiles of AsCTLs
Ar. subalbatus larvae (first, second, third and fourth in star), pupae (early and late stages) and adult females (days 0, 1, 3, 5, 7 and 9) were collected as described previously (Wang et al., 2012) for preparation of total RNAs using TRI reagent (Sigma-Aldrich) following the manufacturer’s instructions. Total RNA was treated with RQ1 RNase-Free DNase (Promega) to remove contaminated genomic DNA and then reverse transcribed to first stand cDNA using M-MLV reverse transcriptase (Promega). The cDNA was diluted twenty-fold as the template for quantitative real-time PCR. Primers used for cloning of AsCTLMA11 (AY441312), AsCTLMA15 (EU206257/AY440709), AsCTLGA5 (EU207651), AsCTL15 (EU205642/AY440422), AsCTL16 (EU206532/AY440644), and AsRPL9 (ribosomal protein L9 gene as an internal control) genes are listed in Table S1. The reaction mixture contained 10 μL 2×SYBR Green/ROX qPCR Master Mix (SABiosciences, Qiagen), 4 μL each of the forward and reverse primers (1 pmole/μL), and 2 μL twenty-fold diluted cDNA template (totally 20μL). The real-time PCR conditions were: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Then dissociation curve analysis was performed. Real-time PCR was performed in an AB7000 qRT-PCR instrument (Applied Biosystems), and the data were output with sequence detection software (SDS-7000 software, Applied Biosystems) and analyzed by a comparative method (2−ΔΔCt). Real-time PCR was repeated with three different biological samples, and each sample was repeated at least three times.
To determine the expression profiles of the five AsCTLs in different tissues of Ar. subalbatus, female adults (4–5 days old) were anesthetized on ice and dissected, hemocytes, ovary, nerve, muscle, Malpighian tubule, fat body, midgut, and cuticle were collected from about 30 mosquitoes following the method described previously (Wang et al., 2012). Total RNAs were extracted from these tissues and reverse transcribed to the first strand cDNAs, which were diluted twenty-fold and used as the templates for real-time PCR as described above. The real-time PCR was performed with three different biological samples, and each sample was repeated at least three times.
2.4 Induced expression of AsCTLs after bacterial challenge
Ar. subalbatus females were reared and separated as described previously (Beerntsen et al., 1989), and female adults (4–5 days old) were used for the experiments. Gram-negative E. coli and Gram-positive M. luteus were cultured overnight in Luria–Bertani medium (10 gtryptone, 5 g yeast extract, and 10 g NaClin l liter distilled water) at 37°C with 200 rpm, pelleted by centrifugation, washed with Aedes saline (154 mM NaCl, 2.68 mM KCl, 1.36 mM CaCl2, 1.19 mM NaHCO3, pH7.0) three times, and resuspended in Aedes saline (McGraw and O’Neill, 2013). Half (0.5)μL of diluted E. coli (OD600=0.1), M. luteus (OD600=0.15) or Aedes saline (as a control) was injected into the thorax of each mosquito. Ten mosquitoes were randomly collected from each group at 3, 6, 12, 24 and 48 h post-injection for preparation of total RNAs, which were reverse transcribed to the first strand cDNAs for real-time PCR analysis as described above. These experiments were repeated with three biological samples, and each sample was repeated three times.
2.5 Expression and purification of recombinant AsCTLs
Total RNA from female adults (4–5 days old) was extracted and reverse transcribed to the first stand cDNA, which was diluted ten-fold and used as the template for the following PCR reactions. Primers used to amplify AsCTLMA11, AsCTLMA15, AsCTLGA5, AsCTL15 and AsCTL16 cDNA sequences encoding mature proteins without putative signal peptides (see Fig. 1) are listed in Table S1.
Fig. 1. Alignment of Ar. subalbatus C-type lectins (AsCTLs).
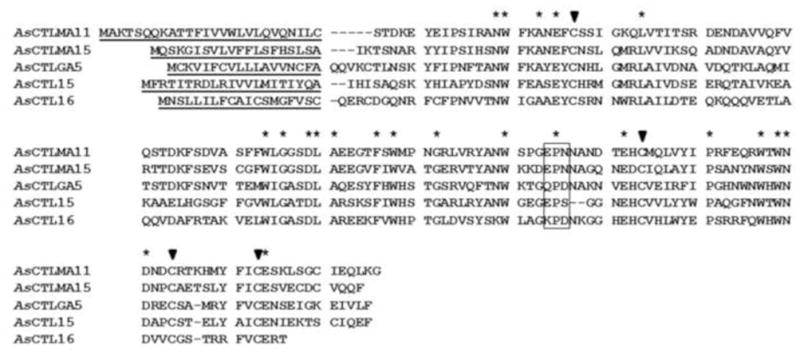
Protein sequences of AsCTLMA11 (AY441312), AsCTLMA15 (EU206257), AsCTLGA5 (EU207651), AsCTL15 (EU205642) and AsCTL16 (EU206532) were aligned by ClustalW, and residues conserved in all five AsCTLs are indicated by asterisks. Predicted signal peptides are underlined, and four highly conserved cysteine residues in the CRDs are indicated by filled triangles. The predicted motifs (EPN, QPD, EPS and KPD) in the CRDs important for carbohydrate binding and calcium coordination are boxed.
The five cDNA fragments were purified and ligated into the NcoI and Hind III sites of the H6-pQE60 vector (Lee et al., 1994) that expresses recombinant proteins with a 6-Histidine tag at the N-terminus. Recombinant AsCTLs were expressed in E. coli XL1-blue cells after induction with 1 mM isopropyl-1-thio-β-D-galactopyranoside (IPTG) at 37°C overnight (Yu et al., 1999).
All five recombinant AsCTLs were expressed as insoluble inclusion bodies and purified in 8 M urea under denaturing conditions by nickel-nitrilotriacetic acid (Ni-NTA) resins (Qiagen) following the manufacturer’s instructions. The purified lectins in 8 M urea were refolded by 3-step dialysis as described previously (Yu et al., 2005) and stored at −80°C for future use.
2.6 Hemagglutination of animal erythrocytes by recombinant AsCTLs
Hemagglutination assays were performed with recombinant AsCTLs as described previously (Yu and Kanost, 2000). Briefly, erythrocytes from human group O (Fisher Scientific), human group B, sheep (Sigma), goat, bovine, horse, and porcine (LampireBiological Labs) were completely washed in Tris-buffered saline (TBS) (25 mM Tris-HCl, 137 mM NaCl, 3 mM KCl, pH 7.0) containing 5 mM CaCl2 and then resuspended in TBS as 2% suspensions. Recombinant AsCTLs were prepared at 100μg/ml in TBS containing 5 mM CaCl2 and serially diluted in wells of a microtiter V-shape plate. Then equal volume of 2% erythrocytes was added to each well and mixed. The plate was incubated for 1 h at room temperature. Agglutinated erythrocytes formed a diffuse mat, whereas unagglutinated erythrocytes formed a clear red dot at the bottom of the well.
To determine ligand binding specificity of AsCTLs, a competitive hemagglutination assay was performed. Recombinant AsCTLGA5 or AsCTL16 (2μg/ml) was pre-incubated with serially diluted polysaccharides or saccharides in the wells of a microtiter V-shape plate at room temperature for 30 min. Then equal volume of 2% sheep erythrocytes was added to each well and mixed and the plate was incubated for 1 h at room temperature. Hemagglutination activity of AsCTLGA5 or AsCTL16 was determined the same as described above.
2.7 Binding of recombinant AsCTLs to microbial cell wall components
Wells of flat-bottom 96-well plates (NuncMaxiSorp, eBioscience) were coated with 2μg/well (50 μL/well of 40μg/mL in water) of microbial cell wall components as described previously (Yu and Kanost, 2000; Yu and Ma, 2006). Purified recombinant AsCTLs were diluted in the binding buffer (50 mM Tris-HCl, 50 mM NaCl, pH8.0) containing 0.1 mg/mL BSA and 5 mM CaCl2 to a final concentration of 10μg/ml and added to each well of the coated plates (50 μL/well). For competitive binding assay, recombinant AsCTLs (10 μg/ml) was pre-incubated with increasing concentrations of free microbial components or saccharides for 1 h at room temperature as described previously (Yu et al., 2005) and then added to LPS (E. coli 026:B6)-coated plates. The plates were incubated at room temperature for 3 h, washed with binding buffer four times (each for 5 min), incubated with mouse monoclonal anti-polyhistidine antibody (Sigma-Aldrich, 1:3000 dilution) (100 μL/well) at 37°C for 2 h, washed with binding buffer again, and incubated with the alkaline phosphatase-conjugated goat anti-mouse IgG (Sigma-Aldrich, 1:2000 dilution) (100 μL/well) at 37°C for another 2 h. Finally, after washing for four times, 50 μL p-nitro-phenyl phosphate (1 mg/mL in 10 mM diethanolamine, 0.5 mM MgCl2) were added to each well, and absorbance at 405 nm was measured every minute for a total of 20 min using a plate reader (BioTek PowerWave XS). The data were analyzed by the GraphPad Prism software.
2.8 Binding of recombinant AsCTLs to microorganisms
Direct binding of recombinant AsCTLs to microorganisms was performed as described previously (Du et al., 2009) with slight modifications. Briefly, Gram-positive bacteria (S. aureus, M. luteus, B. cereus and B. subtilis), Gram-negative bacteria (E. coli DH5α, P. aeruginosa and S. marcescens) and yeast (S. cerevisiae) were cultured in 5 mL LB medium (for bacteria) or in YPD medium (1% yeast extract, 2% peptone and 2% dextrose) (for yeast) and grown to mid-log phase. The microorganisms were pelleted by centrifugation at 6000 g for 5 min, washed twice with TBS, and then thoroughly resuspended in TBS. Purified recombinant AsCTLs and M. sexta cuticle CP36 protein (as a control) (Suderman et al., 2003) in 500 μL buffer A (50 mM Tris-HCl, 5 mM EDTA, pH 8.0) (20μg/mL) were added and incubated with 500 μL bacteria (4×108 cells/mL) or yeast (4×107 cells/mL) in buffer A with rotation for 1 h at room temperature. The microorganisms were pelleted and the supernatants were collected as unbound proteins. These microorganisms were washed four times with TBS, subjected to elution with 7% SDS for 10 min, and washed in 0.5 mL TBS four times. Samples of the unbound proteins, TBS wash, 7% SDS elution and microbial lysates (to detect tightly bound proteins) were subjected to immunoblotting analysis. For immunoblotting, proteins were separated on 12% SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was blocked with 5% bovine serum albumin (BSA) in TBS, incubated with mouse monoclonal anti-polyhistidine antibody (Sigma-Aldrich) (1:3000 dilution in TBS containing 0.1 mg/mL BSA) followed by incubation with alkaline phosphatase conjugated to goat anti-mouse IgG (Sigma-Aldrich) (1:10000 dilution in TBS containing 0.1 mg/mL BSA). Antibody binding was visualized by a color reaction.
2.8 Agglutination of microorganisms by recombinant AsCTLs
Agglutination assays were performed as described previously (Yu et al., 1999, 2006). Fluorescein isothiocyanate (FITC)-labeled S. aureus, E. coli K12 bioparticles (Molecular Probes) and cultured microorganisms (S. marcescens, S. cerevisiae, B. subtilis, B. cereus, P. aeruginosa and M. luteus) were used for the agglutination assays. Aliquots (3.5 μL) of bacteria (3×109 cells/mL) and yeast (2.5×108 cells/mL) suspensions were mixed with purified recombinant AsCTLs or M. sexta CP36 protein (final concentration of 40μg/mL) in a total of 25 μL TBS containing 2 mM CaCl2 and 1 mM MgCl2. The mixtures were incubated at room temperature for 35 minutes and then observed by microscopy or fluorescent microscopy.
2.9 RNA interference experiments
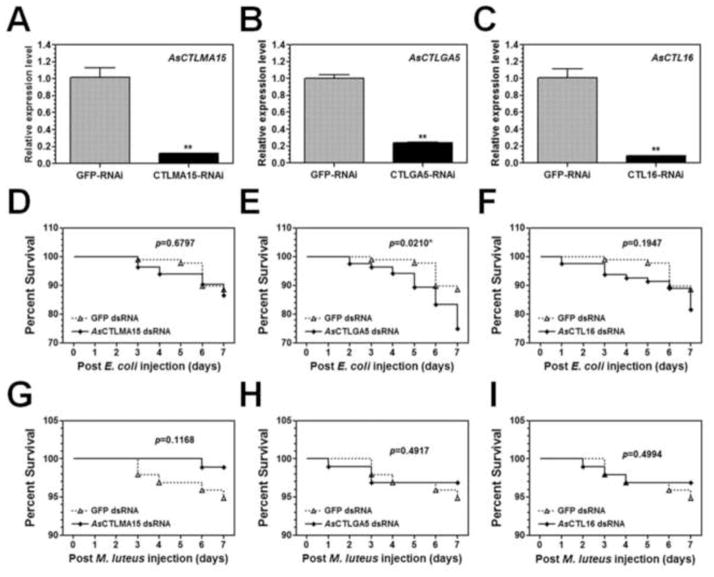
The first strand cDNA was reverse transcribed from total RNA of Ar. subalbatus female adults (4–5 days old) and used as the template for PCR amplification of the five AsCTLs using primers listed in Table S1. The PCR fragments (426, 255, 437, 426 and 407 bp for AsCTLMA11, AsCTLMA15, AsCTLGA5, AsCTL15 and AsCTL16, respectively) were purified by a Gel purification kit (Promega) and used as templates to synthesize double-stranded RNAs (dsRNAs) using MEGAscript® RNAi Kit (Ambion, Life Technologies). Green fluorescent protein (GFP) was used as a control. The final concentrations of dsRNAs were adjusted to 1μg/μL and 0.5 μL of dsRNA was injected into the thorax of each newly emerged Ar. subalbatus female. Four days after dsRNA injection, mosquitoes were collected for preparation of total RNAs for real-time PCR analysis as described above. These RNAi experiments were repeated three times.
2.10 Mosquito survival assay
Newly emerged Ar. subalbatus females were injected with dsRNA to each AsCTL or GFP as described above. Four days after dsRNA injection, mosquitoes in each group were divided into two cartons. Mosquitoes from one carton were injected with 0.5 μL live E. coli (OD600=0.4, resuspended in Aedes saline), and mosquitoes from the other carton were injected with live M. luteus (OD600=1.6, resuspended in Aedes saline). After bacteria injection, mosquitoes were transferred into new cartons and maintained in the same environment. At the same time of every day from day 1 to day 7 after bacteria injection, survival of mosquitoes was checked and the dead mosquitoes were counted and removed from the cartons. The survival rates at each day were calculated and compared between AsCTLs knockdown groups and the GFP control group. These experiments were performed four times (with a total of 80–97 mosquitoes in each treatment from the four experiments), and the survival rate was the combined overall rate.
3. Results
3.1 Sequence analysis of the five Ar. subalbatus C-type lectins
Vertebrate C-type lectins (CTLs) usually contain one CRD with conserved amino acid residues for ligand binding and calcium coordination, and they can be divided into two types based on the conserved ligand binding motif. A mannose-type CTL (CTLMA) contains an EPN (Glu-Pro-Asn) motif in the CRD with predicted ligand specificity for mannose, glucose and fucose, and a galactose-type CTL (CTLGA) contains a QPD (Gln-Pro-Asp) motif in the CRD for galactose and N-acetyl-D-galactosamine (GalNAc) (van Vliet et al., 2008b). CTLs that do not contain a typical EPN or QPD motif in the CRD belong to the other-type and they may bind to different ligands (van Vliet et al., 2008b).
In the Ar. subalbatus database (https://asap.ahabs.wisc.edu/asap/full_text.php), 17 ESTs (not all contain the complete coding sequences) encode single CRDs, and only two CRDs contain the conserved EPN motif and one with the QPD motif. We then chose five ESTs with complete coding sequences that encode secreted CTLs representing the three types of CTLs in this study and named them: AsCTLMA11 (AY441312), AsCTLMA15 (EU206257), AsCTLGA5 (EU207651), AsCTL15 (EU205642), and AsCTL16 (EU206532) based on their similarities to Ae. aegypti homologous AaCTLs (see below). AsCTLMA11 and AsCTLMA15 contain 167 and 161 amino acids with 26- and 20-residue signal peptides, respectively, and both contain an EPN motif in the CRD (Fig. 1), AsCTLGA5 is composed of 162 residues with a 18-residue signal peptide and a QPD motif in the CRD, AsCTL15 and AsCTL16 are 161 and 153 residues long with 21- and 19-residue signal peptides, respectively, and AsCTL15 contains an EPS motif whereas AsCTL16 has a KPD motif in the CRD (Fig. 1).
AsCTLMA11 is most similar to Ae. aegypti AaCTLMA11 (AAEL000543), Culex quinquefasciatus lectin (CPIJ016688), An. gambiae CTLMA6 (AGAP005332) and D. melanogaster CG9134, with 79%, 46%, 41% and 38% identities, respectively. AsCTLMA15 is most similar to AaCTLMA15 (AAEL000563), C. quinquefasciatus lectin (CPIJ017075), An. gambiae CTLMA6 and D. melanogaster CG9134, with 68%, 49%, 37% and 38% identities, respectively. AsCTLGA5 is most similar to AaCTLGA5 (AAEL005641), C. quinquefasciatus lectin (CPIJ017075), An. gambiae CTLMA1 (AGAP007411) and D. melanogaster CG9134, with 77%, 61%, 37% and 39% identities, respectively. AsCTL15 is most similar to AaCTL15 (AAEL012353), C. quinquefasciatus lectin (CPIJ007869), An. gambiae CTLMA6 and D. melanogaster CG9134, with 79%, 54%, 38% and 37% identities, respectively. AsCTL16 is most similar to AaCTL16 (AAEL000533), C. quinquefasciatus lectin (CPIJ017075), An. gambiae CTLMA6 and D. melanogaster CG9134, with 77%, 47%, 33% and 31% identities, respectively. All five AsCTLs also show 30–40% identity to the Bombyx mori macrophage mannose receptor 1-like protein (XP_004931579).
3.2 Tissue distribution, developmental and induced expression profiles
To determine tissue distribution of AsCTLs in Ar. subalbatus females, real-time PCR was performed. The transcripts of AsCTLMA15, AsCTLGA5 and AsCTL15 were highly expressed in hemocytes, and AsCTLMA15 and AsCTL15 mRNAs were also expressed at relatively high levels in fat body, cuticle and muscle (Fig. 2). AsCTLMA11 mRNA was highly expressed in both hemocytes and fat body, and at a relatively high level in muscle. However, AsCTL16 transcript was highly expressed in fat body and also at a relatively high level in midgut (Fig. 2). These results suggest that AsCTLMA15, AsCTLGA5 and AsCTL15 were mainly expressed in hemocytes, and AsCTLMA11 was expressed in both hemocytes and fat body, but AsCTL16 was mainly expressed in fat body.
Fig. 2. Tissue distribution of AsCTLs in Ar. subalbatus females.
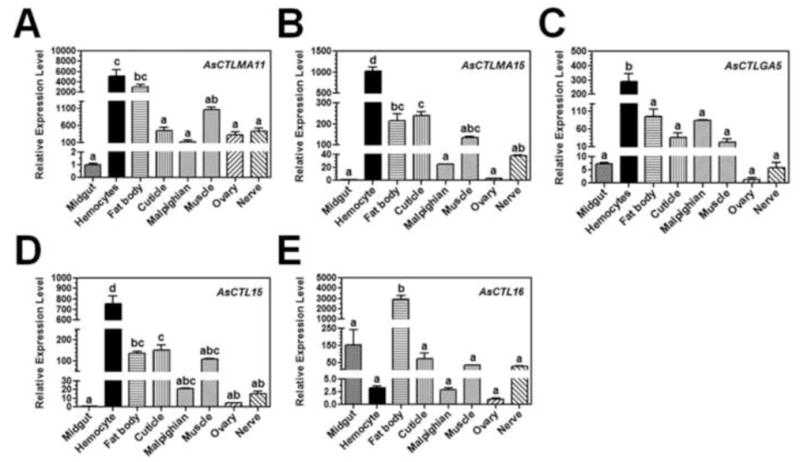
Midgut, hemocytes, fat body, cuticle, Malpighian tubule, muscle, ovary and nerve were dissected from female mosquitoes (4–5 days old) and used for preparation of total RNAs. Expressions of AsCTLs transcripts were determined by real-time PCR. AsRPL9 gene was used as an internal control gene. Each bar represents the mean of three individual measurements ± SEM. Identical letters are not significant difference (p>0.05), while different letters indicate significant difference (p<0.05) determined by one way ANOVA followed by a Tukey’s multiple comparison test among different tissues.
Developmental expression profiles of AsCTLs were also determined by real-time PCR. AsCTLMA11 mRNA was expressed in the late larval stage and early pupal stage, and its expression maintained at a high level in the adult stage (day 0 and days 3–9); AsCTLMA15 transcript was mainly expressed in the late larval stage (L3 and L4) and was also expressed in day 7 adults; AsCTLGA5 transcript was expressed in the late larval stage and early pupal stage; AsCTL15 mRNA was expressed only at the early larval and early pupal stages; and AsCTL16 mRNA was highly expressed in the adult stage (days 0–9) (Fig. 3).
Fig. 3. Developmental expression of AsCTLs.
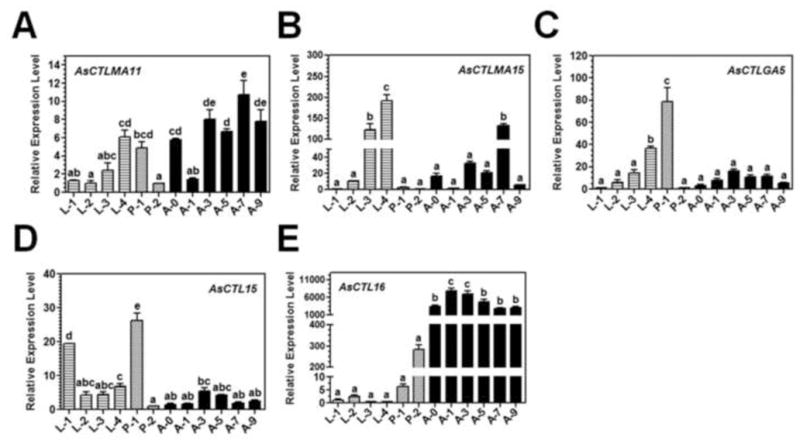
Total RNAs were prepared from first (L-1), second (L-2), third (L-4) and four (L-4) instar larvae, early (P-1) and late (P-2) stage pupae, days 0–9 (A-0 to A-9) Ar. subalbatus adult females and expression of AsCTLs transcripts and the AsRPL9 control gene was determined by real-time PCR. Each bar represents the mean of three individual measurements ± SEM. Identical letters are not significant difference (p>0.05), while different letters indicate significant difference (p<0.05) determined by one way ANOVA followed by a Tukey’s multiple comparison test among developmental stages.
To determine induced expression of AsCTLs in the female adults by Gram-negative E. coli and Gram-positive M. luteus, real-time PCR was also performed. AsCTLMA11 was induced by E. coli at 3 and 24 h post-injection, although the induced expression level was not high but was significant compared to the saline injection control (Fig. 4A), and AsCTLMA15 was induced by E. coli at 3, 24 and 48 h post-injection (Fig. 4B). However, expression of both AsCTLMA11 and AsCTLMA15 was not induced by M. luteus. Expression of AsCTLGA5 and AsCTL15 transcripts was induced by both E. coli and M. luteus starting at 3 h post-injection, reached a peak at 24 h and started to decline at 48 h post-injection. AsCTL16 was induced by M. luteus at 6 h post-injection, but was not induced by E. coli (Fig. 4).
Fig. 4. Induced expression of AsCTLs in Ar. subalbatus females after bacterial infection.
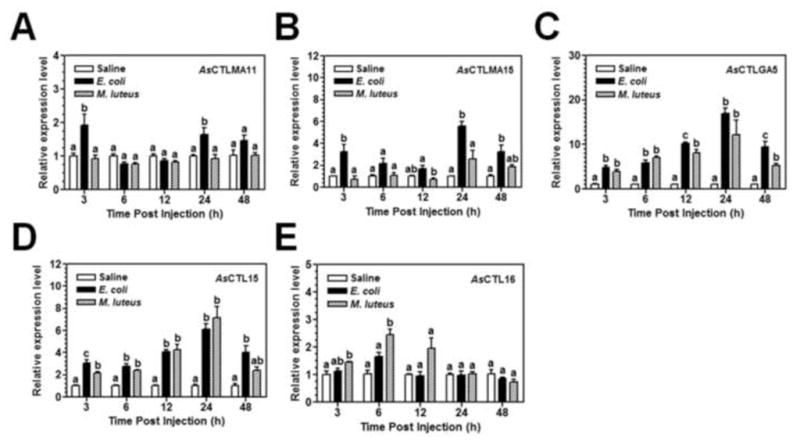
Adult female mosquitoes (4–5 days old) were injected with Aedes saline, E. coli (OD600=0.1), or M. luteus (OD600=0.15) (0.5 μL for each mosquito). Five mosquitoes were randomly collected at 3, 6, 12, 24, and 48 h post-injection for preparation of total RNAs, and expression of AsCTLs transcripts and the AsRPL9 control gene was determined by real-time PCR. Each bar represents the mean of three individual measurements ± SEM. Identical letters are not significant difference (p>0.05), while different letters indicate significant difference (p<0.05) determined by one way ANOVA followed by a Tukey’s multiple comparison test among different treatments at each time point.
3.3 Hemagglutination of animal erythrocytes by recombinant AsCTLs
The five AsCTLs were expressed as recombinant proteins with a His-tag at the N-terminus in bacteria, purified as inclusion bodies under denaturing conditions, and refolded by 3-step dialysis (Yu et al., 2005). Recombinant M. sexta cuticle protein CP36 with a His-tag was also expressed in bacteria and purified as a control protein (Suderman et al., 2003). These proteins could be recognized by a monoclonal mouse anti-polyhistidine antibody (Fig. S1).
Hemagglutination assasy has been used to determined ligand binding specificity of animal C-type lectins (Yu and Kanost, 2000). We performed hemagglutination assay with the five recombinant AsCTLs using erythrocytes from several mammals and found that only AsCTLGA5 and AsCTL16 showed high hemagglutinating activity against sheep and human group O erythrocytes, and AsCTLGA5 also showed high hemagglutinating activity against porcine erythrocytes (Table 1). But AsCTLMA11, AsCTLMA15 and AsCTL15 at 50 μg/ml did not show hemagglutinating activity against any of the erytherocytes tested (Table 1).
Table 1.
Hemagglutination of animal red blood cells by recombinant Ar. subalbatus C-type lectins (AsCTLs)
| Red Blood Cells | Recombinant Ar. subalbatus C-type lectins (μg/ml) | ||||
|---|---|---|---|---|---|
|
| |||||
| AsCTLMA11 | AsCTLMA15 | AsCTLGA5 | AsCTL16 | AsCTL16 | |
| Human Group B | - | - | - | - | - |
| Human Group O | - | - | 0.78 | - | 3.12 |
| Sheep | - | - | 0.78 | - | 0.39 |
| Goat | - | - | - | - | - |
| Bovine | - | - | - | - | - |
| Porcine | - | - | 0.39 | - | - |
| Horse | - | - | - | - | - |
(−): no hemagglutination at 50 μg/ml of AsCTLs
To test ligand binding specificity of AsCTLGA5 and AsCTL16, competitive hemagglutination assay was performed in the presence of different polysaccharides (microbial cell wall components) or saccharides using sheep erythrocytes. Our results showed that both AsCTLGA5 and AsCTL16 showed high binding affinity for smooth LPS from E. coli (026:B6), S. marcescens, P. aeruginosa, and S. enterica, LTA and PG from S. aureus, and weak affinity for Ra mutant of LPS (Ra-LPS)(Table 2). AsCTLGA5 also showed high affinity for mannan and GalNac, and weak affinity for zymosan and sucrose (Table 2).
Table 2.
Effects of saccharides/polysaccharides on hemagglutinating activities of AsCTLs
| Saccharides/Polysaccharides | MIC of Saccharides/Polysaccharides | |
|---|---|---|
| AsCTLGA5 | AsCTL16 | |
| D-Galactose | NIa | NIa |
| L-Galactose | NIa | NIa |
| D-GalNac | 3.9 mM | NIa |
| D-Glucose | NIa | NIa |
| L-Fucose | NIa | NIa |
| L-Rhamnose | NIa | NIa |
| Talose | NIa | NIa |
| Xylose | NIa | NIa |
| Lactose | NIa | NIa |
| Sucrose | 250 mM | NIa |
| Maltose | NIa | NIa |
| Melibiose | NIa | NIa |
| Chitotriose | NIa | NIa |
| Lipid A (mono-P) | NIb | NIb |
| Lipid A (di-P) | NIb | NIb |
| LPS (E. coli 055:B5) | NIb | NIb |
| LPS (E. coli 026:B6) | 7.8 μg/mL | 7.8 μg/mL |
| LPS (S. marcescens) | 3.9 μg/mL | 2.0 μg/mL |
| LPS (S. enterica) | 3.9 μg/mL | 2.0 μg/mL |
| LPS (P. aeruginosa) | 3.9 μg/mL | 3.9 μg/mL |
| Ra-LPS | 250 μg/mL | 250 μg/mL |
| Rc-LPS | NIb | NIb |
| Re-LPS | NIb | NIb |
| Chitin | NIb | NIb |
| LTA (S. aureus) | 1.0 μg/mL | 1.0 μg/mL |
| PG (S. aureus) | 7.8 μg/mL | 31.3 μg/mL |
| Laminarin | NIb | NIb |
| Curdlan | NIb | NIb |
| Mannan | 2.0 μg/mL | NIb |
| Zymosan | 125 μg/mL | NIb |
NIa: not inhibited at 250 mM; NIb: not inhibited at 250 μg/mL
3.4 Binding of recombinant AsCTLs to microbial cell wall components
To determine direct binding of recombinant AsCTLs to different microbial cell wall components, including LPS, LTA, PG, zymosan and laminarin, plate ELISA assays were performed. Our results showed that all five recombinant AsCTLs bound to these microbial cell wall components to a certain extent compared to the control CP36 protein (Fig. S2A–E). More AsCTLs bound to PG-K12 (from E. coli K12), PG-BS (from B. subtilis), PG-SA (from S. aureus) and zymosan than to LTA-BS, LTA-SA and laminarin, and all five AsCTLs also bound to LPS-K12 (Fig. S2A–E).
Smooth LPS is composed of three moieties: O-specific antigen, core carbohydrate, and lipid A(Raetz, 1990). To determine the moieties of LPS that can be recognized by recombinant AsCTLs, plate ELISA assays were also performed with smooth LPS, different rough mutants of LPS (Ra-, Rc-, Rd- and Re-LPS), as well as mono- and di-phosphoryl lipid A. Our results showed that almost similar amounts of AsCTLs bound to smooth LPS from S. enterica and E. coli, Ra-, Rc-, Rd- and Re-LPS, as well as lipid A (Fig. S2 F–J), except that more AsCTLMA15 bound to di-phosphoryl lipid A (Fig. S2G), more AsCTLGA5 bound to Rd-LPS (Fig. S2H), and more AsCTL16 bound to mono- and di-phosphoryl lipid A (Fig. S2J).
To confirm that binding of AsCTLs to microbial components or saccharides is specific, competitive binding assay was performed. Binding of AsCTLMA11 to LPS (from E. coli 026:B6) was competed well by free LPS but only slightly competed by free LTA-SA, PG-SA and mannan (Fig. 5A), binding of AsCTLMA15 and AsCTL15 to LPS was competed well by free LPS and LTA-SA but only slightly by PG-SA and mannan (Fig. 5B and D), binding of AsCTLGA5 to LPS was competed well by free LPS, LTA-SA and mannan but slightly by PG-SA (Fig. 5C), while binding of AsCTL16 to LPS was competed well by free LPS, LTA-SA, PG-SA and mannan (Fig. 5E). GalNac (at 60 mM and 100 mM) but not glucose also significantly decreased binding of AsCTLGA5 to LPS (Fig. 5F), but neither GalNac nor glucose could inhibit binding of AsCTLMA11, AsCTLMA15, AsCTL15 or AsCTL16 to LPS (Fig. S3).
Fig. 5. Binding of recombinant AsCTLs to LPS in the presence of free microbial components or saccharides.
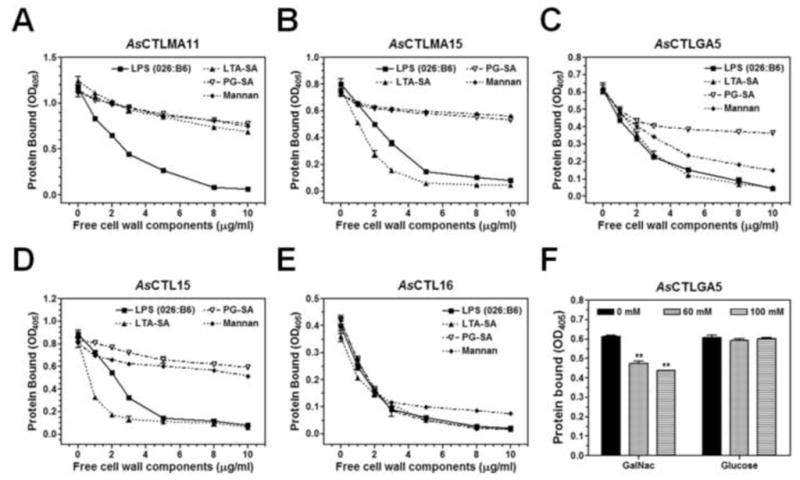
Purified recombinant AsCTLs were diluted in the binding buffer (50 mM Tris-HCl, 50 mM NaCl, pH8.0) containing 5 mM CaCl2 and 0.1 mg/ml BSA to 10μg/ml and pre-incubated with increasing concentrations of free microbial components (A–E) or saccharides (F), and the mixtures were then added to the 96-well plates coated with LPS from E. coli 026:B6. Binding of recombinant proteins to LPS was determined by ELISA assay using anti-polyhistidine antibody as described in the Materials and Methods. The figure showed total binding of recombinant proteins to LPS. Each point (or bar) represents the mean of four individual measurements ± SEM.“**” indicates significant difference (p<0.01) in binding of AsCTLGA5 to LPS (F) between no competitor (0 mM) and with competitor (60 or 100 mM) determined by the unpaired t-test.
3.5 Direct binding of recombinant AsCTLs to microorganisms
We showed that all five recombinant AsCTLs could bind to different microbial cell wall components (Figs. 5 and S2). To test whether the select in scan bind to different microorganisms, a direct binding assay was performed. We first tested binding of recombinant proteins to E. coli, and the results showed that recombinant AsCTLs could bind to E. coli and were eluted by SDS (Fig. 6B–F, lanes 4), but some control CP36 protein also bound to E. coli (Fig. 6A, lane 4). We then tested binding of AsCTLs and CP36 to different microorganisms, and only SDS eluted fractions were analyzed by Western blot. Our results showed that the control CP36 protein did not bind to Gram-negative P. aeruginosa, Gram-positive S. aureus, M. luteus, B. subtilis, or B. cereus (Fig. 6G), but all five AsCTLs bound to P. aeruginosa, S. aureus, M. luteus, B. subtilis, and B. cereus (Fig. 6H–L). Although some control CP36 protein bound to Gram-negative S. marcescens and yeast (S. cerevisiae) (Fig. 6G), more recombinant AsCTLs bound to S. marcescens and S. cerevisiae (Fig. 6H–L), indicating that all five AsCTLs could also bind to the two microorganisms. Comparing the five AsCTLs, more AsCTLGA5 (Fig. 6J), AsCTL15 (Fig. 6K) and AsCTLMA11 (Fig. 6H), but less AsCTLMA15 (Fig. 6I) and AsCTL16 (Fig. 6L) bound to these microorganisms.
Fig. 6. Direct binding of recombinant AsCTLs to microorganisms.
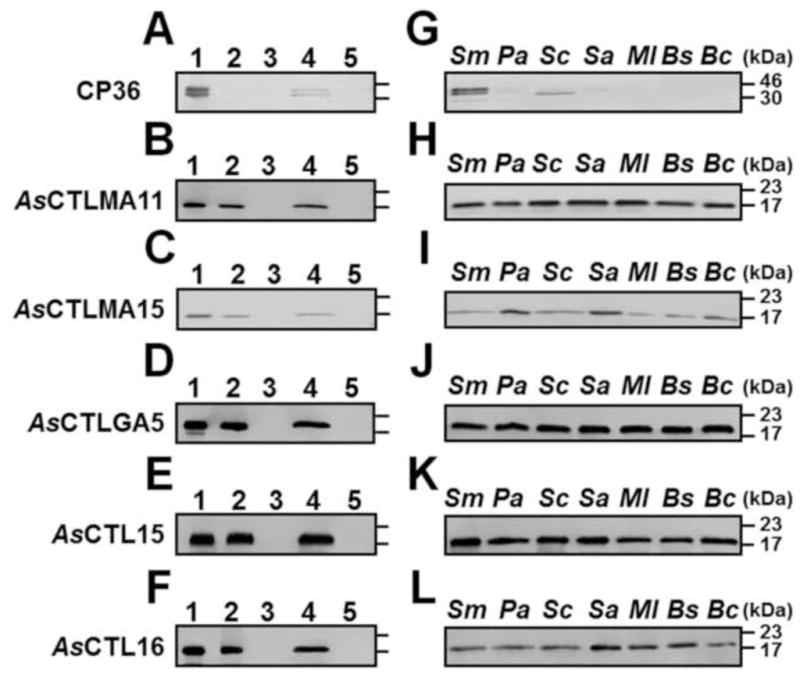
Equal volumes of microorganisms (4×108 cells/mL for bacteria and 4×107 cells/mL for yeast) and recombinant proteins (20 μg/mL) were incubated at room temperature for 1 h, and proteins bound to microorganisms were eluted with 7% SDS. Binding of recombinant proteins to microorganisms was confirmed by Western blot using mouse monoclonal anti-polyhistidine antibody. A–F: Binding of recombinant AsCLTs and CP36 to E. coli: lane 1: purified recombinant proteins (0.5μg each); lane 2: unbound proteins; lane 3: the fourth TBS wash; lane 4: 7% SDS elution; lane 5: E. coli lysates. In lanes 2–5, equivalent volumes to lane 1 were loaded in each lane. G–L: Binding of AsCTLs and CP36 to various microorganisms. Only SDS elution fractions were analyzed. Sm:S. marcescens; Pa: P. aeruginosa; Sc:S. cerevisiae (yeast); Sa:S. aureus; Ml:M. luteus; Bs:B. subtilis; Bc: B. cereus.
3.6 Agglutination of bacterial cells by recombinant AsCTLs
Binding of AsCTLs to microorganisms may cause agglutination of microbial cells. In vitro agglutination assays showed that AsCTLMA15 could agglutinate Gram-positive S. aureus and M. luteus, AsCTLGA5 agglutinated Gram-positive S. aureus and B. cereus, AsCTL15 had agglutinating activity against Gram-positive S. aureus, B. subtilis, B. cereus and Gram-negative E. coli, S. marcescens and P. aeruginosa, AsCTL16 could cause aggregation of S. aureus, M. luteus, B. subtilis, B. cereus, S. marcescens and P. aeruginosa, but AsCTLMA11 did not agglutinate any of the seven bacteria tested (Fig. 7 and Table 3). None of the five AsCTLs could agglutinate yeast (S. cerevisiae) (Table 3). These results further confirmed binding of recombinant AsCTLs to bacteria.
Fig. 7. Agglutination of microorganisms by recombinant AsCTLs.
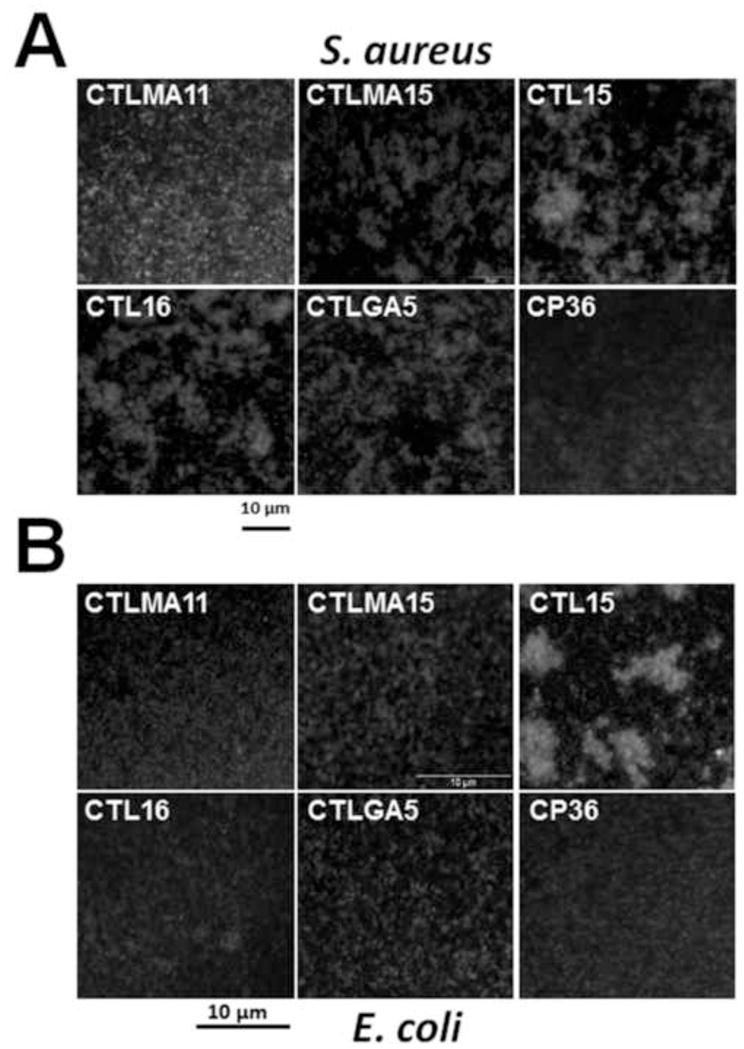
Purified recombinant AsCTL or CP36 (40μg/mL final concentration) was incubated with FITC-labelled S. aureusor E. coli bioparticles in TBS containing 2 mM CaCl2 and 1 mM MgCl2 at room temperature for 35 min, samples of bacterial cells were examined by fluorescence microscopy (or microscopy for microorganisms that were not FITC-labeled in Table 3).
Table 3.
Agglutination of microorganisms by recombinant Ar. subalbatus C-type lectins (AsCTLs)
| Microorganisms | Recombinant Ar. subalbatus C-type lectins | ||||
|---|---|---|---|---|---|
|
| |||||
| Gram-positive bacteria | AsCTLMA15 | AsCTLMA11 | AsCTL15 | AsCTL16 | AsCTLGA5 |
| S. aureus | + | − | + | + | + |
| M. luteus | + | − | − | + | − |
| B. subtilis | − | − | + | + | − |
| B. cereus | − | − | + | + | + |
|
| |||||
| Gram-negative bacteria | |||||
| E. coli | − | − | + | − | − |
| S. marcescens | − | − | + | + | − |
| P. aeruginosa | − | − | + | + | − |
|
| |||||
| Fungi | |||||
| S. cerevisiae | − | − | − | − | − |
(+): with agglutination; (−): without agglutination
3.7 RNAi knockdown of AsCTLs genes and mosquito survival after bacterial infection
To determine in vivo functions of AsCTLs in Ar. subalbatus females, expression of each AsCTL gene was knocked down by dsRNA, and the survival of dsRNA-treated mosquitoes was determined after injection of live E. coli or M. luteus. Real-time PCR results showed that expression of each AsCTL mRNA was significantly reduced by 70–90% four days after dsRNA injection compared to the control GFP-dsRNA injection (Figs. 8A–C, S4A and D), indicating that RNAi was effective in Ar. subalbatus. When these dsRNA-treated mosquitoes were injected with live E. coli or M. luteus, the survival rate of mosquitoes was decreased after E. coli injection in the AsCTL RNAi groups compared to the control group (GFP-RNAi) (Figs. 8D–F, S4B and E). Particularly, the survival rate of mosquitoes treated with dsRNA to AsCTLGA5 was significantly decreased (p=0.0210) after E. coli injection (Fig. 8E). However, the survival rates of mosquitoes did not decrease in the AsCTL RNAi groups after injection of M. luteus compared to the control group (Figs. 8G–I, S4C and F). Instead, the survival rate of mosquitoes treated with AsCTLMA15 actually increased (though not significantly) after M. luteus injection (Fig. 8G).
Fig. 8. RNAi knockdown of AsCTLs and survival of Ar. subalbatus females after bacterial infection.
Newly emerged mosquito females were injected with dsRNA to each AsCTL or GFP (as a control), and expression of AsCTLs transcripts in the dsRNA-injected mosquitoes was determined by real-time PCR four days after dsRNA injection (A–C). Each bar represents the mean of three individual measurements ± SEM.“**” indicates significant difference in the levels of mRNAs between the control (GFP-RNAi) and AsCTL-RNAi groups determined by the unpaired t-test. Four days after dsRNA injection, the mosquitoes were injected with live E. coli (D–F) or live M. luteus (G–I), and the survival of mosquitoes was recorded for 7 days after bacteria injection. The survival curves were generated by Kaplan-Meier method and the statistical analysis was performed by the log rank test (p<0.05 indicates significant difference).
4. Discussion
CTLs are a superfamily of carbohydrate binding proteins found in almost all metazoans. Animal CTLs can function as pattern recognition receptors and play important roles in innate immunity. Mammalian CTLs, such as mannose-binding lectins (MBLs), can opsonize microorganisms and activate the lectin pathway of the complement system (Fujita et al., 2004; Ip et al., 2009; Jack and Turner, 2003), and they are also active against cancer cells (Nakagawa et al., 2003). Insect CTLs, such as those from lepidopteran species, have been shown to participate in both cellular and humoral innate immune responses (Ling and Yu, 2006; Seufi et al., 2012; Tian et al., 2009; Watanabe et al., 2006; Yu and Kanost, 2000, 2003, 2004). In Drosophila and mosquitoes, only a few CTLs have been reported to be involved in innate immunity (Ao et al., 2007; Keebaugh and Schlenke, 2012; Schnitger et al., 2009; Tanji et al., 2006). Interestingly, a CTL (mosGCTL-1 or AaCTLMA15) from Ae. aegypti can facilitate West Nile virus infection (Cheng et al., 2010), and two CTLs (AgCTL4 and AgCTLMA2) from An. gambiae can protect Plasmodium parasites from melanization (Osta et al., 2004a), and they are also required for clearance of Gram-negative bacteria (Schnitger et al., 2009). In the mosquito Ar. subalbatus, a natural vector of filarial nematodes, there are 17 ESTs encoding proteins with CTLDs, but no study on functions of AsCTLs in innate immunity has been reported so far. In this study, we selected five Ar. subalbatus CTLs (AsCTLs) and investigated their functions against bacteria.
The five AsCTLs include two mannose-types (AsCTLMA11 and AsCTLMA15), one galactose-type (AsCTLGA5), and two other-types (AsCTL15 and AsCTL16). They are highly similar to homologous AaCTLs from Ae. aegypti with 68–79% identities. AsCTLMA15, AsCTLGA5 and AsCTL15 were mainly expressed in hemocytes (Fig. 2B–D) and at low levels in adult females (Fig. 3B–D), AsCTL16 mRNA was mainly expressed in fat body (Fig. 2E) and highly expressed in the adult stage (Fig. 3E). AsCTLMA11 mRNA was expressed in both hemocytes and fat body (Fig. 2A) and also at a high level in the adult stage (Fig. 3A). AsCTLGA5 and AsCTL15 mRNAs were induced by both E. coli and M. luteus (Fig. 4C and D), AsCTLMA11 and AsCTLMA15 were induced by E. coli but not by M. luteus (Fig. 4A and B), whereas AsCTL16 transcript was induced by M. luteus (at 6 and 12 h) but not by E. coli (Fig. 4E). The induced expression of AsCTLs by E. coli and/or M. luteus is consistent with that of a lectin containing a fibrinogen-like domain from Ar. subalbatus (AL-1), which is up-regulated by both E. coli and M. luteus (Wang et al., 2004). An. gambiaeAgCTL4 and AgCTLMA2 are also induced by E. coli and S. aureus (Schnitger et al., 2009).
Hemagglutination assays showed that AsCTLGA5 and AsCTL16, but not the other three AsCTLs, could agglutinate erythrocytes (Table 1). AsCTLGA5 and AsCTL16 showed binding affinity for smooth LPS, LTA-SA and PG-SA (Table 2), and AsCTLGA5 also had binding affinity for mannan and GalNac (Table 2). Binding of AsCTLGA5 to GalNac is consistent with its predicted ligand specificity of the galactose-type. In vitro plate ELISA assays confirmed binding of recombinant AsCTLs to different microbial components, including smooth LPS, rough mutants of LPS, lipid A, LTA, PG, mannan, zymosan and laminarin (Figs. 5A–E and S2), indicating a broad binding spectrum of AsCTLs. Recombinant AsCTLs could also directly bind to several Gram-negative and Gram-positive bacteria (Fig. 6). Broad binding spectrum is common to invertebrate CTLs, for example, insect and crustacean CTLs containing an EPN motif (mannose-type) have a broad spectrum of agglutinating, opsonizing and microbicidal activities (Seufi et al., 2012; Sun et al., 2008; Tian et al., 2009; Watanabe et al., 2006; Yu et al., 2005, 2006; Zhang et al., 2009). But mammalian CTLs such as mannose-binding lectins (MBLs) have more restricted binding specificity consistent with the predicted binding motif (Drickamer, 1992).
AsCTLs could bind to Gram-positive and Gram-negative bacteria, as well as to yeast (S. cerevisiae) to a lesser extent (Fig. 6). Our in vitro agglutination assays showed that AsCTL15 and AsCTL16 could agglutinate six of the seven Gram-positive and Gram-negative bacteria tested (Fig. 7 and Table 3), AsCTLMA15 and AsCTLGA5 only agglutinated two Gram-positive bacteria, and AsCTLMA11 did not agglutinate any of the seven bacteria tested (Fig. 7 and Table 3). None of the five recombinant AsCTLs agglutinated yeast (S. cerevisiae) (Table 3). The five AsCTLs all contain a single CRD. In order to agglutinate microorganisms, these AsCTLs need to be able to cross-link microbial cells, and thus the five AsCTLs may differ in formation of oligomers and/or cross-linking microbial cells. Invertebrate CTLs may not require calcium for ligand binding (Shin et al., 2000; Yu and Ma, 2006). Our previous study showed that calcium is not required for immulectin-2 (a C-type lectin with dual CRDs) binding to microbial components but is required to protect immulectin-2 from proteinase digestion, probably by enhancing formation of a more compact structure or oligomers (Yu and Ma, 2006). AsCTL15 contains an EPS motif in the CRD, which is similar to that of a shrimp CTL (FcLec3), and FcLec3 has similar agglutinating activity as AsCTL15 (Wang et al., 2009). AsCTL16 contains a KPD motif in the CRD, and there has been no report about functions of CTLs with a KPD motif so far.
RNAi experiments showed that dsRNAs to AsCTL genes could significantly knock down expressions of AsCTL transcripts by 70–90% in Ar. subalbatus females (Figs. 8A–C, S4A and D). In the dsRNA-treated mosquitoes, the survival of mosquitoes was decreased after injection of live E. coli (Figs. 8D–F, S4B and E), in particular, the survival of mosquitoes treated with dsRNA to AsCTLGA5 was significantly decreased (p=0.0210) compared to the control group (dsRNA to GFP) (Fig. 8E). But the survival of dsRNA-treated mosquitoes did not decrease after injection of live M. luteus (Figs. 8G–I, S4C and F). These results suggest that AsCTLGA5 may play a role in defense against E. coli infection. Knockdown expression of each AsCTL gene by RNAi did not always have a significant effect on mosquito survival (except AsCTLGA5). This may be because there are multiple AsCTL genes in Ar. subalbatus, and each AsCTL may have some effect on mosquito immunity, but the overall effect of multiple AsCTLs could be significant. It is also possible that some AsCTLs are involved in innate immunity against filarial parasites. We did try RNAi experiments followed by injection of filarial parasites, but failed to obtain results due to technical difficulties. Interestingly, the survival of mosquitoes treated with dsRNA to AsCTLMA15 was actually increased (though not significantly) after M. luteus injection (Fig. 8G). It has been reported that decrease in the expression of a CTL from Pierisrapae can down-regulate expressions of some immune-related genes including cecropin A (Fang et al., 2011). We also determined the expression of some AMP genes in the dsRNA-treated mosquitoes and found that M. luteus-induced expression of Ar. subalbatus cecropin, defensin-A and defensin-C was up-regulated after knockdown expression of AsCTLs compared to the control group (dsRNA to GFP) (Shi XZ and Yu XQ, unpublished results). Increase in the expressions of AMPs may compensate for the decrease in AsCTLs expression, and thus increase the survival of mosquitoes after M. luteus injection. We think that CTLs can serve as PRRs and bind to different pathogens. Most CTLs can bind to microorganisms/microbial components but do not interact with receptors in the signaling pathways, and thus they can enhance phagocytosis, nodule formation, encapsulation and melanization. Some CTLs, after binding to microorganisms or microbial components, may interact with receptors in the signaling pathways, thus can modulate (stimulate or inhibit) the signaling pathway to regulate expression of immune-related genes, including AMP genes.
The five AsCTLs are homologous to Ae. aegyptiAaCTLs (68–79% identities), but have low similarities to An. gambiaeAgCTLs. AsCTLMA15 is 68% identical to AaCTLMA15 (mosGCTL-1), which has been shown to interact with West Nile virus and facilitate virus infection (Cheng et al., 2010). AgCTL4 and AgCTLMA2 can protect Plasmodium parasites from melanization (Osta et al., 2004a). Future study is to investigate whether AsCTLs play a role in interaction with filarial nematodes in Ar. subalbatus.
Supplementary Material
Armigeres subalbatus C-type lectins (AsCTLs) transcripts were expressed mainly in hemocytes and/or fat body.
Recombinant AsCTLs bound to several microbial components, such as LPS, peptidoglycan and lipoteichoic acid.
AsCTLs directly bound to several Gram-negative and Gram-positive bacteria and agglutinated bacterial cells.
Injection of dsRNAs to AsCTLs in to female mosquitoes effectively knocked down expression of AsCTLs transcripts.
RNAi knockdown of AsCTLGA5 significantly decreased the survival of mosquitoes after E. coli infection.
Acknowledgments
This work was supported by National Institutes of Health Grant AI082253 and a grant (FEB12) from the University of Missouri Research Board (UMRB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aliota MT, Fuchs JF, Mayhew GF, Chen CC, Christensen BM. Mosquito transcriptome changes and filarial worm resistance in Armigeres subalbatus. BMC Genomics. 2007;8:463. doi: 10.1186/1471-2164-8-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao J, Ling E, Yu XQ. Drosophila C-type lectins enhance cellular encapsulation. Mol Immunol. 2007;44:2541–2548. doi: 10.1016/j.molimm.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerntsen BT, Luckhart S, Christensen BM. Brugia malayi and Brugia pahangi: inherent difference in immune activation in the mosquitoes Armigeres subalbatus and Aedes aegypti. J Parasitol. 1989;75:76–81. [PubMed] [Google Scholar]
- Chen WJ, Dong CF, Chiou LY, Chuang WL. Potential role of Armigeres subalbatus (Diptera: Culicidae) in the transmission of Japanese encephalitis virus in the absence of rice culture on Liu-chiu islet, Taiwan. J Med Entomol. 2000;37:108–113. doi: 10.1603/0022-2585-37.1.108. [DOI] [PubMed] [Google Scholar]
- Cheng G, Cox J, Wang P, Krishnan MN, Dai J, Qian F, Anderson JF, Fikrig E. A C-type lectin collaborates with a CD45 phosphatase homolog to facilitate West Nile virus infection of mosquitoes. Cell. 2010;142:714–725. doi: 10.1016/j.cell.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen BM, Li J, Chen CC, Nappi AJ. Melanization immune responses in mosquito vectors. Trends Parasitol. 2005;21:192–199. doi: 10.1016/j.pt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Muller HM, Osta MA, Paskewitz SM, Reichhart JM, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, von Mering C, Xu J, Zheng L, Bork P, Kafatos FC. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Cirimotich CM, Dong Y, Garver LS, Sim S, Dimopoulos G. Mosquito immune defenses against Plasmodium infection. Dev Comp Immunol. 2010;34:387–395. doi: 10.1016/j.dci.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd RB, Drickamer K. Lectin-like proteins in model organisms: implications for evolution of carbohydrate-binding activity. Glycobiology. 2001;11:71R–79R. doi: 10.1093/glycob/11.5.71r. [DOI] [PubMed] [Google Scholar]
- Drickamer K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature. 1992;360:183–186. doi: 10.1038/360183a0. [DOI] [PubMed] [Google Scholar]
- Du ZQ, Ren Q, Zhao XF, Wang JX. A double WAP domain (DWD)-containing protein with proteinase inhibitory activity in Chinese white shrimp, Fenneropenaeus chinensis. Comp Biochem Physiol B Biochem Mol Biol. 2009;154:203–210. doi: 10.1016/j.cbpb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Fang Q, Wang F, Gatehouse JA, Gatehouse AM, Chen XX, Hu C, Ye GY. Venom of parasitoid, Pteromaluspuparum, suppresses host, Pierisrapae, immune promotion by decreasing host C-type lectin gene expression. PloS one. 2011;6:e26888. doi: 10.1371/journal.pone.0026888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Matsushita M, Endo Y. The lectin-complement pathway--its role in innate immunity and evolution. Immunol Rev. 2004;198:185–202. doi: 10.1111/j.0105-2896.2004.0123.x. [DOI] [PubMed] [Google Scholar]
- Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 2012;13:817–822. doi: 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyer JF. Mosquito immunity. Adv Exp Med Biol. 2010;708:218–238. doi: 10.1007/978-1-4419-8059-5_12. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. Mannose-binding lectin and innate immunity. Immunol Rev. 2009;230:9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- Iwanaga S, Lee BL. Recent advances in the innate immunity of invertebrate animals. J Biochem Mol Biol. 2005;38:128–150. doi: 10.5483/bmbrep.2005.38.2.128. [DOI] [PubMed] [Google Scholar]
- Jack DL, Turner MW. Anti-microbial activities of mannose-binding lectin. Biochem Soc Trans. 2003;31:753–757. doi: 10.1042/bst0310753. [DOI] [PubMed] [Google Scholar]
- Kanojia PC, Geevarghese G. New mosquito records of an area known for Japanese encephalitis hyperendemicity, Gorakhpur District, Uttar Pradesh, India. J Am Mosq Control Assoc. 2005;21:1–4. doi: 10.2987/8756-971X(2005)21[1:NMROAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Jiang H, Yu XQ. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- Keebaugh ES, Schlenke TA. Adaptive evolution of a novel Drosophila lectin induced by parasitic wasp attack. Mol Biol Evol. 2012;29:565–577. doi: 10.1093/molbev/msr191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan AM, Brown GD. Syk-coupled C-type lectins in immunity. Trends Immunol. 2011;32:151–156. doi: 10.1016/j.it.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingeter LM, Lin X. C-type lectin receptor-induced NF-kappaB activation in innate immune and inflammatory responses. Cell Mol Immunol. 2012;9:105–112. doi: 10.1038/cmi.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Linder ME, Gilman AG. Expression of G-protein alpha subunits in Escherichia coli. Methods Enzymol. 1994;237:146–164. doi: 10.1016/s0076-6879(94)37059-1. [DOI] [PubMed] [Google Scholar]
- Ling E, Yu XQ. Cellular encapsulation and melanization are enhanced by immulectins, pattern recognition receptors from the tobacco hornworm Manduca sexta. Dev Comp Immunol. 2006;30:289–299. doi: 10.1016/j.dci.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Mayhew GF, Bartholomay LC, Kou HY, Rocheleau TA, Fuchs JF, Aliota MT, Tsao IY, Huang CY, Liu TT, Hsiao KJ, Tsai SF, Yang UC, Perna NT, Cho WL, Christensen BM, Chen CC. Construction and characterization of an expressed sequenced tag library for the mosquito vector Armigeres subalbatus. BMC Genomics. 2007;8:462. doi: 10.1186/1471-2164-8-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw EA, O’Neill SL. Beyond insecticides: new thinking on an ancient problem. Nature reviews Microbiology. 2013;11:181–193. doi: 10.1038/nrmicro2968. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kawasaki N, Ma Y, Uemura K, Kawasaki T. Antitumor activity of mannan-binding protein. Methods Enzymol. 2003;363:26–33. doi: 10.1016/S0076-6879(03)01041-3. [DOI] [PubMed] [Google Scholar]
- Osta MA, Christophides GK, Kafatos FC. Effects of mosquito genes on Plasmodium development. Science. 2004a;303:2030–2032. doi: 10.1126/science.1091789. [DOI] [PubMed] [Google Scholar]
- Osta MA, Christophides GK, Vlachou D, Kafatos FC. Innate immunity in the malaria vector Anopheles gambiae: comparative and functional genomics. J Exp Biol. 2004b;207:2551–2563. doi: 10.1242/jeb.01066. [DOI] [PubMed] [Google Scholar]
- Pal S, Wu LP. Lessons from the fly: pattern recognition in Drosophila melanogaster. Adv Exp Med Biol. 2009;653:162–174. doi: 10.1007/978-1-4419-0901-5_11. [DOI] [PubMed] [Google Scholar]
- Raetz CR. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Surendran SN. Global climate change and its potential impact on disease transmission by salinity-tolerant mosquito vectors in coastal zones. Frontiers in physiology. 2012;3:198. doi: 10.3389/fphys.2012.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L. Mosquitoes and disease. Science. 2002;298:82–83. doi: 10.1126/science.298.5591.82. [DOI] [PubMed] [Google Scholar]
- Schnitger AK, Yassine H, Kafatos FC, Osta MA. Two C-type lectins cooperate to defend Anopheles gambiae against Gram-negative bacteria. J Biol Chem. 2009;284:17616–17624. doi: 10.1074/jbc.M808298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufi AM, Galal FH, Hafez EE. Characterization of multisugar-binding C-type lectin (SpliLec) from a bacterial-challenged cotton leafworm, Spodoptera littoralis. PloS one. 2012;7:e42795. doi: 10.1371/journal.pone.0042795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SW, Park DS, Kim SC, Park HY. Two carbohydrate recognition domains of Hyphantriacunea lectin bind to bacterial lipopolysaccharides through O-specific chain. FEBS lett. 2000;467:70–74. doi: 10.1016/s0014-5793(00)01127-3. [DOI] [PubMed] [Google Scholar]
- Suderman RJ, Andersen SO, Hopkins TL, Kanost MR, Kramer KJ. Characterization and cDNA cloning of three major proteins from pharate pupal cuticle of Manduca sexta. Insect Biochem Mol Biol. 2003;33:331–343. doi: 10.1016/s0965-1748(02)00247-3. [DOI] [PubMed] [Google Scholar]
- Sun YD, Fu LD, Jia YP, Du XJ, Wang Q, Wang YH, Zhao XF, Yu XQ, Wang JX. A hepatopancreas-specific C-type lectin from the Chinese shrimp Fenneropenaeus chinensis exhibits antimicrobial activity. Mol Immunol. 2008;45:348–361. doi: 10.1016/j.molimm.2007.06.355. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tanji T, Ohashi-Kobayashi A, Natori S. Participation of a galactose-specific C-type lectin in Drosophila immunity. Biochem J. 2006;396:127–138. doi: 10.1042/BJ20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian YY, Liu Y, Zhao XF, Wang JX. Characterization of a C-type lectin from the cotton bollworm, Helicoverpa armigera. Dev Comp Immunol. 2009;33:772–779. doi: 10.1016/j.dci.2009.01.002. [DOI] [PubMed] [Google Scholar]
- van den Berg LM, Gringhuis SI, Geijtenbeek TB. An evolutionary perspective on C-type lectins in infection and immunity. Ann N Y Acad Sci. 2012;1253:149–158. doi: 10.1111/j.1749-6632.2011.06392.x. [DOI] [PubMed] [Google Scholar]
- vanVliet SJ, Garcia-Vallejo JJ, van Kooyk Y. Dendritic cells and C-type lectin receptors: coupling innate to adaptive immune responses. Immunol Cell Biol. 2008a;86:580–587. doi: 10.1038/icb.2008.55. [DOI] [PubMed] [Google Scholar]
- vanVliet SJ, Saeland E, van Kooyk Y. Sweet preferences of MGL: carbohydrate specificity and function. Trends Immunol. 2008b;29:83–90. doi: 10.1016/j.it.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Wang S, Conant GC, Ou R, Beerntsen BT. Cloning and characterization of the peptidoglycan recognition protein genes in the mosquito, Armigeres subalbatus (Diptera: Culicidae) J Med Entomol. 2012;49:656–671. doi: 10.1603/me11165. [DOI] [PubMed] [Google Scholar]
- Wang X, Rocheleau TA, Fuchs JF, Hillyer JF, Chen CC, Christensen BM. A novel lectin with a fibrinogen-like domain and its potential involvement in the innate immune response of Armigeres subalbatus against bacteria. Insect Mol Biol. 2004;13:273–282. doi: 10.1111/j.0962-1075.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- Wang XW, Xu WT, Zhang XW, Zhao XF, Yu XQ, Wang JX. A C-type lectin is involved in the innate immune response of Chinese white shrimp. Fish Shellfish Immunol. 2009;27:556–562. doi: 10.1016/j.fsi.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Miyazawa S, Kitami M, Tabunoki H, Ueda K, Sato R. Characterization of a novel C-type lectin, Bombyx mori multibinding protein, from the B. mori hemolymph: mechanism of wide-range microorganism recognition and role in immunity. J Immunol. 2006;177:4594–4604. doi: 10.4049/jimmunol.177.7.4594. [DOI] [PubMed] [Google Scholar]
- Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian G, Blandin S, Christensen BM, Dong Y, Jiang H, Kanost MR, Koutsos AC, Levashina EA, Li J, Ligoxygakis P, Maccallum RM, Mayhew GF, Mendes A, Michel K, Osta MA, Paskewitz S, Shin SW, Vlachou D, Wang L, Wei W, Zheng L, Zou Z, Severson DW, Raikhel AS, Kafatos FC, Dimopoulos G, Zdobnov EM, Christophides GK. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science. 2007;316:1738–1743. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassine H, Osta MA. Anopheles gambiae innate immunity. Cell Microbiol. 2010;12:1–9. doi: 10.1111/j.1462-5822.2009.01388.x. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Gan H, Kanost MR. Immulectin, an inducible C-type lectin from an insect, Manduca sexta, stimulates activation of plasma prophenol oxidase. Insect Biochem Mol Biol. 1999;29:585–597. doi: 10.1016/s0965-1748(99)00036-3. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Kanost MR. Immulectin-2, a lipopolysaccharide-specific lectin from an insect, Manduca sexta, is induced in response to gram-negative bacteria. J Biol Chem. 2000;275:37373–37381. doi: 10.1074/jbc.M003021200. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Kanost MR. Manduca sexta lipopolysaccharide-specific immulectin-2 protects larvae from bacterial infection. Dev Comp Immunol. 2003;27:189–196. doi: 10.1016/s0145-305x(02)00099-x. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Kanost MR. Immulectin-2, a pattern recognition receptor that stimulates hemocyte encapsulation and melanization in the tobacco hornworm, Manduca sexta. Dev Comp Immunol. 2004;28:891–900. doi: 10.1016/j.dci.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Ling E, Tracy ME, Zhu Y. Immulectin-4 from the tobacco hornworm Manduca sexta binds to lipopolysaccharide and lipoteichoic acid. Insect Mol Biol. 2006;15:119–128. doi: 10.1111/j.1365-2583.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Ma Y. Calcium is not required for immulectin-2 binding, but protects the protein from proteinase digestion. Insect Biochem Mol Biol. 2006;36:505–516. doi: 10.1016/j.ibmb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Tracy ME, Ling E, Scholz FR, Trenczek T. A novel C-type immulectin-3 from Manduca sexta is translocated from hemolymph into the cytoplasm of hemocytes. Insect Biochem Mol Biol. 2005;35:285–295. doi: 10.1016/j.ibmb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- Zhang XW, Xu WT, Wang XW, Mu Y, Zhao XF, Yu XQ, Wang JX. A novel C-type lectin with two CRD domains from Chinese shrimp Fenneropenaeus chinensis functions as a pattern recognition protein. Mol Immunol. 2009;46:1626–1637. doi: 10.1016/j.molimm.2009.02.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.