Abstract
Detection of cytoplasmic DNA represents one of the most fundamental mechanisms of the innate immune system to sense the presence of microbial pathogens1. Moreover, erroneous detection of endogenous DNA by the same sensing mechanisms has an important pathophysiological role in certain sterile inflammatory conditions2, 3. The endoplasmic-reticulum-resident protein STING is critically required for the initiation of type I interferon signalling upon detection of cytosolic DNA of both exogenous and endogenous origin4, 5, 6, 7, 8. Next to its pivotal role in DNA sensing, STING also serves as a direct receptor for the detection of cyclic dinucleotides, which function as second messenger molecules in bacteria9, 10, 11, 12, 13. DNA recognition, however, is triggered in an indirect fashion that depends on a recently characterized cytoplasmic nucleotidyl transferase, termed cGAMP synthase (cGAS), which upon interaction with DNA synthesizes a dinucleotide molecule that in turn binds to and activates STING14, 15. We here show in vivo and in vitro that the cGAS-catalysed reaction product is distinct from previously characterized cyclic dinucleotides. Using a combinatorial approach based on mass spectrometry, enzymatic digestion, NMR analysis and chemical synthesis we demonstrate that cGAS produces a cyclic GMP-AMP dinucleotide, which comprises a 2′-5′ and a 3′-5′ phosphodiester linkage >Gp(2′-5′)Ap(3′-5′)>. We found that the presence of this 2′-5′ linkage was required to exert potent activation of human STING. Moreover, we show that cGAS first catalyses the synthesis of a linear 2′-5′-linked dinucleotide, which is then subject to cGAS-dependent cyclization in a second step through a 3′-5′ phosphodiester linkage. This 13-membered ring structure defines a novel class of second messenger molecules, extending the family of 2′-5′-linked antiviral biomolecules.
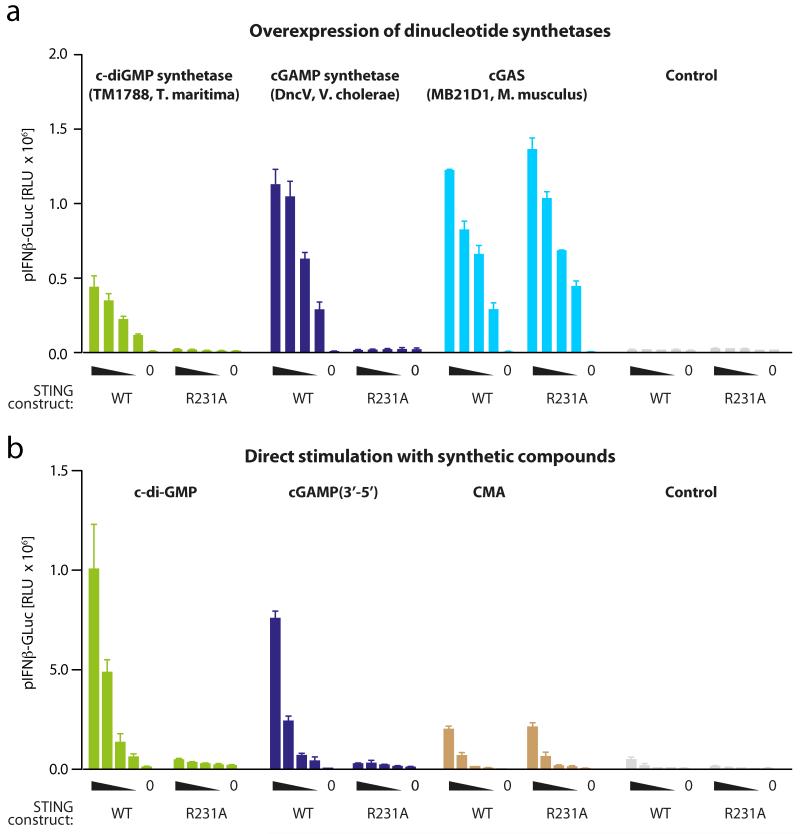
Recently, it has been demonstrated that upon intracellular DNA delivery, a cytoplasmic enzyme dubbed cyclic GMP-AMP synthase (cGAS) produces a ribo-dinucleotide, which in turn binds to and activates STING14, 15. Given the striking analogy to bacterial cyclic dinucleotide recognition and its determined molecular mass, it was suggested that this molecule constitutes a cyclic adenosine monophosphate-guanosine monophosphate (cGAMP) with a symmetric 12-membered ring formed by 3′-5′ linked nucleotide residues (>Gp(3′-5′)Ap(3′-5′)>, cGAMP(3′-5′)). On the other hand, it was shown that STING-dependent DNA sensing can be differentiated from bacterial cyclic di-GMP recognition through a point mutation at a conserved arginine residue (R231A) within the lid region of murine STING9. R231 functions to indirectly bind the phosphate of the phosphodiester bond of cyclic di-GMP/AMP through a Mg2+ or H2O molecule, yet this coordination seems to be dispensable for STING activation in response to DNA transfection. We have recently identified a novel STING ligand (10-carboxymethyl-9-acridanone, CMA) that also triggers STING activation independently of the R231 residue16. In fact, the crystal structure of CMA bound to murine STING revealed that the lid region binds CMA differently than cyclic di-GMP and that R231 is not involved in CMA binding. We were intrigued by the differential role of R231 for DNA and cyclic di-GMP sensing, given the fact that modelling studies using cGAMP(3′-5′) instead of cyclic di-GMP could not readily explain the reported differential role of this residue at the structural level. To explore this further, we expressed cGAS in HEK293T cells together with either wild-type murine STING or its R231A mutant. As a control, we induced endogenous cyclic di-GMP production using a codon-optimized version of the thermophilic diguanylate cyclase domain (tDGC) (amino acids 83-248) of Thermotoga maritima17 and a codon-optimized version of the recently discovered bacterial cGAMP(3′-5′) synthetase (DncV) from Vibrio cholerae18. As expected, overexpression of the cyclic di-GMP synthetase, the cGAMP synthetase and cGAS induced a robust type I interferon (IFN) response in HEK293T cells expressing wild-type murine STING. Moreover, in line with previous reports, expression of the R231A point mutant completely abolished type I IFN production in response to endogenous cyclic di-GMP production, but not upon overexpression of cGAS (Fig. 1a and Supplementary Fig. 1). Surprisingly, however, induction of endogenous cGAMP production using DncV was also completely blunted for the R231A mutant. Next we stimulated HEK293T cells overexpressing wild-type murine STING or the R231A mutant directly with synthetic compounds. As previously reported, CMA-mediated activation of STING did not require coordination through R231 and in accordance with the synthetase data from above, synthetic cyclic di-GMP only activated cells expressing wild-type murine STING, but not the R231A mutant (Fig. 1b). Unexpectedly, synthetic cGAMP(3′-5′) was also completely blunted in its stimulatory activity when transfected into cells expressing STING(R231A). Altogether, these results confirmed previous reports on DNA/cGAS-mediated STING activation being distinct from cyclic dinucleotide sensing with regards to the involvement of the lid region of STING. At the same time, however, these results questioned the concept of cGAMP(3′-5′) being the cGAS-dependent second messenger molecule activating STING.
Figure 1. The R231A STING mutant uncouples cyclic di-GMP sensing from cGAS-induced activation.
a, Overexpression of dinucleotide synthetases. HEK293T cells were transfected with different dinucleotide synthetases (100 ng) together with decreasing amounts of wild-type (WT) mmSTING or the R231A mutant (10, 5, 2.5, 1.25 and 0 ng) and a pIFNβ-luciferase reporter (pIFNβ-GLuc). Reporter activity was measured 16 h after transfection. RLU, relative light units. b, Direct stimulation with synthetic compounds. HEK293T cells were transfected with WT mmSTING or the R231A mutant in conjunction with pIFNβ-GLuc. The next day CMA was added or synthetic cyclic di-GMP or synthetic cGAMP(3′-5′) was transfected as indicated and pIFNβ-GLuc activity was assayed 16 h later. Representative data of two (a) or three (b) independent experiments are shown (mean values + s.e.m.).
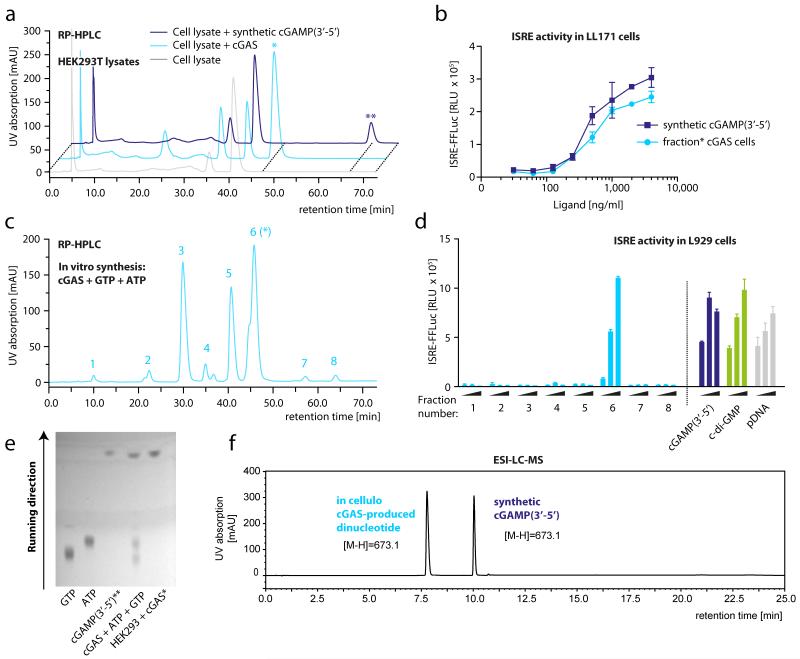
To follow up on this observation, we generated cytoplasmic lysates from cGAS overexpressing HEK293T cells and untreated HEK293T cells and subjected the protein-depleted, low-molecular-weight fraction to reversed-phase high-performance liquid chromatography (RP-HPLC). In comparison to untreated HEK293T cells, cGAS-overexpressing HEK293T cells showed an additional, unique peak with a retention time of 46 min (Fig. 2a, *), whereas synthetic cGAMP(3′-5′) spiked into cell lysate eluted at a far higher retention time (Fig. 2a, **). Comparing endogenously produced cyclic di-GMP to synthetic cyclic di-GMP under the same conditions revealed no difference in retention time, excluding the possibility of the purification process affecting the physiochemical properties of the compounds (Supplementary Fig. 2). Fractionation of the cell-derived, cGAS-specific low-molecular-weight product (*) and transfer into STING competent LL171 cells revealed potent stimulatory activity, within the same range as synthetic cGAMP(3′-5′) (Fig. 2b and Supplementary Fig. 3). Analogous results were obtained when purified cGAS was incubated in vitro with GTP and ATP (Fig. 2c). A cGAS-dependent peak could be detected at the same retention time as in cell lysates from cGAS overexpressing HEK293T cells and only this peak exerted stimulatory activity in LL171 cells (Fig. 2d). Thin-layer chromatography (TLC) as an alternative separation technique revealed that the cell-derived and the in-vitro-synthesized cGAS product showed a similar chromatographic mobility to that of the synthetic cGAMP(3′-5′) (Fig. 2e). Despite the big difference in chromatographic properties under RP-HPLC conditions, electrospray ionization-liquid chromatography-mass spectrometry (ESI-LC-MS) analysis revealed the same molecular mass (m/z (M-H) = 673.1) for both the cell-derived cGAS product and synthetic cGAMP(3′-5′) (Fig. 2f). In addition, while the MS/MS fragmentation pattern of the cGAS-derived molecule was consistent with a ribo-dinucleotide made up of guanosine and adenosine, these studies reproducibly displayed a clear difference compared to synthetic cGAMP(3′-5′) (Supplementary Fig. 4). Most intriguingly, the MS/MS fragmentation studies pointed to the presence of a 2′-5′ phosphodiester bond between guanosine and adenosine (Supplementary Figs 4-6 and Supplementary Notes 1 and 2).
Figure 2. The cGAS reaction product is distinct from cGAMP(3′-5′).
a, RP-HPLC chromatograms of lysates of untreated HEK293T cells (grey), of cGAS overexpressing HEK293T cells (light blue) or of synthetic cGAMP(3′-5′) spiked into untreated HEK293T lysate (dark blue). Asterisks highlight differential elution peaks. b, IFN-stimulated response element (ISRE) activity in LL171 cells. Endogenous cGAS product was purified from a and transfected into LL171 cells, whereas synthetic cGAMP(3′-5′) served as a control. ISRE-reporter activity was measured 14 h later. c, Chromatogram of an in vitro cGAS assay. The asterisk indicates the fraction that elutes at the same retention time as the endogenous product from a. d, ISRE activity in LL171 cells. Peaks 1-8 from c were fractionated and transfected into LL171 cells that were then studied for ISRE-reporter activity using respective control stimuli. e, TLC analysis of in-vitro- and in-vivo-synthesized cGAS product with ATP, GTP and synthetic cGAMP(3′-5′) as controls. f, ESI-LC-MS analysis of in-vivo-produced cGAS product and synthetic cGAMP(3′-5′). Representative data of two (e) or three (a-d, f) independent experiments are shown (mean values + s.e.m.).
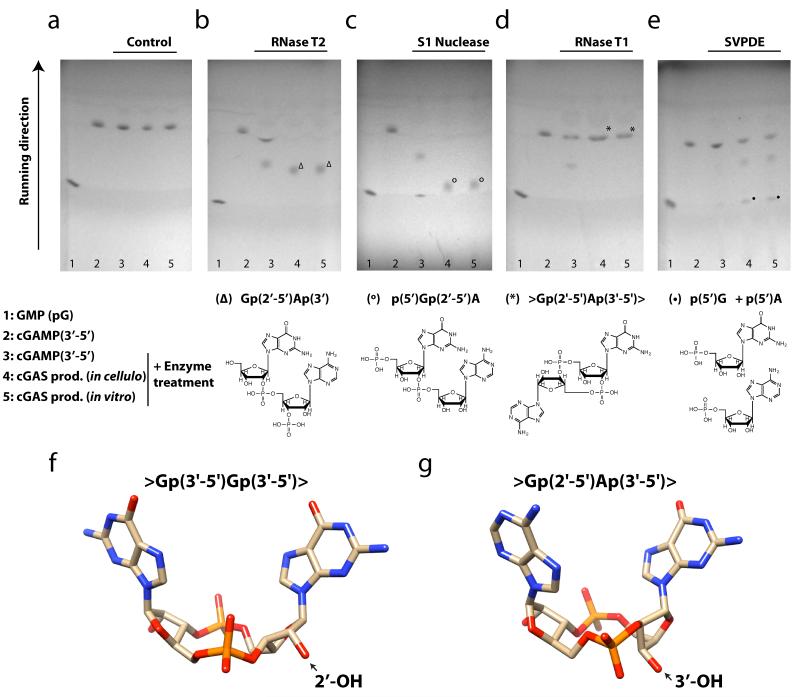
On the basis of these observations, we considered several candidate molecules as products of cGAS (Supplementary Fig. 7). Among these, a cyclic dinucleotide with one or two 2′-5′ phosphodiester bonds seemed to be most likely. To address this hypothesis, we performed a series of enzyme digests coupled to TLC and ESI-LC-MS. First, we treated synthetic cGAMP(3′-5′) and the in-vivo- and in-vitro-synthesized cGAS product using S1 nuclease and ribonuclease T2. Both enzymes can cleave internal 3′-5′ phosphodiester linkages. Synthetic cGAMP(3′-5′) could be processed into mononucleotides by both enzymes, whereas the cGAS-derived cyclic dinucleotide was only cleaved into a linear dinucleotide (Fig. 3a-c and Supplementary Fig. 9a, b). These results suggested that one of the internucleotide bonds was not a 3′-5′ phosphodiester. To address which one of the two phosphodiester bonds was not hydrolysable by the enzymes above, we took advantage of the nucleotide specificity of ribonuclease T1, which catalyses the endonucleolytic cleavage of 3′-5′ phosphodiester bonds only after guanosine. As expected, ribonuclease T1 processed synthetic cGAMP(3′-5′) into a linear dinucleotide, consistent with the presence of a Gp(3′-5′)A phosphodiester bond (Fig. 3d and Supplementary Fig. 9c). The cGAS-derived dinucleotide, however, was not processed, indicating that the GpA phosphodiester bond was not 3′-5′. In line with this notion, substitution of GTP by 2′dGTP during the in vitro enzymatic reaction completely blunted synthesis of dinucleotides by cGAS, whereas addition of 3′dGTP gave rise to small, but consistent amounts of a cGAMP product (Supplementary Fig. 10). In a reverse approach, we made use of snake venom phosphodiesterase I (SVPDE), which can hydrolyse 5′-mononucleotides from 3′-hydroxy-terminated ribo-oligonucleotides. Consistent with its two internal 3′-5′ phosphodiester bonds, synthetic cGAMP was not processed by SVPDE, whereas the cGAS-derived product was hydrolysed in a two-step process into its mononucleotide components (Fig. 3e and Supplementary Fig. 9d). Altogether, these results clearly identified the cGAS-derived dinucleotide product as a cyclic GA dinucleotide with a 2′-5′ phosphodiester linkage between guanosine and adenosine and a 3′-5′ phosphodiester linkage between adenosine and guanosine >Gp(2′-5′)Ap(3′-5′)> (cGAMP(2′-5′)). Comparison of synthetic cGAMP(3′-5′) and the cGAS-derived product by 1H-NMR spectroscopy supported the notion of differing 3′-5′/2′-5′ linkages between guanosine and adenosine within the cyclic GA dinucleotide and revealed crucial information on the ribose conformation of the distinct nucleotide elements in both molecules (Supplementary Fig. 11 and Supplementary Note 3). Relying on these data we were able to formulate a model of cGAMP(2′-5′) based on the previously determined structure of cyclic di-GMP bound to STING (Fig. 3f, g and Supplementary Note 3). To provide an additional proof of cGAMP(2′-5′) being the actual cGAS-derived second messenger molecule, we chemically synthesized >Gp(2′-5′)Ap(3′-5′)> and its isomer >Gp(3′-5′)Ap(3′-5′)> (Supplementary Fig. 12). As expected, synthetic cGAMP(2′-5′) had the same physiochemical properties as the in vitro cGAS-generated dinucleotide (Supplementary Fig. 13).
Figure 3. The second messenger produced by cGAS is >Gp(2′-5′)Ap(3′-5′)>.
a-e, TLC analysis of GMP (lane 1), synthetic cGAMP(3′-5′) (lane 2) and enzyme-treated synthetic cGAMP(3′-5′) (lane 3), in-vivo-synthesized cGAS product (lane 4) and in-vitro-synthesized cGAS product (lane 5). Enzyme treatments of molecules analysed in lanes 3-5 were control (a), RNase T2 (b), S1 nuclease (c), RNase T1 (d) and SVPDE (e). The resulting reaction products from lanes 4 and 5 as confirmed by ESI-LC-MS analysis are depicted below. Representative data out of two independent experiments are shown. f, g, Comparison of the structure of cyclic di-GMP (4F9G.pdb) and a model for cGAMP(2′-5′) based on NMR-derived ribose conformations.
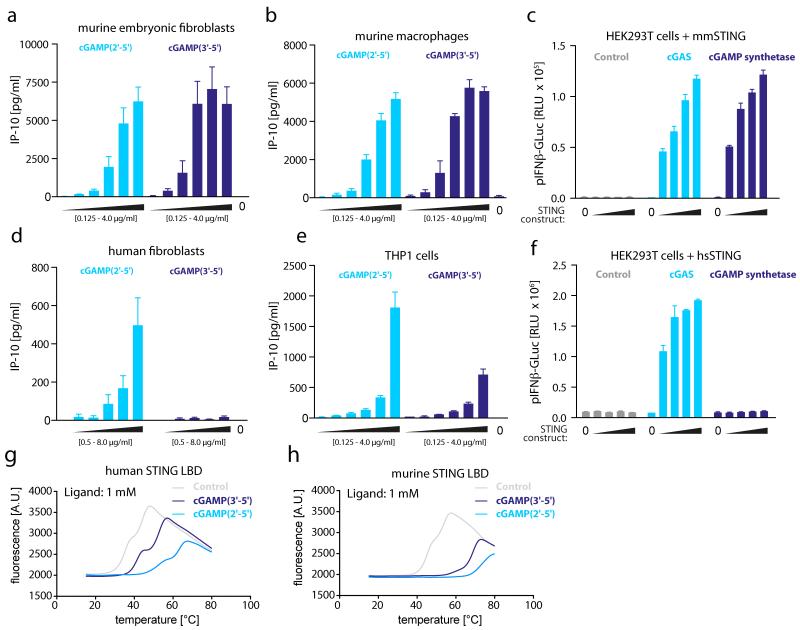
Transfection of both DNA and cyclic dinucleotides (3′-5′linked) has been shown to induce indistinguishable transcriptional responses in murine cells in a STING-dependent manner. However, despite being equally responsive towards DNA challenge, recent reports indicated that human cells are less responsive to intracellular delivery of cyclic dinucleotides or other STING ligands19. To determine whether these species-specific properties of STING would also apply for the recognition of cGAMP, we first sought to compare both cGAMP isomers with regard to their biological activity in human and murine cells. Transfection of both cGAMP(2′-5′) and cGAMP(3′-5′) into murine embryonic fibroblasts and macrophages strongly induced production of the antiviral cytokine IP-10, confirming previous reports on the stimulatory potency of cyclic dinucleotides for murine STING (Fig. 4a, b). However, to our surprise, we reproducibly observed a marked difference in responsiveness towards the two cGAMP isomers, when we tested them for functional activity in human fibroblasts and the monocytic cell line THP-1 (Fig. 4d, e). Here, cGAMP(2′-5′) was more active than cGAMP(3′-5′) with regards to production of IP-10. In fact, human fibroblasts were almost unresponsive towards transfection with cGAMP(3′-5′), even at high concentrations of the cyclic dinucleotide being delivered. Similar results were obtained when we studied HEK293T cells overexpressing cGAS or the cGAMP synthetase DncV derived from V. cholerae (Fig. 4c and f). Whereas expression of murine STING rendered HEK293T responsive towards both the cGAS and the cGAMP synthetase products, only cGAS expression was able to activate human STING. Finally, we performed binding studies with the carboxy-terminal ligand-binding domain (LBD) of mouse and human STING using differential scanning fluorometry (DSF). These data revealed that cGAMP(2′-5′) showed stronger complex formation with both human and mouse STING compared to cGAMP(3′-5′) (Fig. 4g, h). Of note, this preference for cGAMP(2′-5′) over cGAMP(3′-5′) was more prominent when the LBD of human STING was tested. Together, these data indicate that, in contrast to cGAMP(3′-5′), cGAMP(2′-5′) is highly potent in the human system and, like DNA, is not affected by species-specific properties of STING.
Figure 4. cGAMP(2′-5′) is a potent activator of human and murine STING.
a, b, d, e, IP-10 production of murine embryonic fibroblasts, murine macrophages, human fibroblasts and THP1 cells transfected with increasing amounts of cGAMP(2′-5′) or cGAMP(3′-5′). c, f, HEK293T cells transfected with human or murine STING (0, 3.13, 6.25, 12.5 and 25 ng) as indicated, together with cGAS or DncV cGAMP synthetase (100 ng) subsequently analysed for pIFNβ-GLuc activity. g, h, Interaction of the human and murine STING LBD with 1 mM cGAMP(2′-5′) or cGAMP(3′-5′) analysed by DSF. Mean + s.e.m. of two (e) or three (a, b, d) independent experiments or one representative experiment out of three independent experiments (c, f, g, h) are depicted (c, f, mean values + s.e.m.).
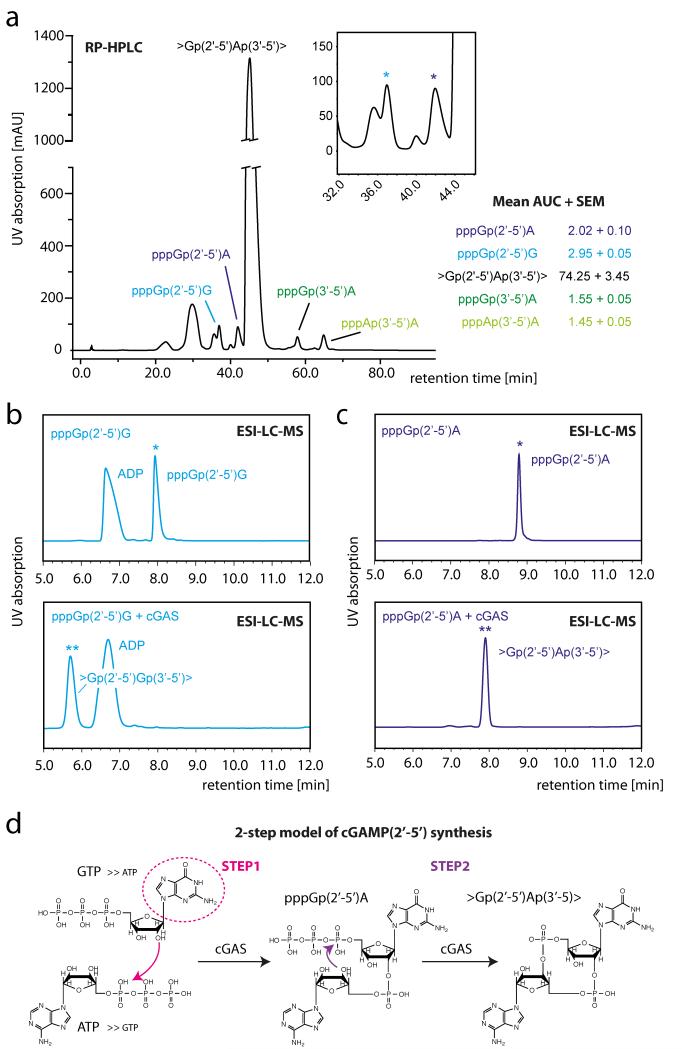
Next we wanted to delineate the mechanism of cGAS-dependent cyclic dinucleotide synthesis. Enzymatic cGAS reactions in the presence of excess substrate (ATP/GTP) displayed, among other dinucleotide species, one predominant peak in ESI-LC-MS analysis, which was represented by pppGp(2′-5′)A (Supplementary Fig. 10). However, upon termination of the reaction we identified four distinct linear dinucleotide species at almost similar quantities, next to >Gp(2′-5′)Ap(3′-5)> as the major species (Fig. 5a). These molecules included: pppGp(2′-5′)A, pppGp(3′-5′)A, pppGp(2′-5′)G and pppAp(3′-5′)A (Fig. 5a). When we incubated ATP or GTP alone with cGAS, either pppAp(3′-5′)A or pppGp(2′-5′)G predominated by approximately 10:1 over their respective phosphodiester linkage isomers (Supplementary Fig. 14). Of note, whereas GTP by itself gave rise to substantial amounts of a cyclic dinucleotide (>Gp(2′-5′)Gp(3′-5′)>), ATP by itself was unable to trigger synthesis of detectable amounts of a cyclic dinucleotide. This observation indicated that a 2′-5′ dinucleotide constitutes the substrate for the subsequent cyclization reaction and that pppGp(2′-5′)A represented the precursor molecule for cGAMP(2′-5′). To prove this hypothesis, we fractionated the four major dinucleotide species obtained during enzymatic cGAS reactions and incubated them again with cGAS. Interestingly, we found that both 2′-5′-linked dinucleotide species were quantitatively converted into cyclic dinucleotides, whereas the 3′-5′-linked dinucleotides were only scarcely, if at all, converted (Fig. 5b and Supplementary Fig. 15). Interestingly, the second phosphodiester bond was linked exclusively via 3′-5′ for all cyclization reactions. Together these results unequivocally identified pppGp(2′-5′)A as the precursor of cGAS-dependent cGAMP(2′-5′) synthesis.
Figure 5. >Gp(2′-5′)Ap(3′-5′)> is synthesized in a two-step process.
a, RP-HPLC chromatogram of a cGAS+ATP+GTP in vitro reaction upon termination of the reaction. The insertion represents an enlargement of the chromatogram indicating the position of pppGp(2′-5′)G and pppGp(2′-5′)A. Right panel demonstrates mean area under the curve (AUC) + s.e.m. for depicted dinucleotides out of two independent experiments. b, c, ESI-LC-MS chromatograms before (top) and after (bottom) in vitro incubation of pppGp(2′-5′)G (b) and pppGp(2′-5′)A (c) with cGAS. Asterisks indicate the position of the substrates (*) and the resulting products (**). Note that the fraction of pppGp(2′-5′)G was contaminated with ADP. Data are representative of two independent experiments. d, Schematic model of the two-step process of cGAS-catalysed cyclic dinucleotide synthesis.
On the basis of these results we postulate the following two-step synthesis model (Fig. 5d). (1) In the presence of ATP and GTP cGAS first catalyses the generation of a linear dinucleotide, with the attacking nucleotide determining the type of phosphodiester bond being generated. 5′-GTP preferentially results in a 2′-5′ linkage, whereas 5′-ATP results in a 3′-5′ linkage leading to either pppGp(2′-5′)R or pppAp(3′-5′)R, respectively. In this first synthesis step, cGAS shows a preference for GTP over ATP being the attacking nucleotide, and ATP over GTP for the nucleotide being attacked. (2) Whereas pppGp(2′-5′)R species are quantitatively cyclized by cGAS in a second step, pppRp(3′-5′)A dinucleotides are poor, if at all, substrates for cyclization. Of note, this second step exclusively generates a 3′-5′ linkage, at least for the dinucleotide species studied. The fact that only scarce amounts of cyclic di-GMP are found during in vitro reactions might be attributed to lower supply of its precursor molecule pppGp(2′-5′)G and presumably the preference of pppGp(2′-5′)A over pppGp(2′-5′)G during the cyclization step. All in all, this model explains the nearly exclusive generation of >Gp(2′-5′)Ap(3′-5)> by cGAS in the presence of ATP and GTP.
Previously it has been shown that the response of STING towards DNA and cyclic dinucleotides can be uncoupled9. This observation can now be rationalized by our finding that cGAS produces a novel class of second messenger being a 2′-5′/3′-5′-linked cyclic dinucleotide, which is structurally and physiochemically distinct from bacteria-derived cyclic dinucleotides. In fact, this report describes the enzymatic production of a cyclic 2′-5′/3′-5′-linked dinucleotide and thereby adds, at the functional level, cGAS to the oligoadenylate synthetase (OAS) family of enzymes that are unique in their ability to synthesize 2′-5′ phosphodiester bonds. Indeed, this functional similarity seems quite plausible given the sequence homology of cGAS to OAS1, which produces 2′-5′-linked oligoadenylates upon binding double stranded RNA15, 20. Another striking analogy is that cGAS as well as the OAS enzymes both require nucleic acid binding to be activated to synthesize their products in a template-independent fashion. Thus, our results now unequivocally unify these two innate sensing systems and suggest both processes to be evolutionary linked.
The unorthodox chemical linkage within cGAMP(2′-5′) provides a unique feature that may be targeted by specific cellular regulation mechanisms. At the same time, the cGAS-dependent, two-step synthesis of cGAMP(2′-5′) could be amenable for the development of specific inhibitors for the treatment of autoimmune diseases that engage the cGAS-STING axis.
Methods
Reagents
Cyclic di-GMP and cyclic GAMP(3′-5′) were obtained from Biolog. DNA oligonucleotides corresponding to ISD were obtained from Metabion and annealed in PBS. 10-carboxymethyl-9-acridanone was purchased from Sigma Aldrich. ATP and GTP were obtained from Fermentas.
Cell culture
HEK293T cells, THP1 cells, human fibroblasts (hTERT-BJ1 cells), mouse embryonic fibroblasts, bone marrow-derived macrophages and LL171 cells (L929 cells containing a stable IFN-stimulated response element-luciferase reporter plasmid (ISRE-Luc)) were cultured in DMEM supplemented with 10% (v/v) FCS, sodium pyruvate (all Life Technologies) and Ciprofloxacin (Bayer Schering Pharma). All mouse cells used in this study and human hTERT-BJ1 cells and THP1 cells show responsiveness towards DNA stimulation and thus could be used for the exploration of DNA sensing pathways.
Plasmids
Expression plasmids coding for murine STING (amino-terminal green fluorescent protein (GFP)-tag)16, murine STING R231A16 and murine cGAS are based on pEFBOS23. Murine cGAS was amplified from cDNA by PCR (forward 5′-ATTACTCGAGATGGAAGATCCGCGTAGA-3′ and reverse 5′-ATTAAGATCTCTATCAAAGCTTGTCAAAAATTGGAAACCC-3′) and cloned into pEFBOS using XhoI and BglII/BamHI. A codon-optimized version of the diguanylate cyclase domain (amino acids 83-248) of TM1788 (Thermotoga maritima MSB8) harbouring a point mutation (R158A) to enhance c-diGMP production was cloned into pEFBOS-C-term-Flag/His using XhoI and BamHI17. In addition, a codon-optimized version of the Vibrio cholerae cGAMP synthetase (DncV; amino acids 1-438) was cloned into pEFBOS-C-term-Flag/His using XhoI and BamHI18.
Immunoblotting
Cells were lysed in 1× Laemmli buffer and denatured at 95 °C for 5 min. Cell lysates were separated by 10% SDS-PAGE and transferred onto nitrocellulose membranes. Blots were incubated with anti-β-actin-IgG-horseradish peroxidase (HRP) and anti-GFP-IgG/anti-rabbit-IgG-HRP (all Santa Cruz Biotechnology).
Cell stimulation
LL171 cells (0.15 × 106 per ml), murine BMDM (1 × 106 per ml), MEFs (0.15 × 106 per ml), hTERT-BJ1 cells (0.2 × 106 per ml) and THP1 cells (0.6 × 106 per ml) were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Plasmid DNA (empty pCI vector) was transfected at a final concentration of 1.33 μg ml−1. Unless otherwise indicated, cyclic dinucleotides were transfected at a final concentration of 2 μg ml−1. Cells were stimulated 14 h before final read-out was performed.
Luciferase assay
LL171 cells were lysed in 5× passive lysis buffer (Promega) for 10 min at room temperature. The total cell lysate was incubated with firefly luciferase substrate at a 1:1 ratio and luminescence was measured on an EnVision 2104 Multilabel Reader (Perkin Elmer). pIFNβ-GLuc activity was measured in HEK293T cell supernatants using Coelenterazine as a substrate.
ELISA
Cell culture supernatants were assayed for mouse IP-10 (R&D Systems) and human IP-10 (BD Biosciences) according to the manufacturer’s instructions.
In vitro assay for cGAS activity
For in vitro synthesis of the cGAS reaction product 2 μM recombinant cGAS was mixed with 3 μM dsDNA (ISD) in Buffer A (100 mM NaCl, 40 mM Tris pH 7.5, 10 mM MgCl2). Reaction was started by addition of 1 mM ATP and 1 mM GTP. After 2-4 h incubation at 37 °C the reaction was stopped and filtered using Amicon Ultra-15 filter devices (10,000 or 30,000 relative molecular mass cut-off).
Preparation of HEK293T cell lysates
HEK293T cells (0.33 × 106 per ml) were transfected with 3.2 μg plasmid using GeneJuice (Novagen). After 20 h cells were collected, washed twice with PBS and pelleted by centrifugation at 500g at 4 °C. The cell pellet was lysed (lysis buffer: 1 mM CaCl2, 3 mM MgCl2, 1 mM EDTA, 1% Triton × 100, 10 mM Tris pH 7.5) for 20 min at 4 °C. The cell lysate was briefly centrifuged (1,000g, 10 min, 4 °C) and the resultant supernatant was further purified via two sequential rounds of phenol-chloroform extraction. The extract was then filtered by centrifugation using Amicon Ultra-15 filter devices (10,000 or 30,000 relative molecular mass cut-off). In some experiments the final extract was concentrated via centrifugation under vacuum (Eppendorf Vacufuge).
Reversed phase-HPLC
Cell lysates and enzymatic reaction mixtures were applied to a 4.1 × 250 mm PRP-1 column (Hamilton) and separated in a linear gradient of 0% buffer B for 8 min, followed by an increase of buffer B from 0 to 75% in 62 min at a flow rate of 1 ml min−1. Buffer A was 20 mM TEAB and buffer B 20 mM TEAB in 20% methanol. The product fractions were collected, evaporated and desalted by repeated co-evaporation with methanol. The residue was dissolved in PBS and the product concentration was determined by measuring ultraviolet absorbance (A260). This HPLC method was mainly employed for preparative runs of cell lysates or in vitro synthesis products. Please note the differing retention times of this method compared to the analytical ESI-LC-MS runs.
Enzymatic reactions
0.07 A260 of cGAMP(3′-5′) and cGAMP generated either in vivo or in vitro were dissolved in 6.5 μl incubation buffer and treated with 1 μl of the following enzymes: RNaseT1 (Fermentas, 100 mM Tris-HCl pH 7.4, 10 mM EDTA, 1 h, 37 °C), S1 nuclease (Fermentas, 50 mM NaOAc pH 4.5, 300 mM NaCl, 2 mM ZnSO4, 1 h, 37 °C), RNase T2 (MoBiTec, 125 mM NH4 Ac pH 4.5, 1 h, 37 °C), SVPDE (Sigma, isolated from Crotalus adamanteus, 50 mM Tris-HCl pH 8.8, 10 mM MgCl2, 30 min, 37 °C). The digestion products were analysed by TLC and ESI-LC-MS.
Thin layer chromatography (TLC)
TLC was performed on 5 × 10 cm LuxPlate Si60 silica-covered glass plates (Merck). The samples (1-2 μl) were spotted onto the plate and separation was performed in n-propanol/ammonium hydroxide/water (11:7:2 v/v/v). The plate was air-dried and bands were visualized with a short-wavelength (254 nm) ultraviolet light source.
ESI-LC-MS and ESI-LC-MS/MS
All reagents used were purchased from Sigma Aldrich. The ESI-LC/MS analysis was performed using a Dionex Ultimate 3000 RS system (Thermo Fisher Scientific) coupled to an IonTrap mass spectrometer (LCQ Deca XP+, Thermo Finnigan) equipped with an electrospray source operating in negative ionization mode. The ionization source parameters were set to: ion transfer capillary temperature 310 °C, spray voltage 4 kV and internal source fragmentation 15 kV. All samples were chromatographed on a Waters XBridge C18 OST column (2.1 × 50 mm; 2.5 μm particle size) at 30 °C column temperature. Separation of the analytes was achieved using a gradient of 10 mM TEAB in water as eluent A and 10 mM TEAB in 20% MeOH as eluent B with a flow rate of 0.25 ml min−1. The HPLC gradient starts at 0% B, hold for 3 min and then increases over 16.5 min to 90% B.
Full-scan mass spectrometry spectra were acquired in a mass range from m/z 150 to 1,000 with isotopic resolution for the singly charged molecular ions. Tandem MS-MS and MS-MS-MS spectra were recorded from isolated ions in the ion trap applying collision induced dissociation (CID) applying helium as collision gas. For tandem MS-MS spectra the singly charged molecular ion was isolated and subsequently fragmented with 28% normalized collision energy. For tandem MS-MS-MS spectra the G-depurinated daughter ion of the cyclic dinucleotides with m/z = 522.0 (-guanine base) generated in the first CID fragmentation stage, was isolated and subsequently fragmented with 30% normalized collision energy.
Periodate oxidation assay
0.1 mg ml−1 of the dinucleotide fractionated from cell culture lysates was incubated with 20 mM sodium periodate for 60 min at room temperature in the dark. After the incubation 10-vol% of 2 M triethylammonium acetate was added to the mixture that then was analysed by ESI-LC-MS.
2′→3′ isomeriza on assay
Approximately 0.1 mg ml−1 of the dinucleotide fractionated from cell culture lysates was incubated for 2 h at 90 °C in the presence of 10 mM EDTA and 20 mM Tris-HCl at pH 8. After the incubation 10-vol% of 2 M triethylammonium acetate was added to the mixture that then was analysed by LC-MS.
Chemical synthesis of >Gp(2′-5′)Ap(3′-5′)> and >Gp(3′-5′)Ap(3′-5′)>
The chemical synthesis of >Gp(2′-5′)Ap(3′-5′)> and >Gp(3′-5′)Ap(3′-5′)> was performed according to the strategy described in ref. 24 using the commercially available 3′-TBDMS protected 2′-guanosine phosphoramidite (Chemgenes) or 2′-TBDMS protected 3′-guanosine phosphoramidite (Sigma-Aldrich) for introduction of the 2′-5′ and 3′-5′ phosphodiester bond linkage, respectively. The 3′-adenosine phosphoramidite and all other reagents were purchased from Sigma-Aldrich. After base deprotection and removal of the TBDMS protecting groups >Gp(2′-5′)Ap(3′-5′)> or >Gp(3′-5′)Ap(3′-5′)> was purified by RP-HPLC as described above and the product was verified by ESI-LC-MS/MS.
Differential scanning fluorometry
Purification of human and murine STING ligand binding domains and differential scanning fluorometry to evaluate their thermal stabilization by nucleotide ligands was performed as previously described16.
Supplementary Material
Acknowledgements
We thank M. Pelegrin for providing us with LL171 cells. K.-P.H. is supported by the National Institutes of Health (U19AI083025), the European Research Council Advanced Grant 322869, and the Center for Integrated Protein Science Munich (CIPSM). A.A. and V.H. are members of the excellence cluster ImmunoSensation. V.H. is supported by grants from the German Research Foundation (SFB670) and the European Research Council (ERC 243046).
Footnotes
References
- 1.Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010;10:123–130. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- 2.Gall A, et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36:120–131. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong B, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Jin L, et al. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28:5014–5026. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun W, et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci U S A. 2009;106:8653–8658. doi: 10.1073/pnas.0900850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdette DL, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YH, Liu XY, Du XX, Jiang ZF, Su XD. The structural basis for the sensing and binding of cyclic di-GMP by STING. Nature structural and molecular biology. 2012;19:728–730. doi: 10.1038/nsmb.2333. [DOI] [PubMed] [Google Scholar]
- 11.Ouyang S, et al. Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity. 2012;36:1073–1086. doi: 10.1016/j.immuni.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang G, et al. Crystal structures of STING protein reveal basis for recognition of cyclic di-GMP. Nature structural and molecular biology. 2012;19:725–727. doi: 10.1038/nsmb.2332. [DOI] [PubMed] [Google Scholar]
- 13.Shu C, Yi G, Watts T, Kao CC, Li P. Structure of STING bound to cyclic di-GMP reveals the mechanism of cyclic dinucleotide recognition by the immune system. Nature structural and molecular biology. 2012;19:722–724. doi: 10.1038/nsmb.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavlar T, Deimling T, Ablasser A, Hopfner KP, Hornung V. Species-specific detection of the antiviral small-molecule compound CMA by STING. The EMBO Journal. 2013;32:1440–1450. doi: 10.1038/emboj.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao F, et al. Enzymatic synthesis of c-di-GMP using a thermophilic diguanylate cyclase. Anal Biochem. 2009;389:138–142. doi: 10.1016/j.ab.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Davies BW, Bogard RW, Young TS, Mekalanos JJ. Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell. 2012;149:358–370. doi: 10.1016/j.cell.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conlon J, et al. Mouse, but not Human STING, Binds and Signals in Response to the Vascular Disrupting Agent 5,6-Dimethylxanthenone-4-Acetic Acid. J. Immunol. 2013;190:5216–5225. doi: 10.4049/jimmunol.1300097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristiansen H, Gad HH, Eskildsen-Larsen S, Despres P, Hartmann R. The oligoadenylate synthetase family: an ancient protein family with multiple antiviral activities. J Interferon Cytokine Res. 2011;31:41–47. doi: 10.1089/jir.2010.0107. [DOI] [PubMed] [Google Scholar]
- 21.Gao P, et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013;153:1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diner EJ. The innate immune DNA sensory cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 2013;3:1355–1361. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaffney BL, Veliath E, Zhao J, Jones RA. One-flask syntheses of c-di-GMP and the [Rp,Rp] and [Rp,Sp] thiophosphate analogues. Org. Lett. 2010;12:3269–3271. doi: 10.1021/ol101236b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.