Abstract
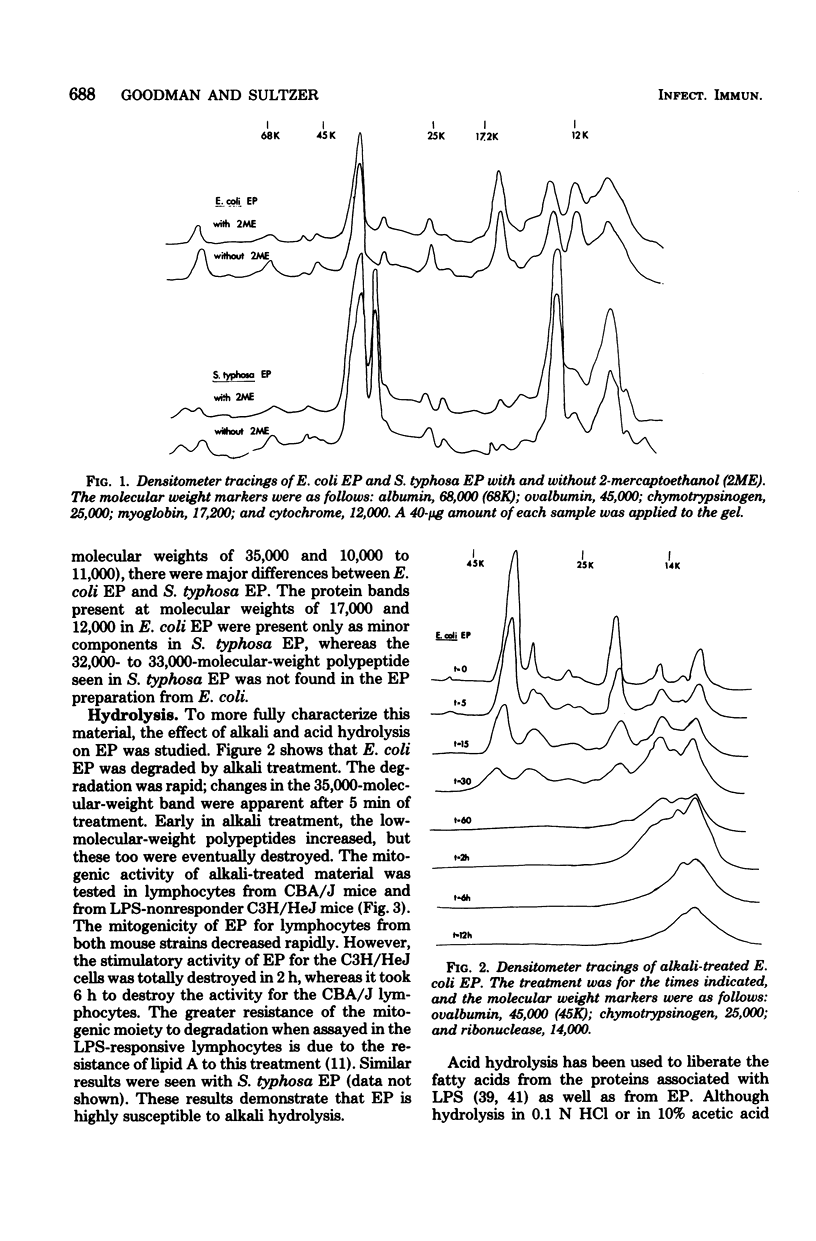
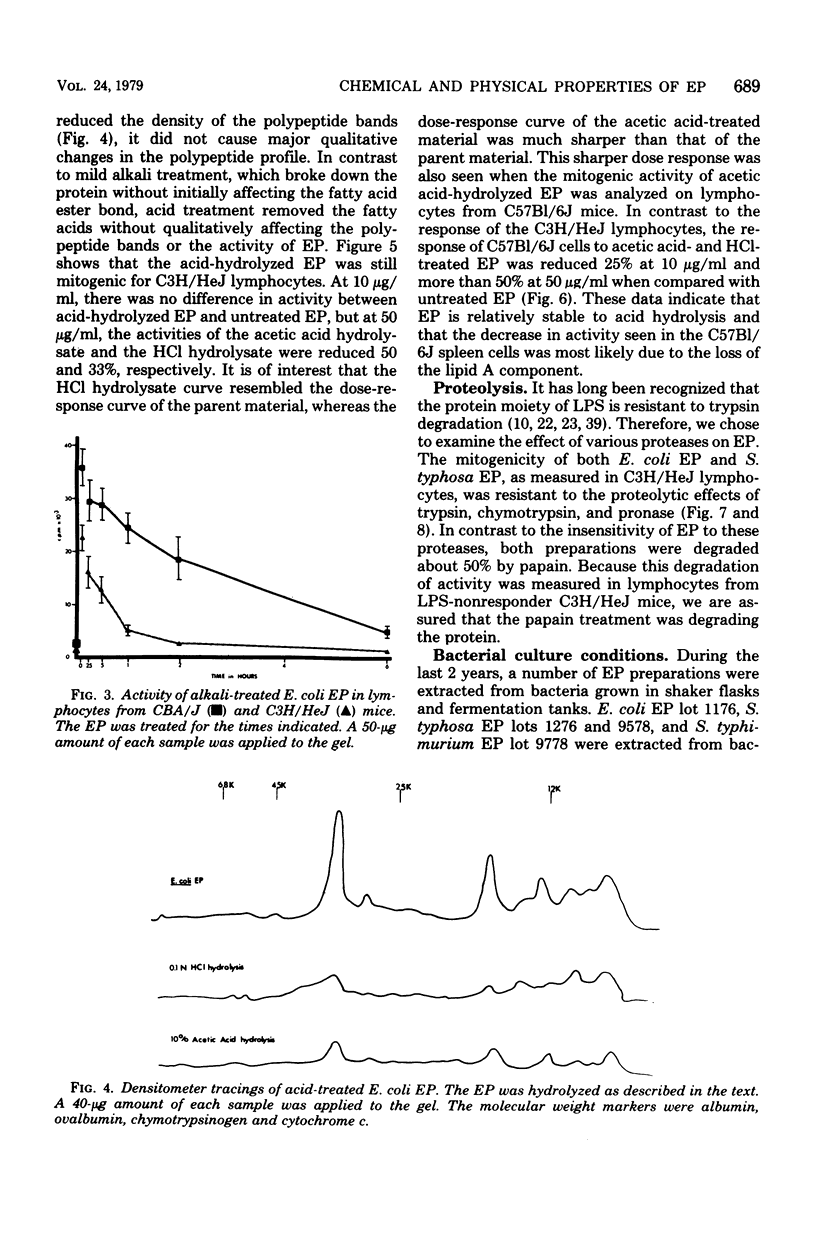
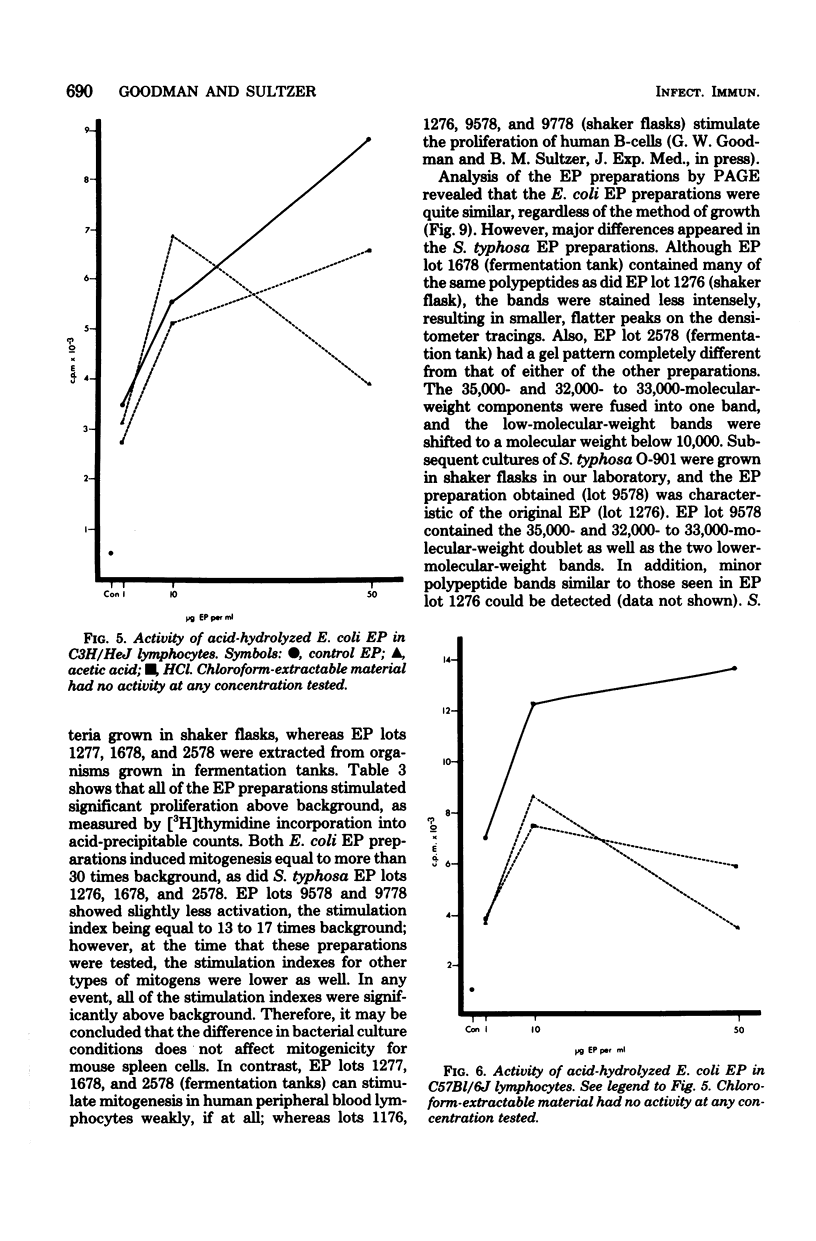
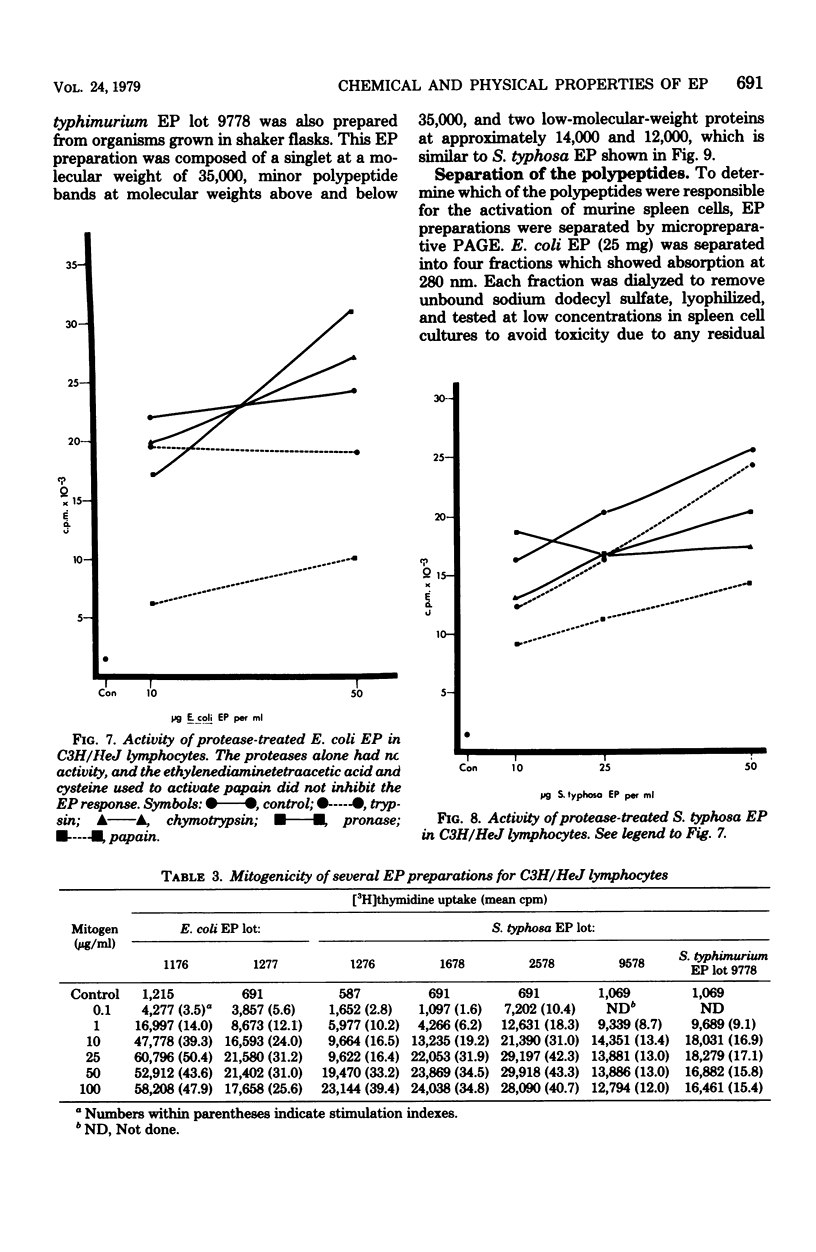
Endotoxin protein, a novel mouse B-lymphocyte mitogen, is a hydrophobic acidic compound composed of approximately 85% protein and 2.2% glucosamine, but no 2-keto-3-deoxyoctonate. Endotoxin protein also contains lipid, and analysis of the fatty acids in this material demonstrated the presence of beta-hydroxymyristate, a marker for lipid A. In addition, analysis of endotoxin protein by polyacrylamide gel electrophoresis showed that it is heterogeneous, containing four or five major polypeptides, depending upon the bacterial species from which it was isolated. The mitogenicity of endotoxin protein was diminished by alkaline hydrolysis, but not by treatment with hydrochloric or acetic acid. Furthermore, its activity was resistant to digestion with trypsin, chymotrypsin, and pronase and was only partially degraded by papain.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz S. J., Morrison D. C. Chemical and biologic properties of a protein-rich fraction of bacterial lipopolysaccharides. I. The in vitro murine lymphocyte response. J Immunol. 1977 Oct;119(4):1475–1481. [PubMed] [Google Scholar]
- Braun V., Bosch V. Sequence of the murein-lipoprotein and the attachment site of the lipid. Eur J Biochem. 1972 Jun 23;28(1):51–69. doi: 10.1111/j.1432-1033.1972.tb01883.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K., Wolff H. Supramolecular structure of the rigid layer of the cell wall of Salmonella, Serratia, Proteus, and Pseudomonas fluorescens. Number of lipoprotein molecules in a membrane layer. Biochemistry. 1970 Dec 22;9(26):5041–5049. doi: 10.1021/bi00828a001. [DOI] [PubMed] [Google Scholar]
- Braun V., Wolff H. The murein-lipoprotein linkage in the cell wall of Escherichia coli. Eur J Biochem. 1970 Jun;14(2):387–391. doi: 10.1111/j.1432-1033.1970.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Freedman H. H., Fox A. E., Willis R. S., Schwartz B. S. Role of protein component of endotoxin in modification of host reactivity. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1316–1320. doi: 10.3181/00379727-125-32346. [DOI] [PubMed] [Google Scholar]
- Freeman G. G., Anderson T. H. The hydrolytic degradation of the antigenic complex of Bact. typhosum Ty 2. Biochem J. 1941 Apr;35(4):564–577. doi: 10.1042/bj0350564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. G., Challinor S. W., Wilson J. The use of a synthetic medium in the isolation of the somatic antigens of Bact. typhi-murium and Bact. typhosum. Biochem J. 1940 Mar;34(3):307–324. doi: 10.1042/bj0340307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman G. W., Sultzer B. M. Mild alkaline hydrolysis of lipopolysaccharide endotoxin enhances its mitogencity for murine B cells. Infect Immun. 1977 Jul;17(1):205–214. doi: 10.1128/iai.17.1.205-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S., Hirashima A., Inouye M. Existence of a free form of a specific membrane lipoprotein in gram-negative bacteria. J Bacteriol. 1974 Dec;120(3):1204–1208. doi: 10.1128/jb.120.3.1204-1208.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima A., Wu H. C., Venkateswaran P. S., Inouye M. Two forms of a structural lipoprotein in the envelope of Escherichia coli. Further characterization of the free form. J Biol Chem. 1973 Aug 25;248(16):5654–5659. [PubMed] [Google Scholar]
- Homma J. Y. The protein moiety of the endotoxin of Pseudomonas aeruginosa. Z Allg Mikrobiol. 1968;8(3):227–248. doi: 10.1002/jobm.3630080310. [DOI] [PubMed] [Google Scholar]
- KIM Y. B., WATSON D. W. INACTIVATION OF GRAM-NEGATIVE BACTERIAL ENDOTOXINS BY PAPAIN. Proc Soc Exp Biol Med. 1964 Jan;115:140–142. doi: 10.3181/00379727-115-28852. [DOI] [PubMed] [Google Scholar]
- Kasai N. Chemical studies on the lipid component of endotoxin, with special emphasis on its relation to biological activities. Ann N Y Acad Sci. 1966 Jun 30;133(2):486–507. doi: 10.1111/j.1749-6632.1966.tb52385.x. [DOI] [PubMed] [Google Scholar]
- Kim Y. B., Watson D. W. Role of antibodies in reactions to gram-negative bacterial endotoxins. Ann N Y Acad Sci. 1966 Jun 30;133(2):727–745. doi: 10.1111/j.1749-6632.1966.tb52402.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Melchers F., Braun V., Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975 Aug 1;142(2):473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. T., Partridge S. M. Studies in immunochemistry: The fractionation and nature of antigenic material isolated from Bact. dysenteriae (Shiga). Biochem J. 1940 Feb;34(2):169–191. doi: 10.1042/bj0340169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J., Jacobs D. M. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976 Sep 1;144(3):840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOWOTNY A. M., THOMAS S., DURON O. S., NOWOTNY A. Relation of structure to function in bacterial O antigens. I. Isolation methods. J Bacteriol. 1963 Feb;85:418–426. doi: 10.1128/jb.85.2.418-426.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B. S., Sultzer B. M., Bullock W. W. Purified protein derivative of tuberculin induces immunoglobulin production in normal mouse spleen cells. J Exp Med. 1973 Jan 1;137(1):127–139. doi: 10.1084/jem.137.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Goodman G. W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976 Sep 1;144(3):821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]
- Tsang J. C., Wang C. S., Alaupovic P. Degradative effect of phenol on endotoxin and lipopolysaccharide preparations from Serratia marcescens. J Bacteriol. 1974 Feb;117(2):786–795. doi: 10.1128/jb.117.2.786-795.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal O. Bacterial endotoxins. The second Carl Prausnitz Memorial Lecture. Int Arch Allergy Appl Immunol. 1975;49(1-2):1–43. [PubMed] [Google Scholar]
- Wober W., Alaupović P. Studies on the protein moiety of endotoxin from gram-negative bacteria. Characterization of the protein moiety isolated by acetic acid hydrolysis of endotoxin from Serratia marcescens 08. Eur J Biochem. 1971 Apr;19(3):357–367. doi: 10.1111/j.1432-1033.1971.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Wu M. C., Heath E. C. Isolation and characterization of lipopolysaccharide protein from Escherichia coli. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2572–2576. doi: 10.1073/pnas.70.9.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]



