Abstract
Introduction
Calcium aluminate cements have shown little affinity for bacterial growth, low toxicity, and immunogenicity when used as a restoration material, but calcium aluminate cements have not been tested in vivo in pulpotomy procedures.
Methods
To address this question, a calcium aluminate cement (Quick-Set) was tested along with 2 mineral trioxide aggregates, ProRoot MTA and MTA Plus. These cements were used as a capping agent after pulpotomy. Control rats had no pulpotomy, or the pulpotomy was not capped. Proinflammatory cytokines interleukin (IL)-1β and IL-1α were measured, and histology was performed at 30 and 60 days after capping. The nociceptive response was determined by measuring the lengthening of the rat's meal duration.
Results
and Conclusions: IL-1β and IL-1α concentrations were reduced in the capped teeth, but no differences were observed among the 3 cements. Dentinal bridging could be detected at both 30 and 60 days with each of the 3 cements, and the pulps were still vital 60 days after capping. Meal duration significantly shortened after placement of the 3 different cements, indicating a nociceptive response, but there were no differences among the materials. Calcium aluminate cements had similar properties to mineral trioxide aggregates and is a viable option for pulpotomy procedures.
Keywords: Calcium aluminate cement, calcium silicate, endodontic, mineral trioxide aggregate, pain, tricalcium silicate
Calcium aluminate cements show little immunogenicity, toxicity, or affinity for bacterial growth when tested as a restoration material (1-3). Unfortunately, calcium aluminate cements have a higher failure rate than commonly used restoration materials (4-6). Recently, calcium aluminate cements have been tested in vitro and in vivo as an endodontic material (7-9). In these studies calcium aluminate cement showed little immunogenicity or affinity for bacterial growth, but to date, no study has tested calcium aluminate cements as a pulp-capping material in vivo. In addition to studying immunogenicity and bacterial static properties, pain after capping pulpotomies with mineral trioxide aggregates (MTAs) was measured in patients (10). To our knowledge, no study has compared the nociceptive response after capping pulpotomies with different hydraulic cements (a cement that hardens on hydration), and a pain study has not included calcium aluminate cement. To address this knowledge gap, we hypothesized that calcium aluminate cements are a viable pulp-capping material that shows low immunogenicity and little pain and has a high biocompatibility when in contact with tooth pulp.
In this study, a calcium aluminosilicate cement and MTAs were used as a capping material after pulpotomy, and the inflammatory, biocompatibility, and nociceptive responses were measured after placement of these cements. Inflammation was measured by quantifying proinflammatory cytokines interleukin (IL)-1β and IL-1α in the pulp of the treated teeth. Histology was also performed to assess dentinal bridging, the presence of bacteria, and pulp vitality. The nociceptive response was measured by using a behavioral assay, specifically the rat's meal duration. Previous studies have shown that a lengthening of the rat's meal duration correlates to orofacial pain in rats (11-16), and that meal duration has been shown to be significantly longer after pulpotomy (12).
Materials and Methods
Animals
All animal experiments were approved by the Baylor College of Dentistry Institutional Animal Care and Use Committee in accordance with the guidelines of the United States Department of Agriculture, National Institutes of Health Office of Laboratory Animal Welfare, and National Research Council's “Guide for Care and Use of Laboratory Animals.” Male Sprague-Dawley rats (250–300 g) were purchased from Harlan Industries, Houston, TX. On arrival, the animals were housed individually in a temperature-controlled room (23°C) and kept on a 14:10 light/dark cycle with lights on at 6:00 am. The rats were given chow (Harlan Industries, Indianapolis, IN) and water ad libitum.
Pulpotomy and Cement Placement
After administering ketamine (90 mg/kg) and xylazine (9 mg/kg), an occlusal pulpotomy on the 6 maxillary molars was completed by a board-certified endodontist by using a ½-round carbide bur. The teeth were immediately capped with 1 of 3 cement materials: ProRoot MTA (Dentsply, Tulsa Dental Specialties, York, PA) mixed 3:1 powder:water, Quick-Set (Avalon Biomed Inc, Bradenton, FL, patent pending) (17), or MTA Plus (Avalon Biomed Inc) mixed at 2.5:1 powder:gel by weight with their respective gels. The mixed cements were placed in the cavity created by the iatrogenic pulpotomy. A self-adhering flowable composite resin (VertiseFlow; Kerr Corporation, Orange, CA) was placed immediately over the cements, and the resin was ultraviolet cured (18). The negative control group had no pulpotomy, and an untreated pulpotomy was used as a positive control. Previous studies had groups with exposed untreated pulp for 28 days or longer (19, 20). Treatment groups in this study included the control, the exposed pulps, the exposed pulps capped with ProRoot MTA, the exposed pulps capped with MTA Plus, and the exposed pulps capped with Quick-Set. Twelve animals were in each of these treatment groups. Six animals in each treatment group were killed at 30 days, and 6 animals were killed at 60 days after capping. Death was completed by exposure to CO2. After breathing was observed to stop, the animals were decapitated. The maxillae were isolated and fractured along the midline; half of the maxilla was placed in 4% paraformaldehyde and 1 × phosphate-buffered saline for histology, and the other half was placed in liquid nitrogen for storage and later analysis. The maxilla that was either fixed or frozen was randomly chosen for each animal in each treatment group.
Enzyme-linked Immunosorbent Assay
IL-1α was quantified because this cytokine was elevated after pulpotomy, whereas the common proinflammatory cytokine IL-1β was not found to be elevated in some instances (21).
Three to four out of a total of 6 animals from each treatment group were chosen randomly for measurement of the cytokine levels (pg/mL) by enzyme-linked immunosorbent assay (ELISA). To quantitate the cytokines in the pulp tissue, a maxilla from the rat was removed from the liquid nitrogen, and the molars (3 molars per maxilla) were extracted. The teeth were ground and placed in 300 μL T-Per tissue protein extraction reagent containing Halt Protease Inhibitor (Thermo Scientific, Rockford, IL). The lysates were frozen, thawed, vortexed, and centrifuged for 10 minutes at 4°C, and the supernatant was decanted. Total protein in the supernatant was determined in each sample by using a BCA protein assay (Thermo Scientific, Waltham, MA). Quantitation was completed on duplicate 50-μL samples of supernatant by using ELISA (R&D Systems, Minneapolis, MN) following the manufacturer's directions. Values were given as pg IL-1α or IL-1β per μg total protein.
Histology
Histology was performed by using a randomly chosen maxilla from 3 rats per treatment group. First, the maxilla was immersed in 4% paraformaldehyde and 1 × phosphate-buffered saline continuously for 1 week, and then the samples were demineralized in 0.5 mol/L EDTA until radiographic examination revealed an absence of radiopaque structures. The demineralized samples were dehydrated and embedded in paraffin blocks. Serial sections, 6 μm thick, were sliced with a Leitz 1512 rotary microtome (Leica, Buffalo Grove, IL) in a buccolingual longitudinal orientation. Every 20th section was collected and stained with hematoxylin-eosin or a Brown and Hopps stain. Briefly, paraffin was removed with xylene, and the slides were hydrated in a series of ethanol/water treatments. A portion of the slides were immersed in hematoxylin, rinsed, immersed in eosin, rinsed, and dehydrated, and a non-aqueous mounting medium was added. Alternate slides were immersed in crystal violet, rinsed, immersed in Gram iodine, rinsed, and finally immersed in fuchsin and then rinsed and mounted. For the Brown and Hopps stain, gram-positive bacteria were blue, and the gram-negative bacteria were red. Two examiners blinded to tooth position and treatment scored the teeth for immune cell infiltrate, presence of bacteria, or dentinal bridging. Imaging was completed with a Zeiss Axioplan microscope (Thornwood, NY) and an Insight 2 Spot camera (SPOT Imaging Solutions, Sterling Heights, MI). Images were captured and analyzed with Spot Advanced software (SPOT Imaging Solutions).
Bacteria and Pulp Vitality Measurements
Every 20th section was collected for the maxilla of 3 rats in each treatment group, and the sections were stained with either Brown and Hopps stain or hematoxylin-eosin stain. After the Brown and Hopps staining, the bacteria on each slide were counted, and all the bacteria for all the sections of a single maxilla were added together. This total bacteria count per maxilla was reported for each of the 3 rats per treatment group. After the hematoxylin-eosin staining, the percentage of pulp vitality remaining after treatment for each rat was calculated by dividing the area of the vital pulp in the maxillary molars by the average area of vital pulp in the 3 control rats, and then the result was multiplied by 100.
Meal Duration
Meal duration was measured before and after pulpotomy by placing rats in individual, sound-attenuated chambers equipped with computer-activated pellet feeders (Med Assoc Inc, East Fairfield, VT). The feeder units dispensed 45 mg rodent chow pellets (product no. FO 165; Bioserv, Frenchtown, NJ). When a rat removed a pellet from the feeder trough, a photo beam placed at the bottom of the trough was no longer blocked, signaling the computer-controlled system to drop another pellet. The computer recorded the date and time each pellet was dropped, and the computer kept a running tally of the total daily food consumption. A record of the pellets dropped over time established the meal duration. Meal duration was monitored in this study 2 days before and for 9 days after the pulpotomy. Nine days after each pulpotomy there was no significant difference compared with the control group. Meal duration was measured in the 12 animals in each treatment group.
Statistics
Meal duration and cytokine data were analyzed by two-way analysis of variance, with treatment and time as independent variables and meal duration and cytokine concentration as dependent variables. Bonferroni post hoc tests were completed on the groups that showed significant main effects. Values were the means ± standard error of the mean.
Results
Proinflammatory cytokines were quantified from 3 extracted teeth per rat. Thirty days after capping and composite overlay, IL-1β and IL-1α levels were significantly higher in the rats with exposed pulps versus rats that had the pulps capped or the control group (Fig. 1A: F4,28 = 6.7, P < .001, n = 4 and Fig. 1B: F4,24 = 10.95, P < .01, n = 3). Sixty days after placing the cement, all the groups had significantly higher amounts of proinflammatory cytokines versus the controls (Fig. 1). The control group was not significantly different when comparing the 30-day and 60-day groups for either IL-1β or IL-1α. Bacteria and immune cells that can stimulate and produce these cytokines were present in the exposed pulps (Fig. 2A–C). Few bacteria were observed in the capped teeth (Fig. 2G); however, clusters of immune cells were present in the margins, and this was noted at the interface between the hydraulic cement and the dentin of less than 20% of the teeth (Fig. 2D–F, open arrows).
Figure 1.
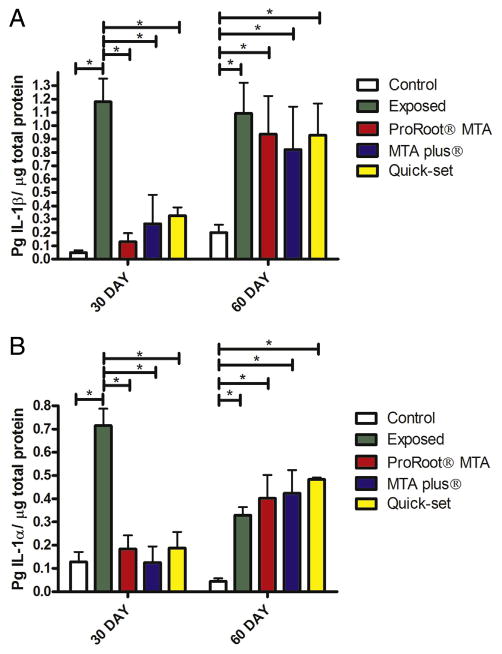
Cytokine levels were analyzed in extracted teeth after pulpotomy and capping with hydraulic cements. A pulpotomy was performed, and the defect was filled with endodontic cement (ie, ProRoot MTA, MTA Plus, or Quick-Set). Control group was anesthetized but did not have the pulp exposed, and exposed group had the pulp exposed but no material was placed. Data for proinflammatory cytokine IL-1β are shown in (A), and data for IL-1α are shown in (B). *P < .05 comparing either exposed or control with other treatment groups within that treatment time period.
Figure 2.
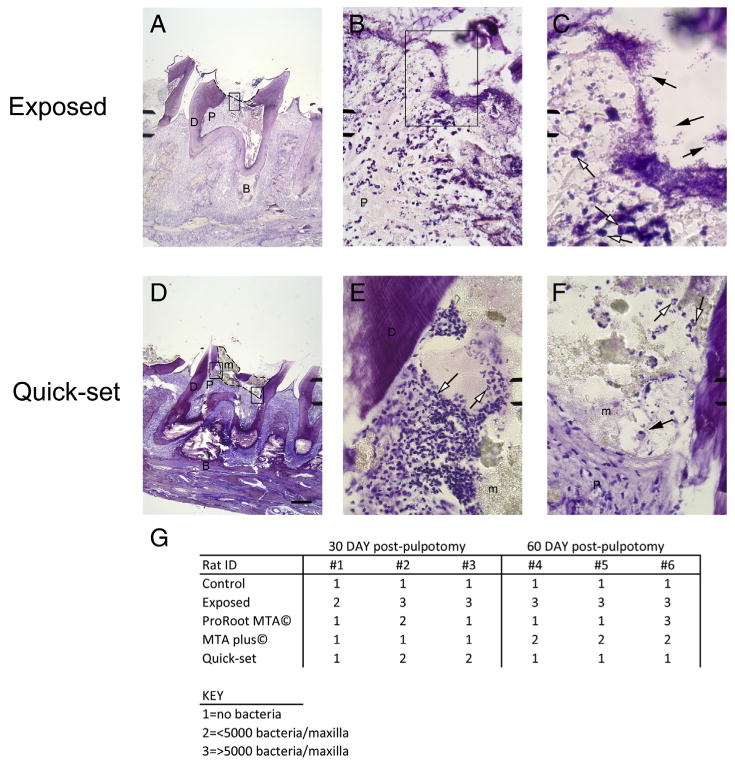
Staining and measurement of bacteria in tooth pulp. Brown and Hopps stain of maxillary sagittal sections 30 days after capping are shown in (A–F). (A–C) Sections are from exposed tooth, and (D–F) sections are from Quick-Set capped tooth. Open arrows point to representative immune cells in the population, and arrows point to representative bacteria present in the section. B, bone; D, dentin; m, hydraulic cement material; P, pulp. A defect is shown by dotted line in (A), and material border is shown by dotted line in (D). Scale bar = 400 μm for (A and D), 25 μm for (B, E, and F), and 10 μm for (C). (G) Bacteria levels in pulp for 3 rats in each treatment group at both 30 and 60 days.
After 60 days, 1.6 ± 0.26 of the 6 teeth that were capped with the hydraulic cement ProRoot MTA, on average, lost this material (5 animals were analyzed in each treatment group, each with 6 molars for analysis). On average, 2.6 ± 0.71 of the 6 teeth that were capped with MTA Plus lost this material (n = 5), and 2.0 ± 0.44 of the 6 teeth capped with Quick-Set lost the material (n = 5). Loss of material was likely because of physical forces applied during mastication. Histology of the teeth indicated that pulp cells were viable when in contact with the hydraulic cements at both 30 and 60 days (Fig. 3, arrows; Table 1). The finer particle size of the MTA Plus and Quick-Set groups compared with the ProRoot MTA cement group can be observed (compare regions labeled “m” in Fig. 3). Exposed teeth exhibited large areas of dead pulp at 30 days, and often these teeth exhibited the absence of viable pulp tissue after 60 days (Fig. 3, second row of panels; Table 1). Dentinal bridging appeared as early as 30 days after placing the hydraulic cements (Fig. 3, open arrows), and this new dentin was present after capping with each of the 3 cements at 30 and 60 days (Fig. 3).
Figure 3.
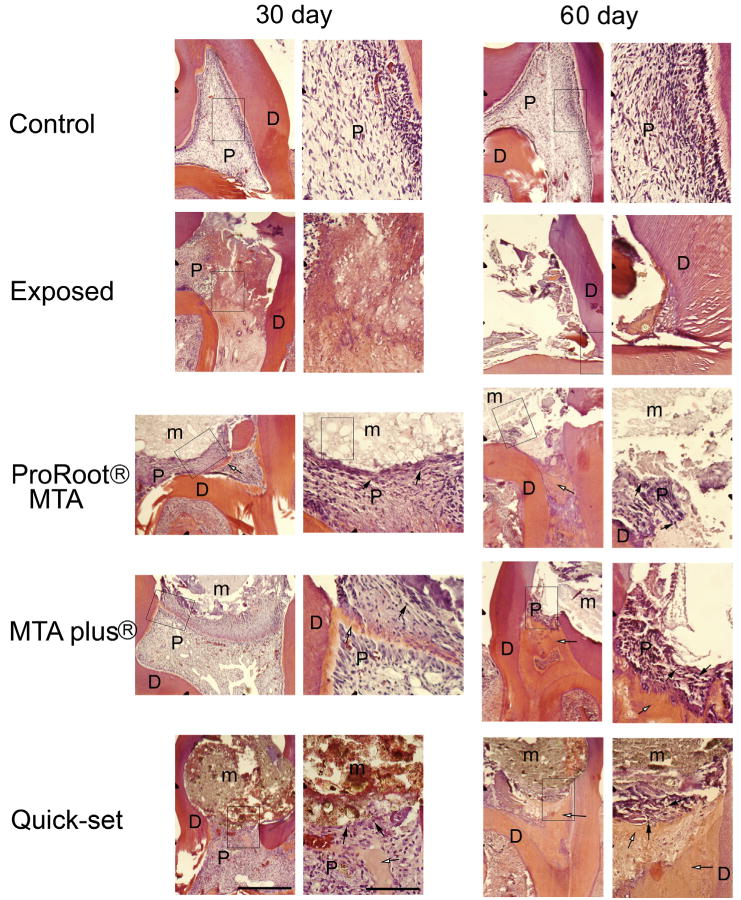
Stained histologic sections of molars from maxilla of rats capped with different hydraulic cements. Sagittal maxillary sections were stained with hematoxylin-eosin. Figure 1 shows treatment groups. Boxed region in left panel is enlarged in adjacent right-hand panel. D, dentin; m, hydraulic cement material; P, pulp. Open arrows point to representative dentinal bridge, and arrows point to representative vital cells in the pulp tissue. Scale bar = 100 μm for lower magnification image and 200 μm for enlarged images.
Table 1. Vital Cells after Pulp Treatment.
| Rat ID | 30 days after pulpotomy | 60 days after pulpotomy | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| #1 | #2 | #3 | #4 | #5 | #6 | |
| Control | High | High | High | High | High | High |
| Exposed | Medium | Low | Medium | Medium | Low | Low |
| ProRoot MTA | Medium | Medium | Medium | Medium | Medium | Low |
| MTA Plus | Medium | Medium | Medium | Medium | Medium | Low |
| Quick-Set | Medium | Medium | Medium | Low | Medium | Low |
High, >95% of pulp vital; low, <10% pulp vital; medium, >10% but <95% pulp vital.
Meal duration (ie, the nociceptive response) was significantly reduced, F4,66 = 10.8, P < .001 (n = 12), after capping with hydraulic cement versus animals with exposed pulp (Fig. 4). Comparison between the 3 hydraulic cements showed no significant difference in meal duration (Fig. 4); however, the nociceptive response significantly increased for 3 days (P < .05) in animals that received a cement pulp cap compared with untreated control animals (Fig. 4).
Figure 4.
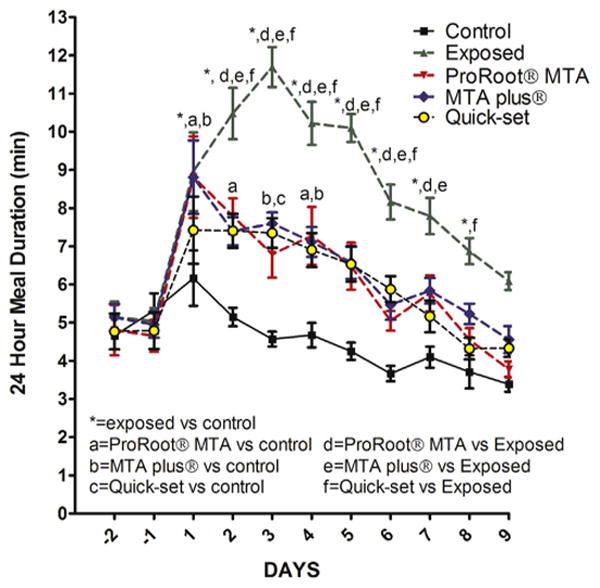
Twenty-four meal duration of rats treated with 3 different hydraulic cements. Meal duration was measured 2 days before pulpotomy, and these pretreatment days were numbered −2 and −1. Days after pulpotomy were numbered 1–9. Figure 1 shows treatment groups. Significant differences of P < .05 are indicated on the graph with * and by letters.
Discussion
Despite the differences in composition, the 3 hydraulic cements had equivalent inflammatory (IL-1β, IL-1α), cellular (dentinal bridging and cellular viability), and nociceptive responses (meal duration). This suggests an equivalent efficacy when the cements were used for capping a pulpotomy procedure in the clinic. In a previous study, pain was reported to be similar for MTA and zinc oxide–eugenol cement when placing a retrofill (22), and similarly in our study, we found that different materials do not affect the pain response. Pain has also been measured in humans when placing MTA cement in a pulpotomy procedure, but to date, no study has measured pain associated with the multiple cements used in endodontic procedures (10).
A very relevant danger for dental pulp tissue after direct pulp capping is a bacterial infection, and bacterial infections of vital pulp tissue can cause inflammation and pulp necrosis (23–25). In rats, the oral bacterial flora is more comparable to that in humans than other commonly used research species (26–29). An improper seal resulting in greater microorganism infiltration at the margins of the material and dentin interface would be indicated by the presence of immune cells. Quick-Set is a calcium aluminate cement with a lower pH (10.9 ± 0.5) than MTA (11.6 ± 0.5), and it demonstrates greater infiltration of the dentinal tubules by the cement (17). Tubule penetration by MTA blocks bacterial access to the nutrients of the pulp, and this blockage can inhibit bacterial growth, reducing inflammation and possibly infection (24, 25, 30, 31). Thus, we expected Quick-Set would have improved bacteriostatic properties compared with MTAs. Thirty days after capping, Quick-Set did not show improved infection control when compared with the other cements. It is more difficult to compare the materials (ie, infection/vitality) after 60 days because on average, 2 of the 6 capped teeth lost the cement, leading to bacterial access and infection of the pulp. Bacterial infection caused by a poor cement/dentin interface and loss of cement likely resulted in the high inflammatory cytokine levels 60 days after capping. Minimizing this infection would enhance dentinal bridging and improve pulp-capping success (23). A future experiment would be to place a permanent restorative material such as amalgam after pulp capping. Permanent restorative materials have been shown to improve the seal after pulp capping and reduce infection and inflammation (32).
When vital pulp tissue is exposed in the rat, an inflammatory reaction results that is very similar to the reaction in humans; however, the biological response is faster in rats than in humans (33). Determining the inflammatory and healing properties after pulp capping a mechanical lesion in a rat model would be expected to provide results representative of human patients. The hydraulic cements all decreased inflammation 30 days after pulp capping a mechanical lesion. This decrease in inflammation was associated with a decrease in orofacial pain, which is consistent with previous studies (11,13–16). Future studies could determine whether a reduction in postoperative pain, an indicator of reduced pulpal damage, can be correlated to a reduction in the 30% failure rate resulting from pulp capping of mechanical lesions (32). Note that no differences were measured in the nociceptive response between the 3 cements in this study; therefore, we could not correlate the quality of pain to differences in healing.
Reparative dentin found in the tested rats after pulp capping was shown to be identical to the reparative dentin found in human teeth (34). In this study we observed signs of dentinal regeneration for all 3 materials at 30 and 60 days. These results are consistent with previous work that showed that reparative hard tissue formation can be detected in 83% of rat molar teeth 28 days after pulp capping (35). Consistent with our results, dentinal bridging has been shown to occur with tricalcium silicates (36), and bioactivity has been shown for calcium aluminates (37) such as the calcium aluminosilicate Quick-Set (18).
Another factor measured in this study was the nociceptive response. The response was significantly higher in rats that had cement placed over the exposed pulps compared with the control teeth. One explanation for the higher nociceptive response would be compression and pressure on the pulp that resulted when the cement was placed. Such compression (eg, vasoconstriction) might lead to inflammation, although studies suggest this pressure should be reduced by fluid leaving the pulp chamber through the vasculature (38, 39).
In conclusion, hydraulic cements improve the inflammatory and nociceptive responses after pulp caps, and the newly developed cement, calcium aluminosilicate or Quick-Set, has equivalent properties to older MTAs that are used for endodontic pulp-capping procedures.
Acknowledgments
The authors thank Connie Tillberg, Mallika Prakesh, Jose Aldana, Alicia Cox, and Brent Herrington for their technical support.
These studies were supported by NIDCR grant DE020204.
Footnotes
The authors report the following potential conflict of interest: Dr Primus owns the intellectual property for the dental materials MTA Plus and Quick-Set materials and has a financial interest in Avalon Biomed Inc.
References
- 1.Konradsson K, van Dijken JW. Interleukin-1 levels in gingival crevicular fluid adjacent to restorations of calcium aluminate cement and resin composite. J Clin Periodontol. 2005;32:462–6. doi: 10.1111/j.1600-051X.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- 2.Franz A, Konradsson K, Konig F, et al. Cytotoxicity of a calcium aluminate cement in comparison with other dental cements and resin-based materials. Acta Odontol Scand. 2006;64:1–8. doi: 10.1080/00016350500279568. [DOI] [PubMed] [Google Scholar]
- 3.Konradsson K, Claesson R, van Dijken JW. Mutans streptococci and lactobacilli in plaque on a leucite-reinforced dental ceramic and on a calcium aluminate cement. Clin Oral Investig. 2006;10:175–80. doi: 10.1007/s00784-006-0045-4. [DOI] [PubMed] [Google Scholar]
- 4.van Dijken JW, Sunnegardh-Gronberg K. A two-year clinical evaluation of a new calcium aluminate cement in Class II cavities. Acta Odontol Scand. 2003;61:235–40. doi: 10.1080/00016350310004575. [DOI] [PubMed] [Google Scholar]
- 5.Berglund A, Hulterstrom AK, Gruffman E, van Dijken JW. Dimensional change of a calcium aluminate cement for posterior restorations in aqueous and dry media. Dent Mater. 2006;22:470–6. doi: 10.1016/j.dental.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 6.van Dijken JW, Sunnegardh-Gronberg K. A calcium aluminate cement as restorative material in Class V cavities. Swed Dent J. 2004;28:111–8. [PubMed] [Google Scholar]
- 7.Aminozarbian MG, Barati M, Salehi I, Mousavi SB. Biocompatibility of mineral trioxide aggregate and three new endodontic cements: an animal study. Dent Res J. 2012;9:54–9. doi: 10.4103/1735-3327.92944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobovitz M, Vianna ME, Pandolfelli VC, et al. Root canal filling with cements based on mineral aggregates: an in vitro analysis of bacterial microleakage. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:140–4. doi: 10.1016/j.tripleo.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Aguilar FG, Roberti Garcia LF, Panzeri Pires-de-Souza FC. Biocompatibility of new calcium aluminate cement (EndoBinder) J Endod. 2012;38:367–71. doi: 10.1016/j.joen.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Asgary S, Eghbal MJ. A clinical trial of pulpotomy vs root canal therapy of mature molars. J Dent Res. 2010;89:1080–5. doi: 10.1177/0022034510374057. [DOI] [PubMed] [Google Scholar]
- 11.Kerins CA, Carlson DS, Hinton RJ, et al. Specificity of meal pattern analysis as an animal model of determining temporomandibular joint inflammation/pain. Int J Oral Maxiollofac Surg. 2005;34:425–31. doi: 10.1016/j.ijom.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Bellinger LL, He L, Kramer PR. Meal Duration: A Measure of Tooth and Temporomadibular Joint Nociception. Washington, DC: American Association of Dental Research; 2010. CD-ROM 1267.1265/AA1231. [Google Scholar]
- 13.Kramer PR, Kerins CA, Schneiderman E, Bellinger LL. Measuring persistent temporomandibular joint nociception in rats and two mice strains. Physiol Behav. 2010;99:669–78. doi: 10.1016/j.physbeh.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellinger LL, Spears R, King CM, et al. Capsaicin sensitive neurons role in the inflamed TMJ acute nociceptive response of female and male rats. Physiol Behav. 2007;90:782–9. doi: 10.1016/j.physbeh.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Guan G, Kerins CC, Bellinger LL, Kramer PR. Estrogenic effect on swelling and monocytic receptor expression in an arthritic temporomandibular joint model. J Steroid Biochem Mol Biol. 2005;97:241–50. doi: 10.1016/j.jsbmb.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Kramer PR, Bellinger LL. The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception. Endocrinology. 2009;150:3680–9. doi: 10.1210/en.2008-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bird DC, Komabayashi T, Guo L, et al. In vitro evaluation of dentinal tubule penetration and biomineralization ability of a new root-end filling material. J Endod. 2012;38:1093–6. doi: 10.1016/j.joen.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsujimoto M, Tsujimoto Y, Ookubo A, et al. Timing for composite resin placement on mineral trioxide aggregate. J Endod. 2013;39:1167–70. doi: 10.1016/j.joen.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germfree and conventional laboratory rats. J South Calif Dent Assoc. 1966;34:449–51. [PubMed] [Google Scholar]
- 20.Khayat BG, Byers MR, Taylor PE, et al. Responses of nerve fibers to pulpal inflammation and periapical lesions in rat molars demonstrated by calcitonin gene-related peptide immunocytochemistry. J Endod. 1988;14:577–87. doi: 10.1016/S0099-2399(88)80054-2. [DOI] [PubMed] [Google Scholar]
- 21.Stashenko P, Wang CY, Tani-Ishii N, Yu SM. Pathogenesis of induced rat periapical lesions. Oral Surg Oral Med Oral Pathol. 1994;78:494–502. doi: 10.1016/0030-4220(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 22.Chong BS, Pitt Ford TR. Postoperative pain after root-end resection and filling. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:762–6. doi: 10.1016/j.tripleo.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Kakehashi S, Stanely HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–9. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 24.Love RM, Jenkinson HF. Invasion of dentinal tubules by oral bacteria. Crit Rev Oral Biol Med. 2002;13:171–83. doi: 10.1177/154411130201300207. [DOI] [PubMed] [Google Scholar]
- 25.Bergenholtz G. Effect of bacterial products on inflammatory reactions in the dental pulp. Scand J Dent Res. 1977;85:122–9. [PubMed] [Google Scholar]
- 26.Watts A, Paterson RC, Cohen BD, Combe EC. Pulp response to a novel adhesive calcium hydroxide based cement. Eur J Prosthodont Restor Dent. 1994;3:27–32. [PubMed] [Google Scholar]
- 27.Huxley HG. The histology of rat molar tooth fissure plaque. Arch Oral Biol. 1971;16:1311–28. doi: 10.1016/0003-9969(71)90034-3. [DOI] [PubMed] [Google Scholar]
- 28.Huxley HG. The recovery of microorganisms from the fissures of rat molar teeth. Arch Oral Biol. 1972;17:1481–5. doi: 10.1016/0003-9969(72)90108-2. [DOI] [PubMed] [Google Scholar]
- 29.Wunder JA, Briner WW, Calkins GP. Identification of the cultivable bacteria in dental plaque from the beagle dog. J Dent Res. 1976;55:1097–102. doi: 10.1177/00220345760550061601. [DOI] [PubMed] [Google Scholar]
- 30.Kina JR, Kina J, Kina EF, et al. Presence of bacteria in dentinal tubules. J Appl Oral Sci. 2008;16:205–8. doi: 10.1590/S1678-77572008000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assouline LS, Fuss Z, Mazor Y, Weiss EI. Bacterial penetration and proliferation in root canal dentinal tubules after applying dentin adhesives in vitro. J Endod. 2001;27:398–400. doi: 10.1097/00004770-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Al-Hiyasat AS, Barrieshi-Nusair KM, Al-Omari MA. The radiographic outcomes of direct pulp-capping procedures performed by dental students: a retrospective study. J Am Dent Assoc. 2006;137:1699–705. doi: 10.14219/jada.archive.2006.0116. [DOI] [PubMed] [Google Scholar]
- 33.Muruzabal M, Erausquin J. Discussion of: methods and criteria in evaluation of periapical response. Int Dent J. 1970;20:539–54. [PubMed] [Google Scholar]
- 34.D'Souza RN, Bachman T, Baumgardner KR, et al. Characterization of cellular responses involved in reparative dentinogenesis in rat molars. J Dent Res. 1995;74:702–9. doi: 10.1177/00220345950740021301. [DOI] [PubMed] [Google Scholar]
- 35.Watts A, Paterson RC. A comparison of pulp responses to two different materials in the dog and the rat. Oral Surg Oral Med Oral Pathol. 1981;52:648–52. doi: 10.1016/0030-4220(81)90085-2. [DOI] [PubMed] [Google Scholar]
- 36.Maroto M, Barberia E, Planells P, Garcia Godoy F. Dentin bridge formation after mineral trioxide aggregate (MTA) pulpotomies in primary teeth. Am J Dentistry. 2005;18:151–4. [PubMed] [Google Scholar]
- 37.Oliveira IR, Andrade TL, Jacobovitz M, Pandolfelli VC. Bioactivity of calcium aluminate endodontic cement. J Endod. 2013;39:774–8. doi: 10.1016/j.joen.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Heyeraas KJ, Berggreen E. Interstitial fluid pressure in normal and inflamed pulp. Crit Rev Oral Biol Med. 1999;10:328–36. doi: 10.1177/10454411990100030501. [DOI] [PubMed] [Google Scholar]
- 39.Donaldson LF. Understanding pulpitis. J Physiol. 2006;573(Pt 1):2–3. doi: 10.1113/jphysiol.2006.110049. [DOI] [PMC free article] [PubMed] [Google Scholar]


