ABSTRACT
Bacterial surface proteins play key roles in virulence and often contribute to bacterial adhesion and invasion. We discovered that the Streptococcus suis type 2 (SS2) gene SSU0587 encodes a protein of 1,491 amino acids that possesses β-galactosidase activity. The surface association of the protein was dependent upon sortase activity. Deleting SSU0587 from clinical SS2 isolate JX081101 caused a loss of both β-galactosidase activity and adherence to microvascular endothelial cells. Deleting SSU0587 had no measurable impact on either invasion of microvascular endothelial cells or on virulence in a murine infection model, although the concentration of JX081101ΔSSU0587 was reduced in the brains of infected mice, as compared with the pathogen loads of the wild-type strain.
Keywords: beta-galactosidase, sortase, Streptococcus suis
Streptococcus suis is an important and prevalent swine pathogen and is also considered an important zoonotic agent [9]. There are 33 recognized serotypes, of which S. suis serotype 2 (SS2) is the most prevalent and virulent in pigs and humans in European and Asian countries. Two large outbreaks of SS2 in China were associated with clinical manifestations of toxic shock syndrome and high lethality. Research during the last ten years has identified many virulence-associated factors, such as suilysin [1], SrtA [11], PgdA [2], SodA [8], CcpA [7], the two-component signal transduction system SalK-SalR [4] and the Rgg transcriptional regulator [14]. However, little is known about the underlying mechanisms of SS2 pathogenicity.
Relatively little information is available on the mechanisms by which SS2 colonization of host cells is established and maintained. Most eukaryotic cell surfaces and secreted molecules are glycosylated. Bacterial glycosyl hydrolases have been extensively researched for their role in both glycoprotein degradation and virulence. Bacteria obtain nutrients from the metabolism of host glycosides by glycosyl hydrolases, and an ability to degrade host glycoproteins is important to bacterial virulence [10]. Some surface exoglycosidases, such as BgaA [5, 10, 13], NanA and StrH [3], in Streptococcus pneumoniae have been documented to be involved in virulence. β-Galactosidase in Streptococcus pneumoniae was believed to regulate both lactose metabolism and virulence [5, 10, 13]. In this study, we describe the characterization of a similar gene (SSU0587) in SS2 and demonstrate that the gene encodes a β-Galactosidase that is involved in SS2 adherence.
The bacterial strains and plasmids used are listed in Table 1. SS2 strain JX081101 (ST7) was isolated from the lung of a diseased pig [9]. Unless otherwise indicated, SS2 strains were grown in Brain Heart Infusion (BHI) at 37°C. E. coli strains were grown in Luria-Bertani broth or agar at 37°C. Antibiotics (Sigma, St. Louis. MO. U.S.A.) were added, when necessary, to the culture media at the following concentrations: spectinomycin (Spc) at 100 µg/ml and chloramphenicol (Cm) at 4 µg/ml for SS2 and Spc at 50 µg/ml Cm at 8 µg/ml, and ampicillin at 100 µg/ml for E. coli.
Table 1. Strains and plasmids used in this study.
| Strains/plasmids | Characteristics/function | Source/reference |
|---|---|---|
| Bacterial strains | ||
| SS2 JX081101 | Virulent SS2 strain isolated from a dead pig, (ST7) | This study [9] |
| E. coli DH5α | Cloning strain | Promega |
| ΔSSU0587 | SSU0587 deletion in JX081101, CmR | This study |
| ΔsrtA | srtA deletion in JX081101, CmR | This study |
| Plasmids | ||
| pMD-18T | Cloning vector; AmpR | Takara |
| pSET4s | Thermosensitive vector SpcR | [7] |
| pSET6s | Thermosensitive vector CmR | [7] |
| pSET4s:ΔSSU0587 | pSET4s for SSU0587 allelic replacement | This study |
| pSET4s:ΔsrtA | pSET4s for srtA allelic replacement | This study |
To construct SSU0587 and srtA deletion mutants in SS2 strain JX081101, the 5′ and 3′ flanking regions of SSU0587 and srtA ORF were PCR-amplified with the primer pairs SSU0587-1F/SSU0587-1R and SSU0587-2F/SSU0587-2R (for SSU0587) and TsrtA-1F/TsrtA-1R and TsrtA-2F/TsrtA-2R (for srtA OFR; Table 2), respectively. The PCR products of SSU0587 were digested with EcoRI and EcoT22I or EcoT22I and PstI, fused at the EcoT22I site and cloned into the EcoRI and PstI sites of pSET4s. The PCR products of srtA were digested with EcoRI and EcoT22I or EcoT22I and HindIII, fused at the EcoT22I site and cloned into the EcoRI and HindIII sites of pSET4s. The unique EcoT22I site was then used to introduce the chloramphenicol acetyltransferase gene (cat) amplified from pSET6s using the primers 6s-F and 6s-R (Table 2). The resulting insert was subcloned into pSET4s, generating the vectors pSET4s-ΔSSU0587 and pSET4s-ΔsrtA.
Table 2. PCR primers. Restriction sites used for cloning are underlined.
| Primers | Sequence (5′-3′) | Description |
|---|---|---|
| SSU0587-1F | TTAGAATTCAGACCCTGCTGGCTATTA | 5′ SSU0587 flanking, EcoRI |
| SSU0587-1R | TATCCTGTATGCATTATTTCCTCCTATAAAGT | 5′ SSU0587 region, EcoT22I |
| SSU0587-2F | AGGAAATAATGCATACAGGATAGTGAGTACTT | 3′ SSU0587 region, EcoT22I |
| SSU0587-2R | CGACTGCAGTATTAAGAATTTTATGCC | 5′ SSU0587 flanking, PstI |
| TsrtA-1F | ATAGAATTCAACAAATGGCCAAGCAGACA | 5′ srtA flanking, EcoRI |
| TsrtA-1R | TAGATGTTATGCATGACTATTTATTCCTTTTCTT | 5′ srtA region, EcoT22I |
| TsrtA-2F | AAATAGTCATGCATAACATCTATATATTAAGGAT | 3′ srtA region, EcoT22I |
| TsrtA-2R | TATAAGCTTTACGTCCCAACTTCTCACTT | 5′ srtA flanking, HindIII |
| 6s-F | TAGTATGCATTAATTCGATGGGTTCCGAGG | 5′ end of cat, EcoT221 |
| 6s-R | TCACATGCATCACCGAACTAGAGCTTGATG | 3′ end of cat, EcoT221 |
| SSU0587-F | TGGGGACGGGGGCATTAT | 5′ SSU0587 segment |
| SSU0587-R | GCGCTTTAGAGTTCTTCGGAGTT | 3′ SSU0587 segment |
| srtA-F | CAAGAGATGGGGAAAGGAAA | 5′ srtA segment |
| srtA-R | TGTCCATAATCATACTGATT | 3′ srtA segment |
| spc-F | GTTCGTGAATACATGTTATA | 5′ spc segment |
| spc-R | GTTTTCTAAAATCTGAT | 3′ spc segment |
The procedures for mutant selection were carried out as described previously [7]. The mutant ΔSSU0587 strain was verified by PCR using three pairs of primers, SSU0587-F/SSU0587-R (between SSU0587), SSU0587-1F/SSU0587-2R (flanking SSU0587) and Spc-F/Spc-R (pSET4s resistance gene), and direct DNA sequencing of mutation sites using genomic DNA. Verification of the ΔsrtA mutant was performed similarly.
To prepare bacteria for enzyme analysis, overnight bacterial cultures in BHI agar were inoculated into fresh media and were incubated at 37°C for 6–8 hr. To distinguish between intracellular and surface localization of β-galactosidase, intact cells were harvested by centrifugation in the exponential phase, the supernatant was removed, and cells were washed once with sodium phosphate buffer. Cell lysates were lysed in sodium phosphate buffer containing 0.1% Triton X-100 and incubated for 5 min at 37°C, after which the supernatants were collected after centrifugation. β-Galactosidase activity was determined as described previously [13] using o-nitrophenyl-β-D-galactopyranoside (ONPG) as the substrate.
bEnd.3 endothelial cells derived from mouse brains were grown in 24-well tissue culture plates in RPMI 1640 culture medium (Invitrogen, Carslbad, CA. U.S.A.) supplemented with NaHCO3, 10% fetal calf serum (FCS) and L-glutamine. SS2 strains were cultured in BHI for 6 hr at 37°C, harvested by centrifugation, washed twice in PBS and resuspended in fresh RPMI. Confluent monolayers of bEnd.3 cells at 105 cells/well were infected with SS2 at a multiplicity of infection (MOI) of 1:100. For adhesion assays, the plates were centrifuged at 800×g for 10 min and incubated for 1 hr at 37°C and 5% CO2. Monolayers were then washed 4 times with PBS, trypsinized and disrupted by gentle pipetting. Viable bacterial cells were determined by plating suitable dilutions of the lysates onto BHI agar. For invasion assays, the infected monolayers were incubated for 1 hr at 37°C and 5% CO2 and then subjected to antibiotic killing of extracellular bacteria (100 µg/ml gentamicin and 5 µg/ml of penicillin G). Viable intracellular bacteria were counted as described above.
The Zhejiang University Committee for Laboratory Animal Management approved all animal experiments. BALB/c mice (3 weeks old) were divided into 7 groups of 10 mice/group. Groups I and II were inoculated intraperitoneally with 0.3 ml suspensions of the wild-type (WT) strain at two different concentrations (low dose=~3.2 × 108 CFU/mouse; high dose=~6.4 × 108 CFU/mouse), while groups III and IV and groups V and VI received similar doses of ΔSSU0587 and ΔsrtA mutants, respectively. Mice in the control group, group VII were injected with 0.3 ml PBS. Mortality data were recorded twice a day during the 5-day period post-inoculation (p.i.). Surviving animals were euthanized on day 6. In a separate experiment, the bacterial burdens in the brain, kidney, liver and spleen of mice (6 mice/strain) that survived 30 hr after challenge with ~3.7 × 108 of the ΔSSU0587 and ΔsrtA mutants were quantified. The tissues were removed and homogenized in PBS containing 20% glycerol. The homogenates were appropriately diluted and spread on BHI agar plates for enumeration.
Unless otherwise specified, all data are expressed as means ± SD and analyzed using two-tailed Student’s t-tests. Mice survival data were analyzed using log-rank tests. P values <0.05 were considered significant.
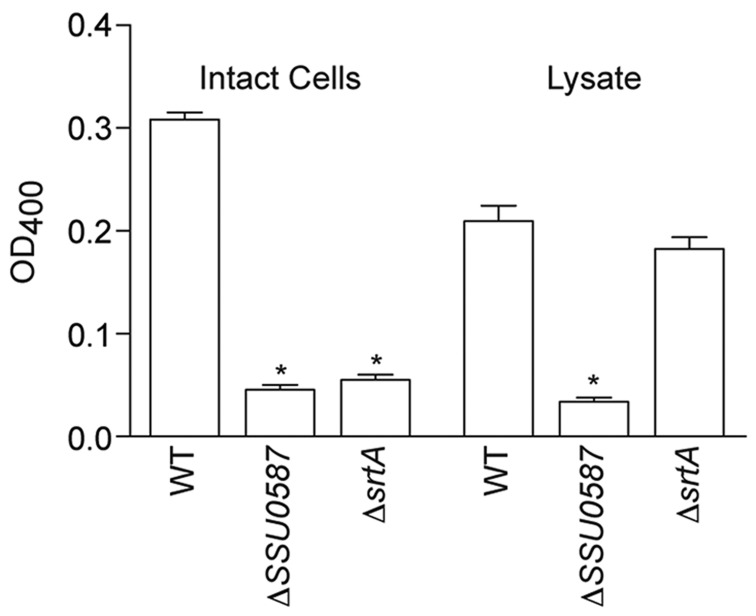
SSU0587 encodes a putative β-galactosidase of 1,419 amino acids. The protein is predicted, based on BLASTP searches, to contain an N-terminal signal peptide (AAs 1–33), two conserved glycosyl hydrolase motifs and a C-terminal cell wall-anchoring LPXTG motif. To analyze whether SSU0587 encodes a functional β-galactosidase, the cat gene from pSET6s was inserted into SSU0587, resulting in disruption of the open reading frame. SS2 β-galactosidase activity was significantly reduced in both intact cell preparations, as well as in bacterial cell lysates (Fig. 1) of ΔSSU0587, as compared with the activity in the WT strain. SSU0587 appeared to be associated with the cell surface in a sortase-dependent manner, as deleting srtA significantly reduced β-galactosidase activity in intact cell preparations but not in bacterial cell lysates (Fig. 1).
Fig. 1.
β-galactosidase activity in intact cells and cell lysates. Data are expressed as the mean + SD of three independent experiments, each in duplicate wells. Statistical differences compared with the WT strain are indicated with an asterisk (t-test).
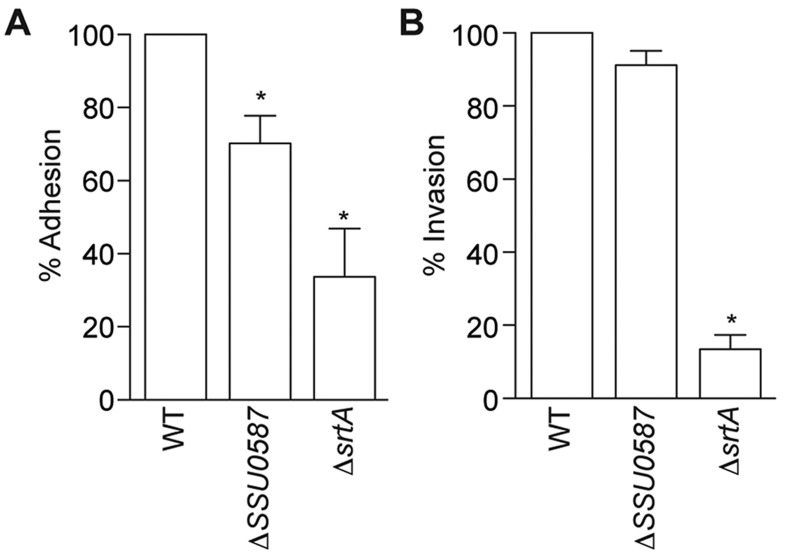
The ΔSSU0587 mutant had significantly lower levels of adherence (70.2%) to bEnd.3 endothelial cells as compared with the WT strain (Fig. 2A). By contrast, deleting SSU0587 had no impact on SS2 invasion (Fig. 2B). As expected, based on previous adhesion studies [11, 12], deleting srtA significantly reduced both SS2 adhesion and invasion into bEnd.3 endothelial cells (Fig. 2A and 2B).
Fig. 2.
Adherence and invasion to bEnd.3 endothelial cell monolayers. Adhesion to (A) and invasion into (B) bEnd.3 endothelial cells by WT, SSU0587 and srtA mutants. Data for the WT strain were normalized to 100%. Data are expressed as the mean + SD of three independent experiments, each in duplicate wells. Statistical differences compared with the WT strain are indicated with an asterisk (t-test).
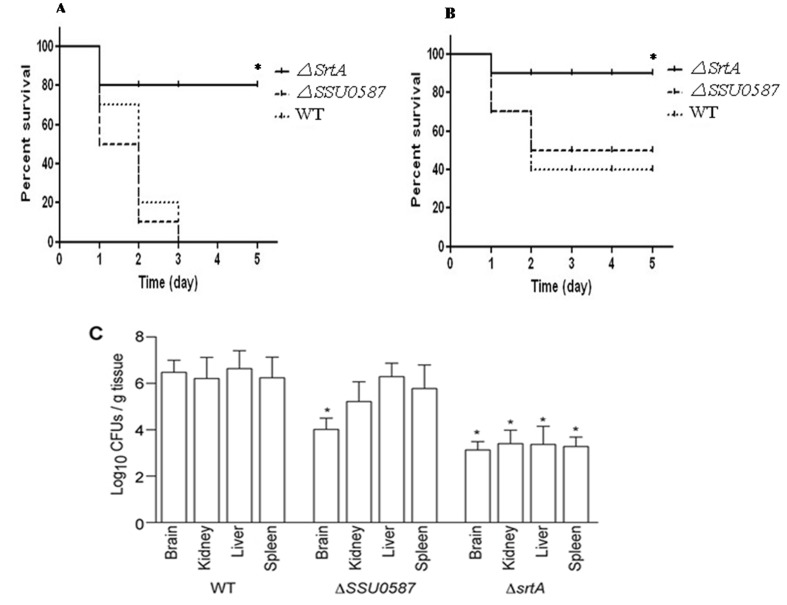
Some surface exoglycosidases contribute to Gram-positive bacterial virulence in animal models [5]. To assess whether SSU0587 contributes to SS2 virulence, we inoculated mice with low- and high-doses of SS2 (WT and mutants) and quantified mouse death and bacterial loads. At high doses (~6.4 × 108 CFU), almost all mice inoculated with either the WT strain or ΔSSU0587 died within 3 days of challenge (Fig. 3A). At low doses, approximately 50% of mice challenged with either the WT strain or ΔSSU0587 survived the trial (Fig. 3B). In neither case, did mouse mortality differ significantly between the WT strain and ΔSSU0587 challenge groups. Pathogen loads were reduced in the brains (but not in the kidney, liver or spleen) of mice infected with ΔSSU0587, as compared with mice infected with the WT strain (Fig. 3C). By contrast, deleting srtA from SS2 significantly reduced mouse mortality at both challenge doses (Fig. 3A and 3B) and reduced pathogen burdens in all organs examined (Fig. 3C).
Fig. 3.
Changes in SS2 virulence attributes in mice due to deletion of SSU0587 and srtA. Survival of mice intraperitoneally inoculated with ~6.4 × 108 CFU (A) and ~3.2 × 108 CFUs (B) of WT, ΔSSU0587 and ΔsrtA mutants. Statistical differences compared with the WT strain are indicated with an asterisk (log-rank test). (C) Bacterial burdens in the brain, kidney, liver and spleen of mice inoculated with 3.2 × 108 CFU of SS2 strains. Six mice in each group that survived the challenge at 30 hr post inoculation were examined. Statistical differences compared with the WT strain are indicated with an asterisk (t-test).
Many bacterial glycosidases contribute to virulence [5, 10]. Streptococcus pneumoniae encodes a β-galactosidase surface protein named BgaA [10]. BgaA contributes to adherence of pneumococcal strains to epithelial cells and promotes resistance to opsonophagocytic killing by reducing complement deposition on the Pneumococcus surface [5]. Here, we identified the gene SSU0587 in SS2, which encodes a protein predicted to have similar structural features as BgaA in that it, SSU0587, has a predicted N-terminal signal peptide, a C-terminal cell wall-anchoring LPXTG motif and 2 glycosyl hydrolase motifs. However, SSU0587 does not have strong sequence similarity and instead shares homology with β-galactosidases encoded by Streptococcus canis and Clostridium perfringens. Deleting SSU0587 significantly reduced SS2 surface β-galactosidase activity. Surface localization of SSU0587 appeared to be dependent on sortase activity.
Several other SS2 surface proteins contribute to adhesion, invasion and immunogenicity, including surface antigen one (SAO), muraminidase-released Protein (MRP) and serum opacity factor (SOF) [6]. Some exoglycosidases have been shown to regulate bacterial adhesion by cleaving sugars to reveal receptors on host cells [5]. We found that deleting SSU0587 resulted in decreased adhesion of SS2 to endothelial cells and reduced pathogen loads in the brains of infected mice. However, deleting SSU0587 had no measurable impact on SS2 invasion or on mouse survival after intraperitoneal inoculation with SS2. It is possible that we did not observe a measurable virulence defect in these studies because of the intraperitoneal route of inoculation used. It is conceivable that future employment of a meningitis model with in the study of SSU0587 might reveal a different impact of this newly characterized Streptococcal β-galactosidase on bacterial virulence and host pathology.
Acknowledgments
This work was funded by the Chinese Ministry of Agriculture (project No. 200803016). We thank Dr Daisuke Takamatsu (National Institute of Animal Health) for providing the pSET vectors.
REFERENCES
- 1.Fittipaldi N., Fuller T. E., Teel J. F., Wilson T. L., Wolfram T. J., Lowery D. E., Gottschalk M.2009. Serotype distribution and production of muramidase-released protein, extracellular factor and suilysin by field strains of Streptococcus suis isolated in the United States. Vet. Microbiol. 139: 310–317. doi: 10.1016/j.vetmic.2009.06.024 [DOI] [PubMed] [Google Scholar]
- 2.Fittipaldi N., Sekizaki T., Takamatsu D., de la Cruz Dominguez-Punaro M., Harel J., Bui N. K., Vollmer W., Gottschalk M.2008. Significant contribution of the pgdA gene to the virulence of Streptococcus suis. Mol. Microbiol. 70: 1120–1135. doi: 10.1111/j.1365-2958.2008.06463.x [DOI] [PubMed] [Google Scholar]
- 3.King S. J., Hippe K. R., Gould J. M., Bae D., Peterson S., Cline R. T., Fasching C., Janoff E. N., Weiser J. N.2004. Phase variable desialylation of host proteins that bind to Streptococcus pneumoniae in vivo and protect the airway. Mol. Microbiol. 54: 159–171. doi: 10.1111/j.1365-2958.2004.04252.x [DOI] [PubMed] [Google Scholar]
- 4.Li M., Wang C., Feng Y., Pan X., Cheng G., Wang J., Ge J., Zheng F., Cao M., Dong Y.2008. SalK/SalR, a two-component signal transduction system, is essential for full virulence of highly invasive Streptococcus suis serotype 2. PLoS ONE 3: e2080. doi: 10.1371/journal.pone.0002080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limoli D. H., Sladek J. A., Fuller L. A., Singh A. K., King S. J.2011. BgaA acts as an adhesin to mediate attachment of some pneumococcal strains to human epithelial cells. Microbiology 157: 2369–2381. doi: 10.1099/mic.0.045609-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takamatsu D., Osaki M., Tharavichitkul P., Takai S., Sekizaki T.2008. Allelic variation and prevalence of serum opacity factor among the Streptococcus suis population. J. Med. Microbiol. 57: 488–494. doi: 10.1099/jmm.0.47755-0 [DOI] [PubMed] [Google Scholar]
- 7.Tang Y., Wu W., Zhang X., Lu Z., Chen J., Fang W.2012. Catabolite control protein A of Streptococcus suis type 2 contributes to sugar metabolism and virulence. J. Microbiol. 50: 994–1002. doi: 10.1007/s12275-012-2035-3 [DOI] [PubMed] [Google Scholar]
- 8.Tang Y., Zhang X., Wu W., Lu Z., Fang W.2012. Inactivation of the sodA gene of Streptococcus suis type 2 encoding superoxide dismutase leads to reduced virulence to mice. Vet. Microbiol. 158: 360–366. doi: 10.1016/j.vetmic.2012.02.028 [DOI] [PubMed] [Google Scholar]
- 9.Tang Y., Zhao H., Wu W., Wu D., Li X., Fang W.2011. Genetic and virulence characterization of Streptococcus suis type 2 isolates from swine in the provinces of Zhejiang and Henan, China. Folia Microbiol. (Praha) 56: 541–548. doi: 10.1007/s12223-011-0077-2 [DOI] [PubMed] [Google Scholar]
- 10.Terra V. S., Homer K. A., Rao S. G., Andrew P. W., Yesilkaya H.2010. Characterization of novel beta-galactosidase activity that contributes to glycoprotein degradation and virulence in Streptococcus pneumoniae. Infect. Immun. 78: 348–357. doi: 10.1128/IAI.00721-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanier G., Sekizaki T., Dominguez-Punaro M. C., Esgleas M., Osaki M., Takamatsu D., Segura M., Gottschalk M.2008. Disruption of srtA gene in Streptococcus suis results in decreased interactions with endothelial cells and extracellular matrix proteins. Vet. Microbiol. 127: 417–424. doi: 10.1016/j.vetmic.2007.08.032 [DOI] [PubMed] [Google Scholar]
- 12.Wang C., Li M., Feng Y. J., Zheng F., Dong Y. Q., Pan X. Z., Cheng G., Dong R. P., Hu D., Feng X. D.2009. The involvement of sortase A in high virulence of STSS-causing Streptococcus suis serotype 2. Arch. Microbiol. 191: 23–33. doi: 10.1007/s00203-008-0425-z [DOI] [PubMed] [Google Scholar]
- 13.Zähner D., Hakenbeck R.2000. The Streptococcus pneumoniae beta-galactosidase is a surface protein. J. Bacteriol. 182: 5919–5921. doi: 10.1128/JB.182.20.5919-5921.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng F., Ji H., Cao M., Wang C., Feng Y., Li M., Pan X., Wang J., Qin Y., Hu F.2011. Contribution of the Rgg transcription regulator to metabolism and virulence of Streptococcus suis serotype 2. Infect. Immun. 79: 1319–1328. doi: 10.1128/IAI.00193-10 [DOI] [PMC free article] [PubMed] [Google Scholar]