Abstract
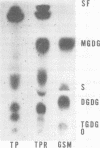
The lipid composition of Treponema pallidum (Nichols virulent strain) was determined after purification of the organisms from the infected testes of corticosteroid-treated rabbits by differential centrifugation, filtration through Nuclepore membranes, and sedimentation in Hypaque density gradients. The total lipids were comprised of 32.2% neutral lipids, mainly cholesterol, and 67.8% phospholipids consisting of phosphatidylcholine (32.1%), sphingomyelin (14.8%), cardiolipin (13.0%), phosphatidylethanolamine (6.2%), phosphatidylinositol-serine (1.2%), and lysophosphatidylcholine (0.4%). Monoglycosyldiglyceride, a glycolipid comprising 25 to 50% of thetotal lipid of all Treponema previously examined, was not detected. The fatty acid composition was similar but quntitatively distinct from that of the infected testes tissue.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. L., Johnson R. C., Peterson D. Metabolism of Common Substrates by the Reiter Strain of Treponema pallidum. Infect Immun. 1971 Jun;3(6):727–734. doi: 10.1128/iai.3.6.727-734.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Nichols J. C., Rumpp J. W., Hayes N. S. Purification of Treponema pallidum from Infected Rabbit Tissue: Resolution into Two Treponemal Populations. Infect Immun. 1974 Nov;10(5):1062–1067. doi: 10.1128/iai.10.5.1062-1067.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Coulon-Morelec M. J., Dupouey P., Marechal J. Les lipides des tréponèmes. Sur la présence d'un galactolipide dans Treponema reiteri. C R Acad Sci Hebd Seances Acad Sci D. 1969 Aug 25;269(8):854–857. [PubMed] [Google Scholar]
- Dupouey P., Betz A. Etude d'un antigène glycolipidique de Treponema reiteri et de l'anticorps correspondant contenu dans un sérum humain. Ann Inst Pasteur (Paris) 1969 Sep;117(3):395–407. [PubMed] [Google Scholar]
- Hardy P. H., Jr, Nell E. E. Isolation and purification of Treponema pallidum from syphilitic lesions in rabbits. Infect Immun. 1975 Jun;11(6):1296–1299. doi: 10.1128/iai.11.6.1296-1299.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Eggebraten L. M. Fatty Acid Requirements of the Kazan 5 and Reiter Strains of Treponema pallidum. Infect Immun. 1971 Jun;3(6):723–726. doi: 10.1128/iai.3.6.723-726.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Livermore B. P., Jenkin H. M., Eggebraten L. Lipids of Treponema pallidum Kazan 5. Infect Immun. 1970 Nov;2(5):606–609. doi: 10.1128/iai.2.5.606-609.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyon J. M., Harris D. L., Glock R. D. Enteropathogenicity of various isolates of Treponema hyodysenteriae. Infect Immun. 1977 Feb;15(2):638–646. doi: 10.1128/iai.15.2.638-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Klingman J. D., Wicher K. Host response to Treponema pallidum infection. I. Quantitative changes of lipids in rabbit organs. Int Arch Allergy Appl Immunol. 1977;55(1-6):476–480. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Livermore B. P., Bey R. F., Johnson R. C. Lipid metabolism of Borrelia hermsi. Infect Immun. 1978 Apr;20(1):215–220. doi: 10.1128/iai.20.1.215-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore B. P., Johnson R. C. Isolation and characterization of a glycolipid from Treponema pallidum, Kazan 5. Biochim Biophys Acta. 1970 Jul 14;210(2):315–318. doi: 10.1016/0005-2760(70)90176-1. [DOI] [PubMed] [Google Scholar]
- Livermore B. P., Johnson R. C. Lipids of the Spirochaetales: comparison of the lipids of several members of the genera Spirochaeta, Treponema, and Leptospira. J Bacteriol. 1974 Dec;120(3):1268–1273. doi: 10.1128/jb.120.3.1268-1273.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. M., Jenkin H. M., Crilly K., Sandok P. L. Effects of fatty acids on motility retention by Treponema pallidum in vitro. Infect Immun. 1978 Mar;19(3):814–821. doi: 10.1128/iai.19.3.814-821.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Meyer F. Lipid metabolism in the parasitic and free-living spirochetes Treponema pallidum (Reiter) and Treponema zuelzerae. Biochim Biophys Acta. 1971 Feb 2;231(1):93–106. doi: 10.1016/0005-2760(71)90257-8. [DOI] [PubMed] [Google Scholar]
- Miao R., Fieldsteel A. H. Genetics of Treponema: relationship between Treponema pallidum and five cultivable treponemes. J Bacteriol. 1978 Jan;133(1):101–107. doi: 10.1128/jb.133.1.101-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J. C., Baseman J. B. Carbon sources utilized by virulent Treponema pallidum. Infect Immun. 1975 Nov;12(5):1044–1050. doi: 10.1128/iai.12.5.1044-1050.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OYAMA V. I., STEINMAN H. G., EAGLE H. The nutritional requirements of treponemata. V. A detoxified lipide as the essential growth factor supplied by crystallized serum albumin. J Bacteriol. 1953 May;65(5):609–616. doi: 10.1128/jb.65.5.609-616.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajković A. D. Cultivation of the Noguchi strain of Treponema pallidum in a lipid medium in the absence of serum. Z Med Mikrobiol Immunol. 1967;153(4):297–312. doi: 10.1007/BF02123751. [DOI] [PubMed] [Google Scholar]
- Sandok P. L., Knight S. T., Jenkin H. M. Examination of various cell culture techniques for co-incubation of virulent Treponema pallidum (Nichols I strain) under anaerobic conditions. J Clin Microbiol. 1976 Oct;4(4):360–371. doi: 10.1128/jcm.4.4.360-371.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L., Cox C. D. Catabolism of glucose and fatty acids by virulent Treponema pallidum. Infect Immun. 1977 Apr;16(1):60–68. doi: 10.1128/iai.16.1.60-68.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váczi L., Király K., Réthy A. Lipid composition of treponemal strains. Acta Microbiol Acad Sci Hung. 1966;13(1):79–84. [PubMed] [Google Scholar]