ABSTRACT
This study aimed to evaluate the immunogenicity of a recombinant Mycobacterium smegmatis vaccine expressing the CMX fusion protein composed of immunodominant epitopes Ag85C, MPT51 and HspX of Mycobacterium tuberculosis, which are important mycobacteria virulence factors. A group of Nelore heifers that were 10 to 12 months of age and negative for the tuberculin skin test (TST) were immunized with four doses of the recombinant vaccine mc2-CMX (M. smegmatis-Ag85C-MPT51-HspX) during a period of one year. Before each immunization, blood was collected to obtain sera for antibody analysis. Serological analysis demonstrated that mc2-CMX was able to induce a humoral response with increased levels of specific IgG antibodies against CMX, despite minimum antibody levels being detected for individual Ag85C, MPT51 or HspX recombinant antigens. However, there was no significant increase in specific CD4+ IFN-γ-positive T cells. Lymphadenomegaly was observed in superficial cervical lymph nodes adjacent to the site of vaccination among mc2-CMX-vaccinated bovines, and the histopathological analysis demonstrated follicular hyperplasia without inflammatory infiltrate or granuloma formation. Animals remained negative for the TST until the end of the experiments, showing no cross-reactivity with the recombinant vaccine and tuberculin proteins. We discuss the potential of mc2-CMX to induce an immune response in cattle.
Keywords: environmental mycobacteria, immunological response, Mycobacterium bovis, public health, zoonosis
Tuberculosis (TB) is an infectious disease of great public health impact worldwide, as every year millions of new cases and deaths occur as a consequence of infection. According to the World Health Organization (WHO), it is estimated that one-third of the world’s population is infected with Mycobacterium tuberculosis (Mtb), the bacilli causing the disease [51].
Mycobacterium bovis is another important mycobacteria, a member of the Mtb Complex responsible for tuberculosis in cattle (bTB) as well as humans. M. bovis can infect a broader range of hosts compared with other mycobacteria within the Mtb complex [48]. This agent can infect humans by means of non-pasteurized milk or derivatives infected with bacteria, or by contact with infected cattle. bTB impairs cattle industry development pertaining to both dairy and beef products, by directly interfering with international trading of these products. Additionally, the costs of diagnostic tests and slaughtering of positive animals result in loss of commercial trade deals, animal transportation restrictions and the need to maintain control and/or eradication programs, all of which lead to significant economic impacts on agri-food businesses worldwide [39, 48].
To improve disease prevention/control, many obstacles must be overcome, one of them being the development of a more efficient vaccine, as the only vaccine in use for humans today, M. bovis BCG (Bacillus Calmette-Guérin, an attenuated strain of M. bovis), does not provide satisfactory protection against human tuberculosis in adults and has restrictions regarding its use in highly endemic areas [3, 6, 7]. Additionally, M. bovis BCG confers a variable degree of protection in bovine, in which its efficacy depends on animal age, dosage and strain type of the vaccine, vaccination regimen and inoculation route [2, 10, 11, 28, 49]. Despite previous studies showing a reduction in disease severity among bovine vaccinated previously with BCG, the vaccine was not capable of inducing effective protection against a virulent M. bovis challenge [12, 23, 28]. One of the hypotheses explaining the low BCG efficacy is the interference of environmental mycobacteria [7, 9, 14]. This theory is based on the fact that vaccination with BCG is less efficient in regions where the population is more exposed to environmental mycobacteria [17]. Another problem related to BCG use in cattle is its interference with the tuberculin skin test, as most antigens present in purified protein derivative (PPD) are also present in M. bovis BCG [47].
At present, there is no vaccine capable of conferring protection in bovines against M. bovis infection, and consequently animals testing positive for the tuberculin skin test (TST) must be slaughtered. Nevertheless, many studies are being conducted using this animal model, as well as mice, guinea pigs and humans. The TB research advances achieved in human and other animal models are far superior to those with bTB, and consequently, the research with the vaccine against M. tuberculosis has surpassed that with M. bovis. Bovine studies can bring many beneficial insights for control of the disease in animals and humans, but the lack of sufficient financial support and high cost of study facilities limits their potential use in TB and bTB studies. The bovine model is better suited to develop vaccine candidates for bTB, and consequently, testing vaccine candidates in this model should provide more accurate and applicable responses [48].
Considering tuberculosis a zoonosis and a disease of serious world public health concern, the necessity to develop new and improved vaccines against tuberculosis with efficacy among different host species, diverse populations and environmental regions is evident.
Mycobacterium smegmatis is a non-pathogenic member of the mycobacteria family that presents rapid growth and can efficiently transform different genes in vitro [20]. Consequently, this species is a resourceful prototype for the development of studies of vaccines against tuberculosis [46, 53].
The immunogenicity and antigenicity of CMX (Ag85C-MPT51-HspX), a recombinant fusion protein from Mtb, has previously been shown in both mouse and human models [15]. CMX contains immunodominant epitopes that constitute significant virulence factors of mycobacteria [4, 29, 43,44,45].
Antigen 85 is a complex of proteins (Ag85A, Ag85B and Ag85C) secreted by Mtb and is an important mycobacteria virulence factor. Ag85C and the other Ag85 complex proteins are crucial for cell wall synthesis playing an important role in bacteria growth and survival [5]. Besides, Ag85C can be recognized by CD4+ and CD8+ T cells from individuals with active tuberculosis [16].
The protein MPT51 seems to be involved in bacterial adhesion to the extracellular matrix. Studies that analyzed the humoral and cellular immune response to MPT51 verified discrimination between patients with active TB from healthy individuals [36].
HspX is a protein expressed during the latent phase of the Mtb infection [52] and when bacteria are confined inside granulomas structures or macrophages under hypoxia and limited energy conditions. Moreover, HspX epitopes can be recognized by CD4+ and CD8+ T cells from active TB patients [36, 42].
In the present study, the immunogenicity of a recombinant M. smegmatis vaccine expressing the protein CMX, formed by the fusion of immunodominant epitopes from Ag85C and MPT51 proteins and the entire HspX protein, was evaluated in a group of tuberculin-negative bovines.
MATERIALS AND METHODS
The Ethics Committee of Goiás Federal University approved this study under protocol # 020/2008.
Recombinant Mycobacterium smegmatis production: M. smegmatis mc2 155 (kindly provided by Dr. Luciana Leite of the Butantan Institute, Brazil) was grown in Middlebrook 7H9 broth (Himedia, Mumbai, India) supplemented with 0.05% of Tween 80 at 37°C for three days. Cells were washed in 10% glycerol, and electrocompetent cells were aliquoted in 100 µl volumes in cryotubes at −80°C until use.
The recombinant plasmid containing the gene of the fusion protein CMX constructed in our lab (pLA71/ CMX) [24] and the empty plasmid (pLA71, kindly provided by Dr. Brigitte Gicquel, from Pasteur Institute, France) were introduced into M. smegmatis mc2 155 (mc2-CMX and mc2-pLA71, respectively) by electroporation.
Construction of the recombinant M. smegmatis-CMX (mc2-CMX) was described by Junqueira-Kipnis et al. [24]. Briefly, the DNA sequence containing the immunodominant epitopes from Ag85C and MPT51 and the entire HspX gene, described previously by De Sousa et al. [15], was used as template for PCR amplification using a set of primers to create flanking restriction enzyme sites and facilitate posterior cloning. The product of this amplification was cloned into pGEM-T Easy Vector (Promega, Madison, WIS, U.S.A.). The recombinant pGEM-T easy vector containing the fused gene (CMX) was digested with KpnI and NotI for elution of the CMX gene. The eluted CMX/KpnI/NotI gene was ligated to the mycobacteria/E. coli shuttle vector pLA71 digested with the same enzymes, the size of which was 12,248 pb, and as a selective marker, pLA71 contains a kanamycin resistance gene [27]. The CMX gene was inserted downstream of the β-lactamase gene (blaF*) promoter. This promoter codes the 32 amino acids of the mature β-lactamase signal sequence and the first 5 amino acids of the mature protein for expression and export of CMX protein. The recombinant pLA71/CMX vector was sequenced to check for mutations. pLA71/CMX and pLA71 vectors were transformed into mc2 and screened on media with kanamycin, to produce the recombinant vaccine mc2-CMX and mc2-pLA71 control vaccines, respectively.
Animals and experimental design: Thirty Nelore calves that ranged in age from 10 to 12 months and were negative for the tuberculin skin test (from the Goiano Federal Institute − Urutaí Campus) were separated into three groups randomly. Vaccination was performed subcutaneously three times at 21-day intervals. Group 1 received 1.0 ml (1 × 107 CFU/ml) of the recombinant vaccine (M. smegmatis mc2-CMX); group 2 received 1.0 ml (1 × 107 CFU/ml) of the empty vector vaccine (M. smegmatis mc2-pLA71); and group 3 received 1.0 ml of PBS. Prior to each immunization, blood collection was performed such that there were blood samples corresponding to the basal level, the levels at 21 days after the first and second immunizations and the level at 30 days after the last immunization.
In order to test the maintenance of memory immune responses, the animals were revaccinated a fourth time at 200 days after the third vaccination. Blood was collected 30 days after the booster.
For immunoenzymatic assays, blood was collected from the tail artery/vein without an anti-coagulant. Sera were obtained after centrifugation at 2,500 g for 10 min at 4°C and stored at −20°C until use.
Blood was also collected with heparin as an anti-coagulant for cytometric analysis. Peripheral blood mononuclear cell (PBMC) was obtained after centrifugation at 2,500 g for 15 min at 4°C, treatment with erythrocytes lysis solution and resuspension in RPMI 1640 media (GIBCOTM, Carlsbad, CA, U.S.A.) supplemented with 24 mM of sodium bicarbonate, 10% heat-inactivated fetal calf serum, 2 mM of L-glutamine (Sigma-Aldrich®, St Louis, MO, U.S.A.), 1% of penicillin-streptomycin (Sigma-Aldrich®), 1 mM sodium pyruvate (Sigma-Aldrich®) and nonessential amino acids (Sigma-Aldrich®). Cells were counted in a hemocytometer.
Immunoenzymatic assay (ELISA): Ninety-six-well polystyrene plates (Santa Cruz Biotechnology, Dallas, TX, U.S.A.) were coated with the lysates of mc2-CMX vaccine or purified recombinant protein (rCMX, rAg85C, rMPT51 or rHspX, 10 µg/ml) diluted in 15 mM carbonate/bicarbonate buffer (pH 9.8). After 18 hr of incubation at 4°C, the plates were blocked with carbonate/bicarbonate buffer containing 1% gelatin for 2 hr at 37°C. Serum, diluted (1:320) in PBS containing 0.1% gelatin, was added and incubated for 2 hr at 37°C. Extensive washing with PBS Tween (0.05%) was performed followed by addition of an anti-bovine IgG conjugate (Jackson ImmunoResearch Laboratories, Inc., London, U.K.) diluted in PBS 0.1% gelatin (1:10,000) and incubation for 1 hr at 37°C. After additional washing with PBS Tween (0.05%), citrate buffer (pH 5.0) containing o-phenylenediamine (OPD) and hydrogen peroxide was added. The reaction was stopped with 2N H2SO4 solution. Samples were analyzed in a Multiskan Plus (Thermo Scientific, Walthan, MA, U.S.A.) ELISA reader at 492 nm.
Flow cytometry: Flow cytometry analysis was performed after stimulation of 106 PBMCs with rCMX (10 µg/ml) or phytohemagglutinin (PHA 1 µg/ml) for 6 hr at 37°C in 5% CO2. Cells were further incubated with monensin (eBioscience, ,San Diego, CA, U.S.A.) for 4 hr at 37°C in 5% CO2. Cell staining was performed with antibodies conjugated with surface membrane or intracellular markers, PE-CD8, PercP-IFN-γ and APC-CD4, for 30 min, washed with PBS containing 0.1% sodium azide, fixed and permeabilized with Perm Fix/Perm Wash (BD PharMingen, SanJose, CA, U.S.A.). Cell selection after flow cytometry acquisition of 50,000 events (BD Biosciences, San Diego, CA, U.S.A. FACSCanto II) was based on size (FSC) and granularity (SSC) to gate lymphocytes using BD FACSDiva software.
Histopathological analysis: Ninety days after the booster immunization, superficial cervical lymph node biopsies were performed at the site of vaccine injections. Biopsy samples were conditioned in 10% buffered formalin, posteriorly submitted to routine processing and stained with hematoxylin eosin (HE) [18] and Fite’s acid fast staining [37].
Single cervical tuberculin skin test: All animals were submitted to tuberculin skin testing prior to beginning the experiments and after the end of all immunizations. The test was performed by intradermally injecting 0.1 ml of M. bovis standard PPD (0.1 mg of M. bovis strain AN5) in the mid-cervical area. The animals were examined for a hypersensitivity response 72 hr post injection [8].
Statistical analysis: The results were analyzed using the Microsoft Excel (version 14.3.4, 2011) and GraphPad (Prism 4.0) software. Differences between groups were analyzed using ANOVA test. Individual groups were compared by the nonparametric Kruskal-Wallis test followed by Dunn’s test. Results with a p-value of less than 0.05% were considered statistically significant.
RESULTS
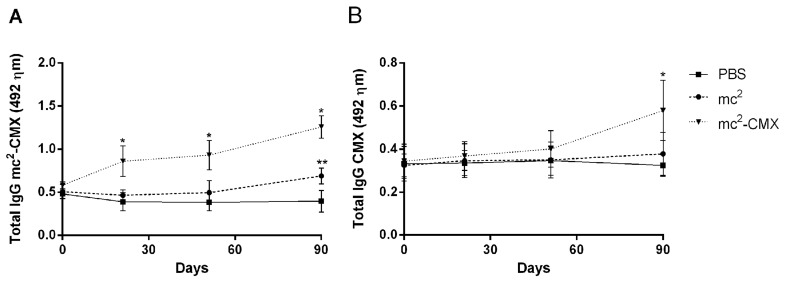
In order to verify if mc2-CMX was able to induce an immune humoral response in bovine, an ELISA was developed to detect the presence of vaccine-specific antibodies (Fig. 1A). A progressive increase in IgG antibody levels was observed as immunizations were performed, and there was a significant difference in mc2-CMX-specific antibody levels at the latter time points of immunizations compared with at the initial time points, as well as compared with the control groups immunized with mc2 or PBS. An ELISA was also performed to detect the presence of rCMX-specific antibodies (Fig. 1B), and the results were similar to those of the first test. A progressive increase in IgG antibodiy levels occurred, with higher titers being observed at latter time points of immunization. However, no response was observed to the individual recombinant antigens (rAg85C, rMPT51 or rHspX, data not shown).
Fig. 1.
Immune humoral response induced by the recombinant vaccine. (A) Serum levels of anti-mc2-CMX antibodies among bovine immunized with mc2-CMX, mc2 and PBS. (B) Serum levels of anti-rCMX antibodies among bovine immunized with mc2CMX, mc2 and PBS. Animals were immunized with 1 × 107 CFU/ml of each vaccine subcutaneously. Sera were obtained at different time points following vaccination and analyzed by ELISA. Each time point represents the mean and standard error of the mean of the optical density from 10 animals per group. * Statistically significant difference between the mc2-CMX and PBS groups (P<0.05). ** Statistically significant difference between the mc2 and PBS groups (P<0.05).
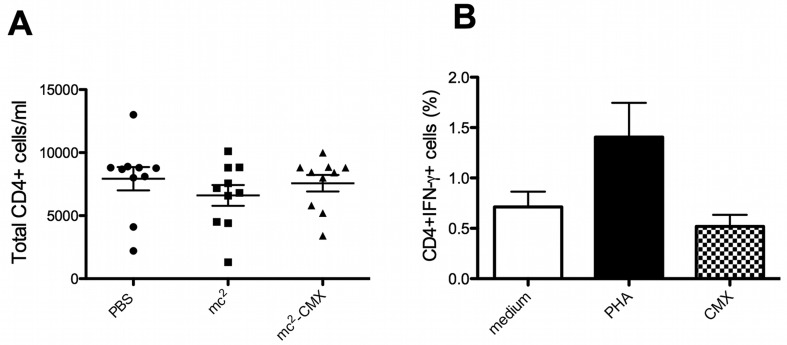
To evaluate if vaccination altered the number of CD4+ T cells in the peripheral blood, PBMCs were analyzed, and the total number of CD4+ T cells from each animal was obtained. No differences were observed among the studied groups (Fig. 2A). Since it is well known that the cellular immune response to bTB is crucial for protection, after the booster vaccination to induce reactivation of memory cells, IFN-γ-producing CD4+ T lymphocyte populations were quantified. Stimulation of PBMCs from mc2-CMX-vaccinated bovine with recombinant rCMX did not reveal an increase in the amount of IFN-γ-producing CD4+ T lymphocytes, regardless of the animal immunization status (Fig. 2B and data not shown).
Fig. 2.
Induction of IFN-γ by CD4+ T cells. Cattle were revaccinated 200 days after the third immunization, and PBMCs were obtained. (A) Total CD4+ T cells from the vaccinated group were quantified by flow cytometry. The dots represent the number of CD4+ T cell/ml from each animal. The horizontal lines represent the average and standard deviation. (B) PBMCs from mc2-CMX-vaccinated animals were stimulated in vitro with medium, PHA or CMX, and the percentage of CD4+IFN-γ-positive T cells was analyzed. The bars represent the average and standard deviation per group. No significant statistical difference was observed.
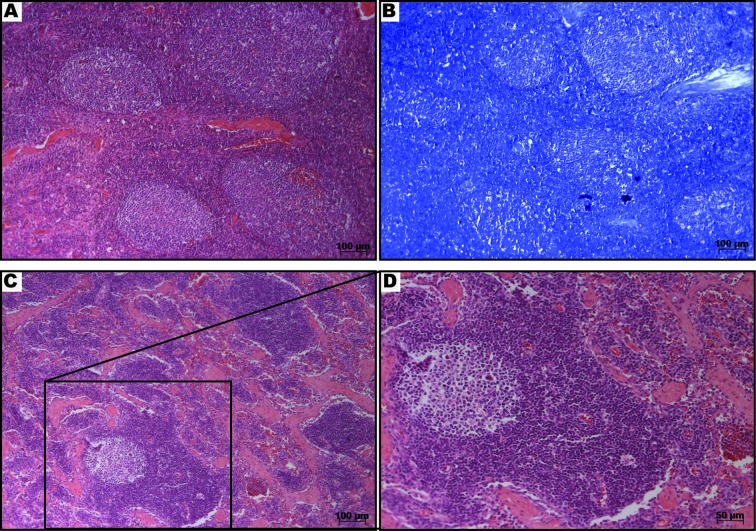
After the second immunization, animals that received the mc2-CMX vaccine presented accentuated lymphadenomegaly in superficial cervical lymph nodes. The lymphadenomegaly was associated with follicular hyperplasia. Neither necrosis nor inflammatory infiltrate or granuloma formation was found within the analyzed biopsy samples (Fig. 3). No acid-fast bacilli were detected within the analyzed tissues (Fig. 3B).
Fig. 3.
Histopathological analysis of a superficial cervical lymph node from the mc2-CMX vaccinated group. The lymph node from a representative animal was sectioned and stained with hematoxylin eosin (H&E) (A, C and D) and Fite’s acid fast stain (B) 90 days after the last immunization of the group. Note the absence of inflammatory infiltrate or granuloma formation or necrosis (A). Fite’s acid fast staining revealed no presence of acid-fast bacilli (B). The structure of a normal lymph node with a germinal center, medular region and blood vessels can be seen (C and D).
All animals remained negative for the tuberculin skin test until the end of the experimental period.
DISCUSSION
In the present study, we investigated the capacity of a recombinant vaccine, mc2-CMX, to induce specific IgG antibodies in bovine. The recombinant vaccine was able to induce high titers of IgG antibodies. However, it was not able to stimulate specific CD4+ T lymphocytes. The cervical lymph nodes of mc2-CMX-vaccinated animals presented follicular hyperplasia characteristic of cellular proliferation. The animals remained negative in the tuberculin skin test throughout the duration of the study.
In recent years, several approaches have been made to increase the protective efficacy of BCG, in addition to the search of new immunogenic antigens from Mtb as well as from other mycobacteria, in order to develop improved vaccines. An effective vaccine against TB or bTB must be capable of preventing infection and establishing a long-lasting protection. Consequently, it is essential that a robust induction of effector and memory cells take place and that a significant level of antibodies is raised following vaccination [40]. It is known that antibodies play an important role in mycobacterial infections, as they can induce microbicidal activities of macrophages and attract monocytes, neutrophils and NK cells to the site of infection, in addition to activating dendritic cells and macrophages, enabling efficient antigen processing and presentation by these cells [19, 25, 39, 41].
In this study, a significant increase in specific IgG levels against mc2-CMX and rCMX was observed in mc2-CMX-vaccinated animals (Fig. 1). Despite the observation of an initial response among animals vaccinated with M. smegmatis (mc2) alone, there was a significant increase in the antibody level in animals receiving mc2-CMX after the second time point of vaccination (P<0.05). This cross-reaction observed among the control groups could be explained by the presence of Ag85C and MPT51 analog proteins in M. smegmatis [5] and by the constant exposure of animals to environmental mycobacteria as well. Bovines vaccinated with mc2-CMX could not produce IgG antibodies against individual Ag85C, MPT51 or HspX recombinant antigens when tested in an indirect ELISA (data not shown), reflecting the immunogenicity of the rCMX protein in the bovine model. Similar results were observed when mice were immunized with mc2-CMX or rCMX [15, 24]. A possible explanation for these observations could involve the tertiary structure of the secreted fusion protein produced by the recombinant M. smegmatis vaccine hiding or exposing unique epitopes not present in the single antigens. To circumvent the absence of response observed to the individual wild-type antigens, further engineering of the recombinant fusion protein is needed, such as replacing the hinge regions with bigger and/or different amino acid sequences to allow independence of each epitope exposition and folding.
Similarly, we have shown that rCMX, a recombinant fusion protein, induces production of IgG1 and IgG2a in mice when used as a subunit vaccine in addition to the ability of this recombinant protein to detect high levels of specific IgG and IgM in the sera of active TB individuals [15]. These results corroborate with immunogenic potential of the recombinant protein in the context of the vaccine. A recent report showed the ability of a recombinant M. smegmatis vaccine expressing a fusion protein comprised of the antigens ESAT- 6 and CFP-10 from Mtb to induce a high titer of antibodies in the mouse [53]. Thus, the fusion protein CMX can be considered a candidate to be used in the production of recombinant or subunit vaccines, as they presented antigenicity and immunogenicity in different study host models. Further development of the fusion protein should be performed before this vaccine is considered a candidate to protect bTB.
The essential role of cell-mediated immune response in the protection against TB has been well characterized [13, 30, 34]. CD4+ T lymphocytes have an important role against Mycobacterium sp., as they produce IFN-γ and TNF-α, which are key cytokines in the immune response against mycobacterial infections [32]. In addition, CD8+ T lymphocytes also contribute to a long-lasting protection against TB [28, 31, 33], as they can use a diverse repertoire of mechanisms against infected cells, especially phagocytes, the main target of mycobacteria, and non-phagocytic cells that can also be infected by this pathogen [21]. Here, we show that there was no significant increase in specific CD4+ IFN-γ-positive T cells (Fig. 2; and data not shown). This phenomena could be explained by a) the majority of the effector/ memory T cell being localized in the draining lymph nodes instead of in peripheral blood [26, 35], b) 30 days after the booster possibly being too long for the persistence of this population of cells in the periphery [26, 39] and c) CMX not having been presented by bovine antigen-presenting cells [22]. All of the above hypotheses must be further investigated.
It is well known that mycobacteria can be drained into the lymph node by infected resident macrophages [1]. Consequently, we sought to understand if the observed lymphadenomegaly in superficial cervical lymph nodes was an inflammatory process caused by M. smegmatis proliferation. The histopathological analysis revealed neither necrosis nor inflammatory infiltrate or granuloma formation within the analyzed biopsy samples. Additionally, no acid-fast bacilli were detected within the analyzed tissues (Fig. 3B). Thus, the observed lymphadenomegaly was probably due to cellular proliferation as a result of a lymphocytic activation. It is possible that the observed hyperplasia was strictly correlated with higher levels of antibodies induced against CMX, although activation of CD4+ or CD8+ T cells was not observed.
Previous studies stated that bovine have a dichotomous response to mycobacteria, that is, alternating high levels of antibody production with cellular immune response [35, 38, 50]. In line with this, we can infer that a humoral immune response was elicited in response to the fusion protein CMX and that a cellular response was directed only toward M. smegmatis, and thus, it was not observed in our assays. An inflammatory response or the presence of bacteria was not observed in the lymphoid tissue (Fig. 3). Furthermore, the maintenance of a negative TST status among animals indicates that the vaccine did not elicit a cross-reaction with the test.
Explanations for pitfalls in human and animal TB vaccines are encountered throughout the literature. Some studies affirm that previous contact with environmental mycobacteria can reduce the immune response induced by TB vaccines. Different animal model studies have already proven the interference of sensitization to other mycobacteria in the immune response against tuberculosis [7, 14, 30]. A study with cattle demonstrated that, prior to BCG immunization, bovines exhibited high levels of IFN-γ in response to PPD from Mycobacterium avium, and these animals showed an IFN-γ response to PPD from M. bovis for only one week after vaccination with BCG; on the other hand, groups that received a newly derived attenuated M. bovis strain maintained this response for two weeks [9]. Failure in BCG vaccination among animal models is consistent with studies in humans, where it has been shown that previous contact with other mycobacteria could block multiplication of the vaccine and induction of an immune response. Regarding humans, a study with populations from Malawi and the United Kingdom showed that people from Malawi had lower levels of IFN-γ one year after BCG vaccination, which was attributed to a preexposure to environmental mycobacteria [6].
The development of new therapies against infectious diseases that affect human populations is a constant challenge for the scientific community. In regard to TB, a zoonosis causing a significant death toll in humans and important economic losses worldwide, these challenges are even greater. Studies using bovine are very expensive and of great complexity. An important limitation of this study was the necessity to validate the real potential of mc2-CMX as a vaccine in a protective study in bovines challenged with M. bovis. This pitfall does not diminish the importance of our findings, as we showed for the first time the bovine immune response to a recombinant fusion protein used as a bTB vaccine.
In conclusion, this work shows that the mc2-CMX vaccine was able to induce a humoral immune response and did not induce cross-reactivity in the TST, indicating that it can potentially be further tested as a vaccine for bovine tuberculosis.
Acknowledgments
This work received financial support from CNPq, Dinter-CAPES and Federal Goiano Institute − Urutaí Campus.
REFERENCES
- 1.Abadie V., Badell E., Douillard P., Ensergueix D., Leenen P. J. M., Tanguy M., Fiette L., Saeland S., Gicquel B., Winter N.2005. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood 106: 1843–1850. doi: 10.1182/blood-2005-03-1281 [DOI] [PubMed] [Google Scholar]
- 2.Ameni G., Vordermeier M., Aseffa A., Young D. B., Hewinson R. G.2010. Field evaluation of the efficacy of Mycobacterium bovis bacillus Calmette–Guerin against bovine tuberculosis in neonatal calves in Ethiopia. Clin. Vaccine Immunol. 17: 1533–1538. doi: 10.1128/CVI.00222-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen P., Doherty T. M.2005. The success and failure of BCG — implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 3: 656–662. doi: 10.1038/nrmicro1211 [DOI] [PubMed] [Google Scholar]
- 4.Backus K. M., Boshoff H. I., Barry C. S., Boutureira O., Patel M. K.2011. Uptake of unnatural trehalose analogs as a reporter for Mycobacterium tuberculosis. Nat. Chem. Biol. 7: 228–235. doi: 10.1038/nchembio.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belisle J. T., Vissa V. D., Sievert T., Takayama K., Brennan P. J., Besra G. S.1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276: 1420–1422. doi: 10.1126/science.276.5317.1420 [DOI] [PubMed] [Google Scholar]
- 6.Black G. F., Weir R. E., Floyd S., Bliss L., Warndorff D. K., Crampin A. C., Ngwira B., Sichali L., Nazareth B., Blackwell J. M., Branson K., Chaguluka S. D., Donovan L., Jarman E., King E., Fine P. E. M., Dockrell H. M.2002. BCG-induced increase in interferon gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359: 1393–1401. doi: 10.1016/S0140-6736(02)08353-8 [DOI] [PubMed] [Google Scholar]
- 7.Brandt L., Cunha J. F., Olsen A. W., Chilima B., Hirsch P., Appelberg R., Andersen P.2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70: 672–678. doi: 10.1128/IAI.70.2.672-678.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Programa Nacional de Controle e Erradicação da Brucelose e da Tuberculose Animal (PNCEBT) / organizadores, Vera Cecilia Ferreira de Figueiredo, José Ricardo Lôbo, Vitor Salvador Picão Gonçalves. − Brasília: MAPA/SDA/DSA, 2006. 188 p. ISBN 85-99851-01-2. [Google Scholar]
- 9.Buddle B. M., Wards B. J., Aldwell F. E., Collins D. M., De Lisle G. W.2002. Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine 20: 1126–1133. doi: 10.1016/S0264-410X(01)00436-4 [DOI] [PubMed] [Google Scholar]
- 10.Buddle B. M., Wedlock D. N., Parlane N. A., Corner L. A., De Lisle G. W., Skinner M. A.2003. Revaccination of neonatal calves with Mycobacterium bovis BCG reduces the level of protection against bovine tuberculosis induced by a single vaccination. Infect. Immun. 71: 6411–6419. doi: 10.1128/IAI.71.11.6411-6419.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buddle B. M., Denis M., Aldwell F. E., Vordermeier H. M., Hewinson R. G., Wedlock D. N.2008. Vaccination of cattle with Mycobacterium bovis BCG by a combination of systemic and oral routes. Tuberculosis (Edinb.) 88: 595–600. doi: 10.1016/j.tube.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 12.Buddle B. M., Aldwell F. E., De Lisle G. W., Vordermeier H. M., Hewinson R. G., Wedlock D. N.2011. Low oral BCG doses fail to protect cattle against an experimental challenge with Mycobacterium bovis. Tuberculosis (Edinb.) 91: 400–405. doi: 10.1016/j.tube.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 13.Cooper A. M.2009. Cell mediate immune responses in tuberculosis. Annu. Rev. Immunol. 27: 393–422. doi: 10.1146/annurev.immunol.021908.132703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lisle G. W., Wards B. J., Buddle B. M., Collins D. M.2005. The efficacy of live tuberculosis vaccines after presensitization with Mycobacterium avium. Tuberculosis (Edinb.) 85: 73–79. doi: 10.1016/j.tube.2004.09.007 [DOI] [PubMed] [Google Scholar]
- 15.de Sousa E. M., Da Costa A. C., Trentini M. M., Araújo Filho J. A., Kipnis A., Junqueira-Kipnis A. P.2012. Immunogenicity of a fusion protein containing immunodominant epitopes of Ag85C, MPT51, and HspX from Mycobacterium tuberculosis in mice and active TB infection. PLoS ONE 7: e47781 doi: 10.1371/journal.pone.0047781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Souza S., Rosseels V., Romano M., Tanghe A., Denis O., Jurion F., Castiglione N., Vanonckelen A., Palfliet K., Huygen K.2003. Mapping of murine Th1 helper T-Cell epitopes of mycolyltransferases Ag85A, Ag85B and Ag85C from Mycobacterium tuberculosis. Infect. Immun. 71: 483–493 doi: 10.1128/IAI.71.1.483-493.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine P. E.1989. The BCG story: lessons from the past and implications for the future. Rev. Infect. Dis. 11: S353–359. doi: 10.1093/clinids/11.Supplement_2.S353 [DOI] [PubMed] [Google Scholar]
- 18.Fischer A. H., Jacobson K. A., Rose J., Zeller R.2008. Hematoxylin and eosin staining of tissue and cell sections. CSH Protocols 2008: . [DOI] [PubMed] [Google Scholar]
- 19.Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., Ley K.2010. Development of monocytes, macrophages, and dendritic cells. Science 327: 656–661. doi: 10.1126/science.1178331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gicquel B.1994. Towards new mycobacterial vaccines. Dev. Biol. Stand. 82: 171–178 [PubMed] [Google Scholar]
- 21.Hernández-Pando R., Jeyanathan M., Mengistu G., Aguilar D., Orozco H., Harboe M., Rook G. A., Bjune G.2000. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet 356: 2133–2138. doi: 10.1016/S0140-6736(00)03493-0 [DOI] [PubMed] [Google Scholar]
- 22.Hope J. C., Thom M. L., McCormick P. A., Howard C. J.2004. Interaction of antigen presenting cells with mycobacteria. Vet. Immunol. Immunopathol. 100: 187–195. doi: 10.1016/j.vetimm.2004.04.007 [DOI] [PubMed] [Google Scholar]
- 23.Hope J. C., Thom M. L., Mc Aulay M., Mead E., Vordermeier H. M., Clifford D., Hewinson R. G., Villarreal-Ramos B.2011. Identification of surrogates and correlates of protection in protective immunity against Mycobacterium bovis infection induced in neonatal calves by vaccination with M. bovis BCG Pasteur and M. bovis BCG Danish. Clin. Vaccine Immunol. 18: 373–379. doi: 10.1128/CVI.00543-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junqueira-Kipnis A. P., Oliveira F. M., Trentini M. M., Tiwari S., Chen B., Resende D. P., Silva B. D. S., Chen M., Tesfa L., Jacobs W. R., Jr, Kipnis A.2013. Prime-boost with Mycobacterium smegmatis recombinant vaccine improves protection in mice infected with Mycobacterium tuberculosis. PLoS ONE 8: e78639. doi: 10.1371/journal.pone.0078639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufmann S. H. E., Ottenhoff T. H. M.2012. Tuberculosis vaccine development: strength lies in tenacity. Trends Immunol. 33: 373–379. doi: 10.1016/j.it.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 26.Kipnis A., Irwin S., Izzo A. A., Basaraba R. J., Orme I.2005. Memory T lymphocytes generated by Mycobacterium bovis BCG vaccination reside within a CD4 CD44lo CD62 ligandhi population. Infect. Immun. 73: 7759–7764. doi: 10.1128/IAI.73.11.7759-7764.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim E. M., Rauzier J., Timm J., Torrea G., Murray A., Gicquel B., Portnoi D.1995. Identification of Mycobacterium tuberculosis DNA sequences encoding exported proteins by using phoA gene fusions. J. Bacteriol. 177: 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Valencia G., Renteria-Evangelista T., Williams J. J., Licea-Navarro A., Mora-Valle A. L., Medina-Basulto G.2010. Field evaluation of the protective efficacy of Mycobacterium bovis BCG vaccine against bovine tuberculosis. Res. Vet. Sci. 88: 44–49. doi: 10.1016/j.rvsc.2009.05.022 [DOI] [PubMed] [Google Scholar]
- 29.Melo Cardoso Almeida C., Vasconcelos A. C., Jr, Kipnis A., Andrade A. L., Junqueira-Kipnis A. P.2008. Humoral immune responses of tuberculosis patients in Brazil indicate recognition of Mycobacterium tuberculosis MPT-51 and GlcB. Clin. Vaccine Immunol. 15: 579–581. doi: 10.1128/CVI.00359-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orme I. M., Roberts A. R., Collins F. M.1986. Lack of evidence for a reduction in the efficacy of subcutaneous BCG vaccination in mice infected with nontuberculous mycobacteria. Tubercle 67: 41–46. doi: 10.1016/0041-3879(86)90030-9 [DOI] [PubMed] [Google Scholar]
- 31.Orme I. M., Miller E. S., Roberts A. D., Furney S. K., Griffin J. P., Dobos K. M., Chi D., Rivoire B., Brennan P. J.1992. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J. Immunol. 148: 189–196 [PubMed] [Google Scholar]
- 32.Ottenhoff T. H. M., Verreck F. A., Lichtenauer-Kaligis E. G., Hoeve M. A., Sanal O., van Dissel J. T.2002. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nat. Genet. 32: 97–105. doi: 10.1038/ng0902-97 [DOI] [PubMed] [Google Scholar]
- 33.Ottenhoff T. H. M., Lewinsohn D. A., Lewinsohn D. M.2008. Human CD4 and CD8 T cell responses to Mycobacterium tuberculosis: antigen specificity, function, implications and applications, pp. 119–156. In: Immunology and Cell Biology (Handbook of tuberculosis) (Wiley-VCH Verlag GmbH & Co.KGaA eds.), Weinheim, Baden-Württemberg. [Google Scholar]
- 34.Ottenhoff T. H. M., Kaufmann S. H.2012. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog. 8: e1002607. doi: 10.1371/journal.ppat.1002607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quevillon E. L., Díaz F., Jaramillo L., Lascurain R., Gutiérrez-Pabello J. A., Castañeda F. A., Arriaga C., Pérez R., González X. E.2013. Comparison of immune peripheral blood cells in tuberculin reactor cattle that are seropositive or seronegative for Mycobacterium bovis antigens. Vet. Immunol. Immunopathol. 153: 194–201. doi: 10.1016/j.vetimm.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 36.Reis M. C. G., Rabahi M. F., Kipnis A., Junqueira-Kipnis A. P.2009. Health care works humoral immune response against GLcB, MPT51 and HspX from Mycobacterium tuberculosis. Braz. J. Infect. Dis. 13: 417–421. doi: 10.1590/S1413-86702009000600006 [DOI] [PubMed] [Google Scholar]
- 37.Reyes Perez A.1963. Modification of the fite-faraco technique for the staining of acid-alcohol fast bacilli in histologic sections. Rev. Latinoam. Anat. Patol. 7: 81–85 [PubMed] [Google Scholar]
- 38.Ritacco V., Lopez B., De Kantor I. N., Barrera L., Errico F., Nader A.1991. Reciprocal cellular and humoral immune responses in bovine tuberculosis. Res. Vet. Sci. 50: 365–367. doi: 10.1016/0034-5288(91)90143-C [DOI] [PubMed] [Google Scholar]
- 39.Rizzi C., Bianco M. V., Bianco F. C., Soria M., Gravisaco M. J., Montenegro V., Vagnoni L., Garbaccio S., Delgado F., Leal K. S., Cataldi A. A., Dellagostin O. A., Bigi F.2012. Vaccination with a BCG strain overexpressing Ag85B protects cattle against Mycobacterium bovis challenge. PLoS ONE 7: e51396. doi: 10.1371/journal.pone.0051396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sallusto F., Lanzavecchia A., Araki K., Ahmed R.2010. From vaccines to memory and back. Immunity 33: 451–463. doi: 10.1016/j.immuni.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuurhuis D. H., Van Montfoort N., Ioan-Facsinay A., Jiawan R., Camps M., Nouta J., Melief C. J., Verbeek J. S., Ossendorp F.2006. Immune complex-loaded dendritic cells are superior to soluble immune complexes as antitumor vaccine. J. Immunol. 176: 4573–4580 [DOI] [PubMed] [Google Scholar]
- 42.Shi C., Chen L. Z., Zhang Y., Zhou Z., Lu J., Fu R., Wang C., Fang Z., Fan X.2010. Enhanced protection against tuberculosis by vaccination with recombinant BCG over-expressing HspX protein. Vaccine 28: 5237–5244. doi: 10.1016/j.vaccine.2010.05.063 [DOI] [PubMed] [Google Scholar]
- 43.Silva B. D. S., Silva E. B., Nascimento I. P., Reis M. C. G., Kipnis A., Junqueira-Kipnis A. P.2009. MPT- 51/ CpG DNA vaccine protects mice against Mycobacterium tuberculosis. Vaccine 27: 4402–4407. doi: 10.1016/j.vaccine.2009.05.049 [DOI] [PubMed] [Google Scholar]
- 44.da Silva E. B., Silva B. D. S., Leon J. R. R., Kipnis A., Santos I. K. F. M., Junqueira-Kipnis A. P.2011. Using BCG, MPT- 51 and Ag85 as antigens in an indirect ELISA for the diagnosis of bovine tuberculosis. Vet. J. 187: 276–278. doi: 10.1016/j.tvjl.2009.11.017 [DOI] [PubMed] [Google Scholar]
- 45.Spratt J. M., Britton W. J., Triccas J. A.2010. In vivo persistence and protective efficacy of the bacille Calmette Guerin vaccine overexpressing the HspX latency antigen. Bioeng. Bugs 1: 61–65. doi: 10.4161/bbug.1.1.10027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sweeney K. A., Dao D. N., Goldberg M. F., Hsu T., Venkataswamy M. M., Tamayo M. H., Ordway D., Sellers R. S., Jain P., Chen B., Chen M., Kim J., Lukose R., Chan J., Orme I. M., Porcelli S. A., Jacobs W. R.2011. A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat. Med. 17: 1261–1268. doi: 10.1038/nm.2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vordermeier M., Gordon S. V., Hewinson R. G.2011. Mycobacterium bovis antigens for the differential diagnosis of vaccinated and infected cattle. Vet. Microbiol. 151: 8–13. doi: 10.1016/j.vetmic.2011.02.020 [DOI] [PubMed] [Google Scholar]
- 48.Waters W. R., Palmer M. V., Buddle B. M., Vordermeier H. M.2012. Bovine tuberculosis vaccine research: historical perspectives and recent advances. Vaccine 30: 2611–2622. doi: 10.1016/j.vaccine.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 49.Wedlock D. N., Denis M., Vordermeier H. M., Hewinson R. G., Buddle B. M.2007. Vaccination of cattle with Danish and Pasteur strains of Mycobacterium bovis BCG induce different levels of IFN gamma post-vaccination, but induce similar levels of protection against bovine tuberculosis. Vet. Immunol. Immunopathol. 118: 50–58. doi: 10.1016/j.vetimm.2007.04.005 [DOI] [PubMed] [Google Scholar]
- 50.Welsh M. D., Cunningham R. T., Corbett D. M., Girvin R. M., McNair J., Skuce R. A., Bryson D. G., Pollock J. M.2005. Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis. Immunology 114: 101–111. doi: 10.1111/j.1365-2567.2004.02003.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization WHO Report 2009: Global Tuberculosis Control: Surveillance, Planning, Financing. Geneva. World Health Organization; 2009 [Google Scholar]
- 52.Yuan Y., Crane D. D., Barry C. E., 3rd1996. Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial alpha-crystallin homolog. J. Bacteriol. 178: 4484–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H., Peng P., Miao S., Zhao Y., Mao F., Wang L., Bai Y., Xu Z., Wei S., Shi C.2010. Recombinant Mycobacterium smegmatis expressing an ESAT6-CFP10 fusion protein induces anti- mycobacterial immune responses and protects against Mycobacterium tuberculosis challenge in mice. Scand. J. Immunol. 72: 349–357. doi: 10.1111/j.1365-3083.2010.02448.x [DOI] [PubMed] [Google Scholar]