Abstract
Introduction
Periapical infections secondary to pulpal necrosis are associated with bacterial contamination of the pulp. Porphyromonas endodontalis, a Gram-negative organism, is considered to be a pulpal pathogen. P. gingivalis is phylogenetically related to P. endodontalis and synthesizes several classes of novel complex lipids that possess biological activity, including the capacity to promote osteoclastogenesis and osteoclast activation. The purpose of this study was to extract and characterize constituent lipids of P. endodontalis, and evaluate their capacity to promote pro-inflammatory secretory responses in the macrophage cell line, RAW 264.7, as well as their capacity to promote osteoclastogenesis and inhibit osteoblast activity.
Methods
Constituent lipids of both organisms were fractionated by HPLC and were structurally characterized using electrospray-mass spectrometry (ESI-MS) or ESI-MS/MS. The virulence potential of P. endodontalis lipids was then compared with known biologically active lipids isolated from P. gingivalis.
Results
P. endodontalis total lipids were shown to promote TNF-α secretion from RAW 264.7 cells and the serine lipid fraction appeared to account for the majority of this effect. P. endodontalis lipid preparations also increased osteoclast formation from RAW 264.7 cells but osteoblast differentiation in culture was inhibited and appeared to be dependent on TLR2 expression.
Conclusions
These effects underscore the importance of P. endodontalis lipids in promoting inflammatory and bone cell activation processes that could lead to periapical pathology.
Keywords: Osteoclastogenesis, Osteoblast, RAW 264.7 cells, P. endodontalis, Electrospray-MS
Introduction
Microorganisms isolated from infected root canals are predominantly Gram-negative and strict anaerobes (1, 2), and Porphyromonas endodontalis is frequently the dominant pathogen in necrotic root canals (3, 4). P. endodontalis is thought to contribute to immune-activated destruction of periapical tissues through the release of virulence factors including lipopolysaccharide (LPS) (5–8). However, several classes of biologically-active complex lipids, including phosphorylated dihydroceramides and serine lipids, have been shown to be produced by P. gingivalis (9–11), an organism that is phylogenetically related to P. endodontalis. Of considerable interest, the serine lipids of P. gingivalis were recently reported to engage human and mouse Toll-like receptor 2 (TLR2) (11). Furthermore, a survey of oral and intestinal organisms of the phylum Bacteroidetes demonstrated that all specimens produce serine lipids of the type recovered in P. gingivalis (11). Because of the close phylogenetic relationship between P. gingivalis and P. endodontalis, the goal of this investigation was to determine whether P. endodontalis produces the same biologically active lipids as those previously identified in P. gingivalis and to determine whether the complex lipids of P. endodontalis promote activation of inflammatory cells or osteoclasts, and whether these lipids inhibit osteoblast function as has been observed with P. gingivalis complex lipids.
Materials and Methods
Bacterial Culture and Preparation of Lipids
P. endodontalis (ATCC 35406, type strain) and P. gingivalis (ATCC 33277, type strain) were grown in batch suspension culture under anaerobic conditions as previously described (9). Bacteria were cultured in Brain Heart Infusion broth under anaerobic conditions and culture purity was confirmed by demonstrating uniform colony formation on blood agar plates. P. endodontalis did not grow on blood agar plates when cultured under aerobic conditions. Gram stain of P. endodontalis samples revealed Gram-negative pleomorphic rods. P. endodontalis and P. gingivalis lipids were extracted using the procedure of Bligh and Dyer (12) and Garbus (13). P. endodontalis and P. gingivalis lipid extracts were fractionated by high-performance liquid chromatography (HPLC) as previously described (9). Replicate HPLC fractionations were pooled and evaluated using electrospray-mass spectrometry (ESI-MS). ESI-MS analysis of lipid fractions was accomplished using a Micromass Quattro II mass spectrometer system (9, 10). The fractions with specific lipid components were pooled and tested for biological activity as described below.
Assessment of Osteoclast Maturation induced by Lipid Fractions
Osteoclast-like cells (OCL) were differentiated from RAW 264.7 cells (American Type Culture collection, Rockville, MD). RAW 264.7 cells were plated at a density of 20,000 cells/well in 24 well plates in alpha modified Eagle medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, California). Cultures of RAW 264.7 derived osteoclasts were treated with the total lipid extracts of either P. endodontalis or P. gingivalis. The bacterial complex lipids were dispersed in culture medium by sonication as described below. After 7 days of culture, cytochemical staining of TRAP-positive cells was performed (14). TRAP-positive cells appeared dark red. Only TRAP-positive cells with more than 3 nuclei were counted as osteoclasts.
RNA Extraction, Quantification, and Reverse Transcription
Total RNA was extracted using TRIZOL reagent (Invitrogen) and phenol/chloroform according to the manufacturer’s instructions. RNA was dissolved in Tris-EDTA (TE), pH 7.4 and the concentration of RNA was determined by measuring absorbance at 260 nm. The concentration of extracted RNA was adjusted to 0.5 µg/µl-1 µg/µl. RNA was treated with DNAse I (Invitrogen) for 15 minutes followed by DNase I inactivation with 25 mM EDTA at 65°C to remove genomic DNA contamination. Reverse transcription was carried out in a 20 µl volume containing about 3 µg of RNA, 1µl of 50 ng/µl random hexamers and 1 µl annealing buffer, 10 µl 2X first-strand reaction mix and 2 µl superscript III/RNase OUT enzyme mix (Invitrogen) at 25°C for 10 minutes and then at 50°C for 50 minutes.
Quantitative Real-Time RT-PCR
Taqman real-time PCR was performed from 1 µl of cDNA using TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) with 100-nM primers and a 50-nM probe. The Taqman Real-time RT-PCR was performed on a Taqman ABI 7500 sequence Detection System (ABSciex). Unlabeled specific primers and the TaqMan MGB probes (6-FAM dye-labeled) for detecting the mouse TRAP gene (Assay ID: Mm00475698 m1); calcitonin receptor gene (Assay ID: Mm00432271 m1) and cathepsin K gene (Assay ID: Mm00484036 m1) were used. A Taqman eukaryotic 18S endogenous control kit was used for housekeeping gene control. Cycling conditions were an initial hold of 2 minutes at 50°C and 10 minutes at 95°C, then the samples were cycled 40 times at 95°C for 15 seconds and 60°C for 1 minute. Each sample was assayed in triplicate.
Preparation of Primary Cultures of Mouse Osteoblasts and Lipid Treatment
Female C57BL/6 (WT) mice were purchased from Jackson Labs (Bar Harbor, ME). Toll-like receptor knockout (TLR2−/−) mice, bred onto a C57BL/6 background, were a generous gift of Dr. S. Akira (Osaka University, Japan) (15). All mice were bred in accordance with University of Connecticut Center for Laboratory Animal Care regulations. Mouse calvaria harvested from 5–7 day old wild-type and TLR2−/− mice were digested with collagenase and trypsin. Calvarial osteoblasts were plated at a cell density of 1.5 × 104/cm2. For the first week, the cells were differentiated in Dulbecco’s minimal essential medium (DMEM). Differentiation in the second and third weeks took place in α-MEM that contained ascorbic acid and β-glycerol phosphate. Treatment groups were divided into vehicle control, 1.2 µg/ml of P. endodontalis total lipid extract and 1.2 µg/ml of P. gingivalis total lipid extract in the form of sonicated liposomes (3 watts for 90 seconds). Lipids were added to osteoblast cultures at 3 day intervals for the second and third weeks of culture based on the previous characterization of P. gingivalis lipid effects on osteoblasts (16). At 21 days, the culture medium was removed and osteoblast differentiation was evaluated by staining for alkaline phosphatase production as well as von Kossa staining for mineral deposition (16).
Preparation and Evaluation of Monocyte Cytokine Production
Monocyte Raw 264.7 cells were plated at a density of 20,000 cells/well and were treated with either vehicle control, 10 µg/ml of P. endodontalis total lipid extract or 10 µg/ml of P. gingivalis total lipid extract in the form of sonicated liposomes. After 24 hours, media samples were collected and analyzed for tumor necrosis factor-alpha (TNF-α) production via ELISA analysis (Duo systems, R&D systems). Specific fractions of P. endodontalis lipids were evaluated in parallel with the total lipid extracts (see below).
Statistical Analysis
Statistical tests included one-factor ANOVA comparing differences between culture treatment groups. Fisher PLSD or Scheffe F-tests were used to evaluate for significant differences between specific pairs of treatment categories. Quantitative data included TRAP-positive multinucleated cell number, PCR gene expression changes and TNF-α secretion. A p value of less than 0.05 was considered significant for statistical comparisons.
Results
Analysis and Comparison of P. endodontalis Lipids with P. gingivalis Lipids
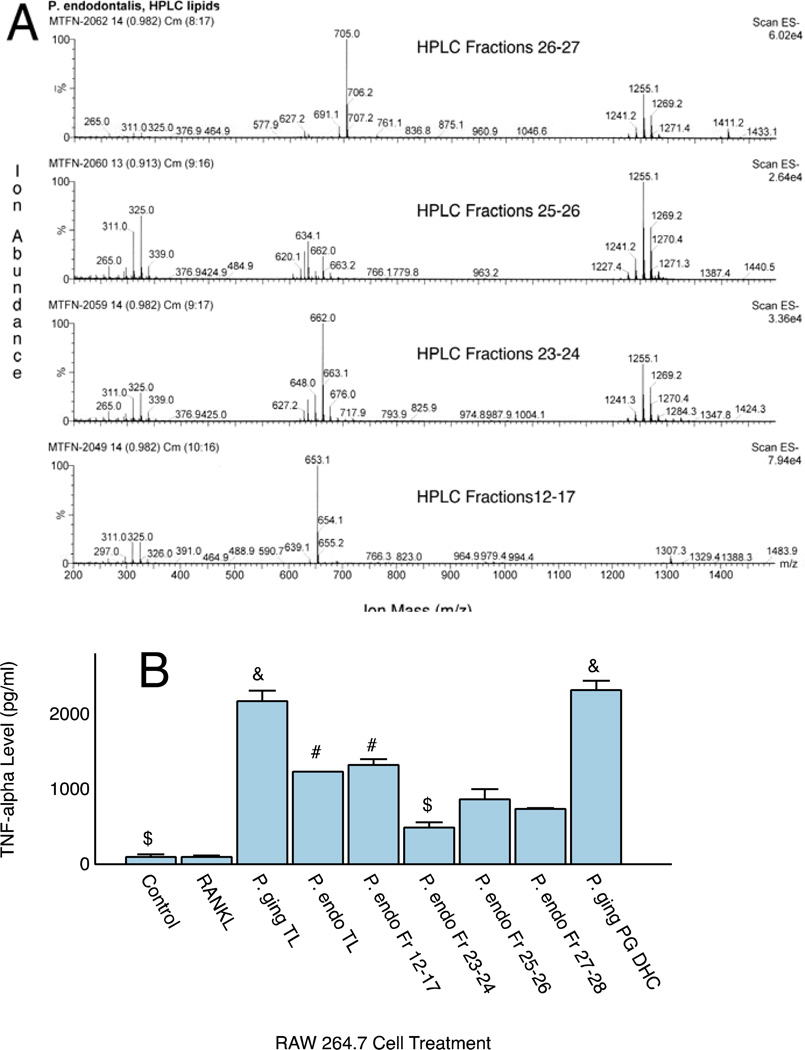
Evaluation of lipid fractions using ESI-MS revealed that P. endodontalis produces most of the complex dihydroceramide lipids that had previously been identified in P. gingivalis, (data not shown). However, the substituted and unsubstituted phosphoglycerol dihydroceramides of P. gingivalis were not observed in the P. endodontalis total lipid extract. Phosphoethanolamine dihydroceramides (PE DHC lipids) and phosphatidylethanolamine phospholipids (PEA lipids) of P. endodontalis were comparable in mass to the same lipid classes previously observed in P. gingivalis (9, 10). Other complex lipids of P. gingivalis were also recovered in P. endodontalis, including the recently characterized serine lipid termed “Lipid 654” (11). Of note, Figure 1A shows that the total lipid extracts or HPLC fractionated lipids of P. endodontalis did not contain common lipid A components characteristic of P. gingivalis LPS (17).
Figure 1.
Mass spectra of pooled HPLC fractions of P. endodontalis lipids and TNF-α Secretory Responses. HPLC fractions of the total lipid extract were pooled based on recovery of specific lipid classes as determined by ESI-MS (Figure 1A). Mass spectra of these pooled HPLC fractions were acquired using negative ion mode ESI-MS as described in the Materials and Methods. HPLC Fractions 12–17 contained the recently reported serine lipid class of P. endodontalis (Lipid 654, centered around m/z 653), HPLC Fractions 23–24 contained mostly PEA lipids (m/z 662 and 648, (10)), HPLC Fractions 25–26 contained mostly high mass lipids of unknown structure (centered around m/z 1255) and HPLC Fractions 27–28 contained mostly PE DHC lipids (m/z 705 and 691, (9)). Figure 1B shows the effect of total lipid extracts from P. gingivalis and P. endodontalis and selected lipid fractions on TNF-α secretion from RAW 264.7 cells in culture. Lipids were sonicated in culture medium at 10 µg/ml and cells were treated for 24 hours with the indicated lipid fractions. TNF-α quantitification was performed with a commercially available ELISA as described in the Methods section. Results are expressed as the mean ± SE (n=3). One factor ANOVA revealed significant differences for all lipid preparations versus controls or RANKL treated cultures (p<0.05 by post hoc pairwise comparisons), &P. gingivalis total lipid (TL) was not significantly different from the P. gingivalis PG DHC lipid, #P. endodontalis total lipid was not significantly different from Fractions 12–17, $P. endodontalis pooled Fraction 23–24 was not significantly different from controls. Control and RANKL treated cultures were not significantly different.
The total lipids of P. endodontalis were fractionated by HPLC and replicate HPLC fractions were pooled by lipid class (Figure 1A) as described in the Figure Legend. Next we examined whether the total lipid extract or specific HPLC lipid fractions from P. endodontalis stimulated TNF-α secretion from macrophages (Figure 1B). RAW 264.7 cells were treated with the medium preparations shown in Figure 1B. TNF-α production by RAW cells was significantly increased with total lipid extracts of P. endodontalis or P. gingivalis when compared with controls or cultures treated with 10 ng/ml of RANKL. Note that the HPLC Fraction 12–17 lipids of P. endodontalis stimulated TNF-α secretion to the same extent as the total lipid extract suggesting that the majority of the stimulatory activity can be attributed to the serine lipid preparation of P. endodontalis. Although the substituted PG DHC lipids of P. gingivalis stimulated TNF-α secretion to the same extent as the total lipid extract, P. endodontalis does not synthesize the PG DHC lipids previously identified in P. gingivalis (9). Therefore, the PG DHC lipids cannot account for the TNF-α responses shown with P. endodontalis lipid preparations.
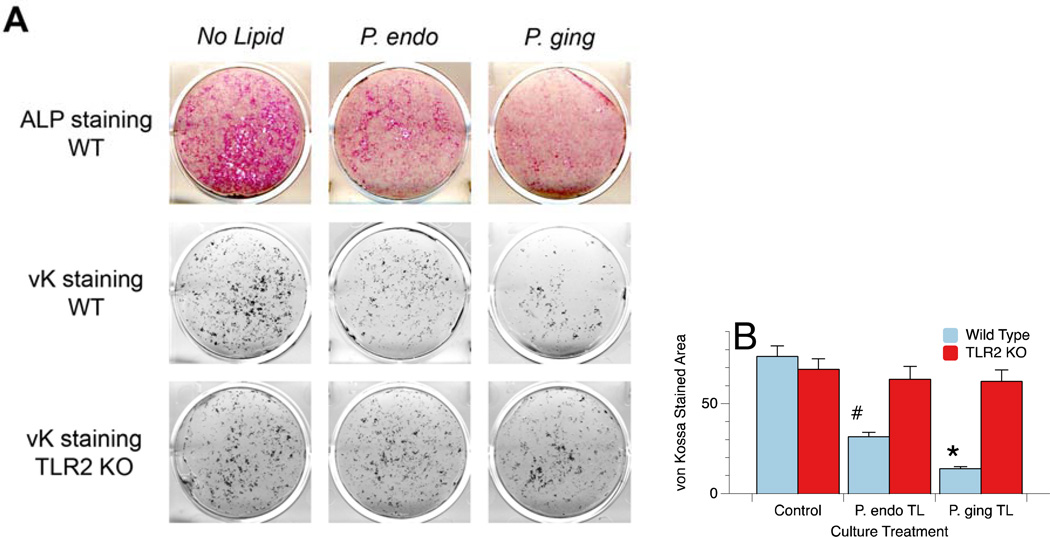
P. endodontalis Lipids Inhibit Osteoblast Differentiation and Mineralization Deposition in vitro
Effects of P. endodontalis lipids on osteoblast maturation and mineralization deposition were determined using alkaline phosphatase staining and formation of mineralization nodules by von Kossa (vK) staining, respectively. Figure 2A shows the decrease in alkaline phosphatase (ALP) expression as well as the diminished mineral deposition (von Kossa stained mineral deposits) by osteoblasts treated with 1.2µg/ml of P. endodontalis or P. gingivalis total lipid extracts for days 7 through 21. Quantification of von Kossa staining using ImageJ software revealed the results shown in Figure 2B. Note that the von Kossa stained mineral deposits were significantly decreased in the osteoblast cultures derived from wild type mice but not TLR2−/− mice. These results indicate that the total lipid extracts of P. endodontalis and P. gingivalis mediate their effects on osteoblasts through engagement of TLR2.
Figure 2.
Wild type (WT) and TLR2−/− (KO) osteoblasts treated with total lipid extracts from P. endodontalis or P. gingivalis and stained for alkaline phosphatase (ALP) or mineralization nodule formation by von Kossa (vK) staining (Figure 2A). Osteoblasts were treated with 1.2µg/ml of P. endodontalis or P. gingivalis total lipid extracts for days 7 through 21. Note that the total lipid extract of both P. endodontalis (P. endo) and P. gingivalis (P. ging) inhibit alkaline phosphatase expression and von Kossa stained mineral deposition in vitro, and the mineral deposition may be TLR2 dependent as has been previously reported for P. gingivalis total lipids (16). Quantitation of the von Kossa (vK) stained mineral deposits was accomplished using ImageJ processing and revealed that in WT cultures, the area of vK staining was significantly less in both P. endo total lipid (TL, #p<0.002) and P. ging TL (*p<0.001) treated osteoblasts cultures (Figure 2B). In contrast, mineral deposition was not significantly affected in osteoblasts derived from TLR2−/− animals.
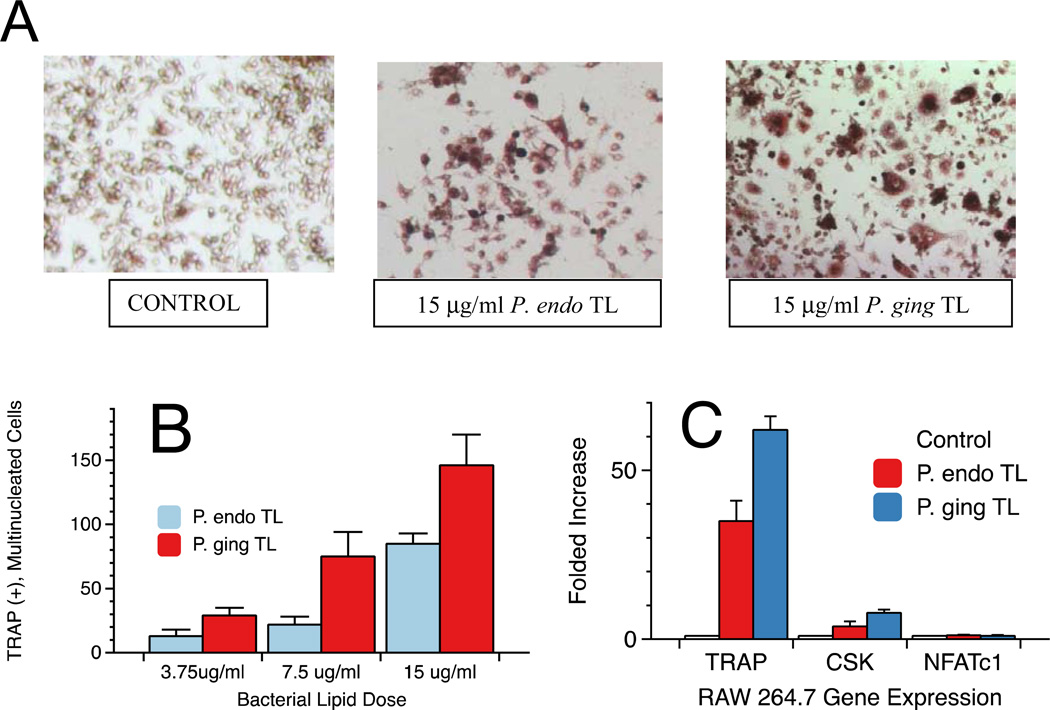
P. endodontalis Lipids Induce Osteoclast-like Cell Formation
Next we evaluated the effect of P. endodontalis total lipid extract on osteoclast cell differentiation and maturation from RAW 264.7 cells. RAW 264.7 cells were plated and treated with control medium, P. endodontalis total lipids or P. gingivalis total lipids for a period of 7 days. TRAP+ cells were visualized by microscopy as shown in Figure 3A. Additional cultures were treated with Receptor activator of nuclear factor kappa-B ligand (RANKL) as positive controls (results not shown). TRAP(+), multinucleated giant cells were visualized by microscopy and were counted in triplicate culture wells. This assessment demonstrated a dose-dependent increase in TRAP-positive osteoclast cells when RAW cells were exposed to increasing concentrations of P. endodontalis or P. gingivalis total lipid extracts (Figure 3B). These dose responses are statistically significant when compared by ANOVA (p<0.05). Next, RNA was isolated from RAW 264.7 cells after exposure to bacterial lipids for 7 days and upregulation of osteoclast-specific genes, including TRAP, cathepsin K (CSK) and NFATc1, were evaluated. Quantitative real-time RT-PCR showed significant increases (p<0.05) in TRAP and cathepsin K gene expression in osteoclasts exposed to total lipid extracts of P. endodontalis or P. gingivalis (Figure 3C). However, NFATc1 upregulation in RAW 264.7 cells was not observed with either total lipid extract.
Figure 3.
Osteoclast formation from RAW 264.7 cells following exposure to total lipids of either P. endodontalis or P. gingivalis. Bacterial lipid effects on the formation of TRAP positive (+), multinucleated cells in cultures were evaluated following treatment with total lipid extracts from either P. endodontalis or P. gingivalis. Bacterial lipids at the indicated concentrations were sonicated in medium before addition to cell cultures. Cells were exposed to lipids for 7 days before staining and microscopic visualization of osteoclasts. Osteoclast formation varied depending on lipid treatment as shown in Figure 3A. TRAP(+), multinucleated giant cells in culture wells were counted and averaged for three wells (Figure 3B). The values were expressed as the mean ± standard error (SE) of triplicate cultures. Control cultures showed no TRAP(+), multinucleated cells. By one factor ANOVA, the number of TRAP(+), multinucleated cells was significantly increased with higher doses of each total lipid preparation when compared with the respective lower doses (p<0.05). Osteoclast-specific gene expression was evaluated in RAW 264.7 cells exposed to 15 µg/ml of P. endodontalis or P. gingivalis total lipids cultured for seven days (Figure 3C). RNA was extracted as described in the Materials and Methods and gene expression quantified by RT-PCR. Each sample was assayed in triplicate.
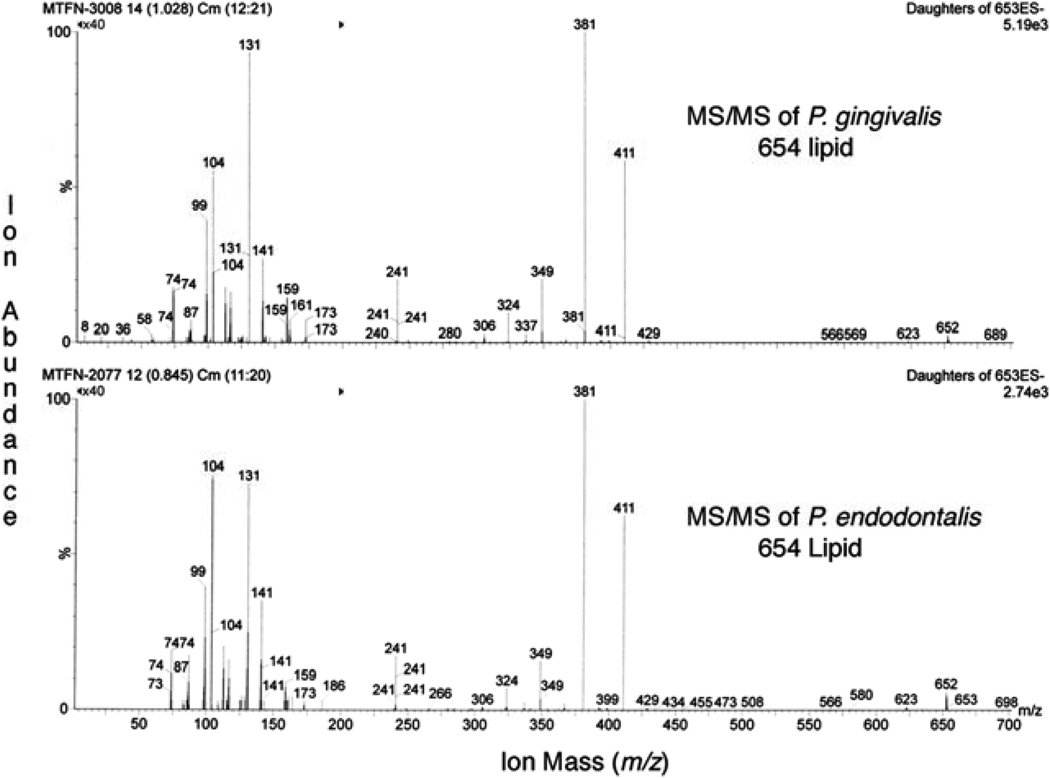
ESI-MS/MS of P. endodontalis and P. gingivalis serine lipids
The serine lipid preparations of P. gingivalis and P. endodonalis were evaluated by electrospray ionization (ESI)-MS/MS using the negative ion mode. The MS/MS spectra demonstrate essentially identical product ions for serine lipid preparations (Lipid 654) from P. gingivalis and P. endodonalis thus confirming identity of the major structural moieties of these serine lipid preparations (see Figure 4).
Figure 4.
MS/MS spectra of serine lipids of P. gingivalis and P. endodontalis. Lipids were extracted and fractionated as described in the Materials and Methods. Highly enriched fractions of the respective serine lipids were analyzed by MS/MS by setting the precursor negative ion to m/z 653 and monitoring product ions from m/z 50 to 700. Note that both MS/MS spectra are essentially identical and are consistent with the previously reported serine lipid, Lipid 654 (11).
Discussion
This study confirmed the presence of unusual complex lipid constituents in P. endodontalis, namely phosphorylated dihydroceramide and serine lipids, that have previously been identified in P. gingivalis (9, 11). We noted that both species of Porphyromonas produce phosphoethanolamine dihydroceramide and phosphatidylethanolamine lipids but also noted the absence of phosphoglycerol dihydroceramide (PGDHC) lipids in P. endodontalis (data not shown). Though the PG DHC lipids of P. gingivalis possess biological activity (see Figure 1B) (9), the absence of this lipid class in P. endodontalis suggests that PG DHC lipids are not participating in the biological responses observed in RAW 264.7 cells or osteoblasts following treatment with P. endodontalis lipid preparations. The presence of PG DHC within P. gingivalis total lipid extracts may account for greater biologic activity observed with P. gingivalis total lipids when compared with P. endodontalis total lipids on osteoblast and osteoclast maturation as well as monocyte TNF-α production.
Serine lipids are recovered in lipid extracts of subgingival calculus and subgingival plaque (data not shown), and from chronic periodontitis sites and human serum samples (11). Though serine lipids as well as phosphorylated dihydroceramide lipids of P. gingivalis are recovered in lipid extracts of subgingival plaque from periodontitis sites, these lipids are more prevalent in lipid extracts of calculus-contaminated teeth (18) suggesting that bacterial lipid adsorption to dental mineralized surfaces is an important consequence of plaque accumulation. Preliminary work has also shown that serine lipids are prevalent in tissue samples recovered from necrotic pulp canals (data not shown). The serine lipids of P. gingivalis (termed “Lipid 654”) were recently shown to mediate responses in cells expressing either mouse or human TLR2 but do not engage TLR4 (11). Engagement of TLR4 is normally attributed to LPS and although P. gingivalis LPS has been reported to engage both TLR2 and TLR4, we have recently shown that P. gingivalis LPS is contaminated with serine lipids which could account for the reported engagement of TLR2 (11). P. gingivalis lipid effects on osteoblast differentiation were also shown to be mediated through TLR2 (16). Our results show that the total lipid extract of P. endodontalis mediates its inhibitory effects on osteoblasts through engagement of TLR2 and this may be attributed to the serine lipid class of P. endodontalis. Future work will clarify this possibility.
NFATc1 is a transcription factor gene induced by RANKL whose presence has been shown to be essential for osteoclastogenesis (19). Our findings suggest that the total lipids of P. endodontalis stimulate osteoclast differentiation and maturation in a manner that is partially independent of the RANKL-stimulated osteoclastogenesis pathway. We noted that the total lipid extract of P. endodontalis does not promote NFATc1 expression in RAW 264.7 cells yet shows the ability to increase the expression of TRAP and Cathepsin K (Figure 5) and to promote osteoclastogenesis (Figure 3). Therefore, NFATc1 expression in RAW 264.7 cells may not be an absolute requirement for osteoclast-like cell formation in culture.
In summary, we have demonstrated that P. endodontalis produces biologically active lipids with the capacity to promote proinflammatory secretory responses and osteoclastogenesis, and yet inhibit osteoblast function. Furthermore, these effects appear to be mediated through engagement of TLR2 as has been shown for P. gingivalis serine lipids (11). The lipid class of P. endodontalis that appears to be most strongly associated with pro-inflammatory reactions is the serine lipid class (Lipid 654) which by mass spectrometric analysis is identical in structure to that produced by P. gingivalis. Future work will clarify which lipids of P. endodontalis produce the most significant deleterious effects to host tissues and will provide additional evidence demonstrating the complex relationship between bacteria, their byproducts and the destructive host immune response that ultimately result in apical periodontitis.
Acknowledgements
We thank Mr. Marvin Thompson of the University of Connecticut Department of Chemistry for his assistance in the mass spectrometric analysis. This research was supported in part by a grant from the American Association of Endodontists Foundation and the NIH grant R01 DE021055-01A1 (FCN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors deny any conflicts of interest.
References
- 1.Fabricius L, Dahlen G, Ohman AE, Moller AJ. Predominant indigenous oral bacteria isolated from infected root canals after varied times of closure. Scand J Dent Res. 1982;90(2):134–144. doi: 10.1111/j.1600-0722.1982.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 2.Farber PA, Seltzer S. Endodontic microbiology. I. Etiology. J Endod. 1988;14(7):363–371. doi: 10.1016/S0099-2399(88)80200-0. [DOI] [PubMed] [Google Scholar]
- 3.Sakamoto M, Rocas IN, Siqueira JF, Jr, Benno Y. Molecular analysis of bacteria in asymptomatic and symptomatic endodontic infections. Oral Microbiol Immunol. 2006;21(2):112–122. doi: 10.1111/j.1399-302X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 4.van Winkelhoff AJ, van Steenbergen TJ, de Graaff J. Porphyromonas (Bacteroides) endodontalis: its role in endodontal infections. J Endod. 1992;18(9):431–434. doi: 10.1016/s0099-2399(06)80843-5. [DOI] [PubMed] [Google Scholar]
- 5.Hosoya S, Matsushima K. Stimulation of interleukin-1 beta production of human dental pulp cells by Porphyromonas endodontalis lipopolysaccharide. J Endod. 1997;23(1):39–42. doi: 10.1016/S0099-2399(97)80205-1. [DOI] [PubMed] [Google Scholar]
- 6.Ko HJ, Lim SS. Production of macrophage inflammatory protein (MIP)-1alpha and MIP-1beta by human polymorphonuclear neutrophils stimulated with Porphyromonas endodontalis lipopolysaccharide. J Endod. 2002;28(11):754–757. doi: 10.1097/00004770-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Ogura N, Shibata Y, Kamino Y, Matsuda U, Hayakawa M, Oikawa T, et al. Stimulation of interleukin-6 production of periodontal ligament cells by Porphyromonas endodontalis lipopolysaccharide. Biochem Med Metab Biol. 1994;53(2):130–136. doi: 10.1006/bmmb.1994.1068. [DOI] [PubMed] [Google Scholar]
- 8.Tang Y, Sun F, Li X, Zhou Y, Yin S, Zhou X. Porphyromonas endodontalis lipopolysaccharides induce RANKL by mouse osteoblast in a way different from that of Escherichia coli lipopolysaccharide. J Endod. 2011;37(12):1653–1658. doi: 10.1016/j.joen.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Nichols FC, Riep B, Mun J, Morton MD, Bojarski MT, Dewhirst FE, et al. Structures and biological activity of phosphorylated dihydroceramides of Porphyromonas gingivalis. J Lipid Res. 2004;45(12):2317–2330. doi: 10.1194/jlr.M400278-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Nichols FC, Riep B, Mun J, Morton MD, Kawai T, Dewhirst FE, et al. Structures and biological activities of novel phosphatidylethanolamine lipids of Porphyromonas gingivalis. J Lipid Res. 2006;47(4):844–853. doi: 10.1194/jlr.M500542-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Clark RB, Cervantes JL, Maciejewski MW, Farrokhi V, Nemati R, Yao X, et al. Serine Lipids of Porphyromonas gingivalis Are Human and Mouse Toll-Like Receptor 2 Ligands. Infect Immun. 2013;81(9):3479–3489. doi: 10.1128/IAI.00803-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 13.Garbus J, DeLuca HF, Loomas ME, Strong FM. Rapid incorporation of phosphate into mitochondrial lipids. J Biol Chem. 1968;238:59–63. [PubMed] [Google Scholar]
- 14.Holliday LS, Welgus HG, Hanna J, Lee BS, Lu M, Jeffrey JJ, et al. Interstitial collagenase activity stimulates the formation of actin rings and ruffled membranes in mouse marrow osteoclasts. Calcif Tissue Int. 2003;72(3):206–214. doi: 10.1007/s00223-002-1008-7. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 16.Wang YH, Jiang J, Zhu Q, Alanezi AZ, Clark RB, Jiang X, et al. Porphyromonas gingivalis Lipids Inhibit Osteoblastic Differentiation and Function. Infect Immun. 2010;78(9):3726–3735. doi: 10.1128/IAI.00225-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols FC, Bajrami B, Clark RB, Housley W, Yao X. Free lipid A isolated from Porphyromonas gingivalis lipopolysaccharide is contaminated with phosphorylated dihydroceramide lipids: recovery in diseased dental samples. Infect Immun. 2012;80(2):860–874. doi: 10.1128/IAI.06180-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols FC, Rojanasomsith K. Porphyromonas gingivalis lipids and diseased dental tissues. Oral Microbiol Immunol. 2006;21(2):84–92. doi: 10.1111/j.1399-302X.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- 19.Negishi-Koga T, Takayanagi H. Ca2+−NFATc1 signaling is an essential axis of osteoclast differentiation. Immunological reviews. 2009;231(1):241–256. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]