Abstract
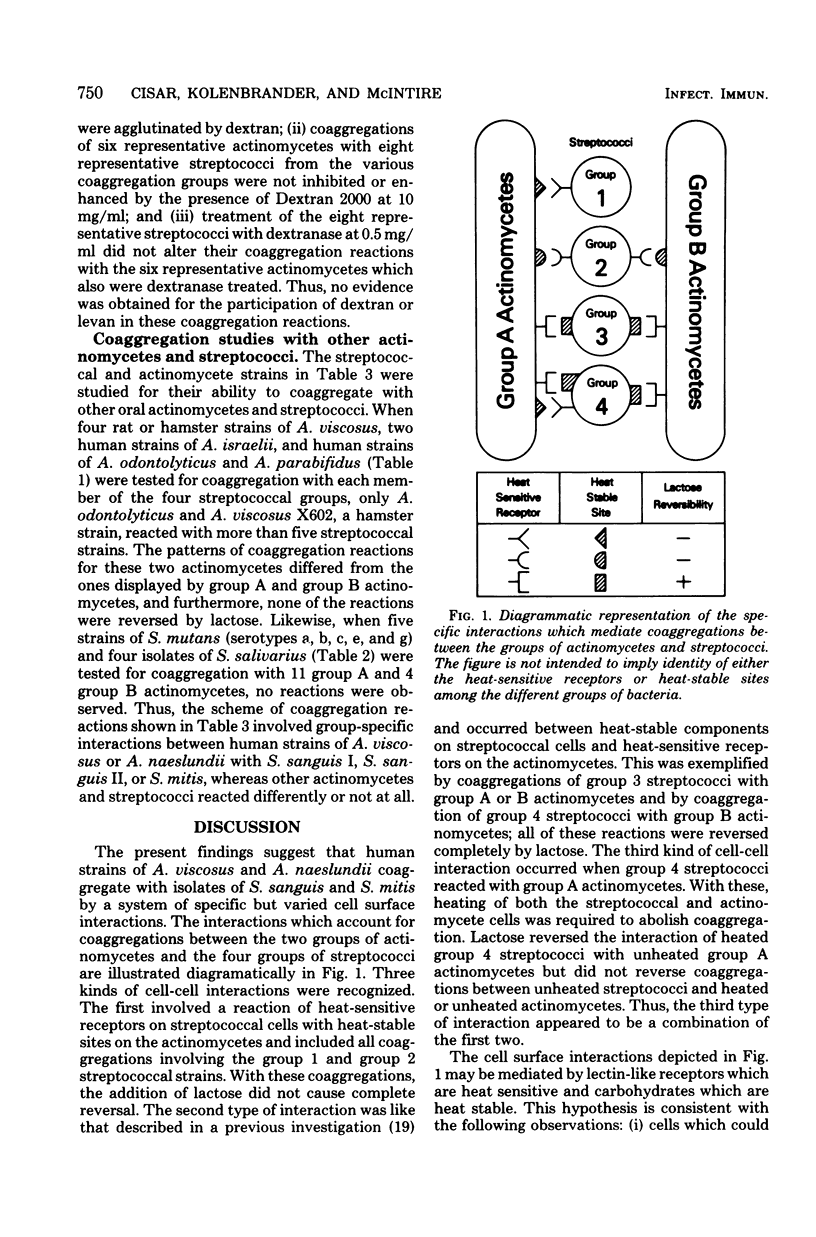
Coaggregation reactions between actinomycete and streptococcal cells occurred frequently when human strains of Actinomyces viscosus or A. naeslundii were mixed with human isolates of Streptococcus sanguis or S. mitis, but were infrequent with other oral actinomycetes and streptococci. Two groups of actinomycetes and four groups of streptococci were defined by the patterns of their coaggregation reactions and by the ability of beta-linked galactosides (i.e., lactose) to reverse these reactions. Coaggregations occurred by one of the following three kinds to cell-cell interactions: (i) coaggregation that was blocked by heating the streptococcus but not the actinomycete and was not reversed by lactose; (ii) coaggregation that was blocked by heating the actinomycete but not the streptococcus and was reversed by lactose; and (iii) coaggregation that was blocked only by heating both cell types. The latter reaction was a combination of the first two since lactose reversed coaggregation between heated streptococci and unheated actinomycetes but did not reverse coaggregations between unheated streptococci and heated actinomycetes. Cells that could be heat inactivated also were inactivated by amino group acetylation or protease digestion, whereas cells that were unaffected by heat were not inactivated by these treatments. Coaggregation reactions of each kind were Ca2+ dependent and insensitive to dextranase treatment. These findings are consistent with the hypothesis that human strains of A. viscosus and A. naeslundii coaggregate with strains of S. sanguis and S. mitis by a system of specific cell surface interactions between protein or glycoprotein receptors on one cell type and carbohydrates on the other type.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgeau G., McBride B. C. Dextran-mediated interbacterial aggregation between dextran-synthesizing streptococci and Actinomyces viscosus. Infect Immun. 1976 Apr;13(4):1228–1234. doi: 10.1128/iai.13.4.1228-1234.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden G. H., Hardie J. M. Commensal and pathogenic Actinomyces species in man. Soc Appl Bacteriol Symp Ser. 1973 Jan;2:277–299. [PubMed] [Google Scholar]
- Boyd H., Leach S. J., Milligan B. N-acylsuccinimides as acylating agents for proteins: the selective acylation of lysine residues. Int J Pept Protein Res. 1972;4(2):117–122. doi: 10.1111/j.1399-3011.1972.tb03407.x. [DOI] [PubMed] [Google Scholar]
- Bratthall D. Immunofluorescent identification of Streptococcus mutans. Odontol Revy. 1972;23(2):181–196. [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G. Lactobacilli and streptococci in the mouth of children. Caries Res. 1975;9(5):333–339. doi: 10.1159/000260166. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Hamada S., Torii M. Effect of sucrose in culture media on the location of glucosyltransferase of Streptococcus mutans and cell adherence to glass surfaces. Infect Immun. 1978 Jun;20(3):592–599. doi: 10.1128/iai.20.3.592-599.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie J. M., Bowden G. H. Physiological classification of oral viridans streptococci. J Dent Res. 1976 Jan;55:A166–A176. doi: 10.1177/002203457605500108011. [DOI] [PubMed] [Google Scholar]
- Horstmeier C., Washington J. A., 2nd Microbiological study of streptococcal bacteremia. Appl Microbiol. 1973 Oct;26(4):589–591. doi: 10.1128/am.26.4.589-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast A. Comparative statistical investigations regarding incidence, etiology and topography of subacute bacterial endocarditis. Jpn Circ J. 1971 Oct;35(10):1203–1212. doi: 10.1253/jcj.35.1203. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Marucha P. T., Keyes P. H., Wittenberger C. L., London J. Rapid method for identification and enumeration of oral Actinomyces. Infect Immun. 1978 Sep;21(3):786–791. doi: 10.1128/iai.21.3.786-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanski J. H., Wittenberger C. L. Mannitol transport in Streptococcus mutans. J Bacteriol. 1975 Dec;124(3):1475–1481. doi: 10.1128/jb.124.3.1475-1481.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Bourgeau G. Dextran-induced aggregation of Actinomyces viscosus. Arch Oral Biol. 1975 Dec;20(12):837–841. doi: 10.1016/0003-9969(75)90063-1. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J. Levan and levansucrase of Actinomyces viscosus. Infect Immun. 1977 Feb;15(2):518–526. doi: 10.1128/iai.15.2.518-526.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Rosan B. Absence of glycerol teichoic acids in certain oral streptococci. Science. 1978 Sep 8;201(4359):918–920. doi: 10.1126/science.684416. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Manganiello A. D., Propas D., Oram V., van Houte J. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res. 1977 Mar;12(2):90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Theilade E., Fejerskov O., Prachyabrued W., Kilian M. Microbiologic study on developing plaque in human fissures. Scand J Dent Res. 1974;82(6):420–427. doi: 10.1111/j.1600-0722.1974.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Tinanoff N., Gross A., Brady J. M. Development of plaque on enamel. Parallel investigations. J Periodontal Res. 1976 Jul;11(4):197–209. doi: 10.1111/j.1600-0765.1976.tb00071.x. [DOI] [PubMed] [Google Scholar]



