Abstract
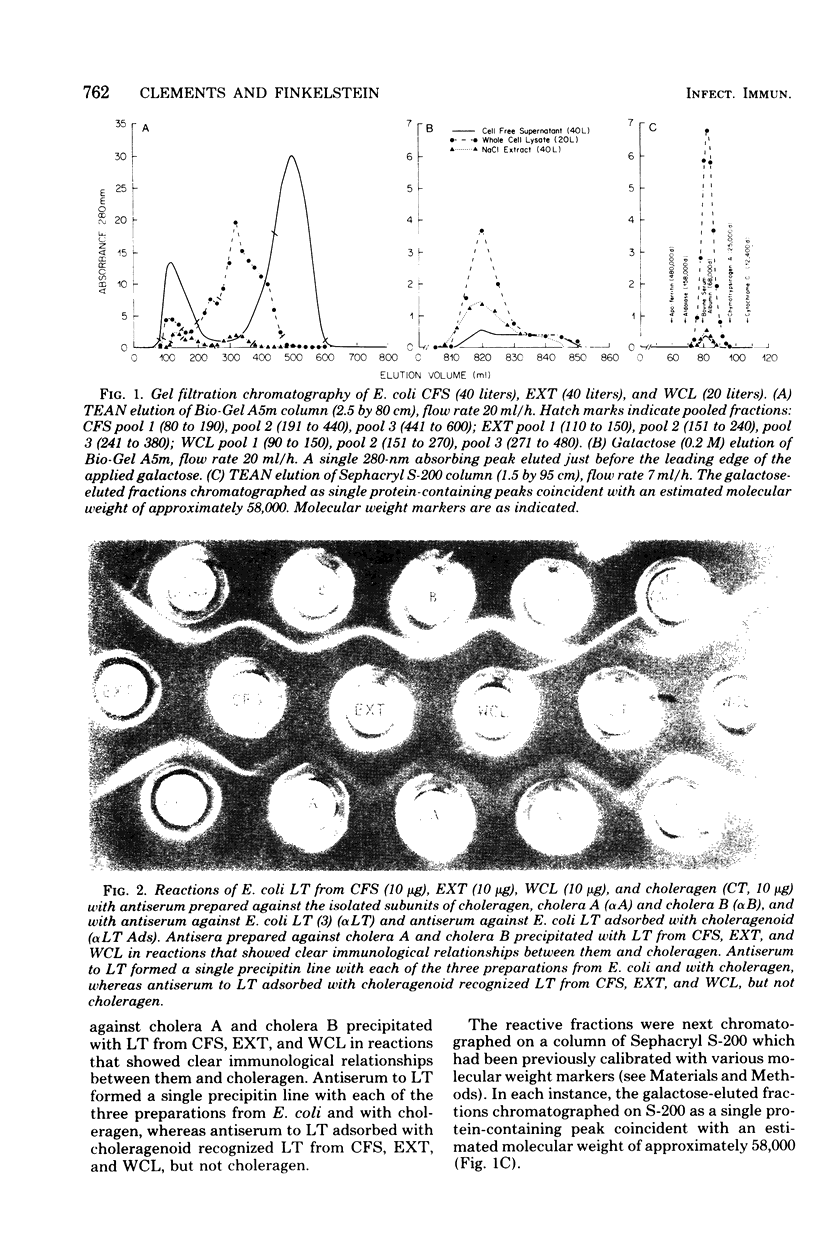
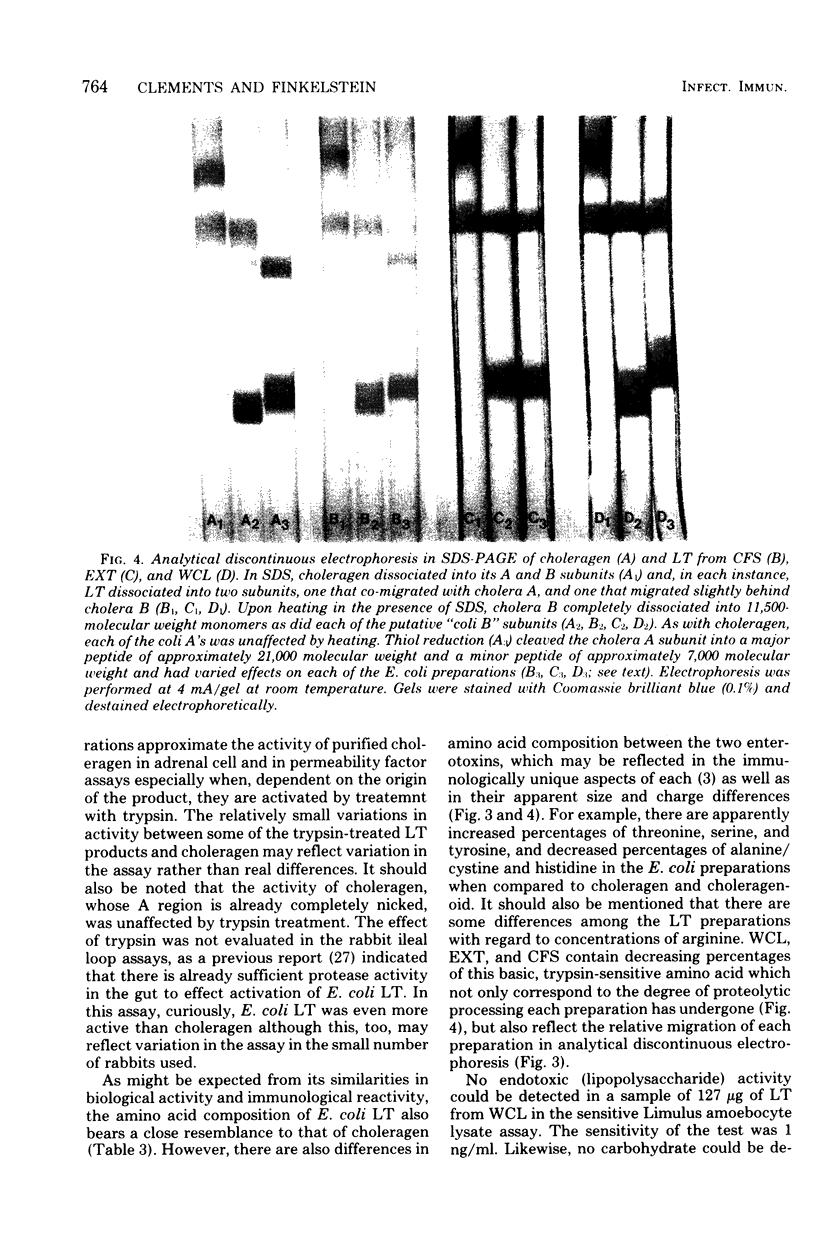
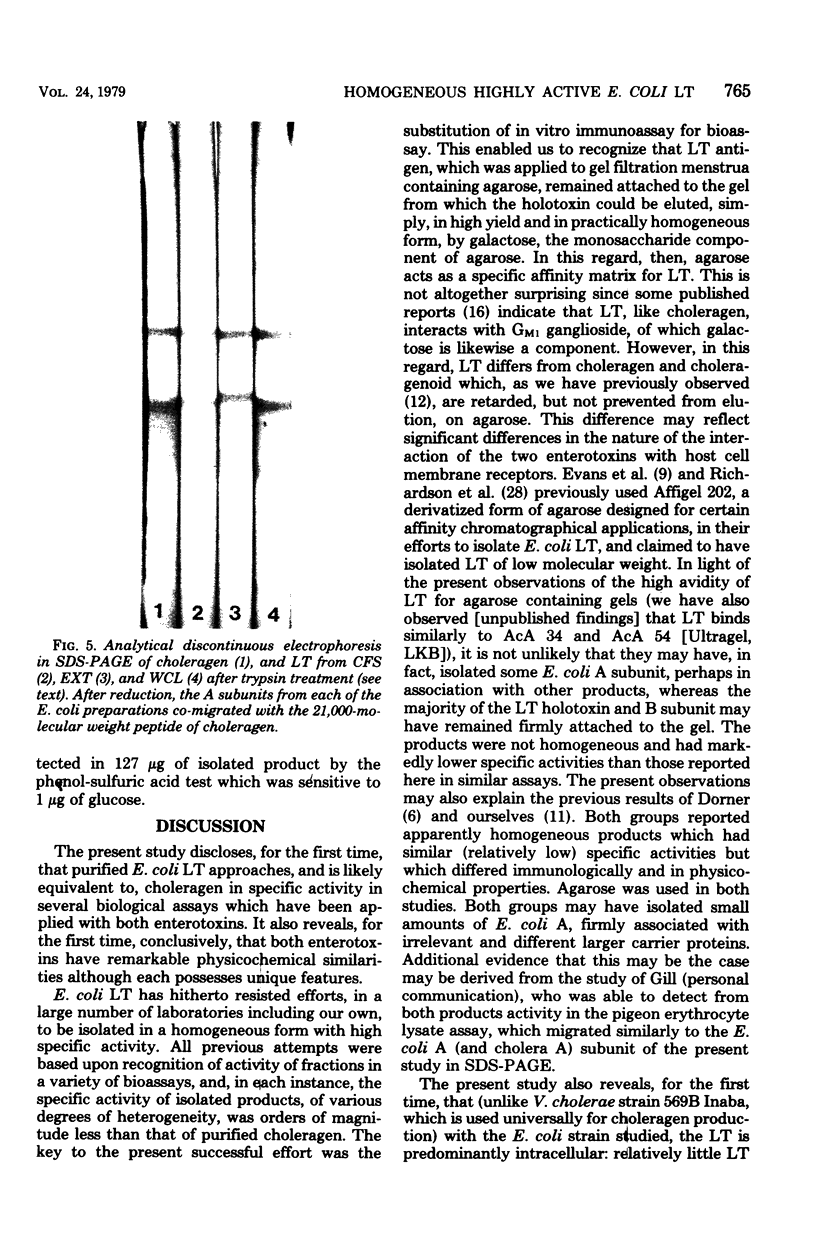
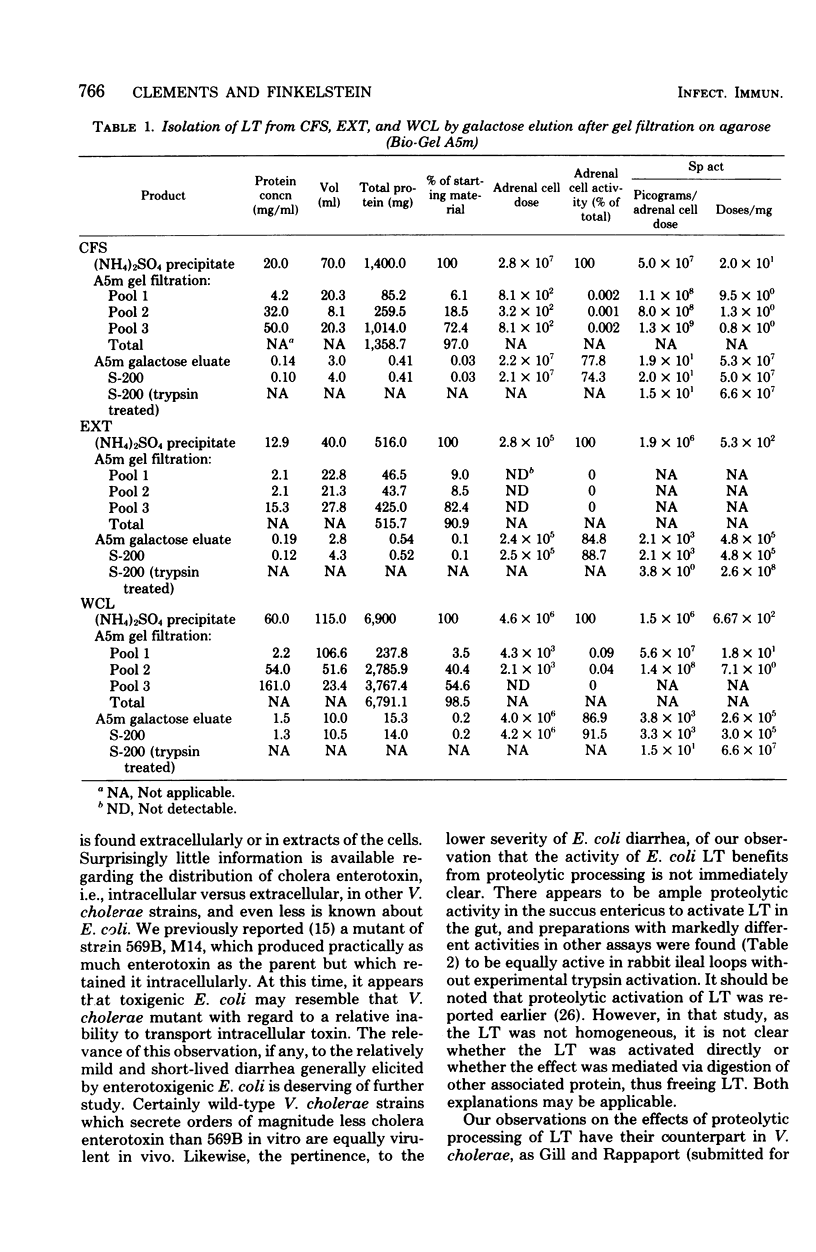
The heat-labile enterotoxin (LT) has been isolated in homogeneous form with high specific activity from three sources: cell-free supernatant, NaCl extract, and whole-cell lysates of an enterotoxigenic Escherichia coli strain. In vitro immunological assays were used in lieu of tedious and highly variable bioassays to recognize fractions with activity. This revealed that the major portion of the LT remained adherent to columns containing agarose, from which it could be eluted quantitatively in practically homogeneous form by galactose. Isolated LT has remarkable similarities to the cholera enterotoxin (choleragen) in both subunit structure and amino acid composition, although there are also notable differences in these two enterotoxins, which are related immunologically and by mode of action. Unlike choleragen, in which the A region is totally nicked, E. coli LT, depending on its source, is activated by proteolytic processing. The activity of LT is equivalent to that of choleragen in bioassays on adrenal cells, in rabbit skin, and in rabbit ileal loops, especially when, depending on the source of material, the LT has been activated by treatment with trypsin. The whole-cell lysate is the richest source of LT.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Demonstration of shared and unique immunological determinants in enterotoxins from Vibrio cholerae and Escherichia coli. Infect Immun. 1978 Dec;22(3):709–713. doi: 10.1128/iai.22.3.709-713.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Immunological cross-reactivity between a heat-labile enterotoxin(s) of Escherichia coli and subunits of Vibrio cholerae enterotoxin. Infect Immun. 1978 Sep;21(3):1036–1039. doi: 10.1128/iai.21.3.1036-1039.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafni Z., Sack R. B., Craig J. P. Purification of heat-labile enterotoxin from four Escherichia coli strains by affinity immunoadsorbent: evidence for similar subunit structure. Infect Immun. 1978 Dec;22(3):852–860. doi: 10.1128/iai.22.3.852-860.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner F. Escherichia coli enterotoxin. Purification and partial characterization. J Biol Chem. 1975 Nov 25;250(22):8712–8719. [PubMed] [Google Scholar]
- Evans D. J., Evans D. G., Gorbach S. L. Polymyxin B-Induced Release of Low-Molecular-Weight, Heat-Labile Enterotoxin from Escherichia coli. Infect Immun. 1974 Nov;10(5):1010–1017. doi: 10.1128/iai.10.5.1010-1017.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Gorbach S. L. Production of vascular permeability factor by enterotoxigenic Escherichia coli isolated from man. Infect Immun. 1973 Nov;8(5):725–730. doi: 10.1128/iai.8.5.725-730.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Vasil M. L., Finkelstein R. A. Studies on toxinogenesis in Vibrio cholerae. III. Characterization of nontoxinogenic mutants in vitro and in experimental animals. J Clin Invest. 1975 Mar;55(3):551–560. doi: 10.1172/JCI107962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973 Dec;8(6):851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T. M., Wu B. J., Braemer A. C., Bidlack D. E. Properties of the enterotoxic component in Escherichia coli enteropathogenic for swine. Infect Immun. 1973 Feb;7(2):178–189. doi: 10.1128/iai.7.2.178-189.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper D. G., Finkelstein R. A., Capra J. D. Subunit structure and N-terminal amino acid sequence of the three chains of cholera enterotoxin. Immunochemistry. 1976 Jul;13(7):605–611. doi: 10.1016/0019-2791(76)90173-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lariviére S., Gyles C. L., Barnum D. A. Preliminary characterization of the heat-labile enterotoxin of Escherichia coli F11(P155). J Infect Dis. 1973 Sep;128(3):312–320. doi: 10.1093/infdis/128.3.312. [DOI] [PubMed] [Google Scholar]
- Mitchell I. D., Tame M. J., Kenworthy R. Separation and purification of enterotoxins from a strain Escherichia coli pathogenic for pigs. J Med Microbiol. 1974 Nov;7(4):439–450. doi: 10.1099/00222615-7-4-439. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Engstrom G. W., Baetz A. L. Response of the rabbit ileal loop to cell-free products from Escherichia coli enteropathogenic for swine. J Infect Dis. 1970 Feb;121(2):182–187. doi: 10.1093/infdis/121.2.182. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Wallace C. K. Stimulation of jejunal secretion by a crude Escherichia coli enterotixin. Gastroenterology. 1972 Sep;63(6):439–448. [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein I., Green R. F., Santos D. S., Maas W. K. Partial purification and characterization of a heat-labile enterotoxin of Escherichia coli. Infect Immun. 1976 Jun;13(6):1710–1720. doi: 10.1128/iai.13.6.1710-1720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlind O., Möllby R., Wadström T. Purification and some properties of a heat-labile enterotoxin from escherichia coli. Zentralbl Bakteriol Orig A. 1974;229(2):190–204. [PubMed] [Google Scholar]
- Vasil M. L., Holmes R. K., Finkelstein R. A. Conjugal transfer of a chromosomal gene determining production of enterotoxin in vibrio cholerae. Science. 1975 Mar 7;187(4179):849–850. doi: 10.1126/science.1114331. [DOI] [PubMed] [Google Scholar]






