Abstract
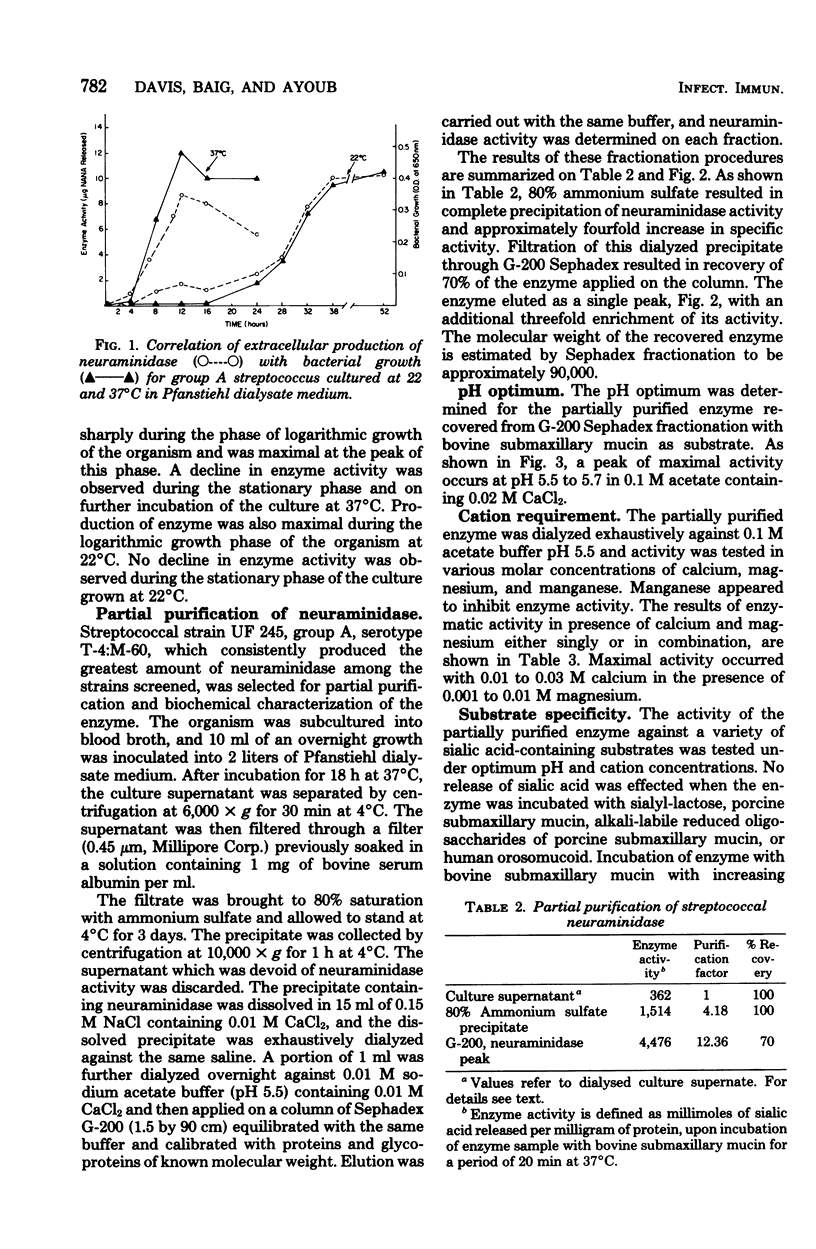
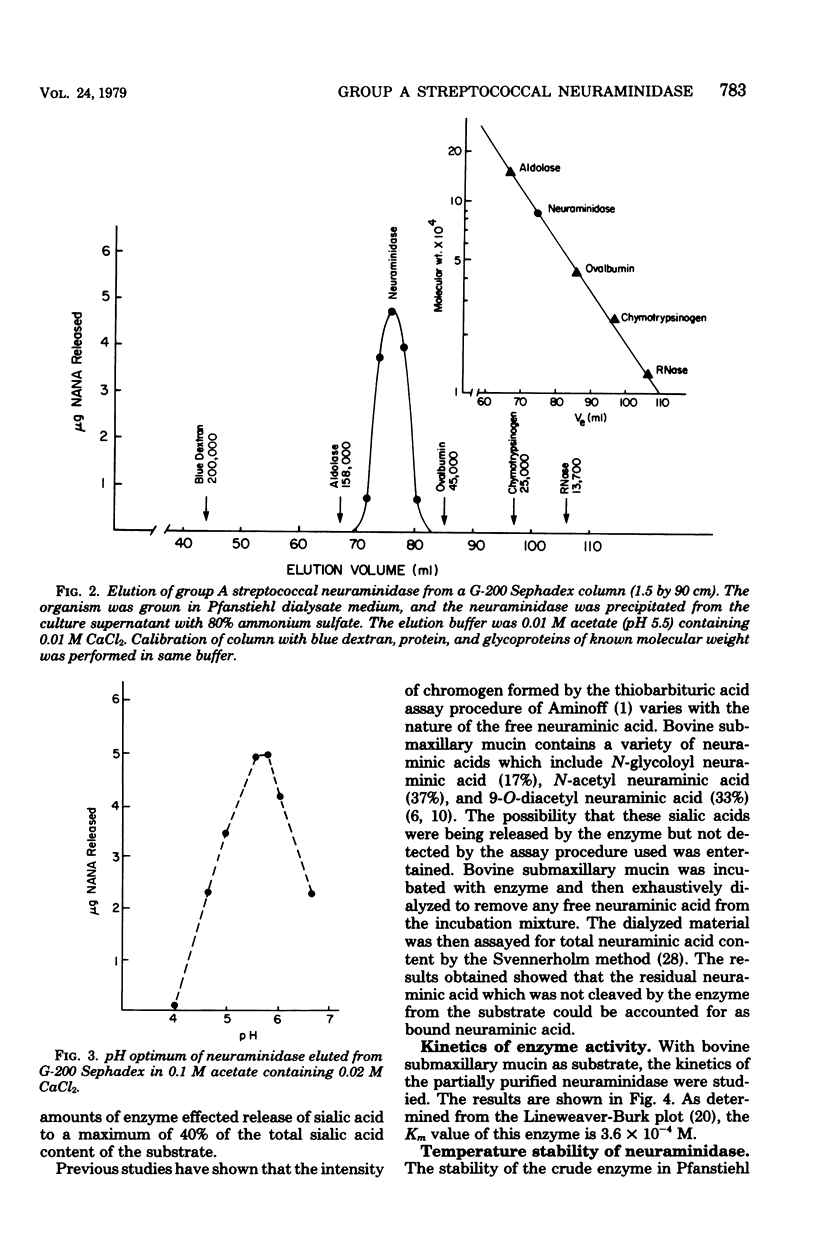
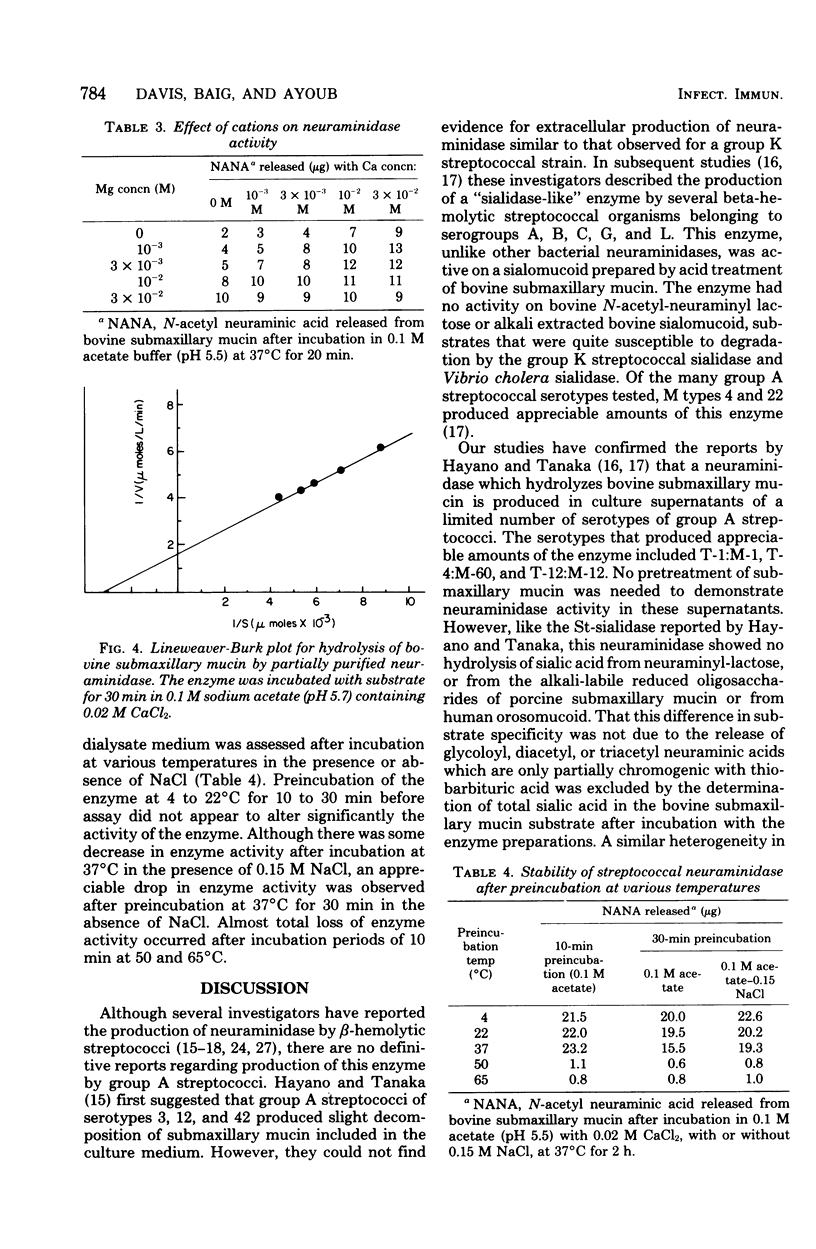
Extracellular neuraminidase production by group A streptococci was examined in 92 strains. Fourteen of these strains produced appreciable amounts of enzyme; 12 of the neuraminidase-producing strains belonged to T types 1, 4, and 12. Production of the enzyme paralleled bacterial growth in culture and was maximal in medium containing 0.2% glucose. The enzyme produced by one of these strains was partially purified by ammonium sulfate fractionation and filtration on G-200 Sephadex. Its molecular weight was estimated at 90,000. Activity was optimal at pH 5.7 and in the presence of 0.01 to 0.03 M calcium and magnesium cations. The enzyme was stable at temperatures of 4 and 37 degrees C for at least 24 h but was inactivated within 10 min at temperatures of 50 and 65 degrees C. The enzyme hydrolyzed 40% of the sialic acid in bovine submaxillary mucin, but was inactive on sialyl-lactose, porcine submaxillary mucin, oligosaccharides derived from porcine mucin, or human orosomucoid. The Km value for this enzyme with bovine submaxillary mucin as substrate was in the order of 3.6 x 10(-4) M.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D., MORGAN W. T. J., WATKINS W. M. The isolation and properties of the human blood-group a substance. Biochem J. 1950 Apr;46(4):426–438. doi: 10.1042/bj0460426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AYOUB E. M., WANNAMAKER L. W. A FACTOR OTHER THAN STREPTOCOCCAL NICOTINAMIDE ADENINE DINUCLEOTIDASE WHICH COMBINES WITH ANTIBODY TO THIS ENZYME: ITS PRODUCTION AND EFFECT ON ANTIBODY DETERMINATIONS. J Immunol. 1963 May;90:793–803. [PubMed] [Google Scholar]
- Arden S. B., Chang W. H., Barksdale L. Distribution of neuraminidase and n-acetylneuraminate lyase activities among corynebacteria, mycobacteria, and nocardias. J Bacteriol. 1972 Dec;112(3):1206–1212. doi: 10.1128/jb.112.3.1206-1212.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig M. M., Aminoff D. Glycoproteins and blood group activity. I. Oligosaccharides of serologically inactive hog submaxillary glycoproteins. J Biol Chem. 1972 Oct 10;247(19):6111–6118. [PubMed] [Google Scholar]
- Buscher H. P., Casals-Stenzel J., Schaufer R. New sialic acids. Identification of N-glycoloyl-O-acetylneuraminic acids and N-acetyl-O-glycoloylneuraminic acids by improved methods for detection of N-acyl and O-acyl groups and by gas-liquid chromatography. Eur J Biochem. 1974 Dec 16;50(1):71–82. doi: 10.1111/j.1432-1033.1974.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Drzeniek R. Substrate specificity of neuraminidases. Histochem J. 1973 May;5(3):271–290. doi: 10.1007/BF01004994. [DOI] [PubMed] [Google Scholar]
- Ferrieri P. Acute post-streptococcal glomerulonephritis and its relationship to the epidemiology of streptococcal infections. Minn Med. 1975 Aug;58(8):598–602. [PubMed] [Google Scholar]
- Fillit H. M., Read S. E., Sherman R. L., Zabriskie J. B., Van de Rijn I. Cellular reactivity to altered glomerular basement membrane in glomerulonephritis. N Engl J Med. 1978 Apr 20;298(16):861–868. doi: 10.1056/NEJM197804202981601. [DOI] [PubMed] [Google Scholar]
- Griswold W. R., McIntosh J. R., Weil R., 3rd, McIntosh R. M. Neuraminidase treated homologous IgG and immune deposit rental disease in inbred rats. Proc Soc Exp Biol Med. 1975 Apr;148(4):1018–1024. doi: 10.3181/00379727-148-38680. [DOI] [PubMed] [Google Scholar]
- Hayano S., Tanaka A., Okuyama Y. Distribution and serological specificity of sialidase produced by various groups of streptococci. J Bacteriol. 1969 Oct;100(1):354–357. doi: 10.1128/jb.100.1.354-357.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano S., Tanaka A. Sialidase-like enzymes produced by group A, B, C, G, and L streptococci and by Streptococcus sanguis. J Bacteriol. 1969 Mar;97(3):1328–1333. doi: 10.1128/jb.97.3.1328-1333.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano S., Tanaka A. Streptococcal sialidase. I. Isolation and properties of sialidase produced by group K Streptococcus. J Bacteriol. 1967 Jun;93(6):1753–1757. doi: 10.1128/jb.93.6.1753-1757.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara T., Terao T., Shioiri-Nakano K., Osawa T. Purification and properties of a neuraminidase from Streptococcus K 6646. Arch Biochem Biophys. 1974 Oct;164(2):575–582. doi: 10.1016/0003-9861(74)90069-1. [DOI] [PubMed] [Google Scholar]
- MOODY M. D., PADULA J., LIZANA D., HALL C. T. EPIDEMIOLOGIC CHARACTERIZATION OF GROUP A STREPTOCOCCI BY T-AGGLUTINATION AND M-PRECIPITATION TESTS IN THE PUBLIC HEALTH LABORATORY. Health Lab Sci. 1965 Jul;2:149–162. [PubMed] [Google Scholar]
- McIntosh R. M., Kaufman D. B., McIntosh J. R., Griswold W. Glomerular lesions produced by autologous serum and autologous IgG modified by treatment with a culture of a -haemolytic streptooccus. J Med Microbiol. 1972 Feb;5(1):1–7. doi: 10.1099/00222615-5-1-1. [DOI] [PubMed] [Google Scholar]
- McIntosh R. M., Kulvinskas C., Kaufman D. B. Alteration of the chemical composition of human immunoglobulin G by Streptococcus pyogenes. J Med Microbiol. 1971 Nov;4(4):535–538. doi: 10.1099/00222615-4-4-535. [DOI] [PubMed] [Google Scholar]
- McIntosh R. M., Kulvinskas C., Kaufman D. B. Cryoglobulins. II. The biological and chemical properties of cryoproteins in acute post-streptococcal glomerulonephritis. Int Arch Allergy Appl Immunol. 1971;41(5):700–715. doi: 10.1159/000230562. [DOI] [PubMed] [Google Scholar]
- Milligan T. W., Straus D. C., Mattingly S. J. Extracellular neuraminidase production by group B streptococci. Infect Immun. 1977 Oct;18(1):189–195. doi: 10.1128/iai.18.1.189-195.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula J. F., Facklam R. R., Moody M. D. Effect of incubation temperature on T-agglutination typing of Streptococcus pyogenes. Appl Microbiol. 1969 Jun;17(6):878–880. doi: 10.1128/am.17.6.878-880.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter J. K., Hayashi J. A., Bahn A. N. Extracellular streptococcal neuraminidase. J Bacteriol. 1968 Apr;95(4):1491–1492. doi: 10.1128/jb.95.4.1491-1492.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- WANNAMAKER L. W. Electrophoretic studies of the extracellular products of group A Streptococci. J Exp Med. 1958 Jun 1;107(6):783–795. doi: 10.1084/jem.107.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]



