Abstract
BACKGROUND
KAE609 (cipargamin; formerly NITD609, Novartis Institute for Tropical Diseases) is a new synthetic antimalarial spiroindolone analogue with potent, dose-dependent antimalarial activity against asexual and sexual stages of Plasmodium falciparum.
METHODS
We conducted a phase 2, open-label study at three centers in Thailand to assess the antimalarial efficacy, safety, and adverse-event profile of KAE609, at a dose of 30 mg per day for 3 days, in two sequential cohorts of adults with uncomplicated P. vivax malaria (10 patients) or P. falciparum malaria (11). The primary end point was the parasite clearance time.
RESULTS
The median parasite clearance time was 12 hours in each cohort (interquartile range, 8 to 16 hours in patients with P. vivax malaria and 10 to 16 hours in those with P. falciparum malaria). The median half-lives for parasite clearance were 0.95 hours (range, 0.68 to 2.01; interquartile range, 0.85 to 1.14) in the patients with P. vivax malaria and 0.90 hours (range, 0.68 to 1.64; interquartile range, 0.78 to 1.07) in those with P. falciparum malaria. By comparison, only 19 of 5076 patients with P. falciparum malaria (<1%) who were treated with oral artesunate in Southeast Asia had a parasite clearance half-life of less than 1 hour. Adverse events were reported in 14 patients (67%), with nausea being the most common. The adverse events were generally mild and did not lead to any discontinuations of the drug. The mean terminal half-life for the elimination of KAE609 was 20.8 hours (range, 11.3 to 37.6), supporting a once-daily oral dosing regimen.
CONCLUSIONS
KAE609, at dose of 30 mg daily for 3 days, cleared parasitemia rapidly in adults with uncomplicated P. vivax or P. falciparum malaria. (Funded by Novartis and others; ClinicalTrials.gov number, NCT01524341.)
The treatment of Plasmodium falciparum malaria has changed considerably over the past decade, with a global change to the rapidly and reliably effective artemisinin-based combination therapies. The deployment of such therapies and insecticide-treated bed nets have been the two main contributors to the substantial recent declines in morbidity and mortality from malaria.1 Unfortunately, artemisinin resistance has emerged in Southeast Asia, threatening these gains2-5 and potentially derailing current control efforts. New medicines are needed,6 but it has proved difficult to develop effective and safe antibacterial and antiparasitic drugs.
The new spiroindolone class of antimalarial agents was identified a few years ago by means of high-throughput phenotypic screening.7 The original hit was subsequently enhanced chemically to improve potency and oral bioavailability. Oral dosing of the resulting synthetic spirotetrahydro–β-carboline candidate KAE609 (cipargamin; formerly NITD609, Novartis Institute for Tropical Diseases) provided a single-dose cure in a P. berghei rodent model of blood-stage malaria.8 Spiroindolones inhibit PfATP4, a parasite plasma membrane Na+-ATPase that regulates sodium and osmotic homeostasis.9 In vitro, KAE609 has potent activity against both the asexual and sexual stages of the malaria parasite.10 In healthy adult volunteers, no safety concerns regarding the use of KAE609 were identified at single doses of up to 300 mg and at multiple doses of up to 150 mg daily for 3 days (Leong J, Novartis Institute for Tropical Diseases: personal communication). A 3-day regimen of 30 mg of KAE609 daily resulted in an exposure level that was predicted to be efficacious in the treatment of malaria and was therefore selected for phase 2 clinical trials.
METHODS
STUDY DESIGN AND OVERSIGHT
The primary objective of this phase 2, open-label study was to assess the initial antimalarial efficacy (parasite clearance), safety, and adverse-event profile of KAE609 at a dose of 30 mg per day for 3 days in adults with uncomplicated P. falciparum or P. vivax malaria.
This study was conducted in three locations in Thailand: the Hospital for Tropical Diseases, Bangkok, and the Mae Sot General Hospital and the Shoklo Malaria Research Unit, both located close to the northwestern border. The study was approved by the ethics committees of the Faculty of Tropical Medicine, Mahidol University, the Thai Ministry of Public Health, and the Institute for the Development of Human Research Protection and by the Oxford Tropical Research Ethics Committee.
All the authors vouch for the completeness of the data and analyses presented and for the fidelity of the study to the protocol. The first author wrote the first and subsequent drafts of the manuscript, with medical writing support from Seren Communications, paid for by Novartis, and with contributions from all the authors.
PATIENTS
Eligible patients were men and women, 20 to 60 years of age, who had a body weight of 40 to 90 kg, fever or a history of fever, and microscopy-confirmed P. vivax or P. falciparum monoinfection with asexual-stage parasite counts between 5000 and 50,000 per cubic millimeter of blood. Women with childbearing potential were not eligible. Exclusion criteria were severe malaria (according to 2010 guidelines from the World Health Organization),11 infection by multiple species, a hemoglobin level of less than 10.0 g per deciliter, schizontemia, severe vomiting, receipt of any antimalarial agent within the previous 14 days or receipt of another investigational drug within the previous 30 days, a history of clinically significant electrocardiographic abnormalities (corrected QT interval [Bazett’s formula], >450 msec in men and >470 msec in women), a history of cancer, chronic liver disease, or severe malnutrition. All the participants provided written informed consent.
TREATMENT AND PROCEDURES
Patients were admitted to an inpatient facility for close monitoring. Study medication (10 mg per capsule of KAE609, for a total dose of 30 mg) was administered orally (without regard to timing of food), with water once daily for 3 days. The dose was repeated if the patient vomited within 45 minutes. Standard antimalarial treatment (artesunate plus mefloquine for P. falciparum malaria and chloroquine plus primaquine for P. vivax malaria) was started on day 5 (or earlier in the event of treatment failure). Acetaminophen (known as paracetamol outside the United States) was administered as required for fever control. Patients were asked to return at any time after discharge on day 6 if they felt unwell.
EFFICACY AND SAFETY ASSESSMENTS
Clinical observations, vital signs, and symptoms were recorded, and thick and thin blood smears were obtained every 4 hours for 24 hours and then every 6 hours until there were two consecutive negative readings. Clearance of fever was defined as two or more consecutive normal (<37.5°C) measurements of body temperature. Thin blood smears were stained by Giemsa or reverse Field’s stains, and parasite counts were reported per 1000 red cells. Thick films were stained with Giemsa or Field’s stains and parasite counts were reported per 200 white cells (or if the count was <10 parasites, counting was continued for up to 500 white cells).
The primary efficacy measure was the parasite clearance time, defined as the interval from the start of treatment to the first of two or more consecutive blood slides that were negative for the parasite. The rates and half-lives of parasite clearance were estimated with the use of the Worldwide Antimalarial Resistance Network parasite clearance estimator.12 In patients with P. falciparum malaria, the gene encoding the P. falciparum kelch protein was amplified and sequenced.5 Safety was evaluated by means of daily assessments of symptoms and clinical examination and by hematologic, blood chemical, and urine testing at screening, on days 2, 3, and 5, and at study completion. Electrocardiograms were obtained at screening, at 3 to 4 hours after the administration of the dose on day 1, before the dose was administered and 3 to 4 hours afterward on days 2 and 3, and at study completion. Adverse events were recorded from the time of consent until study completion (for all adverse events) and until 30 days after study completion (for serious adverse events). The National Cancer Institute Common Terminology Criteria for Adverse Events were used for grading the severity of adverse events.
PHARMACOKINETICS
Blood samples for the measurement of plasma levels of KAE609 were collected in EDTA tubes before dosing (0 hours), at 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 hours after dose 1, and at 0.5, 1, 2, 3, 4, 6, 8, 12, 24, 36, 48, 72, 96, 144, and 192 hours after dose 3 (day 3). Urine volumes were recorded and samples collected on days 1 and 3 before administration of the dose (0 hours), between 0 and 12 hours after the dose was administered, and between 12 and 24 hours after the dose was administered. All samples were stored at −70°C until analysis. The parent-drug concentrations in plasma and urine were analyzed with the use of a validated method of high-performance liquid chromatography with tandem mass spectrometry with a lower limit of quantification of 1 ng per milliliter. Metabolites were not quantified because no metabolites of KAE609 with anti malarial activity have been identified to date. Full details regarding study conduct are provided in the study protocol, available with the full text of this article at NEJM.org.
STATISTICAL ANALYSIS
To determine the sample size, the probability of meeting the primary end point (median parasite clearance time, <96 hours) was calculated across a range of reasonable coefficients of variation (25 to 40%), assuming a true parasite clearance time of less than 80 hours.13,14 We calculated that a cohort of 10 patients who could be evaluated would provide the study with more than 88% power to meet the primary end point. Enrollment of two sequential cohorts of 10 adults with uncomplicated malaria was therefore planned: first a cohort of patients with uncomplicated P. vivax malaria, and then a cohort with uncomplicated P. falciparum malaria.
For the primary efficacy and pharmacodynamic analyses, time-to-event end points were analyzed with the use of the Kaplan–Meier method. We assessed the times to 50% and 99% clearance of asexual parasitemia.15 Parasite clearance half-lives were derived from the log-linear decline in parasite densities after standardized curve fitting.12 Unlike parasite clearance time, the parasite clearance half-life is independent of initial parasitemia and is a better measure for comparison between antimalarial agents.12,15 These parasite-clearance data were compared with historical data (obtained from 2001 through 2013) from 3202 patients with hyperparasitemia,3 an additional 206 patients with lower parasite counts at admission (i.e., similar to the patients in our study) who were studied in the northwest-border area of Thailand, and 1668 patients with P. falciparum malaria who were studied at other locations in Southeast Asia and who were treated with oral artesunate and evaluated similarly. KAE609 pharmacokinetic variables were determined by means of noncompartmental methods with the use of Phoenix WinNonlin, version 6.2 (Certara).
RESULTS
STUDY PATIENTS
This open-label study was conducted from January 10 to June 22, 2012. A total of 21 patients were enrolled: 10 patients with P. vivax malaria and 11 with P. falciparum malaria (Table S1 in the Supplementary Appendix, available at NEJM.org). Baseline parasite densities ranged from 6816 to 53,082 P. vivax parasites per cubic millimeter and from 4139 to 63,745 P. falciparum parasites per cubic millimeter. All 21 patients were included in the efficacy and safety analyses. For the pharmacokinetic analyses, 2 patients were excluded: 1 patient in the P. vivax cohort, who vomited the drug, and 1 in the P. falciparum cohort, who withdrew consent after receiving one dose of KAE609.
RESOLUTION OF FEVER AND PARASITE CLEARANCE
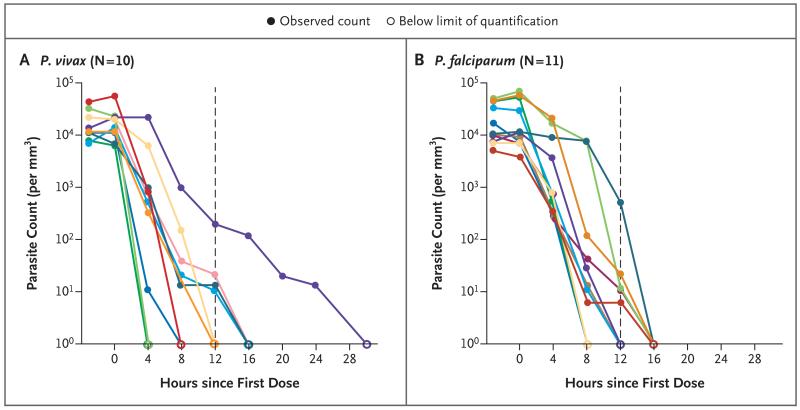
Clinical and parasitologic responses are presented in Table 1. The median times for the resolution of fever were 8 hours in the P. vivax cohort and 12 hours in the P. falciparum cohort. The median parasite clearance time was 12 hours in each cohort (interquartile range, 8 to 16 hours in the P. vivax cohort and 10 to 16 hours in the P. falciparum cohort) (Table 1 and Fig. 1). The median parasite clearance half-life was 0.95 hours (interquartile range, 0.85 to 1.14; range, 0.68 to 2.01) in the P. vivax cohort and 0.90 hours (interquartile range, 0.78 to 1.07; range, 0.68 to 1.64) in the P. falciparum cohort (Fig. 1).
Table 1. Parasite Clearance in Cohorts of Patients with Plasmodium vivax or P. falciparum Infection.
| End Point |
P. vivax Cohort
(N = 10) |
P. falciparum Cohort
(N = 11) |
|---|---|---|
| hours | ||
| Time to clearance of asexual parasitemia | ||
| 50% clearance | 8 | 12 |
| 99% clearance | 12 | 16 |
| 100% clearance | 30 | 16 |
| Time to parasite clearance | ||
| Median | 12 | 12 |
| Interquartile range | 8–16 | 10–16 |
| Parasite clearance half-life | ||
| Median* | 0.95 | 0.90 |
| Interquartile range | 0.85–1.14 | 0.78–1.07 |
| Range | 0.68–2.01 | 0.68–1.64 |
The median parasite clearance half-life was estimated in a separate analysis with the use of the Worldwide Antimalarial Resistance Network parasite clearance estimator.12
Figure 1. Parasite-Clearance Profiles in Individual Patients with Plasmodium vivax or P. falciparum Malaria.
In each panel, the dashed vertical line indicates the median parasite clearance time, and the colored lines represent individual patients.
Five patients with P. falciparum infection had kelch protein mutations that were putatively associated with artemisinin resistance5 (N537I and G538V in one patient each and C580Y in three patients); one of these patients withdrew from the study. The other four patients had parasite clearance that was as rapid as that in the six patients without mutations in the kelch protein (Fig. S1 in the Supplementary Appendix). Gametocytemia was detected in five patients with P. vivax malaria; gametocyte clearance was observed in each of these patients by 8 hours. Gametocytemia was not detected before or after treatment in any patient in the P. falciparum cohort.
SAFETY
No adverse event led to the discontinuation of treatment (Table 2). Two serious adverse events (fever in two patients) were reported (Table 2), and both occurred after parasite clearance and were considered by the site investigator to be unrelated to the study medication (one patient received a concomitant diagnosis of Salmonella typhi infection). Vomiting (also considered by the investigator to be unrelated to the study drug) occurred in three patients (two patients with P. vivax malaria and one with P. falciparum malaria). One of the patients with P. vivax malaria had grade 2 vomiting on day 4 that lasted 48 hours, accompanied by grade 2 nausea (from day 3) that lasted 105 hours, and was treated successfully with dimenhydrinate. The other cases of vomiting (grade 1 in two patients) occurred on days 1 and 2, in the two respective patients, and resolved after 10 to 15 minutes without treatment.
Table 2. Adverse Events.
| Event |
P. vivax Cohort
(N = 10) |
P. falciparum Cohort
(N = 11) |
Total
(N = 21) |
|---|---|---|---|
| number (percent) | |||
| Any adverse event | 8 (80) | 6 (55) | 14 (67) |
| Drug-related adverse event* | 2 (20) | 1 (9) | 3 (14) |
| Treatment discontinuation due to adverse event | 0 | 0 | 0 |
| Serious adverse event† | 1 (10) | 1 (9) | 2 (10) |
| Adverse event of grade ≥2‡ | |||
| Increased alanine aminotransferase level | 1 (10) | 0 | 1 (5) |
| Anorexia | 0 | 1 (9) | 1 (5) |
| Chronic obstructive pulmonary disease | 1 (10) | 0 | 1 (5) |
| Nausea | 1 (10) | 0 | 1 (5) |
| Typhoid fever | 1 (10) | 0 | 1 (5) |
| Vomiting | 1 (10) | 0 | 1 (5) |
Adverse events that were suspected to be related to the study drug were reported in two patients in the P. vivax cohort (increased alanine aminotransferase and aspartate aminotransferase levels in both) and in one patient in the P. falciparum cohort (anorexia, pain in neck muscles, and abdominal pain).
Fever of grade 1 occurred in a 21-year-old patient with P. falciparum malaria (parasite clearance time in this patient, 8 hours), which peaked at a temperature of 39.7°C before resolving on day 3 and which was attributed to malaria. Fever of grade 2 occurred on day 5 in a 35-year-old man with P. vivax malaria in whom blood cultures subsequently grew Salmonella typhi (parasite clearance time in this patient, 12 hours).
Adverse events are listed according to the preferred term in the National Cancer Institute Common Terminology Criteria for Adverse Events. All the events were of grade 2, except for a grade 3 increase in the alanine aminotransferase level in one patient. Patients with multiple occurrences of an adverse event were counted only once in the adverse-event category. Patients with multiple adverse events within a body system were counted only once in the row for any adverse event.
Seven adverse events were suspected to be related to the study drug: increased levels of alanine aminotransferase and aspartate amino-transferase occurred in two patients in the P. vivax cohort (respective peak values: 241 IU per liter and 106 IU per liter on day 9 in one patient, and 78 IU per liter and 63 IU per liter on day 6 in the other [reference ranges for both amino-transferases, 7 to 40 IU per liter]). In addition, one patient with P. falciparum malaria had increased levels of alanine aminotransferase and aspartate aminotransferase (respective peak values: 192 IU per liter and 121 IU per liter on day 6), with an elevated alkaline phosphatase level (peak value: 227 IU per liter [reference range, 40 to 129] on day 6), but these elevations were not considered to be related to the study drug because the values were already slightly elevated at screening (alanine aminotransferase, 54 IU per liter; aspartate aminotransferase, 79 IU per liter; and alkaline phosphatase, 172 IU per liter). No clinically relevant increases in the total bilirubin level were observed in these patients. One patient with P. falciparum malaria had anorexia, muscular neck pain, and abdominal pain.
Grade 1 hyperglycemia with glycosuria occurred in one patient with P. vivax malaria on day 2 and resolved on day 6 without treatment. There was no relationship between the plasma concentrations of KAE609 and changes in the QT interval as assessed electrocardiographically; the median difference in the corrected QT interval (Bazett’s formula) between the value before the administration of the dose and 4 hours afterward was −4 msec.
PHARMACOKINETICS
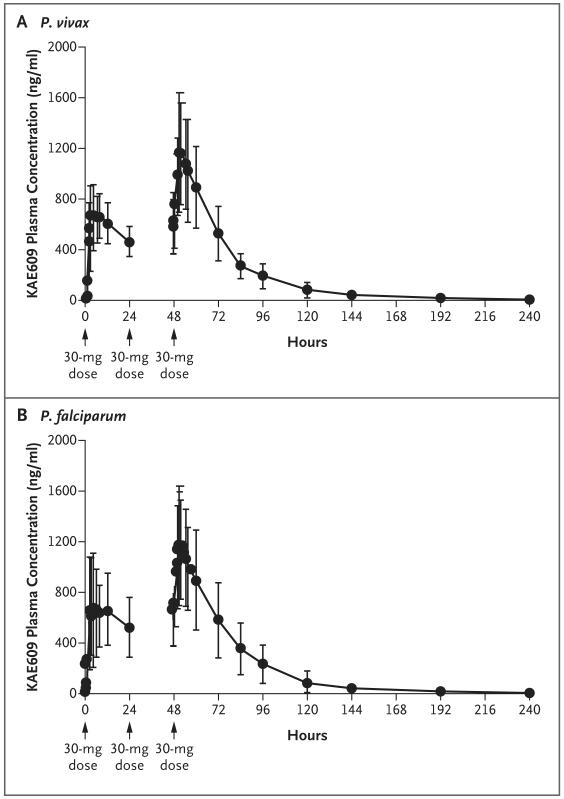
The maximal plasma concentrations (Cmax) of KAE609 were reached in a median of approximately 3 hours (Table 3 and Fig. 2), with moderate variation among patients with regard to absorption (coefficient of variation for the Cmax and the area under the plasma concentration–time curve from time zero to 24 hours [AUC0–24] in the two cohorts, <40%). The mean plasma terminal half-life for the elimination of KAE609 after the final dose was 20.8 hours (range, 11.3 to 37.6 hours in patients with P. vivax malaria and 13.7 to 25.6 hours in those with P. falciparum malaria). There were no substantial differences in the estimates of the pharmacokinetic variables between the two patient cohorts or between the first and last doses. Accumulation ratios, calculated as the AUC0–24 on day 3 divided by the AUC0–24 on day 1, were as expected from the terminal half-life for the elimination of KAE609 (mean ratio, 1.56 in the P. vivax cohort and 1.60 in the P. falciparum cohort). Less than 0.02% of the administered dose was excreted unchanged in urine over a period of 24 hours. Because the therapeutic responses were uniform, no relationship between drug exposure and parasitologic outcome was established (Fig. S1 in the Supplementary Appendix).
Table 3. Pharmacokinetic Variables of KAE609 on Days 1 and 3.*.
| Variable |
P. vivax Cohort (N = 9) |
P. falciparum Cohort (n = 10) |
|---|---|---|
| Day 1 | ||
| AUC0–24† | ||
| Mean (μg × hr/ml) | 13.1±4.2 | 15.5±4.5 |
| Coefficient of variation (%) | 31.9 | 29.0 |
| AUClast | ||
| Mean (μg × hr/ml) | 13.1±3.0 | 14.1±5.0 |
| Coefficient of variation (%) | 23.0 | 35.8 |
| Cmax | ||
| Mean (ng/ml) | 800±193 | 911±349 |
| Coefficient of variation (%) | 24.1 | 38.4 |
| Tmax (hr) | ||
| Median | 4.0 | 4.0 |
| Range | 2.0–12.1 | 2.0–12.2 |
| Day 3 | ||
| AUC0–24‡ | ||
| Mean (μg × hr/ml) | 20.5±6.6 | 22.4±7.5 |
| Coefficient of variation (%) | 32.2 | 33.3 |
| AUClast‡ | ||
| Mean (μg × hr/ml) | 35.0±12.4 | 39.7±17.1 |
| Coefficient of variation (%) | 35.4 | 43.0 |
| Cmax | ||
| Mean (ng/ml) | 1360±451 | 1340±374 |
| Coefficient of variation (%) | 33.3 | 28.0 |
| Tmax (hr) | ||
| Median | 3.0 | 2.5 |
| Range | 2.0–12.0 | 1.0–12.3 |
| Terminal elimination half-life | ||
| Mean (hr) | 23.1±8.0 | 18.5±4.7 |
| Coefficient of variation (%) | 34.5 | 25.5 |
| CL/F | ||
| Mean (ml/hr/kg) | 17.9±6.0 | 17.3±6.6 |
| Coefficient of variation (%) | 33.5 | 38.2 |
| Vz/F | ||
| Mean (ml/kg) | 576±208 | 449±183 |
| Coefficient of variation (%) | 36.1 | 40.6 |
| Accumulation ratioठ| ||
| Mean | 1.6±0.3 | 1.6±0.4 |
| Coefficient of variation (%) | 20.0 | 27.8 |
Plus–minus values are means ±SD. Two patients were excluded from the pharmacokinetic analyses: one patient in the P. vivax cohort, who vomited the drug, and one in the P. falciparum cohort, who withdrew consent after receiving one dose of KAE609. AUC0–24 denotes area under the plasma concentration–time curve from time zero to 24 hours, AUClast area under the plasma concentration–time curve from time zero to the time of the last quantifiable concentration, CL/F apparent systemic (i.e., total body) clearance from plasma after oral administration of the drug, Cmax observed maximum plasma concentration after drug administration, Tmax time to reach the maximum concentration after drug administration, and Vz/F apparent volume of distribution during the terminal elimination phase after drug administration.
Data were missing for four patients in the P. vivax cohort and for three in the P. falciparum cohort.
Data were missing for one patient in the P. falciparum cohort.
The accumulation ratio was calculated as the AUC0–24 on day 3 divided by the AUC0–24 on day 1.
Figure 2. Mean Plasma Concentration–Time Profiles of KAE609.
Patients with Plasmodium vivax or P. falciparum malaria received KAE609 at an oral dose of 30 mg once daily for 3 days. The cumulative time after the first dose of KAE609 is presented. The mean plasma concentration–time profiles after the administration of the drug across all days administered are shown; I bars indicate ±1 SD.
DISCUSSION
The spiroindolone antimalarial agent KAE609 showed rapid efficacy in the treatment of falciparum and vivax malaria in this exploratory phase 2 study conducted in Thailand. To date, the most rapidly acting antimalarial agents identified have been the artemisinins; however, these preliminary data suggest that KAE609 may effect an even faster parasite clearance, including in patients with artemisinin-resistant P. falciparum infection. The median parasite clearance half-life after the administration of KAE609 in the P. falciparum cohort was 0.90 hours (interquartile range, 0.78 to 1.07). By comparison, only 19 of 5076 patients (<1%) with falciparum malaria in Southeast Asia who had been treated with oral artesunate and evaluated similarly had a parasite clearance half-life of less than 1 hour. This finding supports in vitro observations that KAE609 kills ring-stage parasites8 and thus provides many of the pharmacodynamic benefits provided by the artemisinin derivatives, particularly the rapidity of symptom resolution. KAE609 also has P. falciparum gametocytocidal activity in vitro10 and thus may have transmission-blocking properties. Activity against P. vivax was also rapid (median parasite clearance half-life, 0.95 hours; interquartile range, 0.85 to 1.14).
This study was too small for us to derive valid conclusions regarding safety and efficacy. Nausea was the most common reported adverse effect, but its severity was assessed as grade 2 in only one patient. Three patients vomited, which is common in acute malaria. Elevated levels of hepatic enzymes occurred in three patients. Elevated aminotransferase levels may also occur in patients with acute malaria, so much larger studies will be necessary to assess fully the safety and adverse-event profile of this new antimalarial agent. The reliable absorption and mean terminal half-life of 20.8 hours for the elimination of KAE609 supports a once-daily dosing regimen. Exposure (as assessed by means of the Cmax and the area under the curve) was two to three times as high in the study patients as in healthy persons at the same dose.11 The mechanisms underlying the differences in exposure are being investigated. Spiroindolone-resistant P. falciparum isolates bearing mutations in PfATP4 can be selected in vitro,8 and it will be essential to combine KAE609 with a reliably effective second drug to provide protection against the emergence of drug resistance.
In conclusion, the spiroindolone KAE609, a first-in-class agent with a new mechanism of action based on the inhibition of PfATP4, was shown to have rapid antimalarial activity.
Supplementary Material
Acknowledgments
Supported by Novartis. The studies in Bangkok and the Shoklo Malaria Research Unit were part of the Wellcome Trust–Mahidol University–Oxford Tropical Medicine Research Programme, which is supported by the Wellcome Trust of Great Britain. The Wellcome Trust and Medicines for Malaria Venture provided support to the Novartis Institute for Tropical Diseases drug discovery program and for the early clinical development of KAE609.
We thank Kirstin Stricker, Ph.D., C.M.P.P., of Novartis, for critical review of an earlier version of the manuscript; and Rachel Mason, C.M.P.P., of Seren Communications, for medical writing support.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Malaria: fact sheet no. 94. World Health Organization; Geneva: http://www.who.int/mediacentre/factsheets/fs094/en/# [Google Scholar]
- 2.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. doi: 10.1056/NEJMoa0808859. [Erratum, N Engl J Med 2009; 361:1714.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phyo AP, Nkhoma S, Stepniewska K, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–6. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–20. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 5.Ariey F, Witkowski B, Amaratunga C, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–5. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells TNC, Alonso PL, Gutteridge WE. New medicines to improve control and contribute to the eradication of malaria. Nat Rev Drug Discov. 2009;8:879–91. doi: 10.1038/nrd2972. [DOI] [PubMed] [Google Scholar]
- 7.Yeung BK, Zou B, Rottmann M, et al. Spirotetrahydro beta-carbolines (spiroindolones): a new class of potent and orally efficacious compounds for the treatment of malaria. J Med Chem. 2010;53:5155–64. doi: 10.1021/jm100410f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rottmann M, McNamara C, Yeung BKS, et al. Spiroindolones, a potent compound class for the treatment of malaria. Science. 2010;329:1175–80. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spillman NJ, Allen RJW, McNamara CW, et al. Na(+) regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe. 2013;13:227–37. doi: 10.1016/j.chom.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Pelt-Koops JC, Pett HE, Graumans W, et al. The spiroindolone drug candidate NITD609 potently inhibits gametocyto-genesis and blocks Plasmodium falciparum transmission to anopheles mosquito vector. Antimicrob Agents Chemother. 2012;56:3544–8. doi: 10.1128/AAC.06377-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidelines for the treatment of malaria. 2nd ed. World Health Organization; Geneva: 2010. http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf [PubMed] [Google Scholar]
- 12.Flegg JA, Guerin PJ, White NJ, Stepniewska K. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J. 2011;10:339. doi: 10.1186/1475-2875-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White NJ. Antimalarial pharmacokinetics and treatment regimens. Br J Clin Pharmacol. 1992;34:1–10. doi: 10.1111/j.1365-2125.1992.tb04100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pukrittayakamee S, Chantra A, Simpson JA, et al. Therapeutic responses to different antimalarial drugs in vivax malaria. Anti-microb Agents Chemother. 2000;44:1680–5. doi: 10.1128/aac.44.6.1680-1685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White NJ. The parasite clearance curve. Malar J. 2011;10:278. doi: 10.1186/1475-2875-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.